Abstract
The search for a unique biological marker of language-based learning disabilities has so far yielded inconclusive findings. Previous studies have shown a plethora of auditory processing deficits in learning disabilities at both the perceptual and physiological levels. In this study, we investigated the association among brainstem timing, cortical processing of stimulus differences, and literacy skills. To that end, brainstem timing and cortical sensitivity to acoustic change [mismatch negativity (MMN)] were measured in a group of children with learning disabilities and normal-learning children. The learning-disabled (LD) group was further divided into two subgroups with normal and abnormal brainstem timing. MMNs, literacy, and cognitive abilities were compared among the three groups. LD individuals with abnormal brainstem timing were more likely to show reduced processing of acoustic change at the cortical level compared with both normal-learning individuals and LD individuals with normal brainstem timing. This group was also characterized by a more severe form of learning disability manifested by poorer reading, listening comprehension, and general cognitive ability. We conclude that abnormal brainstem timing in learning disabilities is related to higher incidence of reduced cortical sensitivity to acoustic change and to deficient literacy skills. These findings suggest that abnormal brainstem timing may serve as a reliable marker of a subgroup of individuals with learning disabilities. They also suggest that faulty mechanisms of neural timing at the brainstem may be the biological basis of malfunction in this group.
Keywords: brainstem timing, cortical processing, ABR, MMN, learning disability, reading
Introduction
Language-based learning disabilities affect 5-10% of otherwise normally developing children (Torgesen, 1991). Despite decades of research, the underlying causes of learning disabilities remain, at large, a mystery, yet there is agreement that learning disabilities are constitutional in origin (Lyon, 1995).
Auditory processing deficits are thought to underlie language problems by interfering with speech perception and hence the development of adequate phonological representations (Tallal et al., 1993). Indeed, many studies have documented the presence of behavioral auditory processing deficits in 30-40% of learning-disabled (LD) individuals (Tallal, 1980; Reed, 1989; McAnally and Stein, 1996; Wright et al., 1997; Ahissar et al., 2000; Amitay et al., 2002a,b; Ramus et al., 2003; Banai and Ahissar, 2004; Ben-Yehudah et al., 2004).
The two physiological mechanisms considered here in relation to the biological basis of learning disabilities are the mismatch negativity (MMN) at the cortical level and the brainstem response to speech [speech auditory brainstem response (speech-ABR)]. Both are related to processes known to be impaired in subgroups of learning disabilities (fine-grained acoustic discrimination and temporal processing, respectively).
MMN is a cortical response arising as a result of an acoustic change in a repetitive sound sequence (Näätänen, 1992). LD individuals tend to have smaller or absent MMNs in response to both speech (Kraus et al., 1996; Schulte-Korne et al., 1998) and nonspeech (Baldeweg et al., 1999; Renvall and Hari, 2003) acoustic changes, and this has been suggested to account for their behavioral deficits. Yet, the MMN is hard to identify reliably at the individual level (McGee et al., 1997; Dalebout and Fox, 2001), making it unappealing as a diagnostic tool.
The brainstem-evoked response to a brief sound (ABR) is the neural snapshot of the stimulus, faithfully representing the stimulus onset, its fundamental frequency, and harmonics (Moushegian et al., 1973; Boston and Møller, 1985; Galbraith et al., 1995). The speech-ABR is an objective measure, which can be reliably measured at the individual subject level (Russo et al., 2004; Johnson et al., 2005). Of interest here is the transient portion of the speech-ABR, encoding stimulus onset (Kraus and Nicol, 2005). This response is delayed and less precisely timed in a subgroup of LD children (Cunningham et al., 2001; King et al., 2002; Wible et al., 2004), suggesting that their difficulties in higher-level language processes may have roots in the basic representation of sound as low as the brainstem.
What are the functional relationships between brainstem and cortical processing? Recent studies are consistent with the hypothesis that precise brainstem timing in ideal listening conditions is essential for the ability of the cortex to adequately process auditory stimuli under acoustic stress. Thus, Wible et al. (2005) found a strong correlation between the robustness of the P1/N2 cortical response in noise and the degree of precision of the speech-ABR. Moreover, listening training resulted in increased resilience of the cortical response to noise only in children with abnormal brainstem timing (King et al., 2002).
Clarifying the relationship between brainstem timing and cortical processes known to be impaired in many LD individuals is important in trying to account for the role of each (brainstem, cortex) in learning disabilities. It is still not known whether brainstem timing deficits are related to abnormal cortical discrimination of acoustic change, but if abnormal brainstem timing impedes the cortical processing in challenging listening conditions, we would expect that to be the case.
In this study, we explored two questions regarding the possible implications of brainstem timing deficits. We asked (1) whether abnormal brainstem timing is associated with cortical processing of fine speech differences (MMN) and (2) what the possible manifestations of abnormal brainstem timing with respect to literacy and cognitive skills are.
Materials and Methods
Participants
Participants for this study were recruited through advertisements in local newspapers and flyers posted on campus and at Northwestern University speech and learning disabilities clinics. All participants and their guardians gave their informed consent before participation in this study in accordance with the Northwestern Institutional Review Board guidelines. One hundred twenty native-English-speaking children (age, 8-12 years; mean age, 10.1 ± 1.8 years) participated in the study. All children had normal hearing (<20 dB hearing level for octaves between 500 and 4000 Hz), clinically normal click-ABRs, and normal intelligence (standard score >85) as measured by the Brief Cognitive Scale (Woodcock and Johnson, 1989, 1990) or the Test of Nonverbal Intelligence (TONI-3) (Brown et al., 1997). Seventy-four children had been diagnosed with a learning disability by independent clinicians before admission to the study. Forty-six were normal-learning (NL) children with no history of learning problems at school. LD participants were divided into two groups based on brainstem physiology, as described below.
In addition to the independent diagnosis, study internal-measures (see below) were used to measure reading and reading-related abilities in all children. Because reading and reading-related abilities are highly variable among learning-disabled children, and because we were interested in the possible implications of a brainstem timing deficit to literacy, we used these measures to verify the presence of reading-related deficits in the LD group and the absence of such deficits among NL children. To qualify as NL, the child had to score >85 on the reading and spelling tests. To be classified as LD, the child could not score >1.5 SDs above NL average on reading and spelling (i.e., their literacy score had to be <130)
Among the LD children, 19 had a concomitant diagnosis of learning disability and attention deficit disorder. Because these children did not differ from the rest of the learning disabled children on any of the study internal measures, they were, for the purpose of the current study, defined as LD.
Physiological measurements
Stimuli and recording (general)
All stimuli were presented monaurally to the right ear via insert earphones (ER-3; Etymotic Research, Elk Grove Village, IL). During the recording session, children watched a videotape with the sound level set at <40 dB sound pressure level (SPL) to the left ear to promote stillness. Responses were recorded using Ag-AgCl scalp electrodes.
