Abstract
Persistent activation of GABAA receptors by extracellular GABA (tonic inhibition) plays a critical role in signal processing and network excitability in the brain. In hippocampal principal cells, tonic inhibition has been reported to be mediated by α5-subunit-containing GABAA receptors (α5GABAARs). Pharmacological or genetic disruption of these receptors improves cognitive performance, suggesting that tonic inhibition has an adverse effect on information processing. Here, we show that α5GABAARs contribute to tonic currents in pyramidal cells only when ambient GABA concentrations increase (as may occur during increased brain activity). At low ambient GABA concentrations, activation of δ-subunit-containing GABAA receptors predominates. In epileptic tissue, α5GABAARs are downregulated and no longer contribute to tonic currents under conditions of raised extracellular GABA concentrations. Under these conditions, however, the tonic current is greater in pyramidal cells from epileptic tissue than in pyramidal cells from nonepileptic tissue, implying substitution of α5GABAARs by other GABAA receptor subtypes. These results reveal multiple components of tonic GABAA receptor-mediated conductance that are activated by low GABA concentrations. The relative contribution of these components changes after the induction of epilepsy, implying an adaptive plasticity of the tonic current in the presence of spontaneous seizures.
Keywords: tonic, GABA, epilepsy, GABAA receptors, hippocampus, α5
Introduction
GABA mediates fast inhibition by activating synaptic ionotropic GABAA receptors (Mohler et al., 1996; Sperk et al., 1997; Whiting et al., 1999). Low extracellular GABA concentrations ([GABA]o) also generate tonic currents mediated by high-affinity GABAA receptors (Nusser and Mody, 2002; Yeung et al., 2003; Semyanov et al., 2004). Tonic inhibition occurs in brain slices (Brickley et al., 1996; Salin and Prince, 1996; Semyanov et al., 2003), neuronal cultures (Liu et al., 1995; Bai et al., 2001), and in vivo (Chadderton et al., 2004). It is developmentally regulated (Brickley et al., 1996; Wall and Usowicz, 1997; Demarque et al., 2002), depends on synaptic and nonsynaptic GABA release (Rossi et al., 2003), and is modulated by GABA uptake (Semyanov et al., 2003). Its amplitude and pharmacological profile differ among cell types (Semyanov et al., 2003; Stell et al., 2003; Wei et al., 2003). It may play critical roles in regulating network excitability (Semyanov et al., 2003) and information processing (Mitchell and Silver, 2003; Chadderton et al., 2004).
Different GABAA receptor subtypes mediate tonic inhibition, depending on brain region and cell type (Semyanov et al., 2004; Farrant and Nusser, 2005). In cerebellar granule cells, it is mediated by α6δ-containing receptors (Brickley et al., 2001; Stell et al., 2003). Although genetic deletion of the α6 subunit abolishes this tonic GABAA receptor-mediated current, it does not reduce the leak conductance, which is restored by compensatory upregulation of a K+ channel (Brickley et al., 2001). δ-Subunit-containing GABAA receptors also generate tonic currents in dentate granule cells (Stell et al., 2003). In hippocampal pyramidal neurons, tonic inhibition has been reported to be mediated by α5-subunit-containing GABAA receptors (α5GABAARs) (Caraiscos et al., 2004). These receptors are principally extrasynaptic and mainly restricted to the hippocampus (Fritschy et al., 1998; Brunig et al., 2002; Crestani et al., 2002). Deletion and point mutations of the gene encoding for α5 improves cognitive performance (Collinson et al., 2002; Crestani et al., 2002), an effect that is mimicked by an α5-selective benzodiazepine inverse agonist (Chambers et al., 2002). Although these observations suggest that α5 in pyramidal cells (PC) has deleterious effects on neuronal plasticity, its adaptive role may be to protect neurons from excessive excitation by mediating tonic inhibition. Interestingly, experimental models of epilepsy are accompanied by decreases in α5 expression (Fritschy et al., 1999; Houser and Esclapez, 1996, 2003), which has prompted the speculation that reduced tonic inhibition of pyramidal neurons contributes to epileptogenesis.
Here, we show that changing [GABA]o reveals multiple components of tonic current in pyramidal cells and that α5GABAARs increasingly contribute when [GABA]o is raised experimentally within the physiological range. We also show that the downregulation of α5 that occurs in temporal lobe epilepsy is accompanied by a paradoxical increase in tonic currents in CA1 pyramidal neurons under conditions of elevated [GABA]o (5 μm). These results reveal previously unsuspected heterogeneity of tonic GABAA receptor-mediated conductances in the hippocampus and argue that increases in network excitability, such as occur in epilepsy, can engender seemingly compensatory alterations in the tonic current.
Materials and Methods
Epilepsy models. All animal procedures followed the United Kingdom Animal (Scientific Procedures) Act (1986).
Limbic status epilepticus (SE) was induced in adult male Sprague Dawley rats (8 weeks of age; ∼250 g) by intraperitoneal injection of pilocarpine (320 mg/kg) (Turski et al., 1989). So as to lessen peripheral cholinergic effects, 1 mg/kg, i.p. scopolamine methyl nitrate was administered 30 min before and 30 min after pilocarpine. The onset of SE was defined as the appearance of stage 3 (Racine, 1972) seizures followed by continuous clinical seizure activity. Clinically overt SE was terminated after 90 min by 10 mg/kg, i.p. diazepam. The animals were monitored daily for the appearance of spontaneous recurrent seizures. All animals with SE had spontaneous seizures by 2 weeks and were killed after 3 weeks when spontaneous seizures were occurring. Sham control animals were treated in an identical manner except that they received a subconvulsive dose of pilocarpine (32 mg/kg).
In some experiments, SE was induced with a single intraperitoneal injection of 10 mg/kg kainic acid (Schwarzer et al., 1997). SE was terminated by 10 mg/kg, i.p. diazepam 60 min after the first generalized seizure, and the rats were killed at 3 weeks.
Electrophysiology. Transverse hippocampal slices (300 μm thick) were obtained from control rats (8-10 weeks of age), sham control rats, and rats 3 weeks after pilocarpine- or kainate-induced status epilepticus. These were stored in an interface chamber for >1 h before transfer to a submersion recording chamber. The storage and perfusion solution contained the following (in mm): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 26.2 NaHCO3, 1 NaH2PO4, and 22 glucose and was gassed with 95% O2/5% CO2. Whole-cell recordings were made from CA1 stratum radiatum interneurons (IN) and stratum pyramidale pyramidal cells under infrared differential interference contrast imaging as described previously (Semyanov et al., 2003).
Hippocampal cultures were prepared from 0- to 2 d-old postnatal rat pups. The hippocampus was removed, minced, and incubated for 20 min in trypsinated HBSS (Sigma, St. Louis, MO) at 37°C. After washing, neurons were triturated with fire-polished Pasteur pipettes, counted with a hemacytometer, and plated in Neurobasal medium (Invitrogen, San Diego, CA) supplemented with 2% B-27 (Invitrogen), 1.8% HEPES, 1% glutamax (Invitrogen), 1% penicillin/streptomycin (Invitrogen), and 0.2% β-mercaptoethanol, according to a previously described method (Heeroma et al., 2004). Cells were maintained in culture for 14-20 d before use. The following drugs were used in all experiments to block AMPA/kainate, NMDA, and GABAB receptors, respectively: 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f]quinoxaline (25 μm), dl-2-amino-5-phosphonovalerate (50 μm), and CGP52432 (5 μm).
