Abstract
Despite the fact that human Brodmann area 6 (BA6), a traditional “motor” area, is active during higher motor control involving various cognitive operations, the functional specialization within BA6 in the cognitive domain is essentially unknown. Furthermore, its functional relevance in cognition has been questioned because brain activity in BA6 during cognitive tasks has often been explained away as a concomitant, latent motor process. Therefore, we examined the structural-functional relationship of human BA6 in nonmotor cognitive functions and its functional relevance using both functional magnetic resonance imaging (fMRI) and repetitive transcranial magnetic stimulation (rTMS). Subjects performed mental-operation (MO) tasks in which they serially updated verbal and spatial mental representations (MO-v and MO-s). In the fMRI experiments, activity in the medial BA6 was more increased in MO-v, whereas the activity in the lateral BA6 in both hemispheres was more in MO-s. Low-frequency rTMS to the medial BA6 disrupted only the performance of MO-v, whereas rTMS to the lateral BA6 in both hemispheres disrupted only MO-s. Hence the converging results demonstrate a functional double dissociation in which medial BA6 has a critical role in updating verbal information and lateral BA6 has a role in updating spatial information. The present study provides direct physiological evidence of modality-specific cognitive function within human BA6.
Keywords: cognitive, premotor, cortex, magnetic, imaging, stimulation
Introduction
Increasing evidence indicates that some classically designated “motor” areas have roles in both motor and nonmotor cognitive functions (Ito, 1993; Leiner et al., 1993; Middleton and Strick, 1994; Doya, 2000; Imamizu et al., 2000; Picard and Strick, 2001). Brodmann area 6 (BA6), which bridges prefrontal and primary motor cortices, is likely one such cortical area. BA6 has long been recognized as a higher-order motor area (Fulton, 1935; Wise, 1985; Freund, 1990), and its motor functions in relation to anatomical subdivisions have been investigated extensively (Tanji and Shima, 1994; Picard and Strick, 1996; Tanji, 1996).
Recent neuroanatomical evidence has revealed that although the caudal parts of BA6 have a close relationship with primary motor cortex and send massive corticospinal projections, the rostral parts of BA6 have a close connectional relationship with pre-frontal cortex rather than with primary motor cortex and lack a direct projection to the spinal cord (Barbas and Pandya, 1987; Luppino et al., 1993; Lu et al., 1994). These data suggest that the function of the rostral part of BA6 is related more to the functions of prefrontal cortex than those of primary motor cortex. Neuroimaging studies in humans have demonstrated that BA6 is active not only during demanding motor tasks (Roland et al., 1980; Deiber et al., 1991, 1997; Catalan et al., 1998; Grafton et al., 1998), but also during various cognitive tasks (Jonides et al., 1993; Paulesu et al., 1993; Dehaene et al., 1996; Mellet et al., 1996; Lamm et al., 2001; Simon et al., 2002; Hanakawa et al., 2003a,b). Results vary among the studies, however, and the structural-functional relationships within BA6 for cognition are poorly understood compared with those for motor control (Picard and Strick, 2001; Schubotz and von Cramon, 2003). Furthermore, activity in BA6 during cognitive tasks that was revealed using neuroimaging has often been explained as a concomitant, latent motor process such as eye movement or preparation for button pressing, and thus the functional relevance of BA6 activity in cognition has always been questioned (Courtney et al., 1998; Haxby et al., 2000).
The aim of the current study is to clarify the structural-functional relationship within human BA6 for cognition and examine the functional relevance of activity in BA6 during cognitive tasks. Toward this aim, we used a combined approach of functional magnetic resonance imaging (fMRI) and subsequent repetitive transcranial magnetic stimulation (rTMS) to image activity and then transiently inhibit that activity in the same set of subjects performing the same behavioral tasks. This approach enabled us to investigate the functional relevance of brain activity using transient rTMS-induced “virtual lesions” (Hallett, 2000; Pascual-Leone et al., 2000; Sack and Linden, 2003). In the present study, we used verbal and spatial mental-operation (MO) tasks in which subjects were required to sequentially update verbal (MO-v) or spatial (MO-s) representations in memory. It has been reported that broad areas of BA6 are active during such mental operations, even when motor control is strictly excluded (Hanakawa et al., 2002, 2003a).
Materials and Methods
Subjects. Fourteen subjects (10 male and 4 female; mean age 25.4 ± 3.8 years) participated in both fMRI and rTMS studies. All subjects were right-handed as assessed using the Oldfield handedness questionnaire (Oldfield, 1971). None of the subjects had a history of psychiatric or neurological illness. All subjects gave written, informed consent before the experiments. The experiments were approved by the local ethics committee of the National Institute for Physiological Sciences.
