Abstract
Recognition memory can be supported by both the assessment of the familiarity of an item and by recollection of the context in which an item was encountered. Some have hypothesized that the prefrontal cortex (PFC) disproportionately contributes to recollection, whereas an alternative view is that the PFC contributes to both recollection and familiarity. Here, we examined the effects of prefrontal lesions on recollection and familiarity. Patients with unilateral PFC lesions and age-, gender-, and education-matched controls encoded pictures of meaningful objects that were presented briefly to the left or right visual field and subsequently performed recognition tests for centrally presented objects. Laterality effects within the PFC were also assessed in relation to recollection and familiarity processes. Patients with prefrontal lesions showed impaired familiarity-based recognition, and this deficit was specific for objects encoded by the lesioned hemisphere. In addition, recollection of the context in which each item was encountered was impaired independent of the visual field of presentation in patients with left prefrontal lesions. Recollection measured by subjective reports (“remember”) was not impaired in either left or right frontal patients. These findings suggest that the PFC plays a critical role in recognition memory based on familiarity as well as recollection. Furthermore, these results suggest that left PFC regions are critical for source recollection.
Keywords: memory, recollection, familiarity, prefrontal, lesion, source
Introduction
Accumulating behavioral evidence has led many researchers to suggest that recognition memory can be based on the recollection of specific information associated with a previous episode and on the assessment of the familiarity of an item. The neural substrates of these memory processes are controversial. One view is that regions in the medial temporal lobe contribute to both familiarity and recollection and that recollection additionally depends on the prefrontal cortex (PFC) (Knowlton and Squire, 1995; Manns et al., 2003). Consistent with this idea, studies of patients with focal prefrontal lesions have reported disproportionate impairments on tests of recall (Jetter et al., 1986; Janowsky et al., 1989a) and source memory (Janowsky et al., 1989b; Johnson et al., 1997; Simons et al., 2002) that are thought to be sensitive to recollection. An alternative view is that distinct medial temporal regions differentially contribute to familiarity and recollection, whereas the PFC supports both of these processes (Aggleton and Brown, 1999; Yonelinas et al., 2002). This idea draws support from neuroimaging (Henson et al., 1999; Bunge et al., 2004; Ranganath et al., 2004; Yonelinas et al., 2005) and single-unit (Xiang and Brown, 2004) studies in monkeys linking lateral prefrontal activity to both familiarity and recollection.
Results from previous neuropsychological studies are potentially consistent with both hypotheses. Previous results suggest that patients with prefrontal lesions exhibit small but reliable impairments in item recognition (Stuss et al., 1994; Wheeler et al., 1995; Alexander et al., 2003), but it is unclear whether these recognition deficits are attributable to an impairment in familiarity, recollection, or both processes. Another important consideration is that most previous neuropsychological studies have examined memory processes in patients with unilateral lesions. It is possible that spared memory capacities after unilateral lesions might be mediated by the PFC of the intact hemisphere. For example, memory deficits might be most evident when processing within the lesioned hemisphere is taxed more directly (i.e., stimulus presentation contralateral to frontal damage) (Nielsen-Bohlman and Knight, 1999). Accordingly, previous neuropsychological studies using central stimulus presentation might have underestimated prefrontal contributions to recollection and familiarity.
Here, we investigated the effects of unilateral prefrontal lesions on recollection and familiarity during recognition of visually presented objects. Patients and age-, gender-, and education-matched controls encoded objects that were presented briefly to the left or right visual field (Fig. 1). Next, recognition memory for each object was tested using methods to separately estimate the contributions of recollection and familiarity. Because stimulus presentation was lateralized during encoding, we were able to assess memory performance separately for objects processed preferentially by the lesioned versus the spared hemisphere. In addition, prompted by neuroimaging evidence suggesting that there may be some laterality differences within the PFC in regard to recollection and familiarity (Nolde et al., 1998; Kensinger et al., 2003; Dobbins et al., 2004; Mitchell et al., 2004), we characterized the effects of left versus right frontal lesions.