Whole-cell pipettes used to record GABAergic IPSCs contained the following (in mm): 120 CsCl, 8 NaCl, 10 HEPES, 2 EGTA, 0.2 MgCl2, 2 MgATP, 0.3 GTP, and QX-314 (lidocaine N-ethyl bromide quaternary salt), pH 7.2, 290 mOsm. Currents were acquired with an Axopatch 200B amplifier (Molecular Devices, Union City, CA), and records were filtered at 2 kHz, digitized at 5 kHz, and stored on a personal computer. The access and input resistances were monitored throughout the experiments using a-5 mV voltage step. The access resistance was <20 MΩ, and results were discarded if it changed by >20%. To achieve the necessary stability, neurons were recorded at 23-25°C, unless otherwise stated. The current decay after the voltage step was used to calculate the capacitance of the neurons.
Drugs were purchased from Tocris Cookson (Bristol, UK) or Sigma. Data analysis. Spontaneous IPSCs (sIPSCs) and evoked IPSCs (eIPSCs) were analyzed off-line. Data are presented as means ± SEM. Differences were considered significant at p < 0.05, as determined by using Student's paired or unpaired t test (when measures were normally distributed) or Wilcoxon matched-pair or Mann-Whitney U test (when distributions were not normal).
Immunohistochemistry. Immunocytochemistry was performed in three control rats and three epileptic (Ep)rats 3 weeks after SE. The rats were given sodium pentobarbital (Lethobarb; 200 mg, i.p.) and perfused with 50 ml of ice-cold PBS, pH 7.4, followed by 50 ml of 4% paraformaldehyde in PBS. The brains were postfixed for 90 min in the same fixative at 4°C and then put into 20% sucrose in PBS (4°C) for 24 h. Brains were frozen rapidly by immersion in -70°C isopentane (Merck, Darmstadt, Germany) for 3 min. After evaporating the isopentane, they were stored in tightly sealed vials at -70°C.
Horizontal sections (40 μm) were cut at -18°C in a microtome, starting with the ventral surface of the brain, put into Tris-HCl-buffered (50 mm, pH 7.2) saline, 0.1% sodium azide (TBS), and kept at 4-6°C. An affinity-purified rabbit antiserum directed against a synthetic peptide of the α5 subunit (amino acids 2-10) coupled to keyhole limpet hemocyanin was used at a final concentration of 5 μg/ml. The antibody is well characterized (Sperk et al., 1997). Indirect immunocytochemistry was performed on free-floating sections. Sections were rinsed in TBS for 30 min and then preincubated with 10% normal goat serum (Biotrade, Vienna, Austria) in TBS for 90 min. Incubation with the primary antibodies was performed at room temperature for 24 h. Sections were subsequently incubated with horseradish peroxidase-coupled goat anti-rabbit secondary antibodies (P 0448; 1:300; DakoCytomation, Vienna, Austria) at room temperature for 150 min and thereafter reacted with 1.4 mm 3,3′-diaminobenzidine (Fluka; Sigma, Vienna, Austria) and 0.01% H2O2 for 6-10 min. After each incubation step (except the preincubation), three 5 min washes with TBS were performed. All buffers and antibody dilutions, except those for washing and reacting with diaminobenzidine, contained 0.2% Triton X-100.
Results
Reduction of α5 GABAA receptor subunit immunoreactivity in epilepsy
We first confirmed that there is a downregulation of α5 immunoreactivity (IR) in the hippocampus of epileptic animals (Fig. 1A). Strata oriens, radiatum, and lacunosum moleculare of the ventral hippocampus in control rats were labeled most strongly in CA3 and less prominently in CA2 and CA1. In all three rats with chronic epilepsy, after pilocarpine-induced status epilepticus, α5-IR was reduced in CA1, CA3, and entorhinal cortex. This agrees with previous observations not only in the pilocarpine model but also in other epilepsy models (Houser and Esclapez, 1996; Schwarzer et al., 1997; Fritschy et al., 1999; Houser and Esclapez, 2003).
Figure 1.
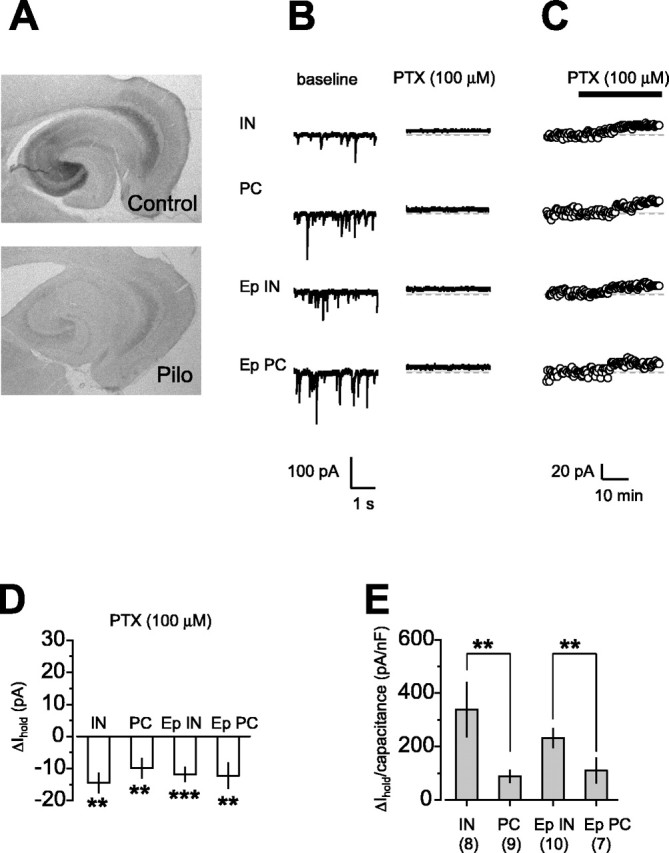
Downregulation of α5GABAA receptor subunit in area CA1 doesnot result in a reduction of the cell-type-specific tonic GABA current. A, Immunohistochemistry for the α5 subunit, showing decreased expression of this subunit in the hippocampus proper and enthorinal cortex of epileptic rats compared with that of control rats. Pilo, Pilocarpine. B, Representative traces obtained from one interneuron and one pyramidal cell from a control and an epileptic rat. Picrotoxin (PTX; 100 μm) abolished spontaneous IPSCs and reduced the baseline Ihold in all the cell types. C, Time course of Ihold before and after picrotoxin in the same cells as in B. D, Summary data of the change in Ihold after picrotoxin application. Ihold was reduced to similar extents in interneurons and pyramidal cells from control and epileptic rats. E, Tonic current density in interneurons and pyramidal cells from control and epileptic rats, measured as the ratio of the picrotoxin-sensitive Ihold to the capacitance of each cell. The tonic current density was significantly larger in interneurons than in pyramidal cells. This cell-type specificity was maintained during epilepsy. **p < 0.01; ***p < 0.001. Error bars represent SEM.
