Abstract
Layer I of the neocortex comprises axonal processes from widespread regions of the brain and a unique population of GABAergic interneurons. Dopamine is known to directly depolarize layer I interneurons, but the underlying mechanism is unclear. Using whole-cell recording techniques in neocortical brain slices, we have examined how dopamine increases excitability of layer I interneurons in postnatal day 7-11 rats. Dopamine (30 μm) caused a 10 mV depolarization of layer I neurons. Paradoxically, neither the D1-like receptor agonist 6-chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide (SKF81297) (1-10 μm) nor the D2-like agonist quinpirole (10 μm) produced a significant depolarization. Depolarization was observed when SKF81297 and quinpirole were coapplied. When G-protein βγ subunits were included in the recording pipette, D1 but not D2 agonists depolarized layer I neurons. Bath application of 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride, a specific blocker of inwardly rectifying hyperpolarization-activated current (Ih) channels, hyperpolarized the neurons and occluded the action of dopamine. Voltage-clamp analysis demonstrated that dopamine increased the amplitude and shifted the voltage dependence of activation of Ih. These results indicate that Ih contributes to the resting potential of layer I interneurons and is subject to modulation by dopamine.
Keywords: neocortex, dopamine, layer I, interneuron, modulation, I h
Introduction
Layer I of the neocortex resides just under the pial surface and is ∼150 μm thick in the adult rat. Neuronal density in layer I is very low, and this layer has been referred to as the molecular or cell-free layer. Virtually all cells in layer I are GABAergic interneurons (DelRio et al., 1994; Li and Schwark, 1994). Studies in vivo and in vitro have begun to explore the function and connectivity of layer I interneurons. Ascending sensory information is relayed to layer I neurons rapidly, arriving 5-7 ms after whisker stimulation. These cells have receptive field properties similar to layer V pyramidal neurons (Zhu and Zhu, 2004), suggesting that these cells play a role in sensory processing. Multiple morphologically distinct types of layer I interneurons have been described previously (Bradford et al., 1978; Marin-Padilla, 1990; Hestrin and Armstrong, 1996; Zhou and Hablitz, 1996b; Christophe et al., 2002; Chu et al., 2003). The axonal arborizations of layer I cells fall into two categories: cells with extensive arbors confined to layer I and interneurons with descending axons reaching as far as layers IV-V (Zhou and Hablitz, 1996b; Christophe et al., 2002). Layer I cells thus appear to be part of a complex cortical circuit and are positioned to participate in information processing.
Layer I receives a dense innervation from dopaminergic fibers (Berger, 1992; Smiley et al., 1992; Sesack et al., 1995). Dopamine has a direct depolarizing effect on layer I interneurons and produces an action potential-dependent, robust enhancement of spontaneous IPSCs (Zhou and Hablitz, 1999). The mechanism underlying the depolarization is unclear. Layer II-IV fast-spiking interneurons are depolarized by dopamine via an effect on an inward-rectifier K+ current and a resting leak K+ current (Gorelova et al., 2002). Dopamine has been reported to modulate an inwardly rectifying hyperpolarization-activated current (Ih) in rat ventral tegmental neurons (Jiang et al., 1993). Identifying the currents and receptor mechanisms involved in depolarization of layer I interneurons is essential for understanding the functional role of these cells and their modulation by dopamine.
Depending on the cell type, Ih in the brain contributes to generation of rhythmic activity (McCormick and Pape, 1990) and determination of the resting membrane potential (Robinson and Siegelbaum, 2003). Four mammalian genes, termed HCN1-HCN4, encode hyperpolarization-activated, nonselective cation channels. Each subunit exhibits distinct patterns of activation and inactivation and varying sensitivities to cyclic nucleotides (Santoro et al., 2000; Wainger et al., 2001). Layer I interneurons display a prominent “sag” response during hyperpolarization, indicative of the presence of HCN channels (Zhou and Hablitz, 1995; Radnikow et al., 2002). Cajal-Retzius cells, another cell type located in layer I, also display robust Ih currents early in development (Kilb and Luhmann, 2000; Radnikow et al., 2002). HCN channels are regulated by intracellular cyclic nucleotide levels (Chen et al., 2001), and dopamine receptor activation is known to alter intracellular cAMP levels (Missale et al., 1998). We therefore examined whether dopamine depolarizes layer I interneurons via an effect on Ih. Our studies demonstrate that dopamine, via a synergistic activation of D1- and D2-like receptors, depolarizes layer I GABAergic interneurons by enhancing Ih.
Materials and Methods
Slice preparation. Neocortical slices were prepared from Sprague Dawley rats (7-11 d old). Animals were handled and housed according to the National Institutes of Health Committee on Laboratory Animal Resources guidelines. All experimental protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee. Every effort was made to minimize pain and discomfort. Rats were anesthetized with ketamine and decapitated. The brain was removed quickly and placed in ice-cold artificial CSF (ACSF), which contained the following (in mm): 125 NaCl, 3.5 KCl, 0.5 CaCl2, 3.5 MgCl2, 26 NaHCO3, and 10 d-glucose. The solution was bubbled with 95%O2/5%CO2 to maintain pH at ∼7.4. Coronal brain slices (300 μm thick) containing the anterior cingulate cortex and the shoulder or Fr2 region of the frontal cortex (Paxinos and Watson, 1997) were cut using a vibratome. Slices were stored for 45 min at 37°C and kept at room temperature until recording. The oxygenated storage solution contained the following (in mm): 125 NaCl, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 2.5 CaCl2, and 1.3 MgCl2. Individual slices were subsequently transferred to a recording chamber continuously perfused (3 ml/min) with oxygenated saline at 32°C. A Zeiss (Thornwood, NY) Axioskop FS microscope equipped with Nomarski optics, a 40× water-immersion lens, and infrared illumination was used to view neurons in the slices. Layer I interneurons were identified by their distance from the pial surface and their firing properties. In addition, cells were intracellularly labeled with biocytin to confirm identification. Labeled cells were processed as described previously (Zhou and Hablitz, 1996b).
