Abstract
Acetylcholine binding to muscarinic acetylcholine receptors activates G-proteins, phospholipase C, and protein kinase C (PKC), which phosphorylates brain Na+ channels and reduces peak Na+ current in hippocampal neurons. Because multiple PKC isozymes with different regulatory properties are expressed in hippocampal neurons, we investigated which ones are responsible for mediating this effect. The diacylglycerol analog oleoylacetylglycerol (OAG) reduced the amplitude of Na+ current in dissociated mouse hippocampal neurons by 28.5 ± 5.3% (p < 0.01). The reduction of peak Na+ current was similar with Ca2+-free internal solution and in 92 nm internal Ca2+, suggesting that calcium-dependent, conventional PKC isozymes were unlikely to mediate this response. Gö6976, which inhibits conventional PKC isozymes, reduced the effect of PKC activators only slightly, whereas rottlerin, which inhibits PKCδ preferentially at 5 μm, had no effect. Ro-31-8425 (20 nm), which inhibits conventional PKC isozymes, did not reduce the response to OAG. However, higher concentrations of Ro-31-8425 (100 nm or 1 μm) that inhibit novel PKC isozymes effectively blocked OAG inhibition of Na+ current. Inclusion of a selective PKCϵ-anchoring inhibitor peptide (PKCϵ-I) in the recording pipette prevented the reduction of peak Na+ current by OAG, whereas an anchoring inhibitor peptide specific for PKCβ and an inactive scrambled PKCϵ-I peptide had no effect. In addition, OAG had no effect on Na+ current in hippocampal neurons from PKCϵ null mice. Overall, our data from four experimental approaches indicate that anchored PKCϵ is the isozyme responsible for PKC-mediated reduction of peak Na+ currents in mouse hippocampal neurons.
Keywords: action potential, channel, excitability, hippocampus, neuromodulation, phosphorylation, protein kinase, pyramidal, sodium [Na], voltage clamp
Introduction
Voltage-gated Na+ channels conduct action potentials in neurons (Hodgkin and Huxley, 1952) and are critical for determining integrative properties, including threshold, frequency of firing, and dendritic excitability (Stuart, 1999). In hippocampal pyramidal neurons, acetylcholine binding to muscarinic receptors activates G-proteins, phospholipase C, and protein kinase C (PKC), which reduces Na+ currents (Cantrell et al., 1996). Modulation of Na+ channels by neurotransmitters acting through PKC has important effects on neuronal activity (for review, see Cantrell and Catterall, 2001). For example, in prefrontal cortex neurons, stimulation of 5-HT2A/C receptors activates PKC, reduces Na+ current, increases spike threshold, and reduces spike train duration (Carr et al., 2002, 2003). The molecular mechanisms for modulation of Na+ channels by PKC are not fully understood, and the PKC isozymes involved have not been identified.
Na+ channels in brain are heteromultimeric, consisting of an α subunit of 260 kDa and β subunits of 33-36 kDa (Catterall, 2000). The α subunit contains four homologous domains, and each domain contains six transmembrane segments and a reentrant pore loop (Catterall, 2000). Expression of the α subunit is sufficient to produce functional Na+ channels (Goldin et al., 1986; Noda et al., 1986), but coexpression of β subunits is required for normal function (Isom et al., 1992, 1995; Morgan et al., 2000; Yu et al., 2003).
Activation of PKC phosphorylates Na+ channel α subunits (Costa and Catterall, 1984) and reduces Na+ current in Xenopus oocytes (Sigel and Baur, 1988; Lotan et al., 1990; Dascal and Lotan, 1991; Schreibmayer et al., 1991), transfected cells (West et al., 1992), and brain neurons (Numann et al., 1991; Cantrell et al., 1996). The sites of PKC phosphorylation are on the α subunit (Costa and Catterall, 1984; Murphy and Catterall, 1992). Serines 554, 573, and 576 in the intracellular linker between domains I and II and serine 1506 in the inactivation gate between domains III and IV are required for modulation (West et al., 1991; Cantrell et al., 2002).
All nine PKC genes are expressed in the brain (Nishizuka, 1992, 1995). PKC isozymes are classified in three groups based on the cofactors required for activation. Conventional PKCs (α, β, and γ) are activated by Ca2+, phosphatidylserine, and diacylglycerol. Novel PKCs (δ, ϵ, θ, and η) are activated by phosphatidylserine and diacylglycerol but not Ca2+. Atypical PKCs (ζ and τ/λ) are insensitive to diacylglycerol and Ca2+ but are activated by other lipid messengers. Additional specificity is provided by specific protein-protein interactions with receptors for activated C kinases (RACKs) that target individual isozymes to substrates (Mochly-Rosen et al., 1990, 1991; Ron et al., 1994). In the experiments described here, we investigated which PKC isozymes are responsible for modulation of Na+ channels in hippocampal neurons using specific activators and inhibitors of PKC activity, inhibitors that disrupt interactions with RACKs, and mice with a targeted deletion of the PKCϵ gene. Our results point to anchored PKCϵ as the primary PKC isozyme responsible for modulation of brain Na+ channels.
Materials and Methods
Materials. The drug Gö6976 was purchased from Calbiochem (Darmstadt, Germany), and the drugs rottlerin and Ro-31-8425 were purchased from Calbiochem (La Jolla, CA). Oleoylacetylglycerol (OAG) was from Sigma (St. Louis, MO). The peptide inhibitor of PKCϵ translocation (EAVSLKPT, PKCϵ-I) and the corresponding scrambled peptide (LSETKPAV) were purchased from Calbiochem. The peptide inhibitor of PKCβ translocation was synthesized by Genemed (San Francisco, CA).
Animal care. C57BL/6J male mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Studies with PKCϵ null mice were performed using male mice on a C57BL/6J × 129/SvJae background. Wild-type littermates were used as controls. The PKCϵ null mutation was generated by homologous recombination, as described previously (Khasar et al., 1999), and maintained in inbred 129/SvJae mice. PKCϵ+/- 129/SvJae male mice were crossed with wild-type C57BL/6J females to generate breeding pairs of F1 generation PKCϵ+/- male and female C57BL/6J × 129/SvJae mice. These PKCϵ+/- F1 hybrids were inter-crossed to generate F2 generation littermates for experiments. Mutant and wild-type mice were housed together in standard Plexiglas cages with food and water available ad libitum. The colony room was maintained on a 12 h light/dark cycle with lights on at 6:00 A.M. Mice were approximately 4 weeks of age at the time of testing. Animal care and handling procedures were in accordance with institutional and National Institutes of Health guidelines.
