Abstract
Rod signals are transmitted to ON retinal ganglion cells by means of gap junctions between AII amacrine cells and ON bipolars. The AII amacrine cells are known to express connexin36 (Cx36), but previous studies of Cx36 in ON cone bipolars have been ambiguous. Here, we studied bipolar cells in a transgenic mouse line that expresses high levels of green fluorescent protein (GFP) in one type of ON cone bipolar cell. We found strong Cx36 immunostaining in the axon terminals of the GFP-labeled type 357 bipolar cells in both vertical sections and whole mounts of the retina. This finding was confirmed by single-cell immunostaining and single-cell reverse transcription-PCR (RT-PCR). As reported previously (Maxeiner et al., 2005), Cx45 was found in some ON bipolar cells, but RT-PCR showed Cx36 and not Cx45 to be expressed by the type 357 bipolar cells. Some of the remaining GFP-negative bipolar cells expressed Cx45 but not Cx36. It appears that different types of ON cone bipolar cells express different connexins at their gap junctions with AII amacrine cells.
Keywords: mouse, GFP, retina, Cx36, ON bipolar cell, gap junctions
Introduction
In mammalian retinas, rod signals generated under scotopic light condition are transmitted to the cone system through the AII amacrine cell (Wässle and Boycott, 1991; Strettoi et al., 1992, 1994; Sterling, 1998). AII amacrine cells make gap junctions with neighboring AII amacrines in the most vitreal region of the inner plexiform layer (IPL) and with axon terminals of cone bipolars in sublamina b of the IPL (Kolb and Famiglietti, 1974; Famiglietti and Kolb, 1975; Strettoi et al., 1992; Massey and Mills, 1999).
Connexin36 (Cx36) expression has been demonstrated in both the outer and inner plexiform layers of the mammalian retina (Feigenspan et al., 2001; Mills et al., 2001). It is located in cone photoreceptors and dendrites of OFF cone bipolars in the outer plexiform layer (Feigenspan et al., 2004) and on the dendrites of AII amacrines in the IPL (Feigenspan et al., 2001; Mills et al., 2001). Cx36 is required for gap-junctional communication between pairs of AII amacrines, because dye coupling between AII amacrines is lost in Cx36 knock-out animals. In addition, Güldenagel et al. (2001) and Deans et al. (2002) have shown a loss of scotopic signaling in Cx36 knock-out mice, indicating a clear functional role for Cx36 in the gap junctions between ON cone bipolars and AII amacrines.
The composition of this junction is unclear. Feigenspan et al. (2001) did not find immunostaining for Cx36 on axon terminals of recoverin-positive bipolar cells, nor did they find immunoreactivity in morphologically identified bipolar cells after dissociation. They concluded that the gap junction between ON bipolar and AII amacrine must be heterotypic in nature, composed of hemichannels containing Cx36 on one side and an as yet unidentified connexin on the other side; a recently identified possibility is Cx45 (Maxeiner et al., 2005). In contrast, a Cx36 reporter in a transgenic mouse was clearly expressed in a subset of bipolar cells with axons terminating in the ON layer of the IPL (Deans et al., 2002). The previous results are thus ambiguous.
The issue is of physiological consequence, because this junction is, under scotopic conditions, a major gateway between the visual stimulus and the output of the retina. We wanted to resolve it by taking advantage of the availability of a transgenic mouse line in which one population of ON cone bipolar cells strongly expresses green fluorescent protein (GFP) (Huang et al., 1999). It appears to be bipolar cell type 7 from the study by Ghosh et al. (2004) and type CB4a from the study by Pignatelli and Strettoi (2004). Given the ability to reliably identify this type of bipolar cell, we could study Cx36 expression in intact tissue, dissociated cells, and a PCR product from picked cells. We found that this particular type of ON cone bipolar expresses Cx36.
Materials and Methods
Retinal preparation. Transgenic mice (357 transgenic mice) were used for the experiments between the ages of 6 and 8 weeks (Huang et al., 1999). Experimental procedures were in accordance with the guidelines of the Subcommittee for Research Animal Care of the Massachusetts General Hospital. Animals were anesthetized with a mixture of ketamine hydrochloride (30-40 mg/kg) and xylazine (3-6 mg/kg). The eyes were quickly enucleated, and the retina was dissected free of the vitreous and sclera in carboxygenated Ames medium (Sigma, St. Louis, MO). The isolated retinas were fixed in 4% paraformaldehyde (PFA) in 0.1 m phosphate buffer (PB), pH 7.4, for 30 min.
