Abstract
Keeping in mind the increased pain complaints reported in anxious or depressive patients, our goal was to investigate in rats the consequences of an experimentally provoked state of anxiety/depression on pain behavior and on its underlying mechanisms. We therefore used a model of social defeat consisting of a 30 min protected confrontation followed by a 15 min physical confrontation, repeated during 4 d, that elicited symptoms close to those observed in humans with anxiety or depression. Indeed, 5 d later, animals subjected to social-defeat confrontation were characterized by a decrease of sweet-water consumption and of body weight, and a hyperactivity of the hypothalamic–pituitary–adrenal axis, suggesting that the social-defeat procedure induced a prolonged state of anxiety.
Rats subjected to the social-defeat procedure showed an enhanced nociceptive behavior to the subcutaneous administration of formalin, 5 d after the last confrontation session. Because chronic treatment with the established anxiolytic chlordiazepoxide (10 mg · kg–1 · d–1) prevented hyperalgesia, this strongly suggested that this experimental procedure might be a suitable animal model of “anxiety-induced hyperalgesia.” Hyperalgesia associated with anxiety not only was related to a significant increase of CCKLM [cholecystokinin (CCK)-like material] in frontal cortex microdialysates but also was prevented by a CCK-B receptor antagonist [4-[[2-[[3-(1H-indol-3-yl)-2-methyl-1-oxo-2[[(tricyclo[3.3[12,17]dec-2-yloxy)-carbonyl]amino]-propyl]amino]-1-phenyethyl]amino]-4-oxo-[R-(R*, R*)]-butanoate N-methyl-d-glucamine (CI-988)] (2 mg/kg), strongly supporting the involvement of central CCKergic systems in these phenomena. Finally, combined treatments with CI-988 and morphine completely suppressed pain-related behavior, supporting the idea that the association of both compounds might represent a new therapeutic approach to reduce the increase of pain complaints highly prevalent among anxious or depressive patients.
Keywords: hyperalgesia, CCK, chronic social defeat, formalin, in vivo microdialysis, frontal cortex
Introduction
Over the past 20 years, there has been growing interest in the relationship between persistent pain and psychiatric pathologies. In humans, anxiety or depression is often believed to influence the perception of pain. Clinical studies have shown that anxiety/depression is associated with an increased frequency of clinical pain complaints (Taenzer et al., 1986; Atkinson et al., 1991; Dworkin et al., 1995; Lautenbacher et al., 1999; Geisser et al., 2000; Kain et al., 2000; Wilson et al., 2001; Campbell et al., 2003). However, the nature and the mechanisms of influence are unclear. Until now, very few animal studies examined the mechanisms underlying the links between pain and depression or anxiety. With respect to the polymorphism of pain as described by humans in terms of sensation and emotion inseparably bound, research on animals has been mainly focused on sensory mechanisms of nociception. In studies of stress and pain, acute exposure to a variety of stressors produced an immediate analgesia, which has been interpreted as an inhibition of pain by fear (Lewis et al., 1980). However, exposure to powerful acute stressors does not model the situation faced by clinicians dealing with pain in anxious or depressed patients. Studies of chronic stress suggest that stress can produce hyperalgesia rather than hypoalgesia (Gamaro et al., 1998; Quintero et al., 2000; Butkevich and Vershinina, 2001; da Silva Torres et al., 2003).
Our objectives were as follows. Objective 1 was to study the relationship between anxiety/depression and the perception of pain. Therefore, the first step was to use an animal model eliciting symptoms close to those observed in humans with anxiety/depression. For this purpose, we used an animal model of chronic social defeat (Becker et al., 2001). Prolonged chemically evoked pain behavior was assessed in the formalin model.
Objective 2 was to document the mechanisms underlying the relationship between anxiety/depression and pain. We chose to study cholecystokinin (CCK), because it has been shown previously to act both in the mechanisms of nociception (Cesselin, 1995; Wiesenfeld-Hallin et al., 1999) and in anxiogenesis (van Megen et al., 1996; Shlik et al., 1997). Microdialysis via a frontal cortex probe was chosen, because it offers the advantage of quantifying the release of CCK from an area both rich in CCK-containing neurons (Beinfeld et al., 1981; Marley et al., 1984; Iadarola et al., 1989) and known to play an important role in the control of anxiety/stress (Damasio, 1997; Davidson, 2002) and in the integration of nociceptive messages (Di Piero et al., 1997; Disbrow et al., 1998). Last, the roles played by anxiety and pain were further documented using morphine as an analgesic, chlordiazepoxide as an anxiolytic, and 4-[[2-[[3-(1H-indol-3-yl)-2-methyl-1-oxo-2[[(tricyclo[3.3[12,17]dec-2-yloxy)-carbonyl]amino]-propyl]amino]-1-phenyethyl]amino]-4-oxo-[R-(R*, R*)]-butanoate N-methyl-d-glucamine(CI-988) as a selective CCK receptor antagonist.
Materials and Methods
Animals
Male Sprague Dawley rats (Centre d'Elevage R. Janvier, Le Genest-St.-Isle, France), weighing 250–300 g, served as experimental intruder animals. They were housed in individual cages (length, 45 cm; width, 25 cm; height, 17 cm) for 10 d before the beginning of the experiments. Long–Evans (LE) rats (Centre d'Elevage R. Janvier), weighing 700–800 g, served as resident rats, in confrontation encounters. They were housed individually in appropriate cages (length, 45 cm; width, 45 cm; height, 17 cm). The same 10 LE rats were used for all the successive series of experiments. All animals were kept under controlled environmental conditions (22 ± 1°C; 60% relative humidity; 12 h light/dark cycle; food and water ad libitum). Procedures involving animals and their care were all performed in conformity with the institutional guidelines, which are in compliance with national and international laws and policies (Council directive 87-848, October 19, 1987, Ministère de l'Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale; permissions 0313 to F. Cesselin and 6180 to J.-J. Benoliel).
Experimental procedures
Social defeat. The behavioral procedure consisted of four daily conditioning sessions that involved the same pairs of residents and intruders. The 45 min conditioning sessions started at 10:00 A.M. They were divided into two consecutive periods. During period I (30 min), intruders were placed singly in a protective cage without cover (length, 25 cm; width, 25 cm; height, 30 cm) inside the resident home cage. The resident home cage had no cover and was surrounded by a 100-cm-high wire-mesh enclosure (length, 45 cm; width, 45 cm). The protective cage allowed unrestricted visual, auditory, and olfactory contacts with the resident but precluded close physical contact. During period II (15 min), the protective cage was either removed with the resident present, allowing physical confrontation (three to four confrontations of ∼10 s each, during which the intruding animal was always dominated by the resident rat) with the intruder (defeated intruders) or removed while the intruder had access to the entire resident home cage (nondefeated intruders). Therefore, the nondefeated intruders were never physically attacked and defeated by the resident. After the fourth conditioning session (i.e., on the fifth day), intruders destined for microdialysis experiments were implanted with a guide cannula (see below). Four days later, the experimental intruders implanted with the microdialysis probe (see below) were subjected to formalin injection (see below).
Body weight. Body weights in defeated and nondefeated rats were measured daily at 9:00 A.M., before (7 d), during (4 d), and after (5 d) the social-defeat procedure.
Adrenal weight. Five days after the end of the fourth conditioning sessions, defeated and nondefeated animals were killed and adrenals were removed, dissected free of adhering fat, and weighed. Organ weights were expressed relative to body weights (in mg/100 g body weight).
Sweet-water consumption. One week before the beginning of the conditioning sessions (days –7 to 1) until 5 d after the end of these sessions, two bottles (one of water and one containing 2% of sucrose) were continuously available to rats. Sucrose and water intakes were measured daily at 9:00 A.M. Bottles were changed from left to right sides of the cage throughout the experiment.
Formalin test: behavioral quantification. Rats were placed in the microdialysis bowl 1 d before microdialysis on a mirror floor to allow an unobstructed view of the paws by the observers.
During microdialysis experiments, 8 min after the collection of fraction 5 (see below), an injection of 0.05 ml of sterile 2.5 or 5% formalin was made just under the skin on the dorsal surface of the rat forepaw. The pain responses were recorded for a period of 70 min. This injection resulted in a pain-induced behavior that can be assessed on a five-level scale in relation to posture: 0, normal posture of the paw; 1, injected paw remaining on the ground but not supporting the animal; 2, injected paw lifted without contact with any surface; 3, injected paw completely raised; 4, injected paw licked, nibbed, or shaken. These pain behaviors were scored using a method based on the scoring method of Dubuisson and Dennis (1977). Behaviors were measured by two experienced observers who were blind to the treatment conditions.