Brainstem recordings (ABR)
Speech ABRs (auditory brainstem-evoked potentials) were elicited using a five-formant speech syllable /da/, generated with a digital speech synthesizer (SenSyn, Somerville, MA). Stimulus duration was 40 ms. Additional details regarding speech synthesis parameters can be found in King et al. (2002). The syllable /da/was presented at an 80 dB SPL in alternating polarities with an interstimulus interval (ISI) of 51 ms. Responses were differentially recorded at sampling rate of 20,000 Hz from Cz (active) to the right earlobe (reference), with the forehead as ground. Responses were bandpass filtered online from 100 to 2000 Hz, 6 dB per octave. Three blocks of 1000 repetitions were collected at each polarity. Responses were on-line averaged (Neuroscan; Compumedics, El Paso, TX) with a 70 ms recording window starting 10 ms before stimulus onset. Trials with eyeblinks >35 μV were rejected on-line. Responses of alternating polarities were added together to minimize contributions from the cochlear microphonic response, a receptor potential generated by cochlear hair cells (Gorga et al., 1985).
Cortical recordings (MMN)
MMNs were evaluated using an oddball paradigm that has been described previously (Kraus et al., 1996, 1999). The stimuli were syllables taken from the /da/-/ga/ speech continuum. Stimuli along this continuum differ in the onset frequency of their third formant (F3). The first stimulus on the continuum (/da/) served as the deviant occurring with a probability of 10% (F3 = 2580 Hz). The standard stimulus, occurring 90% of the time, was the token with F3 = 2500 Hz, representing a barely perceptible difference from the deviant.
Stimuli were presented at 75 dB SPL in a pseudorandom order with an ISI of 490 ms, with at least three standard stimuli in between two deviants. Responses to standard stimuli immediately after a deviant were excluded from analysis. Responses were collected with 590 ms recording window starting 90 ms before stimulus onset. Sampling rate was 1000 Hz. Responses were off-line filtered from 0.1 to 40 Hz (12 dB per octave rolloff). Two hundred fifty responses were collected for the deviant and 2200 for the standard. In addition, 1500 responses were obtained to the deviant stimulus (/da/) presented on its own (termed the alone condition). Responses were recorded from frontal, central, and temporal sites, referenced to the nose tip, with the forehead serving as ground. Data reported in the present paper are from Cz and Fz, where MMN responses are typically the most robust.
Evoked potentials data analysis
Brainstem
Each child's final response was an average of 6000 stimuli. An example of a typical brainstem response to speech is shown in Figure 1. Of interest in the current study was the onset timing of the brainstem response, occurring before 12 ms from stimulus onset and in particular the peaks of positive wave V and negative wave A. These specific peaks were chosen for the following reasons. First, previous studies have identified these peaks as the ones encoding the consonant portion of the /da/ stimulus used in the current study, whereas later peaks encode the more steady-state vowel portion of the stimulus (Russo et al., 2004). Because LD children often have difficulties discriminating consonants, the physiological encoding of the consonant is of particular interest. Second, waves V and A have been found previously to be delayed among children with learning disabilities (King et al., 2002; Wible et al., 2004), and an increased delay between these two waves is correlated with cortical function (Wible et al., 2005).
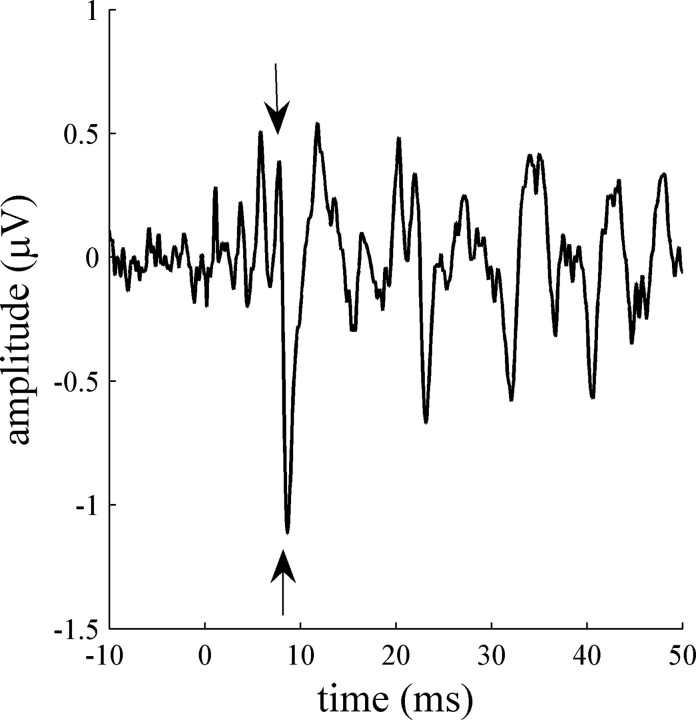
Figure 1.
A typical brainstem response of a NL child to the speech syllable /da/. Onset waves V and A are marked with arrows. Wave V is the positive peak at 7.76 ms, and wave A is the negative trough at 8.61 ms. The VA-complex slope (the transition between the peak and the trough) is defined as the microvolt (amplitude) change per millisecond between wave V and wave A.
The peaks of waves V and A were identified for each subject. Latencies and amplitudes of these peaks were measured. Based on these, the VA complex was characterized. Duration and slope of the V-to-A transition as well as the area of the complex were calculated. Together, the latencies of waves V and A and the VA-complex duration and slope represent the degree of precision and synchrony of the brainstem response to the onset of the stimulus. Later latencies, longer VA duration, and reduced VA slope all indicate a less precise representation of the stimulus onset. Previous studies have shown that the test retest reliability of these timing measures is very good (p > 0.09 for all measures except VA slope, which is more variable) at both the individual and at the group levels (Russo et al., 2004).
A combined brainstem-timing z-score was calculated for each participant in the following way. Each measure was standardized based on the mean and SD of the entire population. These standard scores were then averaged and divided by the SD over the entire population to normalize the resulting score. The resulting z-scores were used to compare brainstem timing between groups.
The LD group was divided into two subgroups based on abnormal scores on the brainstem timing measures (wave V latency, wave A latency, VA duration, VA slope). LD children who scored >2 SDs away (i.e., had delayed latencies, longer VA duration, or reduced VA slope) from the average of the normal learning group (excluding NL subjects outside the 2 SD range) on one of these measures (n = 26) or 1.5 SDs on at least two measures (n = 2) were defined as having abnormal brainstem timing and grouped into the LD-group. The rest of the LD children have normal brainstem timing and were grouped into the LD+ group (n = 46). The 2 SD criterion for inclusion in the LD-group was chosen to create a minimal overlap between the NL and the LD-group to avoid a possible false positive identification of an NL child as having a problem. This is similar to clinically used diagnostic criteria under which children are said to have a reading problem if they score lower than -2 SD from a population mean on a standardized measure of reading.
Cortex
Waveform identification and statistical analysis of the MMN response have been described in detail in previous publications (Kraus et al., 1992, 1995, 1996, 1999; McGee et al., 1997) and are thus only briefly described here.
