Abstract
Neuropathic pain may be primarily driven by immune responses in peripheral nerves. Peripherally released catecholamines may exacerbate neuropathic pain and also modulate immune responses in a complex and sometimes opposing manner by actions on multiple adrenoceptor subtypes. We showed previously that injection of the α2-adrenoceptor agonist clonidine at the site of peripheral nerve injury reduces pain behavior and local tissue pro-inflammatory cytokine content in rats. The current study used a model of acute inflammatory neuritis to test the efficacy and mechanisms of action ofα2-adrenoceptor stimulation to reduce pain. Zymosan, injected on the sciatic nerve, caused hypersensitivity to mechanical stimuli ipsilateral to injection and contralaterally, so-called mirror image pain. Ipsilateral hypersensitivity was inhibited dose-dependently by perineural injection of clonidine. Zymosan increased leukocyte number at the site of injection 3 d later as well as their content of interleukin 1α (IL-1α), IL-1β, and IL-6. Perineural clonidine prevented both the increase in leukocyte number and cytokine expression induced by zymosan. Additionally, clonidine reduced the capacity of leukocytes to express pro-inflammatory cytokines as assessed by treatment of cells ex vivo with lipopolysaccharide, whereas no repression of IL-10 production occurred. Clonidine reduced the number of macrophages and lymphocytes as well as their expression of tumor necrosis factor α. All of the effects of clonidine were prevented by coadministration of anα2A-adrenoceptor-preferring antagonist. These results suggest that α2-adrenoceptor stimulation transforms cytokine gene expression, especially in macrophages and lymphocytes from a pro- to an anti-inflammatory profile in the setting of neuritis, likely relieving neuritis-induced pain by this mechanism.
Keywords: clonidine, neuritis, cytokines, α2-adrenoceptors, neuropathic pain, inflammation
Introduction
Neural immune interactions likely contribute to the generation and maintenance of chronic pain after nerve injury. In the periphery, pro-inflammatory cytokines, especially interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα), exert direct excitatory effects on sensory afferents (Ferreira et al., 1988; Cunha et al., 1992; Sorkin et al., 1997) and are transported centrally where they further sensitize pain transmission (Shubayev and Myers, 2002). In the spinal cord, microglia become activated after peripheral nerve injury, induce central sensitization (DeLeo and Yezierski, 2001), and may underlie extension of pain to the contralateral side, so-called mirror image pain (Twining et al., 2004).
Sympathetic nervous system activity likely exacerbates some neuropathic pain states (Koltzenburg and McMahon, 1991), considered to reflect novel expression of excitatory α-adrenoceptors on nociceptors (Sato and Perl, 1991) and sprouting of sympathetic fibers to surround sensory afferent terminals and cell bodies (McLachlan et al., 1993). Sympathetic nervous system effects on immune cells in pain states have been mostly ignored, although many classes of leukocytes express adrenoceptors (Josefsson et al., 1996) and leukocyte responses to challenge can be modulated by increasing sympathetic nervous system activity or by exogenous adrenoceptor agonists (Moynihan et al., 2004).
The α2-adrenoceptor agonist clonidine reduces hypersensitivity in animals with peripheral nerve injury (Yaksh et al., 1995) and provides analgesia in neuropathic pain patients after spinal injection (Eisenach et al., 1995). The effect of clonidine by this route occurs rapidly and lasts for a few hours. In contrast, when clonidine is injected at the site of peripheral nerve injury, it also reduces hypersensitivity, but with an onset of days and duration of >1 week (Lavand'homme et al., 2002). This slow time course may reflect clonidine-induced changes in recruitment and function of immune cells at the site of inflammation, as supported by the observation that perineural clonidine reduces proinflammatory cytokine content in the injured peripheral nerve when administered at the time of injury (Lavand'homme and Eisenach, 2003). This effect of clonidine is unexpected, because a report indicated that α2-adrenoceptor stimulation increases TNFα production by lipopolysaccharide (LPS)-challenged macrophages (Spengler et al., 1990), which would promote rather than reduce inflammation.
To further understand the regulation of immune responses to nerve injury by α-adrenoceptors, the current study used a model of acute inflammatory neuritis from zymosan injection on the sciatic nerve (Chacur et al., 2001). Zymosan causes hypersensitivity to mechanical stimulation of the hindpaw, which is dependent on expression of IL-1, IL-6, TNFα, and reactive oxygen species and complement at the site of inflammation (Twining et al., 2004) and which is also dependent on activated microglia in the spinal cord (Milligan et al., 2003). Because lymphocytes (Titnchi and Clark, 1984) and macrophages (Spengler et al., 1990) express α2-adrenoceptors, we hypothesized that the phenotype of these leukocytes would be altered by clonidine and thereby repress hypersensitivity. To test this, we determined whether α2-adrenoceptor stimulation would reduce hyperalgesia in acute inflammatory neuritis and, if so, assessed the cell type(s) and cytokine gene expression changes by which these receptors act.
Materials and Methods
Animals and surgery. Male Wistar rats, weighing 200–300 g on the day of surgery, were anesthetized, and a catheter was implanted in the left hindlimb as described previously (Chacur et al., 2001). The catheter consisted of a single distal port SILASTIC tubing attached to a piece of gelfoam that was wrapped loosely around the sciatic nerve at mid-thigh level and anchored to adjacent muscles with 3-0 silk suture. The external end of the catheter was passed subcutaneously to the midline just rostral to the tail base, where it was protected by means of a plastic and aluminum frame and sealed with rubber and silicon glue, as described previously (Milligan et al., 1999). The catheter location was verified at the end of the experiment, and only animals with correctly placed catheters were included in data analysis.
Drugs and treatments. Drugs were administered in the conscious animal through the perisciatic nerve catheter 4–5 d after implantation. The drugs used were zymosan in incomplete Freund's adjuvant (IFA) as vehicle (both from Sigma, St. Louis, MO), clonidine in saline (Roxane Laboratories, Columbus, OH), and the α2A-adrenoceptor-preferring antagonist (+)-2-((4,5-dihydro-1H-imidazol-2-yl)methyl)-2,3-dihydro1-methyl-1H-isoindole (BRL44408) in saline (Tocris, Ellisville, MO).