The results were expressed as follows. First, pain scores in the different figures were expressed with the following formula: pain score = [(0 × T0) + (1 × T1) + (2 × T2) + (3 × T3) + (4 × T4)]/T, in which T0, T1, T2, T3, T4 were the duration (in seconds) spent in levels 0, 1, 2, 3, and 4, respectively, and T was the total duration of the measure intervals (i.e., T0 + T1 + T2 + T3 + T4). Measures were made over 180 s spans during the first 6 min, over 240 s spans for 4 min, and then over 300 s spans until the end of the observation period.
Behavioral pain responses measured during the first 22 min (corresponding to phase I plus interphase) and during the next 30 min (corresponding to phase II) were correlated with CCK-like material (CCKLM) outflows recorded in fractions 5 and 6, respectively, of the microdialysis experiments.
Second, total pain scores were expressed with the following formula: total pain score = [(0 × T0) + (1 × T1) + (2 × T2) + (3 × T3) + (4 × T4)], during the corresponding time fraction (phase I, 1–6 min; interphase, 6–20 min; phase II, 20–70 min).
Microdialysis
The in vitro recovery of CCKLM was estimated as described previously (Nevo et al., 1996; Becker et al., 2001). The microdialysis probes were immersed in a solution containing a known concentration of exogenous CCK. The in vitro recovery was calculated as the percentage of CCKLM quantified in the microdialysates over that present in the original solution. This ratio was 7.8 ± 0.3% (mean ± SEM; n = 28).
Twenty-four hours after the fourth conditioning session, intruders were anesthetized with chloral hydrate (375 mg/kg; i.p.). A stainless-steel guide cannula (CMA/12; CMA Microdialysis, North Chelmsford, MA; outer diameter, 0.7 mm) was placed at the following coordinates so that the tip was just above the frontal cortex: anteroposterior, +2.7 mm from bregma; lateral, –1.7 mm from bregma; horizontal, –0.8 mm from the skull (Paxinos and Watson, 1986). The cannula was then secured with dental cement to the skull, and the skin was sutured. Animals were allowed to recover from the surgery in individual cages for 4 d before the microdialysis experiment.
The day before the experiment, the rats were placed in a Plexiglas microdialysis bowl (35 cm in diameter) with free access to food and water. The next morning, a microdialysis probe (CMA/12; CMA Microdialysis; cutoff, 20,000 Da; outer diameter, 0.5 mm; 2 mm in length) was introduced into the guide cannula so as to protrude by 2 mm into the Fr2 area of the frontal cortex (Nevo et al., 1996). The probe was continuously perfused at a flow rate of 3.0 μl/min with an artificial CSF (aCSF) (Nevo et al., 1996; Becker et al., 2001). To allow dialysis to reach steady state around the membrane probe, perfusion was performed for 90 min (washout period) before collection of the first fraction (Nevo et al., 1996). In all experiments, seven fractions of 90 μl (each corresponding to 30 min of perfusion) were collected at 0°C and then immediately frozen at –30°C until the determination of their CCKLM content using the RIA procedure described below.
At the end of the experiment, placement of microdialysis probes was verified by histological examination. When a probe was incorrectly placed, the corresponding results were discarded (only 4 of the 138 intruders used in the studies).
Treatments during microdialysis experiments. During the microdialysis procedure, 218 min after the probe insertion (i.e., 8 min after the beginning of the fifth fraction), intruders (either defeated or nondefeated) were injected with formalin. Morphine (4 mg/kg, s.c.), CI-988 (2 mg/kg, i.p.), morphine (s.c.) plus CI-988 (i.p.), or vehicle alone was administered to the intruder 40 min before the formalin injection (i.e., 2 min before the beginning of collection of the fourth fraction) during the microdialysis procedure. When rats were implanted with chlordiazepoxide (10 mg · kg–1 · d–1; chronically) or saline pumps, morphine (4 mg/kg, s.c.) was injected according to the same protocol (i.e., 40 min before the formalin injection).
Drugs. The benzodiazepine receptor agonist chlordiazepoxide (10 mg · kg–1 · d–1; F. Hoffmann-La Roche, Basel, Switzerland) was dissolved in distilled water and placed in ALZET pumps (2ML1; Charles River, L'Arbresle, France). Chlordiazepoxide pumps or saline pumps were implanted subcutaneously on the back on the same day as microdialysis surgery (i.e., 4 d before microdialysis experiments). Morphine hydrochloride (2, 4, and 6 mg/kg; Pharmacie Centrale des Hôpitaux, Paris, France) was injected subcutaneously. Formalin or morphine was diluted in saline (0.9% w/v NaCl). CI-988 (2 mg/kg; Pfizer, Paris, France) was prepared as a suspension in acacia gum in distilled water and injected intraperitoneally. Morphine or CI-988 was administered in a volume of 3 ml/kg. Intruders receiving vehicle alone (3 ml/kg) were treated according to the same protocol.
Radioimmunoassay
Corticosterone. At the time of decapitation (5 d after the end of the sessions, at 12:00 P.M.), blood from trunk vessels was collected in chilled tubes. Samples were then centrifuged at 3500 × g for 10 min at 4°C, and the serum was collected to be frozen at –20°C until corticosterone determination. Corticosterone was quantified by radioimmunoassay (ICN Biomedicals, Orsay, France) using 125I-corticosterone as a radiotracer. The detection limit of the assay was 2.5 ng/ml, and half-displacement of 125I-corticosterone bound to antibodies was obtained with ∼250 ng/ml.
Under these RIA conditions, the cross-reactivity was 34% with desoxycorticosterone, 10% with testosterone, 5% with cortisol, 3% with aldosterone, 2% with progesterone, 1% with androstenedione, and <0.01% with pregnenolone, compared with 100% with corticosterone.
The corticosterone content of each fraction was expressed as corticosterone equivalents (i.e., the amount of authentic corticosterone producing the same displacement of the radioiodinated tracer bound to anticorticosterone antibodies as the endogenous material).
CCKLM. The buffer used for preparing 125I-human gastrin solution (2000 Ci/mmol; CIS Bio International, Gif-sur-Yvette, France) and charcoal suspension was 50 mm barbital-HCl, pH 8.5, containing 1 g/L sodium azide and 10 mm MgCl2. The anti-CCK-octapeptide (CCK-8) antiserum was obtained in a rabbit injected repeatedly with CCK-8 coupled to thyroglobulin by glutaraldehyde (Studler et al., 1981). For the measurement of CCKLM in microdialysates, 90 μl fractions were incubated with 100 μl of an anti-CCK antiserum solution (in barbital-HCl buffer containing 3.75 g/L BSA; final dilution, 1:1,500,000) (Benoliel et al., 1992) and 50 μl of barbital-HCl buffer. After a 48 h incubation at 4°C, 50 μl of the 125I-gastrin tracer solution (corresponding to 2000–2500 cpm) were added, and incubation proceeded for a additional 20–24 h. The assay was stopped by adsorbing the free tracer onto active dextran T70-coated charcoal (4 and 40 g/L, respectively, in the barbital-HCl buffer containing 10% horse serum; 1 ml of the suspension per tube). The tubes were immediately centrifuged at 6000 × g for 10 min at 4°C, and the radioactivity in the supernatants was estimated by gamma spectrometry. Standard curves were prepared from RIAs of standard solutions with 0.5–50 pg/tube of authentic sulfated CCK-8 (CCK-8S) in 50 μl of barbital-HCl buffer supplemented with BSA. These aliquots were mixed with 90 μl of aCSF and 100 μl of the antiserum dilution, and assayed as described above. The detection limit of the assay was 0.75 pg of CCK-8S per tube, and half-displacement of 125I-gastrin bound to antibodies was obtained with ∼10 pg of the peptide.
Under these RIA conditions, the cross-reactivity was 233% with nonsulfated CCK-8, 204% with gastrin, 31% with sulfated CCK-7 (CCK-7S), 26% with nonsulfated CCK-7, 25% with CCK-5, 11% with CCK-33, 1% with CCK-4, and undetectable with rat α-CGRP (up to 2.5 μg/tube), compared with 100% for synthetic CCK-8S.
