Abstract
Nicotine, a component of tobacco, is highly addictive but possesses beneficial properties such as cognitive improvements and memory maintenance. Involved in these processes is the neuronal nicotinic acetylcholine receptor (nAChR) α7, whose activation triggers depolarization, intracellular signaling cascades, and synaptic plasticity underlying addiction and cognition. It is therefore important to investigate intracellular mechanisms by which a cell regulates α7 nAChR activity. We have examined the role of phosphorylation by combining molecular biology, biochemistry, and electrophysiology in SH-SY5Y neuroblastoma cells, Xenopus oocytes, rat hippocampal interneurons, and neurons from the supraoptic nucleus, and we found tyrosine phosphorylation of α7 nAChRs. Tyrosine kinase inhibition by genistein decreased α7 nAChR phosphorylation but strongly increased acetylcholine-evoked currents, whereas tyrosine phosphatase inhibition by pervanadate produced opposite effects. Src-family kinases (SFKs) directly interacted with the cytoplasmic loop of α7 nAChRs and phosphorylated the receptors at the plasma membrane. SFK inhibition by PP2 [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine] or SU6656 (2,3-dihydro-N,N-dimethyl-2-oxo-3-[(4,5,6,7-tetrahydro-1H-indol-2-yl)methylene]-1H-indole-5-sulfonamide) increased α7 nAChR-mediated responses, whereas expression of active Src reduced α7 nAChR activity. Mutant α7 nAChRs lacking cytoplasmic loop tyrosine residues because of alanine replacement of Tyr-386 and Tyr-442 were more active than wild-type receptors and insensitive to kinase or phosphatase inhibition. Because the amount of surface α7 receptors was not affected by kinase or phosphatase inhibitors, these data show that functional properties of α7 nAChRs depend on the tyrosine phosphorylation status of the receptor and are the result of a balance between SFKs and tyrosine phosphatases. These findings reveal novel regulatory mechanisms that may help to understand nicotinic receptor-dependent plasticity, addiction, and pathology.
Keywords: nicotinic receptor, α7 nAChR, Src kinase, phosphorylation, phosphatase, regulation
Introduction
Protein phosphorylation and dephosphorylation are key mechanisms to regulate the activity of integral membrane proteins such as ion channels. In brain, tyrosine phosphorylation directs synaptic plasticity, as illustrated by the role of Src-family kinases (SFKs) in hippocampal long-term potentiation (LTP) and spatial learning (Grant et al., 1992; Lu et al., 1998). Although such LTP involves phosphorylation of glutamate receptors (Lu et al., 1998), the hippocampus and other brain areas are rich in other ligandgated channels such as neuronal nicotinic acetylcholine receptors (nAChRs) (Jones et al., 1999). However, regulatory mechanisms of these brain nAChRs such as phosphorylation are still essentially unknown.
One of the most prominent nAChRs is the homomeric α7 receptor, which acts in synaptic plasticity underlying cognitive processing and addiction (Dajas-Bailador and Wonnacott, 2004). In hippocampus, α7 nAChRs are highly expressed in GABAergic interneurons in which they mediate cholinergic synaptic input (Alkondon et al., 1998; Frazier et al., 1998b) and regulate inhibition within the hippocampal network (Alkondon et al., 1997; Jones and Yakel, 1997). Activation of these α7 nAChRs blocks concurrent short-term potentiation and LTP induction in the pyramidal cells innervated by the interneurons (Ji et al., 2001). Inhibition of pyramidal neurons by postsynaptic α7 nAChRs on interneurons also underlies hippocampal auditory gating (Martin et al., 2004). Nicotine-activated presynaptic α7 receptors, because of their high calcium permeability, increase glutamate release in the ventral tegmental area, leading to LTP in dopaminergic neurons, a model for nicotine addiction (Mansvelder and McGehee, 2000)
α7 nAChRs are linked to many normal and pathological conditions. They are important in working memory formation, arousal, and attention (Levin, 2002), nicotine addiction, and nicotine-induced reversal of age-related memory deficits (Picciotto and Zoli, 2002). Their role in hippocampal auditory gating has linked the receptors to schizophrenia (Jones et al., 1999; Martin et al., 2004). The observation that α7 nAChR activation leads to neuroprotection against Aβ-induced neurotoxicity (Kihara et al., 2001; Shaw et al., 2002), together with many other findings (Liu et al., 2001; Picciotto and Zoli, 2002), suggests that the receptor may play a key role in Alzheimer's disease.
These diverse physiological roles of α7 nAChRs are thought to reflect local calcium signaling that triggers downstream pathways, such as activation of cAMP response element-binding protein, protein kinase A, and extracellular signal-regulated kinase 1/2 (Dajas-Bailador et al., 2002; Hu et al., 2002), or phosphatidylinositol 3-kinase, Akt, and Bcl-2 (Kihara et al., 2001). Such mechanisms result in expression of genes important in synaptic plasticity or cell survival (Berg and Conroy, 2002; Dajas-Bailador and Wonnacott, 2004). However, no signaling, scaffolding, or anchoring proteins that directly interact with α7 receptors have as yet been identified (Huh and Fuhrer, 2002).
We began addressing how phosphorylation and intracellular proteins regulate α7 nAChRs. Our results reveal that a balance between kinases and phosphatases regulates tyrosine phosphorylation of the receptors at the cell surface. SFKs are part of these kinases, because SFKs bind and phosphorylate α7 receptors and regulate their activity. Interestingly, dephosphorylated α7 nAChRs cause increased ACh-evoked current, whereas phosphorylated receptors are less active, showing that tyrosine phosphorylation and SFKs negatively regulate receptor activity.
Materials and Methods
Culture of SH-SY5Y α7 cells and isolation of α-bungarotoxin-binding (total) α7 receptors. SH-SY5Y neuroblastoma cells that were stably transfected with human α7 subunits (SH-α7) were maintained in DMEM/Ham's F-12 (1:1) media containing 10% fetal calf serum and 100 μg/ml G418 and were split every 3-4 d. Isolation of α-bungarotoxin (α-BT)-binding α7 receptors was done as described previously for muscle nAChRs from myotubes (Fuhrer and Hall, 1996; Mittaud et al., 2001). Briefly, SH-α7 cells were extracted in lysis buffer containing 1% NP-40, 30 mm triethanolamine, 50 mm NaCl, 5 mm EDTA, 5 mm EGTA, 50 mm NaF, 2 mm Na-orthovanadate, 1 mm N-ethylmaleimide, 1 mm Natetrathionate, 50 μm phenylarsine-oxide, 10 mm p-nitrophenylphosphate, 25 μg/ml aprotinin, 25 μg/ml leupeptin, 2 mm Na-orthovanadate, and 1 mm PMSF. Cell lysates were cleared by centrifugation (refrigerated Eppendorf centrifuge, 14,000 rpm, 5 min at 4°C; Eppendorf Scientific, Westbury, NY), and supernatants were incubated with 200 nm biotinylated α-BT (Invitrogen, Eugene, OR). Streptavidin-agarose beads (Invitrogen) were added to precipitate α7 nAChRs. Nonspecific binding was determined by applying 10 μm free (nonbiotinylated) α-BT before addition of biotinylated α-BT. After precipitation, bead pellets were washed, and bound proteins were eluted and subjected to SDS-PAGE and Western blot analysis. α7 receptors were detected using goat polyclonal antibodies raised against the α7 C terminus (1:200 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) (Drisdel and Green, 2000) or rabbit polyclonal antibodies raised against the α7 N terminus (1:1000 dilution; Abcam, Cambridge, UK). Both antibodies gave the same results. α7 receptors isolated in this manner reflect the total cellular pool of α7 nAChRs. In our figures, α-BT-precipitations (Tox-P) represent such total α7 receptor isolations, unless specified as surface or intracellular precipitation (see below).
Isolation of surface and intracellular α7 nAChRs. To precipitate α7 receptors from the surface of SH-α7 cells, we used a protocol established previously for muscle nAChRs in myotubes (Moransard et al., 2003). Briefly, intact SH-α7 cells were incubated in media with 200 nm biotinylated α-BT for 45 min in the culture incubator. Nonspecific binding was determined by applying 10 μm free (nonbiotinylated) α-BT 30-45 min before adding biotinylated α-BT. Cells were washed twice with PBS and lysed as described above. Streptavidin-agarose beads were added to precipitate surface AChRs.
Intracellular α7 receptors were precipitated as described previously for muscle nAChRs (Moransard et al., 2003). Briefly, intact SH-α7 cells were preincubated for 45 min with 1 μm free α-BT in media in the culture incubator to block surface receptors. Cells were washed twice with PBS to remove unbound α-BT and lysed. Biotinylated α-BT (200 nm) was added to lysates, followed by streptavidin-agarose beads, to isolate intracellular α7 nAChRs.
In these preparations, surface and intracellular α7 receptors were additive to yield total receptors (K. H. Huh and C. Fuhrer, unpublished observations).
Treatment of SH-α7 cells with pervanadate and kinase inhibitors. Pervanadate was prepared as described previously (Meier et al., 1995) and used at a standard concentration of 50 μm in culture medium on SH-α7 cells for 20 min before lysis. To block tyrosine kinases, cells were pretreated with genistein (100 or 250 μm for 10 min), PP2 [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine] (1 or 10 μm for 10 min (Smith et al., 2001), CGP77675; [10 μm for 45-90 min (Missbach et al., 1999)], or 2,3-dihydro-N,N-dimethyl-2-oxo-3-[(4,5,6,7-tetrahydro-1H-indol-2-yl)methylene]-1H-indole-5-sulfonamide [SU6656; a substituted 5,7-diphenyl-pyrrolo[2,3-d]pyrimidine [10 μm for 30 min (Blake et al., 2000)] before addition of pervanadate (50 μm for 20 min). After these treatments, cells were lysed, and α7 receptors were precipitated as described above. Tyrosine phosphorylation of α7 receptors and SFK association were evaluated by Western blotting.
Preparation of rat brain membrane homogenates. Whole brains from postnatal day 5 (P5) rats or forebrains from P30 rats (Lewis) were homogenized in lysis buffer containing 1% NP-40, DTT (2 μm), and pepstatin (5 μg/ml). Homogenized material was incubated at 4°C for 30 min for lysis and centrifuged at 55,000 rpm for 20 min. Cleared soluble lysates were incubated with α-BT directly coupled to Sepharose beads (Fuhrer and Hall, 1996) for 2 h to isolate α7 receptors. For determination of nonspecific binding, these lysates were preincubated with 10 μm free α-BT for 30 min before incubation with α-BT-coupled beads. After incubation, beads were processed as described above. Phosphorylation of the receptors and association of SFKs were determined by Western blot analysis.
Additional antibodies and Western blot analysis. Tyrosine phosphorylation was detected using phosphotyrosine-specific antibodies (4G10; Upstate Biotechnology, Lake Placid, NY). Antibodies against Src-family kinases [pan-Src antibodies (Src-CT), Lyn and Fyn] and glutathione S-transferase (GST) were purchased from Santa Cruz Biotechnology (Fuhrer and Hall, 1996). Specific antibodies against Src were from Oncogene Sciences (Boston, MA) (Fuhrer and Hall, 1996). Blots were probed with antibodies in 4G10 blocking solution (5% BSA, 0.5% NP-40 in TBST (0.1% Tween-20, 20 mm Tris, 137 mm NaCl, pH 7.6)) or in 5% milk in TBST. Proteins were detected by chemiluminescence using a kit from Amersham Biosciences (Little Chalfont, UK). For reprobing, blots were stripped by incubating them for 20 min in 200 mm glycine, 0.1% Tween 20, pH 2.5.
Determination of putative phosphorylation sites. The Netphos 2.0 software (Blom et al., 1999) (http://www.cbs.dtu.dk/services/NetPhos/) was used to determine putative phosphorylation sites in the α7 protein sequence. Tyrosines known to be phosphorylated in nAChR subunits from Torpedo californica (Wagner et al., 1991) were aligned with human α7 nAChRs to further analyze phosphorylation sites. Combination of software prediction and sequence alignment data, also using α7 sequences from a variety of species, was used to predict phosphorylation sites in human α7 receptors.
DNA constructs. Sequences encoding the cytoplasmic loop (amino acids 325-459; GST-α7loop) or the C-terminal region including the fourth transmembrane domain (amino acids 461-502; GST-α7TM4CT) of the human α7 receptor were inserted in frame into pGEX-2T vector encoding glutathione-S-transferase (GST). Tyrosines 386 or 442 in GST-α7loop were mutated to alanine using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) to generate GST-α7(A386), GST-α7(A442), and GST-α7(A386/442). Fusion proteins were expressed in DH5α bacterial strain and purified using glutathione-Sepharose beads as described previously (Fuhrer and Hall, 1996). Mutant α7 2Y-A receptors were constructed accordingly, using QuikChange site-directed mutagenesis kit and the full-length human α7 sequence in a pcDNA3-based expression vector (TOPO vector). Expression vectors for viral Src (vSrc) and vSrc-KD (kinase-dead vSrc) were kindly provided by Dr. Todd Holmes (New York University, New York, NY) (Nitabach et al., 2001, 2002).