Whole-cell recording. Whole-cell current- and voltage-clamp recordings were obtained as described previously (Zhou and Hablitz, 1996b). Tight seals (>2 GΩ before breaking into whole-cell mode) were obtained using patch electrodes having an open-tip resistance of ∼3 MΩ. Series resistance during recording varied from 10 to 20 MΩ and was not compensated. Recordings were terminated whenever significant increases (>20%) in series resistance occurred. The intracellular solution for recording synaptic currents contained the following (in mm): 125 K-gluconate, 10 KCl, 10 HEPES, 2 Mg-ATP, 0.2 Na-GTP, and 0.5 EGTA. The osmolarity and pH were adjusted to 285 mOsm and 7.3, respectively. Recordings were done at 32°C.
Data collection and analysis. Signals were acquired using a Warner PC505A amplifier (Warner Instruments, Hamden, CT) controlled by Clampex 8.0 software (Molecular Devices, Foster City, CA) via a Digidata 1200B interface (Molecular Devices). Current-voltage relationships and repetitive firing properties were studied using computer-generated commands. Responses were filtered at 5 kHz, digitized at 10-20 kHz, and analyzed using Clampfit 8.0 software (Molecular Devices). Data are expressed as mean ± SEM. Statistical analysis of response before, during, and after the addition of dopaminergic agents was performed using a two-tailed Student's t test. p < 0.05 was considered significant.
Drug application. Dopamine (30 μm) was used as the endogenous agonist for dopamine receptors and was bath applied. After establishing whole-cell recording and obtaining control responses, dopamine and dopaminergic agonists were bath applied. After 10 min of drug application, experimental data were collected. Drug-free solution was then applied for 10 min, and wash responses were recorded. Antagonists were applied 20 min before acquiring control records as well as during agonist application. 6-Chloro-2,3,4,5-tetrahydro-1-phenyl-1H-3-benzazepine hydrobromide (SKF81297) and 7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine (SCH23390) were used as selective D1-like agonists and antagonists, respectively. Quinpirole and S(-)eticlopride were used as selective D2-like agonists and antagonists, respectively. Quinpirole and SCH23390 were purchased from Tocris Cookson (Ellisville, MO), whereas SKF81297 and eticlopride were obtained from Sigma-Aldrich (St. Louis, MO). The drugs were stored in frozen stock solution and dissolved in the ACSF before each experiment. Sodium metabisulfite (50 μm Na2S2O5) was used as an anti-oxidant (Sutor and Ten Bruggencate, 1990). The protein kinase A (PKA) peptide inhibitor (PKI 5-24) and bovine brain G-protein βγ (Gβγ) were obtained from Calbiochem (La Jolla, CA). For application, the peptide inhibitor and βγ subunits were included in the patch pipette at a concentration of 1 μm and 20 nm, respectively.
Results
Properties of layer I interneurons
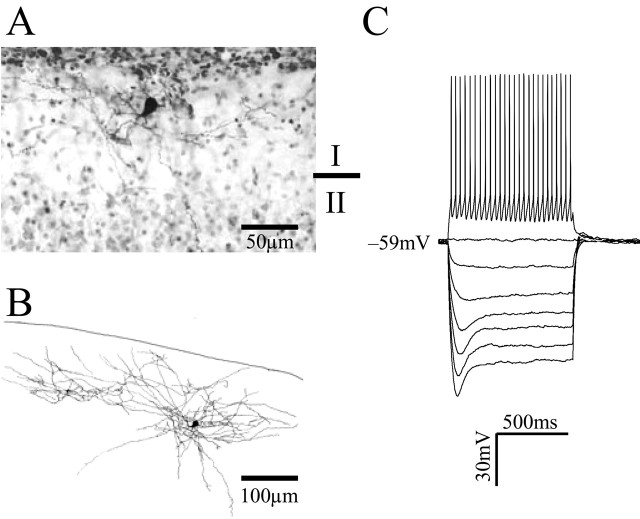
Under direct visualization, recordings were obtained from a total of 74 layer I interneurons from postnatal day 7 (P7) to P11 rats. In current-clamp recordings, the resting membrane potential was -55.8 ± 2 mV (n = 40), and the mean input resistance was 724 ± 73 MΩ (n = 20). A photomicrograph of a typical biocytin-labeled layer I interneuron is shown in Figure 1A. A camera lucida reconstruction of the cell is shown in Figure 1B. This cell was located near the pial surface and had an extensive axonal arbor extending up to 500 μm laterally within layer I. In addition, at least four axon collaterals were observed descending to layers II/III. Dendritic spines were not observed on these cells. Current-clamp recordings from this cell are shown in Figure 1C. A depolarizing current pulse elicited a train of action potentials that showed little or no accommodation. Action potential durations were brief (base duration, ∼2 ms) and were followed by a fast afterhyperpolarization, as described previously (Zhou and Hablitz, 1996a). Hyperpolarizing current pulses evoked a voltage response that reached a peak and sagged back toward rest. Such responses are a hallmark of hyperpolarization-activated, nonspecific cation channels.
Figure 1.
Identification of layer I interneurons in the rat prefrontal cortex. A, Photomicrograph of a biocytin-labeled layer I interneuron. B, Camera lucida reconstruction of the neuron from A. This interneuron had an extensive dendritic tree restricted to the layer I and four axon collaterals that descended to layer II/III. C, Current-clamp recording from the interneuron shown in A. Injection of a depolarizing current elicited a train of action potentials that showed no accommodation. Action potential durations were brief (base duration, ∼2 ms) and were followed by a fast afterhyperpolarization. Hyperpolarizing current pulses induced a hyperpolarization that reached a peak and sagged back toward rest. Such responses are a hallmark of hyperpolarization-activated, nonspecific cation channels.