For preparation of retinal slices, the retina was removed from the sclera and embedded in 2% agarose (Sigma) in Ames medium. Slices were cut at 200 μm on a vibratome and fixed immediately in 2% PFA for 3 min. Intracellular injections were performed with sharp borosilicate microelectrodes filled with 10 mm Alexa Fluor 594 hydrazide (Molecular Probes, Eugene, OR). The dye was injected iontophoretically (5-10 min, -1 nA) into individual AII amacrine cells, using a 63× water-immersion objective (Achroplan; Zeiss, Thornwood, NY). After microinjection, the slice was fixed for 30 min in 2% PFA and rinsed in PB before additional immunostaining for Cx36.
Immunocytochemistry. The following primary antibodies were applied: rabbit anti-Cx36 (1:1000; Zymed, San Francisco, CA) and mouse anti-PKCα clone MC5 (1:100; Amersham Biosciences, Arlington Heights, IL). The retina was preincubated in a solution containing 10% normal goat serum (NGS), 1% bovine serum albumin (BSA), and 0.5% Triton X-100 in PBS, pH 7.4, for 2 h. The primary antibodies were diluted in 5% NGS, 1% BSA, and 0.5% Triton X-100 in PBS and applied for 3-5 d at 4°C. After washes in PBS, secondary antibodies dissolved in 2% NGS and 0.3% Triton X-100 in PB were applied for 2 h at room temperature. Secondary antibodies were conjugated to either Alexa 488, Alexa 594 (Molecular Probes, Eugene, OR), or Cy5 (Jackson ImmunoResearch, West Grove, PA).
Dissociation of the retina. The methods for dissociation of the retina are based on those described previously (Jakobs et al., 2003). In brief, a piece of retina from the 357 mouse was incubated in 2 ml of HBSS containing 0.5 mg/ml papain (Worthington, Lakewood, NJ) for 20 min at 37°C. The reaction was then quenched using 2 ml of minimal essential medium containing 5% horse serum and 200 U/ml DNAseI (Sigma). Dissociated cells were obtained by trituration with a heat-polished Pasteur pipette, collected by centrifugation, and resuspended in Ringer's solution with 0.5% BSA at a density of ∼ 25,000 cells/ml. The cell suspension was deposited on glass pretreated with poly-l-lysine (Sigma). For immunocytochemistry, the cells were washed in PB and fixed in 4% PFA; unfixed cells were used for reverse transcription (RT)-PCR.
Single-cell RT-PCR. The isolation of single bipolar cells from the cell suspension was done on an Axiovert 200 microscope (Zeiss). The GFP-positive bipolars were identified, photographed with a CCD camera (Roper Scientific, Trenton, NJ), aspirated into a microcapillary, and washed in Ringer's solution. Then they were repicked with a fresh microcapillary and transferred into a thin-wall PCR tube. A negative control (50-100 nl of washing medium) was taken for every cell. Whole-retina mRNA served as a positive control. All controls were processed in parallel to the samples.
The single-cell RT-PCR was done in two steps. RT and the first-round PCR was done using the Access PCR kit (Promega, Madison, WI) in the presence of 2 μm primers for Cx36, β-actin as a positive internal control, and rodopsin as a negative internal control. In addition, some samples were assayed for Cx36 and Cx45 simultaneously. The reaction conditions were as follows: 45 min at 48°C for RT and 19 cycles for 1 min at 94°C, 1 min at 60°C, 2 min at 68°C, followed by a final extension step at 68°C for 7 min. The second PCR (31 cycles) was done for each PCR product separately. The reaction products of the second PCR were run on a 2% agarose gel, photographed, eluted from the gel using the Qiaex II kit (Qiagen, Hilden, Germany), and subjected to confirmatory restriction enzyme digestion with XbaI. The primer sequences and lengths of expected amplicons were as follows: Cx36, ccagtaaggagacagaacca (forward), ctgccgaaattgggaacact (reverse), 457 bp, XbaI site at 255; rod-opsin, tacacactcaagcctgaggt (forward), cctggtgggtgaagatgtag (reverse), 265 bp. Two pairs of nested primers were used to amplify Cx45. The sequences of the outer primers were as follows: Cx45, agattgcctacaagcaaaaca (forward 1), gtacatacaaaaactgtccaaca (reverse 1), catcaccaaaacaacccccatg (forward 2), gttcttctctggatctggaagac (reverse 2). The length of the expected final PCR product is 380 bp. The primer sequences for the 255 bp β-actin fragment were described by Paarmann et al. (2000). The primer pairs for β-actin and rod-opsin spanned at least one exon/intron boundary; the primers for Cx36 and Cx45 anneal on the same exon. Therefore, control cells were assayed omitting the RT step or after pretreatment with RNAseA and run in parallel with the samples. Under these conditions, we never observed any bands, showing the RNA dependency of the amplification.