The CCKLM content of each fraction was expressed as CCK equivalents (i.e., the amount of authentic CCK-8S producing the same displacement of the 125I-gastrin bound to anti-CCK antibodies as the endogenous material). These values were not corrected for probe recovery calculated from in vitro experiments with authentic CCK-8S. The levels of CCKLM in each fraction collected after any treatment were expressed as a percentage of the mean level of the peptide in the first three fractions collected immediately after the washout period.
Statistical analysis
Physiological and behavioral data. Differences in body weight, sweetwater consumption, adrenal weight, corticosterone levels, pain scores, or total pain scores were validated using ANOVA followed by pairwise comparisons using the Bonferroni t test. Behavioral data were analyzed by two-way (subjects; treatments) ANOVAs followed by the Bonferroni t test.
Neurochemical data. The CCKLM levels (in percentage) in dialysis samples collected after an injection were compared with baseline levels using the two-tailed, paired Student's t test. The CCKLM levels (in percentage) in control and treated rats during each period of interest were compared independently by two-tailed, unpaired Student's t test.
Results
Effects of social defeat on physiological parameters
Body weight
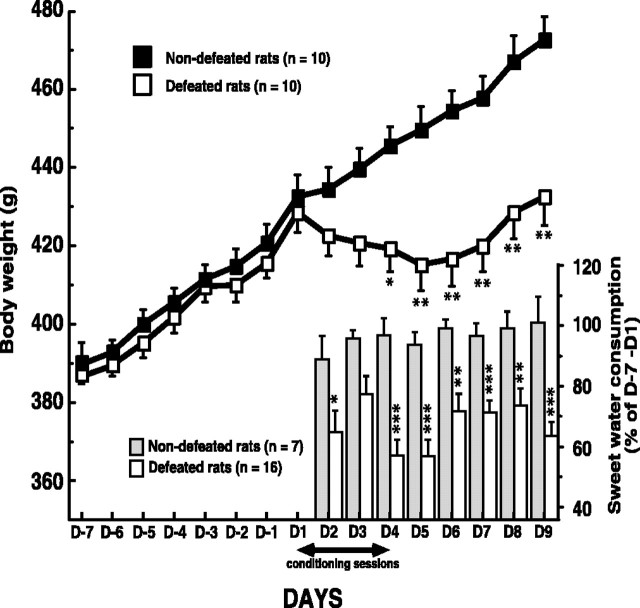
Defeated and nondefeated animals were similar in body weight before starting the social-defeat procedure (day –7; day 1). Changes in body weight as a result of repeated social defeat are shown in Figure 1. Defeated rats weighed less compared with nondefeated rats. This divergence began after the first conditioning session (day 1) and persisted until the end of the experiment (day 9; 432.50 ± 7.32 g vs 472.60 ± 6.13 g; n = 10; F(1,18) = 17.63; p < 0.001).
Figure 1.
Long-term weight change induced by the social-defeat procedure. Daily body weight in nondefeated and defeated rats was measured before, during, and after the fourth days of conditioning sessions. Body weight measured on day 2 is the consequence of the first conditioning session. Each point is the mean ± SEM of data obtained in 10 defeated and 10 nondefeated intruders. *p < 0.002 and **p < 0.001 versus nondefeated intruders. Time course of sweet-water consumption in defeated and nondefeated animals is shown. One week before the beginning of the conditioning sessions (days –7 to 1) until 6 d after the end of these sessions (day 9), the animals had permanent access to two bottles (one of water and one containing 2% sucrose). Sucrose and water intakes were measured daily. The decrease of sweet-water consumption measured on day 2 is the consequence of the first conditioning session. Results are expressed as a percentage of control values (days –7 to 1) for each group. *p < 0.05, **p < 0.01, and ***p < 0.001 versus nondefeated intruders. D, Day.
Sweet-water consumption
During the 7 d before the beginning of conditioning sessions, rats drank significantly more sweet water (∼30 ml/d) than plain water (∼15 ml/d). In nondefeated rats, similar values were measured until the end of the experiment (i.e., 5 d after the end of social-defeat procedure). In contrast, in defeated animals, from the day with the first physical confrontation, sweet-water consumption decreased significantly, whereas that of plain water was unchanged. During all of the experiments, sweet-water consumption in defeated rats averaged ∼70% of that measured in nondefeated rats (Fig. 1).
Hypothalamic–pituitary–adrenal axis
Five days after the end of conditioning sessions, defeated animals exhibited a significant increase in serum corticosterone levels (defeated rats, 56.39 ± 12.74 ng/ml; nondefeated rats, 8.84 ± 2.44 ng/ml; n = 10; F(1,18) = 13.41; p < 0.002) and an obvious increase in adrenal weights compared with nondefeated animals (defeated intruders, 13.65 ± 0.25 mg/100 g of body weight; nondefeated intruders, 9.89 ± 0.41 mg/100 g; n = 10; F(1,18) = 66.85; p < 0.0001).
Effects of formalin (2.5 or 5%) in defeated and nondefeated animals
Behavioral data
In nondefeated rats, formalin injection provoked a vigorous withdrawal of the paw. The affected paw was shaken, licked, or bitten, and generally kept elevated. Phase I began immediately after formalin injection and lasted ∼6 min as shown in Figure 2A. After this period, pain scores were dramatically reduced. During this interval (∼15 min) corresponding to interphase, the rats usually sat still in a corner. Approximately 20 min after formalin injection, the rats exhibited marked signs of pain as expressed by the increase in pain scores. This state persisted for 45–50 min (phase II). In defeated intruders, whatever the formalin doses, pain scores were significantly increased during phase I (2.5% formalin, +22%; 5% formalin, +23%; 1–6 min), interphase (2.5% formalin, +205%; 5% formalin, +100%; 6–20 min) and phase II (2.5% formalin, +47%; 5% formalin, +39%). Actually, in these animals, interphase nearly disappeared and phase II immediately followed phase I (Fig. 2A). The two-way ANOVA showed an effect of formalin doses (interphase, F(1,38) = 7.45, p < 0.01; phase II, F(1,38) = 16.27, p < 0.001), an effect of social defeat (interphase, F(1,38) = 17.18, p < 0.001; phase II, F(1,38) = 23.38, p < 0.0001) without interaction between these two factors (interphase, F(1,38) = 1.03; phase II, F(1,38) = 0.52).
Figure 2.
