Abstract
The basis for the consolidation of memory is a controversial topic, particularly in the case of motor memory. One view is that motor memory is transferred, partially or completely, to a new location during the consolidation process (“systems consolidation”). We investigated this possibility in a primitive motor system, the vestibulo-ocular reflex (VOR). In the simple circuitry of the VOR, there are relatively few possible storage sites for memory. We partially blocked excitatory neurotransmission in the cerebellar cortex of cats with the glutamate antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). If CNQX was injected immediately after 60 min of rotation under conditions that induced a learned decrease in the gain of the VOR, gain was returned to its baseline value. Expression of the new memory could also be disrupted by rotation in darkness, suggesting that consolidation had not taken place; however, after learning had continued for 3 d, expression of the learned change was diminished only slightly by blockade and was unaffected by rotation in darkness. Our interpretation of these results is that learning may take place initially in the cerebellar cortex and that during consolidation, motor memories are converted to a more distributed representation that includes the cerebellar cortex and another site.
Keywords: memory consolidation, cerebellum, motor learning, motor systems, vestibular, oculomotor
Introduction
Motor learning ensures that movements can be performed accurately. In some systems, motor memory clearly becomes less labile over time (Miles and Eighmy, 1980; Scavio et al., 1992; Shadmehr and Holcomb, 1997; Attwell et al., 2002; Kuki et al., 2004); however, the process underlying memory consolidation in motor systems is not completely understood (Attwell et al., 2002; Christian and Thompson 2003; Doyon et al., 2003). In some cases, consolidation may involve shifts in memory location (“systems consolidation”) (Shadmehr and Holcomb, 1997; Medina et al., 2002), but the existence of many possible storage sites has impeded an understanding of this issue. We report a change, coinciding with consolidation, in the role of a specific brain area in the expression of a motor memory, supporting a systems-consolidation view.
The vestibulo-ocular reflex (VOR) uses sensory input from the vestibular labyrinth to move the eyes in a direction opposite to the head, stabilizing gaze during head movements. Circuitry for the VOR is shown in Figure 1. The gain of the VOR is the ratio of the eye speed produced by the reflex to the head speed that evokes it. Under normal visual conditions, perfect gaze stabilization would require a gain of 1.0. When vision is chronically magnified or miniaturized with telescopic spectacles (Miles and Eighmy, 1980), motor learning brings about reversible long-term changes in VOR gain. Although the reflex itself does not require vision, learning is thought to require visual error signals (Ito, 1972; Robinson, 1976).
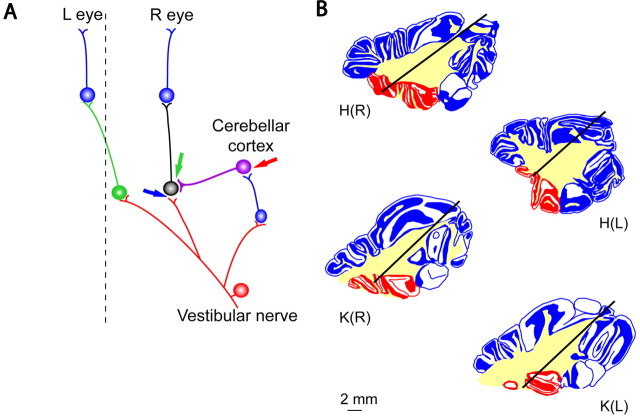
Figure 1.
A, Circuitry for the horizontal rotatory VOR. Primary afferents in the vestibular nerve (red) provide a head velocity signal to excitatory (green) and inhibitory (black) secondary vestibular neurons in the vestibular nuclei, which in turn project to motoneurons (blue). The axon of the excitatory interneuron crosses the midline (dashed line) to the contralateral side. An “inhibitory side loop,” including granule cells (blue) and PCs (violet) of the flocculus, modulates the VOR. The PC output signal is inhibitory. The arrows indicate putative memory sites (see text for details). L, Left; R, right. B, Examples of injection sites. Parasagittal sections through the flocculus (red) and adjacent lobules (blue) are shown. Yellow indicates the extent of the white matter. The cannula tracks are illustrated for the right and left flocculi of cats H and K. Rostral is leftward.
The cerebellar flocculus is necessary for learning (Ito et al., 1974; Robinson, 1976; Rambold et al., 2002), but its exact role is unclear. A popular view is that VOR motor memories are stored at two sites: the parallel fiber–Purkinje cell synapses in the cerebellar cortex (see Fig. 1, red arrow) and the vestibular (noncerebellar) synaptic inputs to VOR interneurons in the brainstem (blue arrow) (Lisberger, 1994; du Lac et al., 1995). After days or weeks of wearing spectacles, the effects of inactivation or removal of the flocculus are consistent with a representation of memory that is distributed between the two sites (Luebke and Robinson, 1994; Pastor et al., 1994; Partsalis et al., 1995). Some results, however, are inconsistent with the distributed-memory model. Floccular inactivation completely abolished motor memory in two studies (McElligott et al., 1998; Nagao and Kitazawa, 2003) in which learning had continued for only 2–3 h, and inactivation of protein kinase C in Purkinje cells (PCs) showed that without cerebellar long-term depression, learning fails to occur (de Zeeuw et al., 1998). The apparent contradiction can be resolved if motor memory is stored at different loci early and late in the learning process, as some have speculated (Galiana, 1986; Peterson et al., 1991; Raymond et al., 1996). To test this hypothesis, we used the glutamate antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block excitatory neurotransmission in the flocculus of cats at different times after learning.