Confocal imaging and image analysis. A Bio-Rad (Hercules, CA) Radiance confocal microscope equipped with a krypton-argon laser, Zeiss Plan Apochromat 25-/0.8 W, and C-Apochromat 40-/1.2 W and 63-/1.2 W lenses were used. Image acquisition was performed sequentially for all channels to rule out cross talk between red, green, and far-red channels.
To quantify the extent of overlap between the axon terminals of the 357 bipolars and the Cx36-immunoreactive puncta, z-stack images from flat-mount retinas were taken. The judgment of x-y-axis of images was based on direct observation. For each region, the Cx36-immunoreactive puncta (red) that were completely surrounded in the X-Y dimensions by a GFP-labeled axon terminal (green) were targeted. For this region (∼20 μm2), the pixel fluorescence intensity in each confocal image of the z-stacks was measured, using the stack arithmetic function of Metaphorph (Universal Imaging Corporation, West Chester, PA), and plotted as a function of z-axis depth for red and green channels.
Results
The major goal in the present study was to address the question of whether or not any ON cone bipolar cells express Cx36, which would make possible homotypic gap junctions with AII amacrine cells. To achieve this goal, we took advantage of availability of a transgenic mouse in which one type of ON cone bipolars (357 bipolar cells) express a high level of GFP (Huang et al., 2003), to perform investigation of Cx36 expression in the axon terminals of this type of ON bipolar.
A polyclonal rabbit anti-Cx36 antibody, the specificity of which had been tested in previous studies (Mills et al., 2001), was used to label Cx36. GFP-expressing 357 bipolars (green, arrowheads) have axons terminating in the inner part (sublamina b) of the IPL, where dense Cx36-immunoreactive puncta (red) appeared (Fig. 1A). In these single-section images, faint labeling of rod bipolars is also observed (Fig. 1A, arrows) but is easily distinguished from cone bipolars. In sections, Cx36-immunoreactive puncta were found to colocalize with axons of the 357 bipolars (Fig. 1A,B, yellow). In flat mounts, strong colocalization was seen along axons of the 357 bipolars (Fig. 1C,D, yellow). We performed a quantitative test to check the correspondence of axons and Cx36 puncta along the z-axis. Examples were selected in which a punctum and axon appeared to be completely colocalized (i.e., a yellow punctum was entirely surrounded in the X-Y dimensions by axonal