SFK association and in vitro phosphorylation of GST-α7 fusion proteins. To study interaction with SFKs (see Fig. 7d), purified glutathione-Sepharose bead-bound fusion proteins were added for 2 h to SH-α7 lysates prepared as described above. After washing with high- and low-salt wash buffers (Fuhrer and Hall, 1996), the resulting precipitates were used to evaluate the association of SFKs by Src-CT Western blotting.
Figure 7.
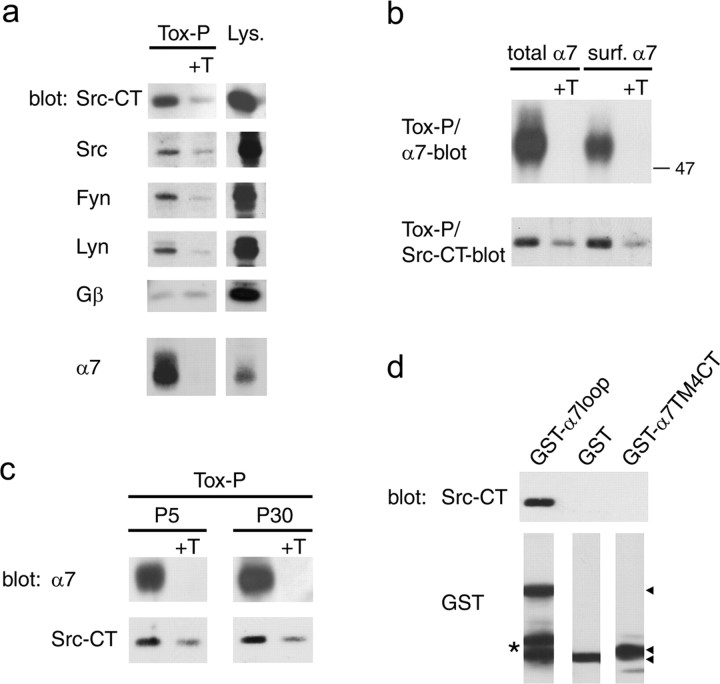
α7 nAChRs interact with SFKs. a, α7 receptors were precipitated from SH-α7 cells using biotinylated α-BT followed by streptavidin-agarose (Tox-P), and associated proteins were identified by immunoblotting. As controls, excess free α-BT was added before precipitation (+T), or a fraction of the lysate was analyzed without precipitation (Lys.). Pan-Src (Src-CT) and single kinase-specific antibodies show that α7 receptors associate with SFKs, including Src, Fyn, and Lyn, whereas no specific association occurs with the Gβ subunit of trimeric G-proteins. b, Surface or total α7 receptors were precipitated from SH-α7 cells and probed with Src-CT antibodies, showing that the association of α7 nAChRs with SFKs is prominent at the cell surface. c, Whole brains of P5 rats and forebrains of P30 rats were homogenized in NP-40-containing buffer, and α7 receptors were isolated using α-BT coupled to Sepharose beads. Src-CT blotting reveals specific association of α7 nAChRs with SFKs in brain. d, Lysates from SH-α7 cells were incubated with glutathione bead-bound GST-fusion proteins containing either the α7 loop (GST-α7loop) or the fourth transmembrane domain and C-terminal rest (GST-α7 TM4CT). Src-CT blotting of precipitated beads shows that SFKs bind specifically to the α7 loop. Arrowheads indicate the GST fusion proteins, and the asterisk denotes degradation products of GST-α7loop.
To examine tyrosine phosphorylation of fusion proteins by SFKs from SH-α7 cells in vitro (see Fig. 8c), cells were pretreated with pervanadate alone or in combination with PP2. Lysates were incubated with purified GST-α7loop fusion proteins, and beads were precipitated and washed. Beads were incubated in phosphorylation assay buffer containing 3 mm ATP (20 mm Tris, pH 7.4, 10 mm MgCl2, 2 mm Na-orthovanadate, and 1 mm PMSF) for 15 min at 37°C or 4°C. Beads were centrifuged at 4°C to remove supernatant, and attached proteins were analyzed by SDS-PAGE and Western blot.
Figure 8.
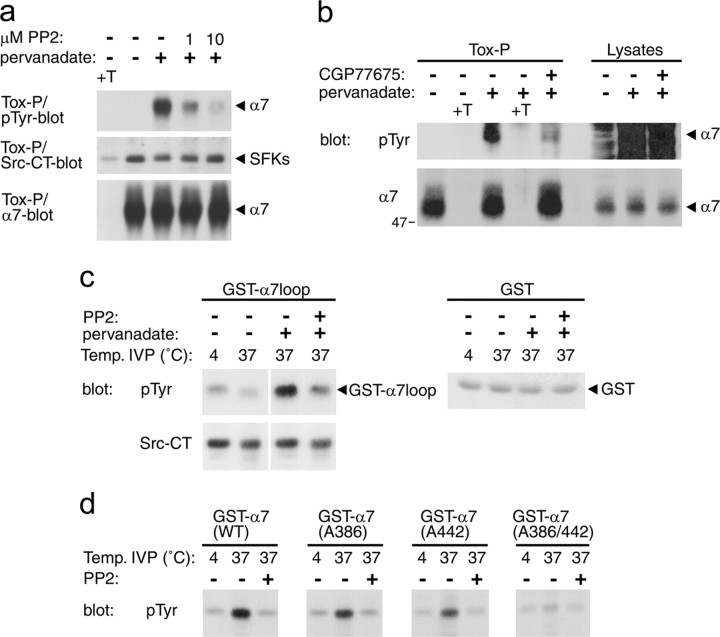
SFKs phosphorylate tyrosine residues in the α7 loop. a, b, SH-α7 cells were treated for 10 min with PP2 or for 45 min with CGP77675, followed by addition of 50 μm pervanadate for 20 min in the presence of the SFK inhibitors. α-BT-precipitated α7 was analyzed by phosphotyrosine and α7 immunoblotting. Fractions of lysates were analyzed without precipitation in b. Pervanadate-induced phosphorylation of α7 nAChRs is reduced by the SFK inhibitors PP2 or CGP77675, showing that SFKs can phosphorylate α7 receptors within SH-α7 cells. c, SH-α7 cells were treated with PP2 and pervanadate as in a, lysed, and incubated with bead-bound GST-α7loop fusion protein or control GST. After precipitation and washing, bead complexes (containing GST fusions and associated proteins, including SFKs) were subjected to in vitro phosphorylation (IVP) reactions in the presence of ATP at 4°C or 37°C. Bead complexes were analyzed by phosphotyrosine and Src-CT immunoblotting, showing that pervanadate-activated SH-α7 cell lysates phosphorylate GST-α7loop proteins and that this phosphorylation originates from SFKs. d, Purified GST-fusion proteins containing the α7 wild-type loop or mutants in which Tyr-386 and/or Tyr-442 are replaced by alanine were incubated with purified human Src kinase. In vitro phosphorylation was assessed in the presence of ATP at 4°C or 37°C, with or without PP2. Phosphotyrosine blotting of reaction products shows that Src phosphorylates both tyrosines in the α7 loop.
To examine tyrosine phosphorylation of fusion proteins by Src kinase in vitro (see Fig. 8d), purified GST-α7loop(WT), GST-α7(A386), GST-α7(A442), and GST-α7(A386/442) proteins were incubated with 1.5 U of purified human Src kinase (c-Src; Upstate Biotechnology). Incubations were performed in phosphorylation assay buffer containing 10 μm ATP (20 mm HEPES, 10 mm MgCl2, and 100 mm NaCl) as described above.
Labeling with Alexa 488-α-BT and fluorescence microscopy. SH-α7 cells were grown for 5 d on l-lysine- and laminin-coated plates in medium supplemented with 20 μm all-trans-retinoic acid to promote neural differentiation. Medium was exchanged by media lacking fetal bovine serum, and 20 ng/ml BDNF was added for 5 d. To stain cells, 100 nm Alexa 488-coupled α-BT (Molecular Probes) was added for 1 h. Nonspecific binding was assessed by incubating cells in 10 μm unlabeled α-BT or 1 mm nicotine 10 min before and during the labeling with Alexa 488-α-BT. Cells were washed in buffer containing 4′,6′-diamidino-2-phenylindole (DAPI) to label nuclei, fixed with cryofix, and mounted on coverslips. Conventional fluorescence imaging was done using a Zeiss (Oberkochen, Germany) Axioskop 2 microscope equipped with a cooled Hamamatsu (Schüpfen, Switzerland) Orca digital camera, using 1000× magnification. Alexa 488-α-BT detected clusters on cell somata and processes, and this signal was abolished by unlabeled α-BT or nicotine (data not shown), confirming that it corresponds to α7 receptors.
Oocyte preparation and cDNA injection. Xenopus oocytes were isolated and prepared as described previously (Eisele et al., 1993; Krause et al., 1998). Two nanograms of expression vectors containing the various cDNAs were injected into the oocyte nucleus. Each oocyte was injected with TOPO expression vector containing wild-type human α7 or mutant α7 2Y-A receptor DNA. For experiments with vSrc kinase, the ratio was 10:1 for α7 versus vSrc or vSrc-KD DNA. Oocytes were incubated for 2-3 d at 18°C in Barth's solution that contained 88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3, 10 mm HEPES, 0.82 mm MgSO4·7H2O, 0.33 mm Ca(NO3)2·4H2O, and 0.41 mm CaCl2·6H2O, at pH 7.4, supplemented with 20 μg/ml kanamycine, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Binding assay with 125I-α-BT. Oocytes labeling experiments were performed 2 d after DNA injection. Oocytes were incubated for 1 h in either control solution or the presence of 10 μm genistein, 125I-α-BT (Amersham Biosciences, Arlington Heights, IL) was added at a final concentration of 10 nm, and cells were incubated for 1 h. After labeling, oocytes were individually washed five times in cold OR2 buffer (for buffer ingredients, see next paragraph). To prevent adsorption of the toxin on the plastic, all solutions were supplemented with 20 μg/ml BSA. Each oocyte was then placed in a vial, and the amount of radioactive 125I-α-BT was determined using a gamma counter (2 min counting mode). Nonspecific binding was determined using 1 μm unlabeled α-BT (1 h preincubation and during radioactive labeling). Thirty oocytes were tested in each condition. Expression rates in oocytes are known to vary considerably (Yoshii et al., 1987; Yu et al., 1991; Cruz-Martin et al., 2001). For this reason, only cells displaying a labeling superior to nonspecific binding were used for the computation, and mean nonspecific values were subtracted. Using a 125I-α-BT standard, the amount of bound toxin was calculated in femtomoles per oocyte.
To label SH-α7 cells, cells were grown on 35 mm dishes to a confluence of 60-100% and incubated with 10 nm 125I-α-BT for 1 h in cell medium. Inhibitor treatments were as in the corresponding biochemical experiments (100 μm genistein was added for 30 min, 10 μm PP2 for 90 min, and 50 μm pervanadate for 20 min before 125I-α-BT), and inhibitors were present during the radioactive labeling. Nonspecific binding was determined by adding 1 μm unlabeled toxin 1 h before and during radioactive labeling. Cells were then washed twice with ice-cold PBS and solubilized in lysis buffer as described above (paragraph about precipitation with biotin-α-BT). Lysates were analyzed in a gamma counter, and ali-quots were taken for protein determination assays. Radioactive signals were robust (30,000-100,000 cpm), the nonspecific background was low (5-10%), and protein levels did not vary between individual cell lysates in an experiment. Cell treatments at 37°C or 21°C produced the same results.
Oocyte electrophysiological recordings. Cells were continuously superfused with OR2 that contained 82.5 mm NaCl, 2.5 mm KCl, 5 mm HEPES, 2.5 mm CaCl2·2H2O, and 1 mm MgCl2.6H2O, pH 7.4, and 0.5 μm atropine was added to prevent possible activation of endogenous muscarinic receptors. All drugs were diluted in the OR2 medium immediately before the experiment. Bovine serum albumin (20 mg/ml) was added to the perfusion and drug media to prevent adsorption of the compounds to the plastic. Electrophysiological recordings were performed using a two-electrode voltage clamp (GENECLAMP amplifier; Axon Instruments, Forster City, CA), and cells were held at -100 mV. The flow rate was ∼6 ml/min, and the volume of the chamber was <100 μl to allow rapid solution exchange.
To minimize contamination by endogenous Ca2+-activated chloride channels, oocytes were incubated with BAPTA-AM (100 μm) for at least 3 h before recording. For preincubation experiments, oocytes were incubated with drugs diluted in Barth's medium in the presence of BAPTA-AM (100 μm). Preincubation periods of 1-2 h were used for genistein and inactive genistein and of 30-60 min for pervanadate. Recordings performed after preincubations were done in the presence of an identical drug concentration to prevent drug washout. To limit possible direct drug effect on the α7 receptor, the drug application was stopped 1 s before and restarted immediately after the acetylcholine test pulse.