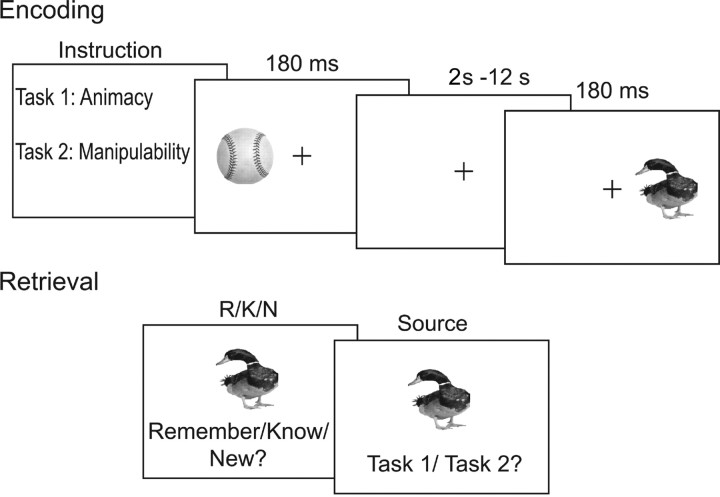
Figure 1.
Example stimuli and task requirements during study and test blocks. Subjects maintained central fixation for both encoding and retrieval. Stimuli were lateralized only at encoding and were presented centrally at retrieval.
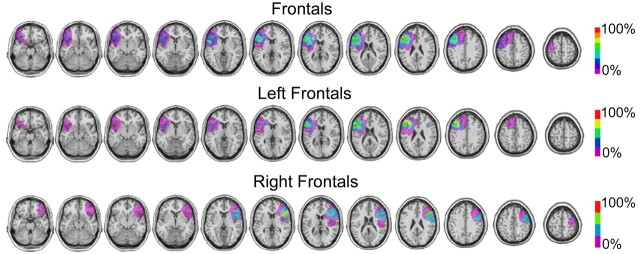
Materials and Methods
Subjects. Nine patients with unilateral PFC lesions (four right) and nine age-matched (61.5 ± 12.1 years), gender-matched (five females), and education-matched (15.2 ± 3.5 years) controls participated. Individual patient demographics can be found in supplemental Table 1 (available at www.jneurosci.org as supplemental material). Each control was selected to be directly age, gender, and education matched to one patient. Additional details of the patients and methods are described in the supplemental Materials and Methods (available at www.jneurosci.org as supplemental material). The average lesion volume was 53 cm3, and the lesion was centered in the dorsolateral PFC (DLPFC) in Brodmann's areas 9, 46, and 6, with >70% overlap, with varying degrees of damage in areas 8, 44, 45, 47, and 10. Lesion overlaps for the overall frontal patient group as well as the left (n = 5) and right (n = 4) frontal patient groups are shown in Figure 2. Lesion volume, central location, and specific Brodmann's area locations for individual patients are shown in supplemental Table 1, and individual patients' lesion reconstructions are shown in supplemental Figure 1 (both available at www.jneurosci.org as supplemental material).
Figure 2.
Lesion overlap for frontal patients and left and right frontal patient groups. Right frontal lesions have been transcribed to the left hemisphere to determine the overlap across all patients. The scale indicates the percentage of patients with lesions in a specific area.
Procedure. Stimuli consisted of 450 grayscale photographs of meaningful objects. Subjects were instructed to fixate centrally throughout stimulus presentation. Subjects were informed that they would be tested on their memory for all studied objects.
