Abstract
Ependymal cells on the walls of brain ventricles play essential roles in the transport of CSF and in brain homeostasis. It has been suggested that ependymal cells also function as stem cells. However, the proliferative capacity of mature ependymal cells remains controversial, and the developmental origin of these cells is not known. Using confocal or electron microscopy (EM) of adult mice that received bromodeoxyuridine (BrdU) or [3H]thymidine for several weeks, we found no evidence that ependymal cells proliferate. In contrast, ependymal cells were labeled by BrdU administration during embryonic development. The majority of them are born between embryonic day 14 (E14) and E16. Interestingly, we found that the maturation of ependymal cells and the formation of cilia occur significantly later, during the first postnatal week. We analyzed the early postnatal ventricular zone at the EM and found a subpopulation of radial glia in various stages of transformation into ependymal cells. These cells often had deuterosomes. To directly test whether radial glia give rise to ependymal cells, we used a Cre-lox recombination strategy to genetically tag radial glia in the neonatal brain and follow their progeny. We found that some radial glia in the lateral ventricular wall transform to give rise to mature ependymal cells. This work identifies the time of birth and early stages in the maturation of ependymal cells and demonstrates that these cells are derived from radial glia. Our results indicate that ependymal cells are born in the embryonic and early postnatal brain and that they do not divide after differentiation. The postmitotic nature of ependymal cells strongly suggests that these cells do not function as neural stem cells in the adult.
Keywords: ependyma, radial glia, proliferation, ventricular zone, lateral ventricle, deuterosomes, cilia
Introduction
In adult mammals, the cerebral ventricles are normally lined by a layer of ependymal cells (Del Bigio, 1995). Most of these cells are cuboidal and multiciliated (Bleier, 1971; Millhouse, 1971). These cells are at the interface between the brain parenchyma and the ventricular cavities and play an essential role in the propulsion of CSF through the ventricular system (Worthington and Cathcart, 1963; Cathcart and Worthington, 1964). The coordinated beating of cilia in ependymal cells creates a current of CSF along the walls of the lateral ventricle; ependymal malfunction leads to disturbances of CSF flow and hydrocephaly (Brody et al., 2000; Taulman et al., 2001; Kobayashi et al., 2002). It has also been suggested that ependymal cells filter brain molecules (Bruni, 1998), insulate the brain from potentially harmful substances in the CSF (Kuchler et al., 1994), move cellular debris in the direction of bulk CSF flow, and optimize the dispersion of neural messengers in the CSF (Roth et al., 1985).
It has been proposed that multiciliated ependymal cells lining the lateral wall of the lateral ventricle in adult mice could act as slowly proliferating neural stem cells (Johansson et al., 1999). However, several groups have not been able to produce multipotent stem cells from purified ependymal cells (Chiasson et al., 1999; Laywell et al., 2000; Capela and Temple, 2002; Doetsch et al., 2002), and the extent to which ependymal cells divide and self-renew in vivo is a matter of debate. Smart (1961) injected 3-d-old mice with [3H]thymidine and found no labeled ependymal cells in the lateral ventricle 32 d later. Imamoto et al. (1978) administered three [3H]thymidine injections at 7 hr intervals to young rats and also found no labeled ependymal cells in the lateral ventricle. After two [3H]thymidine injection in adult rats, none of the ependymal cells were labeled in either the third ventricle (Altman, 1963) or the central canal of the spinal cord (Kerns and Hinsman, 1973). In contrast to these findings, other reports suggest that ependymal cells continue to divide in the adult. It has been suggested that as many as 22% of the ependymal cells in 4-week-old mouse forebrain become labeled after multiple [3H]thymidine injections over a 30 d period (Kraus-Ruppert et al., 1975). Another study suggested that 2.6% of ependymal cells in the anterior lateral ventricle of a 1-d-old rat are labeled by one injection of [3H]thymidine after survival of 1 hr (Chauhan and Lewis, 1979). A few labeled cells were also observed in the walls of the lateral ventricle after [3H]thymidine injection in both 15-d-old and 5- to 7-week-old mice, and these cells were suggested to correspond to dividing ependymal cells (Korr, 1978). When bromodeoxyuridine (BrdU) was continuously supplied in the drinking water over a 2-6 week period, cells lining the lateral wall of the lateral ventricle were found labeled. It was suggested that these cells correspond to ependymal cells (Johansson et al., 1999), but because these labeled cells were not identified using high-resolution techniques, the proliferative capacity of ependymal cells in the adult remains uncertain.
Using light microscopy, we were not able to find evidence of ependymal cells that incorporated BrdU in adult brain. However, this could be because of a low incidence of proliferation in this cell population. We therefore used light and electron microscopy (EM) to identify cells labeled close to the walls of the lateral ventricle after 6 weeks of infusion with [3H]thymidine. We found no evidence that ependymal cells incorporate [3H]thymidine in the adult brain. Instead, we found that the majority of ependymal cells are generated between embryonic day 14 (E14) and E16. Interestingly, ependymal cell differentiation and the formation of ependymal cilia occur later, during the first postnatal week. During this period of ependymal differentiation, we found that a subpopulation of radial glia had prominent deuterosomes in their apical cytoplasm. Because deuterosomes have been associated with the early development of cilia, this suggested that these radial glia serve as progenitors of the new ependymal cells. We confirmed this hypothesis by genetically tagging a subpopulation of radial glia in neonatal mice and showing that some of these cells generated ependymal cells. These results indicate the time of birth and cellular origin of ependymal cells and strongly suggest that these cells do not divide and therefore cannot function as neural stem cells in the adult animal.
Materials and Methods
[3H]thymidine infusions. All animal care was in accordance with the guidelines of the National Institutes of Health and the University of California. [3H]thymidine (specific activity, 6.7 Ci/mm; PerkinElmer Life Sciences, Emeryville, CA) was infused continuously under the skin of adult CD-1 male mice (2-3 months of age) with a mini-osmotic pump (flow rate 0.5 μl/hr; 7 d duration; Alzet model 1007D; Alzet, Cupertino, CA). The pump was replaced every 5 d. After 6 weeks of continuous infusion, the pump was removed, and mice were killed 2 weeks later. In this study, each brain was cut in 200-μm-thick serial coronal vibratome sections, and five vibratome sections from each brain were processed for autoradiography and EM analysis as described previously (Doetsch et al., 1997). Briefly, 1.5-μm-thick semithin sections were cut with a diamond knife and mounted onto slides, dipped in autoradiographic emulsion, exposed for 4 weeks at 4°C, developed in Kodak (Rochester, NY) D-19, and counterstained with 1% toluidine blue.
Cell counts. For each 200-μm-thick vibratome section, nine 1.5-μm-thick semithin sections that were spaced apart by 7.5 μm each were analyzed. A cell was considered labeled if five or more silver grains overlaid the nucleus and the same cell was labeled in three or more consecutive sections. Ependymal cells were identified at the light microscope by their large spherical nuclei, multiciliated somata, and light cytoplasm. Astrocytes were distinguished from ependymal cells by their light nuclei with deep invaginations. Fifteen [3H]thymidine-labeled cells observed in the semithin sections were selected for additional identification at the electron microscope.
