Abstract
The neurotransmitter serotonin (5-HT) plays an important role in the regulation of multiple events in the CNS. We demonstrated recently a coupling between the 5-HT4 receptor and the heterotrimeric G13-protein resulting in RhoA-dependent neurite retraction and cell rounding (Ponimaskin et al., 2002). In the present study, we identified G12 as an additional G-protein that can be activated by another member of serotonin receptors, the 5-HT7 receptor. Expression of 5-HT7 receptor induced constitutive and agonist-dependent activation of a serum response element-mediated gene transcription through G12-mediated activation of small GTPases. In NIH3T3 cells, activation of the 5-HT7 receptor induced filopodia formation via a Cdc42-mediated pathway correlating with RhoA-dependent cell rounding. In mouse hippocampal neurons, activation of the endogenous 5-HT7 receptors significantly increased neurite length, whereas stimulation of 5-HT4 receptors led to a decrease in the length and number of neurites. These data demonstrate distinct roles for 5-HT7R/G12 and 5-HT4R/G13 signaling pathways in neurite outgrowth and retraction, suggesting that serotonin plays a prominent role in regulating the neuronal cytoarchitecture in addition to its classical role as neurotransmitter.
Keywords: 5-HT, serotonin, G-protein-coupled receptor, heterotrimeric G-protein, small GTPases, gene transcription, neuronal morphology
Introduction
Five-hydroxytryptamine (5-HT; serotonin) is an important neuromodulator involved in a wide range of physiological functions. The effects of serotonin are mediated by a large family of receptors, either ionotropic or coupled to second-messenger cascades (Barnes and Sharp, 1999). With the exception of the 5-HT3 receptor, which is a cation channel, all other 5-HT receptors belong to the superfamily of seven transmembrane-spanning receptors that are coupled to multiple heterotrimeric G-proteins. Among these receptors, the most recently cloned is the 5-HT7 type. Initially, the 5-HT7 receptor was determined to be coupled to the Gs-protein to stimulate adenylyl cyclase activity and to produce cAMP, followed by the activation of protein kinase A (PKA) (Shen et al., 1993). Functionally, the 5-HT7 receptor has been associated with a number of physiological and pathophysiological phenomena, such as 5-HT-induced phase shifting of the circadian rhythm or age-dependent changes in circadian timing (Lovenberg et al., 1993; Duncan et al., 1999). A large amount of experimental data suggests that 5-HT7 receptors are involved in the induction of sleep and the development of hypothermia (Hedlund et al., 2003; Thomas et al., 2003).
Heterotrimeric guanine nucleotide-binding proteins (G-proteins) are expressed in all eukaryotic cells and provide a major mechanism for the regulation of cellular responses by transducing signals from the cell surface. The G12-protein family consists of the ubiquitously expressed Gα12 and Gα13 subunits (Strathmann and Simon, 1991), which regulate a variety of cellular responses, including transformation of fibroblasts (Voyno-Yasenetskaya et al., 1994; Xu et al., 1994), activation of Jun N-terminal kinase and serum response element (SRE) (Prasad et al., 1995; Collins et al., 1996; Fromm et al., 1997), stress fiber formation (Buhl et al., 1995), and neurite retraction in PC12 cells (Katoh et al., 1998). Although G12- and G13-proteins share a high amino acid sequence homology (67%), their functional properties are not completely overlapping. For example, Gα13 knock-out mice die during early embryonic development (Offermanns et al., 1997), whereas mice with a disrupted Gα12 gene are viable and fertile (Gu et al., 2002).
Prominent downstream effectors in G12-mediated signaling are the members of the Rho family of small GTPases (Rho, Rac, and Cdc42), which regulate a variety of cellular activities by controlling the actin cytoskeleton or gene expression (Hall, 1998). Rho GTPases are widely expressed in multiple neural tissues and appear to function as key mediators that link the guidance signal to cytoskeletal rearrangements (Yamamoto et al., 1989; Olenik et al., 1999). Marked changes in morphology, motility, and guidance of axons have been observed in response to activation of Rho family GTPases both in vitro and in vivo (Zipkin et al., 1997; Ruchhoeft et al., 1999; Ng et al., 2002). In general, these studies suggest that Rac and Cdc42 are positive regulators promoting neurite extension and growth cone protrusion. Conversely, activation of RhoA induces stress fiber formation, leading to the growth cone collapse and neurite retraction (Sebok et al., 1999; Lee et al., 2000; Li et al., 2000).
We recently demonstrated coupling between the 5-HT4 receptor and G13-protein (Ponimaskin et al., 2002). We also have shown that 5-HT4 receptor-dependent activation of Gα13 leads to RhoA-mediated cell rounding and neurite retraction. In the present study, we identified G12 as an additional G-protein that can be activated by the 5-HT7 receptor. We also revealed that the 5-HT7 receptor may regulate the SRE-mediated gene transcription as well as modulation of neuronal morphology through the Gα12-dependent activation of RhoA and Cdc42 GTPases.
Materials and Methods
Materials. SRE.L-luciferase reporter plasmid was provided by Paul Sternweis (University of Texas Southwestern Medical Center, Dallas, TX). RhoA(N19) was obtained from the Guthrie Research Institute (Sayre, PA). [35S]GTPγS (1300 Ci/mmol) was purchased from Hartmann Analytic (Braunschweig, Germany). An ECL Western blot analysis system and peroxidase-conjugated secondary antibodies were purchased from Amersham Biosciences (Braunschweig, Germany). Enzymes used in molecular cloning were obtained from New England Biolabs (Frankfurt am Main, Germany). 5-HT, 5-carboxyamidotryptamine (5-CT), 8-hydroxy-2(di-n-propylamino)tetralin, and Protein A-Sepharose CL-4B beads were obtained from Sigma (Deisenhofen, Germany). BIMU8 was kindly provided by Boehringer Ingelheim (Ingelheim, Germany). TC-100 insect cell medium, DMEM, fetal calf serum (FCS), 2× YT medium, Cellfectin, and Lipofectamine2000 reagents were purchased from Invitrogen (Karlsruhe, Germany). Anti-myc epitope antibodies as well as Gαi, Gαs, Gα13, Gα12, and Gαq antibodies were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). The Gα12 antibody AS1905 has been described previously (Ponimaskin et al., 1998). The dominant-negative RhoA, Rac1, and Cdc42 were purchased from the Guthrie Research Institute.
Recombinant DNA procedures. All basic DNA procedures were performed as described by Sambrook et al. (1989). The m5-HT7a cDNA was kindly provided by Isabel Bermudes (Oxford Brookes University, Oxford, UK). The m5-HT7 cDNA was amplified by PCR with specific primers to create 11 amino acids Myc-tag (MEQKLISEENL) at the N terminus of the receptor. The PCR fragment was ligated into pcDNA 3.1(-) or pFastBac plasmids (Invitrogen). Recombinant baculoviruses encoding the Myc-5-HT7 receptor were constructed, purified, and amplified as described previously (Veit et al., 1994).
For expression in NIH3T3 cells, murine 5-HT7 cDNA was cloned into the pTracer-CMV2 donor plasmid (Invitrogen). Construction of the 5-HT4-pTracer-CMV2 plasmid has been described previously (Ponimaskin et al., 2002). To create 5-HT7-green fluorescent protein (GFP) and 5-HT4-GFP chimeras with C-terminal GFP fusion, receptor-coding sequences were amplified by PCR and then ligated into the pEGFP-N1 plasmid (Clontech, Cambridge, UK). All constructs were verified by double-stranded dideoxy DNA sequencing at the level of the final plasmid.
Assay for [35S]GTPγS binding in membranes of Sf.9 cells. Agonist-promoted binding of [35S]guanosine 5′-(3-O-thio)triphosphate to different G-proteins induced by stimulation of 5-HT7 receptor was performed according to the study by Ponimaskin et al. (1998).
Adherent cell culture and transient transfection. NIH3T3 cells were grown in DMEM containing 10% FCS and 1% penicillin/streptomycin at 37°C under 5% CO2. For transient transfection, cells were seeded at low density (8 × 105) into 35 mm dishes or into 10 mm coverslips (5 × 105) and transfected with 1 μg of pTracer or pTracer/5-HT7 vectors using Lipofectamine2000 Reagent (Invitrogen) according to the instructions of the manufacturer. In several experiments, plasmids encoding for the dominant-negative mutants RhoA(N19) or Cdc42(N17) were also cotransfected. Ten hours after transfection, cells were starved for different time intervals (16-36 h) before analysis.
Reporter gene assays. SRE-dependent and cAMP response element-binding protein (CREB)-dependent gene expression was determined by the SRE.L reporter and CREB “PathDetect” trans-reporting systems (Stratagene, La Jolla, CA), respectively. Before the experiment, cells were serum starved overnight. The cells were washed twice with PBS and lysed in protein extraction reagent, and the cleared lysates were assayed for luciferase and β-galactosidase (β-gal) activity using the corresponding assay kits (Promega, Madison, WI). Luciferase activities were measured with a Sirius Luminometer Berthold Detection System (Berthold, Bad Wildbad, Germany). Luciferase activity of each sample was normalized to β-galactosidase activity to correct for the differences in transfection efficiency and is expressed as the fold increases over control. In control experiments, cAMP-induced PKA activation was determined by the CREB assay PathDetect system (Stratagene). Data represent mean ± SEM of triplicate determinations.