Dopamine depolarizes interneurons and increases their excitability
Layer I receives a dense dopaminergic input from the ventral tegmental area (Lindvall et al., 1974). Previous studies from our laboratory have shown that dopamine depolarizes and increases the excitability of layer I interneurons (Zhou and Hablitz, 1999). In the present study, bath application of 30 μm dopamine produced a reversible depolarization in all of the tested cells (n = 13). Figure 2A shows an example of a typical membrane depolarization induced by dopamine. The maximum depolarization occurred 6 min after beginning the dopamine application. Responses recovered after a 10-20 min wash period. Reapplication of dopamine produced a similar response in this cell. The mean depolarization induced by dopamine was 10.8 ± 1 mV (n = 5; p < 0.01). Dopamine could potentially increase the release of several neurotransmitters that might indirectly affect the postsynaptic excitability. To determine whether the observed depolarization was mediated by a direct effect of dopamine on the recorded cell, action potential-dependent transmitter release was blocked by adding 1 μm TTX to the bathing medium. As shown in Figure 2B, dopamine depolarized the cell to a similar extent (9.8 ± 2.1 mV; n = 8) under these conditions. In subsequent experiments, 1 μm TTX was routinely added to the recording medium.
Figure 2.
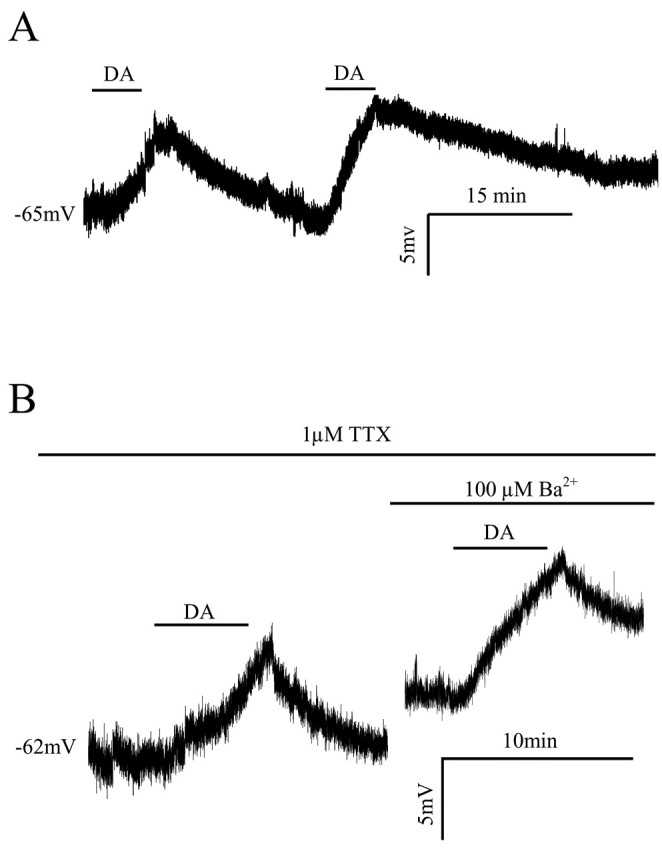
Dopamine depolarizes layer I interneurons in the prefrontal cortex. A, Typical whole-cell current-clamp recording showing that dopamine (50 μm) reversibly depolarizes layer I interneurons. Depolarization response to dopamine recovered after a 10-20 min wash. Reapplication of dopamine induced a similar response in this cell. B, In the presence of 1 μm TTX, dopamine (50 μm) depolarized layer I interneurons to a similar extent as seen in untreated conditions, indicating the depolarization was mediated by a direct effect of dopamine on the recorded cell and is independent of synaptic transmission. In the same interneuron after recovery from the first dopamine application, bath applying 100 μm Ba2+ induced an ∼5 mV depolarization because of the inhibition of a Ba2+-sensitive inward rectifier potassium current. Application of dopamine in the presence of Ba2+ elicited a membrane depolarization comparable with the control condition, suggesting dopamine depolarization of layer I interneurons does not involve modulation of a Ba2+-sensitive inward rectifier current. DA, Dopamine.
Previous studies of prefrontal cortex pyramidal cells (Dong et al., 2004) and interneurons (Gorelova et al., 2002) have shown that dopamine modulates inwardly rectifying potassium currents. We bath applied 0.1 mm Ba2+ to determine whether inward rectifier potassium channels were involved in the dopamine-induced depolarization of layer I interneurons. Ba2+ application depolarized interneurons by 6.4 ± 2 mV (n = 8), indicating the presence of Ba2+-sensitive “leak” currents, as described for layer II/III pyramidal cells (Sutor and Hablitz, 1993). In the presence of 0.1 mm Ba2+ medium, dopamine depolarized layer I cells to an extent comparable with control (11.5 ± 2 mV; n = 3). When Ba2+ was applied in the presence of dopamine, an additional depolarization was observed. These results indicate that dopamine does not modulate a Ba2+-sensitive inward rectifier current in layer I interneurons.
Role of D1- and D2-like receptors in dopamine-mediated depolarizations
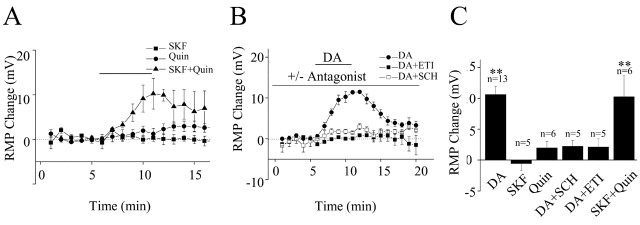
D1- and D2-like receptors are both expressed in superficial cortical layers (Vincent et al., 1993). Subtype-specific agonists and antagonists were used to determine the relative contributions of D1 and D2 receptors to the observed membrane depolarization. Paradoxically, as shown in Figure 3A, neither the D1-like receptor agonist SKF81297 (1-10 μm) nor the D2-like agonist quinpirole (10 μm) produced a significant depolarization (SKF81297: 0.2 ± 0.9 mV, n = 5, p > 0.05; quinpirole: 2.9 ± 1 mV, n = 5, p > 0.05). Behavioral (Ikemoto et al., 1997) and electrophysiological (Hopf et al., 2003) studies suggest that cooperative activation of D1 and D2 receptors is required for some dopamine-dependent behaviors. We therefore tested whether coapplying 1 μm SKF81297 and 10 μm quinpirole could mimic the action of dopamine. This treatment produced a significant depolarization of layer I interneurons (8.1 ± 2 mV; n = 4; p < 0.01) similar to that observed with dopamine. It was then hypothesized that if the dopamine-mediated depolarization required cooperative activation of D1 and D2 receptors, application of either a D1 or a D2 receptor antagonist should block the effect of dopamine. As shown in Figure 3B, it was found that in the presence of the D1 antagonist SCH23390 (10 μm) or the D2 antagonist eticlopride (1 μm), dopamine did not significantly depolarize layer I interneurons (SCH23390, 1.2 ± 0.7 mV; eticlopride, 1.2 ± 1.3 mV; both n = 5; p > 0.05). The pharmacological results are summarized in Figure 3C. These results strongly suggest that the dopamine-induced depolarization requires synergistic activation of both D1- and D2-like receptors.
