Abstract
Addiction is believed to involve glutamate-dependent forms of synaptic plasticity that promote the formation of new habits focused on drug seeking. We used primary cultures of rat prefrontal cortex (PFC) neurons to explore mechanisms by which dopamine-releasing psychomotor stimulants such as cocaine and amphetamine influence synaptic plasticity, focusing on AMPA receptor trafficking because of its key role in long-term potentiation (LTP). Brief stimulation of D1 dopamine receptors increased surface expression of glutamate receptor 1 (GluR1)-containing AMPA receptors through a protein kinase A-dependent mechanism, by increasing their rate of externalization at extrasynaptic sites. Newly externalized GluR1 remained extrasynaptic under basal conditions but could be translocated into synapses by subsequent NMDA receptor activation. These results suggest that D1 receptors may facilitate LTP by increasing the AMPA receptor pool available for synaptic insertion. However, stimulation of D2 receptors decreased surface and synaptic GluR1 expression. These findings are discussed in the context of evidence that D1 and D2 receptors act independently rather than antagonistically in the intact PFC. D1 receptor facilitation of AMPA receptor synaptic insertion helps explain D1 receptor-dependent facilitation of LTP and learning in the normal brain. Abnormal engagement of this mechanism during unregulated dopamine release may account for maladaptive plasticity after repeated exposure to cocaine or amphetamine.
Keywords: addiction, AMPA receptor, dopamine, LTP, plasticity, protein kinase A
Introduction
Addiction is increasingly associated with neuronal plasticity. Therefore, effects of addictive drugs on long-term potentiation (LTP) and long-term depression (LTD) have become a focus of addiction research (Kauer, 2004; Wolf et al., 2004). Dopamine (DA) receptors modulate LTP and LTD in many brain regions important for addiction (Centonze et al., 2003; Jay, 2003; Lovinger et al., 2003), as do DA-releasing psychomotor stimulants such as cocaine (Jones et al., 2000; Thomas et al., 2000, 2001; Ungless et al., 2001; Saal et al., 2003; Dong et al., 2004). However, the mechanisms involved are unclear. This study investigated the possibility that DA receptors in prefrontal cortex (PFC) modulate the synaptic trafficking of AMPA receptors, a critical mechanism for expression of LTP and LTD (Malinow and Malenka, 2002; Song and Huganir, 2002; Bredt and Nicoll, 2003).
In hippocampal pyramidal cells, AMPA receptors containing glutamate receptor 1 (GluR1) or GluR4 subunits are delivered to synapses in an activity-dependent manner during LTP, whereas GluR2/GluR3-containing receptors are constitutively delivered, independent of neuronal activity (Passafaro et al., 2001; Shi et al., 2001). Synaptic GluR1 delivery during LTP requires activation of Ca2+-calmodulin-dependent protein kinase II (CaMKII), although GluR1 is not the relevant substrate (Hayashi et al., 2000). In addition, GluR1 phosphorylation by protein kinase A (PKA) is necessary, but not sufficient, for synaptic delivery (Ehlers, 2000; Esteban et al., 2003; Lee et al., 2003; Lu et al., 2003). D1-class DA receptors are positively coupled to PKA and also regulate Ca2+ signaling (Neve et al., 2004), suggesting cellular mechanisms by which DA may influence LTP. Indeed, the D1 receptor-PKA pathway is important in modulating LTP and memory processes in many brain regions (Jay, 2003).
The PFC plays a well established role in working memory (Goldman-Rakic, 1987), but is also important for addiction-related behaviors such as impulsivity (Jentsch and Taylor, 1999) and reward-related learning (Cardinal et al., 2002). Pyramidal neurons are the most common target of DA terminals in the PFC (Verney et al., 1990) and D1-class DA receptors play a particularly important role in modulating pyramidal cell excitability and plasticity (Seamans and Yang, 2004). This occurs through D1 receptor modulation of synaptic transmission and voltage-sensitive conductances. However, given the convergence of DA and glutamate inputs onto the spines of pyramidal neurons (Sesack et al., 2003) and evidence from studies of primate PFC for proximity of postsynaptic D1 receptors to glutamate synapses (Goldman-Rakic et al., 2000), we hypothesized that DA may also influence plasticity by regulating AMPA receptor trafficking at nearby glutamate synapses. This would be consistent with the key role of AMPA receptor trafficking in LTP and LTD (see above), the importance of the D1 receptor-PKA pathway for regulating LTP and LTD in the PFC (Jay et al., 1998; Gurden et al., 2000; Huang et al., 2004), and the demonstration that D1 receptor-PKA signaling regulates AMPA receptor trafficking in other brain regions (Wolf et al., 2004).
We tested this hypothesis in dissociated cultures prepared from postnatal rat PFC. We found that previous D1 receptor stimulation facilitates AMPA receptor synaptic insertion during NMDA receptor activation, whereas D2 receptors exert opposite effects.
Materials and Methods
Prefrontal cortex cultures. Postnatal day 1 rats were anesthetized by hypothermia on ice. The medial prefrontal cortex was isolated and dissociated with papain (20-25 U/ml; Worthington Biochemical, Lakewood, NJ) at 37°C. Cells were plated onto coverslips coated with poly-d-lysine (100 μg/ml; Sigma, St. Louis, MO) in 24-well culture plates at a density of 20,000 cells per well and grown in Neurobasal medium (Invitrogen, Carlsbad, CA) supplemented with 2 mm glutamine, 0.5% gentamine, and 2% B27. One-half of the medium was replaced with this Neurobasal growth medium every 4 d. Cultures were used for experiments between weeks 2 and 3.
Immunocytochemistry. Cell-surface GluR1 was labeled by incubating live cultures with polyclonal antibody recognizing the extracellular N-terminal domain of GluR1 (amino acids 271-285; RTSDSRDHTRVDWKR; 1:15; Oncogene, Carpinteria, CA) in Neurobasal growth medium for 30 min. Cells were then fixed with 4% paraformaldehyde in PBS for 15 min, blocked with 5% donkey serum in PBS for 60 min, and incubated with donkey anti-rabbit secondary antibody conjugated to cyanine 3 (Cy3) (1:500; Jackson ImmunoResearch, West Grove, PA) for 60 min under nonpermeant conditions. Then, cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min, blocked with 5% donkey serum in PBS for 60 min, and incubated with monoclonal antibody to the synaptic marker synaptobrevin (SB)/vesicle-associated membrane protein 2 (VAMP-2) (1:2000; overnight at 4°C; Synaptic Systems, Goettingen, Germany) followed by donkey anti-mouse secondary antibody conjugated to Alexa 488 (1:2000; 1 h at room temperature; Molecular Probes, Eugene, OR). A punctate pattern of staining was observed for both GluR1 and synaptobrevin. Methods for quantifying total surface GluR1 expression and synaptic GluR1 expression are described below (Data analysis). For cell-surface D1 and D2 receptor staining, live cells were incubated for 30 min with antibody recognizing the extracellular second loop of the D1 receptor or antibody recognizing the extracellular N-terminal domain of the D2 receptor (gifts from Dr. Marjorie Ariano, Rosalind Franklin University of Medicine and Science/The Chicago Medical School). After fixation, donkey anti-rabbit Cy3 antibody (1:500; Jackson ImmunoResearch) was incubated with the cells at room temperature for 1 h.
In experiments with glycine, cultured neurons were treated with glycine for 3 min at room temperature in a bathing solution (140 mm NaCl, 1.3 mm CaCl2, 5.0 mm KCl, 25 mm HEPES, 10 mm glucose, 0.5 μm TTX, pH 7.4) and then transferred to the same solution without any added glycine for 15 min at room temperature (Lu et al., 2001). Cells were then immunostained for GluR1 and synaptobrevin as described above.