For each subject, grand average waveforms were computed based on all available repetitions after artifact rejection for each stimulus (/da/ as a deviant, /da/ alone, frequent /ga/). MMN should be elicited by the deviant /da/ only when it signals an acoustic change. Difference waves were therefore computed for each subject by subtracting the /da/ alone response from the /da/ deviant response. A grand average difference wave was computed for each group. Within groups, a point to point t test was conducted between the /da/ deviant and /da/ alone grand averages to determine whether and when a significant MMN occurred. A p < 0.05 on the t test had to be achieved for at least 20 consecutive milliseconds for the difference to be considered significant, thus fixing the probability of type I error at p = 0.05.
Visual inspection of MMNs. MMNs were identified visually in the individual difference waves as a relative negativity after the P1 response occurring between 100 and 500 ms by two experienced observers, naive to subject identity. Onset, peak, and offset latencies were determined. The onset latency was defined by the first downward deflection in the difference wave after P1 on the original waves. The offset latency was determined as the first upward deflection after the trough of the negative response. Both observers had to agree on the onset and offset latencies. MMN duration was determined by subtracting the onset from the offset latency. MMN area was calculated by integrating the overall response area between onset and offset. MMN area and duration were compared between LDs with abnormal (LD-) and normal (LD+) brainstem timing and normal learning children.
Distribution of MMN sizes. The MMN of each subject was classified into one of three categories based on duration and area. MMN was defined as missing/small if MMN duration was <100 ms or MMN area was <100 μV·ms. If MMN area was >225 μV·ms and MMN duration >175 ms, MMN was defined as robust. Otherwise, if MMN area was >100 μV·ms and MMN duration >100 ms, MMN was defined as intermediate. These criteria were chosen based the previous studies (Kraus et al., 1999) showing that these values result in a similar distribution of MMN scores as observed for the normal learning children in the current study. In particular, both the previous and the current study resulted in small/missing MMNs in ∼10% of NL children.
Cognitive and literacy-related skills
A battery of subtests taken from standardized tests typically used for the diagnosis of learning disabilities, and the evaluation of literacy and phonological abilities was administered.
Reading fluency and orthographic skill were assessed using the reading and spelling subtests of the Wide Range Achievement Test (Wilkinson, 1993) and Word Attack of the Woodcock-Johnson revised battery (Woodcock and Johnson, 1989, 1990). Together, these tests provide information on reading and spelling of familiar words, grapheme-to-phoneme conversion, and phoneme-to-grapheme correspondence. Scores in those three tests are typically highly correlated. A combined literacy score was therefore calculated as the average of single word reading, nonword reading (Word Attack), and spelling scores to represent ortho-phonological ability in a single measure.
Verbal memory was assessed using the Memory for Words subtest (WJR) (Woodcock and Johnson, 1989, 1990).
Phonological abilities were further assessed at the syllabic and subsyllabic levels using a sound blending test (Woodcock and Johnson, 1989, 1990), in which the child hears a series of phonemes and has to combine them to the resulting word (e.g., /d/ /o/ /g/ → dog) and three subtests taken from the Comprehensive Test of Phonological Processing (CTOPP) (Wagner et al., 1999): (1) elision: subjects hear a word and are required to repeat it while omitting a phoneme in the beginning or the middle of the word (e.g., say cat without /k/ → at); (2) phoneme reversal: subjects hear a nonword and are required to say it backwards to produce a real word (e.g., say “oot;” now say it backwards → to); (3) nonword segmentation: subjects hear a nonword and are required to repeat it one sound at a time (e.g., tal → /t/ /a/ /l/).
Verbal and visual processing were assessed using the Listening Comprehension and the Cross Out subtests of the WJR (Woodcock and Johnson, 1989, 1990), respectively. Cross Out is a test of speeded visual processing in which the subject receives a page of small printed drawings printed in horizontal lines across the page. At the beginning of each line, there is a target object and subjects are instructed to cross out all the targets appearing on that line as fast as they can. General cognitive ability was assessed using the Brief Cognitive Scale (Woodcock and Johnson, 1989, 1990), which includes subtests of quantitative problems (e.g., understanding of relationships such as larger than or smaller than), antonyms, and synonyms.
Results
Brainstem timing
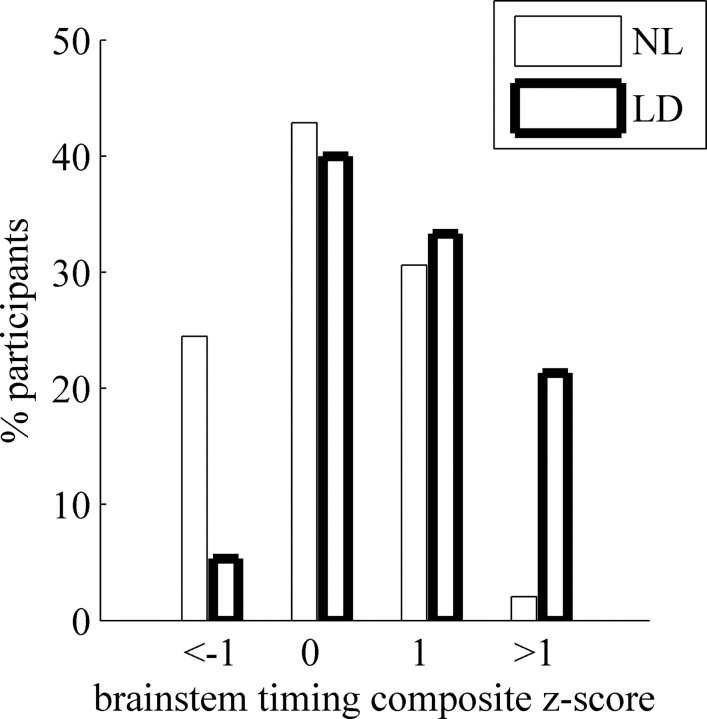
As expected from previous studies, a subgroup of the LD participants exhibited an abnormal brainstem response to the speech syllable /da/. The distribution of the composite timing z-scores of the two groups is shown in Figure 2. Although the distribution over the entire population is more or less normal, the distribution within each of the subgroups is skewed in the opposite direction such that more normal learning individuals populate the left tail of the distribution (good timing), whereas more individuals diagnosed with learning disability populate the right tail (delayed timing). This difference between the groups was highly significant (χ2(3) = 16.6; p = 0.001).
Figure 2.
Distribution of brainstem-timing z-scores.
Furthermore, although there is a significant overlap of brainstem timing scores between the NL and LD groups, a z-score >1 is a good predictor of LD (statistically, 62% of participants are expected to be LD in the current study, and 94% observed are actually observed as LD; χ2 = 6.45; p = 0.011), whereas a z-score less than -1 is a good predictor for normal learning (38% are expected to be NL; 75% are observed to be NL; χ2 = 9.6; p = 0.002).