Tonic current is expressed in CA1 neurons from control and epileptic animals
The decrease in α5-IR leads to the expectation that CA1 pyramidal cells from epileptic animals express a smaller tonic GABAA receptor-mediated current than control neurons. We voltage clamped CA1 interneurons and pyramidal cells at -60 mV in slices from epileptic and control animals in the presence of blockers of ionotropic glutamate receptors and GABAB receptors. We then blocked GABAA receptors with picrotoxin (100 μm) and measured the change in holding current (ΔIhold). This revealed a tonic current in stratum radiatum interneurons and pyramidal cells from control rats at room temperature (23-25°C) (Fig. 1B,C). There was no significant effect on the tonic current of recording at 32°C (17.1 ± 5.5 pA; n = 5; p = 0.24 compared with tonic current at room temperature) (Semyanov et al., 2003). The tonic current recorded under baseline conditions in stratum radiatum interneurons and pyramidal cells in control animals was not significantly different from that detected in epileptic animals (Fig. 1B-D). In all cell types, tonic currents accounted for approximately three-fourths of the total current carried by GABAA receptors (tonic plus phasic currents, calculated as the product of the mean charge transfer and the mean frequency of spontaneous IPSCs: IN, 81 ± 2%, n = 8; PC, 77 ± 4%, n = 9; Ep IN, 75 ± 5%, n = 10; Ep PC, 73 ± 10%, n = 7). The tonic current is thus maintained in epilepsy, despite the downregulation of α5GABAARs.
We asked whether the difference in tonic current between interneurons and pyramidal cells observed previously in the guinea pig (Semyanov et al., 2003) is also present in the rat. The current density (tonic current/cell capacitance) was greater in interneurons than in pyramidal cells in both control and epileptic animals (Fig. 1E). However, there was no significant difference between control and epileptic animals. Thus, the differential expression of tonic current between CA1 interneurons and pyramidal cells normally present in control tissue is preserved in the epileptic tissue despite a profound decrease in the α5 subunit.
The decay of evoked, but not miniature or spontaneous, IPSCs is increased in pyramidal cells from epileptic animals
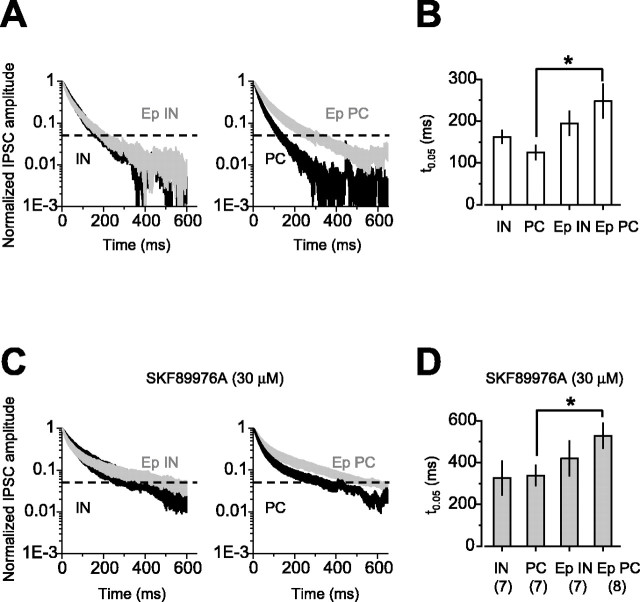
An alternative method of detecting high-affinity, extrasynaptic receptors is to examine the decay kinetics of IPSCs. Small events such as “miniature” IPSCs (mIPSCs) or sIPSCs activate primarily synaptic receptors, whereas synchronous release of GABA from multiple release sites, as occurs with large multiquantal IPSCs, can activate perisynaptic and extrasynaptic GABAA receptors (Overstreet and Westbrook, 2003). If α5GABAARs are mainly expressed extrasynaptically, then loss of these would be expected to result in a change in the late decay of large IPSCs but should have little or no effect on the kinetics of small events. Neither the amplitude nor the decay kinetics of action potential-independent mIPSCs was altered by epilepsy (Fig. 2A-C). There was also no change in the amplitude or decay kinetics of sIPSCs (Fig. 2D-F), arguing against a change in synaptic GABAA receptors in epilepsy. We next examined the decay of large stimulus eIPSCs and measured the time taken for eIPSCs to decay to 5% of the peak amplitude (t(0.05)) (Fig. 3A,B). Loss of α5GABAARs would be expected to lead to a shortening of t(0.05). This was, however, significantly prolonged in pyramidal cells from epileptic animals compared with control animals (p = 0.02). This result is thus unexpected in the context of the decrease in α5.
Figure 2.
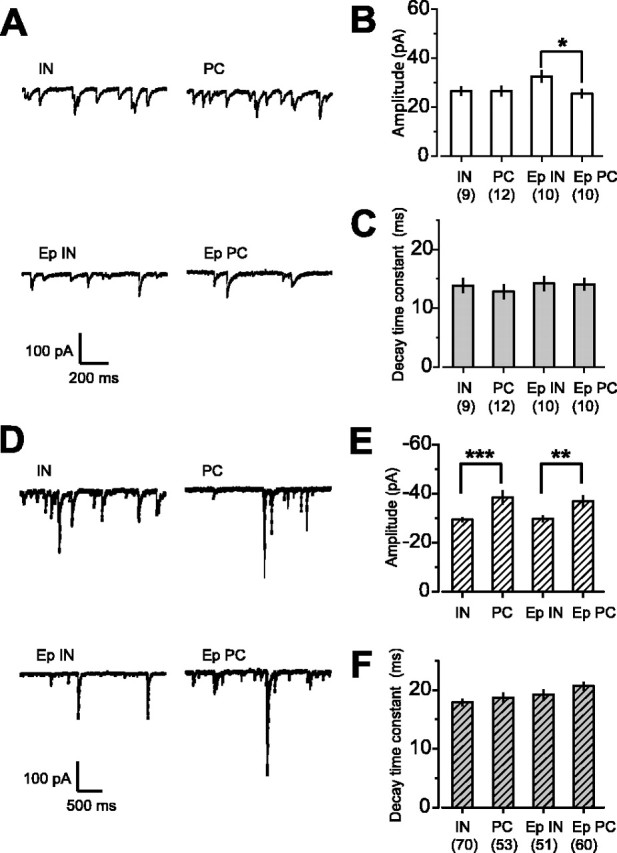
Synaptic GABAA receptor-mediated currents are not altered during epilepsy. A, Representative mIPSC traces recorded from interneurons and pyramidal cells from control and epileptic rats in the presence of tetrodotoxin (1 μm). B, C, Summary histograms of the amplitude and decay time constant of mIPSCs recorded in different cell types. The amplitude of mIPSCs was significantly larger in interneurons than in pyramidal cells in the epileptic hippocampus. No significant change was observed across cell types between control and epileptic rats. D, Representative sIPSC traces recorded from interneurons and pyramidal cells from control and epileptic rats. E, F, Summary histograms of the amplitude and decay time constant of sIPSCs recorded as in D. The amplitude of sIPSCs was larger in control pyramidal cells than in interneurons. This difference was maintained after epilepsy. The monoexponential decay time constant of sIPSCs was similar in all the cell types. *p < 0.05; **p < 0.01; ***p < 0.001. Error bars represent SEM.
