Abstract
The NMDA receptor is an important subtype glutamate receptor that acts as a nonselective cation channel highly permeable to both calcium (Ca2+) and sodium (Na+). The activation of NMDA receptors produces prolonged increases of intracellular Ca2+ concentration ([Ca2+]i) and thereby triggers downstream signaling pathways involved in the regulation of many physiological and pathophysiological processes. Previous studies have focused on how Ca2+ or Na+ affects NMDA receptor activity in isolation. Specifically, [Ca2+]i increase may downregulate NMDA channels and thus is considered an important negative feedback mechanism controlling NMDA receptor activity, whereas an increase in intracellular Na+ concentration ([Na+]i) may upregulate NMDA channel activity. Thus so that the activity-dependent regulation of NMDA receptors and neuroplasticity may be further understood, a critical question that has to be answered is how an individual NMDA receptor may be regulated when both of these ionic species flow into neurons during the same time period via neighboring activated NMDA receptors. Here we report that the gating of a NMDA channel is regulated by the activation of remote NMDA receptors via a functional Na+-Ca2+ interaction and that during the activation of NMDA receptors Na+ influx potentiates Ca2+ influx on one hand and overcomes Ca2+-induced inhibition of NMDA channel gating on the other hand. Furthermore, we have identified that a critical increase (5 ± 1 mm) in [Na+]i is required to mask the effects of Ca2+ on NMDA channel gating in cultured hippocampal neurons. Thus cross talk between NMDA receptors mediated by a functional Na+-Ca2+ interaction is a novel mechanism regulating NMDA receptor activity.
Keywords: NMDA channel gating, intracellular sodium and calcium, single-channel activity, excitability, toxicity, synaptic plasticity
Introduction
The NMDA type glutamate receptor/channel plays important roles in the regulation of fast excitatory synaptic transmission and activity-dependent neuroplasticity (Dingledine et al., 1999). NMDA channels are highly permeable to both sodium (Na+) and calcium (Ca2+) (Mayer and Westbrook, 1987; McBain and Mayer, 1994; Dingledine et al., 1999). The activation of NMDA receptors may produce a prolonged increase in intracellular Ca2+ concentration ([Ca2+]i) and trigger downstream signaling pathways (Mody and MacDonald, 1995; Dingledine et al., 1999). It has been demonstrated that an increase in [Ca2+]i may downregulate NMDA channels in central neurons cultured from embryos and acutely isolated from adult animals. The Ca2+-induced downregulation of NMDA channel activity is considered an important negative feedback mechanism to control NMDA receptor activity (Mayer and Westbrook, 1987; McBain and Mayer, 1994; Kyrozis et al., 1996; Dingledine et al., 1999).
Na+ is the major cation in the extracellular space and can enter cells via a variety of routes, including permeation through ligand-gated (e.g., glutamate) and voltage-gated cation channels and uptake through membrane exchangers as well as through gradient-driven cotransport (Nicholls and Attwell, 1990; Hille, 1992; Rose and Ransom, 1997). In the nervous system Na+ influx regulates neuronal excitability and is responsible for the initiation and propagation of action potentials (Taylor and Dudek, 1982; Hille, 1992). Studies using fluorescence ratio imaging of Na+ indicator dye have reported that electrical activity, particularly bursting activity, dramatically increases [Na+]i in neurons (Callaway and Ross, 1997; Rose and Ransom, 1997; Rose and Konnerth, 2001). Rose and Konnerth (2001) demonstrated that short bursts of synaptic stimulation may cause an increase in [Na+] of up to 40 mm in postsynaptic spines and their adjacent dendrites. During tetanic stimulation that induces synaptic longterm potentiation, [Na+] in synaptically active spines may reach 100 mm (Rose and Konnerth, 2001). They also demonstrated that the [Na+] increases in spines and dendrites induced by synaptic stimulation are mediated mainly by Na+ entry through NMDA channels and are concurrent to synaptic potentials and back-propagating action potentials (Rose and Konnerth, 2001).
Intracellular Na+ has been shown to be an upregulator of NMDA receptors, such that raising [Na+]i or activating Na+-permeable channels may enhance NMDA receptor-mediated currents (Yu and Salter, 1998). Thus the activity of a NMDA receptor may be regulated by other NMDA receptors via the actions of Ca2+ and/or Na+ entering neurons. Because intracellular Na+ and Ca2+ act in an opposing manner on NMDA channel activity and all previous studies have focused only on the effects of either Ca2+ or Na+, a compelling question is how the activity of an individual NMDA channel may be regulated when both ionic species flow into neurons during the same time period through activated remote NMDA receptors. To address this issue, we investigated the regulation of NMDA channel gating by the activation of remote NMDA receptors in cell-attached patches and consequently revealed a novel functional relationship of Na+ and Ca2+ influxes in the regulation of NMDA receptors, which may be important for the understanding of mechanisms underlying activity-dependent neuroplasticity.
Materials and Methods
Primary neuron culture and NMDA single-channel current recordings. Primary cultures from the hippocampus were prepared from fetal Wistar rats (embryonic day 17-19) as described previously (Lei et al., 2002). In brief, hippocampal tissue was dissociated mechanically by trituration and plated onto 35 mm collagen-coated culture dishes at a density of <1 × 106 cells/ml. Hippocampal neurons were used for electrophysiological recordings 8-14 d after plating.
NMDA receptor-mediated single-channel currents were recorded in the cell-attached configuration. Hippocampal cultures were bathed in a standard extracellular solution containing the following (in mm): 100 Na2SO4, 10 Cs2SO4, 1.2 or 4.8 CaCl2, 25 HEPES, 32 glucose, 0.001 TTX, 0.003 glycine, pH 7.35, and 310-320 mOsm. Free Ca2+ concentration of the extracellular solution was at either 0.3 or 1.2 mm as confirmed by measurement with a Ca2+-selective electrode (Thermo Electron, Beverly, MA). The resting potential of neurons bathed with extracellular solutions of these two different Ca2+ concentrations was 60 ± 5mV(n = 4) as measured with sharp electrodes filled with 3 m KCl. A Ca2+-free extracellular solution was made from the standard extracellular solution containing 0.5 mm Na+-BAPTA and no added Ca2+. To reduce noise (McLarnon and Curry, 1990) and prevent cell damage (Choi, 1993; Yu et al., 1997; Yu and Salter, 1998) during NMDA receptor activation, we replaced K+ and Cl- in the solution by Cs+ and SO42-, respectively. Na2SO4 in the solutions was replaced with Cs2SO4 to make extracellular solutions with various Na+ concentrations as indicated. Recording pipettes were made from thin-walled borosilicate glass capillaries (World Precision Instruments, Sarasota, FL) pulled to tips of 1-2 μm and fire polished. The pipettes were filled with the same extracellular solution (except as indicated) used for bathing neurons (DC resistance, 8-10 MΩ) but also containing 10 μm NMDA and 3 μm glycine to evoke NMDA receptor-mediated currents.
During experiments the cultures were placed in a recording chamber on an inverted microscope (Axiovert S100 TV, Carl Zeiss, Göttingen, Germany) equipped with a 64× Varel Relief Contrast System. The image was magnified an additional 30× and displayed on a 17 inch TV monitor so that the morphology of the cell soma and major processes of neurons could be monitored during the recording period. Changes in cell morphology (such as cell swelling) could be detected readily by changing the osmolarity of the extracellular solution and thus lead to alterations in NMDA channel activity (Paoletti and Ascher, 1994). None of the bath solutions and experimental manipulations produced significant changes in size or shape of the cell soma and/or main processes.
Single-channel recording methods and criteria used to ensure that recordings were indeed from NMDA channels have been described in detail previously (Wang et al., 1996; Yu et al., 1997; Yu and Salter, 1998). In brief, NMDA-mediated single-channel currents were recorded with a patch potential of 70 mV from the reversal potential, except where indicated. Currents recorded were filtered at 10 kHz (-3 dB) with an Axo-patch 1D amplifier (Axon Instruments, Foster City, CA), digitized at 33 kHz, and stored onto videotape. The main conductance levels of ∼80% of recorded channels were in the range of 40-60 pS, with the remainder in the range of 10-30 vpS, as typically is observed in our and others' studies (Gibb and Colquhoun, 1992; Stern et al., 1994; Yu et al., 1997; Yu and Salter, 1998). The recorded currents were abolished by the bath application of the lipophilic NMDA channel blocker MK-801 (2 μm), confirming that NMDA receptor-mediated single-channel currents were recorded (Yu and Salter, 1998). After 3 to 5 min of recordings, which served as a control, the tested agents such as l-aspartate or NMDA dissolved in the same extracellular solution were bath applied to patched neurons. NMDA-mediated single-channel currents then were recorded for another 5 to 10 min. Because the bath application of l-aspartate or NMDA to neurons may cause cell depolarization, the holding potential was readjusted to maintain a patch potential of 70 mV from the reversal potential of recorded channels.