In some experiments, the rate of GluR1 externalization was determined by preblocking existing surface receptors with primary antibody and nonconjugated secondary antibody, bringing cells to room temperature to allow receptor externalization, and then detecting newly externalized receptors with a second round of immunostaining (Lu et al., 2001). According to the original method, preblocking steps are conducted at 4°C to minimize GluR1 trafficking (Lu et al., 2001). However, we found that PFC neurons cannot tolerate long incubations (>1 h) at this temperature. They exhibit dendritic blebbing, cell rupture, and nucleus loss (data not shown). Therefore, we modified the method by conducting preblocking steps at a slightly higher temperature (15°C) in a 5% CO2 incubator. Others have used a similar temperature (17°C) to limit AMPA receptor trafficking (Sekine-Aizawa and Huganir, 2004). According to our modified method, live cells were first incubated with the GluR1 antibody (1:15 in Neurobasal growth medium) for 30 min at 15°C in a 5% CO2 refrigerated incubator (Tritech Research, Los Angeles, CA). Cells were then rinsed twice with Neurobasal medium (preequilibrated to 15°C) and then incubated with nonconjugated goat anti-rabbit antibody (5 μg/ml; Sigma) for 30 min at 15°C in a 5% CO2 refrigerated incubator. Then, cells were incubated at room temperature or 37°C, either in control medium or medium containing test drugs, to allow the insertion of new GluR1 subunits into the cell membrane. After this incubation, cultures were rinsed, fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, and rinsed twice with PBS. To detect the newly externalized GluR1, cultures were incubated with N-GluR1 antibody (1:100) at room temperature for 1 h followed by incubation with 5% donkey serum in PBS for 1 h. After three washes with PBS, cells were incubated with Cy3-conjugated donkey anti-rabbit secondary antibody (1:500) at room temperature for 1 h. Because cells are not permeabilized, the second round of immunostaining detects only newly externalized GluR1 subunits. To determine the location of newly externalized GluR1, synapses were immunostained with mouse monoclonal antibody to synaptobrevin/VAMP-2 (1:2000) and Alexa 488 donkey anti-mouse IgG (1:2000) after permeabilization with 0.1% Triton X-100 in PBS for 15 min and incubation with 5% donkey serum in PBS for 1 h.
Data analysis. Images were acquired and analyzed with an imaging system consisting of a Nikon (Melville, NY) inverted microscope, ORCA-ER digital camera and MetaMorph software (Universal Imaging, Downingtown, PA). Images for all experimental groups were taken using identical acquisition parameters. All groups to be compared were run simultaneously using cells from the same culture preparation. For each experimental group, cells from at least four different wells were used, and approximately six cells from each well were analyzed. Processes located about one soma diameter from the soma were selected for analysis under phase contrast imaging to avoid the possibility of experimenter bias based on the intensity of fluorescence staining. The soma was excluded in all measurements. Image analysis was performed using methods similar to those described previously and was based on measuring the area of labeled puncta rather than counting the number of labeled puncta (Beattie et al., 2002). For each image, the total area of fluorescent GluR1-stained puncta was measured automatically using a threshold that was set at least two times higher than the average background fluorescence in processes of untreated control cells. This value was then divided by the total area of the measured processes, which was determined by setting a lower threshold level to measure background fluorescence produced by the fixed cells. The same approach was used for defining the area of SB staining and the area of GluR1/synaptobrevin colocalization (SB + GluR1 area). Synaptic GluR1 incorporation was expressed as the fraction of total SB staining that overlaps with GluR1 staining (SB + GluR1 area/total SB area). For each experimental group, results were normalized to a control group run simultaneously. All values in figures and text refer to mean ± SEM. Independent group t tests were used for comparisons between two experimental groups, and ANOVA was used to compare several groups. When ANOVA indicated significant group differences, a post hoc Dunn's test was used to compare experimental groups to the control group (n, number of fields analyzed).
Results
Prefrontal cortex neurons express cell-surface AMPA receptors, D1 receptors, and D2 receptors
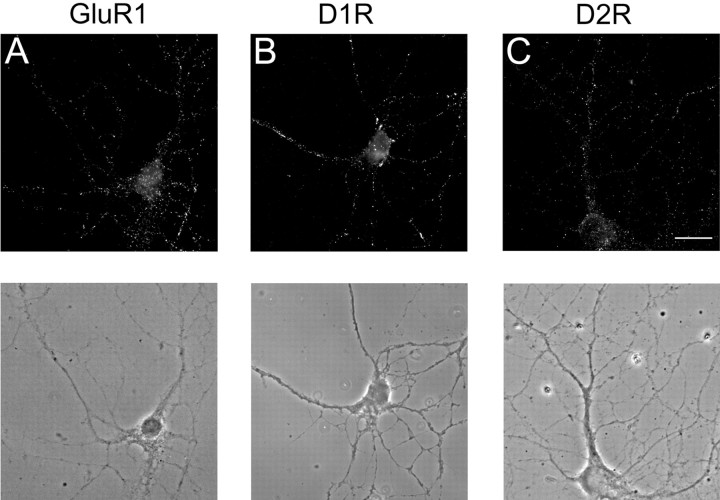
Prefrontal cortex cultures were prepared from rats on postnatal day 1. We focused on pyramidal neurons because they receive convergent DA and glutamate inputs and undergo DA-modulated synaptic plasticity (see Introduction). Experiments were performed after 2-3 weeks in culture, at which time pyramidal neurons show extensive cell-surface staining for AMPA receptors (Fig. 1A), D1 receptors (Fig. 1B), and D2 receptors (Fig. 1C). In the experiments described below, we use DA agonists that stimulate either D1-class (D1/D5) or D2-class (D2/D3/D4) DA receptors but do not distinguish between members of each class. For simplicity, we will use the terms “D1” and “D2” to refer to effects that may involve the D1-class or D2-class of DA receptors, although D5 and D4 receptors are also highly expressed in the rat PFC (Bentivoglio and Morelli, 2005) and may be involved in our observed effects.
Figure 1.
Pyramidal neurons of the PFC exhibit punctate cell-surface expression of AMPA receptors, D1 DA receptors, and D2 DA receptors. A, Cell-surface AMPA receptors were labeled using antibody to the extracellular N terminus of GluR1. B, Cell-surface D1 receptors (D1R) were labeled with antibody to the extracellular second loop of the D1 receptor. C, Cell-surface D2 receptors (D2R) were labeled with antibody to the extracellular N terminus of the D2 receptor. All receptors were visualized with Cy3 fluorescent secondary antibody. The corresponding phase images are shown below each fluorescence image. Pyramidal neurons were identified based on morphological criteria including a pyramidal shaped cell body with dendrites emerging from both the apex and the base, a soma diameter ≥15 μm, and dendrites extending a distance greater than five times the soma diameter. Scale bar, 20 μm.
D1 receptor stimulation increases GluR1 surface expression but not synaptic incorporation
To examine the effect of D1 receptor stimulation on cell-surface and synaptic GluR1 expression, PFC neurons were incubated with medium (control group) or the D1 agonist (±)-6-chloro-7,8-dihydroxy-1-phenyl-2,3,4,5-tetrahydro-1 H-3-benzazepine hydrobromide (SKF 81297) (SKF) for 5 min. Then, live cultures were incubated with antibody recognizing the extracellular N terminus of GluR1, fixed, permeabilized, and incubated with antibody to the synaptic marker synaptobrevin/VAMP-2. GluR1 and synaptobrevin staining were visualized with secondary antibodies conjugated to Cy3 and Alexa 488, respectively. Total surface GluR1 staining was determined by measuring the area of GluR1-positive puncta on processes of pyramidal neurons. Synaptic GluR1 incorporation was defined as the fraction of total synaptobrevin area that overlapped with GluR1 area. Both synaptic and nonsynaptic GluR1 staining had a punctate appearance, consistent with results in hippocampal neurons (Carroll et al., 1999).
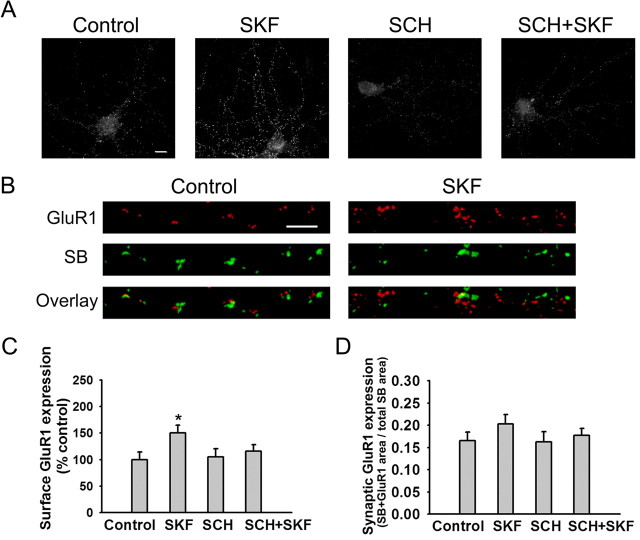
Incubation with the D1 agonist SKF 81297 for 5 min significantly increased GluR1 surface expression in PFC neurons (Fig. 2A, C). This effect was significantly attenuated if the D1 receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1 H-3-benzazepine hydrochloride (SCH 23390) (SCH) (10 μm) was added 5 min before SKF 81297 (Fig. 2A, C). However, SKF 81297 did not produce a significant increase in overlap of synaptobrevin and GluR1 staining (Fig. 2B, D). These results indicate that SKF 81297 increased surface expression of GluR1 but not its synaptic incorporation.