Figure 3.
The late component of eIPSCs increases in pyramidal cells after status epilepticus. A, Average of the decaying phase of eIPSCs normalized by their peak amplitude in interneurons and pyramidal cells from control (black traces; IN, n = 7; PC, n = 7) and epileptic (gray traces; Ep IN, n = 7; Ep PC, n = 8) rats. eIPSCs in pyramidal cells from epileptic animals had a more prolonged time course than in pyramidal cells from control rats. B, Summary data of the time at which the amplitude of the eIPSCs had decayed to 5% of the peak amplitude (t(0.05)). After epilepsy, eIPSCs recorded from pyramidal cells had a slower time course. C, Averaged decaying phases of eIPSCs recorded from the same cells as in A in the presence of SKF89976A (30 μm). D, Summary data of t(0.05) recorded in the presence of SKF89976A. Blocking GABA transporters prolonged the decay of eIPSCs to a similar extent in all the cell types. Even in the presence of SKF89976A, the decay was longer in pyramidal cells from epileptic animals than in pyramidal cells from control rats. *p < 0.05. Error bars represent SEM.
GABA uptake is maintained in CA1 region of the hippocampus in epilepsy
Although the prolonged decay of eIPSCs suggests increased expression of high-affinity GABAA receptors, it could also be explained by a decrease in GABA uptake in epileptic tissue. We repeated the eIPSC decay time measurements in the presence of the GABA transporter inhibitor 1-(4,4-diphenyl-3-butenyl)-3-piperidine carboxylic acid (SKF89976A; 30 μm) (Fig. 3C,D). Inhibiting GABA uptake prolonged t(0.05) by a similar degree in pyramidal cells from epileptic and control animals (Fig. 3D). This argues against impaired GABA uptake as the explanation for the prolonged eIPSC decay time and instead implies changes in perisynaptic and extrasynaptic GABAA receptors in epilepsy.
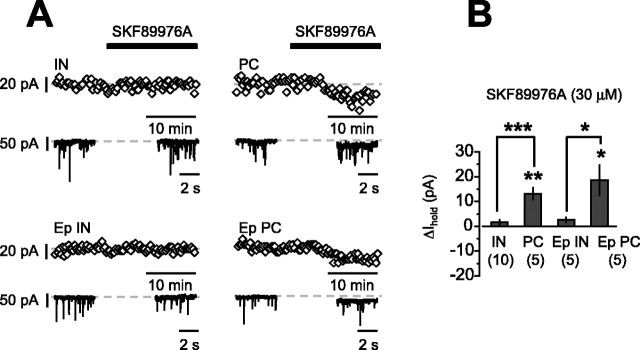
Because GABA uptake differentially regulates tonic currents in CA1 interneurons and pyramidal cells in guinea pigs (Semyanov et al., 2003), we asked whether this was the case in control and epileptic rats. SKF89976A (30 μm) resulted in an increase in Ihold in pyramidal cells from control and epileptic animals and a significantly smaller change in Ihold from interneurons from control and epileptic animals (Fig. 4A,B). If GABA uptake were impaired in CA1 region of epileptic animals, then we would have expected a lesser increase in the tonic current in pyramidal cells from epileptic animals compared with controls; the increase of the tonic current was, however, greater in pyramidal cells from epileptic animals than in those from control animals, although this did not reach significance (PC, 13.2 ± 2.5 pA, n = 5; Ep PC, 18.7 ± 6.2 pA, n = 5; p = 0.44). This indicates that in rats, as in guinea pigs, either GABA uptake has a less pronounced effect on interneurons than pyramidal cells, or the GABAA receptors mediating tonic currents in interneurons are saturated under baseline conditions. This finding further argues against decreased GABA uptake with epileptogenesis.
Figure 4.
The effect of blocking GABA uptake on Ihold in pyramidal cells and interneurons. A, Time course of Ihold recorded from interneurons and pyramidal cells from control and epileptic animals during the application of SKF89976A (30 μm). The insets show representative traces obtained in baseline conditions and at the steady state of the SKF98876A application. B, Summary histogram of the effect of SKF89976A on Ihold. Blocking uptake increased Ihold in pyramidal cells but not in interneurons in both the control and the epileptic hippocampus. *p < 0.05; **p < 0.01; ***p < 0.001. Error bars represent SEM.
Under baseline conditions, tonic currents are not mediated by α5GABAARs
The results presented thus far give rise to a paradox. On the one hand, we and others have observed a robust downregulation of α5 in epileptic animals (Fig. 1A) (Fritschy et al., 1999; Houser and Esclapez, 2003). On the other hand, the tonic current in pyramidal cells of epileptic animals is no smaller than in control animals, and if anything, the decay of large eIPSCs is prolonged and cannot be explained by a change in GABA uptake. A possible resolution of this paradox is that the tonic current (measured without increasing [GABA]o) is not mediated by α5GABAARs. We therefore asked whether the tonic current shows the pharmacological profile of α5GABAARs: low affinity for the benzodiazepine agonist zolpidem and high sensitivity to the α5-specific benzodiazepine inverse agonist L-655,708 (Caraiscos et al., 2004). To be confident of detecting an α5GABAAR component to the tonic current, we used a relatively high concentration of L-655,708 (50 μm), which has been shown previously to be specific for the α5GABAAR component of tonic current in pyramidal cells (Caraiscos et al., 2004). Neither zolpidem (200 nm) nor L-655,708 (50 μm) had a significant effect on Ihold in pyramidal cells from either control or epileptic animals (decrease in Ihold with zolpidem: PC, 0.16 ± 3.6 pA, n = 6, p = 0.96; Ep PC, -5.07 ± 3.94 pA, n = 7, p = 0.24; decrease in Ihold with L-655,708: PC, -3.46 ± 1.89 pA, n = 4, p = 0.16; Ep PC, 4.74 ± 4.29 pA, n = 4, p = 0.35). This suggests that this tonic current is not mediated by α1-3γ- or α5-containing GABAA receptors. We therefore looked for evidence for involvement of δ-subunit-containing receptors, which mediate tonic currents in cerebellar (Brickley et al., 2001; Stell et al., 2003) and dentate granule (Nusser and Mody, 2002; Stell et al., 2003; Wei et al., 2003) cells; these can be potentiated by a low concentration of the neurosteroid allotetrahydrodeoxycorticosterone (THDOC) (Stell et al., 2003). THDOC (10 or 100 nm) significantly increased Ihold in pyramidal cells from both control and epileptic animals in a dose-dependent manner (increase in Ihold with 10 nm THDOC: PC, 7.85 ± 2.27 pA, n = 7, p = 0.01; Ep PC, 10.44 ± 3.05 pA, n = 8, p = 0.01; increase in Ihold with 100 nm THDOC: PC, 20.64 ± 5.40 pA, n = 7, p < 0.01; Ep PC, 23.11 ± 6.75 pA, n = 8, p = 0.01). This result implies that, at low GABA concentrations, the tonic GABAA receptor-mediated current is mediated by δ-containing and not by α5-containing GABAA receptors in pyramidal cells from both control and epileptic rats.