Methods for single-channel data analysis have been described in detail previously (Wang et al., 1996; Yu et al., 1997; Yu and Salter, 1998). In brief, the data stored on videotape were replayed, filtered at 2-5 kHz (-3 dB, 8 pole Bessel), and sampled continuously onto a computer at 20 kHz with pClamp6 software (Axon Instruments). Because only one main conductance level of channel openings was recorded in most patches, the channel open probability and duration of channel openings and closings were determined off-line by using a 50% crossing threshold. Distributions of dwell times were fit with the sum of multiple exponential components by using the Levenberg-Marquardt least-squares method. The usefulness of adding exponential components was assessed by using an F test (De Koninck and Mody, 1994) and also by visual inspection. Channel activity during the entire application period of tested agents (at least 3-5 min), which is from the time after readjustment of patch potential at the same level (70 mV) from the reversal potential after the start of the agent application, was compared with that for a similar period immediately preceding the application. The bursts, clusters, and superclusters of channel openings (Edmonds and Colquhoun, 1995) were identified according to critical times (tc), where each tc is defined by the following:
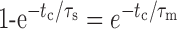 |
τs and τm are the time constants of short and intermediate gap lengths (Colquhoun and Sakmann, 1985; Gibb and Colquhoun, 1992); the durations of bursts, clusters, and superclusters of channel openings were calculated by using pClamp6 software.
The measurement of [Na+]i and [Ca2+]i. [Na+]i and [Ca+]i were measured with the Na+-sensitive and Ca2+-sensitive fluorescent dyes sodium-binding benzofuran isophthalate (SBFI) and fura-2, respectively, using a 346/380 nm pair of excitation wavelengths from monochromators and single-photon counting methods as described previously (Bibby and McCulloch, 1994; Yu and Salter, 1998). Neurons cultured on poly-d-lysine-coated glass coverslips were incubated either with fura-2 AM (5 μm) or SBFI AM (10 μm) mixed with pluronic F-127 (20% w/v; 1:1) and dissolved in the extracellular solutions used for single-channel recordings for 40 or 90 min at room temperature. Then the coverslips were washed and transferred to a perfusion chamber on a Nikon Diaphot II inverted microscope. The 346/380 nm fluorescence ratios were recorded in the soma region of neurons by using Deltascan System (Photon Technology International, South Brunswick, NJ). [Na+]i and [Ca2+]i were measured according to the following equation: [Na+] or [Ca2+] = KD [(R-Rmin)/(Rmax-R)](380max/380min). Calibration of Na+ in neurons required the addition of 10 μm of monensin and 1.5 mm of ouabain [used to equilibrate intracellular with extracellular [Na+] and inhibit the high level of Na+/K+ ATPase activity present in neurons (Rose and Ransom, 1997)] into a calibration solution containing 0, 20, or 150 mm Na+, which was applied sequentially to neurons to determine Rmin, R for 20 mm Na+, and Rmax. The KD of SBFI obtained in this manner from tested neurons was 11.6 mm. Calibration of Ca2+ in neurons required the addition of 6 μm ionomycin [a Ca2+ ionophore (Bibby and McCulloch, 1994; Kyrozis et al., 1996)] into a calibration solution containing 0 or 2 mm Ca2+ sequentially applied to neurons to determine Rmin and Rmax, respectively. The KD of fura-2 in neurons was in accordance with values described previously (Kyrozis et al., 1996). The mean concentrations of intracellular Na+ and Ca2+ were calculated before and during a 300 sec period immediately after NMDA receptor agonist application. All chemicals used were obtained from Sigma (St. Louis, MO) except for ionomycin (Calbiochem, La Jolla, CA) and BAPTA, BAPTA-AM, SBFI-AM, fura-2 AM, and monensin (all of which were purchased from Molecular Probes, Eugene, OR).
Results
Upregulation or downregulation of NMDA channel gating by the activation of remote NMDA receptors is dependent on the interaction of Na+ and Ca2+ influxes
NMDA single-channel activity was recorded in the cell-attached configuration before and during the bath application of NMDA receptor agonist l-aspartate (10-1000 μm), which was used to activate remote NMDA receptors located outside the membrane patch (Fig. 1A). Consistent with previous findings (Edmonds and Colquhoun, 1995; Yu et al., 1997; Yu and Salter, 1998), recordings of single-channel activity before the activation of remote NMDA receptors indicated that the open time of recorded channels ranged from <0.1 msec to longer than several tens of milliseconds, and the shut time ranged from <0.1 msec to longer than several thousands of milliseconds. By fitting the open and shut time distribution histograms, we found three components with time constants (mean ± SE; n = 24 patches) of 0.12 ± 0.01 msec (relative area, 51 ± 4%), 1.6 ± 0.1 msec (29 ± 3%), and 3.7 ± 0.2 msec (20 ± 2%) in the open time and five components with time constants of 0.12 ± 0.01 msec (relative area, 39 ± 3%), 1.0 ± 0.12 msec (17 ± 1%), 14 ± 1.3 msec (10 ± 0.7%), 487 ± 66 msec (16 ± 2%), and 1656 ± 218 msec (18 ± 2%) in the shut time. The overall channel open probability (ratio of total open time vs recording time) and mean open time of recorded NMDA channels were 0.0049 ± 0.0005 and 2.2 ± 0.1 msec, respectively.
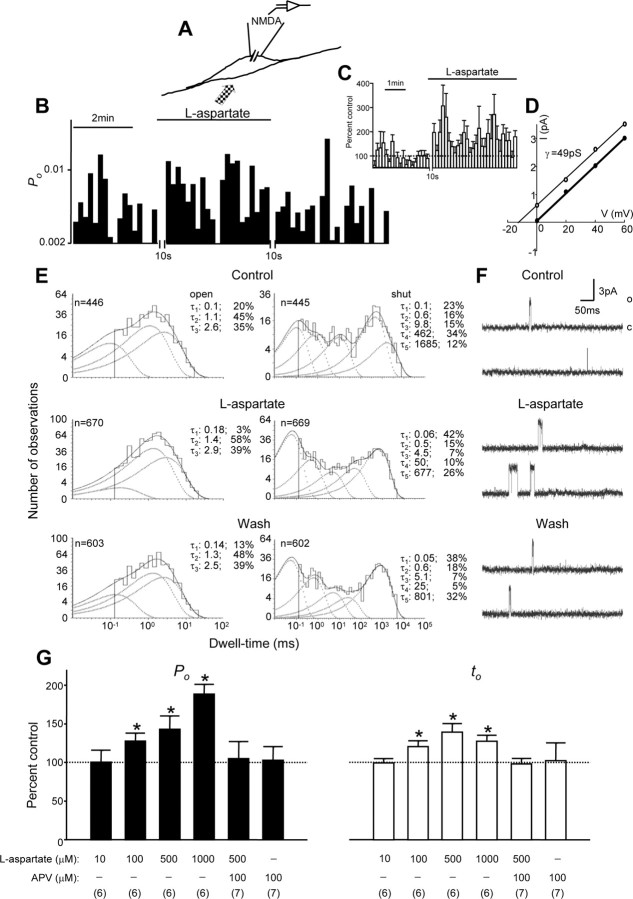
Figure 1.