Mental-operation tasks. Subjects performed MO-v and MO-s tasks requiring the sequential update of verbal or spatial representations in memory according to instruction stimuli (Fig. 1). Trials began with the visual presentation of a prime stimulus for 1.0 sec. For MO-v, the prime stimulus was a Japanese kanji character indicating a day of the week, and for MO-s, the prime stimulus was a marker in one of nine small subdivisions of a square grid. Subsequently, a random series of five to seven instruction stimuli consisting of numerals from 1 to 4 were presented for 0.5 sec each at a rate of 1.0 Hz for both tasks. For MO-v, subjects mentally advanced the day of the week according to instruction stimuli (e.g., the day was advanced from Sunday to Wednesday with an instruction stimulus of 3), and for MO-s, subjects mentally moved the marker clockwise on an imagined grid according to the instruction stimuli (e.g., the marker was moved from the top left corner to the top right corner with an instruction stimulus of 2). After presentation of all instruction stimuli, an answer stimulus was presented for 1.5 sec. The subjects were asked to judge whether the final internal representation from the mental operation matched the presented answer stimulus by pressing one of two response buttons with their right hand. All stimuli subtended a visual angle of 2.0°. The two tasks were identical in that the advancement of each representation was guided by numbers and there was a two-choice response, but they differed in the modality of the updated representation.
Figure 1.
Experimental paradigms for fMRI and rTMS experiments. For both tasks, a trial started with the presentation of a prime stimulus, followed by the presentation of instruction stimuli. Subjects updated a mental representation according to the instruction stimuli and were asked to judge whether the final internal representation from the mental operation matched the presented answer stimulus by pressing one of two response buttons.
fMRI experiment. The fMRI experiment was conducted using a 3.0 tesla MRI scanner (MAGNETOM Allegra, Siemens, Erlangen, Germany). Functional images were acquired using a T2*-weighted echo planar imaging sequence (repetition time/echo time/flip angle/field of view/voxel size/slice number = 2000 msec/30 msec/75°/192 mm/3.0 × 3.0 × 4.0 mm/34 axial slices). A high-resolution structural image was acquired using a magnetization-prepared rapid acquisition in gradient echo (MPRAGE) sequence. Presentation software (Neurobehavioral Systems, Albany, CA) was used for the visual stimulus presentation and to record the responses of the subjects. Stimuli were presented on a screen using a liquid crystal display projector, and subjects viewed the screen though a mirror.
Each experimental session consisted of five trials for each task in a randomized order. The intertrial interval (ITI) ranged from 21 to 23 sec, which allowed the fMRI signal to return to baseline. Each subject completed two experimental sessions with scanning. A total of 155 functional images were collected during each session, and the first 5 images were discarded from data analysis to allow for the stabilization of the magnetization. Before the fMRI experiment, subjects performed five experimental sessions outside the scanner to become familiar with the tasks.
SPM99 software (Wellcome Department of Cognitive Neurology, London, UK) was used for image processing and analysis. To reduce head-motion artifacts, the functional images were realigned to the first functional image (Friston et al., 1995a). For individual analysis, the images were smoothed spatially using an isotropic Gaussian kernel of 8 mm full-width half-maximum to increase the signal-to-noise ratio. A general linear model was used to identify voxels with task-related signal changes (Friston et al., 1995b). The task period was modeled using a boxcar function convolved with a hemodynamic response function, and significant correlations between the observed response and the modeled response were estimated, yielding t-value maps.
Group analysis was performed using anatomical normalization (Friston et al., 1995a) and a random effect model (Friston et al., 1999). The magnitude of the increase in activity in BA6 during the two tasks was compared. The statistical threshold was set to a p value of 0.001 without correction for multiple comparisons (corresponding to t = 3.79).
rTMS experiment. The rTMS experiment was conducted ∼1 week after the fMRI experiment. The tasks used for the rTMS experiment were essentially the same as those for the fMRI experiment except that the ITI was fixed at 1.5 sec. Subjects were seated on a chair ∼110 cm away from the viewing screen and performed the experimental sessions at three different time points (before, immediately after, and 30 min after rTMS). Each experimental session consisted of 15 trials of each task (i.e., 30 trials in total) performed in a random order.
The three locations (medial, left lateral, and right lateral BA6) functionally defined by fMRI for each subject were stimulated during separate sessions, with at least 1 week between each rTMS session. The order in which the locations were stimulated was pseudorandomized and counterbalanced across subjects. Medial BA6 was defined as the activated clusters during MO-v versus MO-s that straddled or were anterior to the vertical anterior commissure line (VAC) (Talairach and Tournoux, 1988; Picard and Strick, 1996), whereas lateral BA6 was defined as the activated clusters during MO-s versus MO-v at the conjunction of the superior frontal and superior precentral sulci (Rizzolatti et al., 1998; Hanakawa et al., 2002). Locations of the TMS targets were fairly consistent across subjects according to the stereotaxic coordinate system by Talairach and Tournoux (1988) as shown in Table 1. The resulting clusters were rendered on the structural image and then co-registered with the subject's head using a frameless stereotaxic system (Evans software, Tomiki Medical Instruments Corporation, Ishikawa, Japan). The coil was fixed on the scalp just above the target location using a mechanical holder (Point Setter, Mitaka Koki Corporation, Tokyo, Japan). The position was monitored continuously during rTMS using the above stereotaxic system.