BrdU administrations. BrdU (Sigma, St. Louis, MO) at 50 μg/gm body weight (10 mg/ml stock, dissolved in 0.9% saline) was injected once at various stages from the 10th day of pregnancy to postnatal day 4 (P4). BrdU was injected intraperitoneally into the mother or subcutaneously into the young mouse postnatally.
BrdU immunocytochemistry. BrdU-treated mice were transcardially perfused with 0.9% saline as adults (2-3 months), and their brains were removed. The lateral walls of the lateral ventricles were dissected as described previously (Doetsch and Alvarez-Buylla, 1996). The resulting whole mounts were fixed overnight in 4% paraformaldehyde (PFA) and washed overnight at 4°C in PBS containing 30% sucrose. Coronal sections (10 μm thick) were cut on a cryostat, and every 10th section was mounted on glass slides. An average of 20 sections per animal were incubated in 60% formamide-2× SSC buffer at 54°C for 20 min, rinsed in 2× SSC for 5 min, incubated in 2N HCl at 37°C for 30 min, and rinsed in 0.1 m boric acid, pH 8.5. Sections were rinsed three times for 10 min each in Tris buffered saline (TBS), pH 7.4, and preblocked for immunocytochemistry (ICC) for 30 min in TBS with 0.1% Triton X-100 and 10% normal horse serum. Sections were incubated for 48 hr at 4°C with rat anti-BrdU monoclonal antibody (mAb) (1:200; Accurate Chemicals, Westbury, NY). BrdU staining was revealed by rhodamine-conjugated anti-rat IgG (1:200; Jackson ImmunoResearch, West Grove, PA) for 2 hr at room temperature and rinsed three times in TBS. Sections were postfixed for 15 min in 3% PFA and incubated for 48 hr at 4°C with rabbit S100β polyclonal antibody (1:500; DakoCytomation, Carpinteria, CA), which is a marker of ependymal cells (de Vitry et al., 1981; Didier et al., 1986; Chiasson et al., 1999; Lim et al., 2000). S100β staining was revealed by cyanine 2-conjugated anti-rabbit mAb (1:200; Jackson ImmunoResearch) for 2 hr at room temperature. For each animal analyzed, the total number of BrdU and S100β double-labeled cells in the ventricular zone (VZ) of the lateral ventricle was counted on 20 coronal sections, and the position of labeled cells was mapped on 10 coronal sections along the rostrocaudal axis using a camera lucida.
S100β and GLAST immunocytochemistry. The lateral walls of the lateral ventricles of P2 and P6 mice were dissected as above. The resulting whole mounts were fixed overnight in 4% PFA and washed overnight at 4°C in PBS containing 30% sucrose. Histological coronal sections were cut at 10 μm on a cryostat and preblocked for ICC for 30 min in TBS with 0.1% Triton X-100 and 10% normal horse serum. Sections were incubated overnight at 4°C with rabbit anti-S100β polyclonal antibody and guinea pig anti-GLAST antibody (1:1000; Chemicon, Temecula, CA). S100β and GLAST staining were revealed by Alexa594- and Alexa488-conjugated anti-rabbit and anti-guinea pig mAb (1:200; Jackson ImmunoResearch), respectively, for 2 hr at room temperature.
Scanning electron microscopy. The lateral walls of the lateral ventricles of E18 embryos and newborn (P0), P2, and P4 mice (three animals per age group) were dissected as above. The resulting whole mounts were fixed in 2.5% glutaraldehyde and 2% PFA overnight, washed twice in 0.1 m phosphate buffer (PB), and incubated for 2 hr in 2% osmium tetroxide (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 m PB. Tissues were dehydrated through an ethanol series, washed twice in 100% ethanol, dried with liquid CO2, mounted, sputter-coated, and viewed using a JEOL (Akishima, Japan) scanning electron microscope.
Anti-acetylated α-tubulin or S100β immunocytochemistry and mapping of multiciliated ependymal cells. The lateral walls of the lateral ventricles of newborn (P0; n = 5), P2 (n = 6), and P4 (n = 5) mice were dissected using the protocol of Doetsch and Alvarez-Buylla (1996). Whole mounts were blocked for 1 hr in 1% bovine serum albumin (BSA) in PBS, incubated for 24 hr at 4°C with 1:10 dilution of anti-acetylated α-tubulin (C3B9) monoclonal antibody (kind gift from Dr. K. Gull, School of Biological Sciences, Manchester, UK) or with 1:500 dilution of S100β antibody (DakoCytomation), and revealed with secondary anti-mouse IgG and anti-rabbit IgG, respectively, coupled to rhodamine secondary antibodies (Jackson ImmunoResearch). The stained walls were washed three times and visualized under fluorescence optics. Controls in which individual primary antibodies were omitted or replaced with other nonspecific monoclonal antibodies resulted in no detectable staining. Multiciliated ependymal cells were mapped using a camera lucida.
Ependymal lineage tracing. Radial glia were labeled in P0 animals by injection of an adenovirus expressing Cre-recombinase into the ventrolateral striatum of 10 R26R (Soriano, 1999) or Z/EG (Novak et al., 2000) Cre reporter mice. In these animals, Cre-mediated recombination results in the expression of LacZ [visualized with β-galactosidase (β-gal) reaction using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal)] or enhanced green florescent protein (eGFP) reporter genes, respectively. Because radial glia are the only cells in the P0 VZ that have processes that extend though the injection site, this method selectively labels this population of cells (Tramontin et al., 2003; Merkle et al., 2004). GFP-expressing mice were used for light microscopic examination of labeled cells and double immunocytochemistry with S100β or CD24. R26R mice were used for ultrastructural analysis, because β-gal precipitate can be readily identified at the EM (Doetsch et al., 1997). Animals were killed 2 d after injection to verify that only radial glia were labeled in the VZ (n = 5 for R26R; n = 6 for Z/EG) and 30 d after injection to examine the progeny of these labeled radial glia (n = 5 for R26R; n = 6 for Z/EG). All animals were killed by transcardial perfusion, and the brains of R26R animals were prepared for β-gal staining and electron microscopy as described previously (Herrera et al., 1999). The brains of Z/EG mice were postfixed for 16 hr in 4% PFA and vibratome sectioned at 50 μm. Sections were preblocked for ICC in 2% BSA plus 8% normal donkey serum in TBS for 1 hr at room temperature. To enhance the eGFP signal, sections were immunostained with rabbit anti-GFP polyclonal (1:1000; Novus Biologicals, Littleton, CO) or sheep anti-GFP polyclonal antibody (1:100; Biogenesis, Kingston, NH) diluted in blocking solution. To confirm the identity of the labeled cells, the sections were counter-stained for the ependymal cell-specific markers S100β (1:1000; DakoCytomation) or CD24 (1:500; BD PharMingen, San Diego, CA) in blocking solution, respectively. Sections were incubated in primary antibodies for 48 hr at 4°C and washed five times for 15 min each in TBS. GFP staining was visualized with Alexa488-conjugated anti-rabbit IgG or anti-sheep IgG (1:400; Molecular Probes, Eugene, OR) and S100β and CD24 were visualized with Alexa594-conjugated anti-rat IgG or anti-rabbit IgG (1: 400; Molecular Probes), respectively, diluted in blocking solution. Sections were stained for 12 hr at 4°C and washed three times for 20 min each in TBS at room temperature before counterstaining for 4′,6′-diamidino-2-phenylindole and mounting. Ependymal cells were identified by their multiciliated cuboid morphology, contact with the ventricle, and double labeling with ependymal markers.