Figure 2.
The D1 receptor agonist SKF 81297 increases GluR1 surface expression but not synaptic incorporation in PFC neurons. A, Representative images of total cell-surface GluR1 staining in control neurons and neurons treated with SKF 81297 (1 μm; 5 min), the D1 receptor antagonist SCH 23390 (10 μm), or SCH plus SKF (SCH+SKF). In the SCH+SKF group, SCH 23390 was added 5 min before SKF. Scale bar, 10 μm. B, Representative images illustrating synaptic GluR1 incorporation, indicated by overlap of GluR1 and SB staining, on processes of control neurons and neurons treated with SKF 81297 (1 μm; 5 min). Scale bar, 5 μm. C, Quantification of total surface GluR1 staining. SKF significantly increased surface GluR1 staining compared with the control group (n = 19-24; Dunn's test; *p < 0.05). Results are presented as the mean area of GluR1 puncta, normalized to the control group. D, Quantification of synaptic GluR1 incorporation, expressed as the fraction of total SB staining area that overlaps with GluR1 staining area, based on analysis of images such as those in B. The SKF, SCH, and SCH+SKF groups were not significantly different from the control group (n = 19-24; ANOVA; p > 0.05). Error bars indicate SEM.
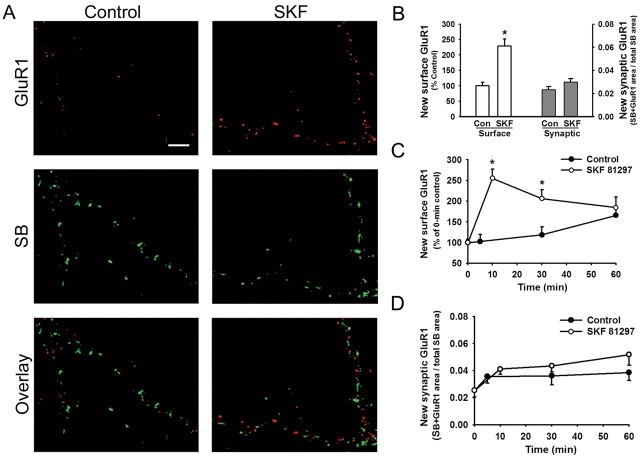
The D1 agonist-induced increase in GluR1 cell-surface expression might arise from accelerated membrane insertion, or, alternatively, the same change could be achieved by slowing the rate of receptor internalization. To distinguish between these mechanisms, we selectively labeled newly inserted GluR1 using a preblocking method modified from Lu et al. (2001). Briefly, preexisting cell-surface GluR1 is masked by preblocking at 15°C with primary antibody and nonconjugated secondary antibody. Then, cells are brought to room temperature for 5 min to enable externalization of new GluR1 subunits. Newly externalized GluR1 is detected with a second round of immunostaining under nonpermeant conditions, this time using a Cy3-conjugated secondary antibody. Finally, cells are stained for synaptobrevin, under permeant conditions, to determine whether the insertion of GluR1 occurred at synapses. Cells treated with SKF 81297 (1 μm) for 5 min showed much higher levels of new surface GluR1 than control cultures, demonstrating that D1 receptor stimulation increases the rate of GluR1 externalization (Fig. 3A; quantitative analysis shown on left side of Fig. 3B). However, very little of this newly externalized GluR1 was colocalized with synaptobrevin (3.7 ± 0.5%). To better quantify synaptic GluR1 incorporation, we determined the percentage of total synaptobrevin area that overlapped with GluR1 area in control and D1 agonist-treated cultures. The percentage was very low in the control group (2.3 ± 0.2%; n = 22) (Fig. 3B, right). This is consistent with a previous study that used a thrombin cleavage assay to demonstrate that GluR1 externalized under basal conditions is found exclusively at extrasynaptic sites during the first 3 min after recovery from thrombin (Passafaro et al., 2001). The percentage of synaptobrevin staining that overlapped with GluR1 staining was slightly higher in cultures treated with SKF 81297 for 5 min (3.0 ± 0.3%; n = 23), but the difference from the control value was not statistically significant (Fig. 3B, right). These results confirm that GluR1 externalized by D1 receptor stimulation is mainly targeted to extrasynaptic sites.
Figure 3.
The D1 agonist SKF 81297 increases the rate of GluR1 externalization, but newly externalized receptors are mainly extrasynaptic. A modification of a preblocking method (Lu et al., 2001) was used to selectively detect newly externalized receptors. A, Examples of staining for SB and newly externalized GluR1 on processes of neurons that were preblocked and then incubated for 5 min at room temperature in control medium or medium containing SKF 81297 (1 μm; 5 min). Scale bar, 5 μm. B, Left two bars, Quantification of the total amount of GluR1 externalized onto the cell surface in the experimental groups illustrated in A (n = 22-23; t test; *p < 0.01). Right two bars, Quantification of the fraction of total SB staining that overlaps with new surface GluR1 staining, indicating that only a small percentage of synapses (2-3%) overlap with the newly externalized GluR1 (n = 22-23; t test; p > 0.05). Con, Control. C, D, Time course experiments were performed to determine whether newly externalized GluR1 remains extrasynaptic after washout of the D1 agonist. Control cultures were preblocked and then returned to 37°C for the indicated time. Cultures in the SKF group were preblocked, incubated with SKF 81297 (1 μm; 5 min), and returned to 37°C for the indicated time. C compares the time course of new surface GluR1 expression in control and SKF-treated cultures (n = 13-22 for each time point; t test; *p < 0.01). Results in C are normalized to a 0 min control group that was fixed immediately after preblocking. D shows the time course of new synaptic GluR1 expression (n = 20-30 for each time point). Control and SKF groups did not differ significantly at any time point (t test; p > 0.05). Results in D are expressed as the percentage of total SB staining that overlaps with new surface GluR1 staining. Error bars indicate SEM.
GluR1 externalized by D1 receptor stimulation remains extrasynaptic for at least 1 h
Although newly externalized GluR1 remained extrasynaptic during the first 5 min of D1 receptor stimulation (see above), it might enter synapses after longer incubation times. To test this, we conducted time course experiments. To examine the basal rate and location of GluR1 externalization, control cultures were preblocked with GluR1 antibody and nonconjugated secondary antibody and then returned to a 37°C CO2 incubator for 5, 30, or 60 min of additional incubation in normal medium. To examine the effect of D1 receptor stimulation, other cultures were incubated with a D1 agonist at 37°C for 5 min, washed to remove the D1 agonist, and then returned to normal medium in a 37°C CO2 incubator for 5, 25, or 55 min (the latter two time points were adjusted so SKF-treated cultures would have the same total incubation time at 37°C as control cultures). Cultures were then stained for cell-surface GluR1, permeabilized, and stained for synaptobrevin.
First, we analyzed the total amount of new cell-surface GluR1 staining (Fig. 3C). Compared with a “0 min control group,” which was fixed immediately after preblocking, surface GluR1 staining increased gradually in control cultures incubated at 37°C in normal medium (∼150% of 0 min control group at 60 min). Exposure to SKF 81297 markedly accelerated GluR1 externalization. When analyzed 5 min after SKF washout, the SKF-treated cultures exhibited significantly higher surface GluR1 staining than control cultures examined after 5 min at 37°C (255 ± 22 and 103 ± 17% of 0 min control group, respectively). At 25 min after SKF washout, surface GluR1 staining had declined but was still significantly greater than in the 30 min control group. At 55 min after SKF washout, the staining was only slightly higher than the 60 min control group (Fig. 3C).
Next, we analyzed the location of newly externalized GluR1 at each time point by quantifying the fraction of total synaptobrevin area that overlapped with GluR1 area. This value was very low for the 0 min control group (2.5 ± 0.5%). However, although total surface GluR1 increased substantially during incubation in control medium at 37°C (Fig. 3C), there was very little translocation to synapses (Fig. 3D). Similarly, although synaptic GluR1 incorporation started at a slightly higher level in the SKF-treated cultures, there was very little translocation to synapses during the remainder of the 37°C incubation and no significant difference in synaptic GluR1 incorporation between the SKF-treated group and the control group at any time point (Fig. 3D). These results indicate that newly externalized GluR1, whether inserted into the membrane under basal conditions or inserted in an accelerated manner in response to D1 receptor stimulation, remains primarily extrasynaptic for at least 1 h. This is consistent with evidence that synaptic insertion of GluR1 requires LTP or CaMKII activation (Shi et al., 1999; Hayashi et al., 2000). The existence of synaptic GluR1 in neurons stained after 2-3 weeks in culture (Fig. 2B) may be explained as the cumulative effect of a slow rate of synaptic insertion during the period in culture.