Animals received a single dose of zymosan (40 μg in 50 μl) or an equivalent volume of IFA. This zymosan dose produces hypersensitivity to mechanical stimulation ipsilateral to injection as well as mirror image hypersensitivity contralaterally (Chacur et al., 2001). Three hours after zymosan or vehicle injection, animals received a perisciatic nerve catheter injection of saline, clonidine (10, 20, or 30 μg), or BRL44408 (30 μg), alone or with clonidine (30 μg), in a total injection volume of 60 μl.
Behavioral testing. The withdrawal threshold was measured twice at 10 min intervals ipsilaterally and contralaterally to drug injection using calibrated von Frey filaments (Stoelting, Wood Dale, IL) and an up–down statistical method (Chaplan et al., 1994), and the average of these values was used for data analysis. The withdrawal threshold was determined in each animal before catheter implantation, 4–5 d after catheter implantation (immediately before zymosan or vehicle injection), 3 h after zymosan or vehicle injection (immediately before clonidine, BRL44408, or saline injection), and 1–3 d thereafter. The groups were IFA plus saline (n = 11), IFA plus clonidine (n = 10), zymosan plus saline (n = 15), zymosan plus clonidine (10, 20, or 30 μg; n = 6, 5, and 13, respectively), and zymosan plus BR44408 plus clonidine (n = 10). The investigator was blinded to drug treatment in all experiments.
Withdrawal thresholds were converted to percentage of maximum possible effect according to the following formula: (withdrawal threshold after drug – withdrawal threshold 3 h after zymosan injection) × 100/(withdrawal threshold before zymosan injection – withdrawal threshold 3 h after zymosan injection).
Leukocyte preparation and quantification. Rats were anesthetized with halothane and decapitated immediately after the last behavioral test. The perisciatic nerve gelfoam was dissected and mechanically dissociated in HBSS (20 ml) by means of micro-forceps in a plastic Petri dish, as described previously (Gazda et al., 2001). Only preparations in which all the gelfoam could be retrieved were used. The suspension was filtered using a 70 μm nylon mesh to separate the leukocytes from the gelfoam. Total leukocyte number per gelfoam insert was determined manually using a hemocytometer, and cell viability was determined by trypan blue exclusion, using 25 μl of 0.4% trypan blue solution (Life Technologies, Grand Island, NY) mixed with 25 μl of cell suspension. Leukocyte suspensions were concentrated to 107 cells/ml in HBSS, and aliquots of 200 μl were frozen for subsequent measurement of cytokine content. The group sizes were IFA plus saline (n = 9), IFA plus clonidine (n = 7), zymosan plus saline (n = 11), zymosan plus clonidine (30 μg; n = 11), and zymosan plus BR44408 plus clonidine (n = 9).
Cytokine measurement. Aliquots were thawed, sonicated for 10 s, and centrifuged at 1500 × g for 10 min at 4°C. Two samples of 50 μl of each supernatant were used immediately for cytokine measurement, and the results were averaged for subsequent data analysis. A nine-plex, bead-based rat cytokine immunoassay kit (Bio-Rad, Hercules, CA) was used for simultaneous detection of granulocyte macrophage–colony-stimulating factor (GM-CSF), interferon γ (IFNγ), IL -10, IL-1α, IL-1β, IL-2, IL-4, IL-6, and TNFα concentrations, following the manufacturer's instructions and as validated previously (Hulse et al., 2005). Multi-wavelength fluorescence and cytokine concentrations were determined with a luminometer (Luminex 100 system; Luminex, Austin, TX) and Bio-Rad software. Concentrations of cytokines in samples were within the linear range of the assay in all cases. Cytokine content in total leukocyte preparations was determined for IFA plus saline (n = 8), zymosan plus saline (n = 9), zymosan plus clonidine (30 μg; n = 9), and zymosan plus BR44408 plus clonidine (n = 8). Values are expressed as nanograms of cytokine/106 leukocytes.
Leukocyte subtype analysis and TNFα expression. Leukocyte subtypes and their TNFα expression were analyzed by flow cytometry using FAC-Scan (Becton Dickinson, Franklin Lakes, NJ) and Cell Quest software (Becton Dickinson). One million cells were incubated for 30 min with surface monoclonal antibodies (mAbs), followed by cell fixation and permeabilization and 30 min of incubation with intracellular mAbs. Cells were kept in 1% paraformaldehyde overnight until flow cytometry was performed. To characterize leukocyte populations, we first identified hematopoietic cells using a mouse anti-rat CD45 mAb conjugated with CyChrome (1 μg/106 cells; BD Biosciences, San Jose, CA) as described previously (Brack et al., 2004). Only presumed viable cells were assessed, using standard forward- and side-scatter analysis. To identify cell type, the cell suspension was incubated with CD45 mAb plus a second mAb conjugated with R-phycoerythrin as follows: mouse anti-rat ED1 for monocytes/macrophages (Brack et al., 2004), mouse anti-rat CD8 for cytotoxic T-lymphocytes (Ikezumi et al., 2004), mouse anti-rat CD4 for helper T-lymphocytes (Katz et al., 1990), mouse anti-rat CD161 for natural killer cells (Durante-Mangoni et al., 2004), mouse anti-rat OX-62 for dendritic cells (Yan et al., 2004), or mouse anti-rat RP-1 for granulocytes (Brack et al., 2004). All mAbs were from Serotec (Raleigh, NC) and used in a volume of 10 μl/106 cells, except for the mAb for RP-1, which was obtained from BD Biosciences and used in a concentration of 1 μg/106 cells. To assess the proportion of each cell type expressing TNFα, cells were incubated with fluorescein isothiocyanate anti-mouse/rat TNFα (1.5 μg/106 cells; eBioscience, San Diego, CA) as characterized previously (Sheehan et al., 1989). For intracellular staining (ED1 and TNFα), cells were fixed and permeabilized with medium (Leucoperm; Serotec) following the instructions of the manufacturer. The specificity of the staining was verified by incubation with appropriate isotype-matched control antibodies. Leukocyte subtype analysis was determined for IFA plus saline (n = 7), zymosan plus saline (n = 8), and zymosan plus clonidine (30 μg; n = 7).