Table 1.
Mean coordinates for the center of the targeted three locations across subjects for rTMS experiment
|
Stimulation location |
Mean coordinates ± SE (mm) |
||||
|---|---|---|---|---|---|
|
|
x
|
y
|
z
|
||
| Medial BA6 | −4 ± 1.1 | 8 ± 2.2 | 65 ± 1.0 | ||
| Left lateral BA6 | −25 ± 1.1 | 3 ± 3.3 | 56 ± 2.0 | ||
| Right lateral BA6 |
23 ± 1.0 |
4 ± 2.8 |
56 ± 2.0 |
||
The actual stimulation locations were determined based on the peak activation in each region individually defined in the fMRI experiment without anatomical normalization. Listed coordinates (x, y, z) were calculated by means of anatomical normalization (Friston et al., 1995a) based on the stereotaxic coordinate system by Talairach and Tournoux (1988). BA, Cytoarchitectonic fields designated by Brodmann.
rTMS was applied using a Magstim 220 (Magstim Company, Whitland, UK) and figure-eight coils, with each wing measuring 70 mm in diameter. During rTMS, subjects received 0.9 Hz biphasic 420 magnetic pulses at 70% of the maximum output of the stimulator. It is known that low-frequency rTMS inhibits cortical excitability for several minutes and temporarily impairs task performance (Chen et al., 1997; Maeda et al., 2000; Robertson et al., 2003). According to methods described previously (Beckers and Zeki, 1995; Corthout et al., 1999; Lewald et al., 2002), we used a fixed intensity defined by the stimulator output, not motor threshold, because previous studies indicated no intra-individual correlation between the excitability of different cortical areas, such as motor and visual cortices (Stewart et al., 2001). By the omission of the measurement of motor threshold, subjects have the advantage of the reduction of both the number of magnetic pulses received and total experimental time.
The transient inhibitory effect of rTMS was observable as an increase in reaction time rather than an increase in errors in the present experiments. Reaction time has proven to be a sensitive index of behavioral performance (Shapiro et al., 2001; Rushworth et al., 2002; Devlin et al., 2003; Kennerley et al., 2004).
Results
fMRI experiment
To measure task-specific BA6 activity, the differences in activity between the two tasks were compared. Activity in medial BA6 increased more during MO-v than during MO-s; conversely, activity in lateral BA6 increased more during MO-s than during MO-v (Fig. 2A). The increase in activity in medial BA6 during MO-v straddled or was anterior to the VAC, whereas that in lateral BA6 during MO-s was at the conjunction of the superior frontal and precentral sulci. These regions correspond to the pre-supplementary motor area (Deiber et al., 1991; Luppino et al., 1993; Picard and Strick, 1996; Tanji, 1996) and the rostral division of dorsal premotor cortex (Preuss et al., 1996) or pre-PMd, the termed used by Picard and Strick (2001). The onset and peak in brain activity in both medial and lateral BA6 preceded the answer stimuli and the subsequent motor responses (Fig. 2B); thus, the activity was likely related to mental manipulation rather than motor preparation or execution. Prefrontal cortex did not exhibit any significant differences in activity between the two tasks (Table 2).
Figure 2.
