Abstract
ATP is released in a vesicular manner from nerve terminals mainly at higher stimulation frequencies. There is a robust expression of ATP (P2) receptors in the brain, but their role is primarily unknown. We report that ATP analogs biphasically modulate the evoked release of glutamate from purified nerve terminals of the rat hippocampus, the facilitation being mediated by P2X1, P2X2/3, and P2X3 [antagonized by 8-(benzamido)naphthalene-1,3,5-trisulfonate and 2′,3′-O-(2,4,6-trinitrophenyl)-ATP] and the inhibition by P2Y1, P2Y2, and/or P2Y4 [antagonized by reactive blue 2 and 2′deoxy-N6-methyladenosine-3′,5′-bisphosphate and mimicked by P1-(urinine 5′-),P4-(inosine 5′-) tetraphosphate and 2-methylthio-ADP] receptors. The combination of single-cell PCR analysis of rat hippocampal pyramidal neurons, Western blot analysis of purified presynaptic active zone fraction, and immunocytochemical analysis of hippocampal glutamatergic terminals revealed that the P2 receptors expressed in glutamatergic neurons, located in the active zone and in glutamatergic terminals, were precisely P2X1, P2X2, and P2X3 subunits and P2Y1, P2Y2, and P2Y4 receptors. This provides coincident functional and molecular evidence that P2 receptors are present and act presynaptically as a modulatory system controlling hippocampal glutamate release.
Keywords: ATP, P2X receptors, P2Y receptors, glutamate release, nerve terminals, rat hippocampus
Introduction
Stimulation of nerve terminals triggers a release of ATP (Sperlágh and Vizi, 1996), which can act directly as an extracellular signaling molecule through activation of P2 (ATP) receptors (Ralevic and Burnstock, 1998) or be converted through ectonucleotidases into adenosine (Zimmermann and Braun, 1996), with subsequent activation of P1 (adenosine) receptors (Fredholm et al., 2005). The P2 receptor family comprises seven ionotropic P2X receptors subunits (P2X1-7), forming either homomeric or heteromeric receptors (North, 2002), and eight metabotropic P2Y receptors (P2Y1,2,4,6,11,12,13,14) (Lazarowski et al., 2003). The brain displays a robust mRNA expression, an intense binding, and immunoreactivity for both P2X and P2Y receptors in neuronal and non-neuronal elements, although the role of central P2 receptors remains ill defined (for review, see Cunha and Ribeiro, 2000). Because an ATPergic contribution to synaptic transmission has only been demonstrated in few central synapses (for review, see Cunha and Ribeiro, 2000; Pankratov et al., 2003), it was proposed that P2 receptors act principally as presynaptic modulators in the brain (Cunha and Ribeiro, 2000). This view is supported by the observed localization of several P2X subunits (for review, see Cunha and Ribeiro, 2000; Deuchars et al., 2001; Diáz-Hernández et al., 2001) and P2Y receptors in brain nerve terminals (Schäfer and Reiser, 1997; Simon et al., 1997) and by functional studies indicating a biphasic P2 receptor modulation of the release of several types of neurotransmitters in different brain regions: inhibition via P2Y and facilitation via P2X receptors (for review, see Cunha and Ribeiro, 2000).
However, it is difficult to unambiguously ascribe P2 receptor-mediated effects to presynaptic modulation, because most functional studies rely solely on the use of the limited number of P2 receptor ligands that have an insufficient selectivity (Ralevic and Burnstock, 1998), and few attempts have been made to complement the studies with molecular or morphological approaches to identify the putative P2 receptors involved. Similarly, limited attempts are made to exclude the involvement of adenosine (formed after extracellular catabolism of ATP by ectonucleotidases) P1 receptors (Dunwiddie et al., 1997; Cunha et al., 1998), which is particularly critical when studying glutamatergic transmission, because adenosine efficiently inhibits excitatory transmission (Fredholm et al., 2005). Finally, it is difficult to unequivocally ascribe the modulation of transmitter release to a presynaptic mechanism, especially when using slices or microdialysis, as a result of indirect circuit- or astrocyte-mediated effects that lead to indirect modulation of synaptic function (Zhang et al., 2003).
Accordingly, we used purified nerve terminals (which allows isolating presynaptic effects) (Raiteri and Raiteri, 2000) to assess whether P2 receptors could presynaptically control the evoked release of glutamate. In parallel, functional and morphological approaches were used to identify the receptor subtypes mediating these effects and to demonstrate their presence in nerve terminals. This combined use of molecular biological, immunological, and pharmacological approaches enabled us to demonstrate that ATP presynaptically modulates the evoked release of glutamate in a biphasic manner through the activation of presynaptic facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and P2Y4 receptors.
Materials and Methods
Animals used in all experiments. Male Wistar rats (6-8 weeks of age; 140-160 g; Harlan Ibérica, Barcelona, Spain) were used throughout this study and were handled according to European Union guidelines for use of experimental animals, the rats being anesthetized under halothane atmosphere before being killed by decapitation.
Glutamate release studies. The release of glutamate from rat hippocampal nerve terminals prepared using a combined sucrose/Percoll centrifugation protocol was performed as described previously (Lopes et al., 2002). Briefly, hippocampal tissue was homogenized in a medium containing 0.32 m sucrose, 1 mm EDTA, 0.1% free bovine serum albumin (BSA), and 10 mm HEPES, pH 7.4. The homogenate was spun for 10 min at 3000 × g at 4°C and the supernatant spun again at 14,000 × g for 12 min. The pellet (P2 fraction) was resuspended in 1 ml of 45% Percoll (v/v) in Krebs'-HEPES-Ringer's (KHR) medium [composed of the following (in mm): 140 NaCl, 1 EDTA, 5 KCl, 5 glucose, and 10 HEPES, pH 7.4] and spun again at 14,000 × g for 2 min. Synaptosomes were then removed from the top layer, washed once with KHR medium, and resuspended in a Krebs' solution [composed of the following (in mm): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 MgSO4, 2 CaCl2, and 10 glucose], which was gassed with a 95% O2 and 5% CO2 mixture. The nerve terminals were equilibrated at 37°C for 10 min, loaded with 0.2 μm [3H]glutamate for 5 min at 37°C, washed, layered over Whatman GF/C filters (VWR, Lisbon, Portugal), and superfused (flow rate, 0.8 ml/min) with Krebs' solution for 20 min before starting collection of the superfusate. The synaptosomes were stimulated with 20 mm K+ (isomolar substitution of NaCl by KCl in the Krebs' solution) at 3 and 9 min after starting sample collection (S1 and S2), triggering a release of tritium in a Ca2+-dependent manner that is mostly [3H]glutamate, gauged by HPLC (Lopes et al., 2002). Tested agonists were added 2 min before S2 onwards and their effect quantified by the modification of S2/S1 ratio versus control (i.e., absence of agonist), whereas antagonists were added from 10 min before starting sample collection onwards, and none of the tested antagonists modified the S2/S1 ratio versus control. Radioactivity was expressed in terms of disintegrations per second per milligram of protein (becquerels per milligram) in each chamber (Lopes et al., 2002).
Intracellular calcium measurements. Intracellular calcium measurements were performed in a partially purified synaptosomal fraction (P2) from the rat hippocampus, essentially as described by Malva et al. (1995). Synaptosomes (3 mg/ml) were incubated with 5 μm fura-2 AM (Molecular Probes, Alfagene, Lisbon, Portugal) and 0.02% Pluronic F-127 (Sigma, Sintra, Portugal) in an incubation medium [composed of the following (in mm): 132 NaCl, 1 KCl, 1 MgCl2, 100 CaCl2, 1.2 H3PO4, 10 glucose, and 10 HEPES-Na, pH 7.4] with 0.1% fatty acid-free BSA for 20 min at 25°C. After this loading period, synaptosomes were pelleted, the nonhydrolyzed probe was removed, and the synaptosomes placed in the same medium plus 1.2 mm CaCl2. Synaptosomes were preincubated with the antagonists 5 min before starting recording at 37°C, and agonists were applied 50 s after starting recording and 150 s before stimulation with 20 mm KCl. The fluorescence was monitored at 37°C, using a computer-assisted Spex Industries (Edison, NJ) Fluoromax spectrofluorometer at 510 nm emission and double excitation at 340 and 380 nm, using 5 nm slits. The calibration was made using 2.5 μm ionomycin (1.33 mm CaCl2; Rmax), at 500 s, and 24 mm EGTA (Rmin), at 600 s. The fluorescence intensities were converted into [Ca2+]i values by using the calibration equation for double excitation wavelength measurements and taking the dissociation constant of the fura-2/Ca2+ complex as 224 nm (Grynkiewicz et al., 1985). Intracellular calcium transients were determined by subtracting the [Ca2+]i basal value immediately before stimulation from the [Ca2+]i value at 24 s after stimulation (peak values).