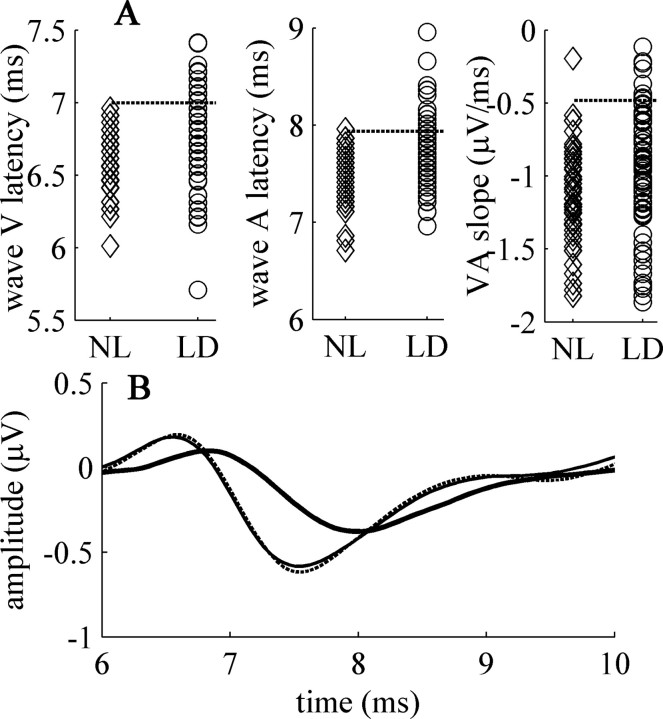
Individual scores on brainstem timing parameters are shown in Figure 3A. As shown, brainstem timing measures of many LD subjects were entirely overlapping with those NL subjects. Forty percent of LD children, however, had brainstem scores completely outside the range spanned by the normal learning group. To further study the possible implications of abnormal brainstem timing with respect to literacy and learning disability, we divided the LD group into two subgroups, now defining abnormal brainstem timing based on the distribution of scores among normal learning individuals (see Materials and Methods for details); one subgroup (LD+) with normal brainstem timing, the other (LD-) with brainstem timing completely outside the ranges span by NL individuals.
Figure 3.
A, Distribution of brainstem-timing parameters. Individual data of (left to right) wave V latency, wave A latency, and the VA complex slope. NL subjects are depicted with diamonds, LD subjects with circles. The dashed lines represent the 2 SD value in the NL group (excluding the outlier on the slope). LD individuals whose data fell above that value on any measureare defined as LD-. B, Grand average speech-ABR waveforms of the three groups. The NL group depicted with a thin line, the LD+ group with a dashed line, and the LD-group with a thick line. Waveforms of the LD+ and NL groups are (by definition) overlapping.
Figure 3B shows grand average speech-ABR waveforms in the range of interest of the 3 groups. By definition, the waveforms of the NL and LD+ groups overlap almost completely, whereas the grand average of the LD-group shows a shift in the latency of waves V and A, as well as the reduced slope of the transition between the two peaks. In what follows, we compare cortical processing and literacy skills among the three groups (see Table 1 for group average literacy, cognitive, and cortical measures).
Table 1.
Literacy, cognitive, and cortical measures (group average ± SD)
|
Task |
NL |
LD+ |
LD− |
|---|---|---|---|
| Literacya | |||
| Word Attack | 114.6 ± 16*** | 97.3 ± 16* | 88.0 ± 12 |
| Word reading | 114.3 ± 13*** | 95.3 ± 14* | 87.7 ± 13 |
| Spelling | 111.2 ± 13*** | 94.6 ± 13* | 86.2 ± 10 |
| Literacy score | 113.5 ± 13*** | 95.7 ± 13* | 87.1 ± 10 |
| Phonological abilities | |||
| Sound blendinga | 98.5 ± 12** | 92.0 ± 13 | 90.0 ± 11 |
| Elisionb | 11.6 ± 2** | 9.2 ± 3 | 8.3 ± 2 |
| Phoneme reversalb | 10.8 ± 2*** | 8.1 ± 2 | 7.3 ± 2 |
| Nonwords segmentationb | 10.7 ± 2* | 9.3 ± 3 | 8.6 ± 2 |
| Other cognitive abilitiesa | |||
| Listening comprehension | 118.7 ± 17** | 113.1 ± 18 | 103 ± 19 |
| Verbal memory | 105.0 ± 13 | 95.4 ± 13 | 97.7 ± 11 |
| Visual processing | 111.9 ± 15*** | 105.3 ± 14* | 94.6 ± 17 |
| Brief Cognitive Scale | 120.7 ± 12** | 108.3 ± 14* | 100.1 ± 15 |
| MMN (area μV·ms) | |||
| Fz | 312 ± 211** | 239 ± 160 | 183 ± 148 |
| Cz | 377 ± 248*** | 239 ± 145 | 190 ± 143 |
| Duration (ms) | |||
| Fz | 203 ± 69** | 173 ± 62 | 142 ± 94 |
| Cz |
222 ± 75***
|
176 ± 74 |
135 ± 87 |
*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 in a Scheffé post hoc comparison with the LD- group. For F values, see Results.
Standard scores. Average in the general population is 100 ± 15.
CTOPP scores.
Relationships between brainstem timing deficits and cortical processing of acoustic change (MMN)
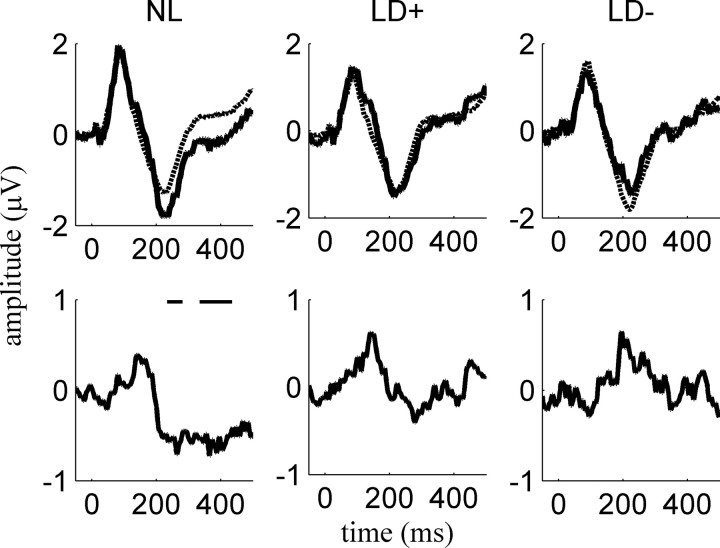
First, grand average MMN responses in each subgroup were calculated as shown in Figure 4. A significant MMN was found in the NL group only (p < 0.05 in a point-to-point t test; 234-281 and 335-435 ms). No significant MMN was observed in either LD group. Yet, no group differences were found in the basic cortical response (P1/N1) to the /da/ stimulus itself (/da/ alone condition), suggesting that the cortical response in LD children is not simply degraded to begin with but that the deficit is specific to the neural representation of fine stimulus differences.
Figure 4.
MMN. Top row, Grand average responses to a rare /da/ presented amid a frequent /ga/ (solid line) versus /da/ presented alone (dashed line). No group differences were found in the responses to /da/ alone. Bottom row, The difference between the response to the rare /da/ and the response to /da/ presented alone. The black horizontal line indicates periods of significant MMN. Data shown are from Fz. The same pattern was seen at Cz.