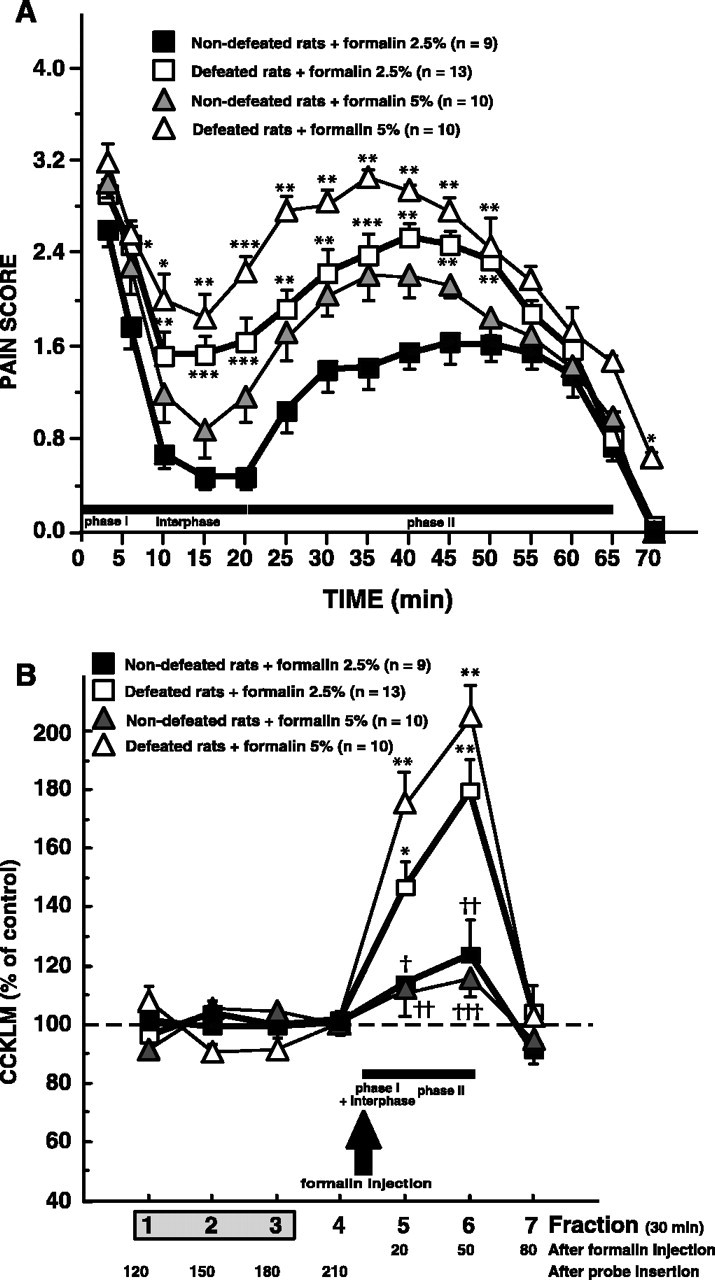
A, Time course of pain scores in defeated and nondefeated animals. Behavioral pain scores were recorded after injection of 2.5 or 5% formalin (50 μl) into the dorsal surface of the right hindpaw. Each point is the mean ± SEM of behavioral score obtained in 10–13 defeated and 9–10 nondefeated intruders. *p < 0.05, **p < 0.01, and ***p < 0.001 versus corresponding nondefeated intruders. B, Effects of 2.5 or 5% formalin injection on dialysate cortical levels of CCKLM in defeated or nondefeated intruders. Cortical CCKLM levels were measured in 30 min collected fractions (90 μl). Fraction 1 was the first fraction collected after a 90 min washout period after microdialysis probe insertion. Formalin (2.5 or 5%; 50 μl) was injected into the dorsal surface of the right hindpaw of defeated or nondefeated intruders, 8 min after the beginning of fraction 5 (arrow). Data are the means ± SEM of CCKLM contents in collected fractions (30 min each), expressed as percentages of basal values, taken as the mean of fractions 1–3. *p < 0.001 and **p < 0.0001 compared with basal values (100%). †p < 0.02, ††p < 0.01, and †††p < 0.001 versus corresponding defeated intruders.
Dialysate cortical levels of CCKLM
The spontaneous cortical CCKLM outflow (i.e., fractions 1–3) in defeated intruders was not significantly different from that measured in nondefeated intruders (mean ± SEM, 1.45 ± 0.08 pg CCK equivalents/fraction, n = 13, vs 1.48 ± 0.06 pg CCK equivalents/fraction, n = 9, respectively).
In defeated and nondefeated rats, injection of saline in the rat's forepaw did not modify CCKLM outflow (data not shown). In nondefeated intruders, formalin injection (2.5 and 5%) did not significantly modify cortical CCKLM outflow. In contrast, in defeated rats, subcutaneous formalin injections on the rat's forepaw induced a significant elevation of dialysate CCKLM levels observed in the corresponding (fifth) microdialysate fraction (+46 ± 8%, t(12) =–4.30, p < 0.001, n = 13; +75 ± 9%, t(9) = –7.96, p < 0.0001, n = 10, respectively; after 2.5 or 5% formalin injection) and in the following fraction (+79 ± 10%, t(12) = –5.69, p < 0.0001, n = 13; +105 ± 9%, t(9) =–5.58, p < 0.0001, n = 10, respectively; after 2.5 or 5% formalin injection). Subsequently, CCKLM levels returned to basal values (Fig. 2B).
Because, at the dose of 2.5% formalin, as well as at 5% formalin, pain scores and CCKLM release were significantly increased in defeated animals compared with nondefeated rats, for ethical reasons, the following experiments were performed at 2.5% formalin.
Effects of morphine pretreatment on 2.5% formalin-induced data
Behavioral data
As illustrated in Figure 3A, injection of saline 40 min before formalin administration did not significantly affect the pain behavior of both defeated and nondefeated intruders, compared with noninjected intruders treated with formalin (compare Figs. 2 A, 3A).
Figure 3.
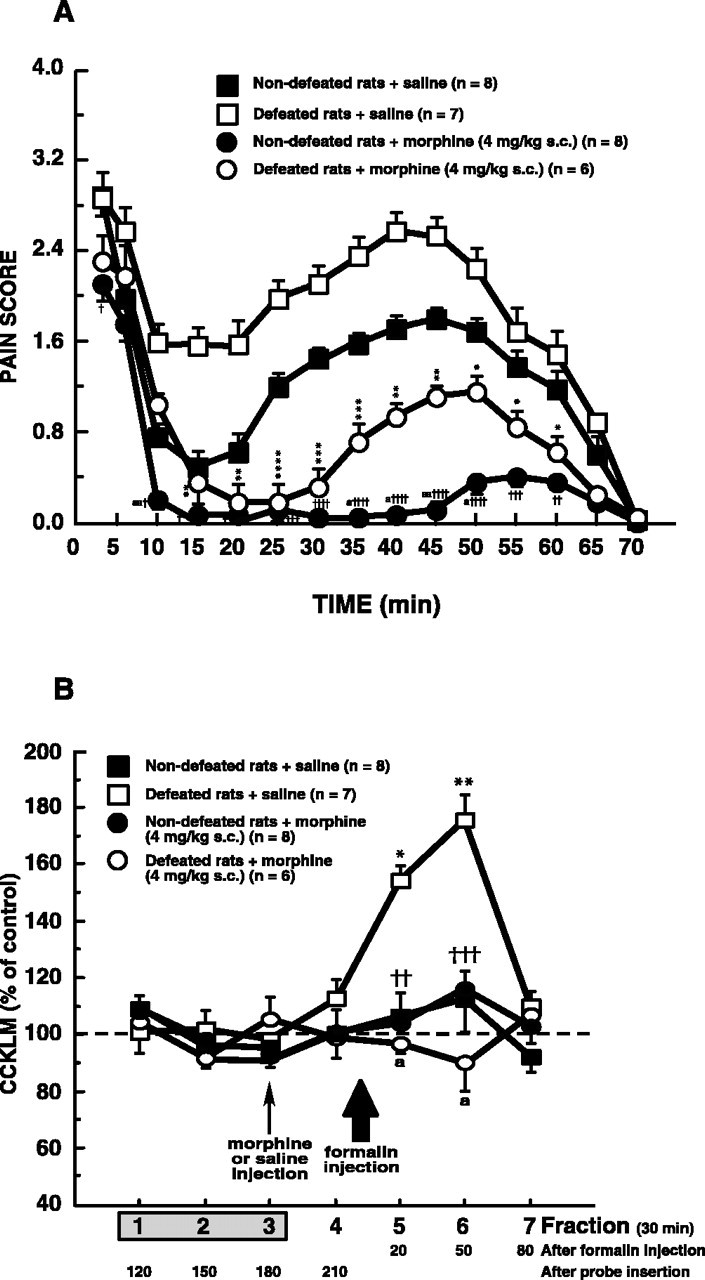
A, Morphine effects on behavioral pain scores recorded after 2.5% formalin injection in defeated or nondefeated intruders. Morphine (4 mg/kg, s.c.) or saline was administered 40 min before injection of 2.5% formalin in defeated or nondefeated intruders. Each point is the mean ± SEM of behavioral score. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 versus defeated intruders injected with saline. †p < 0.05, ††p < 0.01, †††p < 0.001, and ††††p < 0.0001 versus nondefeated intruders injected with saline. a, p < 0.05; aa, p < 0.01 versus defeated intruders injected with morphine. B, Morphine effects on dialysate cortical CCKLM outflow in defeated and nondefeated intruders injected with formalin. Morphine (4 mg/kg, s.c.) or its vehicle (saline) was administered 40 min (i.e., 2 min before fraction 4; small arrow) before injection of 2.5% formalin (corresponding to 8 min after the beginning of fraction 5; large arrow) in defeated or nondefeated intruders. Data are the means ± SEM of CCKLM contents of collected fractions, expressed as percentages of basal values, taken as the mean of fractions 1–3. Fraction 1 was the first sample collected after a 90 min washout period after probe insertion. *p < 0.001 and **p < 0.0001 compared with basal values (100%). ††p < 0.01 and †††p < 0.001 versus defeated intruders injected with saline. a, p < 0.0001 versus defeated intruders injected with saline.