Single-cell reverse transcription-PCR analysis of P2 receptor expression in laser-dissected hippocampal pyramidal neurons. Coronal sections (6 and 10 μm) were obtained from frozen rat brains (between -4.52 and -2.80 mm from bregma). These sections were dehydrated in 100% ethanol and rehydrated gradually (100-50% ethanol), stained with Nissl stain, and rehydrated again with xylene. CA1 or CA3 pyramidal cell bodies were microdissected with laser with a microscope (LCM 210 PixCell II; Arc-turus, Genetic Research Instrumentation, Essex, UK) with a spot size of 7.5 μm (Lopes et al., 2003). The total RNA was then extracted according to StrataPrep Total RNA micropep kit instructions from Stratagene (La Jolla, CA) and subjected to reverse transcription (RT) and cDNA amplification using a three prime end amplification PCR (TPEA-PCR) protocol, as described previously (Richardson et al., 2000). Purification of cDNA was achieved using QIA Quick PCR purification kit (Qiagen, West Sussex, UK). Once obtained, samples of the preamplified cDNA were subjected to 45 rounds of PCR in 20 μl of 45 mm Tris-HCl, pH 8.1, 12.5% (w/v) sucrose, 12 mm (NH4)2SO4, 3.5 mm MgCl2, and 0.5 mm deoxynucleotide triphosphates, with 100 ng of the forward and reverse primers for each P2 receptor cDNA, detailed in Table 1. The cycling conditions were 2.5 min at 92°C (denaturation), 1.5 min at 60°C (annealing), and 1 min at 72°C (extension). After amplification, the products were separated on 2% agarose gels and visualized using ethidium bromide. In all experiments, positive controls for the primers used were electrophoresed in parallel to the gene-specific assays. These routinely contained cDNA derived from 1 ng of whole-brain total RNA.
Table 1.
PCR primers used for detection of P2 receptor mRNA in single hippocampal pyramidal neurons of both CA3 and CA1 areas
|
|
Primers |
|
||
|---|---|---|---|---|
| Name |
Forward (5′-3′) |
Reverse (5′-3′) |
PCR product (bp) |
|
| Ribosomal protein L11 | TTCTATGTGGTGCTGGGTAGG | TTGCCTCCTCTTTGCTGATT | 187 | |
| Glutamate decarboxylase | ATCTTGCTTCAGTAGCCTTTGC | TGTCTTCAAAAACACTTGTGGG | 220 | |
| Glial fibrillary acidic protein | CTGGGCAGGGTACAGG | AAACAGGATGGACGCTTAAA | 222 | |
| Intronic marker | GCCTGCATTCATCTTCATCTGC | AAAGGTGGAACTCGCCCGTTT | 189 | |
| NMDA receptor subunit 1 | TAGTGGCAGTGCTTCAGGG | GTGACACCGGACTGGGGAG | 178 | |
| ATP (P2) receptors | ||||
| rP2X1 | GTTCAGCATGAAGACAGGCA | GAGTATAGATGTGTGAGGGGCC | 136 | |
| rP2X2 | TGTGACTGGGAAACAGAAACC | AGGAGATGGCAGGGAACC | 114 | |
| rP2X3 | AAGAAGGGGCTGCTATTTCTGC | AGGCATGCAAGGGGTAAAG | 126 | |
| rP2X4 | CTGGTGTGCTGGTTGGGCTG | ACCTGAGAGAGCCTCCTTCC | 152 | |
| rP2X5 | ACTTAGGGAAGAGCAAACTCCC | AGCAAGAGCTGAACTGCACA | 156 | |
| rP2X6 | AGGCTAGGGTGAAAGCAACA | GCAGGAATATCAGGTTCTTTGG | 201 | |
| rP2X7 | 1-TAAAGTTTGGATGTGGCTTGG | 1-TCTGTGTGGTGTGTGGTGTGTG | 156 | |
| 2-AGACAAACAAAGTCACCCGG | 2-GGTATACACCTGCCGGTCTGG | 400 | ||
| 3-AGGAGCCCCTTATCAGCTCT | 3-CATTGGTGTACTTGTCGTCC | 711 | ||
| rP2Y1 | CCCTAACTATGATGCAGCTT | GCTGCATCTTATCACCCT | 151 | |
| rP2Y2 | GCCACCTGACTCCCATGCAA | CCGCTGAGCTAAATCCC | 167 | |
| rP2Y4 | GGGGACAAGTATCGAAACCA | GCCCCTGCAGTTAGTTCCCTT | 208 | |
| rP2Y6 | GACACCTGTGTTTCGGGGAC | CCTCTACAGGAGGGGCCTT | 259 | |
| hP2Y11 | ACTGGTGGTTGAGTTCCTGG | TCAGGTGGGAGAAGCTGAGT | 410 | |
| rP2Y12
|
CAGAAATTCCTTGATGAGCA |
ATGTGGTGATTTCCTTGGAG |
175 |
|
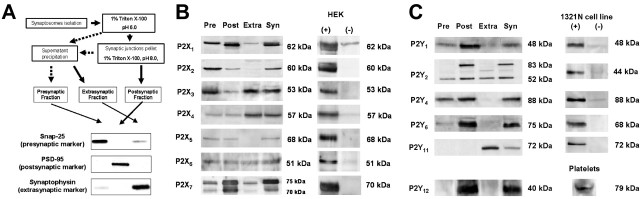
Subcellular fractionation of nerve terminals. The solubilization of extrasynaptic, presynaptic active zone, and postsynaptic fractions from rat hippocampal synaptosomes was performed according to the method described previously (Phillips et al., 2001) with minor modifications. We have confirmed previously that this subsynaptic fractionation method allows a >90% effective separation of active zone [synaptosomal-associated protein of 25 kDa (SNAP25)], postsynaptic density [postsynaptic density-95 (PSD95)], and nonactive zone fraction (synaptophysin) markers and can be used to access the subsynaptic distribution of receptors (Pinheiro et al., 2003; Rebola et al., 2003). Briefly, hippocampi from 10 rats were homogenized at 4°C in 15 ml of isolation solution [0.32 m sucrose, 0.1 mm CaCl2, 1 mm MgCl2, 0.1 mm phenylmethylsulfonylfluoride (PMSF)]. The concentration of sucrose was raised to 1.25 m by addition of 75 ml of 2 m sucrose and 30 ml of 0.1 mm CaCl2 and the suspension divided into 10 ultracentrifuge tubes. The homogenate was overlaid with 8 ml of 1.0 m sucrose, 0.1 mm CaCl2, and with 5 ml of homogenization solution and centrifuged at 100,000 × g for 3 h at 4°C. Synaptosomes were collected at the 1.25/1.0 m sucrose interface, diluted 1:10 in cold 0.32 m sucrose with 0.1 mm CaCl2, and pelleted (15,000 × g for 30 min at 4°C). Pellets were resuspended in 1 ml of 0.32 m sucrose with 0.1 mm CaCl2 and a small sample taken for gel electrophoresis. Then, synaptosomal suspension was diluted 1:10 in cold 0.1 mm CaCl2, and an equal volume of 2× solubilization buffer (2% Triton X-100, 40 mm Tris, pH 6.0) was added to the suspension. The membranes were incubated for 30 min on ice with mild agitation and the insoluble material (synaptic junctions) pelleted (40,000 × g for 30 min at 4°C). The supernatant (extrasynaptic fraction) was decanted and proteins precipitated with 6 vol of acetone at 20°C and recovered by centrifugation (18,000 × g for 30 min at 15°C). The synaptic junctions pellet was washed in pH 6.0 solubilization buffer, resuspended in 10 ml of 1% Triton X-100 and 20 mm Tris, pH 8.0, incubated for 30 min on ice with mild agitation, centrifuged (40,000 × g for 30 min at 4°C), and the supernatant (presynaptic active zone fraction) processed as above. PMSF (1 mm) was added to the suspension in all extraction steps. The pellets from the supernatants and the final insoluble pellet (postsynaptic density fraction) were solubilized in 5% SDS, the protein concentration determined, and the samples added to a ⅙ volume of 6× SDS-PAGE sample buffer for Western blot analysis.
Western blot analysis. The P2X1-7 or P2Y1,2,4,6,11,12 receptors immunoreactivity was evaluated by Western blot analysis as described previously (Rebola et al., 2003) using antibodies against P2X1 (1:500), P2X2 (1: 1000), P2X3 (1:300), P2X4 (1:500), P2X5 (1:100), P2X6 (1:100), P2X7 (1:10,000), P2Y1 (1:200), P2Y2 (1:500), P2Y4 (1:500), P2Y6 (1:300), P2Y11 (1:500), and P2Y12 (1:200). This analysis was conducted in the purified presynaptic active zone, postsynaptic density, and extrasynaptic fractions of rat hippocampal nerve terminals for all P2 receptors. To validate the antibodies, their immunoreactivities were tested in membranes of either human embryonic kidney 293 (HEK293) cells transiently expressing each P2X receptor subunits (P2X1-7) or human astrocytoma 1321N1 cells stably transfected with the cDNAs encoding for P2Y1,2,4,6,11 (see methods below). The immunoreactivity control of the antibody against P2Y12 receptor was evaluated using rat platelet membranes, prepared as described previously (Sugidachi et al., 2001), which have a high density of this receptor (Ralevic and Burnstock, 1998).