Figure 3.
Synergistic activation of D1- and D2-like receptors is required for dopamine-induced membrane depolarization of layer I neurons. A, Summary time course plot of the effect of the specific D1 and D2 agonists SKF81297 and quinpirole, respectively. Neither SKF81297 (1 μm) nor quinpirole (10 μm) depolarized layer I interneurons. Coapplication of SKF81297 (1 μm) and quinpirole (10 μm) elicited depolarizations similar to that produced by dopamine. B, Summary time course plot of the effect of pretreatment with the selective D1-like receptor antagonist SCH23390 (10 μm) or the D2-like receptor antagonist eticlopride (1 μm). Both antagonists blocked the dopamine-induced membrane depolarization. C, Bar graph of averaged membrane potential changes induced by dopamine, SKF81297, quinpirole, dopamine in the presence of SCH23390 or eticlopride, and SKF81297 plus quinpirole. RMP, Resting membrane potential; SKF, SKF81297; Quin, quinpirole; SCH, SCH23390; ETI, eticlopride; DA, dopamine. **p < 0.01.
Dopamine-induced depolarization requires cAMP-dependent PKA activation
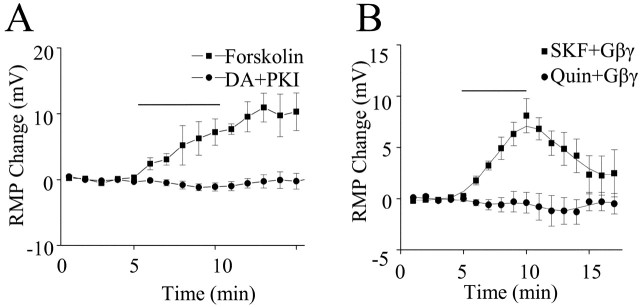
PKA has been shown to play a major role in dopamine signaling. If the dopamine-induced depolarization of layer I interneurons involved PKA, inhibition of PKA should block the depolarization. The addition of 1 μm PKA inhibitory peptide (PKI) in the recording pipette blocked the dopamine-induced depolarization [-1.1 ± 0.6 mV with PKI in the pipette (n = 6; p < 0.01) vs 10.8 ± 1.0 mV with dopamine without PKI], suggesting PKA signaling is involved. To test this further, 10 μm forskolin, a potent nonspecific adenylyl cyclase activator, was bath applied. Forskolin produced a significant depolarization (12.2 ± 3.4 mV; n = 5; p < 0.01 vs control), as shown in Figure 4A. The effect of forskolin was persistent and difficult to reverse after washing.
Figure 4.
Requirement of cAMP-dependent PKA activation for dopamine-induced membrane depolarization. A, Forskolin (10 μm), a potent nonspecific adenylyl cyclase activator, produced a significant membrane depolarization. The effect of forskolin was persistent and difficult to reverse after washing. The addition of 1 μm PKA inhibitory peptide (PKI) in the recording pipette blocked the dopamine (DA)-induced depolarization. In the PKI experiments, dopamine was applied at least 15 min after achieving the whole-cell configuration to allow diffusion of the peptide into the cell. The perfusion of PKI itself did not induce significant membrane potential changes. B, Effect of including purified bovine brain G-protein βγ (Gβγ; 20 nm) in the recording pipette. The D1-like receptor agonist SKF81297 (SKF; 1 μm) produced a significant depolarization when Gβγ was included in the recording pipette; the D2-like antagonist quinpirole (Quin; 10 μm) did not. These results strongly suggest that synergistic activation of D1 and D2 receptors by dopamine produces a depolarization of layer I interneurons via a mechanism involving Gβγ and cAMP signaling. RMP, Resting membrane potential.
Typically, D1 receptors are coupled to Gs and activate adenylate cyclase (AC), whereas D2 receptors are associated with Gi and inhibit AC. Recently, a model was suggested that explains the cooperative interaction of D1 and D2 receptor activation (Hopf et al., 2003). Gβγ subunits released from the Gi/o-linked D2 receptor in combination with Gαs from D1 receptors activate specific adenylyl cyclase subtypes. This kind of Gαs-Gβγ interaction may allow D2-linked Gi/o receptors to enhance, rather than oppose, activation of the AC-PKA signaling pathway. If dopamine-induced depolarizations require cooperative interaction of both D1 and D2 receptors as described above, activation of D1 receptors should produce a depolarization if exogenous Gβγ subunits are included in the patch pipette. As shown in Figure 4B, when purified bovine brain Gβγ was included in the recording pipette, the D1-like receptor agonist SKF81297 (1 μm), but not the D2-like agonist quinpirole (10 μm), produced a significant depolarization (SKF 81297: 8.1 ± 2 mV, n = 7, p < 0.01; 0.4 ± 1.0 mV, n = 4, p > 0.05 vs control).