Enhancement of NMDA channel gating induced by the activation of remote NMDA receptors. A, The recording configuration used to investigate the effects of remote NMDA receptor activation by the bath application of the NMDA receptor agonist l-aspartate on the NMDA channel within the membrane patch. B, A record of NMDA single-channel open probability (Po; bin, 10 sec) before and during stimulation of remote NMDA receptors by the bath application of the NMDA receptor agonist l-aspartate (500 μm). The break during the recording was required for adjusting the patch potential to 70 mV from the NMDA channel reversal potential after l-aspartate was applied to neurons. C, The average single-channel open probability (bin, 10 sec; 7 patches) normalized by the mean values of Po before l-aspartate application. D, The graph shows the I-V relationship for single-channel currents before (open circles) and during l-aspartate application (filled circles). The lines that are depicted represent least-squares regression lines. E, Dwell-time histograms of open and shut times were compiled before (top), during (middle), and after (bottom) the bath application of l-aspartate. In the dwell-time histograms, the solid line shows the Marquardt least-squares fit for the histogram, the dashed lines indicate individual exponential components, and n is the number of events. There were three open components and five closing components.τ, Time constant; percentage values indicate relative areas of each component. F, Representative single-channel current traces are illustrated before, during, and after the application of l-aspartate. o, Open level; c, closed level. G, Summarized data (mean ± SEM) showing that increases in the concentration of l-aspartate applied to remote NMDA receptors increased the Po and mean open time (to) of recorded NMDA channels. Values in parentheses indicate the number of patches tested; *p < 0.05, Wilcoxon test.
We found that bath application of the NMDA receptor agonist l-aspartate may change NMDA channel activity in a concentration-dependent manner (Fig. 1G). A significant increase in NMDA channel gating occurred when 100 μm l-aspartate was bath applied. An example of recorded NMDA channel open probability and averaged open probability of the channels for a period of 10 sec each before and during the bath application of NMDA receptor agonist l-aspartate (500 μm) is shown in Figure 1, B and C, respectively. The bath application of l-aspartate or NMDA to neurons causes cell depolarization and hence a parallel shift of the current-voltage (I-V) relationship (Fig. 1D), indicating that there is no change in single-channel conductance but rather a change in the reversal potential. Therefore, the holding potential was readjusted (the time break shown in Fig. 1B,C) to maintain a patch potential of 70 mV from the reversal potential of recorded channels. We found that the bath application of l-aspartate may increase NMDA channel activity immediately (Fig. 1B,C). During the bath application of l-aspartate the short open component was reduced while the shortest closing component was increased (Fig. 1E). The overall channel open probability and mean open time increased significantly (Fig. 1G).
Consistent with our previous finding, the application of 30 mm K+ to neurons in the presence of the voltage-gated Na+ channel blocker tetrodotoxin (TTX; 1 μm) produced cell depolarization comparable to that induced by the application of 500 μm l-aspartate, but it did not affect NMDA channel activity (Yu and Salter, 1998) (data not shown). Furthermore, we found that, whereas the 500 μm l-aspartate application produced increases in the overall channel open probability in all six recorded patches by 15-100% of that before l-aspartate application (i.e., control), the overall channel open probability during 500 μm l-aspartate application increased by 17-28% of controls in four of seven recorded patches and decreased by 16-20% in the three remaining patches when 100 μm aminophosphonovalerate (APV; a NMDA receptor antagonist) was coapplied. Although 100 μm APV application did not abolish completely the effects produced by l-aspartate application in every patch that was recorded, on average no significant change in NMDA channel activity was noted during l-aspartate application (Fig. 1). Taken together with the finding that the bath application of 100 μm APV alone did not affect recorded NMDA channel activity significantly (Fig. 1G), we conclude that NMDA channel gating can be upregulated via the activation of remote NMDA receptors.
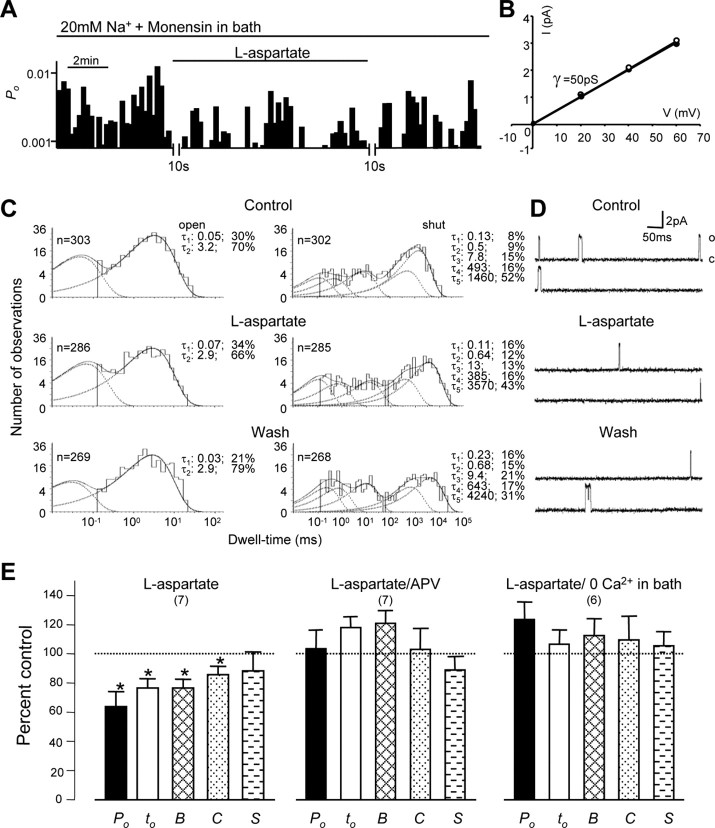
To understand mechanisms underlying the effects of remote NMDA receptor activation, we additionally characterized the functional roles of intracellular Na+ and Ca2+. Our previous study has shown that NMDA channel activity may be downregulated by the activation of remote NMDA receptors when Na+ in the extracellular solution is replaced with the membrane-impermeant cation N-methyl-d-glucamine (NMDG) (Yu and Salter, 1998). However, it remained unclear whether the reduction of NMDA channel gating during the activation of remote NMDA receptors was induced by a reduction of [Na+]i, which can be produced by efflux of intracellular Na+, and/or by some other unknown mechanisms. To answer this question, we investigated the effects of activation of remote NMDA receptors on NMDA channel activity when the Na+ gradient across the cell membrane was decreased by reducing [Na+] to 20 mm and including the Na+ ionophore monensin (10 μm) in the extracellular solution. Figure 2A-D displays an example of NMDA channel activity recorded under these conditions. We found that the application of 500 μm l-aspartate in six of seven recorded patches produced decreases in NMDA channel activity with a reduction of the long open component (Fig. 2C). The decreases in the overall channel open probability varied from 21 to 63% of control. On average, the overall channel open probability and mean open time were reduced to 64 ± 10 and 77 ± 6% of controls, and the duration of bursts and clusters was reduced to 77 ± 5 and 86 ± 6% of controls, respectively (Fig. 2E). In the presence of 100 μm APV, l-aspartate application produced reductions in the overall channel open probability of 27 and 38% in two of seven recorded patches, increases in the overall channel open probability of 27 and 61% of control in two patches, and slight changes (either increases or decreases) of <10% of control in three patches. On average, there was no significant change in NMDA channel activity recorded from neurons bathed with the extracellular solution containing 20 mm Na+ and monensin during l-aspartate application in the presence of 100 μm APV (Fig. 2E). Thus we conclude that the inhibitory effects on NMDA channel gating induced by the bath application of l-aspartate could be blocked by bath coapplication of the NMDA receptor antagonist APV, indicating that the activation of remote NMDA receptors downregulates NMDA channel activity under the condition in which Na+ influx was blocked by reducing the Na+ gradient across the cell membrane. Also, we found that blocking Ca2+ influx by removal of Ca2+ from the extracellular bath solution could prevent the inhibition of NMDA channel gating induced by the activation of remote NMDA receptors. During the activation of remote NMDA receptors the overall channel open probability, mean open time, and the duration of burst, clusters, and superclusters were 124 ± 11, 107 ± 10, and 113 ± 12, 110 ± 16, and 106 ± 10% of controls, respectively (Fig. 2E). These data suggest that Ca2+ influx may play an important role in the downregulation of NMDA channel gating induced by the activation of remote NMDA receptors in neurons bathed with the extracellular solution containing 20 mm Na+ and Na+ ionophore monensin.
Figure 2.