Results
Ependymal cells do not divide in the normal adult brain
To test whether ependymal cells divide in vivo in adult mice, BrdU (1 mg/ml) was given to mice in their drinking water for 2 weeks, and mice were killed 1 week after treatment. Their brains were processed for cryostat sections and double labeled with anti-S100β and anti-BrdU antibodies to identify labeled ependymal cells. No double-labeled cells were observed, which is consistent with other studies using BrdU labeling (data not shown) (Doetsch et al., 1999; Gregg and Weiss, 2003). However, it is possible that ependymal cells divide rarely, escaping detection with the above procedure. We therefore infused [3H]thymidine into adult mice for 6 weeks using mini-osmotic pumps (n = 6). After two additional weeks without [3H]thymidine, mice were killed, and their brains were processed for semithin sections. This technique would label cells that may be dividing infrequently and allows high-resolution, thin-section analysis at the light and electron microscope. Serial 1.5 μm plastic sections were studied at five different levels along the rostrocaudal axis of the lateral ventricular wall. Numerous [3H]thymidine-labeled cells were present close to the lateral wall of the lateral ventricle. Most of these [3H]thymidine-labeled cells were clearly located in the subventricular zone (SVZ) and identified as astrocytes (type-B cells) or type-C cells (Doetsch et al., 1997). The 1.5 μm sections were serially scanned focusing on the ependymal layer for evidence of labeled cells. We scanned 10,032 ependymal cells (large spherical nuclei, multiciliated somata, and light cytoplasm). None of these cells was labeled with [3H]thymidine. A total of 323 labeled nuclei were found close to the ventricle (Table 1), of which 15 appeared to be within the ependymal layer. We resectioned each of these cells at 70 nm and analyzed them by transmission EM (Fig. 1A,B; Table 1). None of them corresponded to multiciliated ependymal cells. Instead, these cells had characteristics of type-B cells as described previously (Doetsch et al., 1997). Some of these cells contacted the ventricle, but others, while within the ependyma, were separated from the ventricle by thin ependymal processes. These results indicate that multiciliated ependymal cells lining the lateral wall of the lateral ventricles do not divide in normal adult mouse brain, suggesting that these cells are produced exclusively during development.
Table 1.
Number of ependymal cells labeled with [3H]thymidine after 6 weeks of infusion
|
Animal |
Labeled ependymal cells/number of ependymal cells examined |
Number of cells studied by TEM |
|---|---|---|
| 1 | 0/1881 | 36 |
| 2 | 0/1608 | 59 |
| 3 | 0/1536 | 52 |
| 4 | 0/1740 | 57 |
| 5 | 0/1674 | 76 |
| 6 |
0/1593 |
43 |
[3H]thymidine was infused for 6 weeks, and mice were killed after 2 weeks without [3H]thymidine. The number of labeled ependymal cells of total ependymal cells analyzed is shown. The number of cells checked by transmission EM (TEM) is also shown.
Figure 1.
Multiciliated ependymal cells do not divide in the adult normal brain. A, In mice infused with [3H]thymidine for 6 weeks and killed 2 weeks after pump removal, we did not see labeled cells that corresponded to ependymal cells. Light microscopy of semithin sections revealed unlabeled ependymal cells (arrowhead) but also labeled cells that were close to the ventricle (arrow). B, All such labeled cells were processed for transmission EM in ultrathin sections. They all corresponded to type-B cells and never to ependymal cells. The cell shown in B (arrow) touches the ventricle (asterisk) but has the characteristics of a type-B cell, including an irregular contour and light cytoplasm, and lacks the cilia, microvilli, and lipid droplets characteristic of ependymal cells.
Birth-dating ependymal cells in the lateral ventricular wall
To determine the birth date of ependymal cells, mice were exposed to BrdU at E10, E12, E14, E16, E18, or P0, P2, and P4 and killed at P90. Brains were collected, sectioned, and double labeled for BrdU and for S100β, a protein expressed by ependymal cells (see Fig. 6A). Analysis was performed on the lateral wall of the lateral ventricle.
Figure 6.
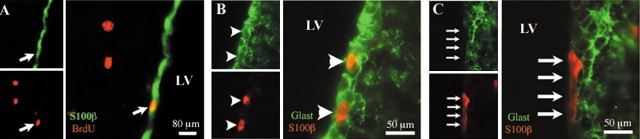
Radial glial transformation into ependymal cells. A, In the adult, some ependymal cells (S100β+) in the lateral ventricular wall are coimmunostained with BrdU (arrow) when mice were exposed to BrdU as embryos (E14). B-C, At P2, S100β+ cells are also GLAST+ and likely correspond to transforming radial glia-ependymal cells (B, arrowheads). In contrast, most S100β+ cells at P6 are GLAST- and correspond to mature ependymal cells (C, arrows). LV, Lateral ventricle.
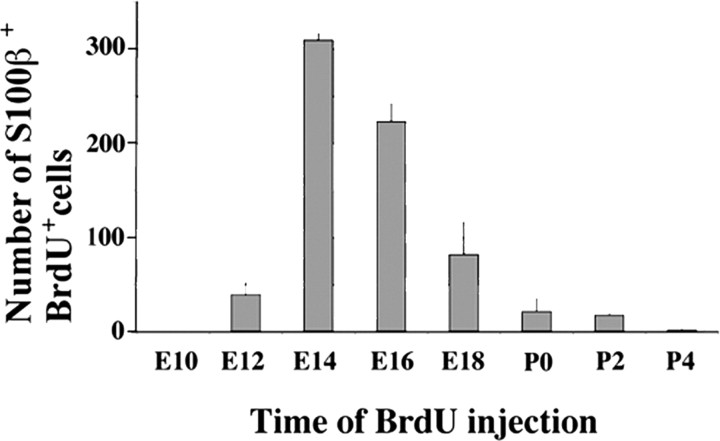
In animals injected at E10 (n = 3), no double-labeled (BrdU+, S100β+) cells were found in the lateral wall of the lateral ventricles. In animals injected on E12 (n = 3), an average of 38 ± 12 labeled BrdU+ ependymal cells were counted on 20 sections per animal. The number of double-labeled ependymal cells peaked at E14 (n = 3) and E16 (n = 3) to an average of 309 ± 7 and 222 ± 19, respectively (Fig. 2). By E18 (n = 4), the number of BrdU+ ependymal cells had decreased to an average of 81 ± 35 labeled cells on 20 sections. In animals injected at P0 (n = 4) and P2 (n = 3), the number of labeled ependymal cells decreased further to an average of 21 ± 13 and 17 ± 2 cells per 20 sections, respectively. In the animals injected at P4 (n = 3), almost no ependymal cells were BrdU labeled. These results indicate that ependymal cells are primarily born in the embryo and that most of these cells are produced between E14 and E16.
Figure 2.