Increased GluR1 surface expression induced by D1 receptor stimulation requires PKA activation
We hypothesized that D1 receptors modulate AMPA receptor trafficking because they are positively coupled to PKA. If this is true, PKA activation should exert the same effects as D1 receptor stimulation on GluR1 surface expression and synaptic expression. To test this, cultures were incubated for 5 min with Sp-adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (SpcAMPS) (10 μm), a membrane-permeable PKA activator. As observed for the D1 agonist, SpcAMPS produced a significant increase in GluR1 surface expression by pyramidal neurons (Fig. 4A,C) but did not produce a significant increase in overlap of GluR1 and synaptobrevin staining (Fig. 4B,D). Thus, PKA activation is sufficient for surface expression of GluR1-containing AMPA receptors but not for synaptic expression.
Figure 4.
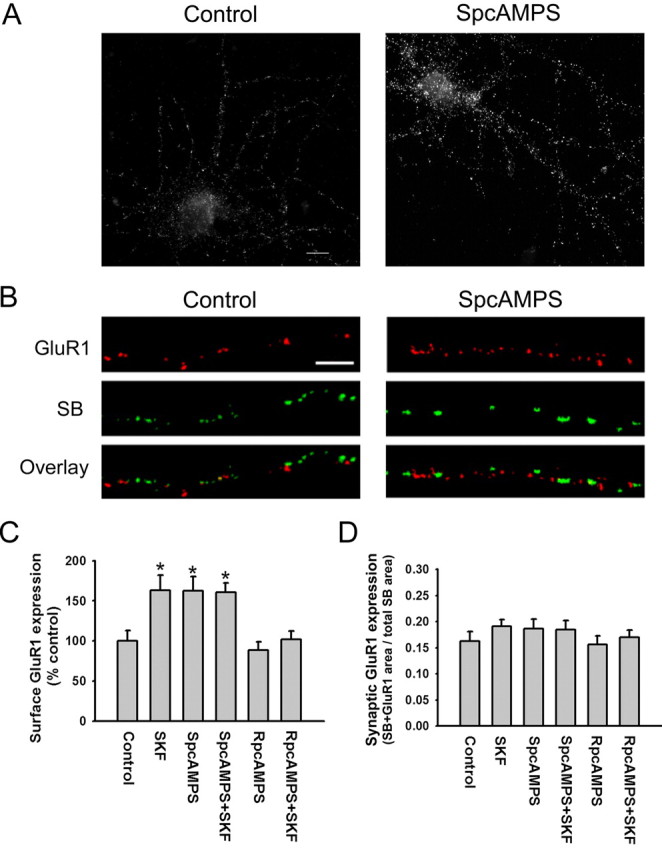
Increased GluR1 surface expression induced by D1 receptor stimulation requires PKA activation. A, Representative images showing that the PKA activator SpcAMPS (10 μm; 5 min) increases total cell-surface GluR1 staining. Scale bar, 10 μm. B, Representative images illustrating that SpcAMPS (10 μm; 5 min) does not alter synaptic GluR1 incorporation, indicated by overlap of GluR1 and SB staining. Scale bar, 5 μm. C, Quantification of the effect of SpcAMPS on GluR1 surface expression (A) and quantification of additional experiments showing that SpcAMPS occludes the effect of the D1 agonist SKF 81297 on GluR1 externalization and that the PKA inhibitor RpcAMPS blocks the increase in GluR1 externalization produced by SKF 81297. For the control group, cells were incubated in medium for 10 min. For the SpcAMPS and RpcAMPS groups, cells were incubated 10 min in 10 μm SpcAMPS or RpcAMPS. For the SpcAMPS+SKF and RpcAMPS+SKF groups, 1 μm SKF 81297 was added during the final 5 min of the incubation. All incubations were at room temperature. Results are presented as the mean area of GluR1 puncta, normalized to the control group. The SKF, SpcAMPS, and SpcAMPS+SKF groups differed significantly from the control group (n = 20-25; Dunn's test; *p < 0.05). D, Quantification of synaptic GluR1 incorporation, expressed as the fraction of total SB staining area that overlaps with GluR1 staining area, for the same experimental groups shown in C. None of the treatments significantly altered synaptic GluR1 incorporation (n = 20-25; ANOVA; p > 0.05). Error bars indicate SEM.
To verify that the PKA pathway is responsible for the D1 receptor-induced increase in GluR1 surface expression, we first examined the ability of PKA inhibitors to prevent this effect. Cultures were incubated for 10 min with Rp-adenosine 3′,5′-cyclic monophosphorothioate triethylammonium salt (RpcAMPS) (10 μm), a membrane-permeable PKA inhibitor, and the D1 agonist SKF 81297 (1 μm) was added for the last 5 min of the incubation. RpcAMPS blocked the D1 agonist-induced increase in GluR1 surface expression, whereas incubation with RpcAMPS alone had no effect (Fig. 4C). Next, we examined whether PKA stimulation occluded the effect of D1 receptor stimulation. Cultures were incubated for 10 min with a maximally effective concentration of the PKA activator SpcAMPS (10 μm), and SKF 81297 (1 μm) was added for the final 5 min of the incubation. After PKA activation, SKF 81297 was unable to further increase GluR1 surface expression (Fig. 4C), confirming that D1 receptors influence AMPA receptor trafficking via the PKA pathway. We also examined the effect of these experimental manipulations on GluR1 synaptic expression. As expected from results shown in Figures 2 and 3, synaptic GluR1 expression was not altered by SKF 81297, SpcAMPS, RpcAMPS, or combined administration of RpcAMPS or SpcAMPS with SKF 81297 (Fig. 4D).
D1 receptor stimulation facilitates synaptic GluR1 insertion during NMDA receptor stimulation
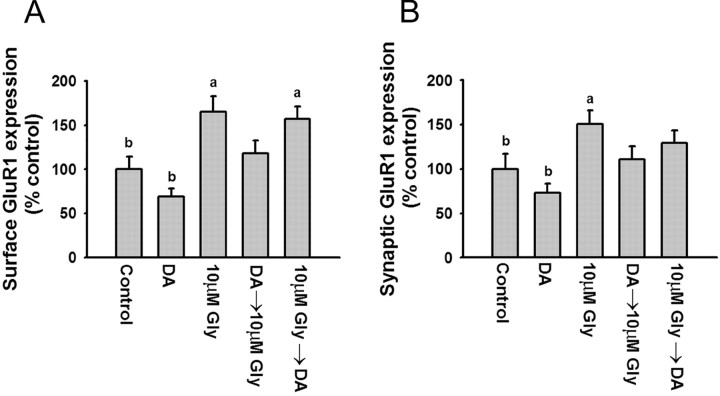
Several studies have found that D1 receptor activation facilitates LTP in the rat PFC through a PKA-dependent mechanism (Jay et al., 1998; Gurden et al., 2000; Huang et al., 2004). We hypothesized that D1 receptor stimulation increases the extrasynaptic cell-surface pool of GluR1 in PFC neurons and thus increases the GluR1 pool available for synaptic insertion during LTP. To test this, we adapted methods from Lu et al. (2001), who used brief exposure to glycine (an obligatory coagonist of the NMDA receptor), to selectively activate synaptic NMDA receptors, leading to LTP of AMPA receptor-mediated miniature EPSCs and increased synaptic GluR1 expression in cultured hippocampal neurons. If our hypothesis regarding D1 receptor activation was correct, we anticipated that previous exposure to a D1 agonist would enable a subthreshold concentration of glycine to produce synaptic GluR1 incorporation.