Effects of different morphine doses on total pain scores. As shown in Table 1, morphine injection at the dose of 2 mg/kg produced a small but significant decrease of total pain scores measured during phase II in nondefeated animals (–31%) as well as in defeated animals (–27%). With higher doses (4 and 6 mg/kg), total pain scores were dramatically decreased during interphase and phase II in nondefeated or defeated intruders (approximately –90% and approximately –70%, respectively). In nondefeated as well as in defeated intruders, total pain scores were not significantly different after 6 mg/kg morphine injection compared with 4 mg/kg morphine. Whatever the morphine dose, two-way ANOVA revealed an effect of morphine (phase II; 2 mg/kg morphine, F(1,23) = 14.40, p < 0.001; 4 mg/kg morphine, F(1,27) = 112.57, p < 0.0001; 6 mg/kg morphine, F(1,24) = 170.24, p < 0.0001), and an effect of social defeat (phase II; 2 mg/kg morphine, F(1,23) = 15.99, p < 0.001; 4 mg/kg morphine, F(1,27) = 23.29, p < 0.0001; 6 mg/kg morphine, F(1,24) = 30.72, p < 0.0001) without interaction between these two factors (2 mg/kg morphine, F(1,23) = 0.11; 4 mg/kg morphine, F(1,27) = 1.22 × 10–4;6 mg/kg morphine, F(1,24) = 0.02).
Table 1.
Effects of different doses of morphine on total pain scores
|
|
Nondefeated rats |
Defeated rats |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
Saline (n = 7) |
Morphine (2 mg/kg) (n = 5) |
Morphine (4 mg/kg) (n = 8) |
Morphine (6 mg/kg) (n = 5) |
Saline (n = 10) |
Morphine (2 mg/kg) (n = 5) |
Morphine (4 mg/kg) (n = 6) |
Morphine (6 mg/kg) (n = 6) |
||||||
| Phase I (1-6 min) | 785 ± 55 | 820 ± 57 | 714 ± 61 | 659 ± 98 | 971 ± 61 | 791 ± 95 | 805 ± 88 | 588 ± 88** | ||||||
| Interphase (6-20 min) | 436 ± 96 | 283 ± 81 | 26 ± 11*** | 3 ± 3***,† | 1313 ± 156 | 1090 ± 225 | 414 ± 103***,† | 68 ± 50****,††† | ||||||
| Phase II (20-70 min) |
3729 ± 293 |
2597 ± 331*
|
384 ± 114****,††††
|
24 ± 24****,††††
|
5372 ± 255 |
3925 ± 557*
|
1960 ± 594****,††
|
1670 ± 309****,††
|
||||||
Morphine (2, 4, or 6 mg/kg) or saline was administered subcutaneously to the nondefeated or defeated intruders 40 min before formalin injection. As described in Materials and Methods, total pain scores were measured during the first 6 min (phase I), after 14 min (interphase), and then until the end of experiment (phase II).
p < 0.02, **p < 0.002, ***p < 0.001, and ****p < 0.0001 comparison versus saline within each group of rats (nondefeated or defeated rats). †p < 0.02, ††p < 0.01, †††p < 0.001, and ††††p < 0.0001 comparison of morphine doses versus 2 mg/kg within each group of rats (nondefeated or defeated rats). Statistical analysis between morphine (4 mg/kg) and morphine (6 mg/kg) showed no significant difference for nondefeated animals. The same results were obtained for defeated intruders.
Time course of pain scores after injection of 4 mg/kg morphine.In nondefeated intruders, pretreatment with morphine (4 mg/kg, s.c.) slightly decreased pain scores during phase I and significantly reduced pain scores measured during interphase and phase II. In defeated animals, also, morphine administration did not affect first phase scores and significantly decreased pain scores recorded during interphase and phase II. However, during phase II, pain scores of defeated animals treated with morphine always remained significantly higher than those measured in nondefeated animals treated with morphine (Fig. 3A).
Dialysate cortical levels of CCKLM
In both nondefeated and defeated intruders, neither saline nor morphine affected the dialysate cortical levels of CCKLM before the injection of formalin (fraction 4) (Fig. 3B).
In defeated or nondefeated intruders, saline did not modify formalin-induced CCKLM overflow (compare Figs. 3B, 2B). In contrast, morphine (4 mg/kg, s.c.), inactive per se in the two groups of intruders (fraction 4), completely abolished the increase in CCKLM contents observed in fractions 5 and 6 in saline-defeated intruders (Fig. 3B).
Effects of CI-988 pretreatment or chlordiazepoxide chronic treatment on 2.5% formalin-induced data
Behavioral data
As illustrated in Figure 4A, after formalin injection, saline pumps or CI-988 vehicle (data not shown) did not significantly affect the pain behavior of both defeated and nondefeated intruders compared with saline-injected intruders (compare Figs. 4A,3A).
Figure 4.
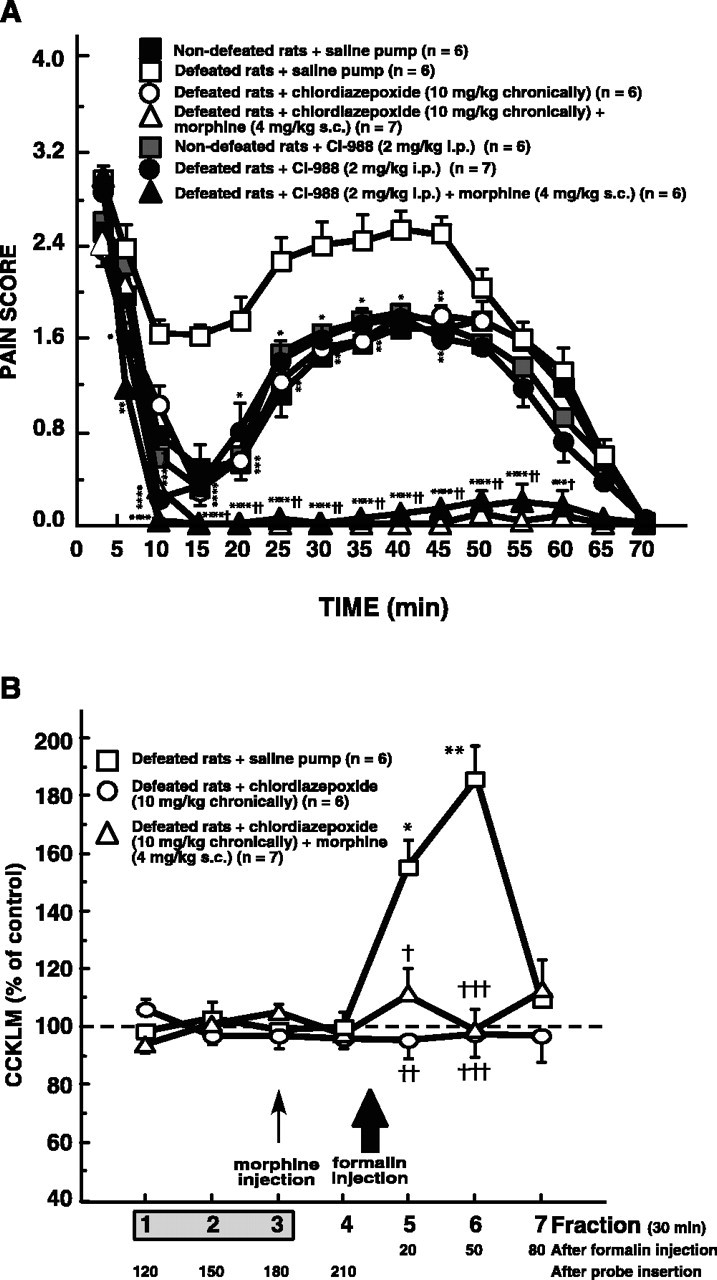
A, Effects of chronic treatment with chlordiazepoxide or CI-988 injection (with or without morphine) on behavioral pain scores recorded in intruders after 2.5% formalin injection. Chlordiazepoxide pumps or saline pumps were implanted subcutaneously on the day of microdialysis surgery for at least 4 d. On the ninth day, during the microdialysis experiment, morphine (4 mg/kg, s.c.), CI-988 (2 mg/kg, i.p.), or a combination of morphine plus CI-988 was administered 40 min before injection of 2.5% formalin. Each point is the mean ± SEM of the behavioral score. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 versus defeated intruders implanted with saline pump. †p < 0.05 and ††p < 0.0001, defeated intruders implanted with chlordiazepoxide pump or injected with CI-988 in association with morphine versus corresponding values for defeated intruders(implanted with chlordiazepoxide pump alone or injected with CI-988 alone). B, Effects of chronic treatment with chlordiazepoxide (with or without morphine) on dialysate cortical CCKLM outflow in formalin-injected intruders. Formalin was injected 8 min after the beginning of fraction 5 (large arrow). Morphine (4 mg/kg, s.c.) was administered 40 min (i.e., 2 min before fraction 4; small arrow) before injection of 2.5% formalin in defeated intruders implanted with a chlordiazepoxide pump. Data are the means ± SEM of CCKLM contents in collected fractions, expressed as percentages of basal values, taken as the mean of fractions 1–3. Fraction 1 was the first sample collected after a 90 min washout period after probe insertion. *p < 0.001 and **p < 0.0001 compared with basal values (100%); †p < 0.05, ††p < 0.01, and †††p < 0.001 versus corresponding values for defeated intruders implanted with saline pump.