For oocyte data analysis, dose-response curves were fit using the empirical Hill equation: Y = 1/1 + (EC50/x)n̂H, where y is the fraction of remaining current, EC50 is the concentration of half-inhibition, nH is the apparent cooperativity, and x is the agonist concentration.
Values indicated throughout the text are given with their respective SEM. For statistical analysis, we used the unpaired, two-tailed Student's t test.
Genistein, inactive genistin (Igen), and sodium orthovanadate were purchased from Sigma (St. Louis, MO), and PP2, PP3 (4-amino-7-phenylpyrazol[3,4-d]pyrimidine), and SU6656 were from Calbiochem (Juro, Lucern, Switzerland). Stock solutions of genistein and its inactive analog were made in DMSO and diluted to their final concentration in OR2. The maximal concentration of DMSO applied to oocytes was 1% (v/v), a concentration that had no effect on the oocyte resting membrane conductance or on the ACh-evoked responses. Pervanadate was prepared as described previously (Meier et al., 1995).
Electrophysiological recordings in SH-α7 cells. Cells were maintained in culture using the same conditions as described for the biochemical experiments. Two to 4 d before the electrophysiological assays, cells were seeded into 35 mm dishes. Cells were washed three times with artificial CSF (in mm: 130 NaCl, 5 KCl, 2 CaCl2, 2 MgCl2, and 10 HEPES, pH adjusted to 7.4 with NaOH) and placed on the stage of an inverted Axiovert 100 microscope (Zeiss). Whole-cell recording patches were achieved using borosilicate tubes pulled with a BB-CH-PC (Mecanex, Nyon, Switzerland). Electrodes were filled with an intracellular solution (in mm: 130 K-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, and 5 EGTA, pH adjusted to 7.4 with KOH) and had a typical resistance of 2-3 MΩ. Currents were recorded using an AxoPatch 200B (Axon Instruments) digitized with a card PCI-1200 from National Instruments (Austin, TX), and data were stored on a Macintosh computer (Apple Computers, Cupertino, CA), using MacDatac software. Drugs were applied using a theta tube mounted on a piezo quartz (Physiks Instruments, Walbronn Germany), as described previously (Buisson and Bertrand, 2001), that allowed very fast drug delivery. Solution exchanges were controlled by electromagnetic valves driven by the data acquisition system.
Preparation of brain slices. Two- to 5-week-old rats [Sprague Dawley; Charles River Wiga (Sulzfeld, Germany), Iffa Credo (L'Arbresle, France)] were anesthetized (pentobarbital, 50 mg/kg, i.p.) and decapitated in accordance with the rules of the Swiss Federal Veterinary Office. The brain was rapidly removed, and coronal hypothalamic or transverse hippocampal slices, 300-400 μm thick, were prepared as described previously (Zaninetti et al., 2000; Ogier and Raggenbass, 2003). A single slice, containing either the supraoptic nucleus or the CA1 region of the hippocampus, was transferred to a thermoregulated recording chamber (32-33°C), submerged and continuously perfused with a solution containing 135 mm NaCl, 15 mm NaHCO3, 5 mm KCl, 1 mm MgCl2, 2 mm CaCl2, and 10 mm glucose and saturated with a gas mixture composed of 95% O2/5% CO2, pH 7.3-7.4. The perfusion solution was supplemented with 2 μm atropine to prevent activation of muscarinic receptors. The recording chamber was set on the stage of an Eclipse E600FN (Nikon, Tokyo, Japan), equipped with a 40×, 0.8 numerical aperture water-immersion objective and differential interference contrast optics, and neurons were visualized using an infrared-sensitive video camera (C2400-07; Hamamatsu).
Whole-cell recordings in brain slices. Patch pipettes were pulled from borosilicate glass capillaries (Harvard Apparatus, Edenbridge, UK) and had tip resistances of 3-6 MΩ. The patch pipette solution contained the following (in mm): 140 K-gluconate, 10 KCl, 10 HEPES, 4 MgCl2, 0.1 BAPTA, 2 Na2-ATP, and 0.4 Na2-GTP, pH 7.2-7.3. The osmolarity of the pipette solution was 285-310 mOsm. Gigaohm seals were made by applying a slight negative pressure to the back of the pipette, and access to the whole-cell configuration was achieved by further applying negative pressure. Access resistance was 10-25 MΩ. Neurons were voltage clamped at -70 mV, and current signals were amplified using an Axopatch 200A (Axon Instruments). They were low-pass filtered at 2 kHz and digitized at 5 kHz. ACh (0.2-1 mm) was applied by pressure ejection from patch-type pipette (30 psi, 40-400 ms) positioned within 10-20 μm from the soma of the recorded neuron.
Results
Tyrosine phosphorylation of α7 nAChRs
nAChRs can be divided in two main groups according to their sensitivity to α-BT (Dani, 2001). The most abundant receptor subtype of the α-BT-sensitive group is the α7 receptor, composed of five identical subunits (Berg and Conroy, 2002). To investigate whether α7 nAChRs are phosphorylated on tyrosine residues, we took advantage of SH-SY5Y cells, a human neuroblastoma cell line used for studying nicotinic receptors. The cells express low levels of functional α7 receptor channels, which specifically bind α-BT (Peng et al., 1994; Puchacz et al., 1994; Groot Kormelink and Luyten, 1997). We stably transfected the cells with human α7 nAChR expression vector to create SH-α7 cells, which showed abundant and saturable α-BT binding. This was demonstrated by α7 receptor precipitation using biotin-α-BT and streptavidin-agarose, followed by α7 Western blot (Fig. 1) and by 125I-α-BT binding (D. Feuerbach, unpublished observations). α7 nAChRs migrated as a major band of ∼50 kDa (Fig. 1) and occasionally as an additional band of ∼55 kDa (see Fig. 5), as observed previously in other cell lines and vertebrate brain (Drisdel and Green, 2000).
Figure 1.
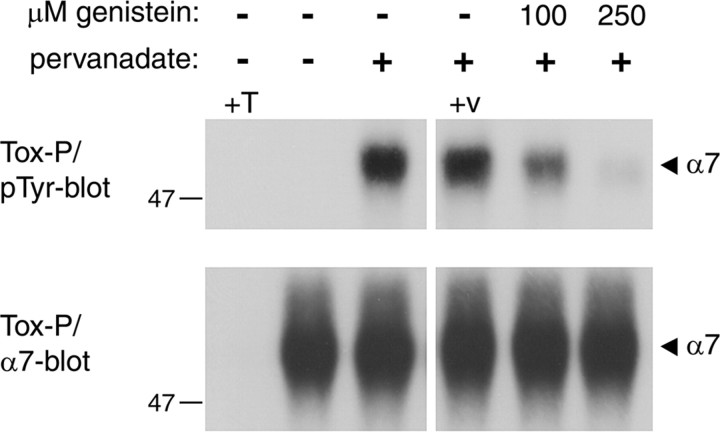
Regulation of α7 receptor tyrosine phosphorylation by genistein and pervanadate. SH-α7 cells were treated for 10 min with genistein, followed by addition of 50 μm pervanadate for 20 min in the presence of genistein. In controls, these compounds were omitted or the vehicle alone (0.25% DMSO, +v) was added. Cells were lysed and α7 receptors were precipitated using biotinylated α-BT and streptavidin-agarose (Tox-P). As a control, an excess of free α-BT was added before precipitation (+T). Immunoblotting using phosphotyrosine-specific antibodies, followed by α7 reprobing, reveals that pervanadate causes strong α7 receptor phosphorylation, which is greatly reduced by genistein. Shorter exposure times and side-by-side alignment of blots confirmed precise overlap between phosphotyrosine and α7 signals (data not shown).
Figure 5.
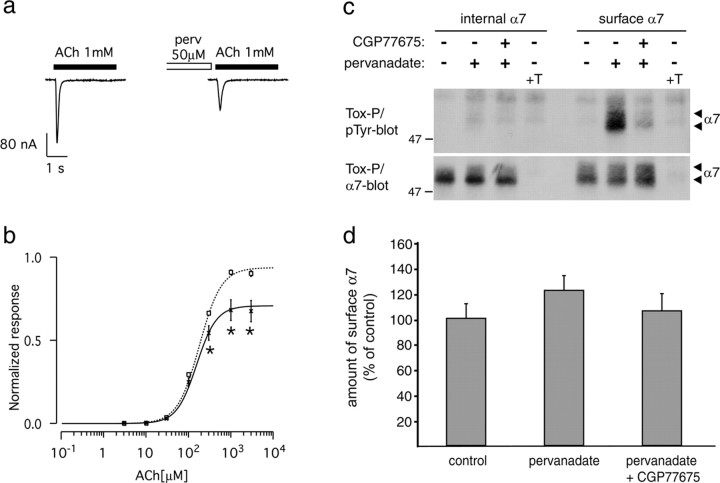
Pervanadate inhibits human α7 nAChRs in Xenopus oocytes. a, ACh-evoked (1 mm, 3 s) current without (left) or after 0.5-1 h incubation with 50 μm pervanadate (perv, right). b, ACh dose-response curve without (squares, dashedline; n = 28) and after pervanadate treatment (crosses, solid line; n = 22). Currents were evoked by successive test pulses (3 s) with increasing ACh concentrations, applied every 90 s. Currents were normalized to 1 at saturating ACh concentration (no perv). Data points are expressed as mean ± SEM, and the lines correspond to the best fit with a Hill equation (supplemental Table 1, available at www.jneurosci.org as supplemental material). Significant differences between pervanadate and control treatments were calculated by unpaired, two-tailed Student's t tests (*p < 0.01). c, SH-α7 cells were treated for 90 min with 10 μm CGP77675, followed by addition of 50 μm pervanadate. In controls, these compounds were omitted or an excess of free α-BT was added before precipitation (+T). Intracellular or surface α7 receptors were precipitated using biotinylated α-BT and analyzed by phosphotyrosine and α7 immunoblotting. Pervanadate causes phosphorylation of surface but not internal α7 receptors, and this phosphorylation originates from SFKs. Pervanadate, alone or with CGP77675, does not decrease the number of surface α7 receptors or affect internal α7. d, α7 blots as shown in c were quantitated by densitometric scanning (mean ± SD; n = 10).
Treatment of SH-α7 cells with the tyrosine phosphatase inhibitor pervanadate for 20 min produced strong phosphorylation of α7 receptors (Fig. 1). Preincubation of cells with the general tyrosine kinase inhibitor genistein resulted in reduction of α7 phosphorylation in a dose-dependent way. These findings demonstrate, for the first time, tyrosine phosphorylation of α-BT-binding α7 nAChRs. This phosphorylation results from a balance between tyrosine kinase and phosphatase activity and can be increased by pervanadate and decreased by genistein. Interestingly, α7 nAChR phosphorylation is under dynamic regulation, with an apparent turnover time of <20 min.
Inhibition of tyrosine kinases by genistein increases α7 nAChR responses
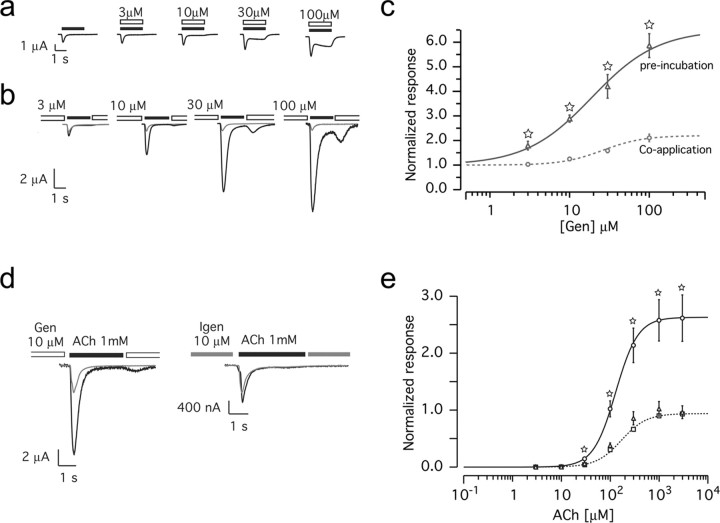
Genistein modulates the activity of ligand-gated channels by both a direct and indirect action (Jassar et al., 1997; Huang et al., 1999; Balduzzi et al., 2002). Although direct effects are thought to arise from an interaction with the channel itself, indirect effects stem from inhibiting intracellular phosphorylation pathways. To examine whether genistein modulates α7 receptors and to distinguish between direct and indirect actions, two experimental protocols were designed and electrophysiological recordings were performed on Xenopus oocytes expressing human α7 nAChRs. First, we assayed the effect of coapplication of increasing genistein concentrations with a fixed ACh test pulse (200 μm, 2 s). Under these circumstances, ACh-evoked currents were slightly increased by genistein, and the plateau phase was modified at high genistein concentration (Fig. 2a). This reflects direct genistein action on the α7 channel because it is very unlikely that modification of phosphorylation pathways occurs within the short ACh test pulse. Second, genistein was preapplied in increasing concentration for 1-2 h, allowing it to interact with intracellular tyrosine kinases. To minimize contamination by genistein during the subsequent ACh test pulse, a brief gap of superfusion with control buffer was introduced (1 s; see Materials and Methods). Under these conditions, ACh-evoked currents were more strongly potentiated (Fig. 2b). We also tested shorter genistein preincubation times, from 10 to 60 min, and found similar effects, albeit with a higher cell-to-cell variation (data not shown).