Isolation of hippocampal neurons. Hippocampal neurons from adult mice (6-12 weeks of age) were acutely isolated using standard procedures (Kay and Wong, 1987; Surmeier et al., 1991; Cantrell et al., 1996). The animals were anesthetized with halothane and decapitated. Brains were quickly removed, iced, and blocked before slicing. Approximately four coronal slices (500 μm), through the level of hippocampus, were cut and transferred to low Ca2+ HEPES-buffer solution containing the following (in mm): 140 Na isethionate, 2 KCl, 4 MgCl2, 0.1 CaCl2, 23 glucose, 15 HEPES, pH 7.4, 300-305 mOsm/L. Slices were then incubated for 1-6 h in NaHCO3 buffered Earle's balanced salt solution (Sigma) bubbled with 95% O2-5% CO2, pH 7.4. Single slices were placed in the low-Ca2+ buffer, and the hippocampus was isolated and placed in a treatment chamber containing protease XIV (Sigma) (1.5 mg/ml) in HEPES-buffered balanced salt solution (Sigma) at 35°C, pH 7.4. After 15 min of enzyme treatment, the tissue was rinsed several times in the low-Ca2+ buffer solution and triturated. The isolated cells settled on a glass coverslip coated with concanavalin A or poly-l-lysine. Within 5 min of plating, the cells adhered firmly to the coated coverslip.
Whole-cell voltage-clamp recording. Recordings from pyramidally shaped hippocampal neurons were made immediately after isolation at room temperature (22-25°C). The electrodes were pulled from VWR micropipettes (VWR Scientific, West Chester, PA) and fire polished (final resistance, 2.5-5.0 MΩ). Approximately 80% of the series resistance was compensated. Unless otherwise indicated, we used an external recording solution consisting of the following (in mm): 20 NaCl, 10 HEPES, 1 MgCl2, 1 CdCl2, 60 CsCl, 150 glucose, pH 7.3 (300-305 mOsm/L). The internal solution contained the following (in mm): 189 N-methyl d-glucamine, 40 HEPES, 4 MgCl2, 0.1 BAPTA, 1.0 NaCl, 25 phosphocreatine, 2 ATP, 0.2 GTP, 0.1 leupeptin, pH 7.2 (270-275 mOsm/L). In some experiments, Ca2+ buffering was altered by replacing BAPTA with 10 mm EGTA and the addition of Ca2+ as noted. Recordings were obtained using an Axopatch 1C Amplifier (Axon Instruments, Union City, CA). Voltage pulses were delivered and currents recorded using a personal computer running Basic FASTLAB software to control an analog-to-digital/digital-to-analog interface (Indec Systems, Mountain View, CA). The voltage-clamp data were filtered at 10 kHz and digitized at 20 μs intervals. The data points displayed after 2.4 ms represent 200 μs intervals and were obtained by averaging groups of 10 points sampled at the 20 μs rate. This reduces the apparent noise after 2.4 ms. Data from cells with high or unstable holding currents were discarded. Voltage-clamp control in cells was assessed by measuring current-voltage relationships after first achieving the whole-cell configuration. Data from cells in which currents activated with enhanced delays, with excessively steep current-voltage relationships or had notches in the records, were omitted from analyses. The measurements of Na+ current are expressed as mean ± SEM, and in some cases, the means were tested for equality using a paired Student's t test. Conductance-voltage curves were generated according to g(V) = I/(V - Vrev), where V is the test pulse voltage and Vrev is the measured reversal potential. The conductance-voltage curve was fit with a Boltzmann function of the following form: g(V) = Gmax/{1 + exp[(V - V1/2)/k]}, where V1/2 is the half-activation voltage, k is a slope factor, and Gmax is the maximum conductance.
Results
Reduction of peak Na+ currents in mouse hippocampal neurons by OAG
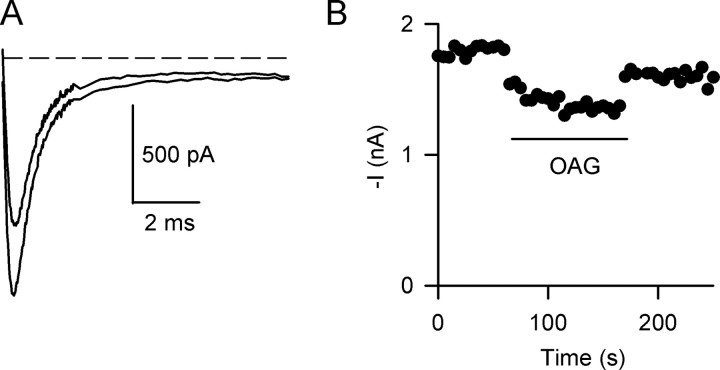
OAG is a membrane-permeant PKC activator that reduces peak Na+ current in cultured brain neurons, acutely isolated rat hippocampal neurons, and Chinese hamster ovary or tsA-201 cells in which Na 1.2α Na+v channels have been expressed (Numann et al., 1991; Cantrell et al., 1996, 2002). We confirmed these results for acutely isolated mouse hippocampal neurons using 50 μm OAG, which produces a maximal effect in rat hippocampal neurons (Cantrell et al., 1996). OAG rapidly reduced peak Na+ current without major changes in the current time course (Fig. 1). The mean reduction after OAG treatment was 27.1 ± 2.4% (n = 8; p < 0.01). To confirm that this effect was caused by activation of PKC, we performed identical experiments with a specific peptide inhibitor of PKC, PKC19-36 (House and Kemp, 1987), in the pipette solution. OAG had little effect in cells dialyzed intracellularly with 2 μm PKC19-36 (<5%; n = 3) (data not shown). This confirms that the effect of OAG is via activation of PKC. Thus, in mouse hippocampal neurons, activation of PKC with OAG reduces Na+ current to a similar extent as has been observed previously in other central neuron preparations (Linden and Routtenberg, 1989; West et al., 1991; Cantrell et al., 1996).
Figure 1.
Reduction of Na+ currents in mouse hippocampal neurons by OAG. Na+ currents were recorded every 5 s in response to a depolarization to -10 mV from a holding potential of -80 mV. A, Na+ current traces in the absence (larger trace) and in the presence (smaller trace) of 50 μm OAG. The dashed line indicates the zero current level. B, Time course of OAG inhibition of Na+ current. Time of OAG application is indicated by the bar.