The downregulation of NMDA channel gating by remote NMDA receptors. A, An example of NMDA channel open probability (Po; bin, 10 sec) recorded from neurons bathed with an extracellular solution in which [Na+] was reduced to 20 mm with Cs+ replacement and the inclusion of the Na+ ionophore monensin (10 μm). B, The graph shows the I-V relationship for single-channel currents before (open circles) and during (filled circles) l-aspartate application. The lines represent least-squares regression lines. C, D well-time histograms of open and shut times were compiled before (top), during (middle), and after (bottom) the bath application of l-aspartate. In the dwell-time histograms, the solid line shows the Marquardt least-squares fit for the histogram, the dashed lines indicate individual exponential components, and n is the number of events. τ, Time constant; the percentage values indicate relative areas of each component. D, Representative single-channel current traces are illustrated before, during, and after the application of l-aspartate. o, Open level; c, closed level. E, Summarized data (mean ± SEM) outlining the inhibitory effects of remote NMDA receptor activation by the bath application of l-aspartate on Po, mean open time (to), burst (B), cluster (C), and supercluster (S) lengths of recorded NMDA channels from neurons bathed with the extracellular solution containing 20 mm Na+ and 10 μm monensin. The inhibitory effects could be prevented by the coapplication of NMDA receptor antagonist APV (100 μm; l-aspartate/APV) or by removal of Ca2+ in the extracellular solution (l-aspartate/0 Ca2+ in bath). Values in parentheses indicate the number of patches tested; *p < 0.05 (Wilcoxon test).
A modest Ca2+ influx may inhibit NMDA receptors when Na+ influx is reduced
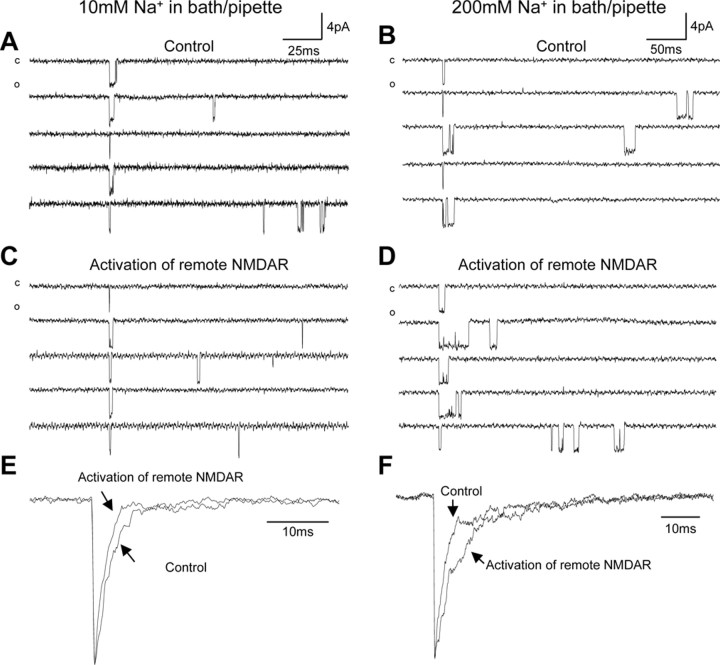
In light of our observations that NMDA receptor/channel gating may be upregulated or downregulated by the activation of remote NMDA receptors via the actions of Na+ or Ca2+ entering neurons, it was of considerable interest to determine how the balance of intracellular Na+ and Ca2+ controls these effects on NMDA channel gating. To this end we measured [Na+]i and [Ca2+]i in the soma region of neurons bathed with an extracellular solution containing 10 mm Na+, 0.3 mm Ca2+, and no Na+ ionophore monensin (Fig. 3A,B). We found that NMDA receptor activation produced [Na+]i increases ranging from 0 to 2 mm (0.8 ± 0.2 mm; n = 9; mean ± SE) and [Ca2+]i increases ranging from 2.7 to 56 nm (35 ± 9 nm; n = 7). Under these conditions we recorded NMDA single-channel activity in cell-attached patches and found that the activation of remote NMDA receptors produced significant decreases in NMDA channel activity (Fig. 3C,E). The overall channel open probability and mean open time were reduced to 62 ± 13 and 69 ± 14% of controls, and the durations of bursts and clusters were reduced to 67 ± 11 and 70 ± 7% of controls, respectively (Fig. 3E). Thus these observations additionally confirmed that NMDA channel gating was inhibited by the activation of remote NMDA receptors when Na+ influx was reduced.
Figure 3.
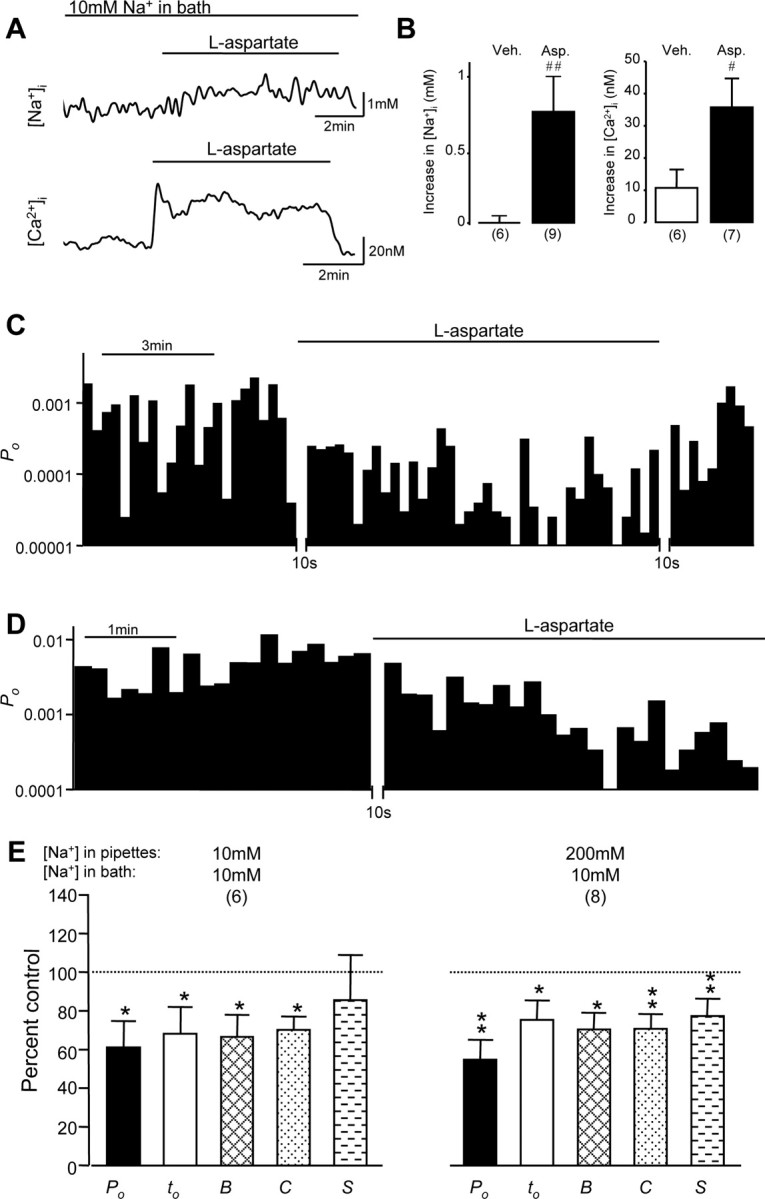
The downregulation of NMDA channel gating by remote NMDA receptors was not regulated by local Na+ influx through recorded channels. A, Examples of traces of [Na+]i (top) and [Ca2+]i (bottom) recorded in the soma region of neurons bathed with the same extracellular solution used from electrophysiological recordings, containing 10 mm Na+ and 0.3 mm Ca2+, before and during l-aspartate (500 μm) application. B, Summarized data indicating changes induced by the application of vehicle (open bars) and l-aspartate (filled bars) on [Na+]i (left) and [Ca2+] (right); #p < 0.05; ##p < 0.01 (Mann-Whitney test) in a comparison between l-aspartate versus vehicle effects. Veh., Vehicle; Asp., l-aspartate. C, An example of NMDA channel open probability (Po; bin, 10 sec) recorded from neurons bathed with the extracellular solution containing 10 mm Na+ but no monensin, using recording pipettes filled with the extracellular solution except that it contained 10 μm NMDA. D, An example of NMDA channel open probability (Po; bin, 10 sec) recorded from neurons bathed with the standard extracellular solution containing 200 mm Na+, using recording pipettes filled with the extracellular solution containing 10 mm Na+ and 10 μm NMDA. E, Summarized data (mean ± SEM) outlining the inhibitory effects of remote NMDA receptor activation produced by the bath application of l-aspartate on Po, mean open time (to), burst (B), cluster (C), and supercluster (S) lengths of NMDA channels recorded by using pipettes filled with the standard extracellular solution containing 200 mm Na+ or with the extracellular solution containing 10 mm Na+, also used to bathe the neurons. Values in parentheses indicate the number of patches tested; *p < 0.05; **p < 0.01 (Wilcoxon test).