Measurement of Rho and CdC42 activity. Direct activation of Rho proteins was determined by using a pull-down assay (Niu et al., 2003). pGEX expression vectors encoding glutathione S-transferase (GST) fusion proteins that contain the isolated GTP-dependent binding domains of the Rac1 and Cdc42 effector p21-activated kinase 1 (PAK1) [amino acids 70-132 of PAK1; PAK Rac-binding domain (RBD)] or the RhoA effector rhotekin (amino acids 7-89 of rhotekin; rhotekin RBD) were used for the bacterial expression of GST fusion proteins. Resulting Western blots were quantified by densitometry.
Morphological analysis of NIH3T3 cells. At 14-16 h after transfection with pTracer vectors cells were washed and incubated for 24-36 h in serum-free DMEM to induce morphological differentiation. Agonist-induced changes in cell shape were monitored using the laser-scanning microscope LSM510 Meta Zeiss (Zeiss, Jena, Germany) at 20× magnification with appropriate GFP filter settings. Experiments were performed at 37°C in bicarbonate/CO2-buffered DMEM. Cells were either scored as rounded, flattened, or flattened with filopodia (“filopodia-bearing”) that reached a length of at least twice the cell body diameter For each transfection, the percentage of rounded, flattened, and filopodia-bearing cells was calculated from ≥300 green cells. Experiments were performed in duplicate per transfection, and morphologies were scored blindly (i.e., without knowledge of experimental conditions). An average percentage was calculated from at least four independent experiments.
Preparation and transfection of hippocampal neurons. Cultures of hippocampal neurons were prepared from 1- to 2-d-old Naval Medical Research Institute mice. Cells were dissociated by trypsin treatment and plated in DMEM supplemented with 10% FCS onto 10 mm glass cover-slips coated with Matrigel (BD Biosciences, Franklin Lake, NJ). After 4 h, the medium was replaced by DMEM supplemented with 5% FCS, 2% B27, 100 mg/L insulin, 100 mg/L transferrin, and 5 μm cytosine arabinoside. Cultures were maintained at 37°C in a humidified incubator gassed with 5% CO2. Cells were transiently transfected either by electroporation with Nucleofector I electroporator (Amaxa, Cologne, Germany) (Dityateva et al., 2003) or by using Lipofectamine2000 reagent (Invitrogen) according to the protocol of the manufacturer.
Neurite outgrowth measurements. Agonists/antagonists of 5-HT receptors were added to cultures 4 or 18 h after cell plating, as indicated in Results. Twenty-four hours after plating, cells were briefly washed with PBS and fixed with 4% formaldehyde in PBS. Untransfected cultures were stained with toluidine blue, and the inverted microscope Axiovert 135 and Kontron imaging system (Kontron Elektronik, Eching, Germany) were used for image acquisition of stained cells and operator-controlled tracing of neurites. For analysis of neurons nucleofected with 5-HT7-GFP or GFP expression vectors (1 μg per 106 cells), cells were visualized and traced using the laser-scanning confocal microscope LSM510-based imaging system (Zeiss). The number, total, and mean lengths of branches per neuron were measured. Statistical evaluation was performed using the paired t test applied to compare the averaged values derived from three independent experiments.
Immunohistochemistry. For immunostaining, cell cultures were fixed by addition of an equivalent volume of formaldehyde (8% in PBS) for 10 min followed by incubation in 4% formaldehyde/PBS for an additional 20 min. Free formaldehyde was quenched with 50 mm glycine for 15 min, and cells were permeabilized with Triton X-100 (0.5% in PBS) for 3 min. After incubation in blocking solution (10% BSA in PBS), cells were exposed to primary antibodies directed against Gα13 or Gα12 at a dilution of 1:100 and 1:500, respectively. Secondary antibody [Alexa Fluor 546 (Molecular Probes, Eugene, OR) diluted 1:1000 or Cy2 (Dianova, Hamburg, Germany) diluted 1:400 in PBS containing 2% BSA] was added to the cells for 1 h. For visualization of F-actin, fixed and permeabilized cells were stained with FITC-conjugated phalloidin (Sigma) for 1 h. Coverslips were mounted in fluorescent mounting medium (DakoCytomation, Ely, UK) and analyzed by laser-scanning confocal microscopy using a63× water-immersion objective.
Results
The 5-HT7 receptor specifically activates the Gα12 subunit
In the present study, we evaluated G-proteins interacting with the 5-HT7 receptor by using the baculovirus expression system. A high-titer baculovirus stock containing cDNA of the murine 5-HT7 receptor that was tagged with a Myc-epitope at the N terminus was used for infection of Sf.9 insect cells.
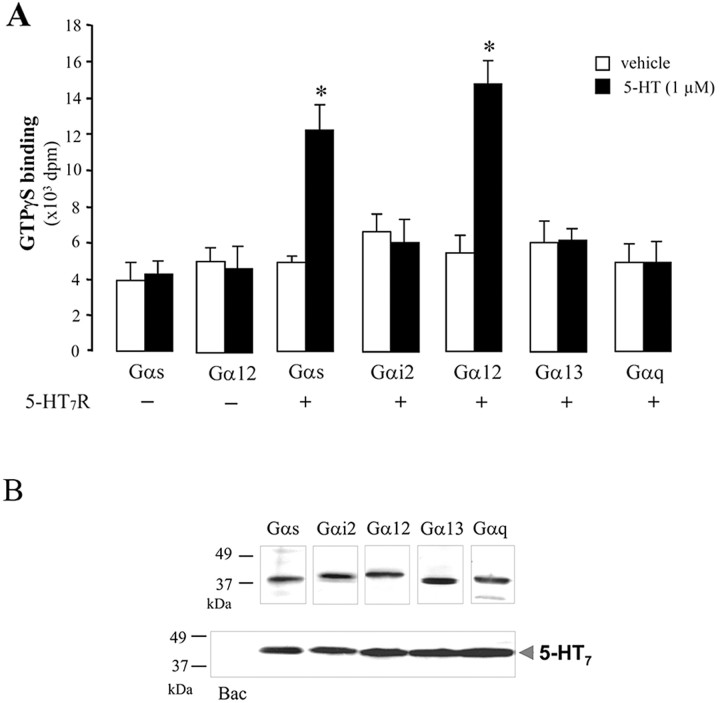
Specific G-protein coupling was evaluated by a [35S]GTPγS-binding assay, which determines the GDP-GTP exchange on the Gα subunit. Membranes from Sf.9 cells coinfected with baculoviruses that encode different mammalian G-proteins (in all cases, the appropriate Gα subunit was coexpressed with β1 and γ2 subunits) and 5-HT7 receptors were incubated in the presence of [35S]GTPγS. To evaluate whether receptor activation could increase GTPγS binding, cells were incubated in the presence or absence of a receptor agonist. Subsequently, Gα-specific antibodies were used to immunoprecipitate appropriate Gα subunits from the detergent extracts. The amount of [35S]GTPγS in the immunoprecipitates was used for verification of Gα subunit activation. Figure 1A shows a set of experiments in which Sf.9 cell membranes containing Gαs, Gαi2, Gα12, Gα13, and Gαq were analyzed for [35S]GTPγS binding. There was no coupling after coexpression of the receptor with Gαi2, Gα13, or Gαq subunits. However, when the 5-HT7 receptor was coexpressed with Gαsor Gα12, we measured an approximately twofold to threefold increase in [35S]GTPγS binding after stimulation with 1 μm 5-HT (Fig. 1A). Omission of the receptor from the assay revealed that Gαs or Gα12 alone did not bind [35S]GTPγS (Fig. 1A). Expression of the 5-HT7 receptor and all Gα subunits in Sf.9 cells was confirmed by Western blot analysis with appropriate antibodies (Fig. 1B). These results demonstrate that the 5-HT7 receptor effectively communicates with G-proteins of the Gs family. Activation of Gα12 by the 5-HT7 receptor was a novel finding that required additional investigation.
Figure 1.
Communication of the 5-HT7 receptor with different G-proteins. A, Membranes were prepared from Sf.9 cells expressing or not expressing the 5-HT7 receptor together with recombinant G-proteins as indicated and then incubated with [35S]GTPγS in the presence of either vehicle (H2O) or 1 μm 5-HT. Immunoprecipitations were performed with appropriate antibodies directed against indicated Gα subunits. Data points represent the means ± SEM from at least four independent experiments performed in duplicate. Statistically significant differences between values obtained with or without stimulation are indicated (*p < 0.01). B, Expression analysis of the 5-HT7 receptor and G-proteins. Sf.9 cells infected with baculoviruses encoding for recombinant, myc-tagged 5-HT7 receptor and for different heterotrimeric G-proteins (Gs, Gi2, G12, G13, and Gq) or with a baculovirus alone (Bac) were subjected to Western blot analysis with anti-myc or appropriate anti-Gα subunit antibodies. The molecular weight marker is indicated to the left.