Role of hyperpolarization-activated currents in dopamine-induced depolarizations
Layer I interneurons display large sag responses during hyperpolarization, indicative of hyperpolarization-activated, nonspecific cation channel activity. Ih, the current underlying sag responses, is modulated by several neurotransmitters and has also been suggested to play a role in regulating the resting membrane potential in some cells (Robinson and Siegelbaum, 2003). We reasoned that if Ih was responsible for the dopamine-induced depolarization, bath application of 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD7288), a specific blocker of Ih channels (Harris and Constanti, 1995), should occlude the action of dopamine. When ZD7288 was applied, a small but reproducible hyperpolarization (-6.5 ± 2 mV; n = 4) was observed. The voltage sag observed with hyperpolarizing current pulses was abolished. An example of the hyperpolarization produced by ZD7288 application is shown in Figure 5A. In the presence of ZD7288, dopamine did not depolarize the cell (Fig. 5A). The results from a group of cells are summarized in Figure 5B. These results strongly suggest that dopamine depolarizes layer I neurons via an action on Ih.
Figure 5.
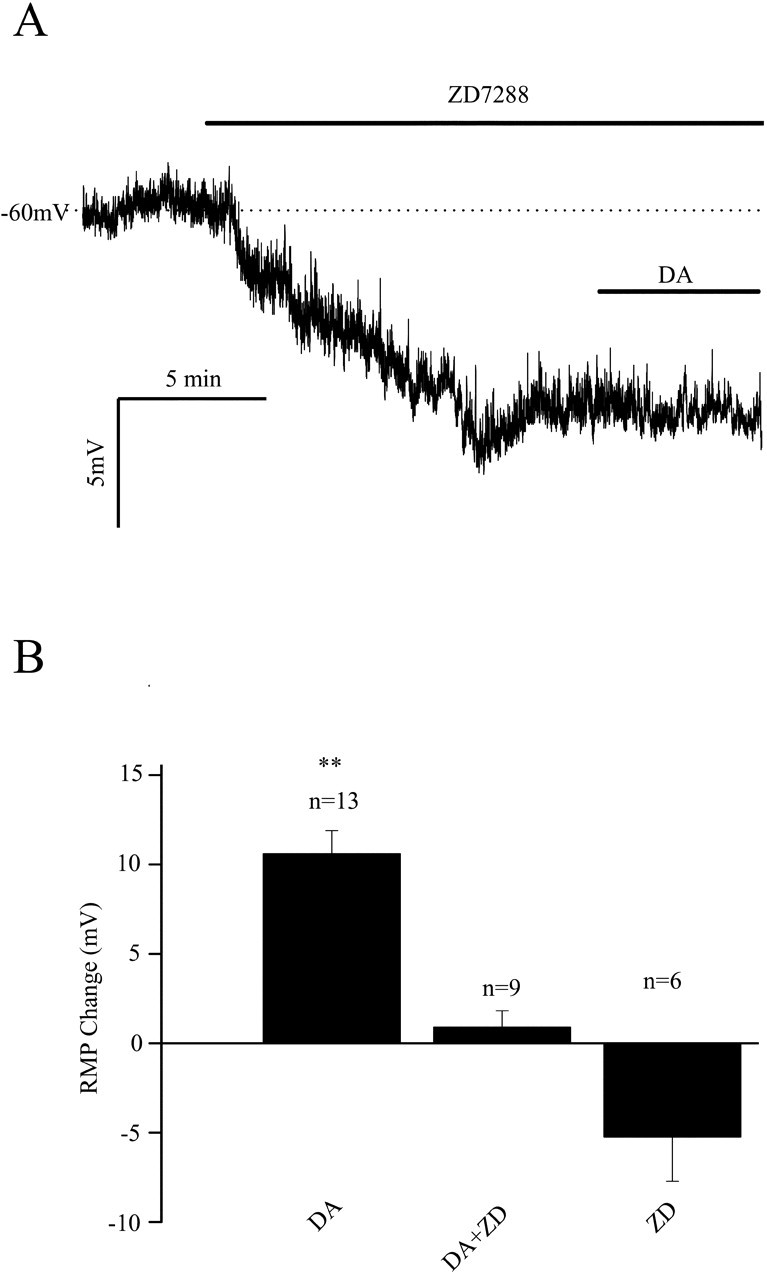
Effects of ZD7288, a specific Ih blocker, on dopamine-induced membrane depolarizations. A, A typical whole-cell current-clamp recording shows the effects of Ih inhibition. When 30 μm ZD7288 was bath applied, a reproducible hyperpolarization was observed, indicating that Ih contributes to the resting membrane potential of these cells. Application of dopamine in the presence of ZD7288 did not produce a depolarization. B, Summary plot of ZD7288 effects. Ih inhibition hyperpolarized interneurons and occluded the depolarizing effect of dopamine. DA, Dopamine; RMP, resting membrane potential; ZD, ZD7288. p < 0.01.
Dopamine enhances Ih in layer I interneurons
To test whether dopamine was affecting Ih, voltage-clamp studies of Ih were conducted. Figure 6A1 shows a typical recording of a hyperpolarization-activated inward current in a layer I interneuron. The neuron was voltage clamped at -50 mV, and a series of hyperpolarizing voltage commands from -110 to -50 in 10 mV steps were applied. A time- and voltage-dependent inward current was activated by steps more negative than -70 mV. In the presence of 30 μm dopamine (Fig. 6A2), Ih amplitude was enhanced and there was a shift in the activation threshold (-70 mV). These changes were partially reversible after washing. Measurements of both the instantaneous (ins) and steady-state (ss) currents were made at the times indicated by the arrows in Figure 6A1. Current-voltage plots are shown in Figure 6B1, where it can be seen that the instantaneous current was linear and not affected by dopamine. The steady-state current showed marked inward rectification and was enhanced by dopamine. The instantaneous current was subtracted from the steady-state current and is plotted in Figure 6B2. A clear enhancement by dopamine is seen. Activation curves for Ih are shown in Figure 6C for a population of cells. Dopamine shifted the curve to the right by changing the V1/2 from -86.0 ± 0.6 mV to -78.9 ± 0.4 mV (n = 19; p < 0.01).
Figure 6.