In vitro challenge. Leukocytes retrieved from the gelfoam of animals treated in vivo 3 d earlier with zymosan plus saline or zymosan plus clonidine were challenged in vitro with LPS (Sigma) or saline. A final concentration of 106 cells/ml was prepared in RPMI medium (RPMI 1640, 10% fetal bovine serum, 1% penicillin, 1% streptomycin, and 1% l-glutamine) and treated with LPS (100 ng/ml final concentration) or saline immediately after preparation of the leukocytes. Cells were incubated overnight (16–18 h) at 37°C in 95% O2/5% CO2. Supernatants were frozen at –80°C until cytokines were measured using the nine-plex, bead-based immunoassay described above.
Statistical analysis. Data are presented as mean ± SEM. The effects of catheter implantation or zymosan or IFA injections on the withdrawal threshold were determined using a repeated-measures, two-way ANOVA, followed by Dunnett's test. Comparisons among groups for cytokine concentrations, leukocyte subtype, and the proportion of each expressing TNFα were performed using t tests, Mann–Whitney U tests, one-way ANOVA followed by the Student–Newman–Keuls test, or the Kruskal–Wallis test as appropriate. p < 0.05 was considered significant.
Results
The effect of clonidine on zymosan-induced hypersensitivity
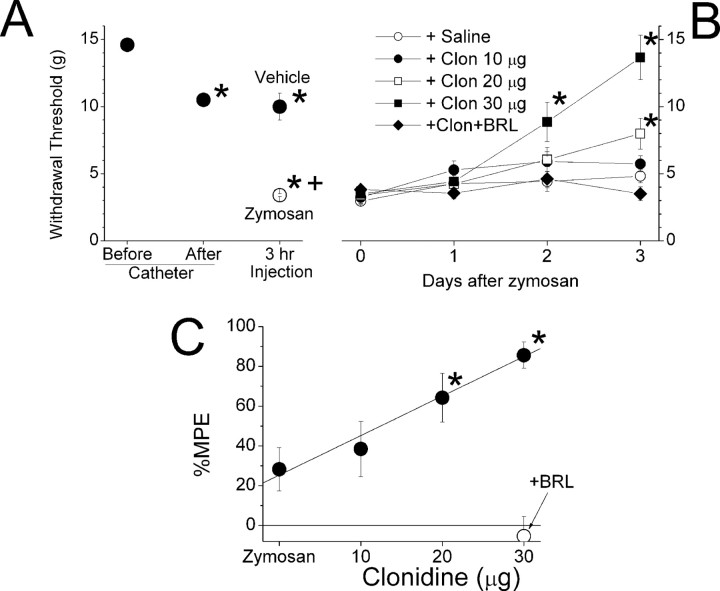
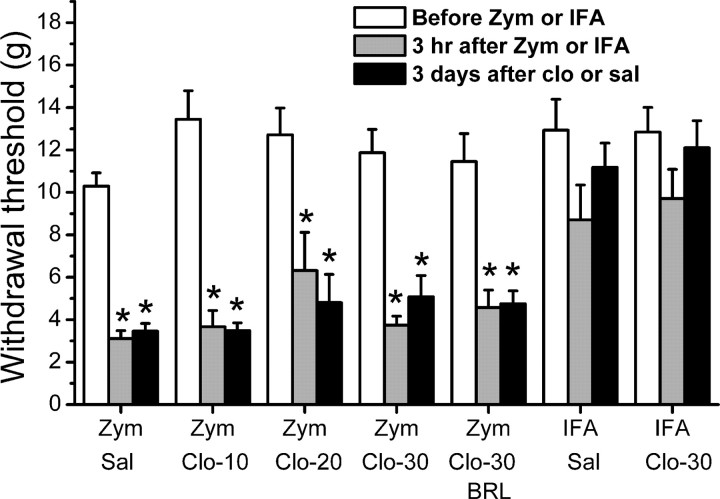
Implantation of the perisciatic catheter and gelfoam resulted in a slightly but significantly reduced withdrawal threshold ipsilateral to surgery (Fig. 1A). In control experiments, IFA alone failed to further reduce withdrawal threshold ipsilateral to injection. In contrast, zymosan significantly reduced the withdrawal threshold 3 h later, with no recovery over a 3 d period (Fig. 1A,B). Clonidine dose-dependently reversed zymosan-induced hypersensitivity ipsilateral to injection, with an onset of ≥48 h after injection (Fig. 1B). Resolution of hypersensitivity beyond 3 d from zymosan injection in control animals precluded study beyond this period (data not shown). However, the clonidine inhibition of hypersensitivity 3 d after injection was dose dependent with a threshold of 20 μg and a linear dose–response (Fig. 1B,C). Coadministration of the α2A-preferring antagonist BRL44408 prevented the reversal of clonidine on zymosan-induced hypersensitivity (Fig. 1B,C).
Figure 1.
Withdrawal threshold ipsilateral to perineural injections. A, The withdrawal threshold to von Frey stimulation ipsilateral to perineural catheterization before and after catheter implantation and 3 h after zymosan or vehicle. *p < 0.05 compared with before the catheter value; +p < 0.05 compared with vehicle. B, The withdrawal threshold to von Frey stimulation ipsilateral to perineural injection of zymosan on day 0, followed in 3 h by saline or different doses of clonidine alone or 30 μg of clonidine plus 30 μg of the α2-adrenoceptor antagonist BRL44408. *p < 0.05 compared with zymosan plus saline; +p < 0.05 compared with zymosan plus 30μg of clonidine. C, The percentage of maximum possible effect (MPE) to return withdrawal threshold to pre-zymosan treatment values 3 d after perineural injection of zymosan alone or zymosan followed by clonidine (10, 20, or 30μg). *p < 0.05 compared with zymosan alone. Each value represents the mean ± SE of 6–15 animals. Zym, Zymosan; sal, saline; clo, clonidine; clo10, clo20, and clo30, 10, 20, and 30μg of clonidine, respectively; BRL, BRL44408.