Materials and Methods
Data from five alert male cats (12–24 months of age) are presented. The behavioral disruption of memory was tested in cats J, K, L, and N, and glutamate receptors were blocked in cats H, K, and N. CNQX injections from five additional cats were omitted because of inaccurate injection placement (one cat) or cerebellar damage (cat J) on at least one side, or because insufficient data were obtained (three cats). Behavioral disruption data were obtained from cat J before the lesion occurred and are included. Animal care guidelines of the Canadian Council of Animal Care were followed throughout.
General methods. Our methods for eye movement recordings and the implantation of head holders have been described previously (Broussard et al., 1999). We measured the gain of the VOR during rotation at 0.2, 0.5, or 2 Hz in complete darkness. Eye velocity was plotted against head velocity for an average of at least 30 cycles of rotation, and VOR gain was defined as the slope of the best linear fit to the data. Gains were normalized across cats to eliminate the consistent differences that we observed between individual cats and to allow comparisons across individuals with respect to relative changes in gain. The non-normalized average baseline gains ranged from 0.73 to 1.01 in different cats.
For VOR cancellation, a black-and-white-patterned screen, covering 180° of the cat's visual field at a 35 cm distance, was fixed to the turntable and illuminated. The percentage of cancellation was calculated as follows: C = Gvor – Gcanc/Gvor, where C is the percentage of cancellation, Gvor is the VOR gain in darkness, and Gcanc is the VOR gain during the cancellation protocol. C is identical to the “cancellation gain” (Zee et al., 1981). The values of C are plotted in Figure 2, F and G. After a few practice sessions, all cats were able to cancel between 60 and 95% of their VOR. We used the cancellation protocol to monitor the efficacy of all CNQX injections.
Figure 2.
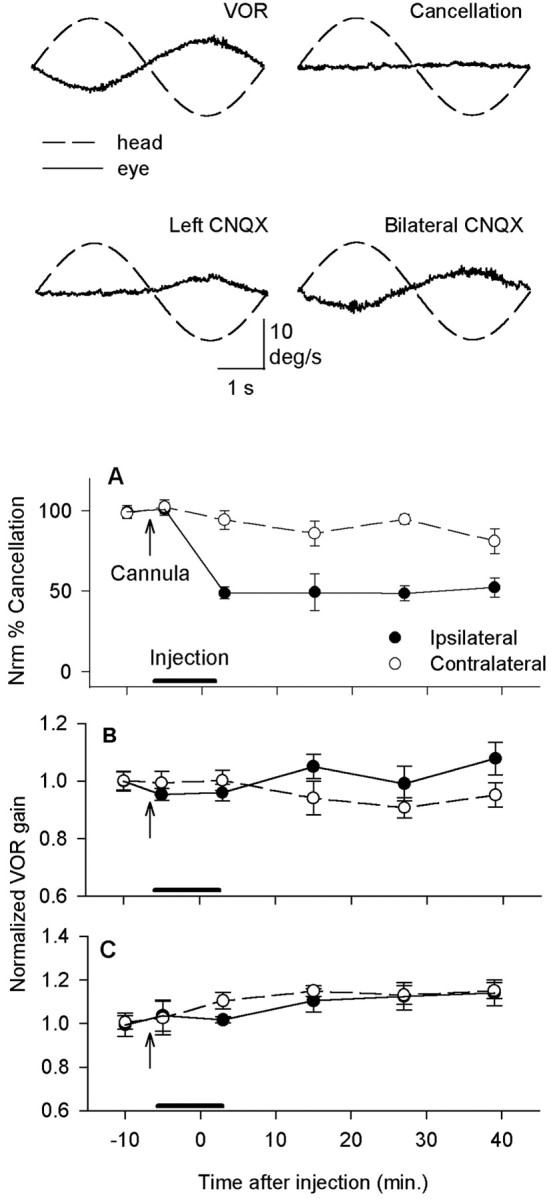
CNQX injections into the flocculus had a powerful effect on VOR cancellation but did not consistently affect the VOR in darkness. Top traces, Before the injection, eye velocity was opposite to head velocity during the VOR at 0.2 Hz but remained near zero during VOR cancellation. After CNQX was injected into the left flocculus, the VOR was not cancelled for leftward rotation. After bilateral CNQX injections, the VOR was not cancelled for either direction. A, When CNQX was injected unilaterally, cancellation, which was normalized to a 100% initial value for each cat, decreased immediately to 50% for ipsilateral rotation. There was no significant effect during contralateral rotation (n = 9; pooled data from cats K, H, and N). B, When unilateral injections were made, there was no effect on the VOR at 0.2 Hz 3 min after the injection (n = 9). An asymmetry appeared over time, with a higher gain for ipsilateral half-cycles. C, At 2 Hz, VOR gain increased slightly for contralateral rotation at 3 min after the injection (n = 5; pooled data from cats K and N). At later times, gain was increased for both directions. Neither effect was statistically significant. In this and all figures, VOR gain was normalized to an initial value of 1.0 for each cat because of individual differences in the baseline gains (see Materials and Methods for details).