Requirement for specific PKC isoforms for reduction of peak Na+ currents by OAG
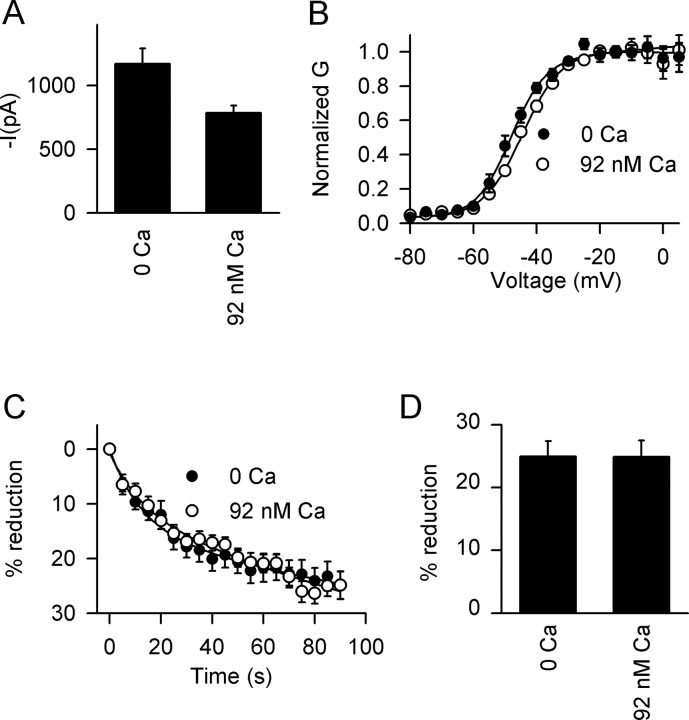
Multiple PKC isozymes with different sensitivities to Ca2+ and lipid activators are expressed in the brain (Nishizuka, 1992, 1995). To examine the requirement for intracellular Ca2+, we measured the effects of OAG in an intracellular solution containing 10 mm EGTA in the absence of added Ca2+ and in 10 mm EGTA plus 4 mm Ca2+ to give 92 nm free Ca2+, a concentration that is close to physiological levels of resting intracellular Ca2+. The amplitude of the mean peak Na+ current was smaller in 92 nm Ca2+ compared with nominally zero Ca2+ conditions (Fig. 2A), but the voltage dependence of activation was similar in the two solutions (Fig. 2B). Importantly, the reduction in peak Na+ current by OAG was similar in both conditions (Fig. 2C,D). These results indicate that the effect of OAG is not Ca2+ dependent and suggest that it is not mediated by the Ca2+-sensitive conventional PKC isozymes α, β, and γ (Nishizuka, 1992; Way et al., 2000).
Figure 2.
Effect of [Ca2+]i on Na+ current and its modulation by OAG in mouse hippocampal neurons. A, Amplitude of Na+ current with the indicated concentrations of free [Ca2+]i. Free Ca2+ was calculated using WebMaxchelator v2.10 with intracellular solutions containing 10 mm EGTA. Mean peak Na+ current was 785 ± 57 pA in 92 nm [Ca2+]i (n = 9) compared with 1169 ± 120 pA (n = 10) in 0 nm. B, Effect of [Ca2+]i on steady-state activation. Half-maximal activation (V1/2) for 0 nm [Ca2+]i was -45.1 ± 1.4 (n = 8) and for 92 nm [Ca2+]i was -44.8 ± 1.9 (n = 5; p > 0.05). C, Time course of modulation by OAG with 0 nm (filled circles) and 92 nm (open circles) [Ca2+]i. The data were normalized to the current measured before OAG application. D, Maximal effect of 50 μm OAG on peak Na+ current as a function of [Ca2+]i. Mean reductions were 24.9 ± 2.5% at 0 nm Ca2+ (n = 14) and 24.9 ± 2.6% at 92 nm Ca2+ (n = 8).
We next used compounds that differentially inhibit different PKC isozymes to further identify those involved in the effect of OAG. Gö6976 inhibits conventional PKCs and the related enzyme PKD1 in the nanomolar concentration range, but novel PKCs are not blocked by even micromolar concentrations (Martiny-Baron et al., 1993; Gschwendt et al., 1996). In the presence of 1 μm Gö6976, OAG inhibited 19.1 ± 0.02% (n = 8; p < 0.01) of the Na+ current compared with 25.4 ± 2.6% (n = 8) in the absence of the inhibitor. The partial (25%) reduction of the OAG effect by 1 μm Gö6976 suggests that, although a small portion of the response may be mediated by conventional PKCs or by PKD1, the response is primarily mediated by novel PKC isozymes.
To further test which nonconventional PKC isoforms are involved in neuromodulation of Na+ channels, we studied OAG inhibition in nominally zero Ca2+ (0.1 mm BAPTA) conditions using additional PKC inhibitors. In the presence of rottlerin, which selectively inhibits PKCδ at 5 μm (Vuong et al., 2000), OAG caused a 35.8 ± 7.1% reduction in peak of Na+ current (data not shown) (n = 3; p > 0.05). This experiment suggested that PKCδ is not important in reducing Na+ currents in mouse hippocampal neurons. Although higher concentrations of rottlerin inhibit other PKC isozymes (Gschwendt et al., 1994), these concentrations proved toxic to our cells.
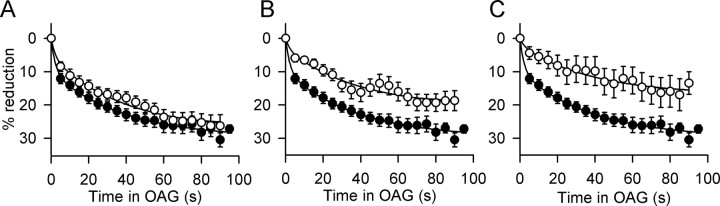
Ro-31-8425 also distinguishes between PKC isozymes (Wilkinson et al., 1993), showing threefold to fourfold selectivity for conventional PKCs (IC50 = 8-14 nm) versus PKCϵ (IC50 = 39 nm). In the presence of 20 nm Ro-31-8425, the effect of OAG on peak Na+ currents was indistinguishable from control (Fig. 3A). When the Ro-31-8425 concentration was raised to 100 nm or 1 μm, the response to OAG was markedly inhibited (Fig. 3B,C). This result is consistent with a primary effect of PKCϵ on Na+ channels in hippocampal neurons.
Figure 3.