The 5-HT7 receptor activates the SRE in a PKA-independent manner
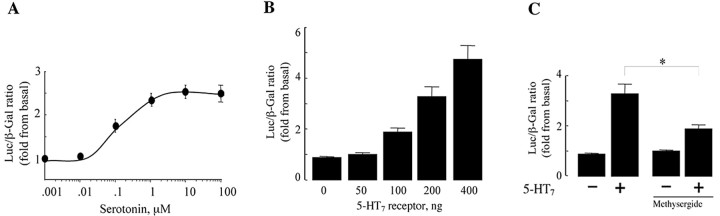
Because the Gα12 subunit is known to regulate gene expression by transcriptional activation of distinct transcriptional control elements such as SRE (Fromm et al., 1997; Mao et al., 1998), we investigated whether the 5-HT7 receptor stimulates SRE activity. To monitor SRE-mediated transcription of a luciferase reporter gene, an altered c-fos SRE, SRE.L, was placed in front of the luciferase gene (Hill et al., 1995). SRE.L binds only to the transcription factor serum response factor (SRF) and not to tertiary complex factor (TCF). NIH3T3 cells were transiently transfected with the 5-HT7 receptor and the SRE-driven luciferase reporter construct, and 5-HT-mediated changes in luciferase activity were measured after 6 h of stimulation, which allow accumulation of translation product to detectable levels (Liu and Wu, 2004). To correct variations in transfection efficiency, an expression vector coding for β-galactosidase was cotransfected, and the expressed β-galactosidase activity was used to normalize SRE luciferase activity. As shown in Figure 2A, serotonin induces a dose-dependent SRE activation in the cells expressing the 5-HT7 receptor with an EC50 value of ∼100 nm. SRE activation was dependent on 5-HT7 receptor expression, because the agonist did not induce SRE activation in the cells transfected only with the SRE reporter vector (data not shown).
Figure 2.
Agonist-induced activation of SRE via 5-HT7 receptor. A, Dose-dependent activation of SRE by serotonin in the NIH3T3 cells expressing the 5-HT7 receptor. NIH3T3 cells seeded onto 24-well plates were transfected with 100 ng of 5-HT7 receptor cDNA, 50 ng of pSRE.L, and 50 ng of pCMV-β-gal vectors. Twenty-four hours after transfection, cells were serum-starved for 16 h and stimulated with indicated concentrations of serotonin for 6 h. Thereafter, activity of SRE was determined. Presented data are the mean ± SEM(n = 4). B, The 5-HT7 receptor activates SRE in a ligand-independent manner. Indicated amounts of the 5-HT7 receptor cDNA were transfected into NIH3T3 cells together with 50 ng of pSRE.L and 50 ng of pCMV-β-gal, and SRE activity was determined. Data points represent the means ± SEM from at least three independent experiments. C, An inverse agonist inhibits 5-HT7 receptor-induced SRE activation. NIH3T3 cells seeded onto 24-well plates were transfected as described in A. Twenty-four hours after transfection, cells were serum-starved for 16 h and treated with 100 nm methysergide. Data points represent the means ± SEM (n = 4). A statistically significant difference between values is indicated (*p < 0.01).
It has been reported that several 5-HT receptors possess a constitutive, agonist-independent activity (Krobert and Levy, 2002). In the case of the 5-HT7 receptor, we found that increased levels of 5-HT7 cDNA (from 20 to 400 ng) resulted in a significant increase of SRE activity (from twofold to fivefold) under non-stimulated conditions (Fig. 2B). This effect was receptor specific because it was blocked by pretreatment of cells expressing the 5-HT7 receptor with 100 nm of the inverse receptor agonist methysergide (Fig. 2C) (Carter et al., 1995).
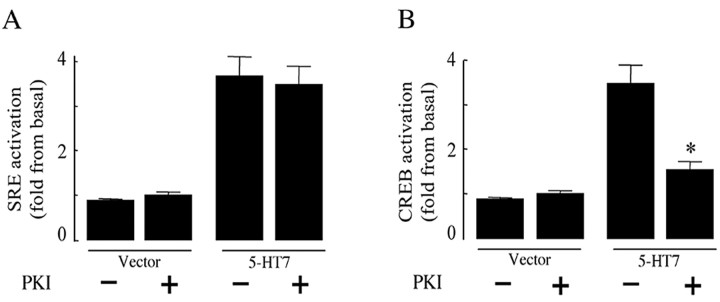
Because the 5-HT7 receptor is also coupled to the Gs-protein, we determined whether receptor-mediated activation of SRE depends on Gs-induced activation of PKA (Fig. 3). In this experiment, cells expressing the 5-HT7 receptor were pretreated with a highly specific, cell-permeable PKA inhibitor, 14-22 amide (PKI), which acts as a PKA pseudosubstrate and inactivates the catalytic subunit of PKA (Harris et al., 1997). Treatment with PKI did not affect 5-HT7 receptor-induced activation of SRE (Fig. 3A). In control experiments, the ability of PKI to inhibit cAMP-induced PKA activation was determined by the CREB assay, which was used as a readout for PKA activation. Activated PKA phosphorylates CREB at serine residue 133 (Gonzalez and Montminy, 1989), leading to activation of luciferase gene transcription from the reporter plasmid. We found that 5-HT7 receptor-induced CREB-dependent expression of luciferase was abolished by treatment with PKI (Fig. 3B), indicating that the 5-HT7 receptor can induce PKA activation. Together, these data indicate that the 5-HT7 receptor induces the SRE activation in a PKA-independent manner.
Figure 3.
5-HT7 receptor-induced SRE activation does not depend on PKA activation. A, NIH3T3 cells were transfected with 100 ng of 5-HT7 receptor cDNA, 50 ng of pSRE.L, and 50 ng of pCMV-β-gal. After serum starvation, cells were treated with 10 μm PKI for 6 h, and SRE activity wasdetermined.B, 5-HT7 receptor-induced CREB activation depends on PKA activation. NIH3T3 cells were transfected with 100 ng of 5-HT7 receptor cDNA or empty pcDNA3 vector together with 75 ng of pFR-luciferase (reporter plasmid) and 4 ng of pFA2-CREB (fusion trans-activator plasmid) for assessment of CREB activation. After serum starvation, cells were treated with 10 μm PKI for 6 h and then CREB activity was determined. Data points represent the means ± SEM from at least three independent experiments. A statistically significant difference between values is indicated (*p < 0.01).
The 5-HT7 receptor activates the serum response element via the Gα12 subunit
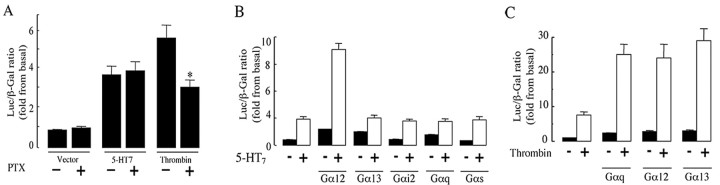
Having shown that the 5-HT7 receptor couples with Gα12 in addition to the Gαs subunit, we examined the identity of the G-proteins that mediate 5-HT7-dependent activation of SRE. To analyze the involvement of the Gi/o-mediated signaling pathway, transfected cells were treated with pertussis toxin (PTX; 400 ng/ml). This treatment did not reduce 5-HT7 receptor-induced SRE activation (Fig. 4A). The ability of PTX to inhibit Gi/o-mediated signaling was verified by stimulation of the endogenously expressed thrombin receptor with 1 U/ml thrombin after pretreatment of NIH3T3 cells with PTX. In this experiment, PTX pretreatment resulted in an ∼50% reduction of thrombin-induced SRE activation (Fig. 4A). These results suggest that pertussis toxin-sensitive G-proteins are not involved in 5-HT7 receptor-induced activation of SRE.
Figure 4.
The 5-HT7 receptor is functionally coupled to Gα12 to induce SRE activation. A, 5-HT7 receptor-dependent SRE activation is PTX independent. NIH3T3 cells were transfected with 100 ng of 5-HT7 receptor cDNA, 50 ng of pSRE.L, and 50 ng of pCMV-βgal, serum-starved, and treated with 400 ng/ml PTX for 4 h. In several experiments, cells were stimulated with 1 U/ml thrombin for 6 h, and SRE activity was determined as described. Data points represent the means ± SEM from at least four independent experiments performed in duplicate. A statistically significant difference between values is indicated (*p < 0.01). B, Gα12 specifically potentiates 5-HT7 receptor-induced SRE activation. NIH3T3 cells were transfected as described in A together with 200 ng of indicated Gα subunit cDNA. Data points represent the means ± SEM from three independent experiments performed in triplicate. C, SRE activation induced by thrombin is potentiated by Gαq, Gα12, and Gα13 subunits. NIH3T3 cells were transfected as described above, serum-starved, and then stimulated with 1 U/ml thrombin for 6 h followed by determination of SRE activity. Data points represent the means ± SEM from at least three independent experiments performed in triplicate. Luc, Luciferase.