As a second stage in the analysis of the cortical data, MMN duration and area were calculated from the individual waveforms and compared among the three groups. In this analysis, significant group differences were observed, as shown in Table 1 (F > 4.4; p < 0.014 for both area and duration; Fz and Cz). Post hoc comparisons revealed that these differences are a result of the LD-group having significantly reduced MMNs compared with NL subjects and from the LD+ group having significantly reduced MMNs at Cz only (p ≤ 0.01). The onset of the MMN response also tended to be later in the LD-group, but this difference just failed to reach significance (F = 2.99; p = 0.055).
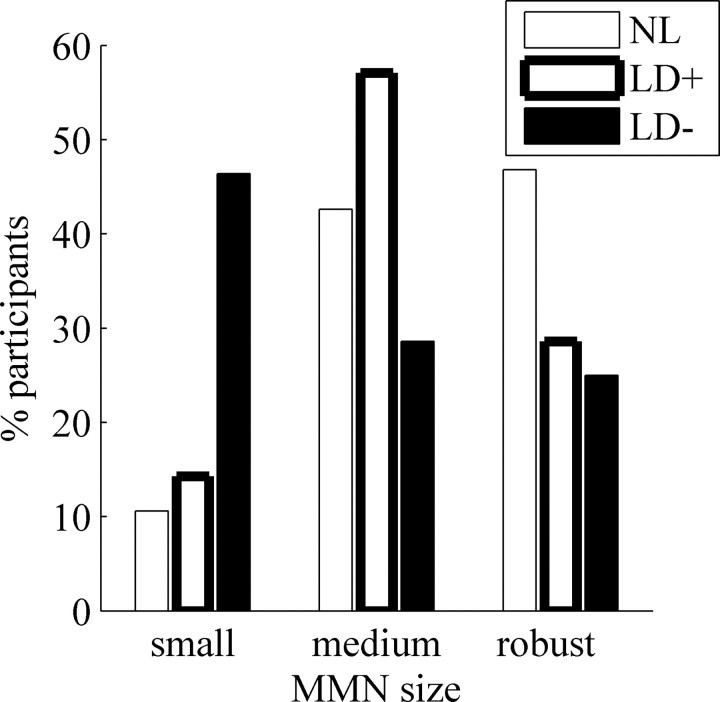
No significant differences in MMN area or duration were observed between the two LD groups, suggesting that both groups have equally impaired MMNs. Alternatively, it may be that more LD-individuals had abnormal MMNs compared with LD+ individuals, yet these differences were not significant at the group level. To disentangle these two alternatives, MMN scores were compared among the three groups by categorically defining each subjects' MMN as small/missing, intermediate, or robust (see Materials and Methods for defining criteria), as shown in Figure 5. This resulted in highly significant group differences (χ2(4) = 18.5; p = 0.001). This last analysis confirms that although MMN values are highly variable, individuals in the LD-group are more likely to have missing or small MMNs compared with the other two groups, whereas the proportion of individuals with missing or small MMNs was similar between the NL and LD+ groups.
Figure 5.
MMN distribution by group. Data are from Fz. MMN size: robust, MMN area >225 μV·ms and MMN duration >175 ms; medium (intermediate), MMN area >100 μV·ms and MMN duration >100 ms; small (small or missing), MMN duration <100 ms or area <100 μV·ms.
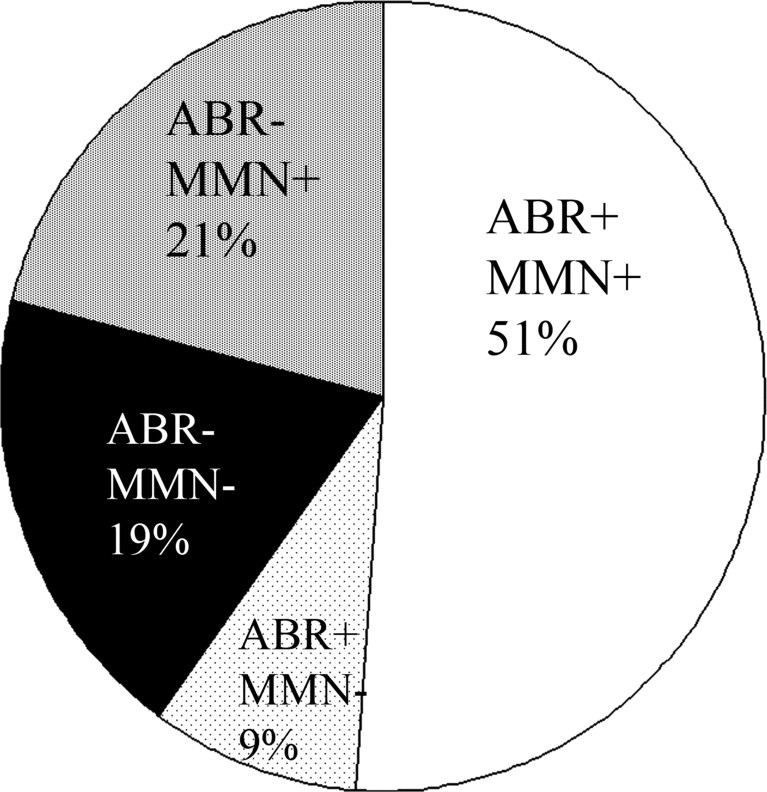
Together, these data suggest that although no significant differences are observed between LD- and LD+ individuals at the group level (Fig. 4), LD-individuals are more likely to have small or absent MMNs compared with NL and LD+ individuals. A schematic summary of the neurophysiological findings among individuals with learning disabilities, at the cortical and brainstem levels, is presented in Figure 6. Overall, 40% of LD individuals exhibited abnormal brainstem timing, suggesting that this measure may serve as a reliable biological marker of a subgroup of LD subjects. Moreover, LD children with abnormal brainstem timing comprised the majority of individuals with an observed physiological deficit in the current study. In the following section, the relationship between abnormal brainstem timing and literacy are considered.
Figure 6.
Physiological abnormalities in individuals with LD. + indicates normal processing, - indicates abnormal processing at the brainstem (ABR) or cortical (MMN) levels. Clockwise from top center, LD subjects with normal physiology (ABR+, MMN+), LD subjects with normal brainstem but abnormal cortical physiology (ABR+, MMN-), abnormal brainstem and abnormal cortical processing (ABR-, MMN-), and abnormal brainstem but normal cortical processing (ABR-, MMN+).
Relationships between brainstem timing deficits and literacy
An interesting question is the extent to which abnormal brainstem timing predicts the severity and type of learning disability. To answer this question, we compared performance of the three groups on a series of measures known to be related to language based learning disabilities (see Table 1 for group means).