Expression of P2X receptors in HEK293 cells and P2Y receptors in human astrocytoma 1321N1 cells. cDNAs encoding for each P2X receptor (P2X1-7) were donated by A. North (University of Sheffield, Sheffield, UK). The HEK293 cells were transiently transfected with the cDNAs using lipofectamine (Invitrogen, Alfagene). cDNA (0.4 μg) was mixed with 5 μl of lipofectamine in 50 μl of Opti-MEM I reduced serum medium (Invitrogen). After 15-45 min of incubation to allow the formation of DNA-liposome complexes, this mixture was overlaid onto 50-80% confluent cells plated in 100 mm dishes and maintained in DMEM with 10% heat-inactivated fetal calf serum (FCS) for 24 h. The cells were then washed twice with PBS medium [containing the following (in mm): 137 NaCl, 2.7 KCl, 1.8 KH2PO4, 10 NaH2PO4, pH 7.4], collected in a hypotonic lysis buffer [containing the following (in mm): 25 HEPES, 2 MgCl2, 1 EDTA, 1 EGTA, pH 7.4], sonicated, and subjected to low-speed centrifugation to remove organelles and nuclei. The protein concentration was determined and the samples were added to a ⅙ vol of 6× SDS-PAGE sample buffer for Western blot analysis.
Human astrocytoma 1321N1 cells stably transfected with cDNAs encoding for P2Y1, P2Y2, P2Y4, P2Y6, or P2Y11 were donated by J. M. Boeynaems (University of Brussels, Brussels, Belgium). Cells were maintained in DMEM nutrient mix supplemented with 10% FCS, 1% mixture of streptomycin (100 U/ml)/penicillin (200 U/ml) (Sigma), and 0.5 mg/ml of geneticin G 418 sulfate (Calbiochem, VWR) in a water-saturated atmosphere of 95% O2 and 5% CO2 at 37°C and passaged when confluent. Wild-type cells were maintained in the same medium but without Geneticin G 418 sulfate. The preparation of membranes and determination of protein concentration for Western blot analysis was performed as described for HEK293 cells.
Immunocytochemical analysis in rat hippocampal nerve terminals. For immunocytochemical analysis, hippocampal synaptosomes were obtained through a discontinuous Percoll gradient, as described previously (Diáz-Hernández et al., 2001; Rodrigues et al., 2005). This procedure for preparation of the synaptosomes is crucial to reduce the amount of postsynaptic density material. In fact, immunocytochemical analysis of the synaptosomes obtained with this discontinuous Percoll gradient showed that <1% of the synaptophysin-positive elements were labeled by an anti-PSD95 antibody (data not shown).
These hippocampal synaptosomes were placed onto coverslips coated previously with poly-l-lysine, fixed with 4% paraformaldehyde for 15 min, and washed twice with PBS medium. The synaptosomes were permeabilized in PBS with 0.2% Triton X-100 for 10 min and then blocked for 1 h in PBS with 3% BSA and 5% normal rat serum. The synaptosomes were then washed twice with PBS and incubated with rabbit anti-P2X1 (1:500), anti-P2X2 (1:1000), anti-P2X4 (1:500), anti-P2X7 (1:10,000), anti-P2Y2 (1:500), anti-P2Y4 (1:500), anti-P2Y11 (1:500), or anti-P2Y12 (1:200) receptors or goat anti-P2X3 (1:300), anti-P2X5 (1:100), anti-P2X6 (1:100), anti-P2Y1 (1:200), or anti-P2Y6 (1:300) receptors and guinea-pig anti-vesicular glutamate transporters type 1 (vGluT1; 1:5000) and anti-vGluT2 (1:5000) for 1 h at room temperature. The synaptosomes were then washed three times with PBS with 3% BSA and incubated for 1 h at room temperature with AlexaFluor-488 (green)-labeled donkey anti-goat or goat anti-rabbit IgG antibodies and AlexaFluor-598 (red)-labeled goat anti-guinea pig IgG antibodies (1:200 for all). After washing and mounting on slides with Prolong Antifade, the preparations were visualized in a Zeiss (Hitec, Sintra, Portugal) Axiovert 200 inverted fluorescence microscope equipped with a cooled CCD camera and analyzed with MetaFluor 5.0 software. Each coverslip was analyzed by counting three different fields and in each field a total amount of 500 individualized elements.
Statistics. The values presented are mean ± SEM of n experiments. To test the significance of the effect of a drug versus control, an unpaired Student's t test was used.
Antibodies. The antibodies against P2X1, P2X2, P2X4, P2X7, P2Y2, P2Y4, and P2Y12 receptors were from Alomone Labs (Jerusalem, Israel); against P2X3, P2X5, P2X6, P2Y1, and P2Y6 receptors were from Santa Cruz Biotechnology (Santa Cruz, CA); against P2Y11 receptor was from Zymed (Quelug, Portugal); the antibodies against vGluT1 and vGluT2 were from Chemicon Europe (Southampton, UK); mouse anti-synaptophysin and mouse anti-SNAP25 were from Sigma; and mouse anti-PSD95 was from Upstate Biotechnology (Lake Placid, NY). All secondary antibodies for immunocytochemical analysis were purchased from Molecular Probes (Alfagene). All secondary alkaline phosphatase-tagged antibodies for Western blot were purchased to Amersham Biosciences (Carnaxide, Portugal).
Drugs. 1,3-Dipropyl-8-cyclopentyladenosine (DPCPX), α,β-methyleneATP (α,β-MeATP), β,γ-ImidoATP (β,γ-ImATP), suramin, brilliant blue G, and pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) (PPADS) were from Sigma; 8-(benzamido)naphthalene-1,3,5-trisulfonate (NF023), 2′deoxy-N6-methyladenosine-3′,5′-bisphosphate (MRS2179), reactive blue 2 (RB2), 2′-3′-O-(4-benzoylbenzoyl)-ATP (Bz-ATP), 2′,3′-O-(2,4,6-trinitrophenyl)-ATP (TNP-ATP), 2-methylthio-ADP, and 4-(2-[7-amino-2-(2-furyl)]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385) were purchased from Tocris Cookson (Bristol, UK); P1,P3-di(uridine 5′-) triphosphate (INS415) and P1-(urinine 5′-),P4-(inosine 5′-) tetraphosphate (INS45973) were provided by J. L. Boyer (Inspire Pharmaceuticals, Durham, NC). All drug stock solutions were made in water at a concentration of 10 mm and diluted directly into the superfusion or batch solution to the appropriate final concentration. [3H]glutamate (specific activity, 45 Ci/mmol) was from Amersham Biosciences.
Results
ATP modulates the evoked release of glutamate in a biphasic manner
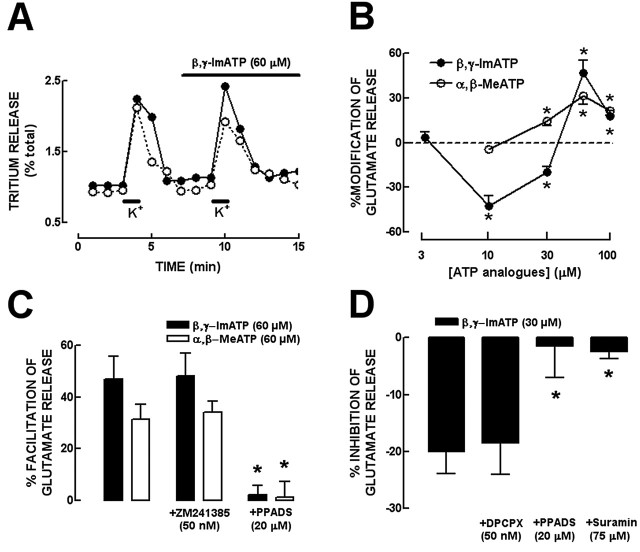
To assess the effect of P2 receptor activation on the evoked release of glutamate from rat hippocampal nerve terminals, we tested two stable ATP analogs, β,γ-ImATP (a nonselective P2 receptor agonist) and α,β-MeATP (a selective agonist of P2 receptors containing P2X1 and P2X3 subunits), because they are poor substrates of both the ectonucleotidases catabolism and ectoprotein kinases (Ralevic and Burnstock, 1998). The chemical stimulation with 20 mm K+ for 30 s of the superfused hippocampal synaptosomes that had been loaded previously with [3H]glutamate triggered a Ca2+-dependent release of [3H]glutamate that represented 90% of the total tritium released (gauged by HPLC), which was considered to represent the evoked release of glutamate (Lopes et al., 2002). Two periods of chemical stimulation (S1 and S2), separated by a 6 min interval, produced a similar evoked release of glutamate (Fig. 1A) with an S2/S1 ratio of 0.88 ± 0.03 (n = 16). β,γ-ImATP (present only in S2) caused a concentration-dependent biphasic effect in the evoked release of glutamate: at the lower concentrations tested, 10 and 30 μm, β,γ-ImATP inhibited glutamate release by 42.9 ± 7.1% (n = 4) and 20.0 ± 4.1% (n = 4), respectively, whereas 60 and 100 μm β,γ-ImATP facilitated glutamate release by 46.9 ± 8.7% (n = 4) and 17.6 ± 4.0% (n = 4) (Fig. 1B). When we tested α,β-MeATP, only facilitatory effects were observed in the concentration range tested (10-100 μm), with a maximal facilitatory effect of 31.4 ± 5.8% (n = 4) observed at 60 μm of α,β-MeATP (Fig. 1B).