Pain scores measured in nondefeated animals treated chronically with chlordiazepoxide (data not shown) or injected with CI-988 (Fig. 4A) were not significantly different from those measured in nondefeated animals receiving saline. Chronic treatment with chlordiazepoxide (10 mg · kg–1 · d–1) or CI-988 (2 mg/kg, i.p.) significantly affected the pain behavior of defeated intruders measured during phase I. During interphase and phase II, these treatments produced a marked decrease of pain scores compared with defeated intruders chronically treated with saline pump (Fig. 4A) or with CI-988 vehicle (data not shown). In all cases, pain scores measured in chlordiazepoxide- or CI-988-treated defeated rats were not significantly different from those recorded in nondefeated animals that received saline pumps (Fig. 4A). Pain scores measured in defeated intruders chronically treated with chlordiazepoxide or pretreated with CI-988 and injected with morphine (4 mg/kg, s.c.) were near to zero during interphase and phase II, and were therefore very significantly different from those recorded in defeated rats treated with saline (Fig. 4A). In defeated intruders, the two-way ANOVA revealed an effect of morphine (interphase, F(1,22) = 21.84, p < 0.0001; phase II, F(1,22) = 67.05, p < 0.0001) and an effect of chlordiazepoxide (interphase, F(1,22) = 17.70, p < 0.001; phase II, F(1,22) = 27.15, p < 0.0001) or CI-988 (interphase, F(1,22) = 16.67, p < 0.001; phase II, F(1,22) = 20.53, p < 0.001) without interaction between the two factors, showing an additive effect between morphine and CI-988 (interphase, F(1,22) = 2.25; phase II, F(1,22) = 0.09) or chlordiazepoxide treatment (interphase, F(1,22) = 3.36; phase II, F(1,22) = 0.02).
Dialysate cortical levels of CCKLM
The basal CCKLM outflow (i.e., fractions 1–3) in defeated intruders treated chronically with chlordiazepoxide was not significantly different from those measured in nondefeated animals or in defeated intruders implanted with saline pumps (mean ± SEM, 1.43 ± 0.10 pg CCK equivalents/fraction, n = 6, vs 1.44 ± 0.09 and 1.49 ± 0.11 pg CCK equivalents/fraction, n = 6, respectively).
In defeated or nondefeated (data not shown) animals, saline pumps did not significantly modify formalin-induced CCKLM outflow (compare Fig. 4B with Figs. 2B, 3B). As displayed in Figure 4B, chronic treatment with chlordiazepoxide associated, or not, with morphine pretreatment completely suppressed the formalin-induced increase of dialysate cortical levels of CCKLM observed in defeated intruders. It has to be noted that CCKLM levels in fraction 4 collected after morphine injection were not different from those observed in “basal” (1–3) fractions in animals treated chronically with chlordiazepoxide.
Discussion
The present study shows the consequences of chronic social defeat on the pain behavior triggered by a prolonged painful stimulation. These data strongly suggest that this experimental procedure could be considered as a suitable model of anxiety-induced hyperalgesia. That a chronic treatment with the established anxiolytic, chlordiazepoxide, may prevent hyperalgesia further supports this hypothesis. Lastly, because anxiety-induced hyperalgesia coexists with an activation of cortical CCKergic systems, and could be prevented by a selective CCK-B receptor antagonist, CI-988, this strongly supports the idea that anxiety-induced hyperalgesia does involve central CCKergic systems.
The social-defeat procedure resulted in profound long-term changes of physiological parameters in subordinate rats. This paradigm resulted in a hyperactivity of hypothalamic–pituitary–adrenal (HPA) axis, as shown by the increase of serum corticosterone levels as well as adrenal weights and a decrease of body weight and sweet-water consumption, suggesting that this procedure induced a prolonged state of anxiety. Previous studies have shown that subordinate animals submitted to social stress have higher glucocorticoid levels, as indices of stress, related to their fearfulness (Chamove and Bowman, 1978). Adrenal weight is a classic sign widely used as a marker of stress reflecting the hyperactivity of the HPA axis (Coenen and Van Luijtelaar, 1985; Alario et al., 1987; Blanchard et al., 1998; Ruis et al., 1999). Numerous studies reported that repeatedly defeated animals gained less weight than undefeated ones (Van De Poll et al., 1982; Willner et al., 1995), which was interpreted as a sign of anxiety or depression.
The procedure used in the present study allowing animals to choose between two bottles (one with sucrose and one with plain water) is original. This experimental design is distinct from those currently used that allow access to one or two bottles during 1 has a rule after a period of water restriction (Willner et al., 1987; Muscat and Willner, 1992; Konkle et al., 2003). Indeed, in the present study, the rats had the choice between these two bottles for 24 h per day (except during the 45 min conditioning session) and during the whole experiment. The decrease in sweet-water consumption in defeated animals is considered as a classical criterion currently used to assess anhedonia in animals (Muscat and Willner, 1992).
All of these results (HPA hyperactivity, body weight loss, and anhedonia) constitute behavioral and biochemical criteria on the basis of their face validity in terms of the target symptoms of clinical generalized anxiety or depression (American Psychiatric Association, 1994; Koolhaas et al., 1995). Thus, intruders were put in a prolonged state of anxiety, allowing us to examine its effects on formalin-evoked pain.
The formalin test is a widely accepted model for the study of nociception (Tjolsen et al., 1992). In nondefeated animals, intraplantar injections of formalin produced a biphasic behavioral reaction as described by Dubuisson and Dennis (1977). In defeated rats, pain scores were significantly increased during the interphase and during the inflammatory phase, suggesting that pain in defeated intruders was more pronounced compared with nondefeated rats. It was shown previously that subchronic swim stress or prenatal stress alters nociceptive behaviors in the formalin test (Quintero et al., 2000; Butkevich and Vershinina, 2003). In line with the present data, pain scores in defeated animals were not significantly different from those of nondefeated rats during phase I, whereas formalin-induced responses were increased during interphase and phase II. Because, in the present study, hyperalgesia was associated with different spontaneous token of anxiety (HPA hyperactivity, body weight loss, anhedonia), the enhanced pain scores can be related to the “anxiety” or “depression” state of animals.
The fact that the hyperalgesia observed after formalin injection in defeated rats was abolished by a chronic treatment with the well known anxiolytic chlordiazepoxide, which at the dose of 10 mg/kg is active but without inducing motor impairment (File and Pellow, 1984; Hodges and Green 1984; Verborgh et al., 1998), further strengthens the anxiety-like interpretation of the situation. This conclusion was evidenced by the lack of antinociceptive effects of chronic treatment with chlordiazepoxide in nondefeated animals. Thus, the experimental procedure used in the present study may be a valuable animal model of anxiety-induced hyperalgesia.