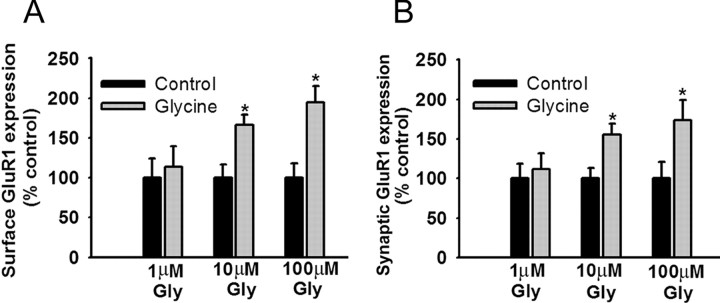
To identify an appropriate concentration of glycine, PFC cultures were incubated with glycine (1, 10, or 100 μm) in bathing solution (see Materials and Methods) for 3 min, rinsed, transferred to the bathing solution without glycine for 15 min, incubated for 30 min with N-terminal GluR1 antibody to label surface GluR1, and then stained for synaptobrevin after fixation and permeabilization. Glycine produced a concentration-dependent increase in both surface GluR1 expression (Fig. 5A) and synaptic GluR1 incorporation (Fig. 5B), with significant increases produced by 10 or 100 μm glycine but not 1 μm glycine. To determine whether previous D1 receptor stimulation would facilitate synaptic GluR1 incorporation, PFC cultures were pretreated for 5 min with the D1 agonist SKF 81297 (1 μm), rinsed, treated for 3 min with the subthreshold concentration of glycine (1 μm), rinsed, transferred to the bathing solution without any added glycine for 15 min, and then stained for GluR1 and synaptobrevin. SKF 81297, either alone or with glycine, increased total surface expression of GluR1 (Fig. 6A). However, neither glycine nor SKF 81297 alone was sufficient to significantly increase GluR1 and synaptobrevin colocalization, whereas D1 agonist pretreatment enabled glycine to produce a significant increase in colocalization (Fig. 6B,C). This was not observed if glycine was applied before the D1 agonist (Fig. 6B,C). Facilitation also failed to occur if the D1 agonist and glycine were applied together, perhaps because D1 agonists have multiple effects on PFC excitability, some of which oppose the D1 receptor-mediated facilitation of synaptic GluR1 incorporation. For example, there is evidence that D1 agonists decrease glutamate transmission through presynaptic mechanisms in the PFC (Gao et al., 2001; Seamans et al., 2001). This would reduce NMDA receptor transmission, despite the presence of glycine, preventing newly externalized AMPA receptors from being inserted into synaptic sites.
Figure 5.
Glycine increases both total surface GluR1 expression and synaptic GluR1 expression in PFC neurons. A, Quantification of the effect of glycine on total cell-surface GluR1 staining. Both 10 and 100 μm glycine significantly increased surface GluR1 expression in PFC neurons compared with their respective control groups (t tests; *p < 0.05), whereas 1 μm glycine did not change GluR1 surface expression (t test; p > 0.05). Results are presented as the mean area of GluR1 puncta, normalized to the control group. B, Quantification of the effect of glycine on synaptic expression of GluR1. Both 10 and 100 μm glycine significantly increased synaptic GluR1 expression in PFC neurons compared with their control groups (t tests; *p < 0.05), whereas 1 μm glycine did not change synaptic GluR1 expression (t test; p > 0.05). Results are presented as the percentage of total SB area that overlaps with GluR1 area, normalized to the control group. Error bars indicate SEM.
Figure 6.
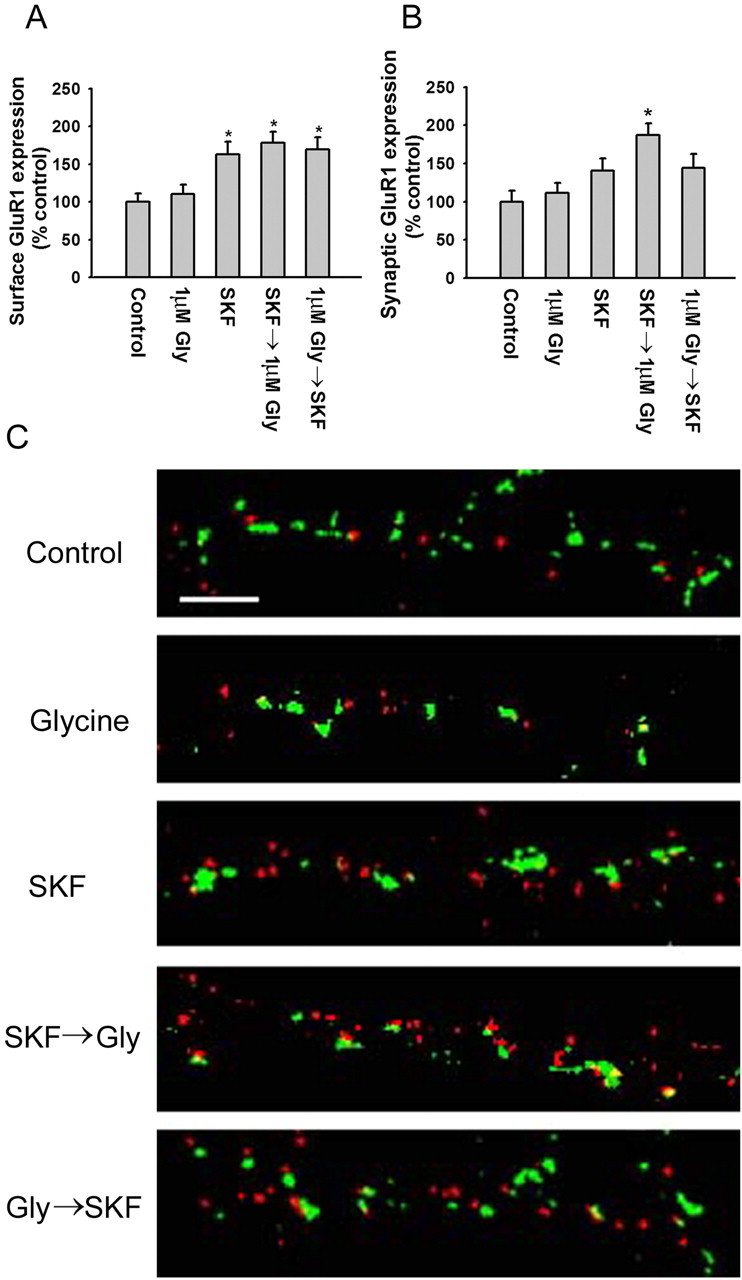
The D1 receptor agonist SKF 81297 facilitates synaptic GluR1 insertion during NMDA receptor stimulation. A, SKF 81297 significantly increases total cell-surface GluR1 expression regardless of whether it is added before or after glycine (Gly). Cultures in the SKF 81297→glycine group were treated for 5 min with 1 μm SKF 81297, rinsed, and treated for 3 min with 1 μm glycine. Cultures in the glycine→ SKF group were treated for 3 min with 1 μm glycine, rinsed, and treated for 5 min with 1 μm SKF 81297. Results are presented as the mean area of GluR1 puncta, normalized to the control group (n = 18-25; Dunn's test; *p < 0.05). B, SKF 81297 significantly increased synaptic GluR1 expression when added before glycine but not after glycine. The SKF, glycine, and glycine→ SKF 81297 groups did not differ significantly from control. Results are presented as the percentage of total SB area that overlaps with GluR1 area, normalized to the control group (n = 18-25; Dunn's test; *p < 0.05). Error bars indicate SEM. C, Examples of colocalization of GluR1 with synaptobrevin on processes of control (Con) neurons and neurons treated with glycine (1 μm; 3 min), SKF 81297 (1 μm; 5 min), SKF 81297→glycine, and glycine→ SKF 81297. Scale bar, 5 μm.
These results show that GluR1 externalized at extrasynaptic sites by D1 receptor stimulation can be translocated to synapses by subsequent NMDA receptor activation. The slight, nonsignificant increase in synaptic GluR1 incorporation produced by SKF alone (Fig. 6B,C) may be attributable to the use of a low Mg2+ bathing solution in these experiments, which would increase NMDA receptor activity to some extent even in the absence of glycine. This might enable a small amount of the GluR1 externalized by the D1 agonist to be translocated to synaptic sites. In support of this interpretation, chemical LTP can be induced by lowering the Mg2+ concentration of the artificial CSF during forskolin/rolipram or SpcAMPS application (Otmakhov et al., 2004).