Short-term protocol. Because gain increases tend to be small and unreliable in cats, we focused on the gain decreases induced by miniaturizing vision. In the short-term experiments, learning was induced by rotation in the light for 60 min while the room was viewed through 0.25× miniaturizing telescopes (Designs for Vision, Ronkonkoma, NY). Opaque frames around the telescopes blocked peripheral vision, and the assembly was attached to a head holder. Angular velocity was a sum-of-sines that alternated several times per minute between two waveforms having three components each: either 0.2, 2.0, and 10 Hz or 0.1, 1, and 5 Hz, with a peak velocity of 5°/s for each component.
We measured the VOR gain at 2 Hz before and after learning. Because the newly achieved VOR gain was labile, a delay of 20 or 40 min was imposed after the end of the learning period and before the preinjection gain measurement. During the delay, the cat was stationary and viewed a featureless screen while wearing the telescopic spectacles. We then injected either a glutamate antagonist or vehicle alone into both flocculi, over a 20 min period (see below, Long-term protocol). A final gain measurement was made 3 min after the end of the injections. At least 24 h were allowed before the next experiment in this repeated-measures design.
Long-term protocol. In the long-term experiments, spectacles were worn continuously for 72 h, and the cat was rotated passively by means of the sum-of-sines (forced rotation) three times, for 60 min each time, at 24 h intervals (see Fig. 2 E, arrows). Between passive rotations, the cats wore spectacles under normal conditions in the animal facility. VOR gain was measured at 0.2 and 2 Hz. At the end of 72 h, there was no forced rotation. Instead, CNQX was injected into the flocculus bilaterally (see below, Implantation of guides and drug injections), and VOR gain was measured a final time. At least 72 h without spectacles were allowed for the return to normal gain before the experiment was repeated.
In a separate set of experiments, we tested for lability of memory by subjecting the cat to the sum-of-sines in total darkness, starting either at the end of the 60 min (short-term) learning protocol (see Fig. 5A–C, G) or at the end of 72 h of spectacle wearing (long-term) (see Fig. 5D–F, H). We measured the VOR gain at 0.5 and 2 Hz at 15 min intervals during the period of dark rotation.
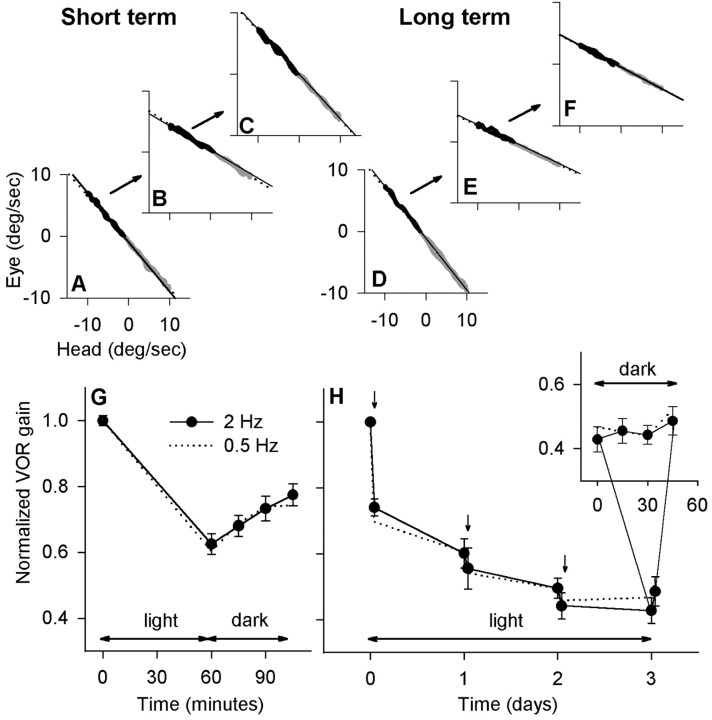
Figure 5.
Rotation in darkness disrupted the learned change in VOR gain after a short learning period but had little effect after 3 d. A–C, Examples of short-term results (cat L). Sixty minutes of learning in the light were followed by 60 min of rotation in darkness. The VOR gain returned to its baseline value. D–F, In the same cat, 60 min of rotation in darkness after 72 h of spectacle wearing had no effect. G, Summary of the short-term experiment. After the end of learning, the VOR gain increased steadily throughout 45 min of rotation in darkness (n = 13; cats J–L). H, Summary of the long-term experiment. Spectacles were worn for 72 h, including forced rotation in the light (arrows). On day 4, the cat was rotated for 45 min in the dark. Inset, Expanded time scale showing the period of rotation in darkness. There was no significant change in VOR gain at either 2 or 0.5 Hz (n = 7; cats L and N).