Perineural zymosan injection also reduced the withdrawal threshold on the contralateral hindpaw (Fig. 2), as described previously (Twining et al., 2004). Perineural clonidine failed to alter contralateral hypersensitivity (Fig. 2), in stark contrast to its efficacy ipsilateral to zymosan injection.
Figure 2.
Withdrawal threshold contralateral to perineural injections. The withdrawal threshold to von Frey stimulation contralateral to perineural catheterization before and 3 h after zymosan (Zym) or IFA injection and then 3 d after perineural injection of saline (sal) or clonidine (clo; 10, 20, or 30 μg alone) or with the α2A-adrenoceptor antagonist BRL44408 (BRL) is shown. Each value represents the mean + SE of 6–15 animals. *p < 0.05 compared with before zymosan or IFA injection. Clo-10, Clo-20, and Clo-30, 10, 20, and 30 μg of clonidine, respectively.
Total leukocyte number and their cytokine expression
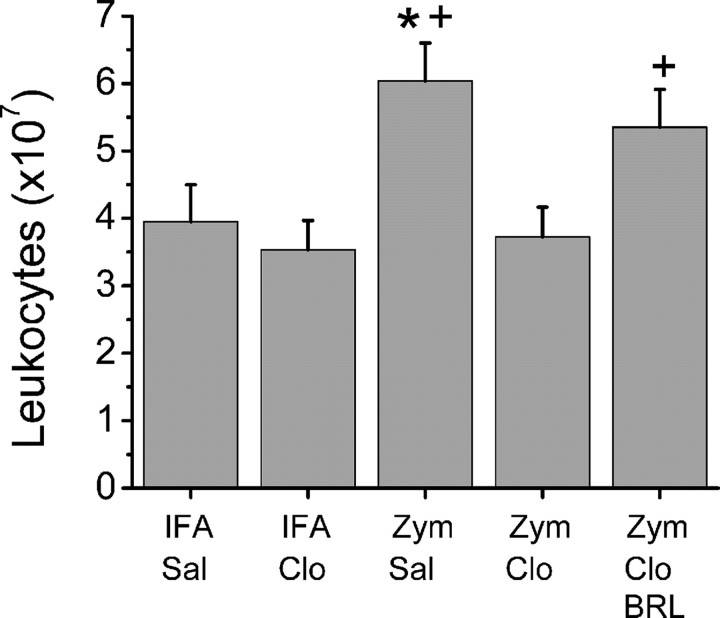
More than 95% of cells prepared from gelfoam in all groups excluded trypan blue and were considered viable. The leukocyte number increased 1.5fold 3 d after perineural injection of zymosan compared with saline control (Fig. 3). Perineural clonidine had no effect on leukocyte number in animals treated previously with saline but completely prevented zymosan-induced leukocytosis (Fig. 3). This effect of clonidine was prevented by coadministration of BRL44408 (Fig. 3).
Figure 3.
Leukocyte number. The number of leukocytes retrieved from gelfoam 3 d after perineural injection of IFA or zymosan (Zym), followed by saline (Sal) or clonidine (Clo; 30μg), alone or with BRL44408 (BRL), is shown. Each bar represents the mean + SE of 7–11 animals. *p < 0.05 compared with IFA plus saline; +p < 0.05 compared with zymosan plus 30 μg of clonidine.
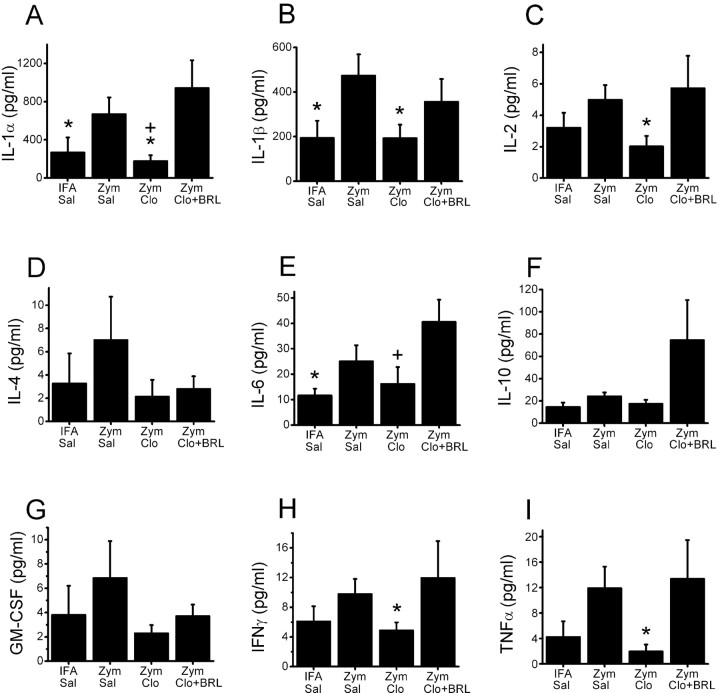
In addition to the effects on leukocyte number, zymosan and clonidine also altered cytokine content of the leukocytes themselves. As such, there was a generalized pattern of increased cytokine expression in leukocytes 3 d after perineural injection of zymosan compared with saline control, with statistically significant increases in pro-inflammatory IL-1α, IL-1β, and IL-6 (Fig. 4). Compared with zymosan plus saline, perineural clonidine reduced cytokine expression in leukocytes to a broad extent, with statistically significant reductions in IL-1α, IL-1β, IL-2, IFNγ, and TNFα (Fig. 4). The effect of clonidine in each case was inhibited by coadministration of BRL44408 (Fig. 4).
Figure 4.