Figure 1.
The ATP analogs β,γ-ImATP and α,β-MeATP biphasically modulate the evoked release of glutamate from rat hippocampal nerve terminals through activation of P2 receptors. A, Time course of tritium release from rat hippocampal synaptosomes challenged with two periods of stimulation with 20 mm K+ for 30 s (S1 and S2), as indicated by the bars above the abscissa. This representative experiment shows that 60 μm β,γ-ImATP (filled symbols), applied through the superfusate in the test chambers 2 min before S2 (as indicated by the top bar), increases glutamate release in S2. B, The nonselective P2X/P2Y receptors agonist β,γ-ImATP caused a concentration-dependent biphasic effect on the evoked release of glutamate, inhibiting at the lower (10-30 μm) and facilitating at higher (60-100 μm) concentrations, whereas the P2X-prefering agonist α,β-MeATP only facilitated the evoked release of glutamate at all concentrations tested. *p < 0.05 compared with 0%. C, The facilitatory effects of both 60 μm β,γ-ImATP and 60 μm α,β-MeATP were not modified in the presence of 50 nm ZM241385 (a selective antagonist of adenosine A2A receptors) but were prevented by 20 μm PPADS (nonselective P2 receptor antagonist). D, The inhibitory effect of 30 μm β,γ-ImATP was not modified in the presence of 50 nm DPCPX (selective antagonist of adenosine A1 receptor) but were prevented by both 75 μm suramin and 20 μm PPADS. *p < 0.05 compared with the effects of ATP analogs. The results are mean ± SEM of four to six experiments.
The biphasic effects of ATP are mediated by P2 receptors
The next necessary step was to test whether these effects of ATP analogs on glutamate release were mediated through activation of P2 receptors or through adenosine (P1) receptors after extracellular catabolism of ATP analogs by the ectonucleotidase pathway (Dunwiddie et al., 1997; Cunha et al., 1998). For that purpose, we tested the ability of P2 and P1 receptor antagonists to modify the effects of β,γ-ImATP and α,β-MeATP. Neither the adenosine A2A receptor antagonist ZM241385 (50 nm) nor the adenosine A1 receptor antagonist DPCPX (50 nm) significantly (p > 0.05) modified the modulatory effects of α,β-MeATP or β,γ-ImATP (Fig. 1C,D). In contrast, these effects were abolished by the P2 receptor antagonists PPADS (20 μm) or suramin (75 μm) (Fig. 1C,D). These results indicate that both the facilitatory effect of α,β-MeATP and the facilitatory and inhibitory effects of β,γ-ImATP on the evoked release of glutamate were mediated through the activation of P2 rather than P1 receptors. It should be noted that DPCPX, ZM241385, PPADS, or suramin alone did not cause any effect on the evoked release of glutamate nor on the S2/S1 ratio, in agreement with the expected absence of tonic effects of endogenously released modulators in superfused synaptosomes. This is probably because any released substance was effectively washed out by superfusion using a flow rate of 0.8 ml/min (Raiteri and Raiteri, 2000).
The inhibitory effects of ATP are mediated by P2Y receptors, whereas the facilitatory effects are mediated by P2X receptors
As illustrated in Figure 2A, the facilitatory effect of 60 μm β,γ-ImATP was reversed in the presence of the selective P2X1,2/3,3 receptor antagonist NF023 (10 μm) (Soto et al., 1999), whereas it was not modified (p > 0.05) by either the P2Y receptor antagonist RB2 (10 μm) or the selective P2Y1 receptor antagonist MRS2179 (10 μm) (Boyer et al., 2002). Furthermore, in the presence of another P2X1-3-selective antagonist, TNP-ATP (100 nm) (North, 2002), the facilitation by α,β-MeATP (60 μm) of the evoked release of glutamate was abolished (3.4 ± 5.5%; n = 5; p < 0.05). On the other hand, the inhibition caused by 10 μm β,γ-ImATP was not modified (p > 0.05) by NF023 (10 μm), was attenuated (p < 0.05) by MRS2179 (10 μm), and prevented (p < 0.05) by RB2 (10 μm) (Fig. 2B). This suggests that P2X receptors were responsible for the facilitatory effects whereas P2Y receptors mediate the inhibitory effect of ATP analogs on the evoked release of glutamate.
Figure 2.
The facilitatory effect of ATP analogs is mediated by P2X receptors, whereas the inhibitory effect is mediated by P2Y receptors. A, The facilitatory effect of 60 μm β,γ-ImATP was reverted in the presence of NF023 (10 μm; a selective P2X1, P2X2/3, and P2X3 receptor antagonist) and not modified by either RB2 (10 μm; a P2Y receptor antagonist) or MRS2179 (10 μm; a selective P2Y1 receptor antagonist). B, The inhibitory effect of 10 μmβ, γ-ImATP was not modified by NF023 but was prevented by RB2 and attenuated by MRS2179. *p < 0.05 compared with the first bar from left in A and B. The results are mean ± SEM of four to six experiments.
Activation of P2X and P2Y receptors also biphasically modulate the evoked [Ca2+]i transients
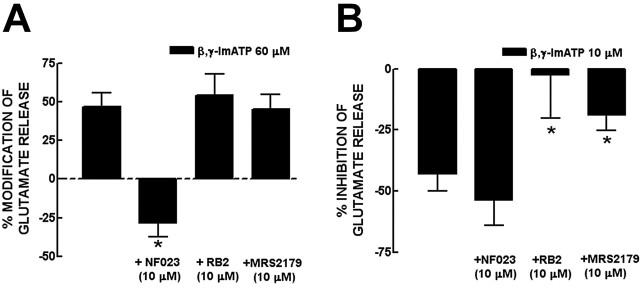
The release of neurotransmitters is triggered by the influx of Ca2+ in the active zone of nerve terminals and the presynaptic modulation of neurotransmitter release often involves the control of this Ca2+ entry, either directly (through ionotropic receptors) or indirectly through modulation of calcium channels (Khakh and Henderson, 2000). Thus, we tested the effect of 30 and 60 μm β,γ-ImATP on the evoked calcium transients, concentrations that had caused inhibitory and facilitatory effects, respectively, on the evoked release of glutamate. Hippocampal nerve terminals were stimulated with 20 mm K+ (as in the previous experiments), resulting in an increase in [Ca2+]i of 331 ± 10 nm over a basal value of 212 ± 6 nm. To exclude the involvement of P1 receptors, all experiments were performed in the presence of ZM241385 (50 nm) and DPCPX (50 nm). As for the control of glutamate release, β,γ-ImATP modulated [Ca2+]i transients in a biphasic manner (Fig. 3A): β,γ-ImATP (60 μm) facilitated [Ca2+]i transients by 14.3 ± 1.7% (n = 6), and β,γ-ImATP (30 μm) inhibited [Ca2+]i transients by 11.0 ± 2.6% (n = 6). Despite their low amplitude, which is expected based on the exponential relationship between [Ca2+]i transients and neurotransmitter release (Augustine, 2001), it is possible to pharmacologically characterize the receptors involved, as performed previously for other presynaptic receptors (Malva et al., 1995; Diáz-Hernández et al., 2001). Both the facilitatory and inhibitory effects of β,γ-ImATP were prevented by PPADS (20 μm), indicating the involvement of P2 receptors. Furthermore, the facilitatory effect of 60 μm β,γ-ImATP was attenuated (p < 0.05) by NF023 (10 μm) but not by 10 μm RB2 (Fig. 3B), whereas the inhibitory effect of 30 μm β,γ-ImATP was attenuated (p < 0.05) by 10 μm RB2 but not by 10 μm NF023 (Fig. 3C). This reinforces the idea that the presynaptic biphasic effects of ATP analogs are mediated by inhibitory P2Y and facilitatory P2X receptors.
Figure 3.