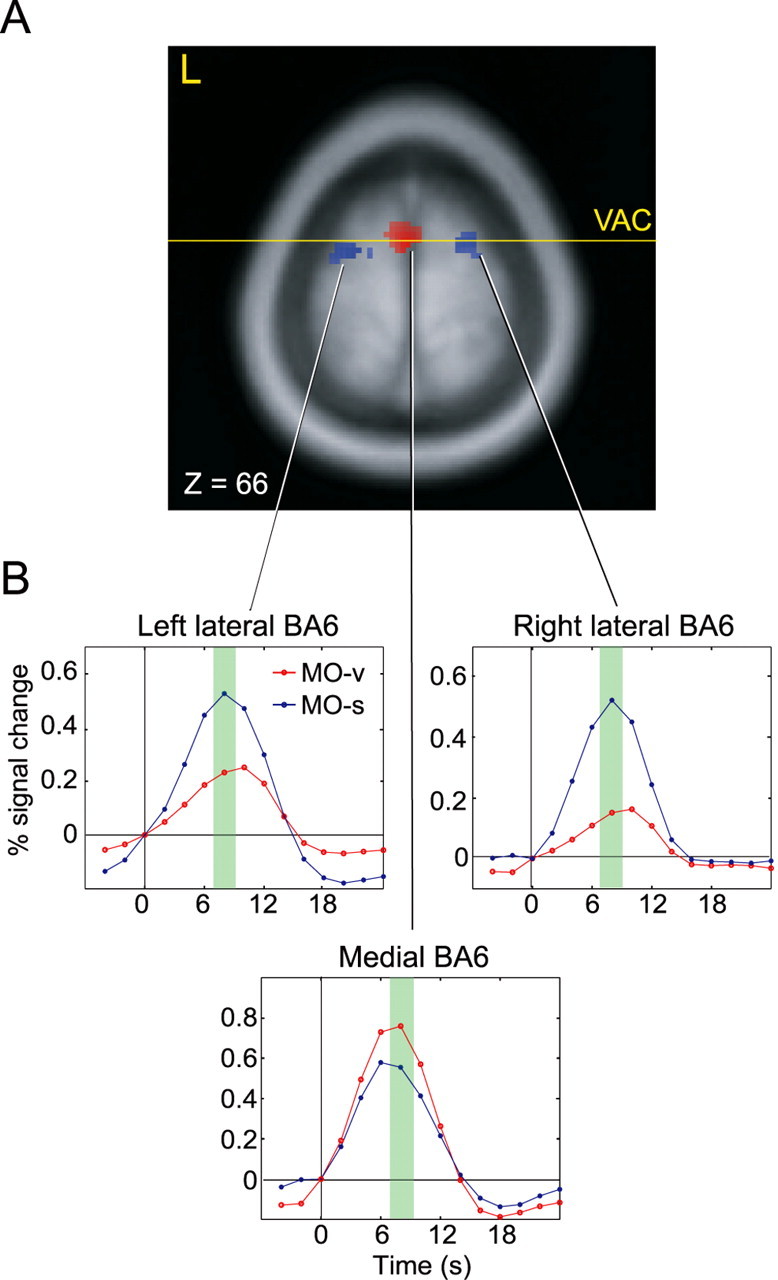
Results of the fMRI experiments. A, Group activation superimposed on a standardized anatomical image. The statistical threshold was set to a p value of 0.001. Medial BA6 (red) was more active during MO-v than MO-s [maximal difference at (x, y, z) = (0, 0, 66) with t = 5.42]. Left and right lateral BA6 (blue) were more active during MO-s than during MO-v [left, t = 6.16 at (-17, -2, 68); right, t = 7.38 at (22, 5, 55)]. VAC, Vertical anterior commissure. B, The time series of the fMRI signal in the voxel with the maximal difference in BA6 across subjects. The horizontal axis represents the time from the presentation of the prime stimulus, and the green shading indicates the time window within which answer stimuli were presented and the motor responses occurred.
Table 2.
Brain regions exhibiting a significant increase in BOLD signal during MO-v versus MO-s and vice versa
|
|
|
Coordinates (mm) |
|
||||
|---|---|---|---|---|---|---|---|
| Regions |
|
X
|
Y
|
Z
|
t value |
||
| MO-v > MO-s | |||||||
| Medial BA6 | 0 | 0 | 66 | 5.42 | |||
| Ventral BA6 | L | −51 | −8 | 41 | 6.21 | ||
| MO-s > MO-v | |||||||
| Lateral BA6 | L | −17 | −2 | 68 | 6.16 | ||
| R | 22 | 5 | 55 | 7.38 | |||
| BA7 (superior parietal lobule) | L | −20 | −65 | 48 | 9.40 | ||
|
|
R |
32 |
−57 |
49 |
11.00 |
||
Coordinates (x, y, z) indicate the voxel of maximal significance in each brain region according to the stereotaxic coordinate system by Talairach and Tournoux (1988). BA, Cytoarchitectonic fields designated by Brodmann. L, Left; R, right.
rTMS experiment
For each subject, the accuracy and median reaction time for the correct responses were calculated. Correlation between the accuracy and reaction time for each task was not significant (p > 0.10 for both tasks). Thus, there was no indication of a speed-accuracy trade-off.
The behavioral effect of rTMS was measured as a change in reaction time, which was calculated as the change in median reaction time immediately or 30 min after rTMS relative to that before rTMS (Fig. 3B). There was an increase in reaction time during MO-v immediately after rTMS, only when medial BA6 was stimulated, whereas there was an increase in reaction time during MO-s only when left or right lateral BA6 was stimulated (p < 0.05; one-sample t test). There was no change in reaction time 30 min after rTMS in any brain region. ANOVA revealed a significant three-way interaction (F(1,13) = 3.70; p < 0.05) among the factors of task, time, and stimulation site. This indicates that the effect of rTMS on the performance of the two tasks was different for each brain region.
Figure 3.

Results of the rTMS experiment. A, Coil position and activity in BA6 in a representative subject. The green bar indicates the position of the coil tangential to the scalp. The white bar indicates the direction of the magnetic pulse from the coil. B, The grand mean change in reaction time (ΔRT) across subjects (±SEM). Asterisks indicate a significant (p < 0.05) increase in reaction time as compared with the baseline reaction time before rTMS.