We then investigated whether the inhibitory effect induced by Ca2+ influx through activated remote NMDA receptors may be affected by local Na+ influx through recorded NMDA channels. NMDA channel activity was recorded by using two different concentrations of Na+ in the recording pipettes. The overall channel open probability (0.0021 ± 0.0007; n = 9 patches) of NMDA channels recorded with pipettes filled with an extracellular solution containing 10 mm Na+ was significantly lower (p < 0.05; Mann-Whitney test) than that of NMDA channels recorded with pipettes filled with an extracellular solution containing 200 mm of Na+ (0.0065 ± 0.0026; n = 10 patches). Regardless of intra-pipette Na+ concentration, the activation of remote NMDA channels produced similar levels of NMDA channel gating inhibition (Fig. 3D,E).
We found that the activation of remote NMDA receptors also could cause an inhibition of ensemble currents. Figure 4 shows examples of alignment and summation of consecutive superclusters recorded from neurons bathed with extracellular solutions containing 10 or 200 mm Na+ before and during the activation of remote NMDA receptors. Compared with the ensemble current before the activation of remote NMDA receptors, there was a shortened decay of the current during the activation of remote NMDA receptors when [Na+]e was reduced to 10 mm (Fig. 4E). In contrast, a prolonged decay of the current during the activation of remote NMDA receptors was found in neurons bathed with the standard extracellular solution containing 200 mm Na+ (Fig. 4F).
Figure 4.
Ensemble NMDA currents were regulated by the activation of remote NMDA receptors. Shown are examples of the alignment of vertically flipped super cluster current traces at the start of the first openings of superclusters recorded before (control) and during the activation of remote NMDA receptors in neurons bathed with an extracellular solution containing 10 mm Na+ (A, C) or 200mm Na+ (B, D). Recording pipettes were filled with the same extracellular solution used for bathing neurons except that it contained 10 μm NMDA. c, Closed level; o, open level. E, The corresponding ensemble currents produced by the summation of 100 and 95 consecutive superclusters recorded before (Control) and during the activation of remote NMDA receptors (NMDAR), examples of which are shown in A and C, respectively. F, The corresponding ensemble currents produced by the summation of 114 and 153 consecutive superclusters recorded before (Control) and during the activation of remote NMDA receptors (NMDAR), examples of which are shown in B and D, respectively. The ensemble currents were normalized by their peak amplitudes and superimposed to show the difference between the currents.
A5 ± 1mm increase in intracellular sodium concentration ([Na+]i) represents a threshold required to mask the downregulation of NMDA channels by remote NMDA receptors
We then investigated the effects of remote NMDA receptor activation when [Na+] in the extracellular bath solution, which contained 0.3 mm Ca2+, was increased to 20 mm. We found that under this condition no significant change in NMDA channel gating could be produced by the activation of remote NMDA receptors (Fig. 5A,B). The overall channel open probability and mean open time were 106 ± 6 and 104 ± 4% of controls, and the durations of bursts, clusters and superclusters were 110 ± 6, 114 ± 11, and 124 ± 16% of controls, respectively (Fig. 5B). Interestingly, even as [Ca2+]e was increased to 1.2 mm, the activation of remote NMDA receptors produced no significant inhibition of channel gating (Fig. 5B). These findings strongly suggest that Na+ influx modulates the regulation of NMDA receptors by intracellular Ca2+ and that possible changes in [Na+]i induced by 20 mm of extracellular Na+ during NMDA receptor activation is critical for controlling the regulation of NMDA receptors.
Figure 5.
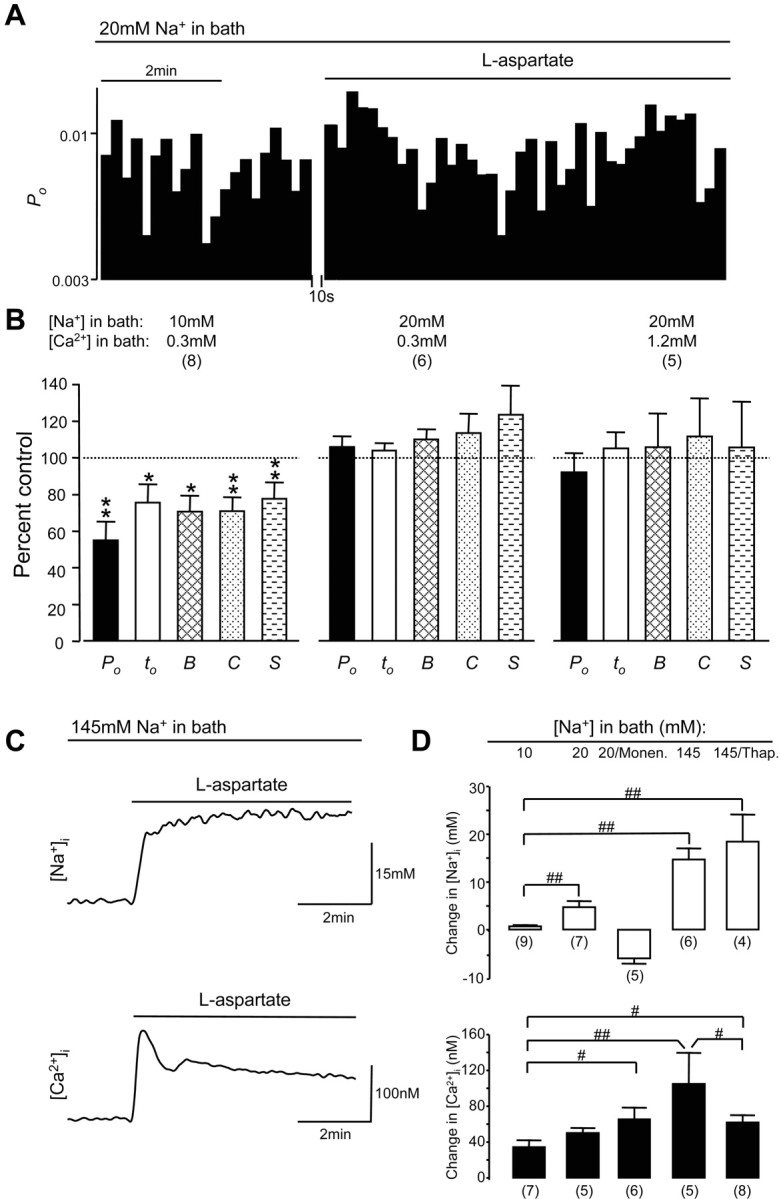
Ca2+ influx-induced downregulation of NMDA channel gating during activation of remote NMDA receptors could be overcome by a modest Na+ influx. A, An example of NMDA channel open probability (Po; bin, 10 sec) recorded from neurons bathed with an extracellular solution containing 20 mm Na+ and 0.3 mm Ca2+. B, Summarized data (mean ± SEM) outlining the effects of remote NMDA receptor activation produced by the bath application of l-aspartate on Po, mean open time (to), burst (B), cluster (C), and supercluster (S) lengths of NMDA channels recorded from neurons bathed with an extracellular solution containing 10 mm Na+ and 0.3 mm Ca2+ or 20 mm Na+ and 0.3 mm Ca2+ or 20 mm Na+ and 1.2 mm Ca2+. Values in parentheses indicate the number of patches tested. *p < 0.05; **p < 0.01 (Wilcoxon test). C, Examples of traces of [Na+]i (top) and [Ca2+]i (bottom) recorded in the soma region of neurons bathed with the extracellular solution containing 0.3 mm Ca2+ and 145 mm Na+ before and during l-aspartate (500 μm) application. D, Summarized data indicating changes (mean ± SEM) in [Na+]i (top) and [Ca2+]i (bottom) induced by the activation of NMDA receptors in neurons bathed with extracellular solutions containing 0.3 mm Ca2+ and 10, 20, or 145 mm Na+ in the absence and presence of monensin (10 μm) or Ca2+-ATPase inhibitor thapsigargin (0.1 μm); # p < 0.05; ## p < 0.01 (Mann-Whitney test) in a comparison between groups as indicated. Values in parentheses indicate the number of neurons tested.