Figure 2.
Genistein potentiates α7 nAChRs in Xenopus oocytes. Oocytes expressing human α7 nAChRs were challenged under two-electrode voltage clamp. a, Recorded current during coapplication of ACh (200 μm, 2 s; filled bars) with increasing concentrations of genistein (open bars). b, ACh-evoked (200 μm, 2 s) current before (gray traces) or after (1-2 h) incubation with increasing concentrations of genistein (black traces). c, Genistein (Gen) dose-response curves obtained from coapplication (a, circles, dashed line) or preincubation (b, triangles, solid line). At each genistein concentration tested, the current enhancement recorded for α7 is much stronger in the case of preincubation than for coapplication. Data are normalized to the ACh-evoked current recorded in absence of genistein (n = 8, for all data points). The EC50 for genistein coapplication is 26 μm and for preincubation is 19 μm. d, Application of ACh (1 mm during 3 s) evoked a current (gray traces) that is enhanced by preincubation with 10 μm genistein (black trace, left) but not with 10 μm Igen (black trace, right). e, ACh dose-response curve. Currents were evoked by successive ACh test pulses (3 s) applied every 90 s, using increasing ACh concentrations. Currents were normalized to 1 at saturating concentration recorded in controls. Squares and dashed line correspond to control (n = 34), circles and solid line were recorded after genistein treatment (10 μm, 1-2 h; n = 16), and triangles correspond to Igen treatment (10 μm, 1-2 h; n = 12). Currents recorded after genistein treatments are significantly different from control, but their EC50 values are not different (supplemental Table 1, available at www.jneurosci.org as supplemental material). For all dose-response curves, data points are mean ± SEM. Lines correspond to the best fit obtained with a Hill equation. Significant differences were calculated by unpaired, two-tailed Student's t tests (☆p < 0.001).
Plots of the peak of the ACh-evoked currents recorded with the two protocols as a function of the genistein concentrations are shown in Figure 2c. Genistein preapplication caused a stronger effect than coapplication. In preapplication, α7 receptor potentiation is independent of the presence of genistein during the ACh test pulse, indicating that most of this genistein effect (at least a threefold potentiation) results from inhibition of intracellular phosphorylation rather than direct action on the α7 channel. We thus used the preapplication protocol for all subsequent experiments. We noticed that the genistein effect is rapidly reversible. After extensively washing oocytes for 10 min, new ACh test pulses evoked responses of normal amplitude and time course (data not shown). This suggests that phosphorylation occurs rapidly, consistent with the rapid turnover of α7 phosphorylation detected in our biochemical experiments in Figure 1.
Specificity of genistein was tested using the biologically inactive derivative Igen. Preapplication of Igen had no effect on ACh-evoked currents (Fig. 2d). ACh dose-response curves revealed a significant increase in the ACh-evoked response attributable to genistein at almost all ACh concentrations, whereas Igen produced no effect (Fig. 2e). The EC50 of ACh was not significantly affected by genistein (supplemental Table 1, available at www.jneurosci.org as supplemental material).
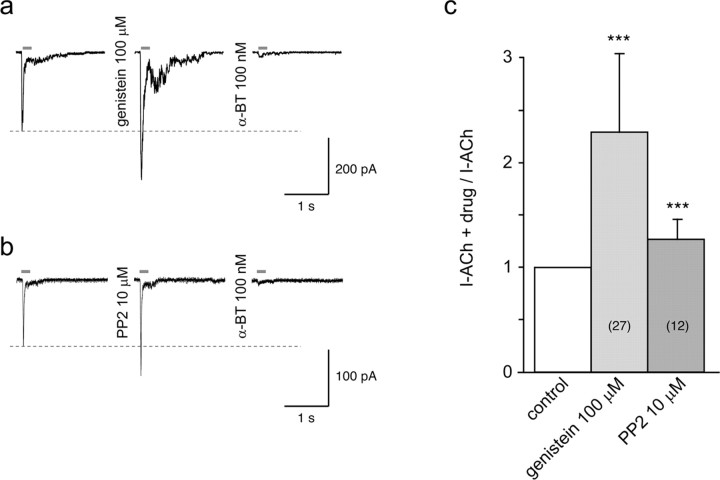
To rule out the possibility that genistein potentiation of α7 responses is restricted to receptors expressed in Xenopus oocytes, electrophysiological experiments were conducted in SH-α7 cells. These cells share several properties with neurons, have endogenous α7 receptors, and thus provide a native environment for α7 nAChRs. Fast agonist applications revealed that these cells display typical ACh-evoked currents and that brief exposure to genistein (1 min) was sufficient to cause a significant increase of the ACh responses in 27 of the 35 cells tested (Fig. 3a). To avoid a direct effect of genistein, cells were washed for 5 s before the ACh pulse. The typical time course of the ACh-evoked current suggests that these responses indeed reflect activation of α7 receptors. This was confirmed by the full blockade of the currents after 3 min exposure to 100 nm α-BT (Fig. 3a). Pooled data showed that the genistein-induced increase in ACh current was on average 2.3-fold (Fig. 3c). Genistein exposure up to 3 min did not cause further potentiation (n = 11 trials; analyzed by paired Student's t tests). Because of the nature of the cell patch for recording, longer genistein incubation times could not be tested.
Figure 3.
Genistein and PP2 potentiate ACh-evoked currents in SH-α7 cells. a, Currents evoked by brief ACh test pulses (1 mm, 200 ms) were recorded in control conditions (left trace), after 1 min exposure to 100 μm genistein (middle trace), and again after 1 min incubation with 100 nm α-BT (right trace). Bars above the traces indicate the duration of ACh application. Dashed line indicates the amplitude of the peak of the ACh-evoked current measured in control. b, Recordings obtained in another cell using the same protocol but with exposure to PP2 (10 μm). To avoid direct effects of genistein or PP2, cells were washed for 5 s before testing with ACh. All recordings were obtained in whole-cell patch-clamp configuration at a holding potential of -100 mV. c, Ratios between ACh-evoked currents recorded after drug treatment and before drug treatment (control), measured for 27 genistein-treated and 12 PP2-treated cells, are represented. Paired Student's t test computations of the current amplitude recorded after exposure to genistein or PP2 versus the value measured in the same cell in control (before drug treatment) indicate a highly significant increase attributable to both inhibitors (***p < 0.001).
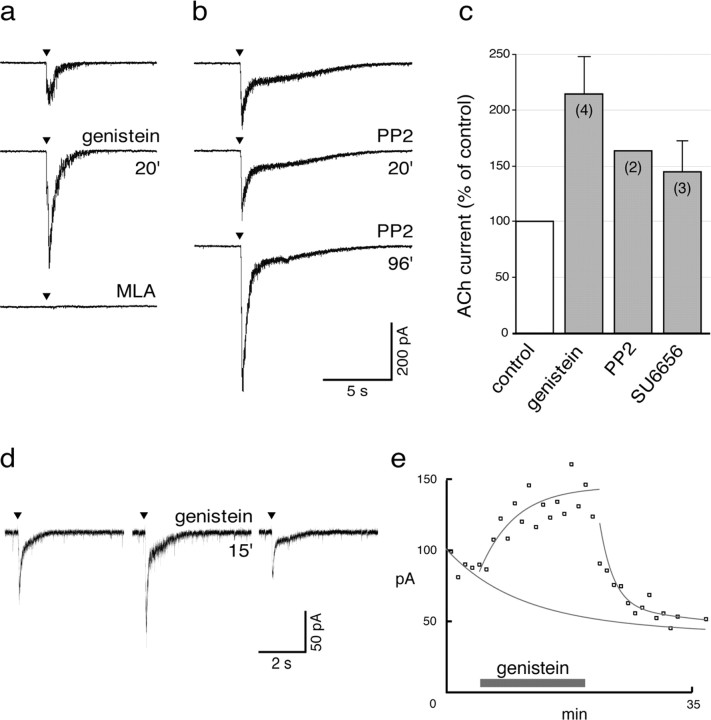
To verify that native α7 nAChRs behave similarly to receptors in SH-SY5Y cells and receptors expressed in Xenopus oocytes, effects of genistein were assayed in α7-expressing neurons in rat brain slices. Whole-cell recordings were performed in either hippocampal interneurons located in the CA1 stratum radiatum or magnocellular neurons located in the hypothalamic supraoptic nucleus. Both classes of neurons express functional α7-containing nAChRs (Alkondon et al., 1997; Jones and Yakel, 1997; Frazier et al., 1998a; Zaninetti et al., 2000, 2002). In hippocampal slices, ACh (0.2 mm) evoked a rapidly activating inward current in all nine interneurons tested. Genistein potentiated the ACh-evoked current by a factor of 2.1 ± 0.4 (n = 4) (Fig. 4a,c). This genistein-enhanced ACh current was sensitive to metyllycaconitine (MLA) (10 nm; n = 3), a competitive antagonist specific for α7 nAChRs (Fig. 4a). Genistein also caused a significant increase of the current evoked by ACh (1 mm) in supraoptic magnocellular neurons (three neurons in three different slices) (Fig. 4d,e). Such currents are known to reflect α7 receptor activity and are blocked by α-BT (Zaninetti et al., 2000). The genistein-induced potentiation was rapidly reversible after removal of the drug (Fig. 4d,e).
Figure 4.
Genistein and specific SFK inhibitors potentiate native α7 nAChRs. a-c, Effect of genistein and of specific SFK inhibitors on hippocampal CA1 interneurons. a represents current traces evoked by brief ACh pulses (0.2 mm; see Materials and Methods) in control conditions (top trace), after genistein application (100 μm, 20 min; middle trace), and in the presence of MLA (10 nm; bottom trace). Genistein causes an increase of the ACh peak current, and MLA abolishes the ACh response. b, Current traces, recorded in another hippocampal interneuron and showing the ACh-evoked current in control conditions (top trace) and after application of PP2 (10 μm) for 20 and 96 min (middle and bottom traces, respectively). PP2 had to be applied for a longer time than genistein before observing a significant potentiation. c, Histogram showing the mean effects of genistein (100 μm), PP2 (10 μm), and SU6656 (0.5 μm) on the ACh-evoked current in hippocampal interneurons. Numbers indicate the number of neurons analyzed. d, e, Effect of genistein on the ACh-evoked current in magnocellular supraoptic neurons. d represents the mean current traces (n = 3) evoked by ACh pulses (1 mm) in control conditions (left trace), after genistein application (100 μm, 15 min; middle trace), and after a 10 min recovery (right trace). e, Peak amplitude of ACh-evoked currents (squares) plotted in absolute value as a function of the recording time. Genistein was added to the perfusion solution for the 15 min period represented by the horizontal gray bar. Lines through the data points are exponential best fits. Note that, despite a progressive decrease in the ACh peak current, probably attributable to receptor desensitization and run down, genistein markedly and reversibly increased the ACh response.
Together, these data show that genistein treatment potentiates α7 ACh-evoked currents. The potentiation is similar (2.1-fold to ∼3-fold), in oocytes, SH-SY5Y cells, and hippocampal interneurons and occurs progressively during a 15 min incubation in supraoptic nucleus slices. Potentiation does not stem from direct genistein action on the α7 receptor channel but most likely occurs from indirect effects, i.e., inhibition of tyrosine kinases. The potentiation is rapidly reversible after drug wash-off in brain, SH-α7 cells, and oocytes, implying that the critical phosphorylation/dephosphorylation steps occur with a rapid turnover shorter than 20 min.
Inhibition of tyrosine phosphatases decreases α7 ACh-evoked responses
To assess the consequences of increased tyrosine phosphorylation for α7 function, we treated oocytes with pervanadate. Pervanadate pretreatment decreased the peak amplitude of ACh-evoked currents (Fig. 5a,b), without substantially affecting current kinetics or the EC50 of ACh (supplemental Table 1, available at www.jneurosci.org as supplemental material). The pervanadate preincubation time could be as short as 30 min and still yielded decreased responses.