D2 receptor activation attenuates the D1 agonist-induced increase in surface GluR1 expression
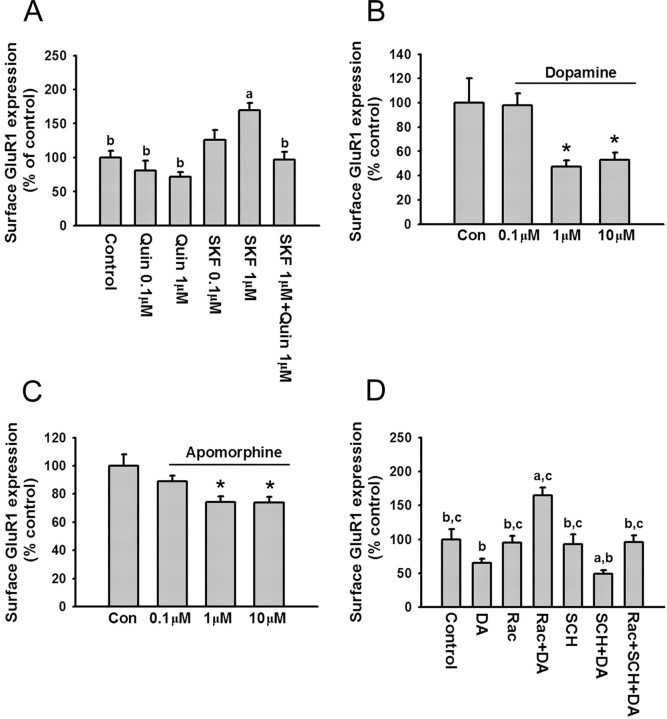
To determine whether D2 receptors also modulate AMPA receptor trafficking, PFC cultures were incubated with the D2 agonist quinpirole (0.1 or 1 μm; 10 min). Quinpirole produced a trend toward decreased surface expression of GluR1 (Fig. 7A). When quinpirole (1 μm) was applied with SKF 81297 (1 μm), quinpirole significantly attenuated the increase in GluR1 surface expression produced by the D1 agonist (Fig. 7A). To further study D1 and D2 receptor interactions, we used DA and the nonselective DA receptor agonist apomorphine. Both DA (1 or 10 μm; 10 min) and apomorphine (1 or 10 μm; 10 min) significantly decreased surface GluR1 expression (Fig. 7B,C). To determine the role of D1 and D2 receptors in the effect of DA, selective D1 and D2 receptor antagonists were applied. The D2 receptor antagonist raclopride, applied 5 min before DA, reversed the inhibitory effects of DA such that GluR1 surface expression was increased over control levels (Fig. 7D). We interpret this to indicate that blocking D2 receptors unmasked the effect of D1 receptor stimulation. When the D1 receptor antagonist SCH 23390 was added 5 min before DA, the inhibitory effect of DA was greater than when DA was added alone (Fig. 7D), presumably because the D2 receptor-mediated effect was no longer opposed by D1 receptor activation. Coapplication of raclopride and SCH 23390 fully blocked the effect of DA on GluR1 surface expression (Fig. 7D). When the antagonists were administered in the absence of DA, they did not significantly influence GluR1 surface expression (Fig. 7D). Together, these results demonstrate that DA decreases GluR1 surface expression by activating D2 receptors, and that D2 receptor activation occludes the effect of D1 receptor activation in dissociated PFC cultures.
Figure 7.
Dopamine decreases surface GluR1 expression on PFC neurons via D2 receptor activation. A, The D2 agonist quinpirole blocks the increased surface GluR1 expression induced by D1 receptor stimulation. Quantification of surface GluR1 expression in control neurons (medium; 10 min) and neurons treated with quinpirole (0.1 or 1 μm; 10 min), SKF 81297 (0.1 or 1 μm; 10 min), or quinpirole (1 μm) plus SKF (1 μm) (a, p < 0.05 compared with control group; b, p < 0.05 compared with SKF 81297 group; Dunn's test; n = 24-39). B, C, Dopamine and apomorphine decrease surface GluR1 expression. Quantification of cell-surface GluR1 staining in control neurons (medium; 10 min) and neurons treated with DA (0.1, 1, or 10 μm; 10 min; n = 28-43) or apomorphine (0.1, 1, or 10 μm; 10 min; n = 26-31). Both drugs significantly decreased surface GluR1 expression (*p < 0.05; Dunn's test). D, Quantification of cell-surface GluR1 staining in control neurons and neurons treated with raclopride (1 μm), raclopride (1 μm) plus DA (1 μm), SCH 23390 (10 μm), SCH 23390 (10 μm) plus DA (1 μm), and raclopride (1 μm) plus SCH 23390 (10 μm) plus DA (1 μm). Cells were incubated with antagonists for 5 min, and then DA was added for another 10 min (a, p < 0.05 compared with control group; b, p < 0.05 compared with raclopride plus DA group; c, p < 0.05 compared with SCH plus DA group; n = 20-37). Error bars indicate SEM. Quin, Quinpirole; Rac, raclopride; Con, control.
Dopamine attenuates synaptic GluR1 expression during NMDA receptor stimulation
Using a subthreshold concentration of glycine (1 μm), we demonstrated that activation of D1 receptors potentiated synaptic insertion of AMPA receptors during NMDA receptor stimulation (Fig. 6). Here, we used a suprathreshold concentration of glycine (10 μm) to determine whether DA decreases AMPA receptor synaptic insertion during NMDA receptor stimulation. This concentration of glycine, applied for 3 min, significantly increased GluR1 surface expression (Fig. 8A) and synaptic incorporation (Fig. 8B), confirming results shown in Figure 5. DA applied alone produced a trend toward decreased GluR1 surface expression and synaptic incorporation that did not reach statistical significance (Fig. 8A,B). PFC cultures incubated with DA (1 μm; 10 min), rinsed, and then treated with glycine (10 μm; 3 min) exhibited levels of GluR1 surface expression and synaptic incorporation that did not differ from controls, indicating that DA attenuated the effect of glycine (Fig. 8A,B). When DA was applied after glycine washout, it did not influence the effect of glycine on GluR1 surface expression but partially overcame its effect on synaptic GluR1 incorporation (Fig. 8A,B). These results indicate that D2 receptor activation, either before or after NMDA receptor stimulation, decreases the ability of NMDA receptor stimulation to produce synaptic insertion of AMPA receptors.
Figure 8.
Dopamine attenuates synaptic GluR1 expression during NMDA receptor stimulation. A, DA attenuates the increase in GluR1 surface expression produced by glycine (10 μm). Cultures were treated with medium, DA (1 μm), or glycine (10 μm). Cultures in the DA→glycine group were treated with DA for 10 min, rinsed, and treated for 3 min with glycine. The order of drug exposure was reversed in the glycine→ DA group (a, p < 0.05 compared with control group; b, p < 0.05 compared with glycine group; Dunn's test; n = 21-34). B, DA attenuates the increase in GluR1 synaptic incorporation produced by glycine (10 μm). Data are from the same experimental groups as in A. Results are presented as the percentage of the total SB area that overlaps with GluR1 area, normalized to the control group (a, p < 0.05 compared with control group; b, p < 0.05 compared with glycine group; Dunn's test; n = 21-34). Error bars indicate SEM.
Discussion
Interaction of D1 and D2 DA receptors in the modulation of AMPA receptor trafficking in the PFC
Activation of the D1 receptor-PKA signaling pathway increased GluR1 surface expression in pyramidal neurons of the PFC by increasing the rate of GluR1 externalization. The new receptors were found at extrasynaptic sites, indicating that D1 receptor stimulation is not sufficient for synaptic GluR1 insertion. However, they could be translocated into synapses by subsequent activation of synaptic NMDA receptors. These results provide direct support for a two-step process of GluR1 synaptic incorporation consisting of insertion into extrasynaptic sites followed by lateral movement into synapses (Passafaro et al., 2001; Bredt and Nicoll, 2003). Furthermore, they support and extend results in organotypic hippocampal slices demonstrating that PKA phosphorylation is necessary but not sufficient to drive GluR1 into synapses; CaMKII must also be activated (Esteban et al., 2003). Other findings also support a role for PKA phosphorylation in AMPA receptor trafficking during LTP (Ehlers, 2000; Lee et al., 2003; Lu et al., 2003). Together, these results suggest that PKA phosphorylation of GluR1 sets the number of AMPA receptors available for synaptic delivery. CaMKII activates the cellular machinery that results in GluR1 synaptic delivery (Lisman et al., 2002).
D2 receptor stimulation exerted opposite effects on GluR1 trafficking to those of D1 agonists, and when both receptors were activated by DA, the D2 effect occluded the D1 effect. Does this mean that the net effect of endogenous DA transmission on AMPA receptor externalization in the intact PFC is inhibitory and that observed effects of D1 receptor signaling are therefore not important? On the contrary, D1 receptor signaling plays a dominant role in the intact PFC, as evidenced by studies showing that optimal working memory performance, the best studied measure of PFC function, depends mainly on D1 receptor activation both in primates (Sawaguchi and Goldman-Rakic, 1991) and rodents (Zahrt et al., 1997; Seamans et al., 1998). Similarly, DA regulates synaptic plasticity in the PFC of the intact rat primarily through activation of D1 receptors (Jay, 2003).