Implantation of guides and drug injections. We used a guidance system composed of a grid fitted inside a cylindrical recording chamber (FHC, Bowdoinham, ME) to position the injection cannula. With the animals under isoflurane anesthesia, bilateral chambers were implanted over the flocculi at a 40° caudal angle, directed 9 mm lateral and 1.4 mm caudal to ear bar zero. To locate possible injection sites, we mapped each flocculus with biphasic current pulses and trains delivered through a bipolar concentric stimulating electrode (Rhodes, Summerland, CA). When ipsiversive smooth eye movements were evoked, we replaced the electrode with a 24 gauge stainless-steel needle and pressure injected 5–20 μl of 3 mm CNQX disodium salt (Sigma, St. Louis, MO) in PBS. Within this range of injection volumes, the efficacy of the injection was not correlated with the injection volume. The total volume was divided between two injection sites that were 1 mm apart along the same track. Mapping was continued with CNQX until we found a site where the injection reduced VOR cancellation to 50% of normal. An effective injection site was always found within 3 mm of the best microstimulation site. The injection cannula was removed after each injection and repositioned at the same location for subsequent injections in the same cat.
Although some of our CNQX injections were large, there was no evidence of diffusion of an effective concentration to the brainstem. The normal VOR in darkness did not decrease; however, we think that a large area of cerebellar cortex was affected. In a previous study (Attwell et al., 1999), 2 μl of 1.54 mm CNQX diffused across folia in the cerebellar cortex to bind to receptors throughout a region ∼5 mm in diameter. In the present study, we injected much larger volumes near the center of the flocculus. Because the flocculus is 6 mm long in cats, the entire structure may have contained bound CNQX. An adjacent structure, the ventral paraflocculus, also contributes to motor learning and pursuit (Rambold et al., 2002). We therefore expected larger injections to have more pronounced effects by affecting both structures; however, this prediction was not verified.
At the end of experiments on each cat, the same volume of pontamine sky blue or neutral red (2% in PBS) was injected at the same site as the CNQX. The brains were then processed for histology using frozen sections and cresyl violet. In all cases, we confirmed that the cannula track entered the flocculus.
Results
Blockade of glutamatergic inputs to PCs in the cerebellar flocculus did not affect the normal performance of the VOR but did prevent the expression of short-term memory and also reduced the cat's ability to cancel its VOR. If a moving target is tracked with head movements, the VOR must be cancelled so that the gaze is not directed away from the target. Cats can perform this task for rotation at 0.2 Hz. We used the effect of CNQX on cancellation of the VOR to estimate the effectiveness of each injection, because cancellation depends on the same regions of the flocculus and adjacent ventral paraflocculus as motor learning (Rambold et al., 2002). Figure 1B shows examples of locations where 15–60 nmol of CNQX was injected into each flocculus. For unilateral injections, blockade reduced cancellation during ipsilateral rotation with a peak effect of ∼50% reduction in cancellation within 3 min after the injection (Fig. 2A). Contralateral rotation was not affected. We also expected CNQX injections to prevent new learning if floccular function was seriously impaired, and preliminary data were consistent with this prediction.
Floccular blockade had small and inconsistent effects on the normal operation of the VOR, in agreement with the view that modulation of PC discharge does not normally contribute to the VOR (Lisberger and Fuchs, 1978; Luebke and Robinson, 1994; McElligott et al., 1998). On average, VOR gain did not change for rotation at 0.2 Hz after unilateral blockade (Fig. 2B) but did increase over time for 2 Hz rotation (Fig. 2C). The increase was not statistically significant (p > 0.1). A Student's t test for paired variates was used for all comparisons unless noted otherwise. Significant asymmetry appeared over time in the gain of the VOR, but only for rotation at 0.2 Hz (p < 0.01). The relative phase of eye and head was not affected at either frequency. Bilateral injections did not affect VOR gain significantly at either frequency. The absence of any gain decrease for rotation toward the injected side indicated that CNQX did not spread effectively to the VOR interneurons in the brainstem.
In contrast to the lack of effect on the normal VOR, recently learned changes in VOR gain were completely reversed by bilateral CNQX injections (Fig. 3A–D). In these and all figures, averaged eye velocity was plotted as a function of head velocity, and VOR gain was defined as the slope of the best linear fit to the data. Gain was calculated separately for the rightward and leftward half-cycles and then averaged, unless noted otherwise. Figure 3D shows the time course of the short-term experiment. The learning period consisted of 60 min of rotation by means of a sum-of-sines waveform (forced rotation) within a complex, stationary visual scene. During learning, the cat wore 0.25× miniaturizing spectacles. The newly modified VOR gain was highly labile; in preliminary experiments, gain drifted back toward normal throughout the first 60 min after learning, even if no injection was given. A delay of 20 or 40 min between learning and VOR measurement improved the stability of subsequent gain measurements (see Materials and Methods). The VOR gain at 2 Hz, 20 min after the end of learning, was reliably 20–25% below its baseline value.
Figure 3.