Birthdays of multiciliated ependymal cells. To determine the birth date of ependymal cells, mice were exposed to BrdU at E10, E12, E14, E16, E18, or P0, P2, and P4 and killed at P90. Bars show the mean number of BrdU and S100β double-labeled cells per 20 sections of the lateral ventricular wall of adult mice.
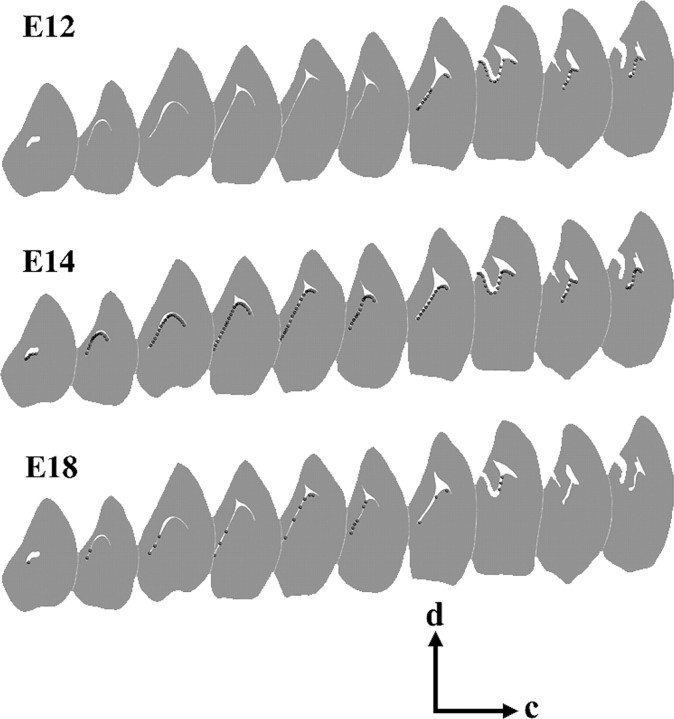
To characterize how ependymal cells arise along the rostrocaudal axis, we mapped the position of double-labeled (BrdU+, S100β+) cells using camera lucida drawings in coronal sections through the lateral ventricular wall of adult mice, each exposed to BrdU on E12, E14, and E18 (Fig. 3). In animals injected at E12, double-labeled cells were exclusively located in caudal regions of the brain. At E14, BrdU+S100β+ cells were found along the entire rostrocaudal axis, and in the animals injected at E18, double-labeled cells were mainly localized in the rostral section of the ventricular wall. These results suggest that ependymal cells are first born in caudal regions of the brain and progressively arise in more rostral regions.
Figure 3.
Birth of ependymal cells along the lateral ventricle. Camera lucida drawings of BrdU and S100β double-labeled cells in coronal sections through adult lateral ventricular wall. Mice received BrdU as embryos at E12, E14, or E18. Double-labeled cells first arise in caudal regions at E12 and progressively appear in more rostral regions at E14 and E18. Dorsal (d) is up, and caudal (c) is right.
Maturation of ependymal cells
To determine the time course of ependymal cell maturation and the location where they first appear in the ventricular wall, we mapped the position of multiciliated ependymal cells using C3B9 (acetylated α-tubulin) or S100β immunocytochemistry and scanning electron microscopy of the lateral wall of the lateral ventricle in animals killed at E16, E18, P0, P2, P4, and P10. A cell was identified as an ependymal cell based on the presence of tufts of cilia on their luminal (apical) surface (Fig. 4C,F,I).
Figure 4.
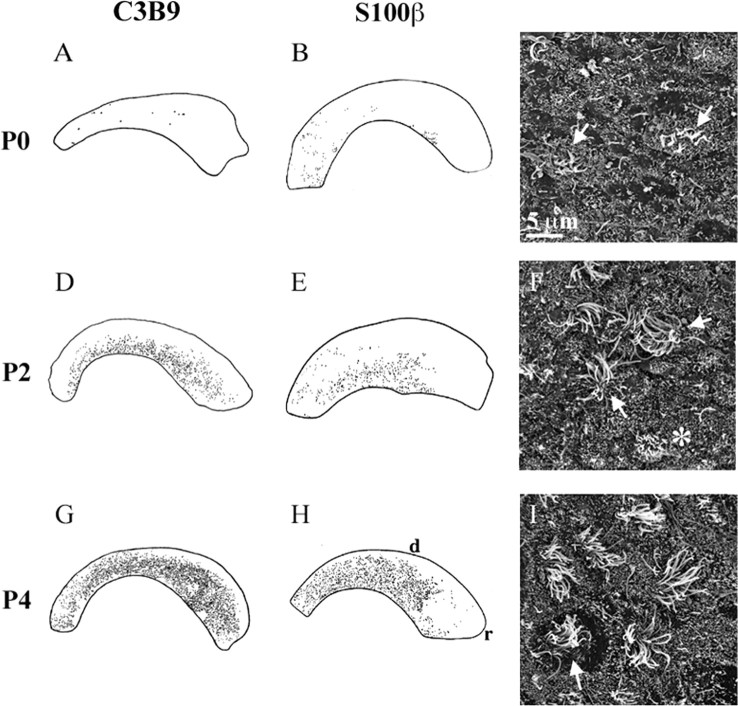
Multiciliated ependymal cells maturate along a caudorostral and ventrodorsal gradient. Camera lucida drawings of C3B9 (A, D, G) and S100β (B, E, H) immunostaining of whole mounts of the lateral ventricular wall show examples of the distribution of multiciliated ependymal cells along the lateral ventricular wall at P0 (A, B), P2 (D, E), and P4 (G, H). Dorsal (d) is up and rostral (r) is right. C, F, I, Scanning electron photomicrographs of the lateral ventricular surface at P0 (C), P2 (F), and P4 (I). At P0, short ependymal cilia start budding out of the ventricular surface (C, arrows). At P2, both immature (F, asterisk) and more mature ependymal cells with longer cilia appear throughout the lateral ventricular wall (F, arrows). Note that the surface of some ependymal cells contains few, if any, microvilli (Fig. 4 I, arrow).
Before birth, no multiciliated ependymal cells could be detected at the scanning electron microscope or with immunocytochemistry (data not shown). In brains analyzed at birth (P0; n = 5), an average of 19 ± 3 multiciliated ependymal cells were observed in the entire lateral wall of the lateral ventricle. These cells were localized in the ventral part of the lateral wall (Fig. 4A,B). At this age, ependymal cilia were short (<5 μm long) (Fig. 4C), suggesting that ciliary budding had just begun. The number of ependymal cells increased rapidly between P0 and P2. At P2 (n = 6), an average of 670 ± 101 ependymal cells were counted on the lateral walls in each brain. The majority of these cells were localized in the ventral and caudal aspect of the lateral wall (Fig. 4D,E). Cilia in ependymal cells on the caudal part of the lateral wall were longer and more numerous than on the rostral aspect of the lateral wall, suggesting that ependymal cells mature first caudally and ventrally. By P4 (n = 5), an average of 4350 ± 276 ependymal cells were counted in each brain and were widely distributed in the ventral and caudal part of the lateral ventricular wall (Fig. 4G,H). After P4, ependymal cells were widespread throughout the lateral wall of the lateral ventricles and too numerous to be counted accurately, because it was not possible to distinguish neighboring ependymal cells.