α5GABAARs are recruited by increasing [GABA]o
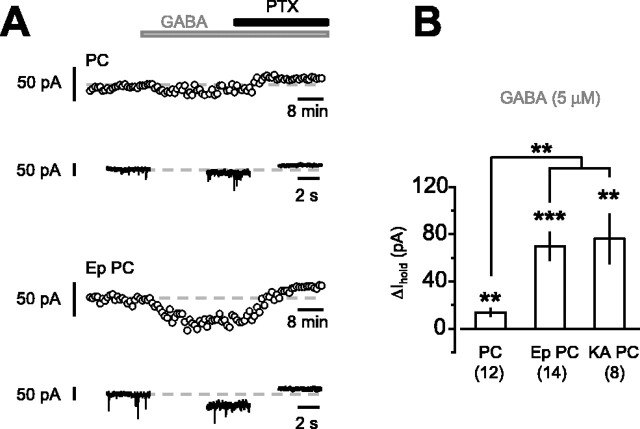
An important difference between the present results and previous studies that have reported a major role for α5 subunits is that we have not increased the ambient GABA concentration (Semyanov et al., 2003; Stell et al., 2003; Wu et al., 2003; Caraiscos et al., 2004). We therefore added 5 μm GABA to the perfusate and again measured Ihold. This manipulation significantly increased Ihold in pyramidal cells from control animals (Fig. 5A,B).
Figure 5.
The effect of increasing [GABA]o on the tonic current in pyramidal cells from epileptic and control animals. A, Time course of Ihold in a pyramidal cell from a control rat and from an epileptic rat after perfusion with 5 μm GABA. Insets show example traces obtained at the steady state of each drug application. PTX, Picrotoxin. B, Histogram summarizing the increase in Ihold in pyramidal cells induced by adding GABA (5 μm) to the perfusing solution in pyramidal cells from control, epileptic animals induced with pilocarpine (Ep PC), and epileptic animals induced with kainic acid (KA PC). The increase was significantly greater in pyramidal cells from epileptic animals (whether generated with pilocarpine or kainic acid) compared with those from control animals. **p < 0.01; ***p < 0.001. Error bars represent SEM.
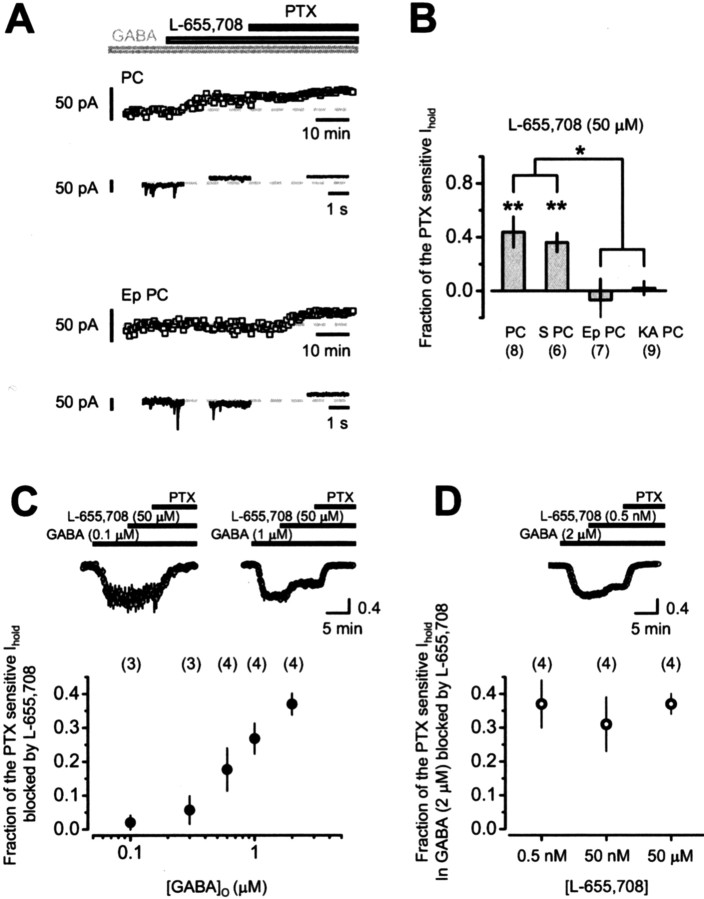
We then determined the proportion of the tonic current in 5 μm GABA perfusion that was attributable to α5GABAARs (Fig. 6A,B). Addition of the α5-specific inverse agonist L-655,708 (50 μm) resulted in a significant reduction in Ihold in pyramidal cells from control rats (p = 0.01), corresponding to 44 ± 11% of the picrotoxin sensitive Ihold (Fig. 6A,B). This observation is consistent with a previous report that tonic inhibition in pyramidal neurons has α5 pharmacology (Caraiscos et al., 2004), although, importantly, in the present study, an elevation of [GABA]o was required to detect it. Thus, tonic currents can be mediated by more than one set of receptors depending on [GABA]o.
Figure 6.
The role of α5-containing GABAA receptors in mediating tonic currents with increases in [GABA]o in pyramidal cells from control and epileptic animals. A, Time course of Ihold in pyramidal cells from control and epileptic animals recorded in the presence of [GABA]o = 5 μm. L-655,708 (50 μm), an inverse agonist of α5GABAARs, reduced Ihold in control but not in epileptic principal cells. Picrotoxin (100 μm) was added at the end of each experiment to estimate the total amount of GABAergic Ihold within each cell. B, Summary histogram of the effect of L-655, 708 on Ihold recorded in the presence of GABA (5 μm). L-655, 708 (50 μm) reduced by 44 and 36% the picrotoxin-sensitive Ihold recorded from control and sham (S PC) pyramidal cells, respectively (p = 0.6 for difference). Consistent with the downregulation of α5 in epilepsy, L-655, 708 changed Ihold by + 7% in pyramidal cells from pilocarpine epilepsy model (EP PC) and by -2% in pyramidal cells from kainic acid epilepsy model (KA PC), indicating that the increase in tonic current induced in these cells by increasing [GABA]o is not mediated by α5GABAARs. Error bars represent SEM. C, The top panel shows representative examples of the time course of Ihold in two cultured hippocampal neurons after the application of different GABA concentrations (left, 0.1 μm; right, 1 μm). Each dot represents the average ± SEM of three consecutive data points acquired every 4 s, normalized by the picrotoxin-sensitive tonic current measured at each [GABA]o. L-655,708 (50 μm) blocks a bigger fraction of the picrotoxin-sensitive Ihold at higher GABA concentrations. The bottom panel summarizes the proportion of the picrotoxin-sensitive Ihold mediated by α5GABAARs at five different GABA concentrations. The contribution of α5GABAARs to Ihold becomes detectable for concentrations of GABA >0.3 μm and progressively increases to ∼40% when [GABA]o = 2 μm. Each point represents the average ± SEM of three or four cells, for a total of 18 recorded neurons. D, The fraction of the picrotoxin-sensitive Ihold in GABA (2 μm) that is sensitive to L-655,708 at three different concentrations, demonstrating exquisite sensitivity of the tonic current to low concentrations of L-655,708 and no change in this sensitivity at higher concentrations, implying that the higher concentrations are not acting at additional GABAA receptor subtypes. The top panel shows a representative example of the time course of applying 0.5 nm L-655,708 followed by picrotoxin. *p < 0.05 for difference between Ep PC and either PC or S PC and p < 0.01 for difference between KA PC and either PC or S PC; **p < 0.01. PTX, Picrotoxin.