Reduction in current amplitude could, in principle, originate from a relocation of surface receptors to intracellular compartments or from a change in the responsiveness of the surface population of α7 nAChRs to ACh. To distinguish between these possibilities, we treated SH-α7 cells with pervanadate and selectively precipitated surface or internal α7 receptors using biotin-α-BT as described previously for muscle nAChRs in myotubes (Moransard et al., 2003) (for details, see Materials and Methods). α7 nAChRs were found to reside both at the surface and in intracellular compartments of SH-α7 cells (Fig. 5c). In untreated cells, 43 ± 3% (mean ± SEM; n = 4 blots) of all α7 receptors were located at the surface. This value is similar to oocytes expressing α7 nAChRs, in which ∼50% of all α7 receptors were recently found to reside at the surface (Cho et al., 2005). In SH-α7 cells, pervanadate caused phosphorylation only of the surface α7 nAChRs and did not reduce the number of surface receptors or alter the ratio between surface and intracellular receptors (Fig. 5c,d). Thus, pervanadate leads to phosphorylation of α7 nAChRs selectively at the cell surface and inhibits α7 function, without detectable change in receptor distribution. The rapid timescale of the pervanadate effect on α7 activity (30 min preincubation) (Fig. 5a,b) is consistent with the rapid turnover of α7 phosphorylation seen in Figure 1.
Mutation of cytoplasmic tyrosine residues increases the α7 nAChR current amplitude
The effects of genistein and pervanadate on α7 receptor function (Figs. 2, 3, 4, 5) could be attributed to changes in levels of tyrosine phosphorylation of α7 nAChRs themselves or indirectly through (de)-phosphorylation of other proteins. To distinguish between these possibilities, we first identified putative tyrosine phosphorylation sites in the α7 protein sequence. Most of the intracellular receptor portion is formed by the long cytoplasmic loop between transmembrane domains TM3 and TM4 (Chini et al., 1994). In human α7, this loop contains two tyrosine residues, Tyr-386 and Tyr-442. Computer algorithms predicted Tyr-442 as a consensus phosphorylation site, whereas Tyr-386 emerged as a phosphorylation site by sequence comparison with known phosphotyrosines in Torpedo nAChR subunits (Chini et al., 1994) (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). The residues surrounding Tyr-386 and Tyr-442 showed a high degree of conservation across species (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Tyr-386 is part of a consensus motif, NXXY, for binding to phosphotyrosine-binding domains in signaling proteins such as the Shc family (Ware et al., 1996). Thus, Tyr-386 and Tyr-442 are strong candidates for phosphorylation.
We therefore substituted both Tyr-386 and Tyr-442 by alanine using site-directed mutagenesis. Oocytes expressing the mutant (α7 2Y-A) receptors displayed significantly larger (∼2.5-fold) ACh-evoked currents than those recorded in sibling oocytes expressing the wild-type receptors (Fig. 6a,b). No significant difference was, however, observed for either the current kinetics or the sensitivity to ACh (supplemental Table 1, available at www.jneurosci.org as supplemental material). Interestingly, the maximal evoked current (Imax) of α7 2Y-A receptors was comparable with the Imax in parallel genistein-treated cells expressing wild-type α7 nAChRs (Fig. 6c). Importantly, the Imax of α7 2Y-A receptors was insensitive to either genistein or pervanadate treatments (Fig. 6c). These data strongly suggest that the modulation of wild-type α7 nAChRs by these compounds originates mostly from changes in tyrosine phosphorylation of α7 receptors themselves. Dephosphorylation of α7 nAChRs, attributable to genistein or mutation of tyrosines, results in receptor potentiation, whereas phosphorylation, evoked by pervanadate, causes inhibition. Tyrosine phosphorylation of α7 thus negatively regulates α7 receptor activity.
Figure 6.
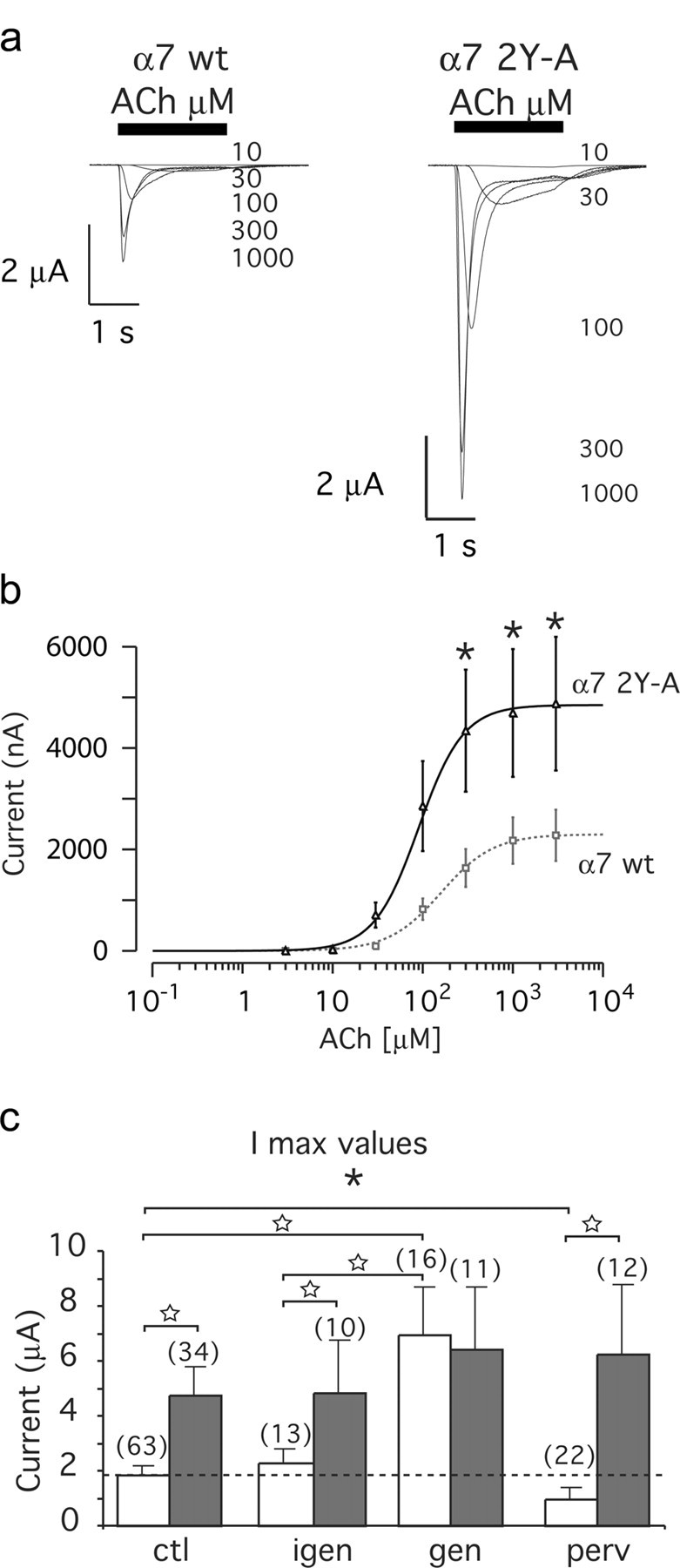
α7 2Y-A mutant nAChRs exhibit larger ACh-evoked currents compared with wild-type α7. Xenopus oocytes expressing wild-type or α7 2Y-A (Y386A, Y442A) receptor were recorded using two-electrode voltage clamp. a, ACh-evoked currents for wild-type and mutant α7 nAChRs. Increasing ACh concentrations are indicated and expressed in micromolar. b, ACh dose-response curves for wild-type α7 (squares, dashed line; n = 63) and mutant α7 2Y-A receptors (triangle, solid line; n = 34). Currents were evoked by successive test pulses (3 s) with increasing ACh concentrations, applied every 90 s. Lines through the data points correspond to the best fit with a Hill equation (supplemental Table 1, available at www.jneurosci.org as supplemental material). c, Maximal recorded currents (Imax) were plotted as a function of the drug applied at wild-type α7 (open bars) or α7 2Y-A mutant receptor (gray bars). Genistein (gen) and genistin (igen) concentrations were 10 μm. The numbers in parentheses indicate the number of cells tested in each condition. Data are expressed as mean ± SEM and show that ACh-evoked currents are higher for α7 2Y-A nAChRs and that these receptors are insensitive to genistein and pervanadate (perv) treatment. Significant differences were calculated by unpaired, two-tailed Student's t tests (*p < 0.01; ★p < 0.001).
SFKs interact with the cytoplasmic loop of α7 nAChRs
To examine which tyrosine kinases phosphorylate and regulate α7 nAChRs, we focused on SFKs, because they, as well as α7 receptors, can modulate synaptic plasticity in the hippocampus (Lu et al., 1998; Ji et al., 2001). Because SFKs usually bind to their substrates, forming a complex (Thomas and Brugge, 1997), we first tested whether they interact with α7 receptors. We prepared lysates of SH-α7 cells and precipitated α7 nAChRs using biotin-α-BT, followed by streptavidin-agarose. We identified α7 nAChR-associated SFKs by immunoblotting with Src-CT (Mittaud et al., 2001), a pan-Src antiserum reactive with several SFKs, including Src, Fyn, and Yes. This assay detected specific association of members of the Src family with α7 nAChRs (Fig. 7a). Using antibodies specific for single SFKs, we identified Src, Fyn, and Lyn as α7-associated kinases. These interactions were specific, because addition of free excess α-BT to the lysates decreased the signal and because no specific association occurred between α7 nAChRs and Gβ subunits of trimeric G-proteins or Yes, despite the expression of these proteins in SH-α7 cells (Fig. 7a and data not shown).
SH-α7 cells express functional α7 receptor channels at the surface (Fig. 3). To determine whether such receptors interact with SFKs, we selectively isolated surface α7 nAChRs from SH-α7 cells by adding biotin-α-BT to intact cells before lysis and indeed found associated SFKs (Fig. 7b). The amount of SFKs on surface α7 receptors was comparable with the amount of SFKs on total α7 nAChRs, suggesting that SFKs preferentially bind to α7 receptors at the cell surface (Fig. 7b).
To verify SFK interaction with functional α7 nAChRs in vivo, we used rat brain, in which α-BT-binding receptors consist of α7 subunits in homomeric complexes (Drisdel and Green, 2000). We prepared brain extracts from P5 and P30 rats, precipitated α7 receptor with α-BT coupled to Sepharose beads (Drisdel and Green, 2000), and detected specific association with SFKs (Fig. 7c). Thus, in both rat brain and human SH-α7 cells, α-BT-binding α7 nAChRs interact with members of the Src family.
Furthermore, we mapped the site on α7 for SFK interaction using bacterially expressed and purified GST fusion proteins (Fuhrer and Hall, 1996) containing portions of human α7 receptor. GST fusion proteins, attached to glutathione-Sepharose beads, were added to lysates of SH-α7 cells, and association of SFKs was analyzed by precipitation and Western blotting using pan-Src antibodies. In these pull-down assays, SFKs from SH-α7 cell extracts interacted with the α7 loop (GST-α7loop protein) but not with an α7 portion comprising transmembrane domain TM4 and the C terminus (GST-α7TM4CT protein) (Fig. 7d). As an additional control, SFKs did not interact with parental GST protein (Fig. 7d). In demonstrating interaction between SFKs and the α7 receptor loop, these experiments confirm the specific α7-SFK association shown by coprecipitation using α-BT (Fig. 7a-c).
Collectively, these data demonstrate that functional, α-BT-binding surface α7 nAChRs interact with SFKs. Most likely, these kinases bind by a direct protein-protein interaction to the intracellular loop between TM3 and TM4.
SFKs phosphorylate α7 nAChRs at the plasma membrane
To determine whether SFKs not only bind to but phosphorylate α7 receptors, we used the Src-family-specific inhibitors PP2 (Smith et al., 2001) and CGP77675 (Missbach et al., 1999; Mittaud et al., 2004). In SH-α7 cells treated with these compounds, pervanadate did not cause efficient phosphorylation of α7 nAChRs, as shown by biotin-α-BT and streptavidin-agarose precipitation, followed by phosphotyrosine immunoblotting (Fig. 8a,b). In SH-α7 cell lysates analyzed without precipitation, phosphorylation of other cellular proteins still occurred in the presence of CGP77675 and pervanadate, showing specificity of the SFK-mediated α7 receptor phosphorylation process (Fig. 8a,b). Use of another SFK-specific inhibitor, SU6656 (Blake et al., 2000), yielded the same result (data not shown). Thus, SFKs can specifically phosphorylate functional, α-BT-binding α7 receptors in SH-α7 cells. It appears that SFK-mediated phosphorylation occurs rapidly, because a 20 min pervanadate treatment is sufficient to visualize it (Fig. 8a,b).
SFK-mediated α7 receptor phosphorylation occurred at the plasma membrane, because CGP77675 blocked pervanadate-induced phosphorylation of surface α7 receptors (Fig. 5c). Furthermore, CGP77675 treatment (when combined with pervanadate) did not affect the number of surface or internal α7 nAChRs (Fig. 5c,d), implying that SFK activity does not affect receptor distribution in SH-α7 cells.