Literacy
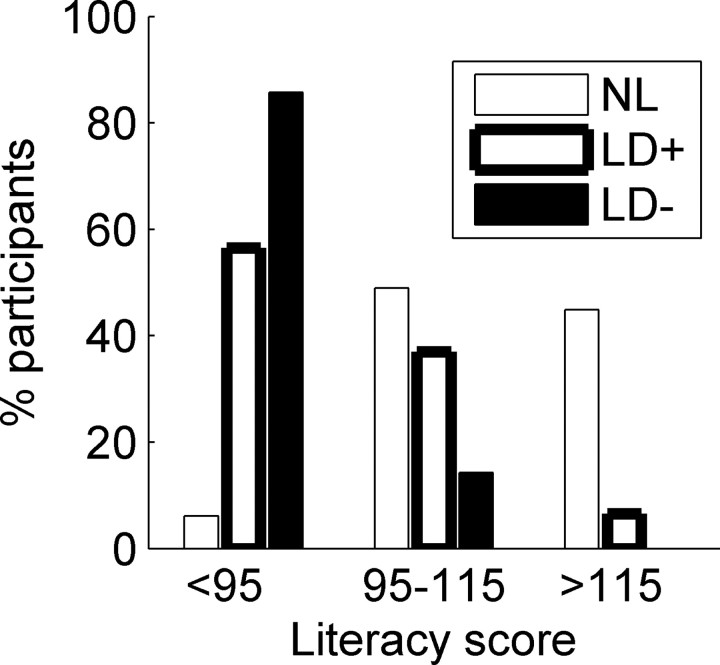
The LD-group had lower literacy scores compared with both the NL and the LD+ groups (F = 46.9; p < 0.001, p < 0.05 in both Scheffé post hoc comparisons). Moreover, as shown in Figure 7, the distribution of literacy scores differed significantly among the groups (χ2(4) = 59; p < 0.001). A discriminant analysis with the literacy score as an independent variable correctly classified 78% of LD-, 40% of LD+, and 77% of NL individuals into their respective groups. These analyses suggest that abnormal brainstem timing may well serve as a marker for reading deficits, although reading difficulties are also found among individuals in the LD+ group (p < 0.05 in the post hoc comparison to NL subjects). The observation that individuals with abnormal brainstem timing are highly unlikely to have good literacy skills suggests that the relationship between brainstem timing and reading related abilities may be more than correlational.
Figure 7.
Distribution of literacy scores.
Other cognitive and verbal abilities
If brainstem timing is functionally related to literacy, we would expect performance on a variety of verbal tasks that rely on phonological processing to be impaired among LD subjects with abnormal brainstem timing. Indeed, performance on all phonological tasks was impaired in the LD-group compared with controls (Table 1), although not compared with the LD+ group (p > 0.5 on all post hoc comparisons), who were also impaired compared with controls (p = 0.01 on sound blending, elision, and phoneme reversal), suggesting that phonological processing may be impaired for different reasons in these two groups.
Furthermore, as shown in Table 1, only the LD-group scored significantly lower than the NL group on measures of visual (Cross Out; F = 9.06; p < 0.001, p < 0.05 on post hoc comparisons to NL and LD+ groups) and verbal (Listening Comprehension; F = 6.3; p = 0.002, p = 0.09 on post hoc comparisons to the NL and LD+ groups) processing. On these two measures, the LD+ group was not significantly different from controls (p > 0.11). Individuals in the LD-group also scored significantly lower on a general measure of cognitive performance compared with the other two groups (brief cognitive scale; F = 17.7; p < 0.001; both post hoc comparisons significant at least p < 0.02). Together, these findings suggest that individuals in the LD-group suffer from a more severe form of learning disability compared with individuals in the LD+ group, yet their general cognitive abilities are well within the normal range.
Discussion
Summary of findings
Abnormal brainstem timing characterized ∼40% of the children with learning disabilities who participated in the current study. Consistent with our expectations, individuals with abnormal brainstem timing were more likely to show reduced cortical sensitivity to acoustic change compared with individuals with normal brainstem timing, although this correlation was far from perfect. Behaviorally, individuals with abnormal brainstem timing were characterized by a more severe form of learning disability and higher incidence of poor literacy compared with LD subjects with intact brainstem timing. Characterization of the physiological and cognitive deficits of LD individuals with normal brainstem timing and a milder form of learning disability is beyond the scope of the current study.
The cooccurrence of brainstem and cortical abnormalities in the current study does not indicate causality. However, because virtually all individuals with abnormal brainstem timing are LD (but not vice versa), meaning that they have higher-level deficits, a possible causal relationship cannot be ignored.
Brainstem timing literacy and intelligence
An intriguing finding of the current study is that brainstem timing deficits are related to lower general cognitive abilities. Although it is tempting to suggest that abnormal brainstem timing may result in a nonspecific cognitive deficit, it should be noted that the Brief Cognitive Scale, which we used as a measure for general intelligence, is highly correlated with verbal abilities and literacy. Although it is hard to envision that “low intelligence” affects auditory processing at the level of the brainstem, it is easy to imagine how noisy auditory input throughout life would contribute to lower performance on tests that rely heavily on verbal skills. Indeed, a comparison of 22 LD- and 35 LD+ individuals (including a subset of 12 LD- and 10 LD+ participants from the current study) on TONI-3 (Brown et al., 1997) revealed no group differences. TONI-3 scores were 103 ± 14 and 106 ± 13 in the LD- and LD+ groups, respectively. These data suggest that the differences between LD individuals with normal and abnormal brainstem timing likely do not reflect differences in nonverbal cognitive ability.
A related question is that of the lack of difference in phonological abilities between the two LD groups. Both groups had phonological deficits compared with NL individuals, but these were not more severe in the LD-group. This is not surprising, given that the majority of learning-disabled individuals have phonological deficits (Snow et al., 1998), and these deficits likely were the cause of their LD diagnosis, but only ∼40% have abnormal brainstem timing. It is plausible that abnormal brainstem timing contributes to phonological deficits in LD-individuals, whereas another factor (maybe verbal memory) contributes to the phonological deficits in the LD+ group.
The organization of the auditory system: bottom-up versus top-down influences
Our findings suggest that abnormal encoding of stimulus onset at the auditory brainstem level (inferior colliculus/lateral lemniscus) is associated with reduced cortical sensitivity to acoustic change (MMN) but not with the basic, obligatory representation (the P1/N1 response) at the cortical level. This pattern of association is consistent with previous studies showing that the cortical responses of children with abnormal brainstem timing in noisy listening conditions are not as robust as those of children with intact brainstem timing (Wible et al., 2005) despite normal cortical representation of speech in quiet (Cunningham et al., 2000).
Given the distribution of brainstem timing and MMN measures in normal-learning children (normal brainstem, 10% small/absent MMN), the observed relationship between abnormal brainstem timing and MMN in individuals with reading disability can be interpreted in (at least) three ways, as follows:
No functional relationship
The observed relationship between abnormal brainstem timing and reduced cortical sensitivity to acoustic change reflects the larger variability typically observed among individuals with learning disabilities. Because their brains are more noisy, more extreme scores are observed at the brainstem, cortex, or both, but these do not reflect any functional relationships. Support for this interpretation comes from the fact that in many cases, pathology was restricted to either the brainstem or the cortex. Yet, we would like to suggest that this interpretation is unlikely given the finding that the proportion of missing/small MMNs was significantly higher among LD-individuals (>45%) compared with either NL or LD-individuals (10-15%).