Leukocyte cytokine expression. Cytokine content, expressed as picograms per milliliter in suspensions of 107 cells/ml of leukocytes retrieved from gelfoam 3 d after perineural injection of IFA or zymosan (Zym), followed by saline (Sal) or clonidine (Clo; 30 μg) alone or with BRL44408 (BRL) is shown. A, IL-1α; B, IL-1β; C, IL-2; D, IL-4; E, IL-6; F, IL-10; G, GM-CSF; H, IFNγ; I, TNFα. Each bar represents the mean + SE of eight to nine animals. *p < 0.05 compared with zymosan plus saline; +p < 0.05 compared with zymosan plus clonidine and BRL.
Leukocyte subtype analysis and TNFα expression
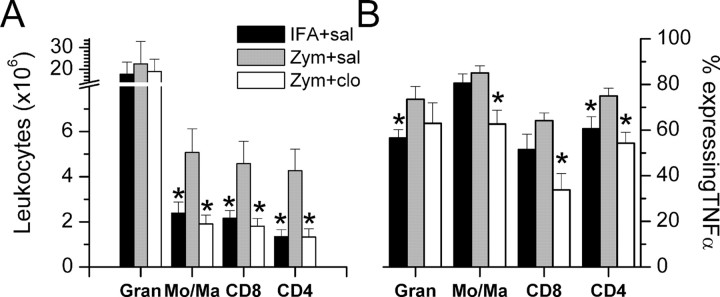
The 1.5-fold increase in total leukocytes in gelfoam 3 d after perineural zymosan injection was attributable to a twofold to threefold increase in monocytes/macrophages and cytotoxic and helper T-lymphocytes and a nonsignificant 30% increase in the number of granulocytes (Fig. 5A). Perineural clonidine did not affect the number of granulocytes after zymosan-induced neuritis and completely blocked the twofold to threefold increase in monocytes/macrophages as well as helper and cytotoxic T-lymphocytes (Fig. 5A).
Figure 5.
The number of leukocyte subsets and their TNFα expression. A, The number of leukocytes per gelfoam isolate identified by flow cytometry to be granulocytes (Gran), monocytes/macrophages (Mo/Ma), CD8 (cytotoxic) T-lymphocytes, and CD4 (helper) T-lymphocytes in animals treated in vivo 3 d earlier with perineural IFA or zymosan (Zym), followed by saline (sal) or 30 μg of clonidine (clo). B, Proportion of leukocytes expressing TNFα of the same subsets. Each bar represents the mean + SE of seven to eight animals. *p < 0.05 compared with zymosan plus saline.
Zymosan treatment produced a general increase in the proportion of leukocytes expressing TNFα, with statistically significant increases in granulocyte and helper T-cell subtypes (Fig. 5B). Clonidine treatment generally reduced TNFα expression, with statistically significant reductions compared with zymosan plus saline in monocytes/macrophages and cytotoxic and helper T-cells (Fig. 5B).
In vitro challenge
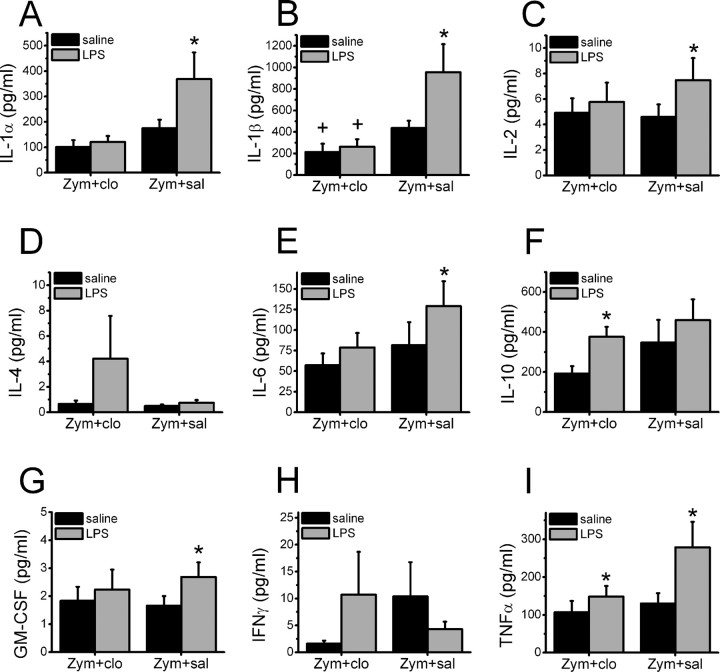
Cells harvested from zymosan plus saline-treated animals responded to the LPS challenge ex vivo with significantly increased production of the pro-inflammatory cytokines IL-1α, IL-1β, IL-2, IL-6, GM-CSF, and TNFα (Fig. 6). For example, TNFα production increased 2.8-fold to the LPS challenge in cells from zymosan plus saline-treated animals but only 1.7-fold to the same challenge in cells that had been exposed to clonidine in vivo. In contrast, cells harvested from zymosan plus clonidine-treated animals responded to the LPS challenge with a significantly induced production of these pro-inflammatory cytokines (Fig. 6I). Additionally, the LPS challenge failed to increase production of the anti-inflammatory cytokine IL-10 in zymosan plus saline-treated animals but significantly increased production of this cytokine in cells from clonidine-treated animals (Fig. 6F). A similar trend occurred with the anti-inflammatory cytokine IL-4 (Fig. 6D).
Figure 6.
Cytokine expression from the LPS challenge. Cytokine concentration after incubation in vitro with saline or LPS in the supernatant of leukocyte suspensions from animals exposed 3 d earlier to perineural zymosan (Zym), followed by saline (sal) or 30 μg of clonidine (clo). Each bar represents the mean + SE of six experiments. A, IL-1α; B, IL-1β; C, IL-2; D, IL-4; E, IL-6; F, IL-10; G, GM-CSF; H, IFNγ; I, TNFα. *p < 0.05 compared with in vitro saline control; +p < 0.05 compared with in vivo zymosan plus saline control.