Measurement of [Na+]i and [Ca2+]i during NMDA receptor activation when neurons were bathed with the extracellular solution containing 20 mm Na+ demonstrated [Na+]i increases ranging from 1.4 to 10 mm (5 ± 1 mm; n = 7), which were significantly more than those found in neurons bathed with 10 mm Na+ extracellular solution and [Ca2+]i increases ranging from 32 to 62 nm (50 ± 5 nm; n = 5) (Fig. 5D). These data demonstrate a novel finding that the inhibitory effects induced by Ca2+ influx through remote NMDA receptors could be overcome by a modest Na+ influx and that the increase in [Na+]i of 5 ± 1mm may represent a critical threshold required to mask the inhibitory effects induced by Ca2+ influx on NMDA channels during the activation of remote NMDA receptors.
This finding also raised the question of why NMDA channel gating was decreased, but not enhanced, by the activation of remote NMDA receptors when basal [Na+]i was increased >5mm as in neurons bathed with an extracellular solution containing 20 mm Na+ and 10 μm monensin (Fig. 2). To answer this question, we measured [Na+]i and [Ca2+]i and found that the basal [Ca2+]i and [Na+]i in these neurons were 84 ± 18 nm and 16 ± 2 mm and that the application of l-aspartate to these neurons produced a significant increase in [Ca2+]i by 66 ± 13 nm (n = 6) as well as a significant decrease in [Na+]i by 5.8 ± 1mm (n = 5) (Fig. 5D) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Taken together with the finding that removal of extracellular Ca2+ prevented the downregulation of NMDA channel gating by remote NMDA receptors, this indicates that the coincident [Ca2+]i increase and [Na+]i decrease induced by the activation of remote NMDA receptors may underlie the inhibition of NMDA channel gating induced by l-aspartate application to neurons bathed with an extracellular solution containing 20 mm Na+ and monensin.
More importantly, we found that increasing [K+]e by 30 mm in the solution containing 200 mm Na+ and 1 μm TTX, which produced no significant changes in NMDA single-channel gating, increased [Na+]i and [Ca2+]i by 7 ± 2mm (n = 7) and 48 ± 19 nm (n = 6), respectively (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). This change in [Na+] and [Ca2+] is similar to measured [Na+]i and [Ca2]i increases (5 ± 1mm and 50 ± 5nm) induced by the activation of remote NMDA receptors, which produced no change in NMDA channel gating (Fig. 5A,B,D). Thus we believe that a Na+ influx that increases [Na+]i by >5 mm is critical for overcoming the effects of Ca2+ influx.
Na+ influx may enhance Ca2+ influx and also may mask the Ca2+-dependent inhibition of NMDA channels induced by the activation of remote NMDA receptors
When extracellular [Na+] was increased additionally to 145 mm, the activation of NMDA receptors produced even greater increases in [Na+]i (15 ± 2mm; n = 6) and [Ca2+]i (105 ± 35 nm; n = 5), which were significantly more than those induced by the activation of NMDA receptors in neurons bathed with extracellular solution containing 10 mm Na+ (Fig. 5C,D). To determine whether the increase in [Ca2+]i was produced by increases in Ca2+ influx, we measured [Ca2+]i during NMDA receptor activation in the presence of the Ca2+-ATPase inhibitor thapsigargin, which depletes intracellular stores of Ca2+ by blocking Ca2+ reuptake into stores. Thapsigargin (0.1 μm) was bath applied to neurons at 20 min before the measurement of [Ca2+]i or [Na+]i and was maintained in the bath solution for the whole recording period. We found that thapsigargin application significantly reduced increases in [Ca2+]i during NMDA receptor activation when compared with those in the absence of thapsigargin (Fig. 5D), but furthermore the increase in [Ca2+]i (62 ± 8nm; n = 8) during NMDA receptor activation in neurons bathed with an extracellular solution containing 145 mm Na+ and thapsigargin was still significantly greater than that found in neurons bathed with an extracellular solution containing 10 mm Na+ (Fig. 5D).
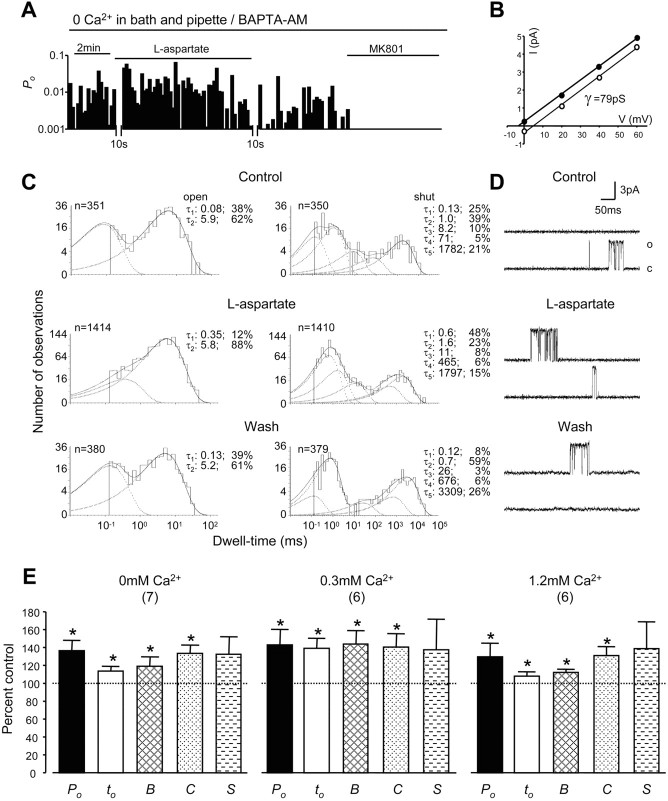
Although our previous study has shown that the upregulation of NMDA channel gating by the activation of remote NMDA receptors is not affected by the removal of Ca2+ in the extracellular bath solution (Yu and Salter, 1998), it remains unclear whether local Ca2+, which flows into recorded NMDA channels and sufficiently activates phosphatase regulation of NMDA receptors (Lieberman and Mody, 1994), or Ca2+ released from intracellular stores during the activation of NMDA receptors (Fig. 5D) may be involved in the regulation of NMDA channel gating. Therefore, we recorded NMDA single-channel activity by using pipettes filled with Ca2+-free extracellular solution from neurons that had been pretreated with BAPTA-AM (10 μm for 4 hr) and bathed in the same extracellular solution containing no Ca2+. Figure 6A-D displays an example of recorded NMDA channel activity. During the activation of remote NMDA receptors the long open component and shortest closing component of NMDA channels were increased. The overall channel open probability and mean open time increased to 137 ± 11 and 114 ± 5% of controls, and the durations of bursts and clusters were increased to 120 ± 10 and 134 ± 9% of controls, respectively (Fig. 6E). Application of the lipophilic NMDA channel blocker MK-801 (2 μm) (Wong and Kemp, 1991; Yu and Salter, 1998; Dingledine et al., 1999) abolished single-channel currents (Fig. 6A) and confirmed that the recorded channel activity was NMDA receptor-mediated. The fact that no significant difference could be found in the effects produced by the activation of remote NMDA receptors as [Ca2+] was changed (from 0 to 0.3-1.2 mm) in the extracellular solutions bathing neurons and filling recording electrodes (Fig. 6E) demonstrates that Na+ influx through activated NMDA receptors not only may be able to enhance Ca2+ influx but also may mask Ca2+-induced inhibitory effects on NMDA channel gating (Fig. 7).
Figure 6.
Role of Ca2+ in the upregulation of NMDA channel gating by remote NMDA receptors. A, An example of NMDA channel open probability (Po; bin, 10 sec) recorded from neurons pretreated with BAPTA-AM (10 μm for 4 hr) and bathed with the Ca2+-free extracellular solution by using recording pipettes filled with the same Ca2+-free extracellular solution except that it contained 10 μm NMDA. The break during the recording was required for adjusting the patch potential to 70 mV from the NMDA channel reversal potential after l-aspartate (500 μm) was applied to neurons. B, The graph shows the I-V relationship for single-channel currents before (open circles) and during (filled circles) l-aspartate application. The depicted lines represent least-squares regression lines. C, Dwell-time histograms of open and shut times were compiled before (top), during (middle), and after (bottom) the bath application of l-aspartate. In the dwell-time histograms, the solid line shows the Marquardt least-squares fit for the histogram, the dashed lines indicate individual exponential components, and n is the number of events. τ, Time constant; the percentage values indicate relative areas of each component. D, Representative single-channel current traces are illustrated before, during, and after the application of l-aspartate. o, Open level; c, closed level. E, Summarized data (mean ± SEM) outlining the effects of remote NMDA receptor activation by the bath application of l-aspartate on Po, mean open time (to), burst (B), cluster (C), and supercluster (S) lengths of NMDA channels recorded in neurons bathed with extracellular solution containing 1.2, 0.3, or 0 mm Ca2+. Recording pipettes were filled with the same extracellular solution used for bathing neurons except that it contained 10 μm NMDA. The neurons bathed with the bath solution containing 0 mm Ca2+ were pretreated with BAPTA-AM for 4 hr. Values in parentheses indicate the number of patches tested; *p < 0.05 (Wilcoxon test).