fluorescence). Fluorescence at that X-Y position was then analyzed quantitatively in the Z dimension. A representative example is shown in Figure 1E, in which the distributions of pixel fluorescence intensity of a Cx36-immunoreactive punctum and the surrounding 357 bipolar terminals almost overlap. Based on this finding, we conclude that Cx36 colocalize with the axons of the 357 bipolars.
Figure 1.
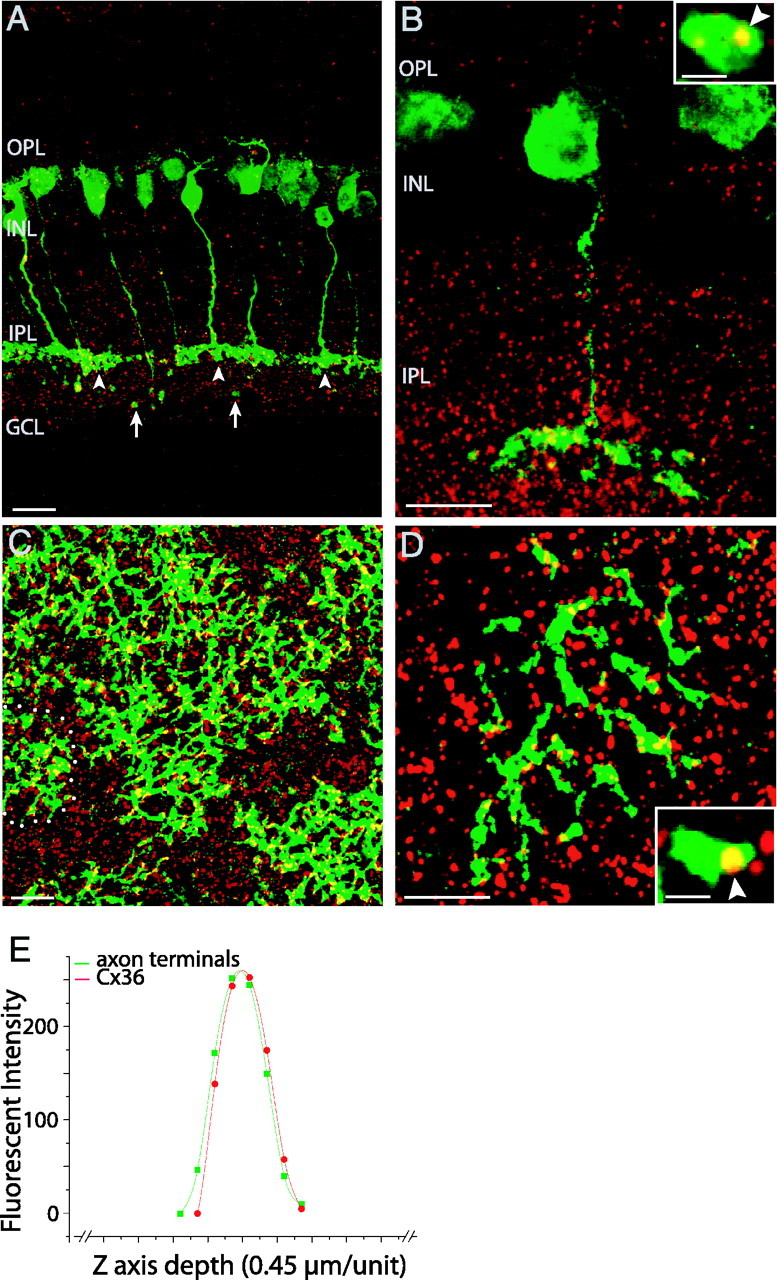
Expression of Cx36 in 357 bipolars of retinas. Strong colocalization (yellow) of Cx36-immunoreactive puncta (red) with axon terminals of the GFP-positive 357 bipolars (green, arrowheads; arrows point to faint labeling of rod bipolars) are shown in low-power (A) and high-power (B, arrowhead in inset) images of vertical sections as well as in low-power (C) and high-power (D, arrowhead in inset) images of flat-mount retinas. Note the low and irregular coverage of the retina by the 357 bipolars. One 357 bipolar (circle) has no overlap with any others, and many overlap on only one side. Scale bars: 20 μm; insets, 2 μm. E, Graph of pixel fluorescence intensity distributions of one Cx36-immunoreactive punctum and 357 bipolars along the z-axis of images as a function of the z-axis depth. OPL, Outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
In these images, Cx36 could belong to AII amacrines or ON cone bipolars, and the density of puncta was so high that it would be difficult to distinguish these colocalizations from chance correspondences. To separate the possibilities, we next labeled isolated individual 357 bipolars with the Cx36 antibody. In a total of 35 isolated 357 bipolars, 11 cells were found to have at least one Cx36-immunoreactive punctum in their remaining axonal terminals. This is most likely an underestimate of true frequency, because of the damage that occurs during dissociation. Figure 2 shows two examples of the isolated 357 bipolars (green) together with Cx36-immunoreactive puncta (Fig. 2A,C, red) and their differential interference contrast (DIC) images (Fig. 2B,D). The 357 bipolars preserved parts of their axon terminals after isolation, and their axonal terminals colocalize with Cx36-immunoreactive puncta (Fig. 2A,C, yellow). Consistent with previous studies (Feigenspan et al., 2001; Mills et al., 2001), isolated rod bipolar cells (n = 20), labeled with PKCα, did not colocalize with Cx36-immunoreactive puncta. Two examples and their DIC images are shown in Figure 2E-H.
Figure 2.