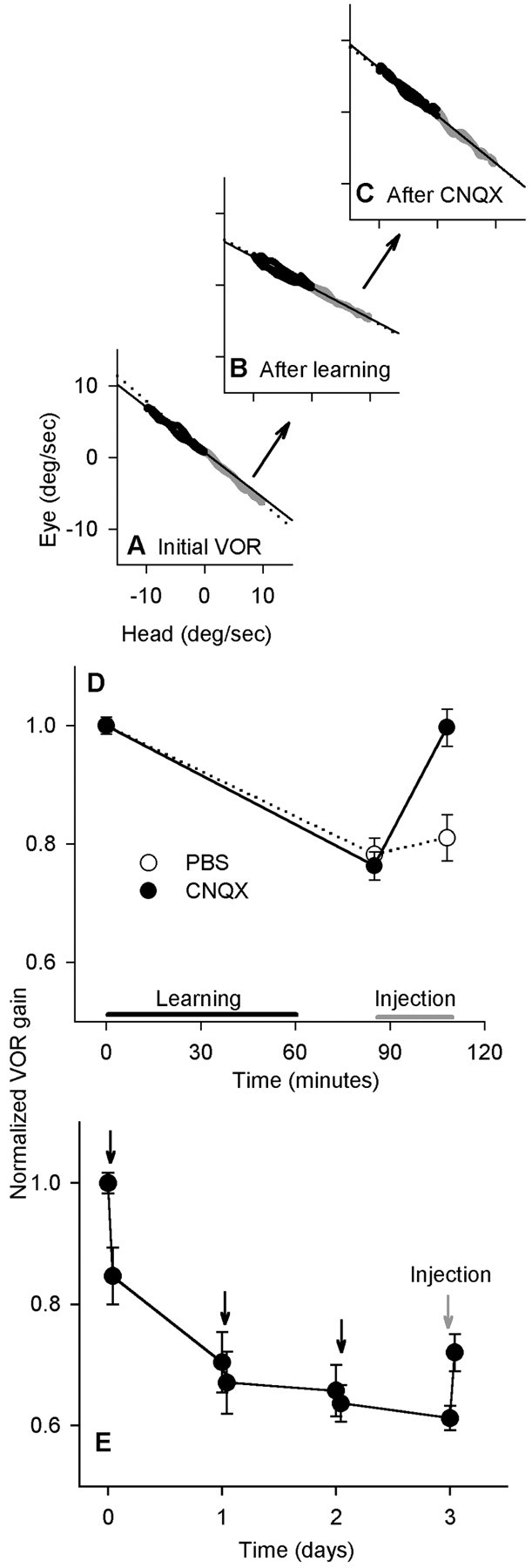
Injections of CNQX into the bilateral flocculi returned VOR gain to normal after the short-term but not the long-term protocol. VOR gain was measured at 2 Hz. A, Eye and head velocity before learning. Leftward (black) and rightward (gray) half-cycles were fit separately. B, After rotation and wearing 0.25× lenses for 60 min, the VOR gain decreased to 67% of baseline. C, After bilateral CNQX injections, the gain increased to 99% of the baseline value. D, Time course of the experiment. VOR gain values were normalized to a mean starting value of 1.0 for each cat; the actual initial gains ranged from 0.62 to 0.82. Sixty minutes of learning (black bar) were followed by injection (graybar) (n = 6 for each group; pooled data from cats H and K). E, In the long-term experiment, spectacles were worn continuously with forced rotation each day (black arrows). The VOR gain was measured at 2 Hz. On day 4, CNQX was injected bilaterally (gray arrow) (n = 6; pooled data from cats H and N).
Bilateral CNQX injections after learning returned the gain to a value that was indistinguishable from baseline (p = 0.38; paired t test; n = 6) (Fig. 3D). PBS vehicle was also injected alone and did not result in a significant change. The difference between the postinjection gains for CNQX and PBS was highly significant (p = 0.004; n = 6).
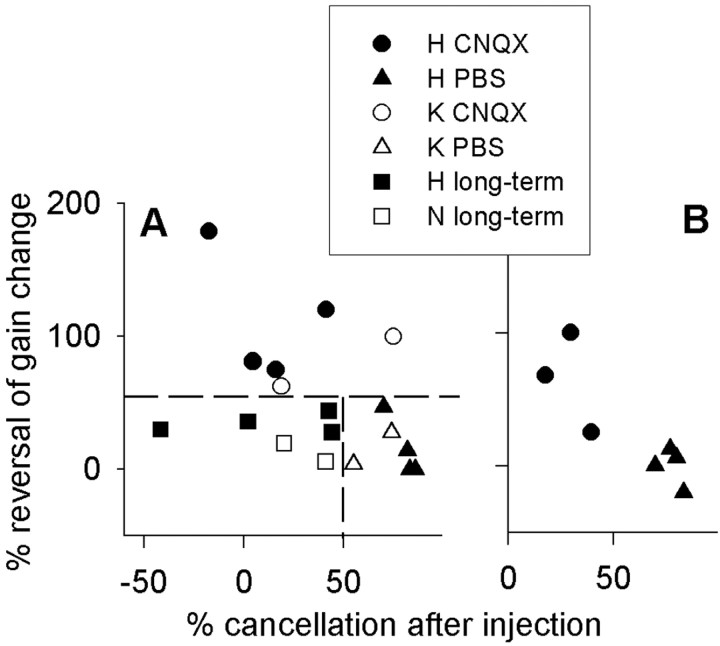
For all of the short-term experiments, the effect of bilateral CNQX injection on VOR cancellation was correlated with the effect on motor learning, represented by the percentage of reversal of the learned change (r = –0.77; n = 18). In Figure 4, the circles indicate CNQX injections, and the triangles indicate PBS injections in the short-term protocol; filled and open symbols are from different cats. Squares represent CNQX injections in the long-term protocol (see following paragraph). Short-term results are shown in Figure 4, A and B, but the delay period was 20 min in A and 40 min in B. There was no overlap of cancellation values between CNQX and PBS injections (Fig. 4A, the horizontal dashed line separates these sets of data). Starting the injection 40 min rather than 20 min after the end of learning did not affect the overall outcome for either blockade of memory or cancellation.