It has been shown that coexpression of a given Gα subunit that binds to the receptor further enhances the receptor-mediated function (Wong et al., 1991). Therefore, NIH3T3 cells with or without the 5-HT7 receptor were cotransfected with the α subunits Gα13, Gα12, Gαi2, Gαq, and Gαs (Fig. 4B). Whereas Gαi2 and Gαs expression alone did not affect SRE activity, expression of Gα13, Gα12, and Gαq induced moderate activation of SRE, in agreement with previous data (Mao et al., 1998). Cotransfection of the 5-HT7 receptor with Gα12 resulted in significant potentiation of SRE activity, whereas cotransfection of the 5-HT7 receptor with other Gα subunits did not affect SRE activity (Fig. 4B). Because the thrombin receptor can activate both Gq and G12 families of G-proteins, we used thrombin-induced SRE activation as a control. In this experiment, SRE activity was markedly enhanced in the cells transfected with Gαq, Gα12, or Gα13 subunits (Fig. 4C). Thus, these data support the observation that the 5-HT7 receptor preferentially couples to Gα12 for SRE activation.
The 5-HT7 receptor activates RhoA and Cdc42 but not Rac1
Because it has been demonstrated that Gα12 modulates SRE activity via small GTPases of the Rho family (Fromm et al., 1997), we analyzed whether the 5-HT7 receptor-induced SRE stimulation is also dependent on Rho activity. For this, the C3 component of botulinum toxin that ADP-ribosylates and, specifically, inactivates different Rho proteins (Aktories et al., 2000) was cotransfected in NIH3T3 cells. C3 toxin significantly inhibited 5-HT7 receptor-induced SRE activation (Fig. 5A). Next, we determined the involvement of specific Rho GTPases in 5-HT7 receptor-dependent SRE activation. Dominant-negative mutants of RhoA, Rac1, and Cdc42 GTPases were coexpressed in NIH3T3 cells, and their ability to inhibit 5-HT7 receptor-induced SRE activation was analyzed. Interestingly, only dominant-negative mutants of RhoA(N19) and Cdc42(N17), but not Rac1(N17), inhibited receptor-induced SRE activation (Fig. 5B). Together, these data suggest that the 5-HT7 receptor operates through modulation of activities of RhoA and Cdc42, but not Rac1.
Figure 5.
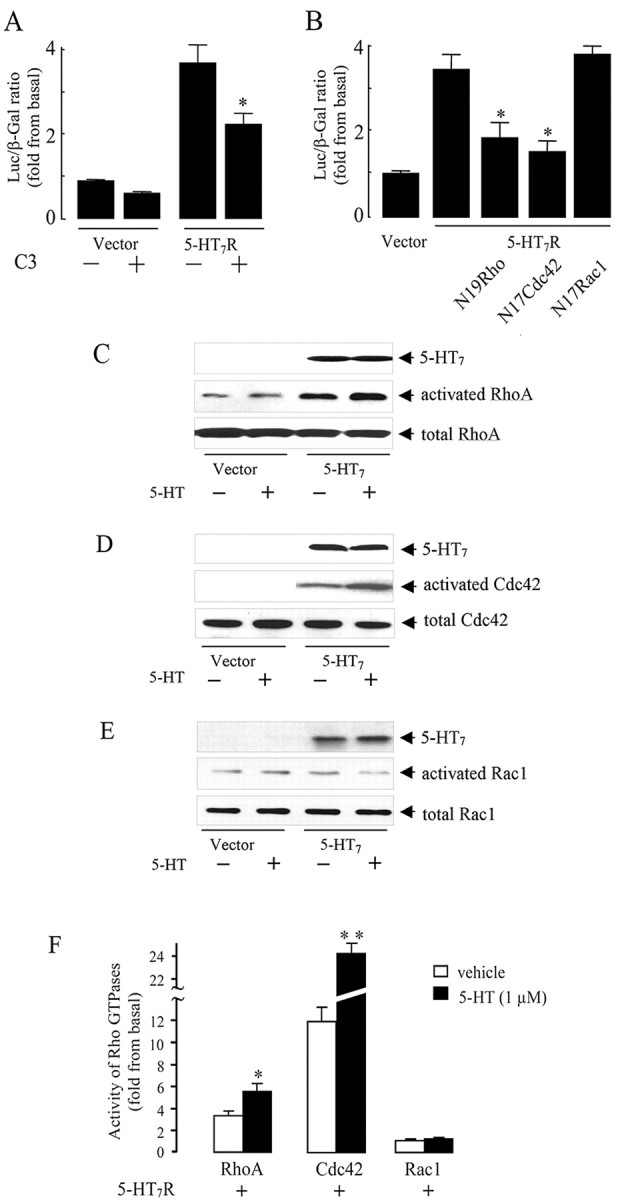
The 5-HT7 receptor stimulates RhoA and Cdc42 but not Rac1 GTPases. A, NIH3T3 cells were transfected with 100 ng of 5-HT7 receptor cDNA, 50 ng of pSRE.L, 50 ng of pCMV-β-gal, and 200 ng of C3 toxin cDNA. SRE activity was determined as described above. Presented data are the mean ± SEM (n = 4). A statistically significant difference between values is indicated (*p < 0.01). B, The 5-HT7 receptor activates SRE via RhoA and Cdc42. NIH3T3 cells were transfected with 50ng of pSRE. L, 50ng of pCMV-β-gal, and 100ng of 5-HT7 receptor cDNA together with 200 ng of each dominant-negative RhoA(N19), Rac1(N17), or Cdc42(N17) mutants as indicated. Activity of SRE was determined as described above. Presented data are the mean ± SEM (n = 3). A statistically significant difference between values is indicated (*p < 0.01). C-E, Rho GTPase activation assay. NIH3T3 cells growing on 100 mm dishes were transfected with 5 μg of RhoV14, RacV12, and Cdc42V12 together with the 5-HT7 receptor cDNA, serum starved, and then stimulated with 1 μm serotonin for 5 min. Rho GTPase activation assay was performed as described in Materials and Methods. C, RhoA activity was determined by the amount of RBD-bound RhoA (middle) normalized to the amount of RhoA in whole-cell lysates (bottom). D, Cdc42 activity was determined by the amount of PBD-bound Cdc42 (middle), normalized to the amount of Cdc42 in whole-cell lysates (bottom). E, Rac1 activity was determined by the normalization of amount of PBD-bound Rac1 (middle) to the amount of Rac1 in whole-cell lysates (middle). In parallel, expression of the 5-HT7 receptor was checked by the Western blots (top). Western blots from representative experiments are shown. F, The intensity of bands shown in C-E was quantified by densitometry, and the amount of active GTPase was normalized to the amount of the same GTPase in total cell lysates. Activities are shown as a fold increase compared with baseline and presented as means ± SEM from at least four independent experiments performed in duplicate. A statistically significant difference between values is indicated (*p < 0.01; **p < 0.001). Luc, Luciferase.
To evaluate the effect of the 5-HT7 receptor on activation of individual Rho GTPases in more detail, we used the Rho-binding domain of RhoA effector, rhotekin, to affinity precipitate active RhoA. We also used the Rac1- and Cdc42-binding domain of Rac1 and Cdc42 effector, PAK serine/threonine kinase, to affinity precipitate active Rac1 and Cdc42 as a direct readout for Rac1 and Cdc42 activation (Fig. 5C-E). Constitutively active mutants of RhoA, Rac1, or Cdc42 were used as a positive control for measuring the activation of the appropriate Rho GTPase. Expression of the 5-HT7 receptor induced an increase in RhoA and Cdc42 activity under basal conditions, and stimulation of the receptor with serotonin resulted in an additional significant activation of these GTPases (Fig. 5C,D,F). Importantly, the 5-HT7 receptor did not activate Rac1 (Fig. 5E,F), indicating the existence of mechanisms regulating the specificity of 5-HT7 receptor action toward different Rho GTPases. The level of the 5-HT7 receptor expression was checked by Western blot analysis, confirming that the different effects obtained for the activation of Rho GTPases were not caused by differences in the amount of expressed protein (Fig. 5). In addition to the results obtained with dominant-negative mutants, these data provide direct evidence for RhoA and Cdc42 activation by the 5-HT7 receptor.
The 5-HT7 receptor induces changes in cell morphology via RhoA and Cdc42
The Rho GTPase protein family plays a very important role in the development of the nervous system by regulation of actin-based motility (Meyer and Feldman, 2002). The Rho GTPases RhoA, Cdc42, and Rac1 reorganize the actin cytoskeleton, leading to stress fiber, filopodium, or lamellipodium formation, respectively.