We also used GST-α7loop protein to assess phosphorylation of the two consensus tyrosine sites by SFKs. In the first approach, GST-α7loop protein was added to lysates prepared from pervanadate- and PP2-treated SH-α7 cells. After pull-down, complexes were subjected to in vitro phosphorylation in the presence of ATP. Without PP2, GST-α7loop protein was phosphorylated as shown by phosphotyrosine immunoblotting (Fig. 8c). PP2 reduced this signal, revealing that SFKs from SH-α7 cells phosphorylated the α7 loop in the assay (Fig. 8c). This was observed for GST fusion proteins containing the human α7 loop (Fig. 8c) or the rat loop sequence (data not shown). In the second approach, wild-type and mutant forms of GST-α7loop protein, lacking Tyr-386 and/or Tyr-442, were incubated with purified Src kinase. Wild-type and single mutants, but not the double mutant, were phosphorylated, illustrating that Src can phosphorylate both tyrosines within the α7 receptor loop (Fig. 8d). In both approaches, a 15 min incubation produced strong in vitro phosphorylation. This rapid phosphorylation suggests that the α7 nAChR is a direct substrate for SFKs.
SFKs regulate α7 receptor function
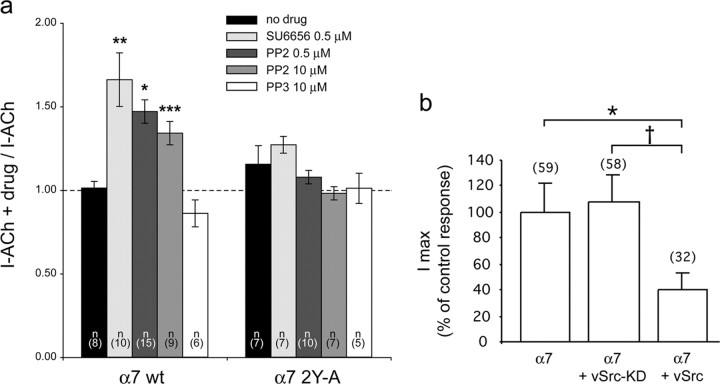
To determine the effect of SFKs on α7 receptor function, we used PP2 and SU6656 on oocytes expressing wild-type human α7 nAChRs. Oocytes were first challenged with brief ACh test pulses (1.25 mm, 1 s) that evoked control currents of maximal amplitude and then incubated for 1 h or longer with either SU6656 or PP2. ACh-evoked currents were recorded again at the end of the incubation using the same pulse parameters. Plots of the ratio between the ACh-evoked currents after and before drug incubation are shown in Figure 9a. A significant increase in the ACh-evoked current was observed after incubation with SU6656 and PP2 but was absent for cells treated with control solution or with the inactive PP3 analog. In agreement with a dephosphorylation-dependent increase of the wild-type α7 receptor activity, none of the compounds tested significantly modified the amplitude of the ACh-evoked currents recorded in oocytes expressing the α7-2YA mutant receptor (Fig. 9a). Thus, inhibiting SFKs increases the α7 nAChR activity, attributable to decreased phosphorylation of α7 receptors themselves.
Figure 9.
SFKs regulate α7 nAChR responses. a, SFK-inhibitors SU6656 and PP2 increase the ACh-evoked currents in wild-type but not mutant α7 nAChRs. ACh-evoked currents were recorded in Xenopus oocytes expressing either wild-type human α7(α7 wt) or the human α7-2YA mutant receptor before and after 1 h incubation with the indicated kinase inhibitor. Ratios between currents recorded after drug treatment and before treatment, measured in a series of cells from two oocyte batches, are represented. n represents the number of cells tested in each condition. Asterisks indicate statistically significant results (compared with no drug control), and error bars correspond to SEM (*p = 0.007; **p = 0.002; ***p = 0.001, by Student's t tests, using two samples with unequal variance and two-tail distribution). b, DNA expression vectors encoding vSrc or viral Src kinase without phosphorylation activity (vSrc-KD) were coinjected with human α7 nAChR vector in Xenopus oocytes. Imax, evoked by 1 mm ACh during 2 s, is reduced by more than 50% when vSrc is present compared with Imax in control, but no effect is observed when vSrc-KD is coexpressed. Data are expressed as mean ± SEM. The number of cells tested is indicated in parentheses. Significant differences were calculated by unpaired, two-tailed Student's t tests (*p < 0.01; †p < 0.05).
We analyzed the effect of PP2 on ACh-evoked currents in SH-α7 cells. PP2 exposure caused a significant increase of the current in 12 of 15 cells tested (Fig. 3b,c) (determined by paired student's t tests). When compared with parallel experiments using genistein, the PP2-induced increase was smaller (Fig. 3b,c), similar to the situation in oocytes.
We verified these findings for native α7 receptors by analyzing the effect of PP2 and SU6656 on ACh-evoked α7 nAChR responses in the hippocampus. Whole-cell recordings of CA1 stratum radiatum interneurons were performed in rat hippocampal slices, using 0.2 mm ACh test pulses. Incubation with PP2 (n = 2 interneurons) or SU6656 (n = 3 interneurons) for periods longer than 1 h caused a significant increase in ACh-evoked inward current, by factors of 1.6 (PP2) and 1.4 ± 0.3 (SU6656) (Fig. 4b,c).
Together, SFK inhibitors significantly potentiate α7 responses, most likely by receptor dephosphorylation. The effects are consistently smaller in the three preparations tested (oocytes, SH-α7 cells, and hippocampal interneurons) when compared with genistein, suggesting that, besides the Src family, other kinases act on α7 nAChRs.
In a gain-of-function approach, we coexpressed human wild-type α7 nAChRs in oocytes together with vSrc, a constitutively active form of Src. Functionally, vSrc corresponds to maximally activated Src kinase in which all inhibitory intramolecular interactions are displaced, leading to open kinase conformation and maximal kinase activity (Thomas and Brugge, 1997). vSrc has therefore been widely used to study regulation of ion channels by SFKs, often in the Xenopus oocyte expression system, and is a model molecule for the fully active cellular Src kinase (Holmes et al., 1996b; Nitabach et al., 2001, 2002).
We observed a significant reduction of the ACh-evoked current in oocytes coinjected with α7 receptor and the vSrc expression vector (Fig. 9b), without noticeable changes in the kinetics of the responses (data not shown). The reduction in Imax by vSrc was comparable with that caused by pervanadate treatment (Fig. 5c). As a control for nonspecific side effects of kinase expression, coinjection of a vSrc mutant (vSrc-KD), lacking kinase activity attributable to a point mutation in the ATP-binding site (Nitabach et al., 2001, 2002), produced no effect (Fig. 9b).
SFKs are antagonized by phosphatases
The pervanadate data show that α7 is a substrate for phosphatases and that α7 phosphorylation has a rapid turnover time (<20 min) revealing dynamic regulation by kinases and phosphatases (Fig. 1). The increased activity attributable to genistein, SU6656, or PP2 stems primarily from dephosphorylation of α7 receptors themselves, because α7 2Y-A receptors are not regulated by these compounds (Figs. 6, 9). Thus, in steady state, the balance between phosphatases and kinases, including SFKs, appears to produce a certain level of basal tyrosine phosphorylation of α7 nAChRs. Our biochemical assays are, however, not sensitive enough to visualize basal α7 phosphorylation (Fig. 1). This most likely originates from persistent phosphatase activity that dephosphorylates α7 receptors during the precipitation with α-BT. Several considerations support this hypothesis. First, in SH-α7 cells, α7 phosphorylation is unstable with rapid turnover. The overall phosphatase activity is pronounced as evidenced by the high level of dephosphorylation, because a 20 min pervanadate treatment produces strong α7 phosphorylation (Fig. 1). Second, although our lysis buffer contains an excess of phosphatase inhibitors (see Materials and Methods), phosphatase activity cannot be completely inactivated in the lysates and is present during the precipitation with α-BT. Over the course of several trial experiments, we have observed that, in lysates made from pervanadate-treated SH-α7 cells, an extension of the biotin-α-BT/streptavidin-agarose precipitation time (normal duration, 2 h) significantly decreases the α7 phosphorylation signal without changing the amount of α7 protein (A. Wiesner and C. Fuhrer, unpublished observations). Third, some of the phosphatases acting on α7 may be associated with the receptor because, in adrenal medulla chromaffin cells, ganglionic-type nAChRs associate with phosphatases in a complex (van Hoek et al., 1997). Such association will increase the efficiency of receptor dephosphorylation and make it hard to block this process during α7 precipitation. Finally, shaker-type voltage-gated potassium channels are also associated and negatively regulated by Src through tyrosine phosphorylation (Holmes et al., 1996a; Nitabach et al., 2002). Tyrosine phosphatases act on these channels, such that their basal tyrosine phosphorylation level is low or undetectable in a Western blot, whereas pervanadate leads to strong channel phosphorylation (Holmes et al., 1996a; Nitabach et al., 2002). The similarity to α7 nAChRs suggests that phosphatase regulation of ion channels may be widespread, antagonize SFKs, and render detection of basal channel phosphorylation difficult.
Together, our data show that increasing α7 receptor phosphorylation by activation of Src or by inactivation of tyrosine phosphatases yields a comparable inhibitory effect on the ACh-evoked currents (Figs. 5, 9). Conversely, decreasing α7 nAChR phosphorylation through inhibition of SFKs causes potentiation of the ACh-triggered response (Figs. 3, 4, 9). Thus, SFKs are counteracted by phosphatases in phosphorylation of α7 receptors, and these kinases modulate the functional activity of α7 nAChRs.
Kinase inhibition does not change amount and clustering of α7 receptors at the cell surface
The increase in current amplitude observed after genistein or PP2 preincubation could be attributed either to an increase in the number of receptors present at the cell surface or to a modification of the receptor properties. Cho et al. (2005) have recently reported that, in Xenopus oocytes expressing rat α7 nAChRs, ∼50% of all receptors reside in intracellular compartments. Genistein caused SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor)-dependent insertion of some of these receptors into the plasma membrane, leading to a twofold increase in the amplitude of ACh-evoked currents, and this also occurred for mutant receptors lacking cytoplasmic tyrosines (Cho et al., 2005). We therefore determined, after genistein treatment, the level of surface receptors in oocytes by labeling with a saturating dose (10 nm) of 125I-α-BT. Data presented in Figure 10a illustrate that genistein incubation does not cause a significant increase in the amount of radioactive labeling for wild-type α7 receptors. In oocytes expressing α7 2Y-A receptors, more labeling was observed for both untreated and genistein-treated oocytes (Fig. 10a). However, given the broad range of expression levels between oocytes, well known from other studies (Yoshii et al., 1987; Corringer et al., 1999; Cruz-Martin et al., 2001), differences between wild-type and mutant receptors and genistein-treated and untreated cells remained nonsignificant.
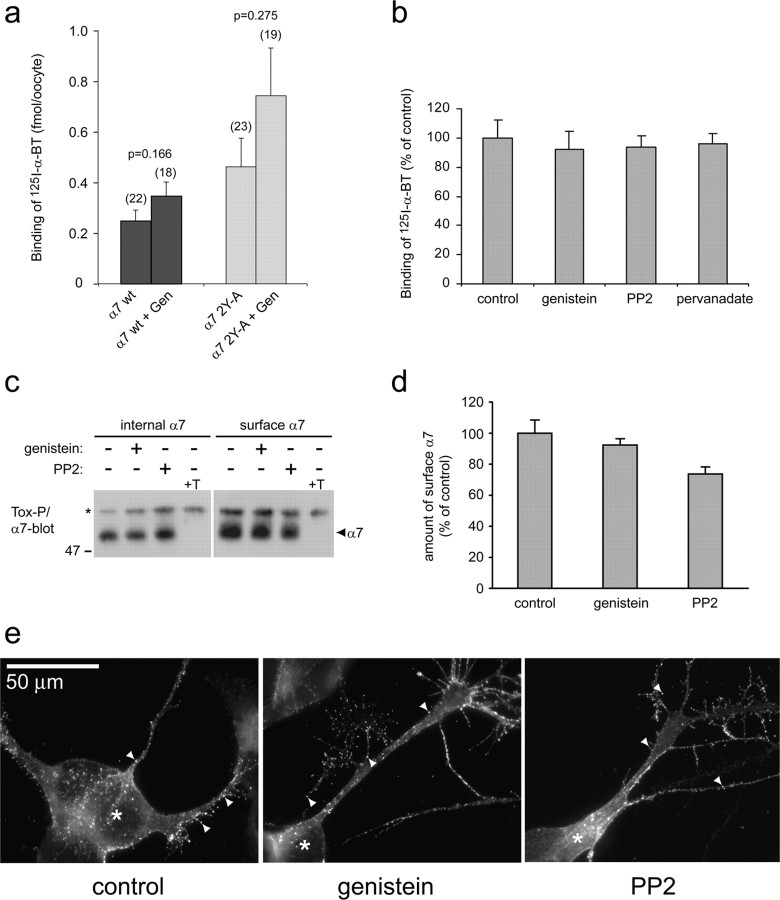
Figure 10.