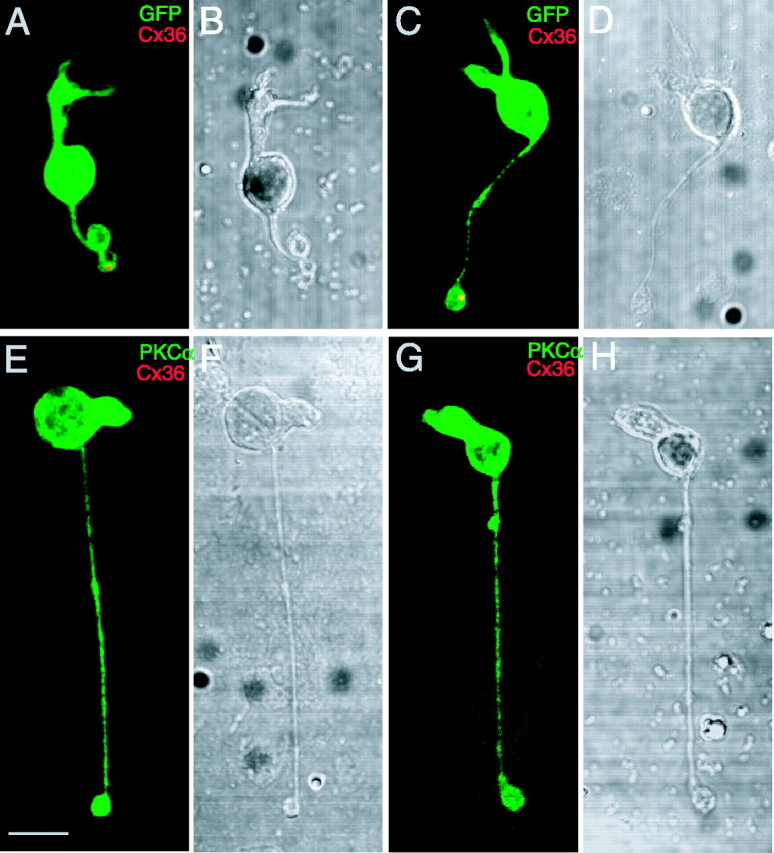
Expression of Cx36 in isolated 357 bipolars, not in isolated rod bipolars. A-D, Two isolated 357 bipolars labeled with the Cx36 antibody. A, C, Axon terminals of the cells (green) colocalized with Cx36-immunoreactive punctate (red). B, D, DIC images of the two same cone bipolar cells. E-H, Two isolated rod bipolars double labeled with PKCα and Cx36 antibodies. E, G, No Cx36-immunoreactive puncta colocalize with the rod bipolars (green). F, H, DIC images of the two same rod bipolars. Scale bar, 5 μm.
We verified Cx36 expression in the 357 bipolars by single-cell RT-PCR. After careful dissociation with papain, it is still possible to identify rod bipolars and cone bipolars by their characteristic morphology. These characteristics allowed us to clearly identify and collect these cells for RT-PCR analysis (Fig. 3A,B).
Figure 3.
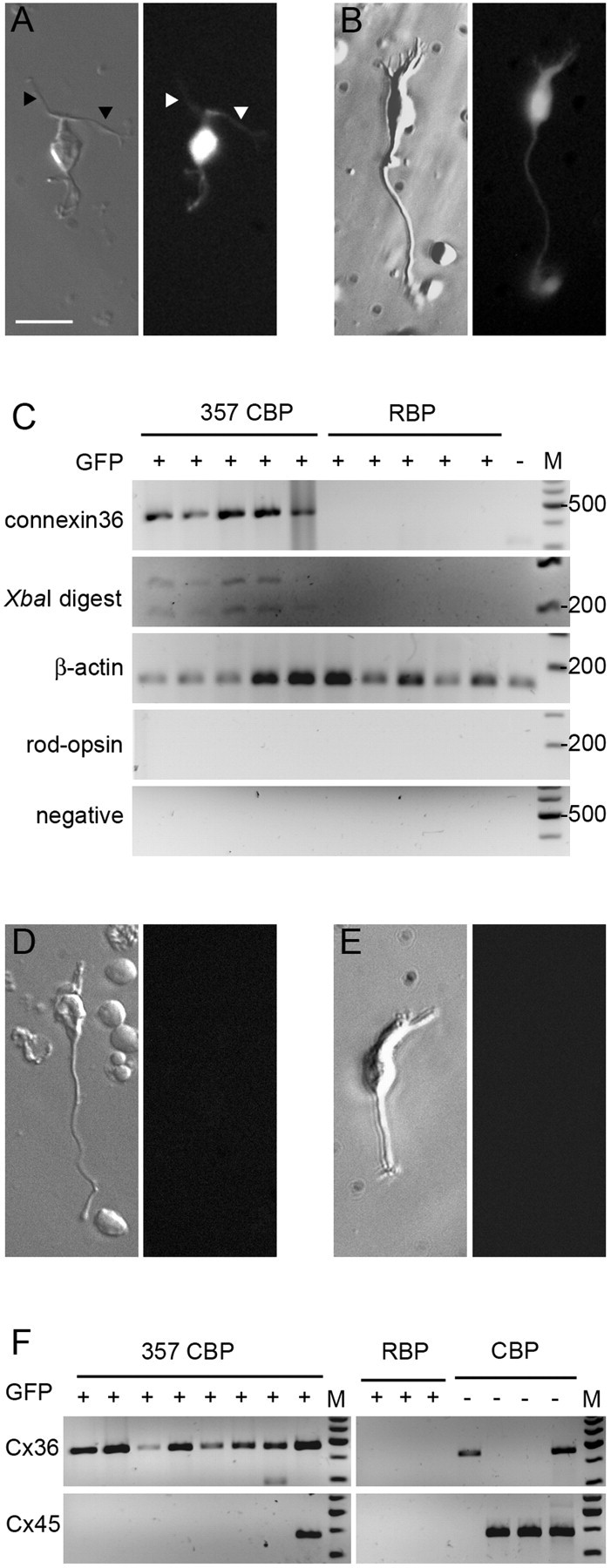
Agarose gel electrophoresis of single-cell RT-PCR products for Cx36 and Cx45 mRNA. A, A typical GFP-expressing 357 bipolar is shown under Hoffman illumination and epifluorescence. The arrowheads indicate the characteristic long, thin dendrites of this cell type. B, A typical rod bipolar cell, also positive for GFP. The long axon and the tuft of dendrites are clearly visible. C, Results of single-cell RT-PCRs from five 357 bipolars, five rod bipolar cells, and one GFP-negative bipolar cell. D, A typical GFP-negative cone bipolar cell that does not express Cx36. E, The only example of a GFP-negative cone bipolar cell in our sample. It was Cx36 positive. F, Simultaneous RT-PCR of Cx36 and Cx45 from different types of bipolars. Scale bar, 20 μm. CBP, Cone bipolar cell; RBP, rod bipolar cell.