Behavioral responses were collected during six blocks of study trials and three blocks of test trials (Fig. 1). Blocks were ordered study–study–test, such that each block of test trials covered the items that were studied in the two preceding study blocks. On each block of study trials, 50 stimuli were presented one at a time 4.15° to the left or right of a central fixation cross for a duration of 180 ms with a randomized stimulus onset asynchrony between 2 and 12 s (mean, 4 s). One-half of the stimuli were presented randomly to the left and one-half of the stimuli were presented randomly to the right of fixation in a pseudorandom sequence. In three of the study blocks, subjects were asked to determine the animacy of each object by pressing 1 for living and 2 for non-living. In the other three study blocks, subjects were asked to determine the manipulability (i.e., movable by one's hands) of each object by pressing 1 if manipulable and 2 if non-manipulable. Subjects completed one of each type of study block and were allowed a few minutes to relax before proceeding to the corresponding test block.
For each test object, subjects made “remember,” “know,” or “new” judgments (Tulving, 1985). Instructions for the test phase included a description of the appropriate use of the remember, know, and new categories (Gardiner and Java, 1991; Rajaram, 1993). The test phase was self-paced, and stimuli were presented full field. Subjects were instructed to respond “remember” if they were certain they had seen the object and could recollect specific associations that occurred at study, to respond “know” if they were certain about previously studying the object but could not recollect any specific associations, and to respond “new” if they were certain they had never studied the object. If a remember or know response was made, a new response cue appeared in place of the previous cue asking the subjects to judge whether the object was studied in the animacy or manipulability task (press 1 = animacy, press 2 = manipulability). This allowed us to verify that remember and know responses were associated with recollection and familiarity processes, respectively (Yonelinas, 2002).
Each block of test trials included 100 objects that were studied in the preceding two test blocks and 50 new objects in a pseudorandom sequence. Objects were all centrally presented above a response cue [press 1 = remember (R), press 2 = know (K), press 3 = new (N)], both of which remained on the screen until a response was made. If subjects responded “new,” a centrally presented fixation cross appeared for 500 ms until the next test stimulus was presented. After study task discriminations were made, a centrally presented fixation cross appeared for 500 ms until the next test stimulus was presented. Trials with reaction times <200 ms were considered trial failures. This resulted in the rejection of <2% of trials.
To correct for the underestimation of familiarity inherent in the remember–know design, estimates of recollection and familiarity were calculated according to the independence remember–know procedure (Yonelinas and Jacoby, 1995): p(recollection) = p(R studied) – p(R unstudied) and p(familiarity) = p(K studied)/(1 – p(R studied)) – p(K unstudied)/(1 – p(R unstudied)). Estimates were calculated separately for contralesionally and ipsilesionally presented studied items.
Recognition performance was assessed as a function of the visual field that each item was encoded in relative to each patient's lesion site. Thus, for patients with left frontal lesions, stimuli that were presented to the right visual field were referred to as contralesional, whereas those presented to the left field were referred to as ipsilesional. For patients with right frontal lesions, stimuli presented to the left visual field were referred to as contralesional, whereas those presented to the right field were referred to as ipsilesional. Each patient was matched to a control subject such that performance for each patient's contralesional and ipsilesional hemifield could be compared with performance in the same hemifield for the corresponding control.
ANOVAs that yielded significant interactions at an α level of p < 0.05 were followed up with planned contrasts (t tests). All ANOVAs included the factor visual field (contralesional vs ipsilesional) to assess memory as a function of field of presentation during encoding. For each analysis, patients were compared with the control group. Subsequently, to examine the effects of frontal laterality, the same analyses were performed for the left and right patient subgroups.