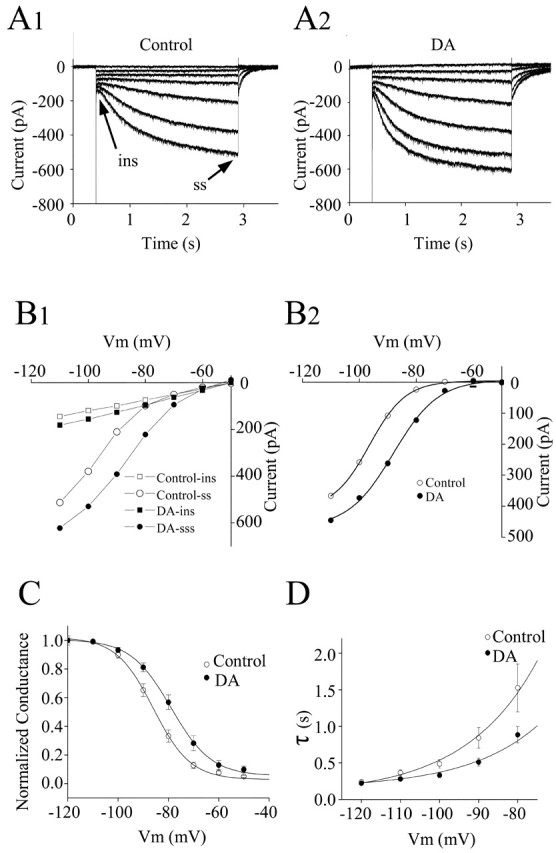
Voltage-clamp analysis of the effects of dopamine on Ih activation. A1, Voltage-clamp recordings of Ih. The neuron was voltage clamped at -50 mV, and a series of hyperpolarizing voltage commands from -110 to -50 in 10 mV steps were applied. A time- and voltage-dependent inward current was activated by steps more negative than -70 mV. The instantaneous current (arrow) was measured just after the decay of the capacitive transient, and the steady-state current (arrow) was measured near the end of each voltage commend. A2, In the presence of 30 μm dopamine, Ih amplitude was enhanced and there was a depolarizing shift in activation threshold (-60 mV). After dopamine application, the holding current increased ∼65 pA corresponding to the depolarization seen in current-clamp recordings. The holding current change has been subtracted. B1, Current-voltage curve showing the instantaneous (▪ and □) and steady-state (○ and •) currents as a function of the commend voltage in control and in the presence of 30 μm dopamine. Dopamine increased the steady-state current at all voltages negative to -60 mV and had little effect on the instantaneous current. B2, Plot of the Ih amplitude measured as the difference between steady-state and instantaneous current under control conditions (•) and in the presence of dopamine (○). C, Dopamine caused a depolarizing shift in the voltage dependence of Ih activation. Ih amplitude from 19 interneurons was converted to conductance (g) with the equation g = Ih/(E - Erev), where E is the command voltage and Erev is the reversal potential of Ih; the value of Erev is -27 mV by calculating the tail current. The conductance g for each cell was normalized by Gmax (g/Gmax); G was taken to be the value of g at -110 mV, which was assumed to reach the maximum activation. The normalized conductance (g/Gmax ± SE) in control (▪) and dopamine (•) were plotted as a function of voltage. The data were fitted with the Boltzman equation [g/G = 1/(1 + e(v-v1/2)/K)]; dopamine shifted the curve to the right by changing the V1/2 from -86.0 ± 0.6 mV to -78.9 ± 0.4 mV (n = 19; p < 0.01). D, Plot of the rate of Ih activation as a function of voltage. ins, Instantaneous current; ss, steady-state current; DA, dopamine; Vm, voltage.
The inward relaxation attributable to Ih was fitted to a single-exponential function. The activation time constant was voltage dependent. The voltage dependence of Ih activation is shown in Figure 6D. In the presence of dopamine, Ih activated more rapidly than control at membrane potentials more positive than -100 mV.
Discussion
The present results demonstrate that dopamine depolarizes layer I interneurons by enhancing the hyperpolarization-activated, nonspecific cationic current Ih. This occurs as a result of a dopamine-induced depolarizing shift in the Ih activation curve. This enhancing action of dopamine requires synergistic activation of D1 and D2 receptors and is blocked by either D1 or D2 receptor antagonists. Intracellularly, the action of dopamine involves Gβγ and cAMP-dependent processes. These results indicate that Ih contributes to the resting potential of layer I interneurons and that modulation of this current alters tonic inhibition in the neocortex.
Ih in layer I neurons
Hyperpolarization-activated, nonspecific cation currents, termed Ih, have been described in a variety of mammalian cell types (Pape, 1996). During early cortical development, neurons in layer I, including interneurons and Cajal-Retzius cells, express a prominent Ih (Zhou and Hablitz, 1995; Kilb and Luhmann, 2000). Similar currents are observed in mature neurons, but with smaller amplitudes. In the present study, we have shown that Ih is also present in P7-P11 layer I interneurons, where it contributes to the resting potential of these cells. In current-clamp recordings, a hyperpolarization-activated voltage sag was observed when the membrane potential reached a value near -70 mV. Stronger hyperpolarizations gave rise to more marked voltage sags and rebound excitation after pulse offset, presumably because of persistent Ih activation. These sag responses and rebound excitations were reduced or blocked by the selective Ih blocker ZD7288. This compound also routinely and reliably produced a membrane hyperpolarization, indicating Ih was active at rest. This is in contrast to Cajal-Retzius cells in layer I where Ih does not contribute to the resting membrane potential (Kilb and Luhmann, 2000). This differential effect on resting potential may represent developmental changes in Ih properties or intrinsic differences between Cajal-Retzius cells and other layer I interneurons.
The present results are based on somatic recordings from layer I interneurons. The dopamine-induced depolarization of these cells results in increased interneuron excitability and spontaneous firing, producing a robust enhancement of spontaneous IPSCs and a tonic inhibition of layer II/III pyramidal cells (Zhou and Hablitz, 1999). In the hippocampus, Ih current density increases markedly from the soma to distal dendrites (Magee, 1998). Ih in distal dendrites serves to dampen dendritic excitability and alters the pattern of local dendritic synaptic integration (Williams and Stuart, 2000a). The presence of Ih currents in the dendrites of layer I interneurons and possible modulation by dopamine has not been examined. If present, dendritic Ih currents would add another level of complexity to the control of interneuron excitability.