Discussion
This study shows the following: (1) zymosan treatment of the sciatic model of neurogenic pain increases hypersensitivity both ipsilaterally and contralaterally; (2) this altered physiological phenotype correlates with an increase in the total leukocyte count assessed in gelfoam; (3) constitutive increases in inflammatory cytokines parallel the increase in leukocyte number; (4) locally administered clonidine by receptor-specific mechanisms abrogates ipsilateral (but not contralateral) hypersensitivity in parallel with reductions in pro-inflammatory mediators with no reduction or apparent increases in anti-inflammatory mediators, further favoring repression of inflammation; (5) clonidine also limits the capacity of exudative leukocytes to express pro-inflammatory genes without concomitant repression of anti-inflammatory products; (6) the effect of clonidine may be cell type specific (macrophages and T-lymphocytes); and (7) contralateral hypersensitivity induced by zymosan produces pain by a mechanism that is not entirely dependent on pro-inflammatory cytokine content of leukocytes surrounding the ipsilateral sciatic nerve. Together, these unexpected results clarify the actions of catecholamines on immune responses and provide the rationale for new approaches to the treatment of neuropathic pain.
Immune response to perineural zymosan
Three days after zymosan injection, the number of leukocytes in the perineural environment increased, as did their activation state, evidenced by increased IL-1 and IL-6 expression. It may appear paradoxical that TNFα, which drives upregulation of IL-1 and -6, is not increased by zymosan. It is possible that TNFα immunoreactivity, measured in the FACS analysis, missed an increase in active cytokine among total immunoreactivity, including proforms, or that the variability in TNFα precluded demonstration of significance from the strong trend (p = 0.07) of increased TNFα. Cytokine expression has not been quantified previously 3 d after zymosan injection in this neuritis model, although others have shown increased TNFα and IL-1β secretion ex vivo from leukocytes recovered from gelfoam at 24 h after perineural zymosan injection (Gazda et al., 2001). Zymosan activates nuclear factor κB by binding to Toll-like receptor 2 (TLR-2) and TLR-6 (Underhill et al., 1999; Young et al., 2001) and likely contributes to pro-inflammatory cytokine gene expression by this mechanism. These cytokines play key roles in the generation of zymosan-induced hypersensitivity, because perisciatic nerve injection of antibodies or receptor antagonists to these cytokines prevents the development of ipsilateral and bilateral hypersensitivity (Twining et al., 2004). Additionally, haplotypes with enhanced IL-6 signaling impart a more than fivefold increased risk in humans for sciatica and pain associated with intervertebral disc disease (Noponen-Hietala et al., 2005), consistent with relevance of this cytokine to clinical pain.
Although the number of perineural leukocytes is not increased 3 and 24 h after zymosan (Gazda et al. 2001), we demonstrate a leukocytosis 3 d after zymosan, probably reflecting the concomitant release of chemokines and cytokines. The population makeup of leukocytes in the gelfoam from control animals typifies a foreign body-induced exudate, and zymosan also increases leukocyte number primarily by increasing T-lymphocytes and monocytes/macrophages. Thus, zymosan increases both the total number of leukocytes and their cellular expression of proinflammatory cytokines in this foreign body, representing a large increase in cytokine content in the environment surrounding the nerve. The parallel time course between the number of proinflammatory leukocytes and hypersensitivity after nerve injury (Sommer and Schafers, 1998; Shamash et al., 2002; Moalem et al., 2004) also supports the importance of zymosan-induced leukocytosis and immune activation to persistent pain in this model. We recognize that intracellular cytokine content, as measured by immunoreactivity in cell homogenates or by FACS in the current study, does not measure released cytokine concentrations, and it is conceivable that cytokine release may differ from expression.
α2-Adrenoceptors transform the immune response to acute neuritis
Perineural clonidine prevented both the zymosan-induced increase in T-lymphocytes and monocytes/macrophages as well as their expression of pro-inflammatory cytokines. The proinflammatory cytokines that were increased after zymosan injection in control animals (IL-1, IL-6, TNFα) attract immune cells, and their reduced expression by clonidine would thereby prevent leukocytosis. α2-Adrenoceptor stimulation induces apoptosis in lymphocytes (Stevenson et al., 2001), providing an alternative explanation for reduced leukocytosis in clonidine-treated animals. This mechanism seems unlikely, however, because clonidine did not alter leukocyte number in gelfoam in the absence of zymosan or the number of leukocytes that failed to exclude trypan blue.
In contrast to its effect on pro-inflammatory mediators, clonidine failed to repress anti-inflammatory cytokine expression. Because pro-inflammatory cytokines generate hypersensitivity and pain (see above) and because IL-10 reduces hypersensitivity and pain (Milligan et al., 2005), we speculate that clonidine produces analgesia by shifting cytokine expression. The mechanisms by which clonidine affects this change require further study. However, T-lymphocytes and monocytes/macrophages constitutively express α2-adrenoceptors (Spengler et al., 1990; Schauenstein et al., 2000) and demonstrate α2A-adrenoceptor immunostaining at the site of nerve injury (Lavand'homme et al., 2002), and therefore likely represent a site of action.
The effects of α2-adrenoceptor activation on macrophages have received little previous attention. In isolated pure preparations of macrophages, α2-adrenoceptor agonists stimulate proinflammatory IL-12 (Kang et al., 2003) and TNFα (Spengler et al., 1990) expression, whereas in a mixed leukocyte preparation from whole-blood, α2-adrenoceptor agonists inhibit LPS-induced TNFα expression (Maes et al., 2000). We propose that α2-adrenoceptor stimulation in the microenvironment of neuritis shifts the macrophage phenotype from pro- to anti-inflammatory. Development of an explant model to mimic this environment would facilitate study of the mechanisms by whichα2-adrenoceptors produce this change.