Effect of Ro-31-8425 on OAG inhibition of Na+ current. Mean time courses for reduction of peak Na+ currents by 50 μm OAG are shown in the absence (filled circles) and presence (open circles) of Ro-31-8425. A, Ro-31-8425 (20 nm) (28.4 ± 2.1% reduction; p < 0.05; n = 8). B, Ro-31-8425 (100 nm) (19.3 ± 2.6% reduction; p < 0.05; n = 8). C, Ro-31-8425 (1 μm) (13.6 ± 3.5% reduction; p < 0.05; n = 7). Without Ro-31-8425, OAG inhibited Na+ currents by 27.1 ± 2.4% (p < 0.01; n = 8).
Effects of peptide inhibitors of PKC anchoring on modulation of Na+ currents
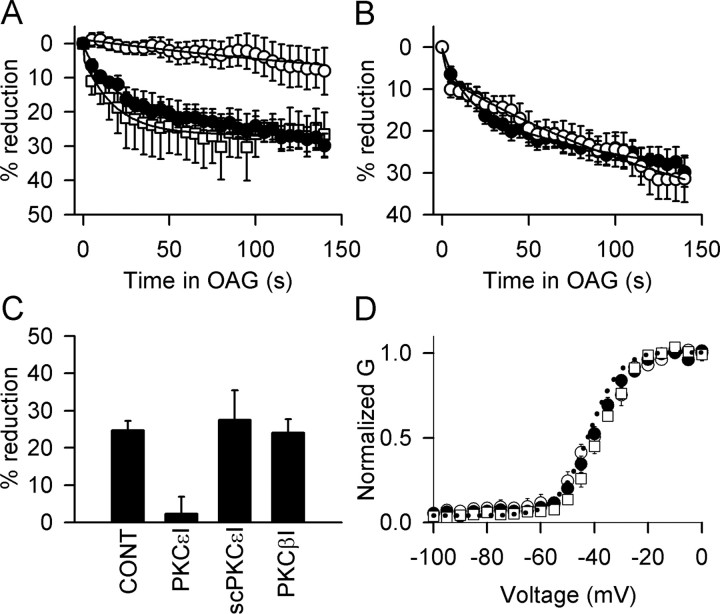
RACKs are postulated to bind PKC isozymes once they are activated and target them to specific membrane compartments and substrates (Mochly-Rosen et al., 1990, 1991; Ron and Mochly-Rosen, 1994). Two such proteins have been identified: RACK1, which binds PKCβ (Ron et al., 1994, 1995), and RACK2, also known as β′COP, a coatamer protein that binds PKCϵ (Csukai et al., 1997). An eight amino acid motif (EAVSLKPT) from PKCϵ binds to RACK2, and a synthetic peptide with this sequence blocks both the translocation and the functional effects of PKCϵ (Ron and Mochly-Rosen, 1994). We included a peptide corresponding to this sequence (PKCϵ-I; 200 μm) in the intracellular recording solution to test its effect on inhibition of Na+ currents by OAG. After allowing 2 min for the peptide to diffuse into the cell through the pipette tip, the effect of OAG was tested. In the presence of this peptide, the response to OAG was reduced to 2.3 ± 4.6%, significantly less than control (p < 0.01; n = 6) (Fig. 4A,C), without effect on the voltage dependence of activation (Fig. 4D). In the presence of a scrambled form of this peptide (LSETKPAV), the average OAG effect was 27.5 ± 7.9% (p < 0.01; n = 4) (Fig. 4C), not significantly different from effects of OAG in the absence of peptide (24.7 ± 2.5%; p > 0.05; n = 14). These data suggest that PKCϵ mediates at least 90% of the OAG effect.
Figure 4.
Effect of peptide inhibitors of interaction with RACKs on the reduction of peak Na+ currents by OAG. A, Time course for OAG reduction of Na+ currents in mouse hippocampal neurons recorded in the absence (filled circles) or presence (open circles) of the PKCϵ translocation inhibitor peptide PKCϵ-I. The scrambled version of this peptide, scPKCϵ-I (open squares) did not block the effect of OAG. B, Time course for OAG inhibition in the absence (filled circles) and presence (open circles) of the PKCβ translocation inhibitor peptide PKCβ-I. C, Summary bar graph showing mean reductions in peak Na+ current for untreated control (CONT) cells (24.7 ± 2.5%; n = 14) and for cells treated with PKCϵ-I (2.3 ± 4.6%; n = 6), scPKCϵ-I (27.5 ± 7.9%; n = 4), and PKCβ-I (24.1 ± 3.6%; n = 5). D, Voltage dependence of activation of the Na+ current; control cells (dotted line; V1/2 = -41.9 ± 1.1 mV; n = 7), PKCϵ-I (open circles; V1/2 = -38.7 ± 1.4 mV; n = 6), PKCβ-I (open squares; V1/2 = -38.4 ± 1.4 mV; n = 6), and scPKCϵ-I (filled circles; V1/2 = -40.8 ± 1.5 mV; n = 7).
PKCβ has been implicated in the modulation of Ca+ current in cardiac myocytes (Zhang et al., 1997). Another peptide (SLNPEWNET, PKCβI) selectively blocks the translocation of PKCβ and thus prevents its effects (Ron et al., 1995). When 200 μm of this peptide was included in the recording pipette, the reduction in Na+ current produced by OAG was indistinguishable from control (Fig. 4B) (24.1 ± 3.6%; n = 5), and there was no effect on the voltage dependence of activation (Fig. 4D). These data confirm that PKCβ is not involved in the reduction of Na+ current by OAG in mouse hippocampal neurons under our recording conditions and demonstrates that block of OAG inhibition is specific for the PKCϵ inhibitor peptide.