β,γ-ImATP modulates the evoked [Ca2+]i transients in a biphasic manner through inhibitory P2Y and facilitatory P2X receptors in rat hippocampal nerve terminals. A, Representative experiment of the increase and decrease of the K+ (20 mm)-evoked [Ca2+]i caused by β, γ-ImATP at 60 and 30 μm, respectively. All experiments were performed in the presence of 50 nm ZM241385 and 50 nm DPCPX to exclude P1 receptor-mediated effects. B, The facilitatory effect of 60 μm β,γ-ImATP was prevented by PPADS (20 μm; a nonselective P2 receptor antagonist) and by NF023 (10 μm; a selective antagonist of P2X1, P2X2/3, and P2X3 receptors) and not modified by RB2 (10 μm; a preferring antagonist of P2Y receptors). C, The inhibitory effect of 30 μm β,γ-ImATP was not modified by NF023 but was prevented by PPADS and attenuated by RB2. The percentage variations of [Ca2+]i are relative to control values (B, C). *p < 0.05 compared with 0%. **p < 0.05 compared with first bar from left in B and C. The results are mean ± SEM of four to six experiments.
P2X7 receptor does not presynaptically control glutamate release
It was described recently that the P2X7 receptor is targeted to glutamatergic nerve terminals in different brain regions (Deuchars et al., 2001; Miras-Portugal et al., 2003), including the hippocampus (Armstrong et al., 2002; Sperlágh et al., 2002). However, some studies cast doubts on the selectivity of the most commonly used antibodies against P2X7 receptors (Sim et al., 2004), and it has been shown that purported P2X7 receptor agonists might also indirectly activate adenosine A1 receptors (Kukley et al., 2004). We report that Bz-ATP (5 μm; a concentration selective for P2X7 or P2X1 receptor activation) (North, 2002) facilitated by 48.5 ± 11.2% (n = 4) the evoked release of glutamate. This facilitatory effect of Bz-ATP was not modified (p > 0.05) by brilliant blue G (200 nm; 51.4 ± 11.5% facilitation; n = 4) but was prevented (p < 0.05) by suramin (75 μm; 5.7 ± 7.6% inhibition; n = 4), indicating the involvement of P2X1 receptors and ruling out the involvement of P2X7 receptors that are sensitive to brilliant blue G (especially in the rat) and insensitive to suramin (North, 2002).
Pharmacological identification of P2Y receptors inhibiting glutamate release
The ability of MRS2179 to attenuate (but not abolish like RB2) the inhibition of glutamate release by ATP analogs indicates that P2Y1 receptors as well as other P2Y receptors are involved. Accordingly, a P2Y2,4 receptor agonist synthesized by Inspire Pharmaceuticals, INS45973 (0.5 μm), inhibited glutamate release by 26.2 ± 6.0% (n = 4), whereas a selective P2Y6 receptor agonist, INS415 (2 μm), was devoid of effects (1.5 ± 7.7%; n = 4) (Shaver et al., 2005). Furthermore, the P2Y1/12/13-selective agonist 2-methylthio-ADP (1 μm) inhibited by 34.7 ± 6.4% (n = 5; p < 0.05) the evoked release of glutamate, an effect that was abolished (p < 0.05) in the presence of 10 μm MRS2179 (3.8 ± 6.1% inhibition; n = 6). These results suggest that, in addition to P2Y1 receptors, P2Y2 and/or P2Y4 receptors, but not P2Y6, P2Y12, or P2Y13 receptors, are also involved in the inhibitory control of the evoked release of glutamate from rat hippocampal nerve terminals.
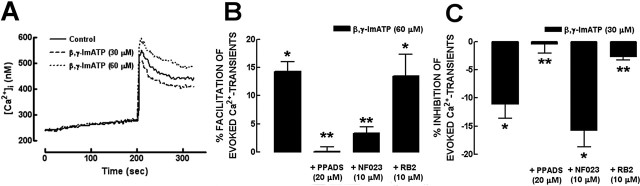
Identification of P2 receptors expressed in single hippocampal pyramidal neurons
The general lack of subtype-selective P2 receptors ligands limits the pharmacological characterization of the P2 receptors involved in the control of glutamate release. Thus, we decided to define which receptors are expressed in single pyramidal neurons (i.e., glutamatergic neurons) to short-list the candidate P2 receptors likely to be involved. We first confirmed the quality of the mRNA extracted from single pyramidal neurons by the lack of PCR product corresponding to an intronic marker (Fig. 4A, lane A), which was present when analyzing genomic DNA (1 ng) (Fig. 4A, lane B). We then selected for analysis 12 laser-dissected pyramidal neurons for which the recovered cDNA obeyed the criteria expected for glutamatergic neurons [i.e., presence of PCR products corresponding to a house-keeping gene, ribosomal protein L11, and to the NR1 subunit of NMDA receptors and absence of PCR products corresponding to glutamate decarboxylase (GAD; a marker of GABAergic neurons) and to glial fibrillary acidic protein (GFAP; a marker of glial cells)] (Fig. 4B). In these 12 samples, we detected PCR products corresponding mainly to P2X3 (6 of 12) but also P2X4 (3 of 12), P2X1, and P2X2 (2 of 12) receptor subunits. We failed to detect in any of the neurons transcripts for P2X5, P2X6, and P2X7, despite our care to use three different sets of primers (Table 1). In relationship to P2Y receptors, we detected PCR products corresponding predominantly to P2Y1 (9 of 12) but also for P2Y2 (6 of 12), P2Y4, P2Y6, P2Y11, and P2Y12 (2 of 12).
Figure 4.
RT-PCR identification of the P2 receptors expressed in single pyramidal neurons of the rat hippocampus. Individual cell bodies of CA1 and CA3 pyramidal neurons were removed under visual microscopic guidance from a Nissl-stained 10-μm-thick hippocampal slice, using a laser dissection slot (7.5 μm). Every sample of mRNA extracted from the cytoplasmic contents harvested from single hippocampal pyramidal neurons was subjected to reverse transcription protocol and amplified using a TPEA. They were then screened by PCR analysis, and the PCR products were resolved on 2-3% agarose gels and visualized by ethidium bromide staining. A, No PCR product was observed in the cDNA samples corresponding to an intronic marker (lane A), which indicates that all post-TPEA samples were DNA free. As positive controls, genomic DNA was always tested in parallel (lane B). B, Every single cDNA sample obtained from the laser-dissected single neurons that matched the PCR criteria for obtaining a single glutamatergic neuron [i.e., positive for a house-keeping gene (ribosomal protein L11) and a glutamatergic marker (NMDA receptor 1 subunit) and negative for both a GABAergic (GAD) and astrocytic (GFAP) markers] was screened by gene-specific PCR analysis for P2X subunits and P2Y receptors (B, bottom gels). In every assay, positive controls were performed using whole-brain cDNA instead (B, top gels). All the primers used are detailed in Table 1. Rib. Prot. L11, Ribosomal protein L11.
Combining these molecular biology data with the pharmacology data, P2X1, P2X2/3, and P2X3 receptors emerge as the leading candidates to mediate P2 receptor facilitation, and P2Y1, P2Y2, and eventually P2Y4 receptors stand as the most likely candidates to mediate P2 receptor inhibition, although P2Y11 receptor should also be considered.
Identification of P2X and P2Y receptors located in the presynaptic active zone
The ability of P2X receptors to facilitate and P2Y receptors to inhibit the evoked release of glutamate allows the prediction that the candidate receptors to mediate these effects should be located in the active zone of rat hippocampal nerve terminals. We took advantage of a recently described fractionation procedure to purify the presynaptic active zone (Phillips et al., 2001) to test by Western blot analysis which P2 receptors were present there. We have previously validated the technique for separation of the presynaptic active zone from the postsynaptic density and from other presynaptic proteins not located in synapses, which allows a >90% efficiency of separation of these fractions (Fig. 5A), and we have confirmed its usefulness to evaluate the subsynaptic distribution of both ionotropic (Pinheiro et al., 2003) and metabotropic (Rebola et al., 2003) receptors.
Figure 5.
Subsynaptic distribution of P2X and P2Y receptors in the hippocampus. A, Selective antibodies for each P2X and P2Y receptors were tested by Western blot analysis in a fraction enriched in the presynaptic active zone, in the postsynaptic density, in nerve terminals outside the active zone, and in the initial synaptosomal fraction from which fractionation began. These fractions were over 90% pure, as illustrated by the ability to recover the immunoreactivity for SNAP25 in the presynaptic active zone fraction, PSD95 in the postsynaptic density fraction, and synaptophysin (a protein located in synaptic vesicles) in the extrasynaptic fraction. B, C, Western blots in these fractions evaluating the subsynaptic distribution of the immunoreactivity of the antibodies selective for each P2X subunit and for each P2Y receptor tested (20-120 μg of protein of each fraction were applied to SDS-PAGE gels). To gauge the selectivity of the antibodies used, their immunoreactivity were tested in membranes of HEK cells transiently transfected with cDNAs encoding for each P2X subunit (B) and of 1321N1 human astrocytoma cell line stably transfected with each P2Y receptor (C). For P2Y12 receptor, rat platelet membranes were used as a positive control. Each blot is representative of at least three blots from different groups of animals with similar results. For each fractionation procedure, Western blot analysis for the markers of each fraction was performed as illustrated in A to assess the efficiency of each fractionation. Pre, Presynaptic active zone; Post, postsynaptic density; Extra, outside the active zone; Syn, synaptosomal.