Bottom-up influence
Poor brainstem timing typically impedes the cortical ability to process sound under acoustic stress (e.g., noise or small differences between stimuli), but under ideal listening conditions, the impoverished input from the brainstem is sufficient to allow for basic cortical representation. Were this not the case, we would expect, for example, the day-to-day speech comprehension difficulties of LD-individuals to be much greater than they are (as is the case with hearing impaired individuals or auditory neuropathy patients). Additional support for this interpretation comes from the higher prevalence of abnormal MMNs in the LD-group in the current study, from the strong correlation between the precision of the brainstem response and the robustness of cortical response in noise (Wible et al., 2005), and from the finding that listening training results in enhanced robustness of the cortical response in individuals with abnormal brainstem timing (King et al., 2002).
The distinction between MMN and P1/N1 responses is consistent with their probable different neural generators (Sams et al., 1991; Korzyukov et al., 1999; Näätänen et al., 2005). Alternatively, MMN may be the outcome of stimulus specific adaptation (to the standard) and facilitation (to the rare stimuli) processes in a single neural population (in primary auditory cortex) (Ulanovsky et al., 2003, 2004). By this account, LD-individuals could be characterized as having normal stimulus specific adaptation (and hence normal P1/N1) but abnormal neural facilitation. Disentangling these two alternatives in beyond the scope of the current study.
Top-down influence
Brainstem timing may be impaired as a result of abnormal feedback from the cortex. Because the auditory brainstem receives efferent inputs from the cortex, it could be argued that abnormal cortical function results in impaired cortical feedback on the brainstem, which ultimately generates an abnormal brainstem response. For example, functional ablation of the auditory cortex in rats results in altered tuning properties and rate-threshold responses in inferior colliculus neurons (Popelar et al., 2003) [for a review of the postulated role of the cortex in reorganization of the auditory system, see Suga and Ma (2003)]. Support for this interpretation comes from the fact that individuals with abnormal brainstem timing to speech stimuli actually have normal ABRs in response to click trains, suggesting that structurally, response generators at the brainstem level may be intact, and therefore abnormal timing could be the result of abnormal cortical feedback.
Additional research is required to disentangle these interpretations. For example, brainstem responses probably mature at an earlier age than cortical responses, so if abnormal brainstem timing can be demonstrated in earlier developmental stages than the cortical abnormalities, this would provide support for the second interpretation. Alternatively, if, after training, changes in the brainstem response will occur only after changes to the cortical response, it would provide support for the third interpretation.
Possible implications for diagnosis and remediation of learning disabilities
Learning disability is a lifelong condition that is difficult to remediate even under the best circumstances. Typically, learning disabilities are diagnosed only several years into schooling, when gaps in performance between the LD child and her peers become sufficiently large. Although earlier diagnosis is highly desirable, research has failed to identify a single physiological marker that could be used as a diagnostic tool. We now suggest that the brainstem response to speech has diagnostic potential. Speech-ABR measures are reliable at the individual subject level. Abnormal brainstem timing cooccurs with language-based learning disability but is rare in the normal learning population, suggesting that the brainstem response can identify learning-disabled individuals with a minimal rate of false alarms. Furthermore, the identified children share a behavioral profile that distinguishes them from the rest of the learning-impaired population (their reading difficulties are more severe).
Given the behavioral and physiological heterogeneity of learning disabilities, this finding is important from a practical stand-point. It suggests that children with abnormal brainstem timing may be defined as a subgroup within the learning disabled population and diagnosed by a unique physiological marker. This group is characterized by more severe literacy deficits than other learning disabilities, yet their phonological abilities overlap with those of the other learning disabilities, suggesting that learning disabilities in these two groups may arise as a result of different underlying causes. This group has also been shown to benefit from listening training more than other learning disabilities (King et al., 2002), indicating that this may be a treatment of choice for the LD-subgroup.
Finally, these findings are significant from a theoretical stand-point. A central debate in the field of language-based learning disabilities has been whether a deficit in “fast temporal processing” (Tallal, 1980; Tallal et al., 1993) could account for the development of speech perception deficits. The present findings link, for the first time to our knowledge, a subcortical source of deficient temporal processing of speech sound onset with abnormal cortical discrimination and literacy skills.
Footnotes
This work was supported by National Institutes of Health-National Institute on Deafness and Other Communication Disorders Grant RO1-01510 and the Hugh Knowles Center for Clinical and Basic Science in Hearing and its Disorders, Northwestern University. We thank all members of the Auditory Neuroscience Laboratory for their help in different stages of the study and Dr. Nachum Ulanovsky and two anonymous reviewers for their helpful comments.
Correspondence should be addressed to Karen Banai, Department of Communication Sciences and Disorders, 2240 North Campus Drive, Evanston, IL 60208. E-mail: k-banai@northwestern.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/259850-08$15.00/0
References
- Ahissar M, Protopapas A, Reid M, Merzenich MM (2000) Auditory processing parallels reading abilities in adults. Proc Natl Acad Sci USA 97: 6832-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitay S, Ahissar M, Nelken I (2002a) Auditory processing deficits in reading disabled adults. J Assoc Res Otolaryngol 3: 302-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitay S, Ben-Yehudah G, Banai K, Ahissar M (2002b) Disabled readers suffer from visual and auditory impairments but not from a specific magnocellular deficit. Brain 125: 2272-2285. [DOI] [PubMed] [Google Scholar]
- Baldeweg T, Richardson A, Watkins S, Foale C, Gruzelier J (1999) Impaired auditory frequency discrimination in dyslexia detected with mismatch evoked potentials. Ann Neurol 45: 495-503. [DOI] [PubMed] [Google Scholar]
- Banai K, Ahissar M (2004) Poor frequency discrimination probes dyslexics with particularly impaired working memory. Audiol Neurootol 9: 328-340. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Banai K, Ahissar M (2004) Patterns of deficit in auditory temporal processing among dyslexic adults. NeuroReport 15: 627-631. [DOI] [PubMed] [Google Scholar]
- Boston JR, Møller AR (1985) Brainstem auditory-evoked potentials. Crit Rev Biomed Eng 13: 97-123. [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK (1997) Test of nonverbal intelligence, Ed 3. Austin, TX: Pro-Ed.