One problem with the experiments thus far is that in slices, the GABA concentration detected by the neurons will differ from the GABA concentration in the perfusate because of GABA uptake and release. Furthermore, this may vary between species and with age. So what range of GABA is being detected by the neurons in the slice, and over what range are α5GABAARs recruited? To address this question, we took advantage of the observation that the qualitative expression and localization of major GABAA receptor subtypes are retained in primary cultures of hippocampal neurons (Brunig et al., 2002). We therefore made whole-cell patch recordings from pyramidal-like neurons in hippocampal cell cultures from rats.
We perfused different concentrations of GABA, which evoked an increase in the holding current, and compared the effect of L-655,708 (50 μm) on the current in each case. By adding picrotoxin (100 μm) at the end of the experiment, this allowed the fraction of the picrotoxin-sensitive current blocked by L-655,708 to be plotted against [GABA]o. Figure 6C shows that, at low micromolar GABA concentrations (<0.3 μm), L-655,708-sensitive receptors made a minimal (<10%) contribution to the tonic current. Indeed, at 0.1 μm GABA, there was a significant tonic current (21 ± 4 pA; n = 3; p = 0.03) to which L-655,708-sensitive receptors made no detectable contribution. Only when [GABA]o was increased to 2 μm did L-655,708 block the tonic current to a similar extent as in the slice preparation perfused with 5 μm GABA (37 ± 3%, n = 4 in cultures; 44 ± 11%, n = 8 in slices; p = 0.66).
These results argue that α5GABAARs are only recruited by GABA concentrations >0.3 μm and, if receptor expression is similar in cultures and acute slices, imply that the ambient GABA concentration in acute slices is less than this concentration. A potential weakness of this argument is that the concentration of L-655,708 used in this study and the study by Caraiscos et al. (2004) was deliberately chosen to be higher than the binding affinity for α5GABAARs (Casula et al., 2001) to maximize the sensitivity of this assay. However, it raises the possibility of nonspecific actions of L-655,708 [although 50 μm L,655-708 had no detectable effect in α5GABAAR-/- mice (Caraiscos et al., 2004) and on the amplitude of sIPSCs in our experiments; p = 0.12]. We therefore repeated experiments on cultured neurons exposed to 2 μm GABA and asked whether applying increasing concentrations of L-655,708 ranging from 0.5 nm to 50 μm blocks an increasing fraction of the picrotoxin current. L-655,708, in fact, had no greater an effect at 50 μm than at low nanomolar concentrations (Fig. 6C), further arguing against a nonspecific effect.
Tonic current in pyramidal cells from epileptic animals
Because there is a downregulation of α5GABAARs in epilepsy (Fig. 1A), we asked whether increasing [GABA]o resulted in a lesser increase in the tonic current in pyramidal cells from epileptic animals. Surprisingly, the increase in current was threefold greater in pyramidal cells from epileptic animals than from control animals (p < 0.01) (Fig. 5A,B), consistent with increased expression of high-affinity GABAA receptors in epileptic animals.
Do α5GABAARs contribute to the increased tonic current in pyramidal neurons from epileptic rat slices perfused with 5 μm GABA? Application of L-655,708 had no significant effect (p = 0.24) on the tonic current in pyramidal cells from epileptic animals recorded during the continued perfusion of 5 μm GABA (Fig. 6A,B). Thus, in the epileptic hippocampus, although the tonic current was increased profoundly by increasing the [GABA]o, this did not show the pharmacological profile of α5GABAAR. Although we found no deficit in GABA uptake when perfusing with a solution containing no GABA (Fig. 3), we checked that the greater increase in tonic current in epileptic tissue was not caused by a deficit in GABA uptake when extracellular GABA was increased. We therefore investigated the effect of blocking GABA uptake with SKF89976A (30 μm) on Ihold during the continued perfusion of 5 μm GABA. Application of SKF89976A (30 μm) resulted in a marked increase in holding current in CA1 pyramidal cells from both control and epileptic tissue. This increase was greater in epileptic tissue, although it did not reach significance (ΔIhold Ep PC, 160.3 ± 33.4 pA, n = 7; PC, 89.6 ± 19.2 pA, n = 8). This finding is not consistent with a deficit of GABA uptake in the CA1 region of epileptic tissue. These results are consistent with downregulation of α5 in epileptic tissue and argue for an increase in other receptor subtype(s), which more than compensate for the loss of α5.
A potential confounding factor is that the handling and housing of the control animals differ from that of the epileptic animals, and handling and social isolation can alter GABAA receptor subtypes (Hsu et al., 2003). We therefore repeated these experiments in tissue from rats that had been treated in an identical manner to the epileptic rats except that they had received a subconvulsive dose of pilocarpine (sham controls; see Materials and Methods). In tissue from the sham controls, the magnitude of the tonic current in 5 μm GABA was not significantly different from that observed in naive rats (naive, 30.6 ± 5.4 pA; sham, 45.4 ± 8.4 pA; p = 0.15 for difference). Furthermore, the proportion of the tonic current mediated by α5GABAARs was no different from that observed in naive controls but was still significantly different from that observed in the epileptic animals (Fig. 6B). These results imply that the changes are not a function of the handling of the animals but are caused by the epileptogenic process per se.
We also asked whether the change in tonic GABAA receptor-mediated signaling is specific to the pilocarpine model of epileptogenesis. We repeated this set of experiments in a different model of temporal lobe epilepsy in which SE was induced with kainic acid (see Materials and Methods). Tissue from these rats demonstrated the same upregulation of tonic current and loss of the α5 component seen in the pilocarpine-treated rats, implying that this phenomenon is not model specific (Figs. 5B, 6B) but again related to epilepsy itself.
Could the increase in the tonic current in high [GABA]o in CA1 pyramidal cells from epileptic animals be mediated by an increase of δ-containing GABAA receptors? To answer this, we applied THDOC (10 nm) in the continued presence of GABA (5 μm). This increased Ihold by no more than was observed at low ambient GABA concentrations (Ep PC, 7.7 ± 4.2 pA, n = 5; 10.4 ± 3.1 pA, n = 8, respectively; p = 0.6 for difference). This implies that the increase in tonic current with higher [GABA]o cannot be explained by the recruitment of more δ-containing GABAA receptors but is consistent with the recruitment of an additional subset of GABAA receptors.
Thus, perfusing GABA reveals an α5GABAAR-mediated tonic current in pyramidal cells from control animals but results in a larger change in Ihold in pyramidal cells from epileptic animals, which is not mediated by α5- or δ-containing GABAA receptors.