We report that the most abundant P2X receptor subunits enriched in the presynaptic active zone fraction are P2X1, P2X2, P2X3, P2X5, and P2X6 (Fig. 5B). P2X1, P2X5, and P2X6 are also abundantly located in the postsynaptic density, and P2X3, P2X4, and P2X6 are abundantly located extrasynaptically in nerve terminals. Concerning the tested P2Y receptors, we report that they are all mainly located in the postsynaptic density, except the P2Y11 receptor that has a predominant extrasynaptic localization (Fig. 5C). The most abundant P2Y receptors in the presynaptic active zone were P2Y1, P2Y2, and P2Y4 receptors, whereas P2Y6 and P2Y12 were nearly exclusively found in the postsynaptic density. The relative subsynaptic distribution of all P2X subunits and P2Y receptors in presynaptic, postsynaptic, and extrasynaptic fractions is detailed in Table 2.
Table 2.
Relative subsynaptic distribution of P2X1-7 subunits and P2Y1,2,4,6,11,12 receptors in the presynaptic, postsynaptic, and extrasynaptic fractions
|
Subsynaptic fraction |
P2X1 |
P2X2 |
P2X3 |
P2X4 |
P2X5 |
P2X6 |
P2X7 |
P2Y1 |
P2Y2 |
P2Y4 |
P2Y6 |
P2Y11 |
P2Y12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Presynaptic | 42.4 ± 2.8 | 75.1 ± 1.4 | 47.0 ± 1.4 | 22.6 ± 1.2 | 55.4 ± 1.7 | 35.4 ± 1.6 | 22.4 ± 4.8 | 28.5 ± 1.5 | 23.1 ± 1.5 | 44.3 ± 2.6 | 5.0 ± 0.7 | 0.8 ± 0.3 | 0.6 ± 0.2 |
| Postsynaptic | 54.5 ± 3.1 | 24.0 ± 1.4 | 14.6 ± 0.7 | 17.1 ± 2.0 | 44.1 ± 1.8 | 33.9 ± 2.4 | 72.8 ± 4.9 | 70.3 ± 1.3 | 53.0 ± 0.8 | 55.0 ± 2.5 | 94.5 ± 0.6 | 0.5 ± 0.2 | 98.6 ± 0.4 |
| Extrasynaptic |
3.1 ± 1.0 |
0.9 ± 0.1 |
38.4 ± 1.5 |
60.2 ± 1.2 |
0.5 ± 0.2 |
30.4 ± 0.8 |
4.8 ± 0.6 |
1.2 ± 0.2 |
23.9 ± 0.9 |
0.7 ± 0.2 |
0.4 ± 0.1 |
98.7 ± 0.5 |
0.8 ± 0.3 |
The data show relative average percentage of immunoreactivity (mean ± SEM) measured by densitometry of three different Western blots from different synaptosomal preparations from different animals.
Identification of P2X and P2Y receptors located in hippocampal glutamatergic terminals
Because the fractionation procedure used above does not allow purification of only the synaptic fractions from glutamatergic terminals, we undertook a complementary double immunocytochemistry study in single nerve terminals aimed to identify the P2X and P2Y receptors located in particular in glutamatergic terminals, identified as the terminals labeled with antibodies against vesicular glutamate transporters type 1 and type 2 (see Fig. 6A as an example for P2X3 of the strategy used to identify its presence in glutamatergic terminals). vGluT1 and/or vGluT2 immunopositive nerve terminals comprised 38.6 ± 0.9% (n = 3) in the total population of hippocampal nerve terminals, identified as synaptophysin-immunoreactive elements. As presented in Figure 6B, the P2X receptor subunits most frequently located in glutamatergic terminals were P2X1, P2X2, P2X3, and P2X4 receptor subunits (Fig. 6B, filled bars). The P2Y receptors most frequently located in glutamatergic terminals were P2Y1, P2Y2, and P2Y4 receptors (Fig. 6B, open bars).
Figure 6.
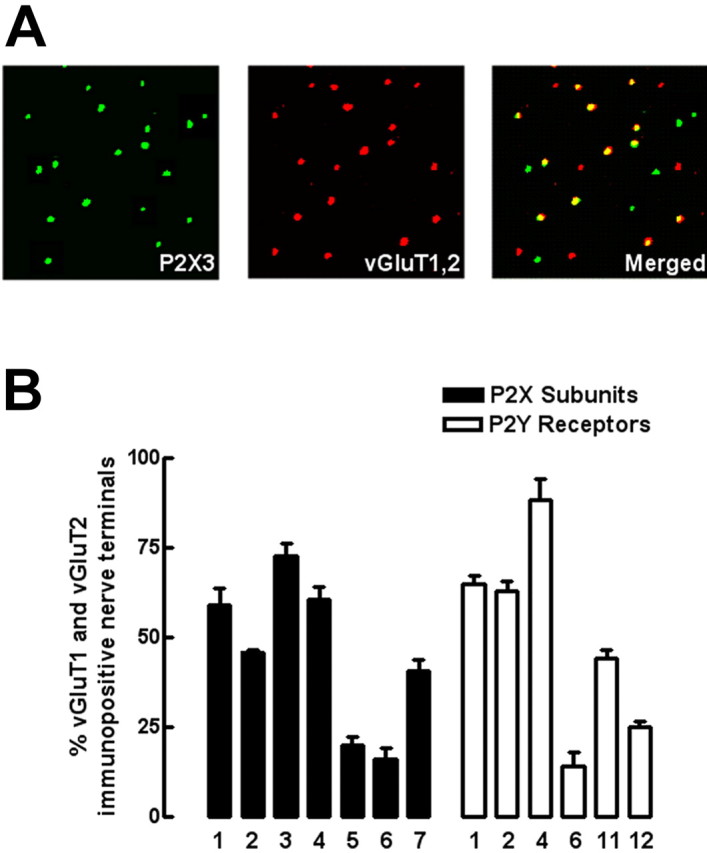
Identification of P2X and P2Y receptors present in rat hippocampal glutamatergic nerve terminals by double immunocytochemical analysis of rat hippocampal single nerve terminals. In A, as an example, the immunocytochemical identification of P2X3 receptors subunit (left) in glutamatergic nerve terminals identified as immunopositive for vesicular glutamate transporters type 1 and type 2 (middle) that comprised 38.6 ± 1.3% (n = 3) of the total synaptosomal population is shown. After merging both images (right), the percentage of rat hippocampal glutamatergic nerve terminals endowed with each P2X subunit and each P2Y receptor was quantified, which is presented in B. The data are mean ± SEM of three or four experiments, and in each experiment, using different synaptosomal preparation from different animals, four different fields acquired from two different coverslips were analyzed.
Crossing this double immunocytochemistry data with the data obtained in the subsynaptic fractionation of the presynaptic active zone, it can conclude that P2X1, P2X2, and P2X3 receptor subunits and P2Y1, P2Y2, and P2Y4 receptors are the most abundantly located in the presynaptic active zone membrane and also the most frequently found in glutamatergic terminals. These receptors are precisely those that are most expressed in pyramidal neurons and the leading candidates based on the pharmacological characterization of the evoked release of glutamate.
Discussion
The present study first shows that ATP can biphasically modulate (i.e., both facilitate and inhibit) the evoked release of glutamate from rat hippocampal nerve terminals through the activation of P2X and P2Y receptors, respectively. However, the main finding is the identification of the presynaptic P2X and P2Y receptors that are responsible for these inhibitory and facilitatory effects. By combining the data obtained in pharmacological studies, single-cell PCR, Western blot after subsynaptic fractionation, and double immunocytochemistry in single nerve terminals, we concluded that the facilitatory effects are caused by P2X1, P2X2/3, and P2X3 receptors and the inhibitory effects are mediated by P2Y1, P2Y2, and/or P2Y4 receptors.