Figure 7.
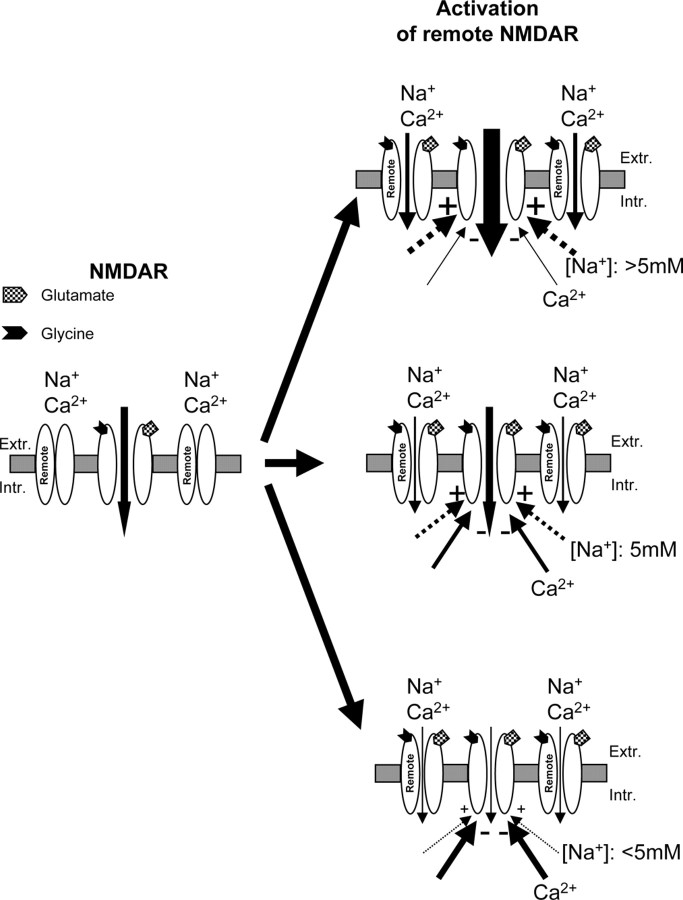
An overview of the effects of Na+ and Ca2+ influx in the regulation of NMDA channel gating by remote NMDA receptors. NMDA channel activity may be upregulated or downregulated by remote NMDA receptors, depending on the amount of Na+ and Ca2+ influx. If Na+ influx is blocked, Ca2+ influx induced by the activation of remote NMDA receptors may inhibit NMDA channel gating. This Ca2+-induced inhibition of NMDA receptors can be prevented by a small Na+ influx, which increases [Na+]i by 5 ± 1 mm. The 5 ± 1 mm increase in [Na+]i is apparently a critical threshold for controlling the Ca2+ influx-induced inhibition of NMDA channel activity during remote NMDA receptor activation. NMDA channel gating may be decreased if Na+ influx during the activation of remote NMDA receptors is less than that required to overcome the effects induced by Ca2+ influx, whereas NMDA channel gating may be potentiated by the activation of remote NMDA receptors if Na+ influx is more than that required to overcome the effects induced by Ca2+ influx. Because there is a large Na+ influx during NMDA receptor activation under physiological conditions, the Na+-dependent upregulation effect plays a dominant role in the regulation of NMDA channel gating by NMDA receptor crosstalk. Remote, Remote NMDA receptors; NMDAR, NMDA receptors; Extr., extracellular; Intr., intracellular.
Discussion
The present study documents that NMDA channel activity may be upregulated or downregulated by remote NMDA receptors, depending on the amount of Na+ and Ca2+ influx. If Na+ influx is blocked, Ca2+ influx induced by the activation of remote NMDA receptors may inhibit NMDA channel gating. However, this inhibitory effect can be overcome by an increase in [Na+]i of >5 mm. Thus there may be a functional Na+-Ca2+ interaction in the regulation of NMDA channels.
Further detailed investigations document that the effects of Na+ and Ca2+ influx on NMDA channel gating during the activation of remote NMDA receptors cannot be explained simply by an algebraic sum of two opposite effects growing monotonically, because (1) Ca2+ influx required to downregulate NMDA receptors under the condition of no Na+ influx is found to be much smaller than that during NMDA receptor activation under normal conditions; (2) a modest Na+ influx, which produces a much smaller increase in [Na+]i than that during NMDA receptor activation under normal conditions, blocks Ca2+ influx-induced inhibition of NMDA channels during the activation of remote NMDA receptors; (3) increases in Na+ influx may enhance Ca2+ influx significantly during the activation of NMDA receptors; and (4) blocking increases in [Ca2+]i both via Ca2+ release from intracellular stores and Ca2+ influx through both local and remote NMDA channels without blocking Na+ influx did not produce any additional increase in NMDA receptor upregulation induced by the activation of remote NMDA receptors when compared with that recorded from neurons under normal conditions.
It has been reported that an ongoing Ca2+ inhibition of NMDA receptors may accompany the activation of NMDA channels and serve as a negative feedback mechanism to control NMDA channels (Mayer and Westbrook, 1987; McBain and Mayer, 1994; Kyrozis et al., 1996; Dingledine et al., 1999). However, there seem to be no significant effects produced by Ca2+ influx on NMDA channel gating during the activation of remote NMDA receptors recorded from neurons bathed with a standard extracellular solution. Interestingly, when Na+ influx was blocked, a modest Ca2+ influx during the activation of remote NMDA receptors produced a 35 ± 9nm increase in [Ca2+]i and significantly inhibited NMDA channel gating (Fig. 7). This Ca2+-induced inhibition of NMDA receptors could be prevented by a modest Na+ influx, which produced an increase in [Na+]i of >5mm (Fig. 7). Thus we have characterized a novel functional relationship of Na+ and Ca2+ influxes in the regulation of NMDA receptor gating in cultured hippocampal neurons.
NMDA receptors are distributed widely in the CNS and are involved in many physiological and pathophysiological processes. Therefore, it will be very interesting to determine whether the functional relationship of Na+ and Ca2+ influx in the regulation of NMDA receptor gating characterized in cultured hippocampal neurons also may be an important mechanism underlying the regulation of neuronal functions in the other regions of the CNS. Because neurons in culture preparations likely may not be representative of the neurons in vivo, the determination of whether a 5 ± 1 mm increase in [Na+]i also represents a threshold increase that enhances Ca2+ influx and also masks the effects of Ca2+ influx in vivo will be critical for understanding the regulation of activity-dependent neuroplasticity in vivo.
A number of mechanisms have been found to underlie the downregulation of NMDA channel gating by intracellular Ca2+: (1) Ca2+-induced α-actinin/cytoskeleton dissociation from the NR1 subunit of NMDA receptors (Krupp et al., 1999), (2) calmodulin activation, which binds to and inhibits NMDA channel activity (Ehlers et al., 1996; Wechsler and Teichberg, 1998; Zhang et al., 1998; Krupp et al., 1999), and (3) activation of the phosphatase calcineurin, which downregulates NMDA channel gating (Lieberman and Mody, 1994; Mulkey et al., 1994; Tong et al., 1995). Our findings suggest that there may be a functional Na+-Ca2+ interaction in the regulation of NMDA receptors (Fig. 7). NMDA channel gating may be decreased if Na+ influx during the activation of remote NMDA receptors is less than that (5 ± 1 mm) required to overcome the effects induced by Ca2+ influx, whereas NMDA channel gating may be potentiated by the activation of remote NMDA receptors if Na+ influx is more than that required to overcome the effects induced by Ca2+ influx (Fig. 7). Our data show that under physiological conditions there is a large Na+ influx during NMDA receptor activation and that the increased Na+ influx may increase Ca2+ influx significantly on one hand but on the other hand also mask Ca2+-induced inhibition of NMDA receptors. Thus the Na+-dependent upregulation effect may play a dominant role in the regulation of the cross talk between NMDA receptors (Fig. 7).