Kinase or phosphatase inhibition does not change the amount and clustering of α7 receptors at the cell surface. a, Oocytes expressing α7 wild-type (wt) or the α7 2Y-A mutant receptor were incubated in control conditions or with 10 μm genistein (Gen) and labeled with 10 nm 125I-α-BT for 1 h. Cells were washed, and bound toxin was determined by gamma counting. Nonspecific binding was assessed by addition of 1 μm unlabeled α-BT and subtracted. Numbers in parentheses indicate the number of cells for calculation of mean ± SEM. Genistein does not affect the number of surface receptors significantly (p values determined by Student's t tests), and the slight increase between wild-type and α7 2Y-A receptors is not significant either. b, SH-α7 cells were treated with 100 μm genistein, 10 μm PP2, or 50 μm pervanadate and labeled with 10 nm 125I-α-BT for 1 h. Washed cells were lysed, and radioactivity was quantitated by gamma counting. Nonspecific binding was assessed by adding 1 μm unlabeled α-BT and subtracted. Inhibitors do not affect levels of surface α7 receptors (mean ± SD; 4 experiments). c, d, SH-α7 cells were incubated with 100 μm genistein or 10 μm PP2, and surface or internal receptors were precipitated with biotin-α-BT (Tox-P) as detailed in Materials and Methods. α7 immunoblotting (c) and quantitation of blots from three experiments by densitometric scanning (d) reveal no increase in the amount of surface receptors attributable to genistein or PP2. +T, Addition of 10 μm free α-BT to cell lysates abolishes the α7 signal. e, SH-α7 cells were incubated with Alexa 488-coupled α-BT to stain surface α7 receptors. Fluorescence microscopy identifies many small (∼1 μm) α7 clusters on soma and cellular processes (arrowheads). Soma (asterisks) were identified by DAPI nuclear staining (data not shown). Genistein (100 μm) or PP2 (10 μm) treatment does not affect number, size, intensity, or distribution of clusters.
We obtained very similar results in SH-α7 cells, because treatment with genistein, PP2, or pervanadate did not change the amount of surface α7 receptors as determined by labeling with 125I-α-BT (Fig. 10b). We also precipitated, using biotin-α-BT, intracellular or surface α7 receptors from these cells (as described before for Fig. 5) and found no increase in the amount of surface receptors when cells were treated with genistein or PP2 (Fig. 10c,d). Finally, intact SH-α7 cells were labeled with Alexa 488-α-BT and examined by fluorescence microscopy to visualize surface α7 receptors. Receptors were visible as clusters on cellular processes and the soma. Some of these clusters appear to be on small filopodiae emanating from the processes (Fig. 10e). The distribution of α7 receptor clusters on soma, processes, and filopodiae is very similar to hippocampal GABAergic interneurons (Kawai et al., 2002). Importantly, genistein and PP2 treatment did not produce any obvious changes in the number, size, intensity, or distribution of α7 clusters (Fig. 10e).
Collectively, these data demonstrate that genistein and PP2 do not significantly affect the amount or distribution of α7 receptor at the surface of SH-α7 cells. However, the same genistein or PP2 treatments significantly increase ACh-evoked current of α7 receptors in oocytes, SH-α7 cells, and hippocampal interneurons (Figs. 2, 3, 4, 9). This increase, thus, does not stem from increased numbers of receptors at the surface but from a modification of the properties of α7 nAChRs present in the plasma membrane.
Discussion
In this work, we have examined the effects of tyrosine phosphorylation on nicotinic receptors in the CNS, focusing on α7 nAChRs. We found that α7 receptors are rapidly phosphorylated and dephosphorylated and that dephosphorylated receptors are more active. Part of the responsible kinases are SFKs, because they bind and phosphorylate α7 nAChRs at the cell surface and modulate their functional properties. The balance between SFKs and phosphatases determines the net phosphorylation of α7 nAChRs, constituting an important regulatory mechanism for the activity of receptors at the plasma membrane and offering an additional capacity for modulation of neuronal network functions.
Changes in α7 nAChR phosphorylation modulate receptor activity
Dephosphorylation of α7 receptors was achieved by genistein treatment. Although we observed that genistein causes at least two effects on the α7 functional activity in oocytes, we found strong potentiation of ACh-evoked currents after prolonged genistein treatment followed by a brief wash. Because genistein was removed during the ACh test pulse, we conclude that potentiation is attributable to inhibition of intracellular phosphorylation rather than direct genistein action on the nAChRs. ACh dose-response curves confirmed α7 receptor potentiation at all ACh concentrations after genistein preapplication but revealed little if any change in receptor sensitivity to ACh. In addition, the increase in current amplitude was not modified by BAPTA treatment, a condition known to minimize the contamination by calcium-activated chloride channels (Devillers-Thiery et al., 1992), further implying that genistein modulates α7 nAChR activity through a more direct mechanism, most likely phosphorylation. Importantly, we found comparable genistein potentiation of α7 responses in neuroblastoma cells, hippocampal CA1 interneurons, and supraoptic magnocellular neurons.
Confirming the genistein effect, mutant α7 2Y-A receptors displayed a potentiation comparable with that of wild-type receptors in genistein-treated cells, and the activity of α7 2Y-A receptors was not affected by genistein or pervanadate. Finally, the importance of phosphorylation for α7 nAChR activity was demonstrated with pervanadate treatment. This caused decreased receptor activity that we attributed to an increase in α7 phosphorylation. Collectively, the data suggest that tyrosine phosphorylation of α7 nAChRs negatively regulates receptor activity.
SFKs phosphorylate and regulate the activity of α7 nAChRs and are antagonized by tyrosine phosphatases
We identified SFKs as tyrosine kinases that interact with the α7 loop and phosphorylate the receptor at the cell surface. Thereby, SFKs are positioned to functionally modulate α7 nAChRs, and we indeed found increased α7 activity during treatment with specific SFK inhibitors, PP2 and SU6656 (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Conversely, coexpression of α7 nAChRs with vSrc reduced amplitudes of ACh-evoked currents in a manner similar to pervanadate.
Although PP2 and SU6656 caused a significant increase of the α7 ACh-evoked currents, their effects were smaller than genistein. This was observed in oocytes, SH-α7 cells, and hippocampal slices. Because genistein is a broad-spectrum tyrosine kinase inhibitor and interferes with additional kinases besides SFKs (Akiyama et al., 1987), these data suggest that α7 receptors are also substrates for other yet unknown tyrosine kinases.
The effects of pervanadate show that α7 nAChR phosphorylation has a rapid turnover time of <20 min, revealing dynamic regulation by kinases and phosphatases. In such a manner, phosphatases could antagonize SFKs and be important control players to determine overall α7 receptor activity. Differences in SFK and/or phosphatase activities between tissue types could lead to greatly different α7 responses and differences in their regulation in various cell types.
Mechanism of α7 modulation by phosphorylation: an effect on receptor function but not number
To interpret how phosphorylation may modulate α7 receptor activity, it is helpful to recall the principle of allosteric modulation. As shown previously for calcium, ivermectin, or 5-hydroxyindole, increase of the ACh-evoked current is accompanied by an increase in receptor sensitivity and apparent cooperativity that are the signatures of positive allosteric effectors (Galzi et al., 1996; Krause et al., 1998). Our observed lack of modification of α7 receptor sensitivity and cooperativity therefore suggests that reduced α7 nAChR phosphorylation by genistein does not act as a classical positive allosteric modulator. Alternative hypotheses include modulation of single-channel properties or increase in the number of responsive (activatable) receptors. Although our data do not reveal obvious changes in response time course, a way to discriminate between these two hypotheses would be to record single-channel activity in different phosphorylation conditions. Technical difficulties and single-channel rundown prevents, however, reliable single α7 channel measurements to be done over time (Alkondon et al., 1994; Buisson et al., 1996; Liu and Berg, 1999).
We find that the number and clustering of receptors at the surface of oocytes and SH-α7 cells is not significantly modified by phosphorylation. Thus, genistein- and PP2-induced potentiation of α7 nAChRs responses must stem from mechanisms other than insertion of additional receptors into the cell membrane. Most likely, phosphorylation affects the number of activatable receptors within the plasma membrane such that dephosphorylated receptors are responsive, whereas phosphorylated receptors are probably nonresponsive (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). In agreement with this hypothesis, mean current amplitudes observed for α7 2Y-A receptors are significantly larger without changes in response time course.
Cho et al. (2005) have recently found a twofold genistein-induced receptor potentiation in oocytes that occurs also for tyrosine-mutated receptors and was attributed to new surface receptor insertion. Although the reasons for the differences between this and our study remain unclear, the following aspect deserves attention. Like in the Cho et al. study, higher genistein concentrations potentiate α7 responses in oocytes threefold up to fivefold. The magnitude of such potentiation is incompatible with the sole hypothesis of receptor insertion. Namely, because only 50% of all receptors are internal (Cho et al., 2005), this could account for at most a twofold potentiation. Therefore, additional mechanisms independent of receptor trafficking must exist, and our data provide first evidences for these mechanisms. This novel hypothesis is supported by various observations (Fig. 10), most importantly by the absence of significant increase in receptor number, detected using 125I-α-BT, in both genistein-treated oocytes and SH-α7 cells.
Previously, phosphorylation was described for nAChRs in the periphery. Phosphorylation on tyrosine or serine/threonine of Torpedo electric organ receptors to a high stoichiometry in vitro causes subtle changes in desensitization kinetics but not in Imax (Huganir et al., 1986; Hopfield et al., 1988). In adrenal medulla chromaffin cells, Src potentiates ganglionic-type heteromeric nAChRs in the secretion of catecholamines (Wang et al., 2004). In mammalian muscle, SFKs interact with the nAChR (Fuhrer and Hall, 1996) and play a role in clustering of nAChRs at the endplate (Mittaud et al., 2001, 2004; Mohamed et al., 2001; Smith et al., 2001). Thus, phosphorylation and SFKs affect peripheral nAChRs in many different ways, depending on tissue, subunit-type of nAChRs, and functional role of the receptors.
Possible roles of SFKs and tyrosine phosphorylation of α7 receptors in CNS neural circuits
Because SFK and phosphatase activities are highly regulated by different signals (Thomas and Brugge, 1997), the control of the end level of phosphorylation offers a possibility of fine-tuning α7 receptor activity. This could play a role in various α7 nAChR-mediated processes. At the cellular and synaptic level, calcium influx through α7 receptors activates various downstream signaling pathways (Dajas-Bailador and Wonnacott, 2004). To control such activation and avoid calcium toxicity, the activity of α7 receptors must be precisely controlled and may involve (de)phosphorylation.
At the neuronal circuit level, it is interesting that phosphorylated α7 nAChRs are less active: this is in contrast to NMDA receptors, which are potentiated by Src and tyrosine phosphorylation (Yu et al., 1997), but similar to shaker-type voltage-gated potassium channels, which are inhibited by vSrc (Holmes et al., 1996b; Nitabach et al., 2002). α7 nAChRs and NMDA receptors have some overlapping functions, because both receptors are highly permeable for calcium and important in CA1 hippocampal LTP (Lu et al., 1998; Dani, 2001; Ji et al., 2001). SFK activation may thus act in LTP induction in several ways: by potentiating NMDA receptors in pyramidal cells (Lu et al., 1998) and by con-comitantly inhibiting postsynaptic α7 nAChRs in GABAergic interneurons, thereby facilitating disinhibition of pyramidal cells. Such a model is supported by our finding that SFK inhibition in hippocampal slices increases the α7 receptor activity in CA1 interneurons. The activity of postsynaptic α7 receptors on hippocampal inhibitory interneurons is also linked to auditory gating (Jones et al., 1999; Martin et al., 2004). It would be interesting to determine whether α7 phosphorylation influences the gating process, changes of which are strongly associated with schizophrenia. Regulation by phosphorylation may also be important for presynaptic α7 nAChRs that control neurotransmitter release.
Such mechanisms, using presynaptic and/or postsynaptic α7 nAChRs, can allow neurons to regulate the weight of their synaptic transmission, allow neural circuits to undergo plasticity, and fine-tune neuronal survival. In this sense, α7 phosphorylation mechanisms could be seen as a higher hierarchical possibility to modulate activity and signaling in neural networks. Such modulation may form a basis for the various emerging roles of α7 nAChRs from synaptic to systems level, including addiction, memory, and neurological disease.
Footnotes
This work was supported by the Dr. Eric Slack-Gyr Foundation and by grants from the Swiss National Science Foundation (C.F., M.R., D.B.). We are grateful to Sonia Bertrand for her help with the oocyte experiments and to Roland Schoeb for help with photography.
Correspondence should be addressed to Dr. Christian Fuhrer, Brain Research Institute, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland. E-mail: chfuhrer@hifo.unizh.ch.
Copyright © 2005 Society for Neuroscience 0270-6474/05/259836-14$15.00/0
E.C., A.W., and K.-H.H. contributed equally to this work.