To further test whether clonidine induces a sustained phenotypic change among the leukocytes, we stimulated cells ex vivo with the TLR-4 ligand LPS 3 d after zymosan injection. LPS-induced increases in production of IL-1, IL-2, IL-6, GM-CSF, and TNFα in cells from zymosan plus saline-treated animals is consistent with a pro-inflammatory profile. Similar responses were obtained with in vitro stimulation by zymosan of leukocytes isolated 3 and 24 h after in vivo zymosan injection in this model (Gazda et al., 2001). In contrast, clonidine treatment not only reduced pro-inflammatory cytokine expression by leukocytes exposed to zymosan in vivo, it also resulted in a phenotype that later responded to the immunological LPS challenge ex vivo by repression of pro-inflammatory cytokine production with sustained production of the anti-inflammatory cytokine IL-10.
α-Adrenoceptors and hypersensitivity from neuritis
The current study provides one explanation for the analgesic efficacy of peripherally administered clonidine in neuropathic pain. Peripheral nerve injury and resultant Wallerian degeneration stimulates an immunological response throughout the length of the nerve, extending to intact peripheral terminals, resulting in pain and hypersensitivity from exposure of these nerve endings to pro-inflammatory products. Thus, intraplantar cyclooxygenase inhibition relieves hypersensitivity and abnormal excitatory neuropeptide expression in afferents and the spinal cord after nerve injury (Ma and Eisenach, 2003). Although the current study used a model of acute neural inflammation, these parallels in presumed mechanism support inhibition of proinflammatory responses to nerve injury as the basis for efficacy of topical clonidine to treat neuropathic pain and suggest that peripherally restricted α2-adrenoceptor agonists such as oxymetazoline (Afrin), which lack sedative and hypotensive effects, could be applied topically or via perineural injection for analgesia in neuropathic pain.
Perineural clonidine failed to reduce mirror-image pain hypersensitivity in the current study. In contrast, perineural injection of agents that block TNFα, IL-6, IL-1, complement activity, or reactive oxygen generation result in a rapid onset repression of bilateral hypersensitivity, lasting for a few hours (Twining et al., 2004). Similarly, acute blockade of microglial function or p38 mitogen-activated protein kinase signaling in the spinal cord immediately after perineural zymosan injection blocks bilateral pain hypersensitivity (Milligan et al., 2003), consistent with a role for activated microglia in contralateral spread of sensitization. Although these studies confirm the relevance of locally produced pro-inflammatory mediators to hypersensitivity, their dramatically different time course compared with clonidine is consistent with acute pharmacological blockade of cytokine action with the former and altered cytokine gene expression by the latter. Additionally, the unilateral blockade of hypersensitivity by clonidine, but not by cytokine blockers, suggests that pro-inflammatory signaling at the site of neuritis is not necessary for the maintenance of contralateral sensitization. Alternatively, proinflammatory cytokine exposure over the 2–3 d before clonidine reduced hypersensitivity could have been sufficient to make mirror-image pain independent of additional peripheral cytokine-driven signaling to the spinal cord.
In summary, a single perineural injection of clonidine gradually alleviates for days hypersensitivity to mechanical stimulation in a neuritis model of pain via actions on α2-adrenoceptors. Unexpectedly, clonidine transforms the macrophage and T-lymphocyte phenotype in neuritis from pro-inflammatory and pain producing to anti-inflammatory and pain reducing. These results provide the rationale for a novel therapeutic strategy for neuropathic pain and a novel mechanism by which catecholamines regulate immune responses and neural-immune interactions.
Footnotes
This work was supported in part by National Institutes of Health Grants GM35523 and NS41386. We thank Julie Wieseler Frank for help with experimental details.
Correspondence should be addressed to Dr. James C. Eisenach, Department of Anesthesiology and Center for the Study of Pharmacologic Plasticity in the Presence of Pain, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1009. E-mail: jim@eisenach.us.
DOI:10.1523/JNEUROSCI.2995-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/258988-07$15.00/0
References
- Brack A, Rittner HL, Machelska H, Leder K, Mousa SA, Schäfer M, Stein C (2004) Control of inflammatory pain by chemokine-mediated recruitment of opioid-containing polymorphonuclear cells. Pain 112: 229–238. [DOI] [PubMed] [Google Scholar]
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR (2001) A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain 94: 231–244. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53: 55–63. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH (1992) The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 107: 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP (2001) The role of neuroinflammation and neuroimmune activation in persistent pain. Pain 90: 1–6. [DOI] [PubMed] [Google Scholar]
- Durante-Mangoni E, Wang R, Shaulov A, He Q, Nasser I, Afdhal N, Koziel MJ, Exley MA (2004) Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol 173: 2159–2166. [DOI] [PubMed] [Google Scholar]
- Eisenach JC, DuPen S, Dubois M, Miguel R, Allin D, Epidural Clonidine Study Group 1995. Epidural clonidine analgesia for intractable cancer pain. Pain 61: 391–399. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S (1988) Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 334: 698–700. [DOI] [PubMed] [Google Scholar]
- Gazda LS, Milligan ED, Hansen MK, Twining CM, Poulos NM, Chacur M, O'Connor KA, Armstrong C, Maier SF, Watkins LR, Myers RR (2001) Sciatic inflammatory neuritis (SIN): behavioral allodynia is paralleled by peri-sciatic proinflammatory cytokine and superoxide production. J Peripher Nerv Syst 6: 111–129. [DOI] [PubMed] [Google Scholar]
- Hulse RF, Kunkler PE, Fedynyshyn JP, Kraig RP (2005) Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J Neurosci Methods 136: 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezumi Y, Kanno K, Karasawa T, Han GD, Ito Y, Koike H, Toyabe S, Uchiyama M, Shimizu F, Kawachi H (2004) The role of lymphocytes in the experimental progressive glomerulonephritis. Kidney Int 66: 1036–1048. [DOI] [PubMed] [Google Scholar]