In nondefeated intruders, 2 mg/kg morphine induced significantly decreased pain scores during interphase and phase II, whereas with 4 and 6 mg/kg doses, morphine almost completely abolished pain behaviors. In contrast, the early phase was not very affected by pretreatment with morphine, whatever the dose. These observations are in excellent agreement with numerous studies showing that morphine was less effective on formalin-evoked pain behaviors during phase I compared with phase II (Dubuisson and Dennis, 1977; Abbott et al., 1995, 1996; Jourdan et al., 1997). During interphase and inflammatory phase, whatever the opioid dose, morphine analgesic effect was similar in defeated and nondefeated rats. This latter observation associated with the fact that, in defeated intruders, 6 mg/kg morphine did not produce an analgesic effect significantly different from that induced by 4 mg/kg, strongly suggests that morphine did not prevent hyperalgesia resulting from the anxiety generated by the experimental procedure of social defeat. To our knowledge, only one recent paper has demonstrated that, in repeatedly stressed animals, morphine effects on nociception were decreased when compared with unstressed controls (da Silva Torres et al., 2003). Because pretreatment with 2 mg/kg morphine produced a partial analgesic effect during interphase and phase II and because 6 mg/kg morphine was not more efficient than 4 mg/kg, the following studies were performed with 4 mg/kg morphine.
In defeated intruders, combinations of chronic treatment with chlordiazepoxide and morphine (4 mg/kg) injection completely nullified pain scores recorded during interphase and inflammatory phase without affecting early-phase pain scores. The effects of chlordiazepoxide and other benzodiazepines, on analgesic effects induced by opioids, have been examined in previous studies and have yielded contradictory results (Weiss, 1969; Fennessy and Sawynok, 1973; Mantegazza et al., 1982; Abbott and Franklin, 1986; Verborgh et al., 1998). Nevertheless, all of these studies were performed in nonstressed animals or in animals submitted to an acute stress that is known to result in stress-induced analgesia, and therefore they cannot be compared with the present experiments. In the light of our results, it could be proposed to prevent the anxiety first and then to use analgesic compounds. However, in humans, the interaction of benzodiazepines and opioids induces deleterious side effects, in particular on the respiratory system (Megarbane et al., 2003). This prompted us to explore a neurotransmission system potentially implicated in these phenomena.
To better understand the neurobiological mechanisms underlying the relation between pain and anxiety/depression, CCK release was measured in the frontal cortex [more exactly the Fr2 region considered as an area of prefrontal cortex (Uylings and van Eden, 1990)] with a microdialysis technique (Nevo et al., 1996; Becker et al., 1999a,b). We have shown previously that CCKLM outflow within the frontal cortex of freely moving rats originates from neurons. This is based on the observation that K+-evoked release is calcium dependant (Nevo et al., 1996). The absolute values of spontaneous cortical CCKLM outflow reported here in nondefeated rats are in agreement with our previous data (Nevo et al., 1996; Becker et al., 1999a,b, 2001).
As reported previously, the long-term state of anxiety obtained in the present study did not modify the basal levels of CCKLM outflow (Becker et al., 2001). Only in defeated animals, formalin injection produced a significant increase of cortical CCKLM release, suggesting a sensitization of CCKergic systems induced by the social-defeat procedure that resulted in the increase of CCK releasing pool. Anyway, these data, linked to the fact that elevated pain scores induced by 5% formalin in nondefeated intruders did not produce an increase of CCKLM release, are in favor of an important role played by CCKergic systems in anxiety-induced hyperalgesia.
A chronic treatment with chlordiazepoxide completely prevented the formalin-induced increase of cortical CCKLM outflow, strongly suggesting that the activation of cortical CCKergic systems is closely linked to the hyperalgesia induced by anxiety. Moreover, because the CCK-B receptor antagonist CI-988 prevented the increase of pain scores induced by chronic social defeat, and because pain scores were not significantly different from those measured in nondefeated rats or defeated rats chronically treated with chlordiazepoxide, this clearly supports the hypothesis of the involvement of CCKergic systems in anxiety-induced hyperalgesia. Because pretreatment with CI-988 did not modify pain scores in nondefeated intruders, the decrease of pain behavior in CI-988-treated defeated rats resulted from an anxiolytic effect rather than from a direct role played by CCKergic systems in pain control.
In conclusion, the present study shows that the experimental social-defeat paradigm should be suitable for studying anxiety-induced hyperalgesia. Furthermore, the high potency of CI-988 and morphine combined treatments to completely suppress pain-related behavior measured in defeated animals, strongly suggests that the association of both compounds could be considered as a new therapeutic approach to reduce the increase of pain complaints highly prevalent among anxious or depressive patients.
Footnotes
This work was supported by grants from Institut National de la Santé et de la Recherche Médicale and Institut UPSA de la Douleur. J.A. was the recipient of a fellowship from the Fondation pour la Recherche Médicale. We are grateful to pharmaceutical companies for generous gifts of chlordiazepoxide (Hoffman-La Roche) and CI-988 (Pfizer). We thank Drs. A. Bogdan and D. Le Bars for statistical assistance.
Correspondence should be addressed to Chrystel Becker, Institut National de la Santé et de la Recherche Médicale E0331, Douleurs et Stress, Faculté de Médecine Pitié-Salpêtrière, 91, boulevard de l'Hôpital, 75634 Paris Cedex 13, France. E-mail: becker@ext.jussieu.fr.
DOI:10.1523/JNEUROSCI.0743-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/257896-09$15.00/0
References
- Abbott FV, Franklin KBJ (1986) Noncompetitive antagonism of morphine analgesia by diazepam in the formalin test. Pharmacol Biochem Behav 24: 319–321. [DOI] [PubMed] [Google Scholar]
- Abbott FV, Franklin KBJ, Westbrook RF (1995) The formalin test: scoring properties of the first and second phases of pain response in rats. Pain 60: 91–102. [DOI] [PubMed] [Google Scholar]
- Abbott FV, Hong Y, Franklin KB (1996) The effect of lesions of the dorsolateral funiculus on formalin pain and morphine analgesia: a doseresponse analysis. Pain 65: 17–23. [DOI] [PubMed] [Google Scholar]
- Alario P, Gamallo A, Beato MJ, Trancho G (1987) Body weight gain, food intake and adrenal development in chronic noise stressed rats. Physiol Behav 40: 29–32. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, Ed 4. Washington, DC: American Psychiatric Association.
- Atkinson JH, Slater MA, Patterson TL, Grant I, Garfin SR (1991) Prevalence, onset, and risk of psychiatric disorders in men with chronic low back pain: a controlled study. Pain 45: 111–121. [DOI] [PubMed] [Google Scholar]
- Becker C, Hamon M, Benoliel J-J (1999a) Prevention by 5-HT1A receptor agonists of restraint stress- and yohimbine-induced release of cholecystokinin in the frontal cortex of the freely moving rat. Neuropharmacology 38: 525–532. [DOI] [PubMed] [Google Scholar]
- Becker C, Hamon M, Cesselin F, Benoliel J-J (1999b) ∂2-opioid receptor mediation of morphine-induced CCK release in the frontal cortex of freely moving rat. Synapse 34: 47–54. [DOI] [PubMed] [Google Scholar]
- Becker C, Thiébot M-H, Touitou Y, Hamon M, Cesselin F, Benoliel J-J (2001) Enhanced cortical extracellular levels of cholecystokinin-like material in a model of anticipation of social defeat in the rat. J Neurosci 21: 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinfeld MC, Meyer DK, Eskay RL, Jensen RT, Brownstein MJ (1981) The distribution of cholecystokinin immunoreactivity in the central nervous system of the rat as determined by radioimmunoassay. Brain Res 212: 51–57. [DOI] [PubMed] [Google Scholar]
- Benoliel J-J, Bourgoin S, Mauborgne A, Pohl M, Legrand JC, Hamon M, Cesselin F (1992) GABA, acting at both GABAA and GABAB receptors, inhibits the release of cholecystokinin-like material from the rat spinal cord in vitro. Brain Res 590: 255–262. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Nikulina JN, Sakai RR, Mckittrick C, Mcewen B, Blanchard CD (1998) Behavioral and endocrine change following chronic predatory stress. Physiol Behav 63: 561–569. [DOI] [PubMed] [Google Scholar]