References
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592-5595. [PubMed] [Google Scholar]
- Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX (1994) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonistelicited whole-cell currents and identification of receptor subunits by in situ hybridization. J Pharmacol Exp Ther 271: 494-506. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX (1997) Neuronal nicotinic acetylcholine receptor activation modulates gammaaminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther 283: 1396-1411. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX (1998) Alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res 810: 257-263. [DOI] [PubMed] [Google Scholar]
- Balduzzi R, Cupello A, Robello M (2002) Modulation of the expression of GABA(A) receptors in rat cerebellar granule cells by protein tyrosine kinases and protein kinase C. Biochim Biophys Acta 1564: 263-270. [DOI] [PubMed] [Google Scholar]
- Berg DK, Conroy WG (2002) Nicotinic alpha 7 receptors: synaptic options and downstream signaling in neurons. J Neurobiol 53: 512-523. [DOI] [PubMed] [Google Scholar]
- Blake RA, Broome MA, Liu X, Wu J, Gishizky M, Sun L, Courtneidge SA (2000) SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20: 9018-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351-1362. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D (2001) Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci 21: 1819-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Gopalakrishnan M, Arneric SP, Sullivan JP, Bertrand D (1996) Human α4β2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: a patch-clamp study. J Neurosci 16: 7880-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini B, Raimond E, Elgoyhen AB, Moralli D, Balzaretti M, Heinemann S (1994) Molecular cloning and chromosomal localization of the human alpha 7-nicotinic receptor subunit gene (CHRNA7). Genomics 19: 379-381. [DOI] [PubMed] [Google Scholar]
- Cho CH, Song W, Leitzell K, Teo E, Meleth AD, Quick MW, Lester RA (2005) Rapid upregulation of α7 nicotinic acetylcholine receptors by tyrosine dephosphorylation. J Neurosci 25: 3712-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corringer PJ, Bertrand S, Galzi JL, Devillers-Thiery A, Changeux JP, Bertrand D (1999) Mutational analysis of the charge selectivity filter of the alpha7 nicotinic acetylcholine receptor. Neuron 22: 831-843. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, Mercado JL, Rojas LV, McNamee MG, Lasalde-Dominicci JA (2001) Tryptophan substitutions at lipid-exposed positions of the gamma M3 transmembrane domain increase the macroscopic ionic current response of the Torpedo californica nicotinic acetylcholine receptor. J Membr Biol 183: 61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador F, Wonnacott S (2004) Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol Sci 25: 317-324. [DOI] [PubMed] [Google Scholar]
- Dajas-Bailador FA, Soliakov L, Wonnacott S (2002) Nicotine activates the extracellular signal-regulated kinase 1/2 via the alpha7 nicotinic acetylcholine receptor and protein kinase A, in SH-SY5Y cells and hippocampal neurones. J Neurochem 80: 520-530. [DOI] [PubMed] [Google Scholar]
- Dani JA (2001) Overview of nicotinic receptors and their roles in the central nervous system. Biol Psychiatry 49: 166-174. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A, Galzi JL, Bertrand S, Changeux JP, Bertrand D (1992) Stratified organization of the nicotinic acetylcholine receptor channel. NeuroReport 3: 1001-1004. [DOI] [PubMed] [Google Scholar]
- Drisdel RC, Green WN (2000) Neuronal α-bungarotoxin receptors are α7 subunit homomers. J Neurosci 20: 133-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisele JL, Bertrand S, Galzi JL, Devillers-Thiery A, Changeux JP, Bertrand D (1993) Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 366: 479-483. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV (1998a) Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci 18: 1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV (1998b) Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J Neurosci 18: 8228-8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrer C, Hall ZW (1996) Functional interaction of Src family kinases with the acetylcholine receptor in C2 myotubes. J Biol Chem 271: 32474-32481. [DOI] [PubMed] [Google Scholar]
- Galzi JL, Edelstein SJ, Changeux J (1996) The multiple phenotypes of allosteric receptor mutants. Proc Natl Acad Sci USA 93: 1853-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER (1992) Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science 258: 1903-1910. [DOI] [PubMed] [Google Scholar]
- Groot Kormelink PJ, Luyten WH (1997) Cloning and sequence of full-length cDNAs encoding the human neuronal nicotinic acetylcholine receptor (nAChR) subunits beta3 and beta4 and expression of seven nAChR subunits in the human neuroblastoma cell line SH-SY5Y and/or IMR-32. FEBS Lett 400: 309-314. [DOI] [PubMed] [Google Scholar]
- Holmes TC, Fadool DA, Levitan IB (1996a) Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci 16: 1581-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TC, Fadool DA, Ren R, Levitan IB (1996b) Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science 274: 2089-2091. [DOI] [PubMed] [Google Scholar]
- Hopfield JF, Tank DW, Greengard P, Huganir RL (1988) Functional modulation of the nicotinic acetylcholine receptor by tyrosine phosphorylation. Nature 336: 677-680. [DOI] [PubMed] [Google Scholar]
- Hu M, Liu QS, Chang KT, Berg DK (2002) Nicotinic regulation of CREB activation in hippocampal neurons by glutamatergic and nonglutamatergic pathways. Mol Cell Neurosci 21: 616-625. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Fang MJ, Dillon GH (1999) The tyrosine kinase inhibitor genistein directly inhibits GABAA receptors. Brain Res Mol Brain Res 67: 177-183. [DOI] [PubMed] [Google Scholar]
- Huganir RL, Delcour AH, Greengard P, Hess GP (1986) Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature 321: 774-776. [DOI] [PubMed] [Google Scholar]
- Huh KH, Fuhrer C (2002) Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses. Mol Neurobiol 25: 79-112. [DOI] [PubMed] [Google Scholar]
- Jassar BS, Ostashewski PM, Jhamandas JH (1997) GABAA receptor modulation by protein tyrosine kinase in the rat diagonal band of Broca. Brain Res 775: 127-133. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA (2001) Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 31: 131-141. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL (1997) Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol (Lond) 504: 603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Sudweeks S, Yakel JL (1999) Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci 22: 555-561. [DOI] [PubMed] [Google Scholar]
- Kawai H, Zago W, Berg DK (2002) Nicotinic α7 receptor clusters on hippocampal GABAergic neurons: regulation by synaptic activity and neurotrophins. J Neurosci 22: 7903-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Shibasaki H, Kume T, Akaike A (2001) alpha 7 nicotinic receptor transduces signals to phosphatidylinositol 3-kinase to block A beta-amyloid-induced neurotoxicity. J Biol Chem 276: 13541-13546. [DOI] [PubMed] [Google Scholar]
- Krause RM, Buisson B, Bertrand S, Corringer PJ, Galzi JL, Changeux JP, Bertrand D (1998) Ivermectin: a positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 53: 283-294. [DOI] [PubMed] [Google Scholar]
- Levin ED (2002) Nicotinic receptor subtypes and cognitive function. J Neurobiol 53: 633-640. [DOI] [PubMed] [Google Scholar]
- Liu Q, Berg DK (1999) Actin filaments and the opposing actions of CaM kinase II and calcineurin in regulating α7-containing nicotinic receptors on chick ciliary ganglion neurons. J Neurosci 19: 10280-10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kawai H, Berg DK (2001) beta-Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci USA 98: 4734-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Roder JC, Davidow J, Salter MW (1998) Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science 279: 1363-1367. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS (2000) Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27: 349-357. [DOI] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R (2004) Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 174: 54-64. [DOI] [PubMed] [Google Scholar]
- Meier T, Perez GM, Wallace BG (1995) Immobilization of nicotinic acetylcholine receptors in mouse C2 myotubes by agrin-induced protein tyrosine phosphorylation. J Cell Biol 131: 441-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missbach M, Jeschke M, Feyen J, Muller K, Glatt M, Green J, Susa M (1999) A novel inhibitor of the tyrosine kinase Src suppresses phosphorylation of its major cellular substrates and reduces bone resorption in vitro and in rodent models in vivo. Bone 24: 437-449. [DOI] [PubMed] [Google Scholar]
- Mittaud P, Marangi PA, Erb-Vogtli S, Fuhrer C (2001) Agrin-induced activation of acetylcholine receptor-bound Src family kinases requires Rapsyn and correlates with acetylcholine receptor clustering. J Biol Chem 276: 14505-14513. [DOI] [PubMed] [Google Scholar]
- Mittaud P, Camilleri AA, Willmann R, Erb-Vogtli S, Burden SJ, Fuhrer C (2004) A single pulse of agrin triggers a pathway that acts to cluster acetylcholine receptors. Mol Cell Biol 24: 7841-7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed AS, Rivas-Plata KA, Kraas JR, Saleh SM, Swope SL (2001) Src-class kinases act within the agrin/MuSK pathway to regulate acetylcholine receptor phosphorylation, cytoskeletal anchoring, and clustering. J Neurosci 21: 3806-3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moransard M, Borges LS, Willmann R, Marangi PA, Brenner HR, Ferns MJ, Fuhrer C (2003) Agrin regulates rapsyn interaction with surface acetylcholine receptors, and this underlies cytoskeletal anchoring and clustering. J Biol Chem 278: 7350-7359. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Llamas DA, Araneda RC, Intile JL, Thompson IJ, Zhou YI, Holmes TC (2001) A mechanism for combinatorial regulation of electrical activity: potassium channel subunits capable of functioning as Src homology 3-dependent adaptors. Proc Natl Acad Sci USA 98: 705-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Llamas DA, Thompson IJ, Collins KA, Holmes TC (2002) Phosphorylation-dependent and phosphorylation-independent modes of modulation of shaker family voltage-gated potassium channels by SRC family protein tyrosine kinases. J Neurosci 22: 7913-7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier R, Raggenbass M (2003) Action of tachykinins in the rat hippocampus: modulation of inhibitory synaptic transmission. Eur J Neurosci 17: 2639-2647. [DOI] [PubMed] [Google Scholar]
- Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J (1994) Human alpha 7 acetylcholine receptor: cloning of the alpha 7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional alpha 7 homomers expressed in Xenopus oocytes. Mol Pharmacol 45: 546-554. [PubMed] [Google Scholar]
- Picciotto MR, Zoli M (2002) Nicotinic receptors in aging and dementia. J Neurobiol 53: 641-655. [DOI] [PubMed] [Google Scholar]
- Puchacz E, Buisson B, Bertrand D, Lukas RJ (1994) Functional expression of nicotinic acetylcholine receptors containing rat alpha 7 subunits in human SH-SY5Y neuroblastoma cells. FEBS Lett 354: 155-159. [DOI] [PubMed] [Google Scholar]
- Shaw S, Bencherif M, Marrero MB (2002) Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against Abeta-(1-42) amyloid. J Biol Chem 277: 44920-44924. [DOI] [PubMed] [Google Scholar]
- Smith CL, Mittaud P, Prescott ED, Fuhrer C, Burden SJ (2001) Src, Fyn, and Yes are not required for neuromuscular synapse formation but are necessary for stabilization of agrin-induced clusters of acetylcholine receptors. J Neurosci 21: 3151-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS (1997) Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol 13: 513-609. [DOI] [PubMed] [Google Scholar]
- van Hoek ML, Allen CS, Parsons SJ (1997) Phosphotyrosine phosphatase activity associated with c-Src in large multimeric complexes isolated from adrenal medullary chromaffin cells. Biochem J 326: 271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K, Edson K, Heginbotham L, Post M, Huganir RL, Czernik AJ (1991) Determination of the tyrosine phosphorylation sites of the nicotinic acetylcholine receptor. J Biol Chem 266: 23784-23789. [PubMed] [Google Scholar]
- Wang K, Hackett JT, Cox ME, Van Hoek M, Lindstrom JM, Parsons SJ (2004) Regulation of the neuronal nicotinic acetylcholine receptor by SRC family tyrosine kinases. J Biol Chem 279: 8779-8786. [DOI] [PubMed] [Google Scholar]
- Ware MD, Rosten P, Damen JE, Liu L, Humphries RK, Krystal G (1996) Cloning and characterization of human SHIP, the 145-kD inositol 5-phosphatase that associates with SHC after cytokine stimulation. Blood 88: 2833-2840. [PubMed] [Google Scholar]
- Yoshii K, Yu L, Mayne KM, Davidson N, Lester HA (1987) Equilibrium properties of mouse-Torpedo acetylcholine receptor hybrids expressed in Xenopus oocytes. J Gen Physiol 90: 553-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Leonard RJ, Davidson N, Lester HA (1991) Single-channel properties of mouse-Torpedo acetylcholine receptor hybrids expressed in Xenopus oocytes. Brain Res Mol Brain Res 10: 203-211. [DOI] [PubMed] [Google Scholar]
- Yu XM, Askalan R, Keil II GJ, Salter MW (1997) NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science 275: 674-678. [DOI] [PubMed] [Google Scholar]
- Zaninetti M, Blanchet C, Tribollet E, Bertrand D, Raggenbass M (2000) Magnocellular neurons of the rat supraoptic nucleus are endowed with functional nicotinic acetylcholine receptors. Neuroscience 95: 319-323. [DOI] [PubMed] [Google Scholar]
- Zaninetti M, Tribollet E, Bertrand D, Raggenbass M (2002) Nicotinic cholinergic activation of magnocellular neurons of the hypothalamic paraventricular nucleus. Neuroscience 110: 287-299. [DOI] [PubMed] [Google Scholar]