Discussion
The observations that α5GABAARs mediate a tonic current in CA1 pyramidal cells (Caraiscos et al., 2004) and that α5 is consistently downregulated in CA1 pyramidal cells in epilepsy (Houser and Esclapez, 1996, 2003; Fritschy et al., 1999) (this study) led to the prediction that tonic inhibition should decrease in epilepsy. We found instead that tonic currents recorded in the absence of added GABA are in fact no smaller in CA1 pyramidal cells from epileptic animals than in cells from control animals. Instead, two lines of evidence point to an increase in GABA sensitivity in epilepsy: the tails of eIPSCs are prolonged, and the tonic current is enhanced by perfusing GABA to a greater extent than in control tissue. These paradoxical results are explained by the discovery that the tonic current has multiple components. In control animals, the current recorded in baseline conditions is sensitive to the δ-subunit-preferring modulator THDOC, but when GABA is elevated, a contribution from α5GABAAR is detected. We confirmed this in hippocampal cell cultures in which we could more accurately clamp [GABA]o. In epileptic tissue, on the other hand, the current that emerges when GABA is elevated remains insensitive to the α5-selective benzodiazepine inverse agonist (consistent with loss of α5 subunits).
GABAA receptor-mediated tonic currents have been variably recorded in CA1 pyramidal cells (Bai et al., 2001; Semyanov et al., 2003; Stell et al., 2003; Wu et al., 2003; Caraiscos et al., 2004). Potential confounding factors in such studies of tonic currents in the hippocampus are that they have been performed with different [GABA]o in different species. In vivo [GABA]o has been estimated to be of the order of 0.8 μm (Lerma et al., 1986) but may rise substantially during behavioral and pathological states (Minamoto et al., 1992; During and Spencer, 1993; Bianchi et al., 2003). In an attempt to reflect the in vivo state in vitro, it has been commonplace to increase [GABA]o by inhibiting GABA break-down (Wu et al., 2003; Caraiscos et al., 2004) uptake (Semyanov et al., 2003) or by perfusing slices with 5 μm GABA (Stell et al., 2003; Wei et al., 2003). However, these manipulations may raise the extracellular GABA concentration surrounding neurons within acute brain slices to different extents, depending on the degree to which transporters are able to clear the neurotransmitter from the extracellular space.
In adult rats, we were able to detect a tonic current in CA1 pyramidal cells in control and epileptic animals under baseline conditions. Although the tonic current is small, it contributes to three-quarters of the total inhibitory input (integrated tonic and phasic GABA currents) onto pyramidal cells in our experimental conditions. Importantly, the tonic current corrected for cell capacitance (cell size) was greater in interneurons than in pyramidal cells, and this difference was preserved in neurons from epileptic animals. This differential expression of the tonic current possibly contributes to homeostatic regulation of network excitability (Semyanov et al., 2003).
At low ambient GABA concentrations in our experiments, α5GABAARs do not contribute to the tonic current in pyramidal cells. The resistance to zolpidem and the sensitivity to THDOC that we observed are consistent with involvement of GABAA receptors containing the δ subunit and/or the α4 subunit (Brown et al., 2002). δ-Containing receptors mediate a tonic current in dentate (Nusser and Mody, 2002; Stell et al., 2003) and cerebellar granule (Brickley et al., 2001; Stell et al., 2003) cells and are ideally suited to detecting submicromolar [GABA]o, because they have a high affinity, slow desensitization rates (Saxena and Macdonald, 1996; Brown et al., 2002), and are expressed extrasynaptically (Nusser et al., 1998). Although α4-containing GABAA receptors are also sensitive to THDOC, their affinity for GABA is substantially increased when they are combined with the δ subunit (Brown et al., 2002). Indeed, the most likely subunit combination mediating the tonic current in CA1 pyramidal cells is α4δ (Liang et al., 2004). These findings are consistent with the findings in cultured hippocampal neurons (Mangan et al., 2005) but, at first hand, appear at odds with the finding of Caraiscos et al. (2004) that the tonic current in CA1 pyramidal cells is primarily mediated by α5GABAARs. The cited study was, however, performed on hippocampal neuronal cultures treated with the GABA transaminase inhibitor vigabatrin for 24 h before recording and thus in conditions of raised [GABA]o. From their results in mice, it is apparent, however, that a proportion of the tonic current is mediated by non-α5-containing GABAA receptors.
When we increased the [GABA]o, the tonic current increased in pyramidal cells from control and epileptic rats. In the control animals, the increase was, as predicted, mediated primarily by α5GABAAR. This result was confirmed in neurons in hippocampal culture and was relevant over the [GABA]o range measured by others in vivo (Cavelier et al., 2005). It thus appears that multiple GABAA receptor subtypes mediate the tonic current. Such heterogeneity may extend the dynamic range over which GABA can be detected (Semyanov et al., 2004). This is important, because GABA concentrations can vary twofold to threefold during physiological activities (e.g., exploration) (Bianchi et al., 2003) and fivefold in pathological states (e.g., during kindling or seizures) (Minamoto et al., 1992; During and Spencer, 1993; Smolders et al., 2004). Thus, α5GABAARs probably minimally contribute to the tonic current in resting conditions and only come into play during periods of activity.
In two animal models of epilepsy, the increase in tonic current in pyramidal cells with increased [GABA]o was significantly larger than that observed in control animals but was mainly mediated by GABAA receptors that do not express the α5 subunit. This increased responsiveness was not attributable to decreased efficacy of GABA uptake, because inhibiting GABA uptake had a similar effect in control and epileptic animals on both tonic current and also the late decay of eIPSCs. This is in keeping with animal and human data, which support a decrease in clearance of synaptically released extracellular GABA in the dentate gyrus (Patrylo et al., 2001) but preserved GABA uptake in the CA1 region in epilepsy (Frahm et al., 2003).
Thus, the increased tonic current is most likely to be mediated by an increase in the expression or activity of high-affinity GABAA receptors. Our observation that there was no change in the mIPSC or sIPSC amplitude and decay time constant in CA1 pyramidal cells from epileptic animals suggests that the main receptor changes occurred extrasynaptically. The decay time constant of small IPSCs is determined by single-channel kinetics and/or diffusion from the cleft, whereas release of GABA from many sites (i.e., during a large evoked IPSC) can result in spillover of GABA onto GABAA receptors beyond the activated synapses (Overstreet and Westbrook, 2003). Thus, our finding of a prolonged decay of large evoked IPSCs with an unchanged decay of smaller spontaneous IPSCs in the epileptic tissue is consistent with an increased expression of high-affinity, extrasynaptic GABAA receptors. Alternatively, it could be explained by the expression of novel extrasynaptic receptors with different kinetics.
What is the functional relevance of the increased tonic current in epileptic animals? Tonic currents modulate both the conductance threshold (and current threshold) for firing and the firing pattern of neurons (Semyanov et al., 2004). When excitation is mediated by random trains of synaptic conductance waveforms, tonic currents act as a multiplicative function on the input-output properties of the neuron (Mitchell and Silver, 2003). Thus, the increase in tonic current that occurs with increased [GABA]o (such as can result during increased network activity) may lead to a compensatory decrease in CA1 neuronal excitability. This could play a role in curtailing seizure activity. An alternative, but as yet untested, hypothesis is that hyperpolarization caused by the tonic current could remove the inactivation of sodium and calcium channels resulting in a paradoxical increase in excitability.