Results
The mean proportions of R and K judgments to contralesionally and ipsilesionally presented studied items and new items are shown for each group in Table 1. Individual patient data can be found in supplemental Table 1 (available at www.jneurosci.org as supplemental material). Results from the remember–know recognition test were used to estimate indices of recollection and familiarity (see Materials and Methods for details). These results for contralesional and ipsilesional visual field stimuli are shown in Figure 3A. For recollection, the visual field (contralesional vs ipsilesional) by group (patients vs controls) ANOVA revealed no significant differences between controls and frontal patients (group: F(1,16) = 1.2, p = 0.28; interaction: F(1,16) < 1). Subsequent analyses in patient subgroups also revealed no significant differences between patients and controls [left frontals (group: F(1,12) < 1; interaction: F(1,12) < 1); right frontals (group: F(1,11) = 2.1, p = 0.18; interaction: F(1,11) < 1)]. For familiarity, there was a significant visual field (contralesional vs ipsilesional) by group interaction in frontal patients (F(1,16) = 8.44; p = 0.01). Follow-up contrasts showed that familiarity for contralesionally presented (t(16) = 2.45; p = 0.02) but not ipsilesionally presented (t(16) = 1.14; p = 0.27) study items was significantly impaired in frontal patients. Subsidiary analyses in patient subgroups revealed a significant interaction in the left frontal patients (F(1,12) = 11.5; p = 0.005) and a marginally significant main effect of group in the right frontal patients (F(1,11) = 3.57; p = 0.08). As shown for the frontal patients above, follow-up contrasts showed that familiarity for contralesionally presented study items was significantly impaired in left frontal patients (t(12) = 2.26; p = 0.04) and marginally impaired in right frontal patients (t(11) = 2.01; p = 0.07), whereas familiarity for ipsilesionally presented study items was intact (all p > 0.3). No other significant main effects or interactions were observed.
Table 1.
Recognition judgments made to studied (contralesionally and ipsilesionally presented during encoding) and new items at retrieval for each group
|
|
Controls (n = 9) |
Frontals (n = 9) |
Left frontals (n = 5) |
Right frontals (n = 4) |
|---|---|---|---|---|
| Studied items (contralesional) | ||||
| Remember | 0.51 (0.16) | 0.46 (0.17) | 0.51 (0.15) | 0.40 (0.17) |
| Know | 0.25 (0.13) | 0.24 (0.13) | 0.19 (0.11) | 0.31 (0.28) |
| Studied items (ipsilesional) | ||||
| Remember | 0.53 (0.16) | 0.46 (0.18) | 0.52 (0.15) | 0.39 (0.20) |
| Know | 0.22 (0.11) | 0.27 (0.21) | 0.22 (0.13) | 0.33 (0.28) |
| New items | ||||
| Remember | 0.06 (0.05) | 0.08 (0.04) | 0.10 (0.03) | 0.06 (0.04) |
| Know |
0.15 (0.07) |
0.23 (0.25) |
0.15 (0.08) |
0.33 (0.36) |
Data are presented as mean (SD).
Figure 3.
Performance for the control and frontal patient groups. A, Recollection and familiarity estimates for objects presented contralesionally and ipsilesionally during encoding. B, Source memory accuracy for objects presented contralesionally and ipsilesionally during encoding. Error bars depict the SEM across subjects. *Marginally significant difference; **significantly different from controls.
We next examined performance on the source memory measure in patients and controls. Previous behavioral studies have shown that source memory accuracy should be higher for items eliciting R rather than K judgments (Perfect et al., 1996). Consistent with this hypothesis, results showed that for all groups, source memory accuracy was significantly higher for R than for K judgments (all p < 0.05). Furthermore, source accuracy was significantly above chance (50%) for R judgments (controls: t(8) = 5.91, p = 0.001; frontals: t(8) = 2.73, p = 0.03; left frontals: t(4) = 2.59, p = 0.05; right frontals: t(3) = 2.99, p = 0.05) but not for K judgments (all p > 0.1). We therefore restricted our comparisons of source memory accuracy between patients and controls to items that elicited R judgments.
Source memory accuracy was calculated as the percentage of remember responses associated with correct source. The visual field (contralesional vs ipsilesional) by group (patients vs controls) ANOVA revealed a marginally significant main effect of group for frontal patients (F(1,16) = 3.37; p = 0.08). However, closer examination of patient subgroups (Fig. 3B) showed that source memory was impaired in patients with left frontal lesions (main group: F(1,12) = 5.97; p = 0.03) but not right frontal lesions (main group: F(1,11) < 1). Closer examination in left frontal patients showed that source accuracy was reliably impaired for contralesionally presented (t(12) = 2.93; p = 0.01) and marginally impaired for ipsilesionally presented (t(12) = 1.98; p = 0.07) study items, as shown in Figure 3B. No other effects were observed.