- Butkevich IP, Vershinina EA (2001) Prenatal stress alters time characteristics and intensity of formalin-induced pain responses in juvenile rats. Brain Res 915: 88–93. [DOI] [PubMed] [Google Scholar]
- Butkevich IP, Vershinina EA (2003) Maternal stress differently alters nociceptive behaviors in the formalin test in adult female and male rats. Brain Res 961: 159–165. [DOI] [PubMed] [Google Scholar]
- Campbell LC, Clauw DJ, Keefe FJ (2003) Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 54: 399–409. [DOI] [PubMed] [Google Scholar]
- Cesselin F (1995) Opioid and anti-opioid peptides. Fund Clin Pharmacol 9: 409–433. [DOI] [PubMed] [Google Scholar]
- Chamove AS, Bowman RE (1978) Rhesus plasma cortisol response at four dominance positions. Aggress Behav 4: 43–55. [Google Scholar]
- Coenen A, Van Luijtelaar EL (1985) Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav 35: 501–504. [DOI] [PubMed] [Google Scholar]
- da Silva Torres IL, Cucco SN, Bassani M, Duarte MS, Silveira PP, Vasconcellos AP, Tabajara AS, Dantas G, Fontella FU, Dalmaz C, Ferreira MB (2003) Long-lasting delayed hyperalgesia after chronic restraint stress in rats—effect of morphine administration. Neurosci Res 45: 277–283. [DOI] [PubMed] [Google Scholar]
- Damasio AR (1997) Towards a neuropathology of emotion and mood. Nature 386: 769–770. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (2002) Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry 51: 68–80. [DOI] [PubMed] [Google Scholar]
- Di Piero V, Fiacco F, Tombari D, Pantano P (1997) Tonic pain: a SPET study in normal subjects and cluster headache patients. Pain 70: 185–191. [DOI] [PubMed] [Google Scholar]
- Disbrow E, Buonocore M, Antognini J, Carstens E, Rowley HA (1998) Somatosensory cortex: a comparison of the response to noxious thermal, mechanical, and electrical stimuli using functional magnetic resonance imaging. Hum Brain Mapp 6: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuisson D, Dennis SG (1977) The formalin test: a quantitative study of the analgesic effects of morphine, meperidine and brain stem stimulation in rats and cats. Pain 4: 161–174. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Clark WC, Lipsitz JD (1995) Pain responsivity in major depression and bipolar disorder. Psychiatry Res 56: 173–181. [DOI] [PubMed] [Google Scholar]
- Fennessy MR, Sawynok J (1973) The effect of benzodiazepines on the analgesic effect of morphine and sodium salicylate. Arch Int Pharmacodyn Ther 204: 77–85. [PubMed] [Google Scholar]
- File SE, Pellow S (1984) The anxiogenic action of Ro 15-1788 is reversed by chronic, but not by acute, treatment with chlordiazepoxide. Brain Res 310: 154–156. [DOI] [PubMed] [Google Scholar]
- Gamaro GD, Xavier MH, Denardin JD, Pilger JA, Ely DR, Ferreira MB, Dalmaz C (1998) The effects of acute and repeated restraint stress on the nociceptive response in rats. Physiol Behav 63: 693–697. [DOI] [PubMed] [Google Scholar]
- Geisser ME, Roth RS, Theisen ME, Robinson ME, Riley JL (2000) Negative affect, self-report of depressive symptoms, and clinical depression: relation to the experience of chronic pain. Clin J Pain 16: 110–120. [DOI] [PubMed] [Google Scholar]
- Hodges HM, Green S (1984) Evidence for the involvement of brain GABA and serotonin systems in the anticonflict effects of chlordiazepoxide in rats. Behav Neural Biol 40: 127–154. [DOI] [PubMed] [Google Scholar]
- Iadarola MJ, Naranjo JR, Duchemin AM, Quach TT (1989) Expression of cholecystokinin and enkephalin mRNA in discrete brain regions. Peptides 10: 687–692. [DOI] [PubMed] [Google Scholar]
- Jourdan D, Ardid D, Bardin L, Bardin M, Neuzeret D, Lanphouthacoul L, Eschalier A (1997) A new automated method of pain scoring in the formalin test in rats. Pain 7: 265–270. [DOI] [PubMed] [Google Scholar]
- Kain ZN, Sevarino F, Alexander GM, Pincus S, Mayes LC (2000) Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated-measures design. J Psychosom Res 49: 417–422. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C (2003) Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res 992: 227–238. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Meerlo P, De Boer SF, Strubbe JH, Bohus B (1995) Social stress in rats: an animal model of depression? Acta Neuropsychiatrica 7: 27–29. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Spernal J, Schreiber W, Krieg JC (1999) Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. Psychosom Med 61: 822–827. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Cannon JT, Liebeskind JC (1980) Opioid and nonopioid mechanisms of stress analgesia. Science 208: 623–625. [DOI] [PubMed] [Google Scholar]
- Mantegazza P, Parenti M, Tammiso R, Vita P, Zambotti F, Zonta N (1982) Modification of the antinociceptive effect of morphine by centrally administered diazepam and midazolam. Br J Pharmacol 75: 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marley PD, Rehfeld JF, Emson PC (1984) Distribution and chromatographic characterisation of gastrin and cholecystokinin in the rat central nervous system. J Neurochem 42: 1523–1535. [DOI] [PubMed] [Google Scholar]
- Megarbane B, Gueye P, Baud F (2003) Interactions between benzodiazepines and opioids. Ann Med Interne (Paris) 154: S64–S72. [PubMed] [Google Scholar]
- Muscat R, Willner P (1992) Suppression of sucrose drinking by chronic mild unpredictable stress: a methodological analysis. Neurosci Biobehav Rev 16: 507–517. [DOI] [PubMed] [Google Scholar]
- Nevo I, Becker C, Hamon M, Benoliel J-J (1996) Stress- and yohimbine-induced release of cholecystokinin in the frontal cortex of freely moving rat: prevention by diazepam but not ondansetron. J Neurochem 66: 2041–2049. [DOI] [PubMed] [Google Scholar]
- Paxinos P, Watson C (1986) The rat brain in stereotaxic coordinates, Ed 2. Sydney: Academic. [DOI] [PubMed]
- Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H (2000) Long-lasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav 67: 449–458. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, Buwalda B, De Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JM (1999) Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology 24: 285–300. [DOI] [PubMed] [Google Scholar]
- Shlik J, Vasar E, Bradwejn J (1997) Cholecystokinin and psychiatric disorders. Role in aetiology and potential of receptor antagonists in therapy. CNS Drugs 8: 134–152. [DOI] [PubMed] [Google Scholar]
- Studler JM, Simon H, Cesselin F, Legrand JC, Glowinski J, Tassin JP (1981) Biochemical investigation on the localization of the cholecystokinin octapeptide in dopaminergic neurons originating from the ventral tegmental area of the rat. Neuropeptides 2: 131–139. [Google Scholar]
- Taenzer P, Melzack R, Jeans ME (1986) Influence of psychological factors on postoperative pain, mood and analgesic requirements. Pain 24: 331–342. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge O-G, Hunskaar S, Rosland JH, Hole K (1992) The formalin test: an evaluation of the method. Pain 51: 5–17. [DOI] [PubMed] [Google Scholar]
- Uylings HB, van Eden CG (1990) Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates including humans. Prog Brain Res 85: 31–62. [DOI] [PubMed] [Google Scholar]
- Van De Poll NE, De Jonge F, Van Oyen HG, Van Pelt J (1982) Aggressive behavior in rats: effects of winning or losing on subsequent aggressive interactions. Behav Process 7: 143–145. [DOI] [PubMed] [Google Scholar]
- van Megen HJGM, Westenberg HGM, den Boer JA, Kahn RS (1996) Cholecystokinin in anxiety. Eur Neuropsychopharmacol 6: 263–280. [DOI] [PubMed] [Google Scholar]
- Verborgh C, De Coster R, D'Haese J, Camu F, Meert TF (1998) Effects of chlordiazepoxide on opioid-induced antinociception and respiratory depression in restrained rats. Pharmacol Biochem Behav 59: 663–670. [DOI] [PubMed] [Google Scholar]
- Weiss J (1969) Morphine antagonistic effect of chlordiazepoxide (Librium). Experientia 25: 381. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, de Arauja Lucas G, Alster P, Xu XJ, Hokfelt T (1999) Cholecystokinin/opioid interactions. Brain Res 848: 78–89. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R (1987) Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93: 358–364. [DOI] [PubMed] [Google Scholar]
- Willner P, D'Aquila PS, Coventry T, Brain P (1995) Loss of social status: preliminary evaluation of a novel animal model of depression. J Psychopharmacol 9: 207–213. [DOI] [PubMed] [Google Scholar]
- Wilson KG, Mikail SF, D'Eon JL, Minns JE (2001) Alternative diagnostic criteria for major depressive disorder in patients with chronic pain. Pain 91: 227–234. [DOI] [PubMed] [Google Scholar]