Our results have important implications for drugs selective for specific GABAA receptors. Drugs that act specifically on α5GABAARs may have a maximal action during active physiological states. Furthermore, modulators of GABAA receptors (e.g., neurosteroids, zinc) may only affect specific components of the tonic current. The change in the subunits mediating tonic current at high GABA concentrations in epileptic animals impacts on the treatment of epilepsy and on the use of drugs that target α5GABAARs in patients with epilepsy. Furthermore, the enhanced sensitivity to GABA of the tonic current in pyramidal cells from epileptic animals may impair learning and memory in these animals. Thus, although the enhanced pyramidal cell tonic current in epilepsy may prevent or curtail seizure activity, it could be detrimental to cognitive performance and may contribute to the cognitive deficits and decline observed in human temporal lobe epilepsy.
Footnotes
This work was supported by the Epilepsy Research Foundation, the Medical Research Council, the Wellcome Trust, and the Austrian Science Foundation (Grant P17203-B13). We are grateful for J. Heeroma and K. Volynski for help with cell cultures and to R. W. Tsien and members of the laboratory for helpful comments.
Correspondence should be addressed to Matthew Walker, Department of Clinical and Experimental Epilepsy, Institute of Neurology, University College London, Queen Square, London WC1N 3BG, UK. E-mail: mwalker@ion.ucl.ac.uk.
Copyright © 2005 Society for Neuroscience 0270-6474/05/2510016-09$15.00/0
References
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA (2001) Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol 59: 814-824. [DOI] [PubMed] [Google Scholar]
- Bianchi L, Ballini C, Colivicchi MA, Della Corte L, Giovannini MG, Pepeu G (2003) Investigation on acetylcholine, aspartate, glutamate and GABA extracellular levels from ventral hippocampus during repeated exploratory activity in the rat. Neurochem Res 28: 565-573. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M (1996) Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 497: 753-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M (2001) Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409: 88-92. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002) Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol 136: 965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM (2002) Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol 443: 43-55. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA (2004) Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci USA 101: 3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula MA, Bromidge FA, Pillai GV, Wingrove PB, Martin K, Maubach K, Seabrook GR, Whiting PJ, Hadingham KL (2001) Identification of amino acid residues responsible for the alpha5 subunit binding selectivity of L-655,708, a benzodiazepine binding site ligand at the GABA(A) receptor. J Neurochem 77: 445-451. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D (2005) Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol 87: 3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Hausser M (2004) Integration of quanta in cerebellar granule cells during sensory processing. Nature 428: 856-860. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Bromidge FA, Broughton HB, Cook S, Dawson GR, Hobbs SC, Maubach KA, Reeve AJ, Seabrook GR, Wafford K, MacLeod AM (2002) 6,7-Dihydro-2-benzothiophen-4(5H)-ones: a novel class of GABA-A alpha5 receptor inverse agonists. J Med Chem 45: 1176-1179. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW (2002) Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci 22: 5572-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U (2002) Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA 99: 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L (2002) Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron 36: 1051-1061. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet 341: 1607-1610. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6: 215-229. [DOI] [PubMed] [Google Scholar]
- Frahm C, Stief F, Zuschratter W, Draguhn A (2003) Unaltered control of extracellular GABA-concentration through GAT-1 in the hippocampus of rats after pilocarpine-induced status epilepticus. Epilepsy Res 52: 243-252. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Johnson DK, Mohler H, Rudolph U (1998) Independent assembly and subcellular targeting of GABA(A)-receptor subtypes demonstrated in mouse hippocampal and olfactory neurons in vivo. Neurosci Lett 249: 99-102. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Kiener T, Bouilleret V, Loup F (1999) GABAergic neurons and GABA(A)-receptors in temporal lobe epilepsy. Neurochem Int 34: 435-445. [DOI] [PubMed] [Google Scholar]
- Heeroma JH, Roelandse M, Wierda K, van Aerde KI, Toonen RF, Hensbroek RA, Brussaard A, Matus A, Verhage M (2004) Trophic support delays but does not prevent cell-intrinsic degeneration of neurons deficient for munc18-1. Eur J Neurosci 20: 623-634. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M (1996) Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures. Epilepsy Res 26: 207-218. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M (2003) Downregulation of the alpha5 subunit of the GABA(A) receptor in the pilocarpine model of temporal lobe epilepsy. Hippocampus 13: 633-645. [DOI] [PubMed] [Google Scholar]
- Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR (2003) Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc Natl Acad Sci USA 100: 12213-12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384: 145-155. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I (2004) Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther 310: 1234-1245. [DOI] [PubMed] [Google Scholar]
- Liu QY, Vautrin J, Tang KM, Barker JL (1995) Exogenous GABA persistently opens Cl- channels in cultured embryonic rat thalamic neurons. J Membr Biol 145: 279-284. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J (2005) Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol 67: 775-788. [DOI] [PubMed] [Google Scholar]
- Minamoto Y, Itano T, Tokuda M, Matsui H, Janjua NA, Hosokawa K, Okada Y, Murakami TH, Negi T, Hatase O (1992) In vivo microdialysis of amino acid neurotransmitters in the hippocampus in amygdaloid kindled rat. Brain Res 573: 345-348. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA (2003) Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron 38: 433-445. [DOI] [PubMed] [Google Scholar]
- Mohler H, Fritschy JM, Luscher B, Rudolph U, Benson J, Benke D (1996) The GABAA receptors. From subunits to diverse functions. Ion Channels 4: 89-113. [PubMed] [Google Scholar]
- Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol 87: 2624-2628. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P (1998) Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 18: 1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Westbrook GL (2003) Synapse density regulates independence at unitary inhibitory synapses. J Neurosci 23: 2618-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Spencer DD, Williamson A (2001) GABA uptake and hetero-transport are impaired in the dentate gyrus of epileptic rats and humans with temporal lobe sclerosis. J Neurophysiol 85: 1533-1542. [DOI] [PubMed] [Google Scholar]
- Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32: 281-294. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D (2003) Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol (Lond) 548: 97-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Prince DA (1996) Spontaneous GABAA receptor-mediated inhibitory currents in adult rat somatosensory cortex. J Neurophysiol 75: 1573-1588. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL (1996) Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol 49: 567-579. [PubMed] [Google Scholar]
- Schwarzer C, Tsunashima K, Wanzenbock C, Fuchs K, Sieghart W, Sperk G (1997) GABA(A) receptor subunits in the rat hippocampus II: altered distribution in kainic acid-induced temporal lobe epilepsy. Neuroscience 80: 1001-1017. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM (2003) GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6: 484-490. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262-269. [DOI] [PubMed] [Google Scholar]
- Smolders I, Lindekens H, Clinckers R, Meurs A, O'Neill MJ, Lodge D, Ebinger G, Michotte Y (2004) In vivo modulation of extracellular hippocampal glutamate and GABA levels and limbic seizures by group I and II metabotropic glutamate receptor ligands. J Neurochem 88: 1068-1077. [DOI] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W (1997) GABA(A) receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience 80: 987-1000. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100: 14439-14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA (1989) Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse 3: 154-171. [DOI] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM (1997) Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci 9: 533-548. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I (2003) Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23: 10650-10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA (1999) Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann NY Acad Sci 868: 645-653. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Richerson GB (2003) Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol 89: 2021-2034. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA (2003) Tonically activated GABAA receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol 63: 2-8. [DOI] [PubMed] [Google Scholar]