The baseline reaction time during MO-v was longer than during MO-s (MO-v, 703 msec; MO-s, 608 msec; p < 0.01), although task accuracy was comparable (MO-v, 95%; MO-s, 93%; NS); thus, the possibility exists that the task-specific rTMS effect in medial BA6 was caused by an increase in attentional load related to task difficulty (Pardo et al., 1990). To exclude this possibility, we examined the correlation of the difference in baseline reaction time between the two tasks (reaction time during MO-v minus reaction time during MO-s before rTMS, as a parameter for the difference in attentional load) with the difference in rTMS-evoked change in reaction time between the two tasks (change in reaction time during MO-v minus change in reaction time during MO-s, as a parameter for the rTMS effect). There was no significant correlation between these parameters (r = 0.025; p = 0.94).
Discussion
The results of the present study provide converging physiological evidence that the subdivisions of human BA6 have a critical role in cognitive processing in a modality-specific manner: medial and lateral BA6 are preferentially involved in the cognitive update of verbal and spatial representations, respectively. This suggests that the function of at least a part of this motor area is not restricted to motor control but relevant to nonmotor cognition. This is similar to the idea that subdivisions of the basal ganglia and cerebellum, previously regarded as pure motor areas, have cognitive functions (Ito, 1993; Leiner et al., 1993; Middleton and Strick, 1994; Schmahmann, 1997; Doya, 2000).
One advantage of the present study using both fMRI identification of activity and rTMS inhibition of that activity is that the functional relevance of brain activity in BA6 was demonstrated directly. In the fMRI experiments, region-specific brain activity was measured while subjects performed different cognitive tasks. Then, in the rTMS experiments, task performance was evaluated while magnetic stimulation interfered with region-specific brain activity. Thus, the dependent and independent variables were counterchanged between the two experiments, and the bidirectional investigation yielded more reliable information about the brain-behavior relationship than a single modality approach. Another advantage is that the “virtual lesion” effect induced by rTMS in normal subjects enabled us to test the structural-functional relationship in a more experimentally controlled way (Walsh and Rushworth, 1999) than is possible using clinical case studies on patients with specific pathological lesions (Sawamoto et al., 2002).
The double dissociation observed in the same group of subjects provides evidence against the possibility that the results are caused by artifactual effects of rTMS, such as the spreading of effects to neighboring regions or individual differences in cortical excitability. These data also speak against the idea that rTMS inhibited motor responses, because the required judgment, preparation, and motor response were identical in both tasks. Regarding task difficulty, there was no significant correlation between difference in attentional load for the two tasks and in the degree of rTMS effect on the performance of the two tasks. Thus, it is unlikely that the task-specific effect of rTMS in medial BA6 during MO-v was related simply to an increase in general attentional load.
Medial BA6 has been known to be involved in the motor expression of language process (Brickner, 1940; Penfield and Welch, 1951; Fried et al., 1991). Recent neuroimaging studies have suggested that medial BA6 is also involved in temporal maintenance or update of verbal information that is not used for speech but for solving nonmotor cognitive tasks (Paulesu et al., 1993; Fiez et al., 1996; Smith et al., 1998). Lateral BA6 has also long been known to be involved in higher-order motor processes, especially those related to visuomotor control (Moll and Kuypers, 1977; Weinrich and Wise, 1982; Wise et al., 1983; Halsband and Passingham, 1985). Wise and his colleague showed that the activity in some neurons in the rostral part of dorsal premotor cortex reflects the orientation of selective spatial attention as opposed to the target of a reaching movement, eye position, and saccade direction (Boussaoud and Wise, 1993; Boussaoud, 2001; Lebedev and Wise, 2001). In addition to these neurophysiological studies, some human neuroimaging studies have also suggested that lateral BA6 is involved in cognitive processes such as spatial working memory or spatial attention, although such activity in BA6 during cognitive tasks is often dismissed because it is located within the premotor cortex or frontal eye field and thus considered to be related to hand or eye movements (Jonides et al., 1993; Mellet et al., 1996; Courtney et al., 1998; Simon et al., 2002). The present results, which are consistent with these previous observations, provide systematic, strong evidence that activity in lateral and medial BA6 was functionally relevant for different cognitive processing and such differential roles originated from a difference in the cognitive representations subjected to mental update, namely verbal and spatial representations.
The present results fit well within the structural-functional framework that has been proposed for the motor domain of BA6: internally generated and externally guided motor control involves the medial and lateral regions, respectively, of BA6 (Goldberg, 1985; Wessel et al., 1997; Crosson et al., 2001). The innate properties of verbal and spatial representations are consistent with the concepts of “internal” and “external,” respectively, in that verbal representations are more abstract and decoupled from the physical world, whereas spatial representations are more concrete and directly connected to the physical world. Such a difference in the relationship between the inner brain and the outer physical world may be reflected not only in motor control but also in cognitive operations and thus may be processed in different areas of BA6.