Modulation of Na+ currents in hippocampal neurons from PKCϵ-/- mice
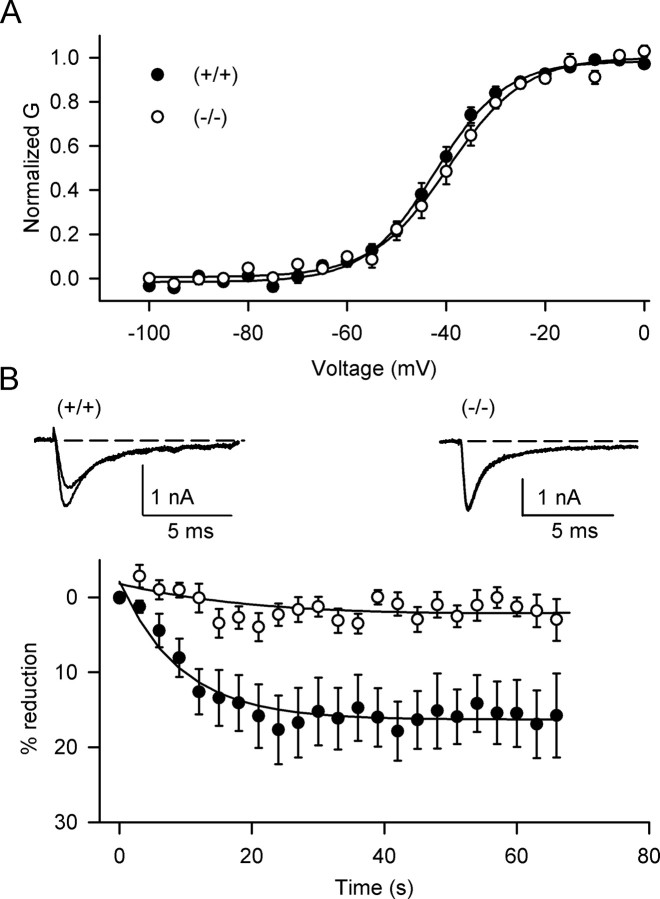
The preceding experiments implicated PKCϵ in modulation of Na+ channels in hippocampal neurons. PKCϵ-/- mice (Khasar et al., 1999) provide an additional stringent test of the role of PKCϵ in Na+ channel modulation. The voltage for half-activation of peak Na+ current in hippocampal neurons from PKCϵ-/- mice was -43.7 ± 1.6 mV (n = 7), indistinguishable from -46.1 ± 2.2 mV in neurons from PKCϵ+/+ mice (p > 0.05; n = 7) (Fig. 5A). The voltage dependence of half-inactivation of Na+ currents in PKCϵ+/+ (-60.4 ± 0.6 mV) and PKCϵ-/- (-65.0 ± 1.6 mV) neurons was also similar (p > 0.05; n = 7).
Figure 5.
Reduction of peak Na+ currents in PKCϵ-/- mice by OAG. A, Voltage dependence of activation of the Na+ current in hippocampal neurons; PKCϵ+/+ (V1/2 = -46.1 ± 2.2 mV; n = 7), PKCϵ-/- (V1/2 = -43.7 ± 1.6 mV; n = 7). B, Inset, Representative Na+ currents in the absence and presence of 50 μm OAG from wild-type (left) and PKCϵ-/- mice (right). Dashed lines indicate zero current level. The smaller current was recorded during treatment with OAG. Bottom, Time course of OAG effect on Na+ currents. PKCϵ-/- mice, Open circles; PKCϵ+/+ mice, filled symbols. The mean maximal inhibition of Na+ current by OAG in PKC-/- mice was 1.28 ± 1.25% (n = 7) and for wild-type littermates was 16.9 ± 4.5% (n = 7; p < 0.01).
We compared the effect of OAG on hippocampal neurons from PKCϵ-/- mice and their wild-type siblings. OAG reduced peak Na+ current in PKCϵ+/+ neurons by 16.9 ± 4.5% (n = 7) (Fig. 5B). In contrast, OAG produced no significant effect on Na+ current in the PKCϵ-/- neurons analyzed in parallel (1.28 ± 1.25%; n = 7; p < 0.01). Thus, absence of PKCϵ had no effect on basal Na+ channel properties but completely prevented OAG-induced inhibition of Na+ currents in hippocampal neurons.
It was surprising that the effect of OAG on neurons from wild-type littermates of PKCϵ-/- mice was smaller (16.9 ± 4.5%; p < 0.05) than with neurons from C57BL/6J mice (27.1 ± 2.4%) (Figs. 1, 2, 3, 4) or from C57BL/6J × 129/Sv mice studied contemporaneously. Because wild-type and PKCϵ-/- littermates used in Figure 5 were C57BL/6J × 129/SvJae, we suspect that the difference in the effect of OAG is attributable to differences in genetic background.
Converging evidence for modulation of Na+ channels by PKCϵ
Previous studies showed that acetylcholine acts via activation of muscarinic acetylcholine receptors and consequent activation of PKC to reduce Na+ currents in hippocampal neurons (see Introduction). We used multiple approaches to identify the PKC isozyme(s) responsible for modulation of Na+ currents: differential activation by calcium and diacylglycerols, selective inhibition by PKC inhibitors and anchoring inhibitor peptides, and targeted gene deletion. These approaches have different strengths and weaknesses, but the results considered together provide strong support for an essential role for anchored PKCϵ in modulation of Na+ channels in hippocampal neurons. These points and the physiological significance of modulation of Na+ channels by PKCϵ are considered in the Discussion.
Discussion
PKCϵ specifically modulates Na+ channels in hippocampal neurons
The diacylglycerol analog OAG effectively activates endogenous PKC isozymes that are responsible for modulation of Na+ channels by muscarinic acetylcholine receptors (Cantrell et al., 1996, 2002). Because conventional (α, β, and γ) and novel (δ, ϵ, θ, and η) isozymes respond to OAG, whereas atypical isozymes (ζ and τ/λ) do not, these results suggest that atypical PKCs are not involved in modulation of Na+ channels. Similarly, because activation of conventional isozymes is enhanced by increased intracellular Ca2+, whereas activation of novel and atypical isozymes is not (Nishizuka, 1992), our experiments showing similar OAG-induced modulation at nominally zero intracellular Ca2+ and 92 nm Ca2+ argue against a requirement for conventional PKCs and narrow the focus to the novel isozymes δ, ϵ, θ, and η.
Gö6976 is widely used to distinguish conventional and novel PKC isozymes, because it inhibits conventional PKCs in the nanomolar range but does not inhibit novel PKCs, even at micromolar concentrations (Martiny-Baron et al., 1993; Gschwendt et al., 1996). We found that Gö6976 inhibited <25% of the OAG effect, which is consistent with a primary role for the novel PKC isozymes in Na+ channel modulation. Similarly, the requirement for 100 nm or 1 μm Ro-31-8425 for inhibition of the effect of OAG favored a requirement for PKCϵ rather than conventional PKCs (Wilkinson et al., 1993). The lack of effect of 5 μm rottlerin suggested that PKCδ was not involved (Vuong et al., 2000), leaving PKCϵ, θ, and η as primary candidates. Because PKCϵ is highly expressed in hippocampal neurons (Naik et al., 2000; Nishizuka, 1995), these pharmacological results make it a likely candidate for modulation of Na+ channels.