Discussion
The goal of the present study was to understand the effects of prefrontal lesions on familiarity and recollection. As noted, previous neuropsychological studies have shown that prefrontal lesions are associated with small but reliable deficits in recognition memory tests (Stuss et al., 1994; Wheeler et al., 1995; Alexander et al., 2003). Other studies have shown spared-item recognition and impaired temporal source memory in patients with PFC lesions (Shimamura et al., 1990; Simons et al., 2002). The interpretation of these data are complicated by the fact that item recognition and temporal source memory judgments could be supported by familiarity strength and/or recollection (Jacoby and Dallas, 1981; Quamme et al., 2002; Yonelinas and Levy, 2002).
The present results, however, suggest that the PFC may be essential for normal familiarity-based recognition and that preserved familiarity in previous studies might have reflected spared processing in the intact hemisphere. More specifically, we found that patients with prefrontal lesions are impaired in familiarity-based recognition, but that this impairment was specifically observed when encoding processing was biased toward the lesioned hemisphere.
There are a few potential explanations why the familiarity deficit in PFC patients was specific to items encoded in the contralesional hemifield. One possibility is that prefrontal mechanisms that contribute to familiarity are strongly lateralized, to the point that damage to one hemisphere compromises familiarity-relevant processing specifically or disproportionately in the lesioned hemisphere. For instance, one previous study from our group showed that unilateral prefrontal lesion patients exhibited impaired visual detection performance and reduced neuronal activity within the lesioned hemisphere for contralesionally presented stimuli (Barcelo et al., 2000). If familiarity-based processing is similarly lateralized, it might follow that familiarity would be most affected for stimuli processed by the lesioned hemisphere (contralesionally presented). An alternative possibility is that neural networks that contribute to familiarity encoding are reorganized after unilateral lesions such that processing is channeled toward the intact hemisphere. Lateralizing stimuli to the contralesional hemifield might have compromised this compensatory process. This idea is consistent with recent findings showing recovery of function substantiated by the intact hemisphere in unilateral lesion patients (Blasi et al., 2002). In this study, verbal learning in aphasic patients was associated with modulation of neural activity within the right hemisphere in left frontal patients but not healthy controls. Without physiological data in the present study to support the reorganization of function idea, it is difficult to distinguish between these hypotheses. Future investigations of unilateral PFC patients combining episodic memory tasks and physiological measures, such as event-related potential (Nielsen-Bohlman and Knight, 1999; Barcelo et al., 2000) or functional imaging (Blasi et al., 2002), will be useful in this regard.
Contrary to the view that the PFC is essential for episodic recollection (Knowlton and Squire, 1995; Manns et al., 2003), the present results revealed a more complex role of lateral PFC in recollective processing. We found that the subjective experience of recollection, indexed by remember judgments, was not impaired in patients with lateral PFC lesions. However, objective recollection, indexed by source memory accuracy, was impaired in patients with left lateralized PFC lesions. This latter result is consistent with previous reports showing impaired source memory accuracy in patients with PFC lesions (Janowsky et al., 1989b; Johnson et al., 1997). There are several possible explanations for this pattern of results.
One potential interpretation might be that patients with PFC lesions did not understand the remember–know instructions. To minimize this possibility, we took special care to ensure that subjects understood the procedure by instructing them to make know judgments when they were confident they had studied the items but could not recollect any details and remember judgments when they could additionally recollect the context. Analyses of source memory accuracy independently confirmed the validity of RK judgments in our patients. Indeed, these patients, like controls, showed above-chance source memory accuracy only for R judgments and significantly higher source accuracy for R than for K judgments. These findings confirm that both patients and controls based their R judgments on the recollection of specific information. Furthermore, a lack of understanding of the RK instructions cannot explain the fact that familiarity was impaired specifically for contralesionally encoded stimuli.