Figure 4.
A, For short-term experiments, the percentage of the learned change that was blocked was correlated with the percentage of cancellation at 0.2 Hz after bilateral injection of CNQX (circles) or PBS alone (triangles). The squares indicate where CNQX was injected for long-term experiments. Filled symbols represent data from c at H, open triangles and circles represent data from cat K, and open squares represent data from cat N. After CNQX injections in the long-term experiments, cancellation was affected but memory was not. Dashed lines emphasize the lack of overlap between conditions. B, Similar results were obtained in short-term experiments if the delay period between learning and injection was 40 min rather than 20 min.
After the learned change in VOR gain was allowed to approach an asymptote, bilateral floccular blockade had a relatively smaller effect on the learned change in gain (Fig. 3E). The 1 h periods of forced rotation during 3 d of continuous spectacle wearing brought the VOR gain near its asymptote for the 2 and 0.2 Hz rotations. On the final day, CNQX was injected bilaterally into the flocculi at the same sites and with the same volume as in the short-term experiment. Immediately after the injections, the VOR gain increased significantly at both rotation frequencies, partially reversing the learned change (for 2 Hz, p < 0.01; n = 6); however, gain remained significantly lower than baseline (p < 0.0001). The effect on VOR cancellation was similar to that in the short-term experiments, but there was no overlap in reversal of the learned gain change (Fig. 4A, vertical dashed line). The VOR was tested at both 2 and 0.2 Hz, with similar results.
After miniaturizing lenses or reversing prisms have been worn for ≥1 week, both visual and vestibular inputs are required to reverse the learned change in gain (Robinson, 1976; Miles and Eighmy, 1980). The return to normal is prevented if either visual or vestibular sensory signals are absent. Motor learning starting from normal gain also requires a combination of either visual and vestibular or visual and oculomotor signals; however, recent results have indicated that vestibular signals alone could reverse part of the learned change (Cohen et al., 2004). Consistent with these results, we found that rotation in darkness induced a rapid reduction in the expression of short-term memory. Sixty minutes of rotation in darkness immediately after 60 min of learning appeared to reverse learning in cat L (Fig. 5A–C). Across three subjects, when VOR gain was measured at 15 min intervals (Fig. 5G), the gain had increased significantly from its postlearning value after only 30 min (p < 0.01; n = 13) and continued to increase at a constant rate throughout rotation. These results indicate that memory was labile in our short-term protocol.
In the long-term protocol, VOR gain approached an asymptote over 72 h of spectacle wearing, including forced rotations (Fig. 5H). As the asymptote is approached, little new learning occurs, and we predicted that the existing memory would consolidate. In the same cat for which short-term data are shown in Figure 5A–C, four 15 min periods of rotation in darkness on day 4 of spectacle wearing failed to cause any change in gain, supporting our prediction (Fig. 5D–F). Across two subjects, 45 min of dark rotation after 72 h failed to change the gain significantly (p = 0.09; n = 7). After the spectacles were removed, VOR gain returned to normal (data not shown). In summary, during the initial phase of VOR motor learning, memory could be disrupted by the vestibular stimulus presented alone and therefore fit the commonly accepted definition of unconsolidated memory (Shadmehr and Holcomb, 1997). After 3 d of spectacle wearing, memory appeared to be consolidated.
Discussion
The VOR circuitry is relatively simple, with components located in the cerebellar cortex and brainstem. Both of these structures are believed to participate in memory storage (Lisberger, 1994; du Lac et al., 1995) and may also participate in the consolidation of motor memory. Systematic investigations of consolidation in this simple system have begun only recently (Broussard and Kassardjian, 2004; Kuki et al., 2004). Here, we present evidence that VOR motor memory consolidates and that this occurs concurrently with a change in the location of memory storage.
Our results showed that blocking AMPA–kainate inputs bilaterally in the floccular cortex powerfully affected the expression of short-term memory for decreases in VOR gain. The direct effect of blockade of glutamate receptors on both PCs and interneurons in our preparation was probably to render them incapable of responding to input from either parallel or climbing fibers. CNQX blocks the AMPA and kainate types of glutamate receptors, which are believed to mediate the excitatory inputs to PC dendrites and to the interneurons that inhibit PCs. Given the observation that PCs and interneurons both fire spontaneously at high rates when their excitatory inputs are blocked in slice preparations (Hausser and Clark 1997; Edgerton and Reinhart, 2003), it is highly unlikely that blocking the same inputs in vivo silenced PCs; however, we cannot rule out increases or decreases in the PC resting rates, which would represent quantitative differences from the situation in the slice. The implications of this are discussed below.