An alternative or additional interpretation for the double dissociation observed in the present study is the difference in the types of sequences in which the two representations were arranged. In the present study, subjects had to monitor the current position in verbal sequence or spatial alignment and to update the position according to a number instruction and a predetermined rule in both tasks. The verbal representation of “week” is organized in a temporal and serial sequence, whereas the representation of “location” is organized in spatial and parallel alignment. Thus, the medial and lateral dissociation may be attributable to the difference between temporal sequence and spatial alignment to be updated in the two tasks. This idea is supported partly by previous findings that control of serial ordered movements, including speech, involve medial BA6 (Penfield and Welch, 1951; Shima et al., 1996; Kennerley et al., 2004), and some neurons in the rostral part of dorsal premotor cortex are involved in processing the sequence of spatial cues and motor sequences (Ohbayashi et al., 2003).
During MO-v, left ventral premotor cortex was preferentially active in addition to medial BA6 (Table 2). Some previous experiments have reported brain activation and an effect of TMS inhibition in this region during verbal tasks (Herwig et al., 2003; Longcamp et al., 2003; McDermott et al., 2003; Wilson et al., 2004). This region was clearly distinct from the left rostral part of dorsal premotor cortex, which exhibited selective activity and TMS inhibition during MO-s in the present study. Thus, lateral BA6 may be divided into additional subdivisions according to cognitive functions as well as motor control (Muakkassa and Strick, 1979; He et al., 1993; Godschalk et al., 1995; Preuss et al., 1996; Hoshi and Tanji, 2002).
In summary, the present study demonstrates that medial BA6 has a critical role in the update of verbal representations and lateral BA6 has a role in the update of spatial representations. These results provide direct physiological evidence of modality-specific cognitive function within human BA6. One methodological problem of low-frequency rTMS (≤1 Hz) experiments is that there is considerable individual variability of the effect (Maeda et al., 2000), and the results may underestimate the function of a stimulated area. Thus, the possibility remains that the cognitive function of BA6 may be even more extensive than that demonstrated here.
Footnotes
This work was partly supported by a grant-in-aid for Scientific Research on Priority Areas, Advanced Brain Science from Japan Ministry of Education, Culture, Sports, Science and Technology, and Research Fellowships for Young Scientists from Japan Society for the Promotion of Science. We thank Dr. J. Kahle for skillful English editing.
Correspondence should be addressed to Dr. Manabu Honda, Division of Cerebral Integration, National Institute for Physiological Sciences, Japan, 38 Nishigonaka, Myodaiji, Okazaki 444-8585, Japan. E-mail: honda@nips.ac.jp.
Copyright © 2005 Society for Neuroscience 0270-6474/05/250496-06$15.00/0
References
- Barbas H, Pandya DN (1987) Architecture and frontal cortical connections of the premotor cortex (area 6) in the rhesus monkey. J Comp Neurol 256: 211-228. [DOI] [PubMed] [Google Scholar]
- Beckers G, Zeki S (1995) The consequences of inactivating areas V1 and V5 on visual motion perception. Brain 118: 49-60. [DOI] [PubMed] [Google Scholar]
- Boussaoud D (2001) Attention versus intention in the primate premotor cortex. NeuroImage 14: S40-45. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Wise SP (1993) Primate frontal cortex: neuronal activity following attentional versus intentional cues. Exp Brain Res 95: 15-27. [DOI] [PubMed] [Google Scholar]
- Brickner RM (1940) A human cortical area producing repetitive phenomena when stimulated. J Neurophysiol 3: 128-130. [Google Scholar]
- Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M (1998) The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain 121: 253-264. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG (1997) Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398-1403. [DOI] [PubMed] [Google Scholar]
- Corthout E, Uttl B, Walsh V, Hallett M, Cowey A (1999) Timing of activity in early visual cortex as revealed by transcranial magnetic stimulation. NeuroReport 10: 2631-2634. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV (1998) An area specialized for spatial working memory in human frontal cortex. Science 279: 1347-1351. [DOI] [PubMed] [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gokcay D, Mohr CM, Auerbach EJ, Freeman AJ, Leonard CM, Briggs RW (2001) Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. J Cognit Neurosci 13: 272-283. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Tzourio N, Frak V, Raynaud L, Cohen L, Mehler J, Mazoyer B (1996) Cerebral activations during number multiplication and comparison: a PET study. Neuropsychologia 34: 1097-1106. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RS (1991) Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res 84: 393-402. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Wise SP, Honda M, Catalan MJ, Grafman J, Hallett M (1997) Frontal and parietal networks for conditional motor learning: a positron emission tomography study. J Neurophysiol 78: 977-991. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF (2003) Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cognit Neurosci 15: 71-84. [DOI] [PubMed] [Google Scholar]