RT-PCR is vulnerable to false-negative and false-positive results because of cell loss during transfer and carryover of contaminating mRNA or cell debris, respectively. Therefore, every individual bipolar cell chosen for analysis was aspirated, expelled into fresh medium, repicked, and expelled into the PCR tube. Primers for positive (β-actin) and negative (rod-opsin) internal controls were always included, and negative controls were taken from the washing medium.
Five 357 bipolars were assayed for Cx36 expression. Five rod bipolars and one GFP-negative bipolar were also analyzed as controls. We found Cx36 expression in all 357 bipolars but in none of the other cells (Fig. 3C). We analyzed six additional GFP-negative bipolars. One GFP-negative cone bipolar expressed Cx36, and others were negative. Two typical cells are shown in Fig. 3, D and E.
We wanted to see whether 357 bipolars expressed Cx45 in addition to Cx36. Eight additional 357 bipolars, three rod bipolars, and four GFP-negative cone bipolars were assayed for both connexins simultaneously. All of the 357 bipolars expressed Cx36. One 357 bipolar was found to be also positive for Cx45. Although this could, in principle, indicate heterogeneity of 357 bipolars with respect to Cx45 expression, the more likely explanation is that we inadvertently collected more than one cell in this one case. In agreement with previous findings (Deans et al., 2002; Maxeiner et al., 2005), rod bipolars were negative for both connexins. Of the four GFP-negative cone bipolars, assayed under identical conditions, three were found positive for Cx45 (Fig. 3F). Together, this indicates that rod bipolars express neither Cx36 nor Cx45 and that the situation is more complex for cone bipolar cells: 357 bipolars are Cx36 positive and Cx45 negative, whereas Cx45 was clearly detectable in some other cone bipolars, in accordance with the results of Maxeiner et al. (2005). A more detailed fractionation of the other cone bipolars (i.e., a correlation of their morphology with connexin expression) was not undertaken.
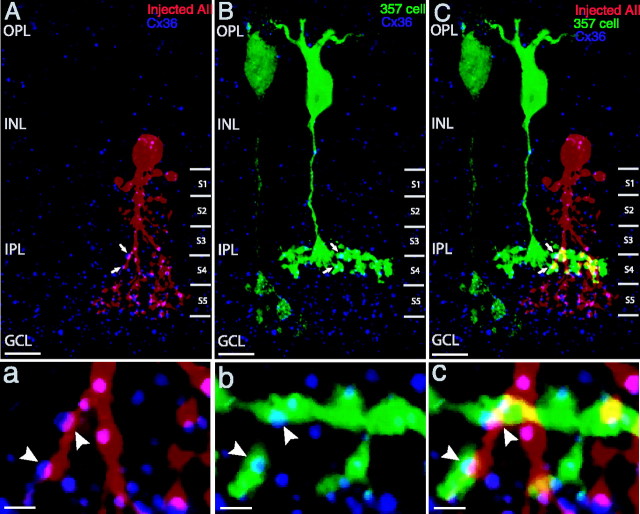
Finally, we microinjected individual AII amacrine cells in retinal slices of the 357 mice, using sharp microelectrodes filled with Alexa Fluor 594. AII amacrine cells that were close to a GFP-positive 357 cell were chosen for microinjection. Figure 4 shows one sample of injected AII amacrine cells (red) that has characteristic lobular appendages in sublamina a of the IPL and fine dendrites in the sublamina b, together with a GFP-positive 357 cell (green) and Cx36 immunoreactivity (blue) in a slice. Consistent with the previous studies, Cx36 immunoreactivity is associated with the dendrites of the AII amacrine cell (Fig. 4A, arrows), and Cx36 puncta appear in the dendrites of the AII amacrine cell (Fig. 4A, arrows) and with the axonal terminals of the 357 bipolar (Fig. 4B, arrows). High-magnification views of single optical sections demonstrate Cx36 puncta colocalized with both the AII amacrine cell dendrites (Fig. 4a, arrowheads) and the axonal terminals of the 357 bipolar (Fig. 4b, arrowheads) at their sites of contact (Fig. 4c, arrowheads). Because of limits to the resolution of light microscopy, it is not possible unequivocally to assign these puncta to one cell or the other: because the 357 bipolars and AII both express Cx36, they are most likely homotypic Cx36-Cx36 junctions, but they could, in principle, be junctions that contain Cx36 and some other hemichannel.