How can the dominant role for D1 receptors be reconciled with the ability of D2 agonists to occlude the effect of D1 agonists in PFC cultures? A solution is suggested by evidence that D1 and D2 receptors signal independently, rather than antagonistically, in the intact PFC. One reason is that low concentrations of DA (nanomolar) selectively activate D1 receptors in the PFC, whereas higher concentrations (micromolar) are required to activate D2 receptors (Zheng et al., 1999; Trantham-Davidson et al., 2004). The lower, D1-preferring, concentrations of DA correspond to those measured in the PFC during working memory tasks in rats (Phillips et al., 2004). DA levels may reach micromolar concentrations near release sites before diffusing away to nanomolar concentrations in the extracellular space (Kawagoe et al., 1992; Garris and Wightman, 1994). High micromolar concentrations associated with particularly alerting stimuli or stress may selectively activate D2 receptors closer to release sites, rather than the predominantly extrasynaptic D1 receptors (Smiley et al., 1994; Yung et al., 1995; Caille et al., 1996). Independent D1 and D2 receptor signaling may also reflect the abundance of D1-like receptors in the PFC (Bentivoglio and Morelli, 2005) and different patterns of cellular localization for D1 and D2 receptors in the PFC (Vincent et al., 1993; Gaspar et al., 1995). The important point is that physiological DA levels in the PFC exert important functional effects by activating D1 receptor signaling in isolation from D2 receptor signaling, justifying consideration of the functional consequences of independently activating the D1 receptor signaling pathway.
D1 receptors and plasticity in the PFC
Experiments in anesthetized rats have found that tetanic stimulation of hippocampal projections to the PFC produces NMDA receptor-dependent LTP that is enhanced by locally applied DA or by stimulation of the ventral tegmental area (VTA) and reduced by VTA lesions (Gurden et al., 1999). This effect of DA is mediated via activation of D1 receptors through a PKA-dependent mechanism (Jay et al., 1998; Gurden et al., 2000). Our results suggest an explanation for these observations, that is, D1 receptor stimulation increases the extrasynaptic pool of AMPA receptors available for synaptic insertion in response to NMDA receptor stimulation during the tetanus. This mechanism may also underlie a different type of DA-induced plasticity in the PFC. In PFC slices, bath application of DA normally favors the emergence of LTD (Law-Tho et al., 1995; Otani et al., 1998). However, if DA is applied, washed out, and then added to the bath again in conjunction with high-frequency stimulation, LTP is induced instead of LTD (Blond et al., 2002). This may reflect a priming effect of D1 receptor stimulation during the first bath application of DA on AMPA receptor synaptic insertion during the second stimulation period. Other results support the idea that D1 receptors can facilitate both LTD and LTP in PFC neurons under appropriate experimental conditions (Huang et al., 2004).
Extrapolating to the behavioral level, our results help explain the requirement for coordinated D1-PKA signaling and NMDA receptor activation in the PFC during appetitive learning (Baldwin et al., 2002) and perhaps in working memory (Jay, 2003; Seamans and Yang, 2004), although it should be noted that the D1-PKA pathway has other important cellular targets in the PFC. The D1-PKA signaling pathway also contributes to learning and memory processes in other brain regions, including the hippocampus and the striatal complex (Berke and Hyman, 2000; Jay, 2003; Beninger and Gerdjikov, 2004; Kelley, 2004). Facilitation of synaptic GluR1 insertion may contribute to D1 receptor effects in these regions, based on the PKA dependence of AMPA receptor externalization in hippocampus (see above) and our results showing that D1 receptor stimulation accelerates AMPA receptor externalization in nucleus accumbens neurons (Chao et al., 2002; Mangiavacchi and Wolf, 2004).
Our results may also be relevant to the role of DA in regulating the excitability of PFC pyramidal neurons. This role is very complex, because DA receptors are located postsynaptically but also on terminals and interneurons, and have many effects on voltage-gated and synaptic currents (Seamans and Yang, 2004). One important variable in determining the effect of DA is the time elapsed between applying DA and the electrophysiological test (Otani et al., 2003; Seamans and Yang, 2004). Experiments using intracellular current injection to assess the excitability of deep-layer pyramidal neurons in rodent PFC have found a transient depression in excitability that is primarily D2 receptor-mediated and a delayed but prolonged increase in excitability that is mediated by D1 receptors (Yang and Seamans, 1996; Gulledge and Jaffe, 1998, 2001; Gorelova and Yang, 2000; Seamans and Yang, 2004) (for review, see Otani et al., 2003). Similarly, D2 receptor agonists reduce NMDA receptor currents in PFC neurons, whereas D1 agonists increase them (Zheng et al., 1999; Seamans et al., 2001). The prolonged increase in excitability produced by D1 agonists helps explain their role in maintaining the depolarized up state in pyramidal neurons, a state that facilitates action potentials and plasticity (O'Donnell, 2003), and may contribute to self-sustaining activity in PFC neurons during working memory (Durstewitz and Seamans, 2002; Seamans and Yang, 2004).
D1 receptors increase the excitability of PFC pyramidal neurons by modulating voltage-sensitive conductances (Seamans and Yang, 2004). However, D1 receptor facilitation of AMPA receptor externalization may also contribute. By increasing the number of AMPA receptors on the cell surface, D1 receptor stimulation would ultimately lead to increased excitatory transmission, provided that NMDA receptor stimulation is sufficient to produce synaptic insertion of AMPA receptors once they have been externalized by D1 receptor stimulation. This mechanism predicts an activity-dependent increase in excitability that would manifest slowly, consistent with electrophysiological studies of D1 receptor function (see above). When levels of glutamate transmission rise, this same mechanism may help to facilitate the induction of LTP, as discussed above. It is interesting to speculate that synaptic targeting of AMPA receptors after D1 receptor stimulation may be facilitated by D1 receptor-induced increases in the sensitivity of postsynaptic NMDA receptors (Seamans et al., 2001; Wang and O'Donnell, 2001).
The ability of D1 receptors to facilitate AMPA receptor synaptic targeting in PFC cultures was dependent on the temporal relationship between activation of D1 and NMDA receptors. Facilitation occurred when D1 receptor stimulation preceded NMDA receptor stimulation, but not when NMDA receptor stimulation occurred first, presumably because the increase in the extrasynaptic pool of AMPA receptors must precede NMDA receptor stimulation. Strikingly similar temporal requirements were observed in experiments showing that brief exposure to a novel environment enhanced the ability of a weak tetanus to induce LTP in hippocampal CA1 neurons (Li et al., 2003). Novelty-induced facilitation of LTP was mediated by D1 receptor-PKA signaling and occurred only if exposure to the novel environment preceded the weak tetanus, not if the order was reversed, and could be abolished by introducing a delay between novelty and the weak tetanus. Summing up, there appears to be a temporal window shortly after D1 receptor stimulation during which neuronal activation is more likely to produce synaptic insertion of AMPA receptors leading to LTP.
D2 receptors and plasticity in the PFC
In contrast to D1 receptors, the role of D2 receptors in PFC function is less clear. Recent evidence suggests that they modulate specific components of working memory (Wang et al., 2004). As mentioned above, one consequence of activating D2 receptors in the PFC is a rapid depression of the excitability of PFC pyramidal neurons that results from activation of GABA transmission and modulation of a Na+ conductance (Gulledge and Jaffe, 1998, 2001). These effects may contribute to the important role of D2 receptors in facilitating LTD induction in PFC slices (Law-Tho et al., 1995; Otani et al., 1998, 1999). Our results, showing that D2 receptors decrease AMPA receptor surface and synaptic expression, suggest an additional mechanism by which D2 receptors may depress neuronal excitability and plasticity. Inhibition of PKA is unlikely to account for this effect of D2 receptor activation, because a PKA inhibitor did not reproduce the effect of a D2 agonist on AMPA receptor trafficking (Fig. 4). D2-class receptors influence many signaling molecules in addition to adenylyl cyclase, including ion channels, MAP (mitogen-activated protein) kinases, and phospholipases (Neve et al., 2004).
Conclusions
Our results suggest that DA receptors may regulate synaptic plasticity by modulating AMPA receptor surface and synaptic expression. Under normal circumstances, this may contribute to the role of DA in learning adaptive behaviors important for survival (Kelley and Berridge, 2002). During repeated cocaine exposure, unregulated DA receptor signaling may lead to inappropriate modulation of AMPA receptor trafficking and abnormal synaptic plasticity, which in turn may trigger inappropriate synaptic remodeling (Robinson and Kolb, 1999). These processes may contribute to the rewiring of neuronal circuits that underlies the transition from casual to compulsive drug use.
Footnotes
This work was supported by National Institute on Drug Abuse Grants DA015835 and DA00453. We are grateful to Dr. Jeremy Seamans for helpful discussions and comments on this manuscript.