Our observations can be explained if, in the short term, memory is encoded as a change in synaptic transmission or neuronal excitability in the cerebellar cortex (Fig. 1, red arrow). This would result in changes in the discharge patterns of PCs during rotation. CNQX injections, by blocking AMPA transmission, would be expected to disrupt the learned pattern. A cortical locus of short-term memory would be consistent with a large body of previous data (Raymond et al., 1996; Ito, 1972; Sakurai, 1987; McElligott et al., 1998; Nagao and Kitazawa, 2003).
An alternative interpretation of our results is that short-term memory is stored as a modification in the inhibitory connection between PCs and the VOR interneurons in the brainstem (Fig. 1, green arrow). Previous results indicated that long-term motor memory is not stored at any site between the PC and the extraocular muscle (Lisberger, 1994). The possibility of short-term memory storage at such locations has not been investigated; however, to be consistent with the previous data as well as those presented here, this explanation would require memory to be shifted from the PC–interneuron synapse to a long-term, distributed representation in the brainstem and cerebellar cortex that specifically does not include the PC–interneuron synapse. This would not be a parsimonious interpretation.
A third possibility, that short-term memory is stored in the brainstem VOR pathway but also requires a tonic cerebellar output signal representing a set value of VOR gain, would also be consistent with our data. PCs powerfully inhibit VOR interneurons, and changes in tonic rates could affect signal transmission by these interneurons. This hypothesis predicts a tight correlation between VOR gain and resting rates of PCs. A recent study revealed a weak correlation between the resting rates of floccular PCs and VOR gain in the short term (Hirata and Highstein, 2001). At the same time, sensitivities of floccular PCs to vestibular input changed significantly. These results are consistent with changes at input synapses on PCs (Fig. 1, red arrow) as well as with changes at other locations. They do not support the notion that PC resting rate controls VOR gain.
When CNQX was injected bilaterally after 3 d of learning, the blockade of excitatory synapses (as confirmed by its effect on VOR cancellation behavior) had a much smaller effect on the expression of motor memory. This outcome was consistent with the storage of long-term motor memory as modifications at the synapses providing vestibular input to both the floccular cortex and the brainstem (Fig. 1, red and blue arrows) (Lisberger, 1994). Together, our results suggest that the location of the memory for decreases in VOR gain is shifted as it becomes consolidated. A limitation on this interpretation is that mechanistic differences exist between learned increases and decreases in gain, with learned, high-gain states generally more labile than low gain in the long term (Miles and Eighmy, 1980; Boyden and Raymond, 2003; Kuki et al., 2004). We did not study gain increases, which tend to be small in cats; therefore, our results cannot be extrapolated to the robust gain increases that occur in other species.
Changes in storage location during consolidation have been proposed for other memory systems. Time-limited retrograde amnesia is one possible manifestation of a shift in location (Zola-Morgan and Squire, 1990). Experimental evidence supports such shifts in location after motor-skill learning (Shadmehr and Holcomb, 1997), conditioned eye blinks (Kim et al., 1995; Medina et al., 2002), and fear conditioning (Medina et al., 2002); however, memory shifts are not universally accepted (Nadel and Moscovitch, 1997; Doyon et al., 2003). The VOR is a primitive motor system that is conserved across vertebrate classes. Our results suggest that even in this simple system, motor memory may form initially in the cerebellar cortex and become distributed, during consolidation, between two or more sites. One possibility is that short-term learning takes place in the cortex, after which an appropriate error signal is generated by PCs to guide changes in the brainstem during consolidation (Broussard and Kassardjian, 2004). This does not rule out cortical processes such as synaptogenesis, which may also contribute to consolidation. Additional experiments are necessary to determine whether consolidation and the change in memory location are the same process or whether they merely coexist within the same time frame.
Footnotes
This work was supported by the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada. We thank Dr. J. L. Raymond for many helpful conversations and Drs. C. Yeo, D. Tweed, and E. Stanley for insightful comments on a previous version of this manuscript. We thank H. Xiao for technical assistance.
Correspondence should be addressed to Dr. Dianne M. Broussard, MP12-318, Toronto Western Hospital, 399 Bathurst Street, Toronto, Ontario, Canada M5T 2S8. E-mail: dianne@uhnres.utoronto.ca.