Figure 4.
Cx36 appears to be present at contacts of a 357 bipolar with AII amacrine cells. A-C, Confocal projected images. Cx36 (blue) is seen in the dendrites of an injected AII amacrine cell (red, A) and in the axonal terminals of a 357 bipolar (green, B). A, B, Some of the Cx36-immunoreactive puncta colocalize with both (arrows). C, The merged image of A and B demonstrates that the Cx36 puncta appear at contacts of the 357 bipolar with AII amacrine cell (arrows). a-c, High-magnification views of single optical sections show Cx36 puncta occur at the gap junctions of the 357 bipolar with AII amacrine cell (arrowheads; c) and colocalize with dendrites (arrowheads; a) and axonal terminals (arrowheads; b). Scale bars: A-C, 10 μm; a-c, 2 μm. OPL, Outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Discussion
All of our results indicate that the 357 ON cone bipolar cells in the mouse retina express Cx36. It is conceivable but unlikely that the 357 bipolar cells make Cx36-Cx36 junctions with each other. The sparse mosaic of these bipolar cells, like many others, creates a coverage factor of less than unity (Mills and Massey, 1992; Milam et al., 1993; Massey and Mills, 1996; Brown and Masland, 1999; Chan et al., 2001). A consequence is that most 357 bipolars have slight axonal overlap with another 357 bipolar; indeed, some cells have no overlap at all and thus cannot make gap junctions with another 357 bipolar.
Instead, it seems likely that the type 357 bipolar makes a Cx36-Cx36 junction with the AII amacrine cell. AII amacrine cells are known to express Cx36, type 357 bipolars are now shown to express Cx36, and type 357 bipolars contact AII at a junction at which Cx36 is expressed by at least one of the partners (Fig. 4). Although one can perhaps imagine a scenario in which another type of junction could exist, the most straightforward inference is that the junction is homotypic.
A recent study (Maxeiner et al., 2005) has shown that some ON bipolars express Cx45 but that these are only a subset of the total population of ON bipolars. Furthermore, their results show that glycine diffuses from AII amacrine cells into bipolar cells in a Cx45(-/-) mouse [Maxeiner et al. (2005), their Fig. 7B]. Tissue staining by Deans et al. (2002) demonstrated that some ON cone bipolars, but not all of them, express Cx36 (their Figs. 2, 3). There is no contradiction between these two previous studies, nor do either conflict with the present results: certain type of ON cone bipolar cells appear to express Cx36 at their gap junction with AII amacrine cells, whereas others express Cx45.
The intriguing question raised by this finding is why it would be desirable for different types of ON cone bipolars to use different gap-junctional proteins. A possibility is that the reason has to do with the physiological parallelism of bipolar cell channels. The various types of bipolar cell are believed to have different physiological properties. Similarly, gap junctions that are assembled from different connexins function differently in gating, single-channel conductance, and permeability (for review, see Bennett and Zukin, 2004). Assuming, as seems likely, that most types of ON cone bipolar cells form gap junctions with the AII amacrine cell, a current possibility is that some communicate via Cx36-Cx36 junctions and some via Cx36-Cx45 junctions. Perhaps the two types of gap junction have physiological or regulatory properties (Mills and Massey, 1995, 2000) matched to the specific functions of particular types of bipolar cells.
Footnotes
R.H.M. is a Senior Investigator of Research to Prevent Blindness.