- Josefsson E, Bergquist J, Ekman R, Tarkowski A (1996) Catecholamines are synthesized by mouse lymphocytes and regulate function of these cells by induction of apoptosis. Immunology 88: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BY, Lee SW, Kim TS (2003) Stimulation of interleukin-12 production in mouse macrophages via activation of p38 mitogen-activated protein kinase by alpha2-adrenoceptor agonists. Eur J Pharmacol 467: 223–231. [DOI] [PubMed] [Google Scholar]
- Katz J, Michalek SM, Beagley KW, Eldridge JH (1990) Characterization of rat T helper cell clones specific for Bacteroides gingivalis antigen. Infect Immun 58: 2785–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltzenburg M, McMahon SB (1991) The enigmatic role of the sympathetic nervous system in chronic pain. Trends Pharmacol Sci 12: 399–402. [DOI] [PubMed] [Google Scholar]
- Lavand'homme PM, Eisenach JC (2003) Perioperative administration of the α2-adrenoceptor agonist clonidine at the site of nerve injury reduces the development of mechanical hypersensitivity and modulates local cytokine expression. Pain 105: 247–254. [DOI] [PubMed] [Google Scholar]
- Lavand'homme PM, Ma W, De Kock M, Eisenach JC (2002) Peri-neural α2A-adrenoceptor activation inhibits spinal cord neuroplasticity and tactile allodynia after nerve injury. Anesthesiology 97: 972–980. [DOI] [PubMed] [Google Scholar]
- Ma W, Eisenach JC (2003) Intraplantar injection of a cyclooxygenase inhibitor ketorolac reduces immunoreactivities of substance P calcitonin gene-related peptide, and dynorphin in the dorsal horn of rats with nerve injury or inflammation. Neuroscience 121: 681–690. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Kenis G, Egyed B, Bosmans E (2000) The effects of noradrenaline and alpha-2 adrenoceptor agents on the production of monocytic products. Psychiatry Res 96: 245–253. [DOI] [PubMed] [Google Scholar]
- McLachlan EM, Janig W, Devor M, Michaelis M (1993) Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature 363: 543–546. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Hinde JL, Mehmert KK, Maier SF, Watkins LR (1999) A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods 90: 81–86. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Twining C, Chacur M, Biedenkapp J, O'Connor K, Poole S, Tracey K, Martin D, Maier SF, Watkins LR (2003) Spinal glia and proinflammatory cytokines mediate mirror-image neuropathic pain in rats. J Neurosci 23: 1026–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O'Connor K, Martin D, Forsayeth JR, Maier SF, Johnson K, Chavez RA, Leinwand LA, Watkins LR (2005) Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 21: 2136–2148. [DOI] [PubMed] [Google Scholar]
- Moalem G, Xu K, Yu L (2004) T lymphocytes play a role in neuropathic pain following peripheral nerve injury in rats. Neuroscience 129: 767–777. [DOI] [PubMed] [Google Scholar]
- Moynihan J, Kruszewska B, Madden K, Callahan T (2004) Sympathetic nervous system regulation of immunity. J Neuroimmunol 147: 87–90. [DOI] [PubMed] [Google Scholar]
- Noponen-Hietala N, Virtanen I, Karttunen R, Schwenke S, Jakkula E, Li H, Merikivi R, Barral S, Ott J, Karppinen J, Ala-Kokko L (2005) Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain 114: 186–194. [DOI] [PubMed] [Google Scholar]
- Sato J, Perl ER (1991) Adrenergic excitation of cutaneous pain receptors induced by peripheral nerve injury. Science 251: 1608–1610. [DOI] [PubMed] [Google Scholar]
- Schauenstein K, Felsner P, Rinner I, Liebmann PM, Stevenson JR, Westermann J, Haas HS, Cohen RL, Chambers DA (2000) In vivo immunomodulation by peripheral adrenergic and cholinergic agonists/antagonists in rat and mouse models. Ann NY Acad Sci 917: 618–627. [DOI] [PubMed] [Google Scholar]
- Shamash S, Reichert F, Rotshenker S (2002) The cytokine network of Wallerian degeneration: tumor necrosis factor-α, interleukin-1α, and interleukin-1β. J Neurosci 22: 3052–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan KC, Ruddle NH, Schreiber RD (1989) Generation and characterization of hamster monoclonal antibodeis that neutralize murine tumor necrosis factors. J Immunol 142: 3884–3893. [PubMed] [Google Scholar]
- Shubayev VI, Myers RR (2002) Anterograde TNFα transport from rat dorsal root ganglion to spinal cord and injured sciatic nerve. Neurosci Lett 320: 99–101. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schafers M (1998) Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res 784: 154–162. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao W-H, Wagner R, Myers RR (1997) Tumour necrosis factoralpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 81: 255–262. [DOI] [PubMed] [Google Scholar]
- Spengler RN, Allen RM, Remick DG, Strieter RM, Kunkel SL (1990) Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J Immunol 145: 1430–1434. [PubMed] [Google Scholar]
- Stevenson JR, Westermann J, Liebmann PM, Hortner M, Rinner I, Felsner P, Wolfler A, Schauenstein K (2001) Prolonged alpha-adrenergic stimulation causes changes in leukocyte distribution and lymphocyte apoptosis in the rat. J Neuroimmunol 120: 50–57. [DOI] [PubMed] [Google Scholar]
- Titnchi S, Clark B (1984) Alpha-2 adrenoceptors in human lymphocytes: direct characterisation by [3H]-yohimbine binding. Biochem Biophys Res Commun 121: 1–7. [DOI] [PubMed] [Google Scholar]
- Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR (2004) Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain 110: 299–309. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401: 811–815. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Pogrel JW, Lee YW, Chaplan SR (1995) Reversal of nerve ligation-induced allodynia by spinal alpha-2 adrenoceptor agonists. J Pharmacol Exp Ther 272: 207–214. [PubMed] [Google Scholar]
- Yan H, Miyagi T, Satoh E, Sugiura W, Yamamoto N, Kimura H (2004) Phenotype and function of GM-CSF independent dendritic cells generated by long-term propagation of rat bone marrow cells. Cell Immunol 229: 117–129. [DOI] [PubMed] [Google Scholar]
- Young SH, Ye J, Frazer DG, Shi X, Castranova V (2001) Molecular mechanism of tumor necrosis factor-alpha production in 1→3-beta-glucan (zymosan)-activated macrophages. J Biol Chem 276: 20781–20787. [DOI] [PubMed] [Google Scholar]