DOI:10.1523/JNEUROSCI.2215-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/257979-07$15.00/0
References
- Attwell PJ, Rahman S, Ivarsson M, Yeo CH (1999) Cerebellar cortical AMPA-kainate receptor blockade prevents performance of classically conditioned nictitating membrane responses. J Neurosci 19: RC45(1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell PJE, Cooke SF, Yeo CH (2002) Cerebellar function in consolidation of a motor memory. Neuron 34: 1011–1020. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Raymond JL (2003) Active reversal of motor memories reveals rules governing memory encoding. Neuron 39: 1031–1042. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Kassardjian CD (2004) Learning in a simple motor system. Learn Mem 11: 127–136. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Bhatia JK, Jones GEG (1999) The dynamics of the vestibuloocular reflex after peripheral vestibular damage. I. Frequency-dependent asymmetry. Exp Brain Res 125: 353–364. [DOI] [PubMed] [Google Scholar]
- Christian KM, Thompson RF (2003) Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem 11: 427–455. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Meissner GW, Schafer RJ, Raymond JL (2004) Reversal of motor learning in the vestibulo-ocular reflex in the absence of visual input. Learn Mem 11: 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw CI, Hansel C, Bian F, Koekkoek SKE, van Alphen AM, Linden DJ, Oberdick J (1998) Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20: 495–508. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003) Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41: 252–262. [DOI] [PubMed] [Google Scholar]
- du Lac S, Raymond JL, Sejnowski TJ, Lisberger SG (1995) Learning and memory in the vestibulo-ocular reflex. Annu Rev Neurosci 18: 409–442. [DOI] [PubMed] [Google Scholar]
- Edgerton JR, Reinhart PH (2003) Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol (Lond) 548: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiana HL (1986) A new approach to understanding adaptive visual-vestibular interactions in the central nervous system. J Neurophysiol 55: 349–374. [DOI] [PubMed] [Google Scholar]
- Hausser M, Clark BA (1997) Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron 19: 665–678. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Highstein SM (2001) Acute adaptation of the vestibuloocular reflex: signal processing by floccular and ventral parafloccular Purkinje cells. J Neurophysiol 85: 2267–2288. [DOI] [PubMed] [Google Scholar]
- Ito M (1972) Neural design of the cerebellar motor control system. Brain Res 40: 81–84. [DOI] [PubMed] [Google Scholar]
- Ito M, Shiida T, Yagi N, Yamamoto M (1974) Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res 65: 170–174. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF (1995) Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci 109: 195–203. [DOI] [PubMed] [Google Scholar]
- Kuki Y, Hirata Y, Blazquez PM, Heiney SA, Highstein SM (2004) Memory retention of vestibuloocular reflex motor learning in squirrel monkeys. NeuroReport 15: 1007–1011. [DOI] [PubMed] [Google Scholar]
- Lisberger SG (1994) Neural basis for motor learning in the vestibulo-ocular reflex of primates: III. Computational and behavioral analysis of the sites of learning. J Neurophysiol 72: 974–998. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF (1978) Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol 41: 733–763. [DOI] [PubMed] [Google Scholar]
- Luebke AE, Robinson DA (1994) Gain changes of the cat's vestibulo-ocular reflex after flocculus deactivation. Exp Brain Res 98: 379–390. [DOI] [PubMed] [Google Scholar]
- McElligott JG, Beeton P, Polk J (1998) Effect of cerebellar inactivation by lidocaine microdialysis on the vestibuloocular reflex in goldfish. J Neurophysiol 79: 1286–1294. [DOI] [PubMed] [Google Scholar]
- Medina JF, Repa JC, Mauk MD, LeDoux JE (2002) Parallels between cerebellum- and amygdala-dependent conditioning. Nat Rev Neurosci 3: 122–131. [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB (1980) Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol 43: 1406–1425. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M (1997) Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7: 217–227. [DOI] [PubMed] [Google Scholar]
- Nagao S, Kitazawa H (2003) Effects of reversible shutdown of the monkey flocculus on the retention of adaptation of the horizontal vestibulo-ocular reflex. Neuroscience 118: 563–570. [DOI] [PubMed] [Google Scholar]
- Partsalis AM, Zhang Y, Highstein SM (1995) Dorsal Y group in the squirrel monkey. II. Contribution of the cerebellar flocculus to neuronal responses in normal and adapted animals. J Neurophysiol 73: 632–650. [DOI] [PubMed] [Google Scholar]
- Pastor AM, de la Cruz RR, Baker R (1994) Cerebellar role in adaptation of goldfish vestibuloocular reflex. J Neurophysiol 72: 1383–1394. [DOI] [PubMed] [Google Scholar]
- Peterson BW, Baker JF, Houk JC (1991) A model of adaptive control of vestibuloocular reflex based on properties of cross-axis adaptation. Ann NY Acad Sci 627: 319–337. [DOI] [PubMed] [Google Scholar]
- Rambold H, Churchland A, Selig Y, Jasmin L, Lisberger SG (2002) Partial ablations of the flocculus and ventral paraflocculus in monkeys cause linked deficits in smooth pursuit eye movements and adaptive modification of the VOR. J Neurophysiol 87: 912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD (1996) The cerebellum: a neuronal learning machine? Science 272: 1126–1131. [DOI] [PubMed] [Google Scholar]
- Robinson DA (1976) Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol 39: 954–969. [DOI] [PubMed] [Google Scholar]
- Sakurai M (1987) Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol (Lond) 394: 463–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavio MJ, Clift PS, Wills JC (1992) Posttraining effects of amphetamine, chlorpromazine, ketamine, and scopolamine on the acquisition and extinction of the rabbit's conditioned nictitating membrane response. Behav Neurosci 900–908. [DOI] [PubMed]
- Shadmehr R, Holcomb HH (1997) Neural correlates of motor memory consolidation. Science 277: 821–825. [DOI] [PubMed] [Google Scholar]
- Zee DS, Yamazaki A, Butler PH, Gucer G (1981) Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 46: 878–899. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan SM, Squire LR (1990) The primate hippocampal formation: evidence for a time-limited role in memory storage. Science 250: 288–290. [DOI] [PubMed] [Google Scholar]