These conclusions are based on the observation that the ATP analog β,γ-ImATP modulated the evoked release of glutamate from rat hippocampal nerve terminals in a concentration-dependent biphasic manner, inhibiting at the lower concentrations tested (10-30 μm) and facilitating at concentration of 60-100 μm. These biphasic effects are mediated through P2, but not adenosine P1, receptor activation because the selective antagonists of adenosine A1 or A2A receptors (DPCPX or ZM241385) failed to modify the effects of β,γ-ImATP, which were prevented by the P2 receptor antagonists PPADS and suramin. The pharmacological characterization was developed to show that the facilitatory effects are mediated by P2X receptors and the inhibitory effects by P2Y receptors because: (1) α,β-MeATP, an agonist of P2X1- and P2X3-containing receptors, only facilitated glutamate release at all concentrations tested (10-100 μm); (2) the selective antagonists of P2X1, P2X2/3, and P2X3 receptors, NF023 and TNP-ATP, prevented the facilitatory (but not the inhibitory) effect of ATP analogs; (3) the selective P2Y and P2Y1 antagonists RB2 and MRS2179 prevented and attenuated, respectively, the inhibitory (but not the facilitatory) effect of ATP analogs. This is reinforced by the fact that a similar pharmacological profile was obtained both for the release of glutamate and for calcium transients in the same hippocampal nerve terminals. Furthermore, apart from P2Y1 receptors, we could define the involvement of P2Y2 and P2Y4 receptors and exclude P2Y6 receptors, based on the use of selective agonists provided by Inspire Pharmaceuticals, disregard the involvement of P2Y12 and P2Y13 receptors, based on the ability of MRS2179 to abolish the inhibition caused by 2-methylthio-ADP, and rule out the involvement of P2X7 receptors.
The lack of selective pharmacological tools to further narrow the candidate P2 receptors led us to use alternative approaches to identify the P2X and P2Y receptors presynaptically controlling glutamate release. For that purpose, we used three different molecular approaches from the mRNA to the protein level. The rationale was the following: (1) the receptors should be expressed in hippocampal glutamatergic neurons; (2) they should be located in the active zone; (3) they should be located in glutamatergic terminals. Only the receptor candidates fulfilling these three criteria that also matched the pharmacological characterization should be claimed to be involved in the control of glutamate release. By single-cell RT-PCR, we detected mRNA expression in glutamatergic neurons from CA1 and CA3 areas for P2X1, P2X2, P2X3, and P2X4 subunits and P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y12 receptors. Looking at the subsynaptic distribution at the protein level, we found that P2X1, P2X2, and P2X3 were enriched in the presynaptic active zone, whereas P2Y1, P2Y2, and P2Y4 were the only tested P2Y receptors located at the presynaptic active zone. Restricting the analysis to the glutamatergic nerve terminals, double immunocytochemistry revealed that P2X1, P2X2, P2X3, and P2X4 were present in a higher percentage of glutamatergic nerve terminals (identified as vGluT1/2 positive), whereas P2Y1, P2Y2, and P2Y4 were the most frequently found P2Y receptors in glutamatergic terminals. Thus, combining the data obtained in these molecular studies, the P2 receptors that are expressed in glutamatergic cells, targeted to nerve terminals, and located in the presynaptic active zone are P2X1, P2X2, and P2X3 subunits and P2Y1, P2Y2, and P2Y4 receptors. This is in total agreement with the pharmacological characterization, thus confirming the involvement of P2X1, P2X2/3, and P2X3 receptors in the facilitation and of P2Y1, P2Y2, and P2Y4 receptors in the inhibition of hippocampal glutamate release.
This study constitutes the first attempt to thoroughly identify which P2 receptors controlled glutamate release using combined functional and molecular approaches, which yielded remarkably coincident conclusions. The main conclusion that P2 receptors presynaptically control the release of glutamate, together with the morphological data indicating that a substantial proportion of glutamatergic terminals are endowed with different subtypes of P2 receptors (both P2X and P2Y), confirms our hypothesis that P2 receptors play a major neuromodulatory role at the presynaptic level controlling the release of neurotransmitters rather than acting postsynaptically (Cunha and Ribeiro, 2000). This provides a logical explanation to reconcile the robust expression and density of P2 receptors in the hippocampus with the discrete contribution of P2 receptors to synaptic transmission (Pankratov et al., 1998; Mori et al., 2001). It was also interesting to conclude that receptors for the same ligand (ATP) but with opposite effects were present in hippocampal glutamatergic terminals, as reported to occur in glutamatergic synapses of the rat medial habenula nucleus (Price et al., 2003). In particular, β,γ-ImidoATP caused biphasic effects on glutamate release over a narrow concentration range, in a manner analogous to that observed to occur for the biphasic P2X/P2Y receptor modulation of noradrenaline release from the rat vas deferens (Queiroz et al., 2003). It remains to be determined whether this prevalence of P2X receptor-mediated facilitation over P2Y receptor-mediated inhibition of hippocampal glutamate release with increasing concentrations of ATP analogs results from: (1) a greater efficiency of glutamatergic P2X compared with P2Y receptors; (2) a greater number of glutamatergic terminals equipped with a greater density of P2X compared with P2Y receptors; (3) an interaction between P2X and P2Y receptors so that the former would down-regulate the later. The possible involvement of P2X1 and/or P2X3 receptors in this predominant P2X receptor-mediated facilitation of hippocampal glutamate release at higher concentrations of ATP analogs might also seem surprising in view of their fast desensitizing kinetics observed in heterologous expression systems or when located postsynaptically (North, 2002). However, like for P2X1-3 (present study) (Diáz-Hernández et al., 2001; Queiroz et al., 2003), the activation of other presynaptic ionotropic receptors, such as kainate or nicotinic acetylcholine receptors (Khakh and Henderson, 2000), also causes long-lasting non-desensitizing presynaptic changes, in clear contrast with their fast desensitizing properties when located outside nerve terminals, for reasons still to be unraveled.
Finally, it still remains to be defined in what physiological conditions might this P2 receptor modulation of glutamate release play a significant role. In fact, most attempts to define a possible role for P2 receptor modulation of excitatory synaptic transmission in hippocampal slices essentially concluded that all the effects of exogenously added ATP or ATP analogs were attributable to adenosine P1 receptor activation (Dunwiddie et al., 1997; Cunha et al., 1998; Mendoza-Fernandez et al., 2000; Masino et al., 2002). This is in agreement with the efficiency of ectonucleotidases in these synapses, which are able to convert ATP (and ATP analog) into adenosine in a channeling manner (Cunha et al., 1998) within milliseconds (Dunwiddie et al., 1997), making it possible to conceive a scenario in which exogenously added ATP might never reach synaptic P2 receptors before being degraded by ectonucleotidases (Zimmermann and Braun, 1996). It should also be pointed out that these electrophysiological studies were all performed in the same group of glutamatergic synapses connecting the Schaffer fibers to CA1 pyramidal neurons. When studying other synapses, it was found that P2X2 receptors controlled glutamatergic transmission onto interneurons of the CA1 region (Khakh et al., 2003), P2Y1 receptors controlled interneuron excitability (Kawamura et al., 2004), whereas in mossy fiber/CA3 pyramid synapses, a mixed P2/P1 modulation by exogenously added ATP was concluded (Kukley et al., 2004). Thus, it primarily remains to be tested whether P2 receptors might exert a more important control of glutamate release in other glutamatergic synapses in the hippocampus. A credible alternative might be that the neuromodulatory role of P2 receptors in glutamatergic synapses might not be related to conditions of low-frequency stimulation, in which one would expect calcium influx through P2X receptors to be most effective, but might only come into play during bursts of actions potentials, in which one would expect this P2 receptor-mediated calcium entry to be overshadowed by the massive calcium influx and the effect of other facilitating factor. Accordingly, the most consistent modulatory role of P2 receptors has been found in the control of long-term potentiation (O'Kane and Stone, 2000, Pankratov et al., 2002; Almeida et al., 2003). This is also in agreement with the finding that the release of ATP in hippocampal preparations increases disproportionally with increasing frequencies of nerve stimulation (Wieraszko et al., 1989; Cunha et al., 1996).
In conclusion, the present study provided coincident functional and molecular evidences showing that P2 receptors biphasically modulate the release of glutamate from hippocampal nerve terminals, thus explaining the discrepancy between P2 receptor expression and detected functional activity in this area of the brain. The precise physiological conditions under which this modulation becomes functionally important remain to be determined.
Footnotes
This work was supported by Fundação para a Ciência e Tecnologia (POCTI/36372/1999 and POCTI/43633/ESP/1999). We thank A. L. Carvalho and A. Gomes for helping us in the transfection procedures; J. O. Malva and P. S. Pinheiro for their support in the subcellular fractionation experiments; J. L. Boyer (Inspire Pharmaceuticals) for the kind gift of P2Y2,4,6-selective agonists; A. North (University of Sheffield, Sheffield, UK) for providing the P2X receptor cDNAs; J. M. Boeynaems (University of Brussels, Brussels, Belgium) for providing the human astrocytoma cell lines stably transfected with P2Y receptors; D. Cousens (GlaxoSmithKline UK) for providing P2Y receptors antibodies; and L. Widowson, L. V. Lopes, and J. M. Marques for their help in the PCR studies.
Correspondence should be addressed to Rodrigo A. Cunha, Institute of Biochemistry, Faculty of Medicine, University of Coimbra, 3004-504 Coimbra, Portugal. E-mail: racunha@clix.pt.