Another possible explanation for the dissociation between objective and subjective measures of recollection is that patients with left PFC lesions recollected contextual information, but this information was not relevant to the source memory judgment (i.e., “non-criterial recollection”). Alternately, these patients might have recollected the same contextual information as controls, but they were unable to use this information to make source attributions. This hypothesis is consistent with the findings of impaired source monitoring in frontal patients (Johnson et al., 1997).
A third possible explanation for the dissociation between subjective and objective recollection in left PFC patients may be that these measures are dependent on different PFC subregions. Our patients had lesions centered in the left DLPFC, often extending into the ventrolateral PFC. However, frontopolar regions (at or near Brodmann's area 10) were intact in these patients. One recent study found that patients with frontopolar lesions made fewer remember responses to recognized items than did controls, whereas remember rates were intact in patients with DLPFC lesions (Wheeler and Stuss, 2003). Frontopolar regions may therefore be important for making subjective reports about recollection but not necessarily for the actual retrieval of the recollected information, which may rely more on the left DLPFC. Future investigations directly contrasting performance in such patient groups will be extremely important in substantiating these suggested dissociations.
Recent findings from neuroimaging studies have supported the idea that the left and right PFC may contribute differently to recollection and familiarity processes (Nolde et al., 1998; Cabeza et al., 2003; Kensinger et al., 2003; Dobbins et al., 2004; Mitchell et al., 2004) (for review, see Ranganath, 2004). For example, some researchers have suggested that the left PFC might disproportionately contribute to recollection of specific information (Nolde et al., 1998; Kensinger et al., 2003; Dobbins et al., 2004; Mitchell et al., 2004). Although the present study was not designed to test this model, our results were consistent with this prediction. An important question for future research is whether the left PFC is critical for all forms of source memory (e.g., temporal, spatial, and content-based source decisions) or whether it is primarily involved in source decisions requiring evaluation of specific episodic information.
In conclusion, the present results demonstrate that the PFC makes important contributions to familiarity and recollection. Our results additionally suggest a potential dissociation between the contributions from left and right prefrontal regions to recollection and source monitoring. Finally, this study highlights the importance of biasing processing toward the lesioned hemisphere of unilateral patients to determine the contribution of specific regions to cognitive processes.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS21135 and PO1NS40813 and by the Veterans Administration Research Service. We thank Celina Trujillo for assistance with data acquisition and analysis, Clay Clayworth for lesion reconstructions, and Donatella Scabini for patient recruitment. The authors declare that they have no competing financial interests.
Correspondence should be addressed to Dr. Audrey Duarte, Helen Wills Neuroscience Institute, 132 Barker Hall, MC #3190, University of California at Berkeley, Berkeley, CA 94720-3190. E-mail: aduarte@itsa.ucsf.edu.
DOI:10.1523/JNEUROSCI.1392-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/258333-05$15.00/0
References
- Aggleton JP, Brown MW (1999) Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci 22: 425–444; discussion 444–489. [PubMed] [Google Scholar]
- Alexander MP, Stuss DT, Fansabedian N (2003) California Verbal Learning Test: performance by patients with focal frontal and non-frontal lesions. Brain 126: 1493–1503. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Suwazono S, Knight RT (2000) Prefrontal modulation of visual processing in humans. Nat Neurosci 3: 399–403. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M (2002) Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron 36: 159–170. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD (2004) Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn 56: 141–152. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND (2003) Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J Cogn Neurosci 15: 249–259. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Simons JS, Schacter DL (2004) fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci 16: 908–920. [DOI] [PubMed] [Google Scholar]