In the present study, Ih activation was well described by a single exponential with a time constant of 700 ms at -90 mV. This value is in the range for Ih in thalamic neurons (Santoro et al., 2000), hippocampal interneurons (Santoro et al., 2000), and neocortical pyramidal cells (Williams and Stuart, 2000b) and is consistent with mediation by HCN1-HCN2 subunits. Considerably faster time constants have been described in hippocampal CA1 pyramidal cells (Magee, 1998). HCN1-HCN4 subunits exhibit distinct patterns of activation and inactivation and varying sensitivities to cyclic nucleotides (Santoro et al., 2000; Wainger et al., 2001). HCN1-HCN2 are also highly expressed in layer I and sensitive to cAMP modulation, whereas HCN3-HCN4 are less prominent (Takuya, 2004). The Ih activation time constants and forskolin results in layer I interneurons is consistent with mediation by channels containing HCN1-HCN2 subunits.
Dopamine modulation of intrinsic excitability in the prefrontal cortex
Various effects of dopamine on the membrane properties of individual prefrontal cortex neurons have been reported. An early in vivo study showed that a membrane depolarization accompanied dopamine inhibition of cortical neurons (Bernardi et al., 1982). The number of spikes elicited by depolarizing current pulses in prefrontal cortex neurons in vitro has been reported to be decreased by low concentrations (0.1-10 μm) of dopamine (Geijo-Barrientos and Pastore, 1995). Higher dopamine concentrations (20-100 μm) produce an increase in excitability (Penit-Soria et al., 1987; Shi et al., 1997), perhaps via D1 receptors (Yang and Seamans, 1996). Dopamine also decreases excitability of layer V prefrontal cortex neurons via D2 receptor activation (Gulledge and Jaffe, 1998) but has variable effects on layer II/III prefrontal cortex pyramidal cells (Gonzalez-Islas and Hablitz, 2001).
In contrast to the varied effects on pyramidal cells, dopamine consistently increases the excitability GABAergic interneurons (Zhou and Hablitz, 1999; Gorelova et al., 2002). The present results clearly demonstrate the dopamine-induced increased excitability of layer I neurons is attributable to an enhancement of Ih, whereas a Ba2+-sensitive inward rectifier current was not affected. Dopamine depolarizes and increases the excitability of fast-spiking interneurons in layers II-V via an effect on a Cs+-sensitive inward rectifier K+ current and a resting leak K+ current; other classes of interneurons were not significantly affected (Gorelova et al., 2002). This indicates that dopamine has multiple effects on interneuron excitability that vary by cell type and ion channel. These differences presumably relate to the function of the individual interneuron subtypes in regulation of excitability in local neocortical circuits.
Dopamine-induced depolarization of layer I interneurons required synergistic activation of D1 and D2 receptors. In addition, the effects of dopamine were mimicked by forskolin and blocked by the PKA inhibitory peptide, indicating involvement of cAMP-dependent PKA activation. Because D1 and D2 receptors are conventionally thought to positively and negatively, respectively, couple to cAMP production (Missale et al., 1998), it has been difficult to understand how synergistic activation of both receptors could lead to increased cAMP levels via traditional GS- and GI/O-coupled mechanisms. An alternate signaling pathway involves AC stimulation by Gβγ subunits released from GI/O proteins (Taussig et al., 1993; Watts and Neve, 1997). This allows GI/O-linked D2 receptors to add to the D1 activation of the PKA pathway. Cooperative activation of D1 and D2 receptors involving Gβγ subunits has also been described in nucleus accumbens neurons (Hopf et al., 2003) and may represent a widespread mechanism whereby dopamine modulates intrinsic excitability.
Physiological significance
Information concerning the function and connectivity of layer I interneurons is expanding. Layer I neurons are connected via electrical and chemical synapses (Chu et al., 2003). In addition, layer I cells provide inhibitory inputs to pyramidal cells (Chu et al., 2003). In vivo recordings have shown that ascending sensory information is rapidly relayed to layer I neurons, arriving 5-7 ms after whisker stimulation (Zhu and Zhu, 2004). Excitatory inputs to the apical tufts of pyramidal cells reaching layer I can trigger dendritic action potentials that affect pyramidal cell activity (Schiller et al., 1997; Zhu, 2000). Distal dendritic zones have unique coincidence detection mechanisms that allow subthreshold EPSPs to trigger bursts of calcium-dependent action potentials when it coincides with a back-propagating action potential. This Ca2+ spike firing could be abolished by appropriately timed IPSPs. Thus, the increase in IPSPs resulting from dopamine-induced depolarization could alter distal dendritic integration as well as enhance inhibition in other layers.
Footnotes
This work was supported by National Institutes of Health Grant NS18145.