Identification of maturing ependymal cells: the presence of deuterosomes
The above observations suggest that cells born in the embryo complete their differentiation into multiciliated ependymal cells during the first postnatal week. To identify early stages in the differentiation of ependymal cells, we studied the ultra-structure of VZ cells at various developmental stages. We reported previously on the organization and composition of the VZ in neonatal mice (Tramontin et al., 2003). This pseudostratified epithelium contains the cell bodies of radial glia, most of which contact the ventricle and extend a single 9 + 0 cilium (primary cilium) into the ventricular lumen. The backbone of the ciliary axoneme of a 9 + 0 cilium is formed by nine microtubule doublets arranged in a circle, whereas a 9 + 2 cilium contains two additional microtubule doublets in the center. VZ cells were identified as radial glia at the electron microscope based on their elongated nuclei with lax chromatin, long radial process, and single 9 + 0 cilium (Hinds and Ruffett, 1971). A small proportion of these radial glia also had deeply invaginated nuclei (Fig. 5A), cytoplasmic electron-dense granules or aggregates (Fig. 5B), and deuterosomes between the nucleus and the apical cell surface (Fig. 5D-G).
Figure 5.
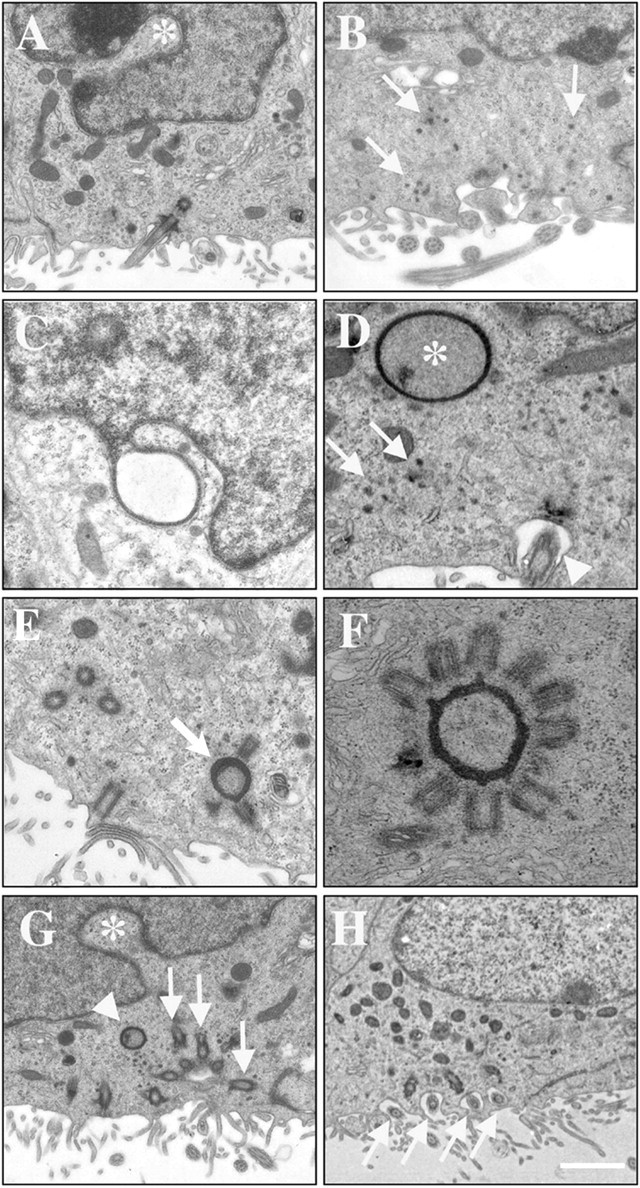
Multiciliated ependymal cells differentiate from radial glial cells. Transmission electron photomicrographs of the putative steps of differentiation of a radial glial cell into a multiciliated ependymal cell are shown. A, B, In neonates, the nuclei of some radial glia are deeply invaginated (A, asterisk), and their cytoplasm contains electron-dense granules between the nucleus and the apical cell surface (B, arrows). These cells still possess a single 9 + 0 cilium that identifies them as radial glia (A, arrowhead). C, D, As development proceeds (P2-P4), structures attached to the nucleus by a very thin process (C, asterisk) or found in the cytoplasm very near the nuclear envelope (D, asterisk) appear similar to deuterosomes and probably evaginate from the nuclear envelope. At this stage, the cells still extend a 9 + 0 cilium at their surface (D, arrowhead) and still contain electron-dense aggregates in the cytoplasm (D, arrows). E, F, Deuterosomes located closer to the apical cell surface (E, arrow) are associated with as many as 12 immature procentrioles (F). G, In immature ependymal cells, the nuclei of the cells still contain invaginations (asterisk), the deuterosome is still present (arrowhead), and basal bodies are found closer to the luminal surface of the cell (arrow). H, Mature multiciliated ependymal cells have a round nucleus and unclumped chromatin. Deuterosomes have disappeared, and mature centrioles now serve as ciliary basal bodies (arrows). Scale bar: A, G, 0.8 μm; B-E, H, 0.5 μm; F, 0.15 μm.
Deuterosomes are large electron-opaque spherical bodies without limiting membranes and are typically found in the cytoplasm between the nucleus and the apical cell surface. They have been described in multiciliated epithelial cells (Sorokin, 1968; Loots and Nel, 1989; Hagiwara et al., 1992) but have not been detected previously in the brain. Deuterosomes are thought to be generated by aggregation and condensation of dense cytoplasmic granules and are thought to serve as the core for centriole formation (Hagiwara et al., 2000). Our observations confirm the finding that deuterosomes are nucleation centers for cilial basal bodies in multiciliated cells (Fig. 5C-F) (Dirksen, 1971); we sometimes observed deuterosomes with multiple basal bodies radiating outward from their surface (Fig. 5F). As development proceeded, we observed cells with many basal bodies close to the apical (luminal) surface of the cell that had apparently dispersed from deuterosomes (Fig. 5G,H). In many cells, we could see small 9 + 2 cilia extending from these basal bodies. These cells had lost the single 9 + 0 cilium that is a hallmark of radial glia. In mature ependymal cells, all cilia have a 9 + 2 organization.
The origin of the deuterosomes is not known and has not been described previously in the developing VZ. Interestingly, in maturing ependymal cells, we often found deuterosome-like structures associated with the nucleus. These structures did not contain chromatin but appeared to be evaginating from the nuclear envelope. In several cases, we found these structures attached to the nucleus by only a very thin process (Fig. 5C). More often, deuterosomes without associated basal bodies were found very near the nuclear envelope. At this stage, the cells still extended a 9 + 0 cilium at their surface (Fig. 5D, arrowhead). Deuterosomes located closer to the apical cell surface were always found in close association with the electron-dense aggregates mentioned above and often had basal bodies radiating from their surface (Fig. 5E,F). These observations suggest that deuterosomes may be derived from the nucleus. Furthermore, the progressive acquisition of ependymal characteristics in cells that initially had radial glial characteristics suggests that radial glia transform into ependymal cells.