Given that 5-HT7 receptor-dependent activation of Gα12 results in activation of RhoA and Cdc42, we used NIH3T3 cells as a model to analyze the possible role of the 5-HT7 receptor in controlling cell morphology. NIH3T3 cells were transiently transfected with the receptor subcloned into the pTracer vector. This vector allows specific scoring of only transfected cells by parallel expression of GFP. After transfection with a control pTracer vector, 70 ± 3% of GFP-expressed cells were flattened, 21 ± 3% were rounded, and another 9 ± 2% displayed filopodia outgrowth. Treatment of cells with serotonin did not cause any changes in cell morphology (Fig. 6A). In contrast, expression of the 5-HT7 receptor induced significant changes in cell morphology by increasing the percentage of rounded and filopodia-bearing cells to 41 ± 2 and 15 ± 2%, respectively (Fig. 6A). This suggests that after overexpression, the 5-HT7 receptor displays G12-mediated constitutive activity. Stimulation of receptor-expressing cells with 5-HT increased the amount of rounded cells to 51 ± 3% and filopodia-bearing cells to 20 ± 1% (Fig. 6A).
Figure 6.
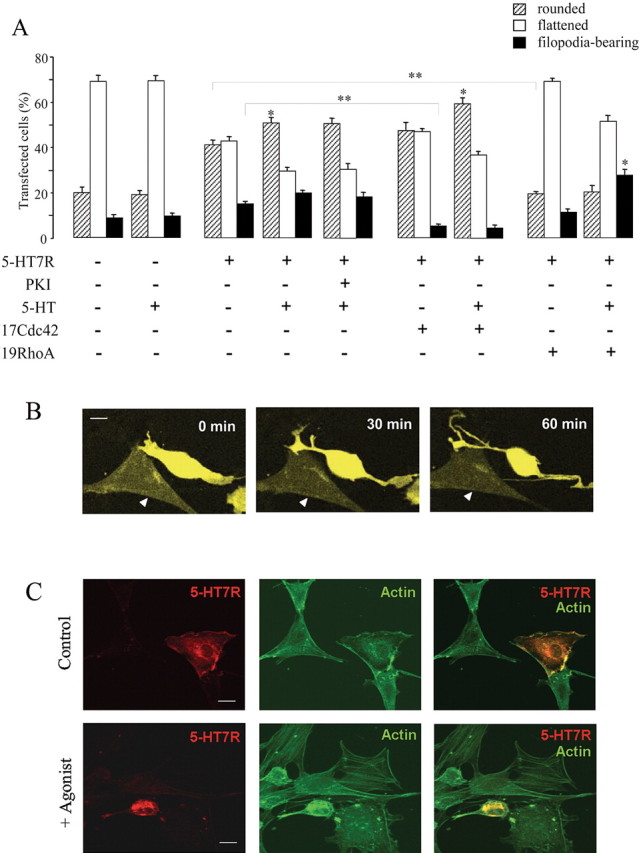
Regulation of cell morphology by the 5-HT7 receptor in NIH3T3 cells. NIH3T3 cells were transiently transfected with either a control (pTracer) vector or with pTracer vector encoding for the 5-HT7 receptor. Cells were cultured in serum-free medium overnight, and morphology was assessed after stimulation with agonists as indicated. A, Effect of the 5-HT7 receptor expression (with or without agonist stimulation) on the morphology of NIH3T3 cells. Dominant-negative mutants RhoA(N19) and Cdc42(N17) were coexpressed with the 5-HT7 receptor, as indicated. In several experiments, cells were treated with 10 μm cell-permeable PKA inhibitor 14-22 amide (PKI). Data points represent the means ± SEM from at least four independent experiments performed in duplicate. A statistically significant difference between values is indicated (*p < 0.05; **p < 0.01). B, Changes in the shape of cells expressing 5-HT7 receptors. NIH3T3 cells were transfected with pTracer vector encoding for the 5-HT7 receptor. Images shown were recorded by the confocal fluorescence microscopy at the beginning (0 min), during (30 min), and at the end (60 min) of a 1 h exposure to the 1 μm 5-HT with LSM510-Meta microscope at 63× magnification. A nontransfected cell is indicated by a triangle. Scale bar, 10 μm. The video is available as supplemental material (available at www.jneurosci.org). C, Analysis of the actin cytoskeleton in 5-HT7 receptor-expressing cells. NIH3T3 cells were transfected with GFP-tagged 5-HT7 receptor, serum starved, and then subjected to FITC-phalloidin staining. Representative confocal images obtained with LSM510-Meta microscope at 63× magnification are shown. Scale bar, 10 μm.
To analyze whether the receptor-induced changes in cell morphology are mediated by Gs-dependent activation of PKA, cells expressing the 5-HT7 receptor were pretreated with a highly specific, cell-permeable PKA inhibitor, 14-22 amide (PKI). Treatment with 10 μm PKI did not significantly affect 5-HT7 receptor-induced changes in the cell shape, demonstrating that the Gs-mediated pathway was not critically involved in such morphological effects (Fig. 6A).
To assess the specific roles of RhoA and Cdc42 in 5-HT7-dependent modulation of cell shape, dominant-negative RhoA(N19) and Cdc42(N17) mutants were cotransfected together with the 5-HT7 receptor. Data showed that, in cells expressing the 5-HT7 receptor, the dominant-negative Cdc42 mutant significantly inhibited receptor-induced filopodia formation from 20 ± 1to6 ± 1% without any effect on cell rounding (Fig. 6A). Interestingly, 5-HT7 receptor-induced cell rounding was reduced from 51 ± 3 to 19 ± 2% in cells expressing the dominant-negative mutant N19RhoA, whereas the percentage of cells with filopodia was not affected (Fig. 6A).
To analyze changes in cell shape induced by the 5-HT7 receptor in real time, NIH3T3 cells were transfected with pTracer vector encoding the 5-HT7 receptor and analyzed by laser-scanning confocal microscopy. Images were scanned every 5 min during 1 h, and 10 μm 5-HT was added to the medium after two scanning cycles. In cells expressing the 5-HT7 receptor, serotonin generated formation of elongated filopodia paralleled by pronounced cell rounding in a time-dependent manner, whereas control cells did not show any changes in morphology (Fig. 6B) (video 1, available at www.jneurosci.org as supplemental material). Notably, 5-HT7 receptor-dependent phenotypes observed in such real-time experiments were similar to those found in fixed cells.
Finally, we analyzed the role of the 5-HT7 receptor in actin reorganization by staining NIH3T3 cells expressing GFP-tagged 5-HT7 receptor with FITC-phalloidin. Functionality of receptor-GFP constructs was assessed by ligand binding, determination of cAMP levels, and SRE assay. In all cases, GFP chimeras demonstrated similar responses as their wild-type counterparts, suggesting their functional activity. Stimulation of receptor-expressing cells with 10 μm serotonin for 60 min led to the contraction of the cortical cytoskeleton forcing cells to round up, in addition to formation of filopodia-like protrusions containing bundled actin (Fig. 6C). In cells that did not express the 5-HT7 receptor, serotonin did not induce changes in the actin cytoskeleton, demonstrating that the observed changes were dependent on the 5-HT7 receptor.
Together, these combined data suggest the importance of the 5-HT7R/Gα12 signaling pathway for serotonin-mediated changes in cell shape and demonstrate that 5-HT7 receptor-dependent filopodia formation is mediated by Cdc42, whereas receptor-dependent cell rounding is mediated by RhoA.
5-HT7/G12 and 5-HT4/G13 signaling pathways are involved in the regulation of neurite outgrowth in hippocampal neurons
We reported recently that the 5-HT4a receptor is coupled to Gα13 and that activation of this signaling pathway causes RhoA-dependent neurite retraction and cell rounding (Ponimaskin et al., 2002). In the present study, we demonstrated that the 5-HT7 receptor activates both RhoA and Cdc42 via Gα12, leading to filopodia formation paralleled by cell rounding. Because both 5-HT4a and 5-HT7 receptors are expressed in neurons in vivo, we analyzed the effect of these receptors on neuronal morphology.
As a model system, we used cultures of dissociated hippocampal neurons prepared from 1- to 2-d-old mice. After 4 h in culture, cells were treated for 20 h with the appropriated agonist and/or antagonist and subjected to morphometric analysis. Exposure of neuronal culture to BIMU8, a 5-HT4 receptor-selective agonist (Eglen et al., 1995), resulted in a significant shortening in the total length of neurites to 56 ± 8%, when compared with untreated control, which was set to 100% (Fig. 7A). It was mainly because of significant reduction in the mean number of neurites per neuron to 63 ± 7%. The mean length of neurites after treatment with BIMU8 was reduced to 87 ± 4% (Fig. 7A). These effects were 5-HT4 receptor specific, because they were completely blocked by application of the highly selective receptor antagonist GR113808 (Fig. 7A). To test for the functional role of the 5-HT7 receptor in the regulation of neurite outgrowth, we analyzed the effects of the specific agonist 5-CT of the receptor. Application of 100 nm 5-CT to the hippocampal culture led to a significant increase in the total and mean length of neurites to 181 ± 14 and to 161 ± 16%, respectively, when compared with the untreated control (Fig. 7A). Parallel treatment of the cells with the selective 5-HT7 receptor antagonist, 100 nm SB269970 (Hagan et al., 2000), blocked this effect, indicating that the increase in the length of neurites was mediated by 5-HT7 receptor activation. The number of neurites was not affected after selective stimulation of the 5-HT7 receptor with 5-CT. Notably, 5-HT7 receptor-mediated effects on neurite outgrowth were obvious already after a 6 h application of 5-CT. The total and mean length of neurites were increased in these experiments to 150 ± 8 and to 141 ± 8%, compared with untreated neurons.