- Gardiner JM, Java RI (1991) Forgetting in recognition memory with and without recollective experience. Mem Cognit 19: 617–623. [DOI] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ (1999) Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci 19: 3962–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M (1981) On the relationship between autobiographical memory and perceptual learning. J Exp Psychol Gen 110: 306–340. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Kritchevsky M, Squire LR (1989a) Cognitive impairment following frontal lobe damage and its relevance to human amnesia. Behav Neurosci 103: 548–560. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR (1989b) Source memory impairment in patients with frontal lobe lesions. Neuropsychologia 27: 1043–1056. [DOI] [PubMed] [Google Scholar]
- Jetter W, Poser U, Freeman Jr RB, Markowitsch HJ (1986) A verbal long term memory deficit in frontal lobe damaged patients. Cortex 22: 229–242. [DOI] [PubMed] [Google Scholar]
- Johnson MK, O'Connor M, Cantor J (1997) Confabulation, memory deficits, and frontal dysfunction. Brain Cogn 34: 189–206. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Clarke RJ, Corkin S (2003) What neural correlates underlie successful encoding and retrieval? A functional magnetic resonance imaging study using a divided attention paradigm. J Neurosci 23: 2407–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR (1995) Remembering and knowing: two different expressions of declarative memory. J Exp Psychol Learn Mem Cogn 21: 699–710. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR (2003) Recognition memory and the human hippocampus. Neuron 37: 171–180. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, Greene EJ (2004) Prefrontal cortex activity associated with source monitoring in a working memory task. J Cogn Neurosci 16: 921–934. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT (1999) Prefrontal cortical involvement in visual working memory. Brain Res Cogn Brain Res 8: 299–310. [DOI] [PubMed] [Google Scholar]
- Nolde SF, Johnson MK, D'Esposito M (1998) Left prefrontal activation during episodic remembering: an event-related fMRI study. NeuroReport 9: 3509–3514. [DOI] [PubMed] [Google Scholar]
- Perfect TJ, Mayes AR, Downes JJ, Van Eijk R (1996) Does context discriminate recollection from familiarity in recognition memory? Q J Exp Psychol A 49: 797–813. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Frederick C, Kroll NE, Yonelinas AP, Dobbins IG (2002) Recognition memory for source and occurrence: the importance of recollection. Mem Cognit 30: 893–907. [DOI] [PubMed] [Google Scholar]
- Rajaram S (1993) Remembering and knowing: two means of access to the personal past. Mem Cognit 21: 89–102. [DOI] [PubMed] [Google Scholar]
- Ranganath C (2004) The 3-D prefrontal cortex: hemispheric asymmetries in prefrontal activity and their relation to memory retrieval processes. J Cogn Neurosci 16: 903–907. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M (2004) Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42: 2–13. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR (1990) Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia 28: 803–813. [DOI] [PubMed] [Google Scholar]
- Simons JS, Verfaellie M, Galton CJ, Miller BL, Hodges JR, Graham KS (2002) Recollection-based memory in frontotemporal dementia: implications for theories of long-term memory. Brain 125: 2523–2536. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Palumbo CL, Buckle L, Sayer L, Pogue J (1994) Organizational strategies of patients with unilateral or bilateral frontal lobe injury in word list learning tasks. Neuropsychology 8: 355–373. [Google Scholar]
- Tulving E (1985) Memory and consciousness. Can Psychol 26: 1–12. [Google Scholar]
- Wheeler MA, Stuss DT (2003) Remembering and knowing in patients with-frontal lobe injuries. Cortex 39: 827–846. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E (1995) Frontal lobe damage produces episodic memory impairment. J Int Neuropsychol Soc 1: 525–536. [DOI] [PubMed] [Google Scholar]
- Xiang JZ, Brown MW (2004) Neuronal responses related to long-term recognition memory processes in prefrontal cortex. Neuron 42: 817–829. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP (2002) The nature of recollection and familiarity: a review of 30 years of research. J Mem Lang 46: 441–517. [Google Scholar]
- Yonelinas AP, Jacoby LL (1995) The relation between remembering and knowing as bases for recognition: effects of size congruency. J Mem Lang 34: 622–643. [Google Scholar]
- Yonelinas AP, Levy BJ (2002) Dissociating familiarity from recollection in human recognition memory: different rates of forgetting over short retention intervals. Psychon Bull Rev 9: 575–582. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT (2002) Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci 5: 1236–1241. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD (2005) Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]