PKC isoforms are translocated to cell membranes after activation and are bound there by interaction with RACKs (Mochly-Rosen et al., 1990, 1991; Ron and Mochly-Rosen, 1994). Inhibitor peptides derived from PKCβ can disrupt its binding to RACK1, and an inhibitor peptide derived from PKCϵ can disrupt its binding to RACK2 (Schechtman and Mochly-Rosen, 2001). These peptides are specific inhibitors of the corresponding PKC isozymes. We found that PKCϵ-I reduced Na+ channel modulation by OAG, but PKCβ-I had no effect, further supporting the primary role of PKCϵ.
To test the role of PKCϵ genetically, we studied PKCϵ-/- mice. These mice have altered nociception (Khasar et al., 1999) and markedly reduced ethanol self-administration (Hodge et al., 1999), indicating that PKCϵ has unique signaling functions in neurons that cannot be fully compensated by other PKC isozymes. Remarkably, the reduction of peak Na+ currents by activation of PKC with OAG was completely lost in hippocampal neurons from these mice. Thus, neurotransmitters that modulate Na+ currents through activation of PKC depend entirely on PKCϵ in hippocampal neurons.
Our results with PKCϵ-I and PKCϵ-/- mice showed complete block of the effect of OAG, consistent with an essential role for PKCϵ in Na+ channel modulation. In contrast, the conventional PKC inhibitor Gö6976 reduced the effect of OAG by 25%. However, Gö6976 is also a potent inhibitor of PKD1, which can be activated by PKCϵ and may lie downstream of PKCϵ in some signaling pathways (Gschwendt et al., 1996; Brandlin et al., 2002). Therefore, our findings indicate that direct phosphorylation by anchored PKCϵ plays the major role in modulating hippocampal Na+ channels, suggesting that PKCϵ has either unique access to, or unique substrate specificity for, the PKC phosphorylation sites on brain Na+ channels. In addition, PKCϵ may have a smaller indirect effect on Na+ channels through regulation of PKD1.
PKCϵ links Na+ channel modulation to neurotransmitter-activated lipid signaling pathways
An important consequence of the essential role for PKCϵ in Na+ channel modulation is the resulting restriction of upstream signaling pathways that can activate regulation. PKCϵ is not activated by Ca2+ but responds to many lipid messengers, including diacylglycerols, free fatty acids such as arachidonic acid, and phosphatidylinositol 1,4,5-trisphosphate (Nishizuka, 1995). The neurotransmitter receptors that activate these lipid signaling pathways, but not those that primarily activate Ca2+ signaling, are now prime candidates for Na+ channel regulation. Activation of specific isozymes is an important determinant of the physiological outcome of PKC signaling. For example, PKCδ exacerbates while PKCϵ protects against cardiac ischemia, and PKCγ increases while PKCϵ inhibits alcohol modulation of GABA receptors (Choi and Messing, 2003). In addition, four different Na+ channels are expressed in the CNS (Goldin, 2001). NaV1.1 channels have virtually identical PKC phosphorylation sites to NaV1.2 (Cantrell et al., 2002), suggesting similar regulation, whereas NaV1.3 and NaV1.6 channels have amino acid changes flanking the potentially phosphorylated serines that may alter their regulation. Future research will likely identify isozyme-specific consequences of PKC regulation of specific subtypes of Na+ channels.
Anchoring PKCϵ enhances modulation of Na+ channels in hippocampal neurons
Because targeting of PKC isozymes plays a critical role in their substrate specificity, the striking requirement for PKCϵ for Na+ channel modulation suggests that it might be specifically targeted to these channels. Consistent with this idea, the translocation inhibitor peptide PKCϵ-I substantially reduced PKC modulation of Na+ channels. Thus, stable anchoring of activated PKCϵ is required for Na+ channel modulation in brain neurons, as previously observed for cardiac cells (Xiao et al., 2001). These results raise the possibility that activated PKCϵ might be targeted to Na+ channels by protein-protein interactions. Previous results on dopamine modulation of Na+ channels by the PKA pathway have shown that direct binding of A kinase-anchoring protein-15 to the intracellular linker connecting domains I and II is required for modulation (Tibbs et al., 1998; Cantrell et al., 1999, 2002). Additional experiments will be required to determine whether protein-protein interactions are also involved in Na+ channel modulation by PKCϵ.
Potential physiological effects of modulation of Na+ channels by anchored PKCϵ
Na+ channels participate in integration of depolarizing synaptic inputs in dendrites and cell bodies and initiate and conduct action potentials in axons and complex nerve terminal ramifications (see Introduction). Modulation of Na+ channels by PKC is likely to affect integration of depolarizing inputs in dendrites and threshold and frequency of firing of action potentials in cell bodies and axonal initial segments (Johnston et al., 1999; Stuart, 1999). Cholinergic input to the hippocampus acts via muscarinic acetylcholine receptors and PKC to inhibit intrinsic bursting activity of CA1 neurons, converting their firing pattern from phasic bursting to tonic firing of single spikes (Azouz et al., 1994). These events are correlated with reduction of persistent Na+ current in CA1 neurons (Alroy et al., 1999), which is likely to be accompanied by a similar reduction in transient Na+ current (Cantrell et al., 1996). Because generation of bursts is a major mechanism of information transmission (Lisman, 1997), these changes in firing pattern are likely to affect input-output relationships in hippocampal neurons. Our results indicate that these modulatory events are mediated specifically by PKCϵ.
Although our experiments have focused on hippocampal neurons, it is likely that Na+ channels are modulated by a similar mechanism in other neurons, because Na+ channels and PKCϵ are widely coexpressed. Muscarinic acetylcholine receptor activation in neocortical neurons inhibits persistent Na+ current evoked by prolonged depolarization (Mittmann and Alzheimer, 1998). Persistent Na+ current is active near critical subthreshold voltages where other ionic currents are small (Mittmann and Alzheimer, 1998), and modulation of its functional properties is thought to contribute to synaptic integration, regulation of intrinsic firing patterns, and active backpropagation of action potentials from the soma into the dendrites. The signaling mechanism underlying modulation of persistent Na+ current by muscarinic receptor activation is likely to be activation of PKC-mediated phosphorylation (Mittmann and Alzheimer, 1998). Similarly, activation of 5-HT2a/c receptors in cortical pyramidal neurons also reduces peak Na+ currents through activation of PKC (Carr et al., 2002). Moreover, in striatal medium spiny neurons and cholinergic interneurons, activation of D2 dopamine receptors reduces Na+ currents through a PKC-dependent mechanism (Maurice et al., 2001). D1 and D2 dopamine receptors are coexpressed and reciprocally modulate Na+ current through cAMP and PKA in a subset of medium spiny neurons (Surmeier et al., 1992; Schiffman et al., 1996; Aizman et al., 2000). However, the primary effect of D agonists on Na+2 channels in medium spiny neurons is inhibitory (Surmeier et al., 1992), and this effect is mediated via a G-protein- and PKC-dependent pathway (Surmeier et al., 1993). Based on our findings, we suspect that these modulatory events in cortical and striatal neurons are also likely to involve phosphorylation of Na+ channels by PKCϵ, but additional experiments will be required to establish this mechanism.