- Doya K (2000) Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10: 732-739. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE (1996) A positron emission tomography study of the short-term maintenance of verbal information. J Neurosci 16: 808-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund HJ (1990) Premotor area and preparation of movement. Rev Neurol 146: 543-547. [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD (1991) Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci 11: 3656-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ (1995a) Spatial registration and normalization of images. Hum Brain Mapp 2: 165-189. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ (1995b) Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2: 189-210. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ (1999) How many subjects constitute a study? NeuroImage 10: 1-5. [DOI] [PubMed] [Google Scholar]
- Fulton JF (1935) Definition of the “motor” and “premotor” areas. Brain 58: 311-316. [Google Scholar]
- Godschalk M, Mitz AR, van Duin B, van der Burg H (1995) Somatotopy of monkey premotor cortex examined with microstimulation. Neurosci Res 23: 269-279. [DOI] [PubMed] [Google Scholar]
- Goldberg G (1985) Supplementary motor area structure and function: review and hypotheses. Behav Brain Sci 8: 567-616. [Google Scholar]
- Grafton ST, Fagg AH, Arbib MA (1998) Dorsal premotor cortex and conditional movement selection: a PET functional mapping study. J Neurophysiol 79: 1092-1097. [DOI] [PubMed] [Google Scholar]
- Hallett M (2000) Transcranial magnetic stimulation and the human brain. Nature 406: 147-150. [DOI] [PubMed] [Google Scholar]
- Halsband U, Passingham RE (1985) Premotor cortex and the conditions for movement in monkeys (Macaca fascicularis). Behav Brain Res 18: 269-277. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Sawamoto N, Okada T, Yonekura Y, Fukuyama H, Shibasaki H (2002) The role of rostral Brodmann area 6 in mental-operation tasks: an integrative neuroimaging approach. Cereb Cortex 12: 1157-1170. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Honda M, Okada T, Fukuyama H, Shibasaki H (2003a) Neural correlates underlying mental calculation in abacus experts: a functional magnetic resonance imaging study. NeuroImage 19: 296-307. [DOI] [PubMed] [Google Scholar]
- Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M (2003b) Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol 89: 989-1002. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Petit L, Ungerleider LG, Courtney SM (2000) Distinguishing the functional roles of multiple regions in distributed neural systems for visual working memory. NeuroImage 11: 145-156. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL (1993) Topographic organization of corticospinal projections from the frontal lobe: motor areas on the lateral surface of the hemisphere. J Neurosci 13: 952-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Abler B, Schonfeldt-Lecuona C, Wunderlich A, Grothe J, Spitzer M, Walter H (2003) Verbal storage in a premotor-parietal network: evidence from fMRI-guided magnetic stimulation. NeuroImage 20: 1032-1041. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tanji J (2002) Contrasting neuronal activity in the dorsal and ventral premotor areas during preparation to reach. J Neurophysiol 87: 1123-1128. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M (2000) Human cerebellar activity reflecting an acquired internal model of a new tool. Nature 403: 192-195. [DOI] [PubMed] [Google Scholar]
- Ito M (1993) Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci 16: 448-450. [DOI] [PubMed] [Google Scholar]
- Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA (1993) Spatial working memory in humans as revealed by PET. Nature 363: 623-625. [DOI] [PubMed] [Google Scholar]
- Kennerley SW, Sakai K, Rushworth MF (2004) Organization of action sequences and the role of the pre-SMA. J Neurophysiol 91: 978-993. [DOI] [PubMed] [Google Scholar]
- Lamm C, Windischberger C, Leodolter U, Moser E, Bauer H (2001) Evidence for premotor cortex activity during dynamic visuospatial imagery from single-trial functional magnetic resonance imaging and event-related slow cortical potentials. NeuroImage 14: 268-283. [DOI] [PubMed] [Google Scholar]
- Lebedev MA, Wise SP (2001) Tuning for the orientation of spatial attention in dorsal premotor cortex. Eur J Neurosci 13: 1002-1008. [DOI] [PubMed] [Google Scholar]
- Leiner HC, Leiner AL, Dow RS (1993) Cognitive and language functions of the human cerebellum. Trends Neurosci 16: 444-447. [DOI] [PubMed] [Google Scholar]
- Lewald J, Foltys H, Topper R (2002) Role of the posterior parietal cortex in spatial hearing. J Neurosci 22: RC207(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcamp M, Anton JL, Roth M, Velay JL (2003) Visual presentation of single letters activates a premotor area involved in writing. NeuroImage 19: 1492-1500. [DOI] [PubMed] [Google Scholar]
- Lu MT, Preston JB, Strick PL (1994) Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J Comp Neurol 341: 375-392. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G (1993) Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol 338: 114-140. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A (2000) Inter-individual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133: 425-430. [DOI] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, Ojemann JG (2003) A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia 41: 293-303. [DOI] [PubMed] [Google Scholar]