- Cunningham J, Nicol T, Zecker S, Kraus N (2000) Speech-evoked neurophysiologic responses in children with learning problems: development and behavioral correlates of perception. Ear Hear 21: 554-568. [DOI] [PubMed] [Google Scholar]
- Cunningham J, Nicol T, Zecker SG, Bradlow A, Kraus N (2001) Neurobiologic responses to speech in noise in children with learning problems: deficits and strategies for improvement. Clin Neurophysiol 112: 758-767. [DOI] [PubMed] [Google Scholar]
- Dalebout SD, Fox LG (2001) Reliability of the mismatch negativity in the responses of individual listeners. J Am Acad Audiol 12: 245-253. [PubMed] [Google Scholar]
- Galbraith GC, Arbagey PW, Branski R, Comerci N, Rector PM (1995) Intelligible speech encoded in the human brain stem frequency-following response. NeuroReport 6: 2363-2367. [DOI] [PubMed] [Google Scholar]
- Gorga M, Abbas P, Worthington D (1985) Stimulus calibration in ABR measurements. In: The auditory brainstem response (Jacobsen J, ed), pp 49-62. San Diego: College-Hill.
- Johnson KL, Nicol T, Kraus N (2005) The brainstem response to speech: a biological marker of auditory processing. Ear Hear 26: 424-434. [DOI] [PubMed] [Google Scholar]
- King C, Warrier CM, Hayes E, Kraus N (2002) Deficits in auditory brainstem pathway encoding of speech sounds in children with learning problems. Neurosci Lett 319: 111-115. [DOI] [PubMed] [Google Scholar]
- Korzyukov O, Alho K, Kujala A, Gumenyuk V, Ilmoniemi RJ, Virtanen J, Kropotov J, Näätänen R (1999) Electromagnetic responses of the human auditory cortex generated by sensory-memory based processing of tone-frequency changes. Neurosci Lett 276: 169-172. [DOI] [PubMed] [Google Scholar]
- Kraus N, Nicol T (2005) Brainstem origins for cortical `what' and `where' pathways in the auditory system. Trends Neurosci 28: 176-181. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Sharma A, Carrell T, Nicol T (1992) Mismatch negativity event-related potential elicited by speech stimuli. Ear Hear 13: 158-164. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee T, Carrell TD, King C, Tremblay K (1995) Central auditory system plasticity associated with speech discrimination training. J Cogn Neurosci 7: 27-34. [DOI] [PubMed] [Google Scholar]
- Kraus N, McGee TJ, Carrell TD, Zecker SG, Nicol TG, Koch DB (1996) Auditory neurophysiologic responses and discrimination deficits in children with learning problems. Science 273: 971-973. [DOI] [PubMed] [Google Scholar]
- Kraus N, Koch DB, McGee TJ, Nicol TG, Cunningham J (1999) Speech-sound discrimination in school-age children: psychophysical and neurophysiologic measures. J Speech Lang Hear Res 42: 1042-1060. [DOI] [PubMed] [Google Scholar]
- Lyon GR (1995) Toward a definition of dyslexia. Ann Dyslexia 45: 3-27. [DOI] [PubMed] [Google Scholar]
- McAnally KI, Stein JF (1996) Auditory temporal coding in dyslexia. Proc R Soc Lond B Biol Sci 263: 961-965. [DOI] [PubMed] [Google Scholar]
- McGee T, Kraus N, Nicol T (1997) Is it really a mismatch negativity? An assessment of methods for determining response validity in individual subjects. Electroencephalogr Clin Neurophysiol 104: 359-368. [DOI] [PubMed] [Google Scholar]
- Moushegian G, Rupert AL, Stillman RD (1973) Scalp-recorded early responses in man to frequencies in the speech range. Electroencephalogr Clin Neurophysiol 35: 665-667. [DOI] [PubMed] [Google Scholar]
- Näätänen R (1992) Attention and brain function. Hillsdale, NJ: Lawrence Erlbaum.
- Näätänen R, Jacobsen T, Winkler I (2005) Memory-based or afferent processes in mismatch negativity (MMN): a review of the evidence. Psychophysiology 42: 25-32. [DOI] [PubMed] [Google Scholar]
- Popelar J, Nwabueze-Ogbo FC, Syka J (2003) Changes in neuronal activity of the inferior colliculus in rat after temporal inactivation of the auditory cortex. Physiol Res 52: 615-628. [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U (2003) Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain 126: 841-865. [DOI] [PubMed] [Google Scholar]
- Reed MA (1989) Speech perception and the discrimination of brief auditory cues in reading disabled children. J Exp Child Psychol 48: 270-292. [DOI] [PubMed] [Google Scholar]
- Renvall H, Hari R (2003) Diminished auditory mismatch fields in dyslexic adults. Ann Neurol 53: 551-557. [DOI] [PubMed] [Google Scholar]
- Russo N, Nicol T, Musacchia G, Kraus N (2004) Brainstem responses to speech syllables. Clin Neurophysiol 115: 2021-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams M, Kaukoranta E, Hamalainen M, Naatanen R (1991) Cortical activity elicited by changes in auditory stimuli: different sources for the magnetic N100m and mismatch responses. Psychophysiology 28: 21-29. [DOI] [PubMed] [Google Scholar]
- Schulte-Korne G, Deimel W, Bartling J, Remschmidt H (1998) Auditory processing and dyslexia: evidence for a specific speech processing deficit. NeuroReport 9: 337-340. [DOI] [PubMed] [Google Scholar]
- Snow CE, Burns MS, Griffin P, eds (1998) Who has reading difficulties? In: Preventing reading difficulties in young children, Chap 3, pp 87-99. Washington, DC: National Academic Press.
- Suga N, Ma X (2003) Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci 4: 783-794. [DOI] [PubMed] [Google Scholar]
- Tallal P (1980) Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang 9: 182-198. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Fitch RH (1993) Neurobiological basis of speech: a case for the preeminence of temporal processing. Ann NY Acad Sci 682: 27-47. [DOI] [PubMed] [Google Scholar]
- Torgesen JK (1991) Learning disabilities: historical and conceptual issues. In: Learning about learning disabilities (Wong B, ed), pp 3-39. San Diego: Academic.
- Ulanovsky N, Las L, Nelken I (2003) Processing of low-probability sounds by cortical neurons. Nat Neurosci 6: 391-398. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Farkas D, Nelken I (2004) Multiple time scales of adaptation in auditory cortex neurons. J Neurosci 24: 10440-10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RK, Torgesen JK, Rachotte CA (1999) Comprehensive test of phonological processing (CTOPP). Austin, TX: Pro-Ed.
- Wible B, Nicol T, Kraus N (2004) Atypical brainstem representation of onset and formant structure of speech sounds in children with language-based learning problems. Biol Psychol 67: 299-317. [DOI] [PubMed] [Google Scholar]
- Wible B, Nicol T, Kraus N (2005) Correlation between brainstem and cortical auditory processes in normal and language-impaired children. Brain 128: 417-423. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS (1993) Wide range achievement test (WRAT3). Wilmington, DE: Wide Range.
- Woodcock RW, Johnson MB (1989, 1990) Woodcock Johnson psycho-educational battery: tests of cognitive ability (WJ-R). Allen, TX: DLM Teaching Resources.
- Wright BA, Lombardino LJ, King WM, Puranik CS, Leonard CM, Merzenich MM (1997) Deficits in auditory temporal and spectral resolution in language-impaired children. Nature 387: 176-178. [DOI] [PubMed] [Google Scholar]