Copyright © 2005 Society for Neuroscience 0270-6474/05/256286-10$15.00/0
References
- Almeida T, Rodrigues RJ, de Mendonça A, Ribeiro JA, Cunha RA (2003) Purinergic P2 receptors trigger adenosine release leading to adenosine A2A receptor activation and facilitation of long-term potentiation in rat hippocampal slices. Neuroscience 122: 111-121. [DOI] [PubMed] [Google Scholar]
- Armstrong JN, Brust TB, Lewis RG, MacVicar BA (2002) Activation of presynaptic P2X7-like receptors depresses mossy fiber-CA3 synaptic transmission through p38 mitogen-activated protein kinase. J Neurosci 22: 5938-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ (2001) How does calcium trigger neurotransmitter release? Curr Opin Neurobiol 11: 320-326. [DOI] [PubMed] [Google Scholar]
- Boyer JL, Adams M, Ravi RG, Jacobson KA, Harden TK (2002) 2-Chloro N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate is a selective P2Y1 receptor antagonist. Br J Pharmacol 135: 2004-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Ribeiro JA (2000) ATP as a presynaptic modulator. Life Sci 68: 119-137. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Vizi ES, Ribeiro JA, Sebastião AM (1996) Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high-but not low-frequency stimulation of rat hippocampal slices. J Neurochem 67: 2180-2187. [DOI] [PubMed] [Google Scholar]
- Cunha RA, Sebastião AM, Ribeiro JA (1998) Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ectonucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci 18: 1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J (2001) Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J Neurosci 21: 7143-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diáz-Hernández M, Gomez-Villafuertes R, Hernando F, Pintor J, Miras-Portugal MT (2001) Presence of different ATP receptors on rat mid-brain single synaptic terminals. Involvement of the P2X3 subunits. Neurosci Lett 301: 159-162. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L, Proctor WR (1997) Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17: 7673-7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningson P, Vaugeois JM (2005) Adenosine and brain function. Int Rev Neurobiol 63: 191-270. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440-3450. [PubMed] [Google Scholar]
- Kawamura M, Gachet C, Inoue K, Kato F (2004) Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J Neurosci 24: 10835-10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Henderson G (2000) Modulation of fast synaptic transmission by presynaptic ligand-gated cation channels. J Auton Nerv Syst 81: 110-121. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Gittermann D, Cockayne DA, Jones A (2003) ATP modulation of excitatory synapses onto interneurons. J Neurosci 23: 7426-7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Stausberg P, Adelman G, Chessell IP, Dietrich D (2004) Ectonucleotidases and nucleoside transporters mediate activation of adenosine receptors on hippocampal mossy fibers by P2X7 receptor agonist 2′-3′-O-(4-benzoylbenzoyl)-ATP. J Neurosci 24: 7128-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK (2003) Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol 64: 785-795. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA (2002) Adenosine A2A receptor facilitation of hippocampal synaptic transmission is dependent on tonic A1 receptor inhibition. Neuroscience 112: 319-329. [DOI] [PubMed] [Google Scholar]
- Lopes LV, Rebola N, Pinheiro PC, Richardson PJ, Oliveira CR, Cunha RA (2003) Adenosine A3 receptors are located in neurons of the rat hippocampus. NeuroReport 14: 1645-1648. [DOI] [PubMed] [Google Scholar]
- Malva JO, Ambrósio AF, Cunha RA, Ribeiro JA, Carvalho AP, Carvalho CM (1995) A functionally active presynaptic high affinity kainate receptor in the rat hippocampal CA3 subregion. Neurosci Lett 185: 83-86. [DOI] [PubMed] [Google Scholar]
- Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Johansson B, Fredholm BB, Dunwiddie TV (2002) Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine A1 receptors. J Pharmacol Exp Ther 303: 356-363. [DOI] [PubMed] [Google Scholar]
- Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C (2000) ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J Pharmacol Exp Ther 293: 172-179. [PubMed] [Google Scholar]
- Miras-Portugal MT, Diáz-Hernández M, Giraldez C, Heras C, Gomez-Villafuertes R, Sen RP, Gualix J, Pintor J (2003) P2X7 receptors in rat brain: presence in synaptic terminals and granule cells. Neurochem Res 28: 1597-1605. [DOI] [PubMed] [Google Scholar]
- Mori M, Heuss C, Gahwiler BH, Gerber U (2001) Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J Physiol (Lond) 535: 115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA (2002) Molecular physiology of P2 receptors. Physiol Rev 82: 1013-1067. [DOI] [PubMed] [Google Scholar]
- O'Kane EM, Stone TW (2000) Characterisation of ATP-induced facilitation of transmission in rat hippocampus. Eur J Pharmacol 409: 159-166. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O (1998) A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci 10: 3898-3902. [DOI] [PubMed] [Google Scholar]
- Pankratov Y, Lalo U, Krishtal O, Verkhratsky A (2003) P2X receptor-mediated excitatory synaptic currents in somatosensory cortex. Mol Cell Neurosci 24: 842-849. [DOI] [PubMed] [Google Scholar]
- Pankratov YV, Lalo UV, Krishtal OA (2002) Role for P2X receptors in long-term potentiation. J Neurosci 22: 8363-8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR (2001) The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron 32: 63-77. [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Rodrigues RJ, Silva AP, Cunha RA, Oliveira CR, Malva JO (2003) Solubilization and immunological identification of presynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in the rat hippocampus. Neurosci Lett 336: 97-100. [DOI] [PubMed] [Google Scholar]
- Price GD, Roberston SJ, Edwards FA (2003) Long-term potentiation of glutamatergic synaptic transmission induced by activation of presynaptic P2Y receptors in the rat medial habenula nucleus. Eur J Neurosci 17: 844-850. [DOI] [PubMed] [Google Scholar]
- Queiroz G, Talaia C, Gonçalves J (2003) ATP modulates noradrenaline release by activation of inhibitory P2Y receptors and facilitatory P2X receptors in the rat vas deferens. J Pharmacol Exp Ther 307: 809-815. [DOI] [PubMed] [Google Scholar]
- Raiteri L, Raiteri M (2000) Synaptosomes still viable after 25 years of superfusion. Neurochem Res 25: 1265-1274. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50: 413-492. [PubMed] [Google Scholar]
- Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA (2003) Subcellular localization of adenosine A1 receptors in nerve terminals and synapses of the rat hippocampus. Brain Res 987: 49-58. [DOI] [PubMed] [Google Scholar]
- Richardson PJ, Dixon AK, Lee K, Bell MI, Cox PJ, Williams R, Pinnock RD, Freeman TC (2000) Correlating physiology with gene expression in striatal cholinergic neurones. J Neurochem 74: 839-846. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro T, Rebola N, Oliveira CR, Cunha RA (2005) Co-localization and functional interaction between adenosine A2A and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem 92: 433-441. [DOI] [PubMed] [Google Scholar]
- Schäfer R, Reiser G (1997) Characterization of [35S]-ATPγS and [3H]-α,β-MeATP binding sites in rat brain cortical synaptosomes: regulation of ligand binding by divalent cations. Br J Pharmacol 121: 913-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaver SR, Rideout JL, Pendergast W, Douglass JG, Brown EG, Boyer JL, Patel RI, Redick CC, Jones AC, Picher M, Yerxa BR (2005) Structure-activity relationships of dinucleotides: potent and selective agonists of P2Y receptors. Purinergic Signal 1: 183-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JA, Young MT, Sung HY, North RA, Surprenant A (2004) Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24: 6307-6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Webb TE, Barnard EA (1997) Distribution of [35S]dATPγS binding sites in the adult rat neuraxis. Neuropharmacology 36: 1243-1251. [DOI] [PubMed] [Google Scholar]
- Soto F, Lambrecht G, Nickel P, Stuhmer W, Busch AE (1999) Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology 38: 141-149. [DOI] [PubMed] [Google Scholar]
- Sperlágh B, Vizi ES (1996) Neuronal synthesis, storage and release of ATP. Sem Neurosci 8: 175-186. [Google Scholar]
- Sperlágh B, Köfalvi A, Deuchars J, Atkinson L, Milligan CJ, Buckley NJ, Vizi ES (2002) Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J Neurochem 81: 1196-1211. [DOI] [PubMed] [Google Scholar]
- Sugidachi A, Asai F, Yoneda K, Iwamura R, Ogawa T, Otsuguro K, Koike H (2001) Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type G(i)-linked P2T antagonist, CS-747. Br J Pharmacol 132: 47-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieraszko A, Goldsmith G, Seyfried TN (1989) Stimulation dependent release of adenosine triphosphate from hippocampal slices. Brain Res 485: 244-250. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CO, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S (2003) ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron 40: 971-982. [DOI] [PubMed] [Google Scholar]
- Zimmermann H, Braun N (1996) Extracellular metabolism of nucleotides in the nervous system. J Auton Pharmacol 16: 397-400. [DOI] [PubMed] [Google Scholar]