Our analysis suggests that radial glia develop two unique ultrastructural characteristics as they transform into multiciliated cells: electron-dense aggregates and deuterosomes. We analyzed the VZ of the lateral ventricle at different ages to determine the proportion of cells that had one or both of these characteristics. We could not find any cells with these characteristics at E10, E12, E14, or E16. At E18, we studied 246 cells and found two cells with electron-dense aggregates (n = 2 brains). At P0, these cells were more prevalent (7 of 173 cells; n = 2 brains), but we did not observe any cell with deuterosomes. At P2, however, 17 of 191 cells had both deuterosomes and aggregates, and more than one-fourth of the cells analyzed (51 of 191) had electron-dense aggregates. By P7, 54 of 164 cells had electron-dense aggregates, 64 had both aggregates and deuterosomes, and 11 had matured into multiciliated ependymal cells (n = 2 brains). By P15, mature ependymal cells dominated the VZ, and we did not observe any cells with electron-dense aggregates or deuterosomes. These results suggest that as radial glia transform into multiciliated cells, they first develop electron-dense aggregates and then form deuterosomes that serve as nucleation centers for the basal bodies of 9 + 2 cilia.
Radial glia give rise to ependymal cells
The results described above suggested that radial glia give rise to ependymal cells. To identify the transformation of radial glial cells to ependymal cells, we performed double-labeling experiments on coronal cryostat sections of the lateral wall of the lateral ventricle with the radial glial cell marker GLAST and the ependymal cell marker S100β at P2 and P6. Most S100β+ cells expressed the radial glia marker GLAST at P2 (Fig. 6B) and became GLAST- by P6 (Fig. 6C). These observations suggest that some radial glial cells transform into ependymal cells by going through a stage in which they express both radial glial and ependymal cell markers.
To confirm these results, we specifically labeled neonatal (P0) striatal radial glia to determine whether they gave rise to ependymal cells. Radial glia can be labeled by an injection of tracer at a distance from the cell bodies in the VZ, in regions traversed by their long processes (Voigt, 1989; Malatesta et al., 2000, 2003). Radial glia are the only cell type in the P0 VZ that have processes that extend through the lateral striatum (Tramontin et al., 2003). We labeled radial glia at their distal processes by injecting an adenovirus encoding Cre recombinase into the ventrolateral striatum of mice with floxed lacZ (R26R mice) (Soriano, 1999) or floxed eGFP (Z/EG mice) (Novak et al., 2000) reporter genes (Merkle et al., 2004). Two days after injection, we observed small groups of labeled radial glia in the lateral ventricular wall (Fig. 7C). We confirmed that these cells were radial glia by immunohistochemistry and by electron microscopy. Four days after injection, cells in the medial ventricular wall expressed the ependymal cell marker S100β, but this expression was very weak in the lateral wall (Fig. 7D). However, some labeled cells in the lateral wall had a radial glial morphology but were multiciliated and coexpressed GFP and the ependymal cell marker CD24 (Fig. 7F). This suggests that radial glia transform into ependymal cells and go through transitional stages in which they display the morphological and antigenic characteristics of both radial glia and ependymal cells. In Z/EG mice examined 30 d after injection, some GFP-labeled cells in the VZ were S100β+ and had a multiciliated, cuboidal morphology (Fig. 7H). We confirmed these observations using electron microscopy on P30 R26R mice injected at P0 to label radial glia as described above. After β-gal staining, LacZ-positive (radial glia derived) cells had electron-dense, perinuclear X-gal deposits. In the ependymal layer, we observed X-gal-positive cells that had the ultrastructural characteristics of ependymal cells, including cilia, microvilli, light cytoplasm, and round nuclei (Fig. 7G). Interestingly, the basal bodies from which these cilia form were sometimes surrounded by X-gal deposits (Fig. 7E). Because basal bodies are associated with deuterosomes, this observation supports the hypothesis that deuterosomes are derived from the nuclear membrane, which is where X-gal deposits are normally found in labeled cells. Together, these observations demonstrate that ependymal cells are derived from radial glial cells.
Figure 7.

Radial glia give rise to ependymal cells. A, B, Schematic depicting the injection site in the ventrolateral striatum at P0 (A) and the resulting labeling pattern at P2 (B). Injection of an adenovirus expressing Cre recombinase results in labeling of infected cells in mice that report Cre recombination with either lacZ (R26R mice) or eGFP (Z/EG mice). Radial glia are infected at their long processes that pass through the striatal injection site. C, The only labeled cells in the VZ are radial glia (region from box in B) for which cell bodies appear to undergo interkinetic nuclear migration (arrow). LV, Lateral ventricle. D, At P4, radial glia in the lateral ventricular wall do not express S100β or only express it very weakly (arrows), although expression is strong in the medial wall. In this example, the ventricle collapsed during histological processing so the medial and lateral walls are touching (dotted line), allowing for direct comparison of S100β expression. Some S100β+ cells have radial processes, suggesting that they are derived from cells with radial processes. These cells can be seen on both the medial (arrowhead) and lateral (data not shown) ventricular walls. E, F, Intermediate radial glia-ependymal stages. Cells with radial glial morphology, including a long radial process (F, arrows), express the ependymal cell marker CD24 and are beginning to produce cilia (arrowhead). The basal bodies from which these cilia form (E, boxed region) are surrounded by X-gal precipitate in R26R animals injected with Ad:Cre at P0 (E, arrows). This demonstrates that immature multiciliated cells in the lateral ventricular wall are derived from radial glia and suggests that basal bodies were associated with deuterosomes derived from the nuclear membrane, where the X-gal precipitate is normally found. G, H, Radial glia give rise to mature ependymal cells. By P30, animals injected with Ad:Cre at P0 in the ventrolateral striatum have ependymal cells in the lateral wall of the lateral ventricle. These cells were localized in a patch in the same region where radial glia were originally labeled. In injected R26R animals, these cells have X-gal precipitate surrounding the nucleus and the basal bodies (G, arrowheads) and have the ultrastructural characteristics of mature ependymal cells including a light cytoplasm, round nucleus, cilia (G, arrow), and microvilli (G, asterisk). In injected Z/EG animals, these cells coexpress GFP (H, arrows) and S100β (H, arrowheads) throughout their cell bodies, including their cilia.
Discussion
We show here that multiciliated ependymal cells in the lateral wall of the lateral ventricle of the brain do not divide in the adult. These cells are derived from radial glial cells, the majority of which undergo their final nuclear DNA synthesis between E14 and E16. Ciliogenesis begins around birth and progresses caudorostrally and ventrodorsally along the lateral wall of the lateral ventricles of the rodent brain. It has been suggested that ependymal cells can function as self-renewing stem cells in the adult brain (Johansson et al., 1999). The proliferative capability of ependymal cells remained controversial (Bruni, 1998) and fueled an ongoing discussion of whether these cells could function as neural stem cells (Chiasson et al., 1999; Doetsch et al., 1999; Johansson et al., 1999; Laywell et al., 2000; Capela and Temple, 2002). It has been argued that multiciliated ependymal cells divide so slowly that 2-6 weeks exposure of proliferation markers is required to detect those that divide (Johansson et al., 1999). We infused [3H]thymidine continuously for 6 weeks and studied [3H]-labeled cells on the walls on the lateral ventricle at five different rostrocaudal levels and specifically focused on those cells that were closest to the ventricle to increase the possibility of detecting a labeled ependymal cell. We found no evidence for ependymal cell division. We conclude that multiciliated ependymal cells are postmitotic in the adult rodent brain.