Correspondence should be addressed to Dr. John J. Hablitz, Department of Neurobiology, University of Alabama at Birmingham, CIRC510, Birmingham, AL 35294. E-mail: jhablitz@uab.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/256322-07$15.00/0
References
- Berger B (1992) Dopaminergic innervation of the frontal cerebral cortex: evolutionary trends and functional implications. In: Advances in neurology (Chauvel P, Delgado-Escueta AV, Haalgren E, Bancaud J, eds), pp 525-544. New York: Raven. [PubMed]
- Bernardi G, Cherubini E, Marciani MG, Mercuri N, Stanzione P (1982) Responses of intracellularly recorded cortical neurons to the iontophoretic application of dopamine. Brain Res 245: 267-274. [DOI] [PubMed] [Google Scholar]
- Bradford R, Parnavelas JG, Lieberman AR (1978) Neurons in layer I of the developing occipital cortex of the rat. J Comp Neurol 176: 121-132. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang J, Siegelbaum SA (2001) Properties of hyperpolarization-activated pacemaker current defined by coassembly of HCN1 and HCN2 subunits and basal modulation by cyclic nucleotide. J Gen Physiol 117: 491-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe E, Roebuck A, Staiger JF, Lavery DJ, Charpak S, Audinat E (2002) Two types of nicotinic receptors mediate an excitation of neocortical layer I interneurons. J Neurophysiol 88: 1318-1327. [DOI] [PubMed] [Google Scholar]
- Chu Z, Galarreta M, Hestrin S (2003) Synaptic interactions of late-spiking neocortical neurons in layer 1. J Neurosci 23: 96-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelRio JA, Soriano E, Ferrer I (1994) Development of GABA immunoreactivity in the neocortex of the mouse. J Comp Neurol 326: 501-526. [DOI] [PubMed] [Google Scholar]
- Dong Y, Cooper D, Nasif F, Hu XT, White FJ (2004) Dopamine modulates inwardly rectifying potassium currents in medial prefrontal cortex pyramidal neurons. J Neurosci 24: 3077-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijo-Barrientos E, Pastore C (1995) The effects of dopamine on the subthreshold electrophysiological responses of rat prefrontal cortex neurons in vitro. Eur J Neurosci 7: 358-366. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ (2001) Dopamine inhibition of evoked IPSCs in rat prefrontal cortex. J Neurophysiol 86: 2911-2918. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR (2002) Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol 88: 3150-3166. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB (1998) Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci 18: 9139-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NC, Constanti A (1995) Mechanism of block by ZD 7288 of the hyperpolarization-activated inward rectifying current in guinea pig substantia nigra neurons in vitro. J Neurophysiol 74: 2366-2378. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Armstrong WE (1996) Morphology and physiology of cortical neurons in layer I. J Neurosci 16: 5290-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A (2003) Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein βγ subunits. J Neurosci 23: 5079-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ (1997) Role of dopamine D1 and D2 receptors in the nucleus accumbens in mediating reward. J Neurosci 17: 8580-8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z-G, Pessia M, North RA (1993) Dopamine and baclofen inhibit the hyperpolarization-activated cation current in rat ventral tegmental neurones. J Physiol (Lond) 462: 753-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W, Luhmann HJ (2000) Characterization of a hyperpolarization-activated inward current in Cajal-Retzius cells in rat neonatal neocortex. J Neurophysiol 84: 1681-1691. [DOI] [PubMed] [Google Scholar]
- Li J, Schwark HD (1994) Distribution and proportions of GABA-immunoreactive neurons in cat primary somatosensory cortex. J Comp Neurol 343: 353-361. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Moore R, Stenevi U (1974) Mesencephalic dopamine neurons projecting to neocortex. Brain Res 81: 325-331. [DOI] [PubMed] [Google Scholar]
- Magee JC (1998) Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613-7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M (1990) Three-dimensional structural organization of layer I of the human cerebral cortex: a Golgi study. J Comp Neurol 299: 89-105. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape CH (1990) Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurons. J Physiol (Lond) 431: 291-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78: 189-225. [DOI] [PubMed] [Google Scholar]
- Pape HC (1996) Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299-327. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. San Diego: Academic.
- Penit-Soria J, Audinat E, Crepel F (1987) Excitation of rat prefrontal cortical neurons by dopamine: an in vitro electrophysiological study. Brain Res 425: 263-274. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D, Lubke J (2002) Axonal projection, input and output synapses, and synaptic physiology of Cajal-Retzius cells in the developing rat neocortex. J Neurosci 22: 6908-6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RB, Siegelbaum SA (2003) Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol 65: 453-480. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA (2000) Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20: 5264-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Schiller Y, Stuart G, Sakmann B (1997) Calcium action potentials restricted to distal apical dendrites of rat neocortical pyramidal neurons. J Physiol (Lond) 505: 605-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA (1995) Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol 363: 264-280. [DOI] [PubMed] [Google Scholar]
- Shi W-X, Zheng P, Lliang X-F, Bunney BS (1997) Characterization of dopamine-induced depolarization of prefrontal cortical neurons. Synapse 26: 415-422. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS (1992) Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol 321: 325-335. [DOI] [PubMed] [Google Scholar]
- Sutor B, Hablitz JJ (1993) Influence of barium on rectification in rat neocortical neurons. Neurosci Lett 157: 62-66. [DOI] [PubMed] [Google Scholar]
- Sutor B, Ten Bruggencate G (1990) Ascorbic acid: a useful reductant to avoid oxidation of catecholamines in electrophysiological experiments in vitro? Neurosci Lett 116: 287-292. [DOI] [PubMed] [Google Scholar]
- Takuya N (2004) Immunohistochemical localization of HCN1-4 channel subunits in the rat brain. J Comp Neurol 471: 241-276. [DOI] [PubMed] [Google Scholar]
- Taussig R, Quarmby LM, Gilman AG (1993) Regulation of purified type I and type II adenylylcyclases by G protein beta gamma subunits. J Biol Chem 268: 9-12. [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM (1993) Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci 13: 2551-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR (2001) Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 411: 805-810. [DOI] [PubMed] [Google Scholar]
- Watts VJ, Neve KA (1997) Activation of type II adenylate cyclase by D2 and D4 but not D3 dopamine receptors. Mol Pharmacol 52: 181-186. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ (2000a) Action potential back propagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J Neurosci 20: 1307-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ (2000b) Site independence of EPSP time course is mediated by dendritic Ih in neocortical pyramidal neurons. J Neurophysiol 83: 3177-3182. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JK (1996) Dopamine D1 receptor actions in layers of V-VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci 16: 1922-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ (1995) Postnatal development of anatomical and electrophysiological properties of layer I neurons in rat neocortex. Soc Neurosci Abstr 21: 2022. [Google Scholar]
- Zhou FM, Hablitz JJ (1996a) Layer I neurons of rat neocortex. I. Action potential and repetitive firing properties. J Neurophysiol 76: 651-667. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ (1996b) Morphological properties of intracellularly labeled layer I neurons in rat neocortex. J Comp Neurol 376: 198-213. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ (1999) Dopamine modulation of membrane and synaptic properties of interneurons in rat cerebral cortex. J Neurophysiol 81: 967-976. [DOI] [PubMed] [Google Scholar]
- Zhu JJ (2000) Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol (Lond) 526: 571-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhu JJ (2004) Rapid arrival and integration of ascending sensory information in layer 1 nonpyramidal neurons and tuft dendrites of layer 5 pyramidal neurons of the neocortex. J Neurosci 24: 1272-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]