- Mellet E, Tzourio N, Crivello F, Joliot M, Denis M, Mazoyer B (1996) Functional anatomy of spatial mental imagery generated from verbal instructions. J Neurosci 16: 6504-6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (1994) Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science 266: 458-461. [DOI] [PubMed] [Google Scholar]
- Moll L, Kuypers HG (1977) Premotor cortical ablations in monkeys: contralateral changes in visually guided reaching behavior. Science 198: 317-319. [DOI] [PubMed] [Google Scholar]
- Muakkassa KF, Strick PL (1979) Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized “premotor” areas. Brain Res 177: 176-182. [DOI] [PubMed] [Google Scholar]
- Ohbayashi M, Ohki K, Miyashita Y (2003) Conversion of working memory to motor sequence in the monkey premotor cortex. Science 301: 233-236. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97-113. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME (1990) The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci USA 87: 256-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J (2000) Transcranial magnetic stimulation in cognitive neuroscience-virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol 10: 232-237. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS (1993) The neural correlates of the verbal component of working memory. Nature 362: 342-345. [DOI] [PubMed] [Google Scholar]
- Penfield W, Welch K (1951) The supplementary motor area of the cerebral cortex: a clinical and experimental study. AMA Arch Neurol Psychiatry 66: 289-317. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (1996) Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex 6: 342-353. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL (2001) Imaging the premotor areas. Curr Opin Neurobiol 11: 663-672. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Stepniewska I, Kaas JH (1996) Movement representation in the dorsal and ventral premotor areas of owl monkeys: a microstimulation study. J Comp Neurol 371: 649-676. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G, Matelli M (1998) The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol 106: 283-296. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual-Leone A (2003) Studies in cognition: the problems solved and created by transcranial magnetic stimulation. J Cognit Neurosci 15: 948-960. [DOI] [PubMed] [Google Scholar]
- Roland PE, Skinhoj E, Lassen NA, Larsen B (1980) Different cortical areas in man in organization of voluntary movements in extrapersonal space. J Neurophysiol 43: 137-150. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Hadland KA, Paus T, Sipila PK (2002) Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol 87: 2577-2592. [DOI] [PubMed] [Google Scholar]
- Sack AT, Linden DE (2003) Combining transcranial magnetic stimulation and functional imaging in cognitive brain research: possibilities and limitations. Brain Res Brain Res Rev 43: 41-56. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Hanakawa T, Fukuyama H, Shibasaki H (2002) Cognitive slowing in Parkinson's disease: a behavioral evaluation independent of motor slowing. J Neurosci 22: 5198-5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann J (1997) The cerebellum and cognition. San Diego: Academic.
- Schubotz RI, von Cramon DY (2003) Functional-anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. NeuroImage 20[Suppl 1]: S120-131. [DOI] [PubMed] [Google Scholar]
- Shapiro KA, Pascual-Leone A, Mottaghy FM, Gangitano M, Caramazza A (2001) Grammatical distinctions in the left frontal cortex. J Cognit Neurosci 13: 713-720. [DOI] [PubMed] [Google Scholar]
- Shima K, Mushiake H, Saito N, Tanji J (1996) Role for cells in the presupplementary motor area in updating motor plans. Proc Natl Acad Sci USA 93: 8694-8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D (2002) Spatial attention and memory versus motor preparation: premotor cortex involvement as revealed by fMRI. J Neurophysiol 88: 2047-2057. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA (1998) Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci USA 95: 876-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart LM, Walsh V, Rothwell JC (2001) Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia 39: 415-419. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988) Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme.
- Tanji J (1996) New concepts of the supplementary motor area. Curr Opin Neurobiol 6: 782-787. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K (1994) Role for supplementary motor area cells in planning several movements ahead. Nature 371: 413-416. [DOI] [PubMed] [Google Scholar]
- Walsh V, Rushworth M (1999) A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia 37: 125-135. [PubMed] [Google Scholar]
- Weinrich M, Wise SP (1982) The premotor cortex of the monkey. J Neurosci 2: 1329-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel K, Zeffiro T, Toro C, Hallett M (1997) Self-paced versus metronome-paced finger movements. A positron emission tomography study. J Neuroimaging 7: 145-151. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M (2004) Listening to speech activates motor areas involved in speech production. Nat Neurosci 7: 701-702. [DOI] [PubMed] [Google Scholar]
- Wise SP (1985) The primate premotor cortex: past, present, and preparatory. Annu Rev Neurosci 8: 1-19. [DOI] [PubMed] [Google Scholar]
- Wise SP, Weinrich M, Mauritz KH (1983) Motor aspects of cue-related neuronal activity in premotor cortex of the rhesus monkey. Brain Res 260: 301-305. [DOI] [PubMed] [Google Scholar]