Differences in the techniques used to identify ependymal cells and/or dividing cells may account for the discrepancies found in the literature (Altman, 1963; Korr, 1980; Johansson et al., 1999). Indeed, some [3H]thymidine or BrdU-positive cells touching the ventricles could be considered ependymal cells on thicker sections when observed at the light microscope. However, when these cells are analyzed at the electron microscope, they are not multiciliated and possess the ultrastructural features of astrocytes (Fig. 1A,B). It has been argued that ependymal cells could still function as stem cells if they transformed into another cell type before division (e.g., an SVZ astrocyte). This is extremely un-likely, because it would result in a progressive depletion of ependymal cells on the walls of the lateral ventricle. Furthermore, if ependymal cells were regenerating from another mitotic cell type, we should have detected labeled ependymal cells in our [3H]thymidine-labeling experiment. The postmitotic nature of mature ependymal cells is consistent with the observation that multiciliated epithelial cells with numerous basal bodies in the apical cytoplasm are postmitotic (Lange et al., 2000) and that the ependymal layer in the mammalian brain does not regenerate when it is injured (Sarnat, 1995).
Work from other groups suggests that ependymal cells develop regionally along a caudal to rostral gradient (Sarnat, 1992; Bruni, 1998). In the mouse, Rakic and Sidman (1968) demonstrated that ependymal cells from the subcommissural organ of the third ventricle undergo their final division between E11 and E13, which is consistent with the present study showing that the majority of multiciliated ependymal cells from the lateral wall of the lateral ventricle arise between E14 and E16 (Fig. 2). Interestingly, there was a lag between final cell division and appearance of cilia on the walls of the lateral ventricles. Ependymal cilia appeared progressively on this wall between P0 and P4. This suggested that the subpopulation of cells that transform into ependymal cells divides very infrequently, if at all, after birth. Close examination of the ventricular wall suggested that these ependymal precursors correspond to radial glial cells that appear to go through intermediate stages as they transform into ependymal cells. Interestingly, we found that these cells frequently contain deuterosomes. Ultrastructural analysis of these cells at different ages suggests that these structures are derived from nuclear evaginations and that they serve as nucleating organelles for the formation of the ependymal basal bodies. To our knowledge, this is the first description of this process.
We present direct evidence that ependymal cells are derived from radial glia. These cells were labeled at their distal process in the ventrolateral striatum of the neonatal brain with an adenovirus. Labeled radial glia were observed soon after viral injection in a restricted patch in the VZ of the dorsal lateral ventricle, corresponding to the cell bodies of radial glia for which processes project through the ventrolateral striatum. Labeled ependymal cells in the same region of the VZ were observed 30 d later, indicating that these cells are derived from radial glia. These observations suggest that radial glia or their progeny do not migrate tangentially before transforming into ependymal cells. The appearance of deuterosomes in cells with radial processes and the ultrastructural characteristics of radial glia suggest that at least some radial glial cells directly transform into ependymal cells. A short, radially oriented process is present in some ependymal cells (Gregg and Weiss, 2003). This process may be a remnant of the long radial glial process. Similar radial processes have been described previously in adult reptiles (Garcia-Verdugo et al., 2002), amphibians, and birds [Alvarez-Buylla et al. (1998) and references therein]. These observations, together with the morphological stages of radial glial transformation we describe, indicate that ependymal cells are derived regionally from subpopulations of radial glia. Interestingly, most of the radial glia destined to become ependymal cells appear to be postmitotic by P0 (Fig. 2). This delay in the transformation from radial glia to ependymal cell could be a reflection of the complex differentiation program and the time it takes to form ependymal cells or could be a mechanism to prevent premature ependymal differentiation. The data presented provide the times when this transformation is occurring and organelles that may be involved in the transformation. This information should help elucidate the molecular mechanisms regulating the early specification and delayed differentiation of ependymal cells.
Radial glial cells have been shown to function as primary precursors in the adult avian brain (Alvarez-Buylla et al., 2002) and in the developing mammalian CNS, giving rise to neurons and/or glia, depending on the age and region of the brain analyzed (Malatesta et al., 2000; Miyata et al., 2001; Noctor et al., 2001; Tamamaki et al., 2001; Merkle et al., 2004). It has been suggested previously, based on anatomical observations that radial glia could serve as precursors for ependymal cells. However, no direct evidence existed to support this claim. The present study provides direct support for this hypothesis. It suggests that some radial glial cells become committed to becoming or producing ependymal cells at the same time many neurons are produced in this same VZ. This finding further supports the central role that radial glial cells play as progenitors during development (Alvarez-Buylla et al., 2001). It will be interesting to investigate what factors induce the ependymal lineage and how radial glia generate the remarkable planar organization of the adult ependymal layer. The identification of the origin of ependymal cells in the present study will increase understanding of how these important cells fail to function correctly in a large number of pathologies, including hydrocephaly.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants NS28478 and HD32116 and by a gift from Frances and John Bowes. N.S. was supported by a Human Frontier Science Program fellowship (LT0423/2000-B). F.T.M. was supported by an Aging and Neurodegenerative Diseases Training Grant (AG00278-01) from NIH-National Institute on Aging. We thank Gail Martin for providing Cre reporter mice and all members of the Alvarez-Buylla laboratory for insightful and stimulating discussions. N.S. thanks Lyess Khouadja for his support and encouragement during this work and for very helpful assistance during the preparation of this manuscript.