Correspondence should be addressed to Dr. Marina E. Wolf, Department of Neuroscience, Rosalind Franklin University of Medicine and Science/The Chicago Medical School, 3333 Green Bay Road, North Chicago, IL 60064. E-mail: marina.wolf@rosalindfranklin.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/257342-10$15.00/0
References
- Baldwin AE, Sadeghian K, Kelley AE (2002) Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci 22: 1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC (2002) Control of synaptic strength by glial TNFα. Science 295: 2282-2285. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Gerdjikov T (2004) The role of signaling molecules in reward-related incentive learning. Neurotox Res 6: 91-104. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Morelli M (2005) The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In: Handbook of chemical neuroanatomy, Vol 21, Dopamine (Dunnett SB, Bentivoglio M, Björklund A, Hökfelt T, eds), pp 1-106. Amsterdam: Elsevier. [Google Scholar]
- Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515-532. [DOI] [PubMed] [Google Scholar]
- Blond O, Crépel F, Otani S (2002) Long-term potentiation in rat prefrontal slices facilitated by phased application of dopamine. Eur J Pharmacol 438: 115-116. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40: 361-379. [DOI] [PubMed] [Google Scholar]
- Caille I, Dumartin B, Bloch B (1996) Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res 730: 17-31. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26: 321-352. [DOI] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC (1999) Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci 2: 454-460. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P (2003) Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci 14: 207-216. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME (2002) D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J Neurochem 83: 704-712. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC (2004) Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA-/- mice. Proc Natl Acad Sci USA 101: 14282-14287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK (2002) The computational role of dopamine D1 receptors in working memory. Neural Netw 15: 561-572. [DOI] [PubMed] [Google Scholar]
- Ehlers MD (2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28: 511-525. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136-143. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS (2001) Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA 98: 295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM (1994) Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci 14: 442-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar P, Bloch B, Le Moine C (1995) D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci 7: 1050-1063. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1987) Circuity of primary prefrontal cortex and regulation of behavior by representational memory. In: Handbook of physiology, the nervous system, higher functions of the brain (Plum F, ed), pp 373-417. Bethesda, MD: American Physiological Society.
- Goldman-Rakic PS, Muly III EC, Williams GV (2000) D1 receptors in prefrontal cells and circuits. Brain Res Rev 31: 295-301. [DOI] [PubMed] [Google Scholar]
- Gorelova NA, Yang CR (2000) Dopamine D1/D5 receptor activation modulates a persistent sodium current in rat prefrontal cortical neurons in vitro. J Neurophysiol 84: 75-87. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB (1998) Dopamine decreases the excitability of layer V pyramidal cells in the rat prefrontal cortex. J Neurosci 18: 9139-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Jaffe DB (2001) Multiple effects of dopamine on layer V pyramidal cell excitability in rat prefrontal cortex. J Neurophysiol 86: 586-595. [DOI] [PubMed] [Google Scholar]
- Gurden H, Tassin JP, Jay TM (1999) Integrity of the mesocortical dopaminergic system is necessary for complete expression of in vivo hippocampal-prefrontal cortex long-term potentiation. Neuroscience 94: 1019-1027. [DOI] [PubMed] [Google Scholar]
- Gurden H, Takita M, Jay TM (2000) Essential role of D1 but not D2 receptors in the NMDA receptor-dependent long-term potentiation at hippocampal-prefrontal cortex synapses in vivo J Neurosci 20: RC106(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262-2267. [DOI] [PubMed] [Google Scholar]
- Huang YY, Simpson E, Kellendonk C, Kandel ER (2004) Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci USA 101: 3236-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM (2003) Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol 69: 375-390. [DOI] [PubMed] [Google Scholar]
- Jay TM, Gurden H, Yamaguchi T (1998) Rapid increase in PKA activity during long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in vivo. Eur J Neurosci 10: 3302-3306. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146: 373-390. [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA (2000) Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J Neurosci 20: 5575-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA (2004) Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol 66: 447-475. [DOI] [PubMed] [Google Scholar]
- Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM (1992) Regulation of transient dopamine concentration gradients in the microenvironment surrounding nerve terminals in the rat striatum. Neuroscience 51: 55-64. [DOI] [PubMed] [Google Scholar]
- Kelley AE (2004) Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44: 161-179. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22: 3306-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law-Tho D, Desce JM, Crepel F (1995) Dopamine favours the emergence of long-term depression versus long-term potentiation in slices of rat prefrontal cortex. Neurosci Lett 188: 125-128. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631-643. [DOI] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ (2003) Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci 6: 526-531. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H (2002) The molecular basis of CaMKII function in synaptic and behavioral memory. Nat Rev Neurosci 3: 175-190. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Partridge JG, Tang KC (2003) Plastic control of striatal glutamatergic transmission by ensemble actions of several neurotransmitters and targets for drugs of abuse. Ann NY Acad Sci 1003: 226-240. [DOI] [PubMed] [Google Scholar]
- Lu HC, She WC, Plas DT, Neumann PE, Janz R, Crair MC (2003) Adenylyl cyclase I regulates AMPA receptor trafficking during mouse cortical “barrel” map development. Nat Neurosci 6: 939-947. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT (2001) Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243-254. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103-126. [DOI] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME (2004) D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J Neurochem 88: 1261-1271. [DOI] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H (2004) Dopamine receptor signaling. J Recept Signal Transduct Res 24: 165-205. [DOI] [PubMed] [Google Scholar]
- O'Donnell P (2003) Dopamine gating of forebrain neural ensembles. Eur J Neurosci 17: 429-435. [DOI] [PubMed] [Google Scholar]
- Otani S, Blond O, Desce JM, Crépel F (1998) Dopamine facilitates long-term depression of glutamatergic transmission in rat prefrontal cortex. Neuroscience 85: 669-676. [DOI] [PubMed] [Google Scholar]
- Otani S, Auclair N, Desce J-M, Roisin MP, Crépel F (1999) Dopamine receptors and group I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinase. J Neurosci 19: 9788-9802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S, Daniel H, Roisin MP, Crépel F (2003) Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb Cortex 13: 1251-1256. [DOI] [PubMed] [Google Scholar]
- Otmakhov N, Khibnik L, Otmakhova N, Carpenter S, Riahi S, Asrican B, Lisman JE (2004) Forskolin-induced LTP in the CA1 hippocampal region is NMDA receptor dependent. J Neurophysiol 91: 1955-1962. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M (2001) Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci 4: 917-926. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB (2004) Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J Neurosci 24: 547-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B (1999) Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11: 1598-1604. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC (2003) Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577-582. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS (1991) D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251: 947-950. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1-58. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG (1998) D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci 18: 1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ (2001) Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA 98: 301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine-Aizawa Y, Huganir RL (2004) Imaging of receptor trafficking by using α-bungarotoxin-binding-site-tagged receptors. Proc Natl Acad Sci USA 101: 17114-17119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A (2003) Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci 1003: 36-52. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R (1999) Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science 284: 1811-1816. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331-343. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS (1994) D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA 91: 5720-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578-588. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, Bonci A (2000) Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci 20: 5581-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC (2001) Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci 4: 1217-1223. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans J (2004) Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci 24: 10652-10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583-587. [DOI] [PubMed] [Google Scholar]
- Verney C, Alvarez C, Geffard M, Berger B (1990) Ultrastructural double-labelling study of dopamine terminals and GABA-containing neurons in rat anteromedial cerebral cortex. Eur J Neurosci 2: 960-972. [DOI] [PubMed] [Google Scholar]
- Vincent SL, Khan Y, Benes FM (1993) Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J Neurosci 13: 2551-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, O'Donnell P (2001) D1 dopamine receptors potentiate NMDA-mediated excitability increase in layer V prefrontal cortical pyramidal neurons. Cereb Cortex 11: 452-462. [DOI] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS (2004) Selective D2 receptor actions on the functional circuitry of working memory. Science 303: 853-856. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ (2004) Psychomotor stimulants and neuronal plasticity. Neuropharmacology 47 [Suppl 1]: 61-79. [DOI] [PubMed] [Google Scholar]
- Yang CR, Seamans JS (1996) Dopamine D1 receptor actions in layer V-VI rat prefrontal cortex neurons in vitro: modulation of dendritic-somatic signal integration. J Neurosci 16: 1922-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KKL, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI (1995) Immunocyochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65: 709-730. [DOI] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF (1997) Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci 17: 8528-8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zhang XX, Bunney BS, Shi WX (1999) Opposite modulation of cortical N-methyl-d-aspartate receptor-mediated responses by low and high concentrations of dopamine. Neuroscience 91: 527-535. [DOI] [PubMed] [Google Scholar]