In contrast to their inhibition of brain Na+ channels, PKA and PKC act synergistically to enhance the activity of slowly inactivating Na+ channels in nociceptive dorsal root ganglion neurons (Gold et al., 1998). Therefore, although PKCϵ has many modulatory targets in neurons, the loss of modulation of Na+ channels in PKCϵ-/- mice may well contribute to the altered nociception reported previously in these animals (Khasar et al., 1999). As these examples illustrate, modulation of Na+ channels by PKCϵ likely has broad functional significance in the nervous system.
Footnotes
This work was supported by National Institutes of Health Research Grants R01 NS15751 (W.A.C.) and K01 MH01669 (A.R.C.).
Correspondence should be addressed to William A. Catterall, Department of Pharmacology, Mailstop 357280, University of Washington, Seattle, WA 98195-7280. E-mail: wcatt@u.washington.edu.
A. R. Cantrell's present address: Department of Neurobiology and Anatomy, University of Tennessee Medical Center, 855 Monroe Avenue, Suite 515, Memphis, TN 38163.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250507-07$15.00/0
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A (2000) Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3: 226-230. [DOI] [PubMed] [Google Scholar]
- Alroy G, Su H, Yaari Y (1999) Protein kinase C mediates muscarinic block of intrinsic bursting in rat hippocampal neurons. J Physiol (Lond) 518: 71-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Jensen MS, Yaari Y (1994) Muscarinic modulation of intrinsic burst firing in rat hippocampal neurons. Eur J Neurosci 6: 961-966. [DOI] [PubMed] [Google Scholar]
- Brandlin I, Eiseler T, Salowsky R, Johannes FJ (2002) Protein kinase Cμ regulation of the JNK pathway is triggered via phosphoinositide-dependent kinase 1 and protein kinase Cϵ. J Biol Chem 277: 45451-45457. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Catterall WA (2001) Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci 2: 397-407. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Ma JY, Scheuer T, Catterall WA (1996) Muscarinic modulation of Na+ current by activation of protein kinase C in rat hippocampal neurons. Neuron 16: 1019-1026. [DOI] [PubMed] [Google Scholar]
- Cantrell AR, Tibbs VC, Scheuer T, Catterall WA (1999) Dopaminergic modulation of voltage-gated Na+ current in rat hippocampal neurons requires anchoring of cAMP-dependent protein kinase. J Neurosci 19: RC21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Tibbs VC, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T (2002) Molecular mechanism of convergent regulation of brain Na+ channels by protein kinase C and protein kinase A anchored to AKAP-15. Mol Cell Neurosci 21: 63-80. [DOI] [PubMed] [Google Scholar]
- Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ (2002) Serotonin receptor activation inhibits Na+ current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci 22: 6846-6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Day M, Cantrell AR, Held J, Scheuer T, Catterall WA, Surmeier DJ (2003) Transmitter modulation of slow, activity-dependent alterations in Na+ channel availability endows neurons with a novel form of cellular plasticity. Neuron 39: 793-806. [DOI] [PubMed] [Google Scholar]
- Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated Na+ channels. Neuron 26: 13-25. [DOI] [PubMed] [Google Scholar]
- Choi DS, Messing RO (2003) Animal models in the study of protein kinase C isozymes. Methods Mol Biol 233: 455-473. [DOI] [PubMed] [Google Scholar]
- Costa MR, Catterall WA (1984) Phosphorylation of the alpha subunit of the Na+ channel by protein kinase C. Cell Mol Neurobiol 4: 291-297. [DOI] [PubMed] [Google Scholar]
- Csukai M, Chen CH, De Matteis MA, Mochly-Rosen D (1997) The coatomer protein β′-COP, a selective binding protein RACK for protein kinase Cϵ. J Biol Chem 272: 29200-29206. [DOI] [PubMed] [Google Scholar]
- Dascal N, Lotan I (1991) Activation of protein kinase C alters voltage dependence of a Na+ channel. Neuron 6: 165-175. [DOI] [PubMed] [Google Scholar]
- Gold MS, Levine JD, Correa AM (1998) Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization in rat sensory neurons in vitro J Neurosci 18: 10345-10355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL (2001) Resurgence of sodium channel research. Annu Rev Physiol 63: 871-894. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Snutch T, Lübbert H, Dowsett A, Marshall J, Auld V, Downey W, Fritz LC, Lester HA, Dunn R, Catterall WA, Davidson N (1986) Messenger RNA coding for only the α subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc Natl Acad Sci USA 83: 7503-7507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F (1994) Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun 199: 93-98. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ (1996) Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett 392: 77-80. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO (1999) Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCϵ. Nat Neurosci 2: 997-1002. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol (Lond) 117: 500-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C, Kemp BE (1987) Protein kinase C contains a pseudosubstrate prototype in its regulatory domain. Science 238: 1726-1728. [DOI] [PubMed] [Google Scholar]
- Isom LL, De Jongh KS, Patton DE, Reber BF, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA (1992) Primary structure and functional expression of the β1 subunit of the rat brain Na+ channel. Science 256: 839-842. [DOI] [PubMed] [Google Scholar]
- Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, Catterall WA (1995) Structure and function of the β2 subunit of brain Na+ channels, a transmembrane glycoprotein with a CAM motif. Cell 83: 433-442. [DOI] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Colbert CM, Magee JC (1999) Regulation of back-propagating action potentials in hippocampal neurons. Curr Opin Neurobiol 9: 288-292. [DOI] [PubMed] [Google Scholar]
- Kay AR, Wong RK (1987) Ca2+ current activation kinetics in isolated pyramidal neurones of the Ca1 region of the mature guinea-pig hippocampus. J Physiol (Lond) 392: 603-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO (1999) A novel nociceptor signaling pathway revealed in protein kinase C epsilon mutant mice. Neuron 24: 253-260. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Routtenberg A (1989) cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol (Lond) 419: 95-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE (1997) Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 20: 38. [DOI] [PubMed] [Google Scholar]