Correspondence should be addressed to Arturo Alvarez-Buylla, Department of Neurosurgery and Program in Developmental and Stem Cell Biology, Box 0525, University of California San Francisco, San Francisco, CA 94143. E-mail: abuylla@itsa.ucsf.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250010-09$15.00/0
References
- Altman J (1963) Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec 145: 573-591. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Mateo AS, Merchant-Larios H (1998) Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. J Neurosci 18: 1020-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2: 287-293. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Seri B, Doetsch F (2002) Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull 57: 751-758. [DOI] [PubMed] [Google Scholar]
- Bleier R (1971) The relations of ependyma to neurons and capillaries in the hypothalamus: a Golgi-Cox study. J Comp Neurol 142: 439-463. [DOI] [PubMed] [Google Scholar]
- Brody SL, Yan XH, Wuerffel MK, Song SK, Shapiro SD (2000) Ciliogenesis and left-right axis defects in forkhead factor HFH-4-null mice. Am J Respir Cell Mol Biol 23: 45-51. [DOI] [PubMed] [Google Scholar]
- Bruni JE (1998) Ependymal development, proliferation, and functions: a review. Microsc Res Tech 41: 2-13. [DOI] [PubMed] [Google Scholar]
- Capela A, Temple S (2002) LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35: 865-875. [DOI] [PubMed] [Google Scholar]
- Cathcart III RS, Worthington Jr WC (1964) Ciliary movement in the rat cerebral ventricles: clearing action and directions of currents. J Neuropathol Exp Neurol 23: 609-618. [DOI] [PubMed] [Google Scholar]
- Chauhan AN, Lewis PD (1979) A quantitative study of cell proliferation in ependyma and choroid plexus in the postnatal rat brain. Neuropathol Appl Neurobiol 5: 303-309. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D (1999) Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci 19: 4462-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bigio MR (1995) The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia 14: 1-13. [DOI] [PubMed] [Google Scholar]
- de Vitry F, Picart R, Jacque C, Tixier-Vidal A (1981) Glial fibrillary acidic protein. A cellular marker of tanycytes in the mouse hypothalamus. Dev Neurosci 4: 457-460. [DOI] [PubMed] [Google Scholar]
- Didier M, Harandi M, Aguera M, Bancel B, Tardy M, Fages C, Calas A, Stagaard M, Mollgard K, Belin MF (1986) Differential immunocytochemical staining for glial fibrillary acidic (GFA) protein, S-100 protein and glutamine synthetase in the rat subcommissural organ, nonspecialized ventricular ependyma and adjacent neuropil. Cell Tissue Res 245: 343-351. [DOI] [PubMed] [Google Scholar]
- Dirksen ER (1971) Centriole morphogenesis in developing ciliated epithelium of the mouse oviduct. J Cell Biol 51: 286-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A (1996) Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci USA 93: 14895-14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A (1997) Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J Neurosci 17: 5046-5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97: 703-716. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A (2002) EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36: 1021-1034. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E (2002) The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull 57: 765-775. [DOI] [PubMed] [Google Scholar]
- Gregg C, Weiss S (2003) Generation of functional radial glial cells by embryonic and adult forebrain neural stem cells. J Neurosci 23: 11587-11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Shibasaki S, Ohwada N (1992) Ciliogenesis in the human oviduct epithelium during the normal menstrual cycle. J Electron Microsc (Tokyo) 41: 321-329. [PubMed] [Google Scholar]
- Hagiwara H, Ohwada N, Aoki T, Takata K (2000) Ciliogenesis and ciliary abnormalities. Med Electron Microsc 33: 109-114. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A (1999) Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann Neurol 46: 867-877. [DOI] [PubMed] [Google Scholar]
- Hinds JW, Ruffett TL (1971) Cell proliferation in the neural tube: an electron microscopic and Golgi analysis in the mouse cerebral vesicle. Z Zellforsch Mikrosk Anat 115: 226-264. [DOI] [PubMed] [Google Scholar]
- Imamoto K, Paterson JA, Leblond CP (1978) Radioautographic investigation of gliogenesis in the corpus callosum of young rats. I. Sequential changes in oligodendrocytes. J Comp Neurol 180: 115-128, 132-137. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J (1999) Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96: 25-34. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Hinsman EJ (1973) Neuroglial response to sciatic neurectomy. I. Light microscopy and autoradiography. J Comp Neurol 151: 237-254. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Watanabe M, Okada Y, Sawa H, Takai H, Nakanishi M, Kawase Y, Suzuki H, Nagashima K, Ikeda K, Motoyama N (2002) Hydrocephalus, situs inversus, chronic sinusitis, and male infertility in DNA polymerase lambda-deficient mice: possible implication for the pathogenesis of immotile cilia syndrome. Mol Cell Biol 22: 2769-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korr H (1978) Combination of metallic impregnation and autoradiography of brain sections. A method for differentiation of proliferating glial cells in the brain of adult rats and mice. Histochemistry 59: 111-116. [DOI] [PubMed] [Google Scholar]
- Korr H (1980) Proliferation of different cell types in the brain. Adv Anat Embryol Cell Biol 61: 1-72. [DOI] [PubMed] [Google Scholar]
- Kraus-Ruppert R, Laissue J, Burki H, Odartchenko N (1975) Kinetic studies on glial, Schwann and capsular cells labelled with [3H] thymidine in cerebrospinal tissue of young mice. J Neurol Sci 26: 555-563. [DOI] [PubMed] [Google Scholar]
- Kuchler S, Graff MN, Gobaille S, Vincendon G, Roche AC, Delaunoy JP, Monsigny M, Zanetta JP (1994) Mannose dependent tightening of the rat ependymal cell barrier. In vivo and in vitro study using neoglycoproteins. Neurochem Int 24: 43-55. [DOI] [PubMed] [Google Scholar]
- Lange BM, Faragher AJ, March P, Gull K (2000) Centriole duplication and maturation in animal cells. Curr Top Dev Biol 49: 235-249. [DOI] [PubMed] [Google Scholar]
- Laywell ED, Rakic P, Kukekov VG, Holland EC, Steindler DA (2000) Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc Natl Acad Sci USA 97: 13883-13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A (2000) Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28: 713-726. [DOI] [PubMed] [Google Scholar]
- Loots GP, Nel PP (1989) Early stages of ciliogenesis in the respiratory epithelium of the nasal cavity of rabbit embryos. Cell Tissue Res 255: 589-594. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hartfuss E, Gotz M (2000) Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127: 5253-5263. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Gotz M (2003) Neuronal or glial progeny: regional differences in radial glia fate. Neuron 37: 751-764. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A (2004) Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA, in press. [DOI] [PMC free article] [PubMed]
- Millhouse OE (1971) A Golgi study of third ventricle tanycytes in the adult rodent brain. Z Zellforsch Mikrosk Anat 121: 1-13. [DOI] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M (2001) Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31: 727-741. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR (2001) Neurons derived from radial glial cells establish radial units in neocortex. Nature 409: 714-720. [DOI] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG (2000) Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28: 147-155. [PubMed] [Google Scholar]
- Rakic P, Sidman RL (1968) Subcommissural organ and adjacent ependyma: autoradiographic study of their origin in the mouse brain. Am J Anat 122: 317-335. [DOI] [PubMed] [Google Scholar]
- Roth Y, Kimhi Y, Edery H, Aharonson E, Priel Z (1985) Ciliary motility in brain ventricular system and trachea of hamsters. Brain Res 330: 291-297. [DOI] [PubMed] [Google Scholar]
- Sarnat HB (1992) Role of human fetal ependyma. Pediatr Neurol 8: 163-178. [DOI] [PubMed] [Google Scholar]
- Sarnat HB (1995) Ependymal reactions to injury. A review. J Neuropathol Exp Neurol 54: 1-15. [DOI] [PubMed] [Google Scholar]
- Smart I (1961) The subependymal layer of the mouse brain and its cell production as shown by radioautography after thymidine-H3 injection. J Comp Neurol 116: 325-338. [Google Scholar]
- Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70-71. [DOI] [PubMed] [Google Scholar]
- Sorokin SP (1968) Centriole formation and ciliogenesis. Aspen Emphysema Conf 11: 213-216. [PubMed] [Google Scholar]
- Tamamaki N, Nakamura K, Okamoto K, Kaneko T (2001) Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res 41: 51-60. [DOI] [PubMed] [Google Scholar]
- Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK (2001) Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell 12: 589-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A (2003) Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex 13: 580-587. [DOI] [PubMed] [Google Scholar]
- Voigt T (1989) Development of glial cells in the cerebral wall of ferrets: direct tracing of their transformation from radial glia into astrocytes. J Comp Neurol 289: 74-88. [DOI] [PubMed] [Google Scholar]
- Worthington Jr WC, Cathcart III RS (1963) Ependymal cilia: distribution and activity in the adult human brain. Science 139: 221-222. [DOI] [PubMed] [Google Scholar]