Figure 7.
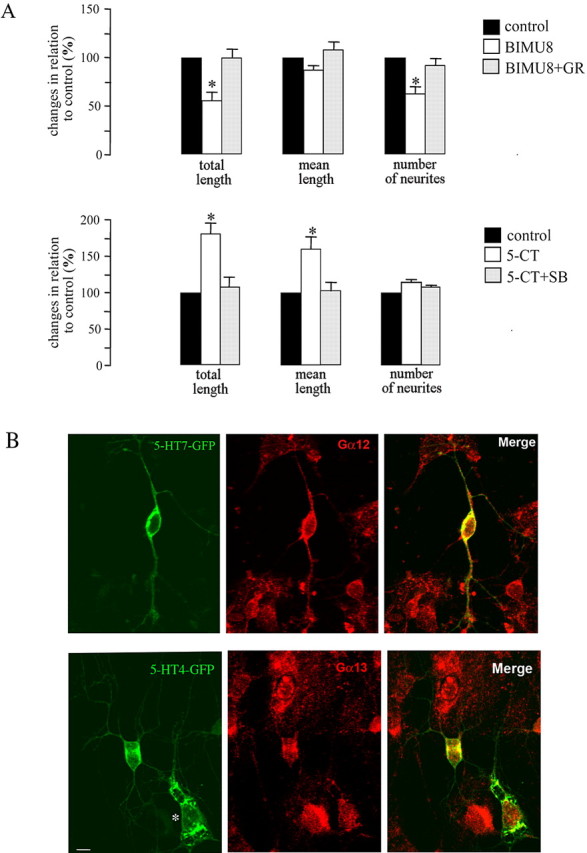
Effect of the 5-HT7R/G12 and 5-HT4R/G13 signaling pathways on neuronal morphology. A, For morphometric analysis of neurite outgrowth, cultured hippocampal neurons were fixed, stained with toluidine blue, and used for operator-controlled tracing of neurites. BIMU8 used at 100 nm is a selective 5-HT4 receptor agonist. GR113808 (GR) used at 2 μm is a highly selective 5-HT4 receptor antagonist. 5-CT used at 100 nm is a selective 5-HT7 receptor agonist. Treatment of the cells with the 5-HT7 receptor antagonist SB269970 (SB) at 100 nm blocked the effect of 5-CT. Data sets were collected after 20 h of treatment with an agonist/antagonist. The bars represent means ± SEM from three independent experiments performed in triplicate. Values obtained for untreated neurons were set to 100%. A statistically significant difference between values is indicated (*p < 0.05). B, Distribution of 5-HT7 and 5-HT4 receptors and Gα12 and Gα13 proteins in hippocampal neurons. The neurons were transfected with either GFP-tagged 5-HT4 or 5-HT7 receptors. The endogenous Gα12 or Gα13 proteins were visualized by immunostaining with specific antibodies. The asterisk indicates a transfected glial cell. Scale bar, 10 μm.
In addition, we examined whether 5-HT treatment of hippocampal neurons transfected with recombinant 5-HT7 receptor would reproduce 5-CT-stimulated neurite outgrowth observed in nontransfected cells. Morphometric analysis revealed that the neurons transfected with the 5-HT7-GFP receptor were not different from GFP-transfected cells in the absence of 5-HT. Treatment of GFP-transfected cultures with 1 μm 5-HT reduced the total length of neurites to 76 ± 7%. However, application of 5-HT to the neurons overexpressing 5-HT7-GFP receptor significantly increased both the total and mean length of neurites to 129 ± 10 and 142 ± 14%, respectively, whereas the number of neurites was not changed significantly.
To determine the spatial distribution of the 5-HT4a and 5-HT7 receptors and Gα12/13 proteins in hippocampal neurons, we performed immunostaining for endogenous Gα12 or Gα13 proteins in neurons transiently transfected with either GFP-tagged 5-HT4a or 5-HT7 receptors. Distribution of GFP-tagged receptors and endogenous Gα subunits was analyzed by confocal microscopy (Fig. 7B). Remarkably, we detected a high degree of colocalization of Gα13 and the 5-HT4a receptor in the somata and of Gα12 and the 5-HT7 receptor in somata and neurites (Fig. 7B). In contrast, when the distribution of Gα12 and the 5-HT4a receptor or Gα13 and the 5-HT7 receptor was studied, we did not detect colocalization (data not shown). We also performed three-dimensional colocalization analysis by LSM510 software and found that the correlation coefficient in the case of 5-HT7R/G12 and 5-HT4R/G13 ranged between 0.28 and 0.56, whereas for 5-HT7R/G13 and 5-HT4R/G12, it was between 0.04 and 0.08. Differential distribution of the Gα12 and Gα13 proteins in neurons was also confirmed by double staining with subunit-specific antibodies (Fig. S1, available at www.jneurosci.org as supplemental material). The combined data suggest that 5-HT7R/G12 and 5-HT4R/G13 signaling pathways have distinct cellular localizations and provide evidence that these pathways may be involved in the regulation of neuronal morphogenesis.
Discussion
The serotonin 5-HT7 receptor is the most recently identified member of the G-protein-coupled 5-HT receptor family. Stimulation of the native as well as heterologously expressed 5-HT7 receptor has been shown to cause Gαs-mediated activation of adenylyl cyclases, leading to increased cAMP production (Vanhoenacker et al., 2000). In the present study, we identified the Gα12 subunit of heterotrimeric G-protein as an additional interaction partner for the 5-HT7 receptor. We also demonstrated that activation of the 5-HT7R/G12 signaling pathway leads to stimulation of small GTPases of the Rho family, resulting in modulation of transcriptional activity and regulation of cell morphology.
5-HT7R/G12 signaling and regulation of gene transcription
One of the physiological consequences of Rho GTPase stimulation is the activation of a transcription factor, SRF, which binds to the SRE. Regulation of SRE activity is mediated by two different signaling pathways: TCF-dependent and TCF-independent pathways. It was determined that only TCF-independent regulation is modulated by small GTPases of the Rho family (Hill et al., 1995). Moreover, Rho-dependent activation of SRE is induced by G12-proteins (Fromm et al., 1997; Dutt et al., 2004). Therefore, we used an altered c-fos SRE, SRE.L, which binds only to the SRF but not to TCF to analyze the effect of the 5-HT7 receptor on SRE activation. Our data demonstrate that the 5-HT7 receptor activates SRE in a dose-dependent manner after agonist stimulation. Involvement of the Gα12 subunit and Rho GTPases in receptor-mediated SRE activation was demonstrated by the following observations: (1) 5-HT7 receptor-induced SRE activation was PKA independent and not inhibited by PTX treatment; (2) expression of Gα12 enhanced 5-HT7 receptor-induced SRE activation; and (3) a C3 toxin significantly inhibited 5-HT7 receptor-induced SRE activation. Interestingly, 5-HT7 receptor-dependent SRE activation was mediated by the activity of RhoA and Cdc42 but not Rac1, suggesting distinct molecular mechanism(s) regulating the specificity of 5-HT7 receptor signaling toward different Rho GTPases.
The finding that the 5-HT7 receptor may activate SRE-mediated gene transfection suggests the importance of this pathway for transcriptional regulation and could therefore explain several effects of serotonin on neuronal development and differentiation. For instance, blockade of 5-HT uptake during embryonic development has been shown to result in alterations in the morphology of the cingulate cortex, which normally receives rich dopaminergic input. In this region, pyramidal neurons produce many apical dendrites after prolonged 5-HT exposure (Levitt et al., 1997). Moreover, a prolonged increase in 5-HT concentrations affects dopamine D1 receptor expression, leading to a persistent imbalance of cortical activity (Levitt et al., 1997; Stanwood et al., 2001). Depletion of neurocortical serotonin during embryonic development permanently impairs cortical circuitry and results in permanent alterations of dendritic arborization (Durig and Hornung, 2000). Moreover, both 5-HT7-like immunoreactivity and c-Fos stimulation colocalize to the same neurons in the rat brain sections (Neumaier et al., 2001). Together, these data suggest that differential modulation of transcriptional activity of immediate early genes by the 5-HT7R/G12 signaling pathway may represent a new mechanism involved in the long-term effects of 5-HT in the CNS.
5-HT7R/G12 signaling and neuronal morphology
The most prominent function of small GTPases is the regulation of the actin cytoskeleton. Over the past several years, it has become evident that members of the Rho family are expressed in the CNS (Olenik et al., 1999; O'Kane et al., 2003) and that Rho-family GTPases are key regulators of neuronal morphology, motility, and axonal pathfinding in vitro and in vivo (Hall, 1998). The combined studies suggest that Rac1 and Cdc42 activities promote neurite extension and branching, whereas RhoA causes neurite retraction and collapse of the growth cone. Although the importance of Rho GTPases in neuronal development is widely accepted, the ligands and receptors involved in Rho-mediated signaling are poorly characterized.