Correspondence should be addressed to Dr. Bin Lin, Massachusetts General Hospital, 50 Blossom Street, Wellman 429, Boston, MA 02114. E-mail: blin@helix.mgh.harvard.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/256696-06$15.00/0
References
- Bennett M, Zukin R (2004) Electrical coupling and neuronal synchronization in the mammalian brain. Neuron 41: 495-511. [DOI] [PubMed] [Google Scholar]
- Brown SP, Masland RH (1999) Costratification of a population of bipolar cells with the direction selective circuitry of the rabbit retina. J Comp Neurol 408: 97-106. [PubMed] [Google Scholar]
- Chan TL, Martin PR, Grünert U (2001) Immunocytochemical identification and analysis of the diffuse bipolar cell type DB6 in macaque monkey retina. Eur J Neurosci 13: 829-832. [DOI] [PubMed] [Google Scholar]
- Deans M, Volgyi B, Goodenough D, Bloomfield S, Paul D (2002) Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron 36: 703-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti EV, Kolb H (1975) A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res 84: 293-300. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Teubner B, Willecke K, Weiler R (2001) Expression of neuronal connexin36 in AII amacrine cells of the mammalian retina. J Neurosci 21: 230-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Janssen-Bienhold U, Hormuzdi S, Monyer H, Degen J, Sohl G, Willecke K, Ammermuller J, Weiler R (2004) Expression of connexin36 in cone pedicles and OFF-cone bipolar cells of the mouse retina. J Neurosci 24: 3325-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Bujan S, Haverkamp S, Feigenspan A, Wassle H (2004) Types of bipolar cells in the mouse retina. J Comp Neurol 469: 70-82. [DOI] [PubMed] [Google Scholar]
- Güldenagel M, Ammermuller J, Feigenspan A, Teubner B, Degen J, Sohl G, Willecke K, Weiler R (2001) Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci 21: 6036-6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF (1999) Gγ13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci 2: 1055-1062. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T (2003) The G protein subunit Gγ13 is co-expressed with Gαo and Gβ3 in retinal On bipolar cells. J Comp Neurol 455: 1-10. [DOI] [PubMed] [Google Scholar]
- Jakobs T, Ben Y, Masland R (2003) CD15 immunoreactive amacrine cells in the mouse retina. J Comp Neurol 465: 361-371. [DOI] [PubMed] [Google Scholar]
- Kolb H, Famiglietti EV (1974) Rod and cone pathways in the inner plexiform layer of the cat retina. Science 186: 47-49. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL (1996) A calbindin-immunoreactive cone bipolar cell type in the rabbit retina. J Comp Neurol 366: 15-33. [DOI] [PubMed] [Google Scholar]
- Massey SC, Mills SL (1999) Gap junctions between AII amacrine cells and calbindin-positive bipolar cells in the rabbit retina. Vis Neurosci 16: 1181-1189. [DOI] [PubMed] [Google Scholar]
- Maxeiner S, Dedek K, Janssen-Bienhold U, Ammermuller J, Brune H, Kirsch T, Pieper M, Degen J, Kruger O, Willecke K, Weiler R (2005) Deletion of connexin45 in mouse retinal neurons disrupts the rod/cone signaling pathway between AII amacrine and ON cone bipolar cells and leads to impaired visual transmission. J Neurosci 25: 566-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam AH, Dacey DM, Dizhoor AM (1993) Recoverin immunoreactivity in mammalian cone bipolar cells. Vis Neurosci 10: 1-12. [DOI] [PubMed] [Google Scholar]
- Mills S, Massey S (2000) A series of biotinylated tracers distinguishes three types of gap junction in retina. J Neurosci 20: 8629-8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC (1992) Morphology of bipolar cells labeled by DAPI in the rabbit retina. J Comp Neurol 321: 133-149. [DOI] [PubMed] [Google Scholar]
- Mills SL, Massey SC (1995) Differential properties of two gap junctional pathways made by AII amacrine cells. Nature 377: 734-737. [DOI] [PubMed] [Google Scholar]
- Mills SL, O'Brien JJ, Li W, O'Brien J, Massey SC (2001) Rod pathways in the mammalian retina use connexin 36. J Comp Neurol 436: 336-350. [PMC free article] [PubMed] [Google Scholar]
- Paarmann I, Frermann D, Keller B, Hollmann M (2000) Expression of 15 glutamate receptor subunits and various splice variants in tissue slices and single neurons of brainstem nuclei and potential functional implications. J Neurochem 1335-1345. [DOI] [PubMed]
- Pignatelli V, Strettoi E (2004) Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol 476: 254-266. [DOI] [PubMed] [Google Scholar]
- Sterling P (1998) Retina. In: The synaptic organization of the brain (Shepherd GM, ed), pp 205-253. New York: Oxford UP.
- Strettoi E, Raviola E, Dacheux RF (1992) Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol 325: 152-168. [DOI] [PubMed] [Google Scholar]
- Strettoi E, Dacheux R, Raviola E (1994) Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. J Comp Neurol 347: 139-149. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB (1991) Functional architecture of the mammalian retina. Physiol Rev 71: 447-480. [DOI] [PubMed] [Google Scholar]