When neurons were bathed with a standard extracellular solution, the activation of remote NMDA receptors significantly increased overall channel open probability, mean open time, and the duration of bursts and clusters. The increases of the long-open and short-closing components during the activation of remote NMDA receptors may underlie the increases in overall channel open probability and burst and cluster activities. Because we found that the bath application of NMDA or l-aspartate produced no change in the number of NMDA receptors detected on the cell surface (data not shown), which was consistent with that reported in a previous study (Nong et al., 2003), and that there was no change in the mean length of a single run of openings with no double openings (Colquhoun and Hawkes, 1990), it is un-likely that the number of NMDA channels on the membrane patch during the upregulation of remote NMDA channels is altered.
NMDA receptors are distributed widely on the cell surface, with locations on both the synaptic and extrasynaptic regions of central neurons. Our observations show that single NMDA channel gating may be regulated by the activation of remote NMDA receptors, which can result in changes in ensemble NMDA currents. Bregestovski and his colleagues (Medina et al., 1996) found that single NMDA channel activity recorded in cell-attached patches may be downregulated after the washout of NMDA, which was bath applied to neurons. These findings support our suggestion that the activity of individual NMDA channels may behave differently when their neighboring NMDA receptors are activated (Fig. 7).
Although technical difficulties preclude the direct observation of remote NMDA receptor activation effects on the activity of an individual NMDA receptor located at the synapse, we were able to assess the regulation of synaptic function by activation of remote NMDA receptors via the use of simulation experiments. Supercluster current traces recorded before and during the activation of remote NMDA receptors were aligned at the start of the first openings and subsequently were summed to produce an ensemble current. The decay of normalized ensemble currents was significantly different before and during the activation of remote NMDA receptors. Depending on the amount of Na+ and Ca2+ influx through activated remote NMDA receptors, there can be a prolonged or shortened decay in ensemble currents. Thus this type of data provides additional evidence to support the concept that the cross talk between NMDA receptors, which is mediated by a functional Na+-Ca2+ interaction, may be a novel mechanism regulating synaptic responses.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (CIHR), the Ontario Neurotrauma Foundation, and the Heart and Stroke Foundation of Ontario to X. -M. Y. and by a CIHR group grant to the CIHR Group in Matrix Dynamics. We thank Drs. Z.-G. Xiong and X.-P. Chu for technical assistance and Drs. M. W. Salter, H. Van Tol, and Z.-P. Feng for critical comments on this manuscript.
Correspondence should be addressed to Xian-Min Yu, College of Medicine, Room 2300-C, Florida State University, 1115 West Call Street, Tallahassee, FL 32306-4300. E-mail: xianmin.yu@med.fsu.edu or xianmin.yu@utoronto.ca.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250139-10$15.00/0
References
- Bibby KJ, McCulloch CA (1994) Regulation of cell volume and [Ca2+]i in attached human fibroblasts responding to anisosmotic buffers. Am J Physiol 266: C1639-C1649. [DOI] [PubMed] [Google Scholar]
- Callaway JC, Ross WN (1997) Spatial distribution of synaptically activated sodium concentration changes in cerebellar Purkinje neurons. J Neurophysiol 77: 145-152. [DOI] [PubMed] [Google Scholar]
- Choi DW (1993) NMDA receptors and AMPA/kainate receptors mediate parallel injury in cerebral cortical cultures subjected to oxygen-glucose deprivation. Prog Brain Res 96: 137-143. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG (1990) Stochastic properties of ion channel openings and bursts in a membrane patch that contains two channels: evidence concerning the number of channels present when a record containing only single openings is observed. Proc R Soc Lond B Biol Sci 240: 453-477. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Sakmann B (1985) Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol (Lond) 369: 501-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y, Mody I (1994) Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol 71: 1318-1335. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7-61. [PubMed] [Google Scholar]
- Edmonds B, Colquhoun D (1995) Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Annu Rev Physiol 57: 495-519. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Zhang S, Bernhardt JP, Huganir RL (1996) Inactivation of NMDA receptors by direct interaction of calmodulin with the NR1 subunit. Cell 84: 745-755. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D (1992) Activation of N-methyl-d-aspartate receptors by l-glutamate in cells dissociated from adult rat hippocampus. J Physiol (Lond) 456: 143-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B (1992) Ionic channels of excitable membranes, Ed 2. Sunderland, MA: Sinauer.
- Krupp JJ, Vissel B, Thomas CG, Heinemann SF, Westbrook GL (1999) Interactions of calmodulin and α-actinin with the NR1 subunit modulate Ca2+-dependent inactivation of NMDA receptors. J Neurosci 19: 1165-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrozis A, Albuquerque C, Gu J, MacDermott AB (1996) Ca2+-dependent inactivation of NMDA receptors: fast kinetics and high Ca2+ sensitivity in rat dorsal horn neurons. J Physiol (Lond) 495: 449-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei G, Xue S, Chery N, Liu Q, Xu J, Kwan CL, Fu Y, Lu YM, Liu M, Harder KH, Yu X-M (2002) Gain control of N-methyl-d-aspartate receptor activity by receptor-like protein tyrosine phosphatase alpha. EMBO J 21: 2977-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DN, Mody I (1994) Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature 369: 235-239. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL (1987) The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol 28: 197-276. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML (1994) N-methyl-d-aspartic acid receptor structure and function. Physiol Rev 74: 723-760. [DOI] [PubMed] [Google Scholar]
- McLarnon JG, Curry K (1990) Single channel properties of the N-methyl-d-aspartate receptor channel using NMDA and NMDA agonists: on-cell recordings. Exp Brain Res 82: 82-88. [DOI] [PubMed] [Google Scholar]
- Medina I, Filippova N, Bakhramov A, Bregestovski P (1996) Calcium-induced inactivation of NMDA receptor channels evolves independently of run-down in cultured rat brain neurones. J Physiol (Lond) 495: 411-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, MacDonald JF (1995) NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol Sci 16: 356-359. [DOI] [PubMed] [Google Scholar]
- Mulkey RM, Endo S, Shenolikar S, Malenka RC (1994) Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature 369: 486-488. [DOI] [PubMed] [Google Scholar]
- Nicholls D, Attwell D (1990) The release and uptake of excitatory amino acids. Trends Pharmacol Sci 11: 462-468. [DOI] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW (2003) Glycine binding primes NMDA receptor internalization. Nature 422: 302-307. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Ascher P (1994) Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron 13: 645-655. [DOI] [PubMed] [Google Scholar]
- Rose CR, Konnerth A (2001) NMDA receptor-mediated Na+ signals in spines and dendrites. J Neurosci 21: 4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Ransom BR (1997) Regulation of intracellular sodium in cultured rat hippocampal neurones. J Physiol (Lond) 499: 573-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P, Cik M, Colquhoun D, Stephenson FA (1994) Single channel properties of cloned NMDA receptors in a human cell line: comparison with results from Xenopus oocytes. J Physiol (Lond) 476: 391-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CP, Dudek FE (1982) Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science 218: 810-812. [DOI] [PubMed] [Google Scholar]
- Tong G, Shepherd D, Jahr CE (1995) Synaptic desensitization of NMDA receptors by calcineurin. Science 267: 1510-1512. [DOI] [PubMed] [Google Scholar]
- Wang YT, Yu X-M, Salter MW (1996) Ca2+-independent reduction of N-methyl-d-aspartate channel activity by protein tyrosine phosphatase. Proc Natl Acad Sci USA 93: 1721-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler A, Teichberg VI (1998) Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium, and calmodulin. EMBO J 17: 3931-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EH, Kemp JA (1991) Sites for antagonism on the N-methyl-d-aspartate receptor channel complex. Annu Rev Pharmacol Toxicol 31: 401-425. [DOI] [PubMed] [Google Scholar]
- Yu X-M, Salter MW (1998) Gain control of NMDA receptor currents by intracellular sodium. Nature 396: 469-474. [DOI] [PubMed] [Google Scholar]
- Yu X-M, Askalan R, Keil GJI, Salter MW (1997) NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275: 674-678. [DOI] [PubMed] [Google Scholar]
- Zhang S, Ehlers MD, Bernhardt JP, Su CT, Huganir RL (1998) Calmodulin mediates calcium-dependent inactivation of N-methyl-d-aspartate receptors. Neuron 21: 443-453. [DOI] [PubMed] [Google Scholar]