Interestingly, several neurotransmitters, including serotonin, have been shown to be involved in many aspects of neuronal development such as neurite outgrowth, growth cone motility, and dendritic spine density in addition to their well-established role in neuronal communication (van Kesteren and Spencer, 2003). For instance, depletion of 5-HT results in a reduction of dendritic length and decreased spine formation in hippocampal neurons of rats (Haring and Yan, 1999; Alves et al., 2002). In contrast, application of 5-HT increases dendritic differentiation in the rat cerebral cortex (Liu and Lauder, 1991) and promotes neurite outgrowth from thalamic neurons (Lieske et al., 1999). Prolonged exposure of rats to the 5-HT reuptake inhibitor also significantly increases dendritic spine density and the length of dendrites in the striatum radiatum (Norrholm and Ouimet, 2000). Although these observations are consistent with the assumption that 5-HT acts to promote neurite outgrowth, other experiments give different results. For example, serotonin induces growth cone collapse and neurite retraction in neurons of chick dorsal root ganglion (Igarashi et al., 1995). The several serotonin receptors, including 5-HT1A, 5-HT2A, and 5-HT2B, have been proposed to be involved in the modulation of such neurotrophic events produced by 5-HT in mammals (Yan et al., 1997; Fiorica-Howells et al., 2000; Azmitia, 2001).
Molecular downstream mechanisms underlying such opposite effects of 5-HT on neurite outgrowth are still poorly understood. It has been reported that activation of the small GTPase RhoA through a G12/13-initiated pathway induces growth cone collapse and neurite retraction (Kranenburg et al., 1999). Similarly, expression of constitutively active Gα12 and Gα13 induced RhoA-dependent neurite retraction in PC12 cells (Katoh et al., 1998). We demonstrated recently that the 5-HT4 receptor activates RhoA GTPase via the Gα13 protein, providing a molecular basis for serotonin-induced RhoA-dependent inhibition of neurite outgrowth. Here, we demonstrate that the 5-HT7 receptor activates RhoA and Cdc42 via Gα12 by using dominant-negative mutants and by direct measurement of Rho activity using rhotekin- and PAK-binding assays. We also show that receptor-dependent activation of Rho GTPases was functional in terms of regulation of cell shape, because expression of the 5-HT7 receptor in NIH3T3 cells resulted in agonist-promoted filopodia formation and cell rounding. Furthermore, our results indicate that receptor-triggered changes in cell shape require both Cdc42 and RhoA, because expression of either dominant-negative Cdc42 or RhoA abolished filopodia formation and cell rounding, respectively, suggesting the existence of cross-talk between the Cdc42 and RhoA pathways that may be mediated through convergent actions of these two GTPases on the downstream effector myosin. Indeed, it has been shown that activation of PAK by Cdc42 results in the reduction of myosin activity (Manser et al., 1994), whereas activation of RhoA leads to an increase of myosin activity either directly by myosin light chain (MLC) phosphorylation (Amano et al., 1996) or indirectly by Rho-associated kinase-induced inhibition of phosphatase (Kawano et al., 1999). Alternatively, the cross-talk between Cdc42 and RhoA may occur at the level of GTPases. Cdc42 and Rho may function in a hierarchical cascade wherein Cdc42 activates Rac1, which in turn activates Rho. In addition, a reciprocal mechanism may exist by which Cdc42 downregulates RhoA activity (Li et al., 2002). Our results are more consistent with the second model, because expression of the dominant-negative mutant Cdc42(N17) resulted not only in inhibition of the appropriate GTPase but also in enhanced activity of RhoA.
The most distinctive finding of the present study is the observation that specific stimulation of endogenously expressed 5-HT4 or 5-HT7 receptors by selective agonists results in distinct morphological changes of hippocampal neurons: treatment of cultured neurons with the 5-HT4 receptor agonist BIMU8 reduces both total length and number of neurites, whereas incubation with the 5-HT7 receptor agonist 5-CT leads to pronounced extension of neurite length. These effects were receptor specific, because they were blocked by application of receptor selective antagonists. Interestingly, we observed a high degree of colocalization between Gα13 and the 5-HT4 receptor and between Gα12 and the 5-HT7 receptor, which may provide a physical basis for the functional effects described above. More importantly, the functional coexistence of 5-HT7R/G12 and 5-HT4R/G13 signaling pathways may provide a molecular link between serotonin, which operates as a soluble guidance ligand, and the Rho GTPase machinery, controlling neuronal morphology and motility. Our data also suggest that depending on the expression level and/or subcellular localization of the 5-HT4 and 5-HT7 receptors, serotonin may differentially regulate neuronal morphology because of opposite effects of these receptors on neurite retraction and extension.
Footnotes
This study was supported by the Deutsche Forschungsgemeinschaft through the Center of Molecular Physiology of the Brain (E.G.P.) and Grant PO 732/1-2. Additional support was provided by National Institutes of Health Grants GM56159 and GM65160 and by grants from the American Heart Association (T.A.V.-Y.), Philip Morris, Inc., and Philip Morris International (M.S., A.D.). T.A.V.-Y. is an Established Investigator of the American Heart Association.
Correspondence should be addressed to Evgeni G. Ponimaskin at the above address. E-mail: eponima@gwdg.de.
Copyright © 2005 Society for Neuroscience 0270-6474/05/257821-10$15.00/0
E.K. and G.L. contributed equally to this work.
References
- Aktories K, Schmidt G, Just I (2000) Rho GTPases as targets of bacterial protein toxins. Biol Chem 381: 421-426. [DOI] [PubMed] [Google Scholar]
- Alves SE, Hoskin E, Lee SJ, Brake WG, Ferguson D, Luine V, Allen PB, Green-gard P, McEwen BS (2002) Serotonin mediates CA1 spine density but is not crucial for ovarian steroid regulation of synaptic plasticity in the adult rat dorsal hippocampus. Synapse 45: 143-151. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271: 20246-20249. [DOI] [PubMed] [Google Scholar]
- Azmitia EC (2001) Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56: 413-424. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38: 1083-1152. [DOI] [PubMed] [Google Scholar]
- Buhl AM, Johnson NL, Dhanasekaran N, Johnson GL (1995) G alpha 12 and G alpha 13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J Biol Chem 270: 24631-24634. [DOI] [PubMed] [Google Scholar]
- Carter D, Champney M, Hwang B, Eglen RM (1995) Characterization of a postjunctional 5-HT receptor mediating relaxation of guinea-pig isolated ileum. Eur J Pharmacol 280: 243-250. [DOI] [PubMed] [Google Scholar]
- Collins LR, Minden A, Karin M, Brown JH (1996) Galpha12 stimulates c-Jun NH2-terminal kinase through the small G proteins Ras and Rac. J Biol Chem 271: 17349-17353. [DOI] [PubMed] [Google Scholar]
- Dityateva G, Hammond M, Thiel C, Ruonala MO, Delling M, Siebenkotten G, Nix M, Dityatev A (2003) Rapid and efficient electroporation-based gene transfer into primary dissociated neurons. J Neurosci Methods 130: 65-73. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Short J, Wheeler DL (1999) Comparison of the effects of aging on 5-HT7 and 5-HT1A receptors in discrete regions of the circadian timing system in hamsters. Brain Res 829: 39-45. [DOI] [PubMed] [Google Scholar]
- Durig J, Hornung JP (2000) Neonatal serotonin depletion affects developing and mature mouse cortical neurons. NeuroReport 11: 833-837. [DOI] [PubMed] [Google Scholar]
- Dutt P, Nguyen N, Toksoz D (2004) Role of Lbc RhoGEF in Galpha12/13-induced signals to Rho GTPase. Cell Signal 16: 201-209. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Wong EH, Dumuis A, Bockaert J (1995) Central 5-HT4 receptors. Trends Pharmacol Sci 16: 391-398. [DOI] [PubMed] [Google Scholar]
- Fiorica-Howells E, Maroteaux L, Gershon MD (2000) Serotonin and the 5-HT2B receptor in the development of enteric neurons. J Neurosci 20: 294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm C, Coso OA, Montaner S, Xu N, Gutkind JS (1997) The small GTP-binding protein Rho links G protein-coupled receptors and Galpha12 to the serum response element and to cellular transformation. Proc Natl Acad Sci USA 94: 10098-10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR (1989) Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 675-680. [DOI] [PubMed] [Google Scholar]
- Gu JL, Muller S, Mancino V, Offermanns S, Simon MI (2002) Interaction of G alpha(12) with G alpha(13) and G alpha(q) signaling pathways. Proc Natl Acad Sci USA 99: 9352-9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR (2000) Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol 130: 539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A (1998) Rho GTPases and the actin cytoskeleton. Science 279: 509-514. [DOI] [PubMed] [Google Scholar]