- Lotan I, Dascal N, Naor Z, Boton R (1990) Modulation of vertebrate brain Na+ and K+ channels by subtypes of protein kinase C. FEBS Lett 267: 25-28. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kopchs G, Hug H, Marme D, Schachtele C (1993) Selective inhibition of protein kinase C isozymes by the indocarbazole Gö6976. J Biol Chem 268: 9194-9197. [PubMed] [Google Scholar]
- Maurice N, Tkatch T, Meisler M, Sprunger LK, Surmeier DJ (2001) D1/D5 dopamine receptor activation differentially modulates rapidly inactivating and persistent Na+ currents in prefrontal cortex pyramidal neurons. J Neurosci 21: 2268-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittmann T, Alzheimer C (1998) Muscarinic inhibition of persistent Na+ current in rat neocortical pyramidal neurons. J Neurophysiol 79: 1579-1582. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D, Henrich CJ, Cheever L, Khaner H, Simpson PC (1990) A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul 1: 693-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D, Khaner H, Lopez J (1991) Identification of intracellular receptor proteins for activated protein kinase C. Proc Natl Acad Sci USA 88: 3997-4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, Lee K, Pinnock RD, Hughes J, Richardson PJ, Mizuguchi K, Jackson AP (2000) β3: An additional auxiliary subunit of the voltage-sensitive Na+ channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci USA 97: 2308-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BJ, Catterall WA (1992) Phosphorylation of purified rat brain Na+ channel reconstituted into phospholipid vesicles by protein kinase C. J Biol Chem 267: 16129-16134. [PubMed] [Google Scholar]
- Naik MU, Benedikz E, Hernandez I, Libien J, Hrabe J, Valsamis M, Dow-Edwards D, Osman M, Sacktor TC (2000) Distribution of protein kinase Mζ and the complete protein kinase C isoform family in rat brain. J Comp Neurol 426: 243-258. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1992) Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258: 607-614. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y (1995) Protein kinase C and lipid signaling for sustained cellular responses. FASEB J 9: 484-496. [PubMed] [Google Scholar]
- Noda M, Ikeda T, Suzuki H, Takeshima H, Takahashi T, Kuno M, Numa S (1986) Expression of functional Na+ channels from cloned cDNA. Nature 322: 826-828. [DOI] [PubMed] [Google Scholar]
- Numann R, Catterall WA, Scheuer T (1991) Functional modulation of brain Na+ channels by protein kinase C phosphorylation. Science 254: 115-118. [DOI] [PubMed] [Google Scholar]
- Ron D, Mochly-Rosen D (1994) Agonists and antagonists of protein kinase C function, derived from its binding proteins. J Biol Chem 269: 21395-21398. [PubMed] [Google Scholar]
- Ron D, Chen CH, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D (1994) Cloning of an intracellular receptor for protein kinase C: a homolog of the β subunit of G proteins. Proc Natl Acad Sci USA 91: 839-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Luo J, Mochly-Rosen D (1995) C2 region-derived peptides inhibit translocation and function of β protein kinase C in vivo J Biol Chem 270: 24180-24187. [DOI] [PubMed] [Google Scholar]
- Schechtman D, Mochly-Rosen D (2001) Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene 20: 6339-6347. [DOI] [PubMed] [Google Scholar]
- Schiffman SN, Lledo P-M, Vincent J-D (1996) Dopamine D1 receptor modulates the voltage-gated Na+ current in rat striatal neurones through a protein kinase A. J Physiol (Lond) 483: 95-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer W, Dascal N, Lotan I, Wallner M, Weigl L (1991) Molecular mechanism of protein kinase C modulation of Na+ channel α-subunits expressed in Xenopus oocytes. FEBS Lett 291: 341-344. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R (1988) Activation of protein kinase C differentially modulates neuronal Na+, Ca2+, and gamma-aminobutyrate type A channels. Proc Natl Acad Sci USA 85: 6192-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G (1999) Voltage-activated Na+ channels amplify inhibition in neocortical pyramidal neurons. Nat Neurosci 2: 144-150. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Stefani A, Foehring RC, Kitai ST (1991) Developmental regulation of a slowly-inactivating potassium conductance in rat neostriatal neurons. Neurosci Lett 122: 41-46. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST (1992) Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA 89: 10178-10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Reiner A, Levine MS, Ariano MA (1993) Are neostriatal dopamine receptors co-localized? Trends Neurosci 16: 299-305. [DOI] [PubMed] [Google Scholar]
- Tibbs VC, Gray PC, Catterall WA, Murphy BJ (1998) AKAP15 anchors cAMP-dependent protein kinase to brain Na+ channels. J Biol Chem 273: 25783-25788. [DOI] [PubMed] [Google Scholar]
- Vuong H, Patterson T, Shapiro P, Kalvakolanu DV, Wu R, Ma WY, Dong Z, Kleeberger SR, Reddy SP (2000) Phorbol ester-induced expression of airway squamous cell differentiation marker, SPRR1B, is regulated by protein kinase Cδ/Ras/MEKK1/MKKK1-dependent AP-1 signal transduction pathway. J Biol Chem 275: 32250-32259. [DOI] [PubMed] [Google Scholar]
- Way KJ, Chou E, King GL (2000) Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci 21: 181-187. [DOI] [PubMed] [Google Scholar]
- West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA (1991) A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science 254: 866-868. [DOI] [PubMed] [Google Scholar]
- West JW, Scheuer T, Maechler L, Catterall WA (1992) Efficient expression of rat brain type IIA Na+ channel α subunits in a somatic cell line. Neuron 8: 59-70. [DOI] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS (1993) Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J 294: 335-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GQ, Qu Y, Sun ZQ, Mochlyh-Rosen D, Boutifir M (2001) Evidence for a functional role for ϵPKC isozyme in the regulation of cardiac sodium channels. Am J Physiol Cell Physiol 281: C1477-C1486. [DOI] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriara H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R (2003) Sodium channel β4, a new disulfide-linked auxiliary subunit with similarity to β2. J Neurosci 23: 7577-7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZH, Johnson JA, Chen L, El-Sherif N, Mochly-Rosen D, Boutjidir M (1997) C2 region-derived peptides of β-protein kinase C regulate cardiac calcium channels. Circ Res 80: 720-729. [DOI] [PubMed] [Google Scholar]