- Haring JH, Yan W (1999) Dentate granule cell function after neonatal treatment with parachloroamphetamine or 5,7-dihydroxytryptamine. Brain Res Dev Brain Res 114: 269-272. [DOI] [PubMed] [Google Scholar]
- Harris TE, Persaud SJ, Jones PM (1997) Pseudosubstrate inhibition of cyclic AMP-dependent protein kinase in intact pancreatic islets: effects on cyclic AMP-dependent and glucose-dependent insulin secretion. Biochem Biophys Res Commun 232: 648-651. [DOI] [PubMed] [Google Scholar]
- Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG (2003) No hypothermic response to serotonin in 5-HT7 receptor knock-out mice. Proc Natl Acad Sci USA 100: 1375-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R (1995) The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81: 1159-1170. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Li WW, Sudo Y, Fishman MC (1995) Ligand-induced growth cone collapse: amplification and blockade by variant GAP-43 peptides. J Neurosci 15: 5660-5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Aoki J, Yamaguchi Y, Kitano Y, Ichikawa A, Negishi M (1998) Constitutively active Galpha12, Galpha13, and Galphaq induce Rho-dependent neurite retraction through different signaling pathways. J Biol Chem 273: 28700-28707. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K (1999) Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol 147: 1023-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH (1999) Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell 10: 1851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krobert KA, Levy FO (2002) The human 5-HT7 serotonin receptor splice variants: constitutive activity and inverse agonist effects. Br J Pharmacol 135: 1563-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T, Winter C, Marticke SS, Lee A, Luo L (2000) Essential roles of Drosophila RhoA in the regulation of neuroblast proliferation and dendritic but not axonal morphogenesis. Neuron 25: 307-316. [DOI] [PubMed] [Google Scholar]
- Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH (1997) New evidence for neurotransmitter influences on brain development. Trends Neurosci 20: 269-274. [DOI] [PubMed] [Google Scholar]
- Li Z, Van Aelst L, Cline HT (2000) Rho GTPases regulate distinct aspects of dendritic arbor growth in Xenopus central neurons in vivo. Nat Neurosci 3: 217-225. [DOI] [PubMed] [Google Scholar]
- Li Z, Aizenman CD, Cline HT (2002) Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron 33: 741-750. [DOI] [PubMed] [Google Scholar]
- Lieske V, Bennett-Clarke CA, Rhoades RW (1999) Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. Neuroscience 90: 967-974. [DOI] [PubMed] [Google Scholar]
- Liu B, Wu D (2004) Analysis of the coupling of G12/13 to G protein-coupled receptors using a luciferase reporter assay. Methods Mol Biol 237: 145-149. [DOI] [PubMed] [Google Scholar]
- Liu JP, Lauder JM (1991) Serotonin and nialamide differentially regulate survival and growth of cultured serotonin and catecholamine neurons. Brain Res Dev Brain Res 62: 297-305. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG (1993) A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11: 449-458. [DOI] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L (1994) A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367: 40-46. [DOI] [PubMed] [Google Scholar]
- Mao J, Yuan H, Xie W, Simon MI, Wu D (1998) Specific involvement of G proteins in regulation of serum response factor-mediated gene transcription by different receptors. J Biol Chem 273: 27118-27123. [DOI] [PubMed] [Google Scholar]
- Meyer G, Feldman EL (2002) Signaling mechanisms that regulate actin-based motility processes in the nervous system. J Neurochem 83: 490-503. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Sexton TJ, Yracheta J, Diaz AM, Brownfield M (2001) Localization of 5-HT(7) receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J Chem Neuroanat 21: 63-73. [DOI] [PubMed] [Google Scholar]
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, Dietzl G, Dickson BJ, Luo L (2002) Rac GTPases control axon growth, guidance and branching. Nature 416: 442-447. [DOI] [PubMed] [Google Scholar]
- Niu J, Profirovic J, Pan H, Vaiskunaite R, Voyno-Yasenetskaya T (2003) G Protein betagamma subunits stimulate p114RhoGEF, a guanine nucleotide exchange factor for RhoA and Rac1: regulation of cell shape and reactive oxygen species production. Circ Res 93: 848-856. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC (2000) Chronic fluoxetine administration to juvenile rats prevents age-associated dendritic spine proliferation in hippocampus. Brain Res 883: 205-215. [DOI] [PubMed] [Google Scholar]
- Offermanns S, Mancino V, Revel JP, Simon MI (1997) Vascular system defects and impaired cell chemokinesis as a result of Galpha13 deficiency. Science 275: 533-536. [DOI] [PubMed] [Google Scholar]
- O'Kane EM, Stone TW, Morris BJ (2003) Distribution of Rho family GT-Pases in the adult rat hippocampus and cerebellum. Brain Res Mol Brain Res 114: 1-8. [DOI] [PubMed] [Google Scholar]
- Olenik C, Aktories K, Meyer DK (1999) Differential expression of the small GTP-binding proteins RhoA, RhoB, Cdc42u and Cdc42b in developing rat neocortex. Brain Res Mol Brain Res 70: 9-17. [DOI] [PubMed] [Google Scholar]
- Ponimaskin E, Harteneck C, Schultz G, Schmidt MF (1998) A cysteine-11 to serine mutant of G alpha12 impairs activation through the thrombin receptor. FEBS Lett 429: 370-374. [DOI] [PubMed] [Google Scholar]
- Ponimaskin EG, Profirovic J, Vaiskunaite R, Richter DW, Voyno-Yasenetskaya TA (2002) 5-Hydroxytryptamine 4(a) receptor is coupled to the Galpha subunit of heterotrimeric G13 protein. J Biol Chem 277: 20812-20819. [DOI] [PubMed] [Google Scholar]
- Prasad MV, Dermott JM, Heasley LE, Johnson GL, Dhanasekaran N (1995) Activation of Jun kinase/stress-activated protein kinase by GTPase-deficient mutants of G alpha 12 and G alpha 13. J Biol Chem 270: 18655-18659. [DOI] [PubMed] [Google Scholar]
- Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA (1999) The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo J Neurosci 19: 8454-8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
- Sebok A, Nusser N, Debreceni B, Guo Z, Santos MF, Szeberenyi J, Tigyi G (1999) Different roles for RhoA during neurite initiation, elongation, and regeneration in PC12 cells. J Neurochem 73: 949-960. [DOI] [PubMed] [Google Scholar]
- Shen Y, Monsma Jr FJ, Metcalf MA, Jose PA, Hamblin MW, Sibley DR (1993) Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J Biol Chem 268: 18200-18204. [PubMed] [Google Scholar]
- Stanwood GD, Washington RA, Levitt P (2001) Identification of a sensitive period of prenatal cocaine exposure that alters the development of the anterior cingulate cortex. Cereb Cortex 11: 430-440. [DOI] [PubMed] [Google Scholar]
- Strathmann MP, Simon MI (1991) G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits. Proc Natl Acad Sci USA 88: 5582-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, Gribble AD, Jeffrey P, Stevens AJ, Deeks NJ, Eddershaw PJ, Fenwick SH, Riley G, Stean T, Scott CM, Hill MJ, Middlemiss DN, Hagan JJ, Price GW, Forbes IT (2003) SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol 139: 705-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren RE, Spencer GE (2003) The role of neurotransmitters in neurite outgrowth and synapse formation. Rev Neurosci 14: 217-231. [DOI] [PubMed] [Google Scholar]
- Vanhoenacker P, Haegeman G, Leysen JE (2000) 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol Sci 21: 70-77. [DOI] [PubMed] [Google Scholar]
- Veit M, Nurnberg B, Spicher K, Harteneck C, Ponimaskin E, Schultz G, Schmidt MF (1994) The alpha-subunits of G-proteins G12 and G13 are palmitoylated, but not amidically myristoylated. FEBS Lett 339: 160-164. [DOI] [PubMed] [Google Scholar]
- Voyno-Yasenetskaya TA, Pace AM, Bourne HR (1994) Mutant alpha subunits of G12 and G13 proteins induce neoplastic transformation of Rat-1 fibroblasts. Oncogene 9: 2559-2565. [PubMed] [Google Scholar]
- Wong YH, Federman A, Pace AM, Zachary I, Evans T, Pouyssegur J, Bourne HR (1991) Mutant alpha subunits of Gi2 inhibit cyclic AMP accumulation. Nature 351: 63-65. [DOI] [PubMed] [Google Scholar]
- Xu N, Voyno-Yasenetskaya T, Gutkind JS (1994) Potent transforming activity of the G13 alpha subunit defines a novel family of oncogenes. Biochem Biophys Res Commun 201: 603-609. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Tanimoto T, Kim S, Kikuchi A, Takai Y (1989) Small molecular weight GTP-binding proteins and signal transduction. Clin Chim Acta 185: 347-355. [DOI] [PubMed] [Google Scholar]
- Yan W, Wilson CC, Haring JH (1997) 5-HT1a receptors mediate the neurotrophic effect of serotonin on developing dentate granule cells. Brain Res Dev Brain Res 98: 185-190. [DOI] [PubMed] [Google Scholar]
- Zipkin ID, Kindt RM, Kenyon CJ (1997) Role of a new Rho family member in cell migration and axon guidance in C. elegans Cell 90: 883-894. [DOI] [PubMed] [Google Scholar]