Abstract
The muscarinic acetylcholine receptor (mAChR) has been considered one of the neurotransmitter receptors regulating hippocampal synaptic plasticity, which likely plays a critical role in learning and memory. In previous studies, however, muscarinic agonists were used at relatively high concentrations, and the subtype selectivity of muscarinic antagonists was not satisfactory. Thus, it remains to be answered whether physiological levels of ACh are involved in the regulation of synaptic plasticity and which mAChR subtypes are responsible for such effects. We found in this study that a low concentration (50 nm) of carbachol enhanced long-term potentiation (LTP) of excitatory synaptic transmission in mouse hippocampal slices. Notably, this enhancing effect was abolished in M1 mAChR knock-out (KO) but not in M3 mAChR KO mice, although LTP itself was intact in both mutant mice. Furthermore, we found that repetitive stimulation in the stratum oriens, which presumably triggered the release of endogenous ACh from cholinergic terminals, could enhance LTP in wild-type mice but not in M1 mAChR KO mice. These results suggest that physiologically released ACh from cholinergic fibers modulates hippocampal synaptic plasticity through the postsynaptic M1 mAChR activation.
Keywords: synaptic transmission, long-term potentiation, LTP, acetylcholine, muscarinic receptor, knock-out mouse, learning
Introduction
The cholinergic system is involved in higher brain functions, including learning and memory (for review, see Blokland, 1996) in which the hippocampus is implicated (for review, see Milner et al., 1998). The hippocampus is extensively innervated by cholinergic fibers mainly originating from the septal nucleus (for review, see Nicoll, 1985), and the action of ACh on learning and memory is mostly mediated by muscarinic acetylcholine receptors (mAChRs) (Blokland, 1996). Five distinct subtypes of mAChRs (M1-M5 subtypes) have been genetically identified by gene cloning studies (for review, see Caulfield and Birdsall, 1998). Among them, M1, M3, and M5 mAChRs are coupled with Gq/11, activating phospholipase C, whereas M2 and M4 mAChRs are coupled with Gi/o, inhibiting adenylate cyclase. The hippocampus expresses all five mAChR subtypes (Levey et al., 1995; Fisahn et al., 2002), in which they modulate various ionic channels and regulate excitability of neurons (for review, see Nicoll et al., 1990). Application of mAChR agonists to hippocampal slices produces network oscillations (Huerta and Lisman, 1993; Fisahn et al., 1998, 2002), which modulate the induction of hippocampal long-term potentiation (LTP) (Williams and Johnston, 1988; Huerta and Lisman, 1993; Shimoshige et al., 1997), a cellular model for learning and memory in the vertebrate CNS (for review, see Lynch, 2004), and oscillations in the whole animal are considered to reflect learning processes (Winson, 1978). In fact, malfunction of the cholinergic system in some mental disorders causes memory impairment (Blokland, 1996). In Alzheimer's disease, it has been reported that cholinergic terminals are lost and mAChR densities decrease in the brain, including the hippocampus (Rodríguez-Puertas et al., 1997). Furthermore, an aging-related decrease in the M1 mAChR density has been found in the hippocampal CA1 region (Tayebati et al., 2002).
Relatively high concentrations of mAChR agonists were used in previous studies (Huerta and Lisman, 1993; Auerbach and Segal, 1996; Shimoshige et al., 1997); however, neural activities in physiological conditions would never raise local ACh concentrations to such a high level in the intact brain, partly because there exist cholinesterase enzymes and ACh is markedly susceptible to hydrolysis by the enzymes. Conversely, most of the cholinergic agonists used in previous studies, such as carbachol [carbamoylcholine (CCh)] or bethanechol (carbamoyl-β-methylcholine), are completely resistant to hydrolysis by cholinesterase and have far longer duration of actions than ACh. Thus, it still remains unclear whether physiological levels of ACh are involved in the regulation of synaptic plasticity and learning. To address this issue, we performed electrophysiological experiments. We found that a low concentration of CCh, a nonselective cholinergic agonist, as well as endogenous ACh released by repetitive stimulation of cholinergic fibers, enhanced LTP in the CA1 region of the hippocampus. This priming effect was absent in mutant mice lacking M1 mAChRs but not in mutant mice lacking M3 mAChRs.
Materials and Methods
All experiments were performed according to the guidelines laid down by the Animal Care and Experimentation Committee of Kobe University and University of Tokyo.
M1 (Ohno-Shosaku et al., 2003) and M3 (Matsui et al., 2000) knockout (KO) mice and KO mice lacking both M1 and M3 mAChRs (M1/M3) (Ohno-Shosaku et al., 2003) were generated in a mixed background between 129/SvJ and C57BL/6J mice as described previously. These hybrid lines were back-crossed to C57BL/6J mice to yield an N4 generation of M1 mAChR KO mice, an N10 generation of M3 mAChR KO mice, and an N3 or N4 generation of M1/M3 mAChR KO mice. For electrophysiology, male C57BL/6J mice and wild-type and mutant mice (6-17 weeks old) were decapitated under deep halothane anesthesia, and hippocampi were quickly removed (Shimuta et al., 2001). Transverse hippocampal slices (400 μm thick) were cut with a vibratome tissue slicer and placed in an interface-type holding chamber for at least 1 h. During the experiments, a single slice was transferred to the recording chamber and submerged beneath a continuously superfusing medium that had been saturated with 95% O2 and 5% CO2. The composition of the medium was as follows (in mm): 119 NaCl, 2.5 KCl, 1 NaH2PO4, 26.2 NaHCO3, 1.3 MgSO4, 2.5 CaCl2, and 11 glucose. The field potential recordings were made with a glass electrode (3 m NaCl) placed in the stratum radiatum of the CA1 region. In wholecell patch-clamp recordings, the pipette solution contained the following (in mm): 140 potassium methansulfonate, 10 HEPES, 8 NaCl, 2 Mg-ATP, and 0.3 Na3-GTP, pH 7.2 adjusted with KOH (290-300 mOsm). An Axopatch 1D amplifier (Axon Instruments, Foster City, CA) was used, and the signal was filtered at 1 kHz, digitized at 10 kHz, and stored on a personal computer. For evoking synaptic responses, a bipolar tungsten stimulating electrode was placed in the stratum radiatum, and Schaffer collateral/commissural fibers were stimulated at 0.1 Hz. The stimulus strength was adjusted so that it gave rise to the slope value of EPSPs between 0.10 and 0.15 mV/ms. Maximal slope values of the initial rising phase of EPSPs were measured to avoid contamination of voltage-dependent components as much as possible. All of the data were accepted except when the baseline responses were unstable and/or the presynaptic fiber volley was changed during the experiment. The magnitude of LTP was calculated as percentage of the averaged EPSP slope value from 50-60 min after the conditioning relative to the baseline EPSP slope value. All experiments were done at 25 ± 0.1°C. The data are expressed as the means ± SEMs. Student's t test (paired or unpaired) and ANOVA were used to determine whether there was a significant difference (p < 0.05) in the mean. In most sets of experiments, the number of slices was the same as the number of animals, but, in some pharmacological experiments, the number of slices or neurons (n) exceeded the number of animals (N). The majority of electrophysiological experiments in which KO mice were used were performed in a blind manner, and the results were essentially identical to those of the nonblind experiments, and thus all the data were pooled. Even if we excluded the nonblind data, the result of statistical analyses was not affected in any set of experiments. Carbachol, atropine sulfate, and eserine hemisulfate were purchased from Sigma (St. Louis, MO).
Results
Muscarinic regulation of hippocampal synaptic transmission and plasticity
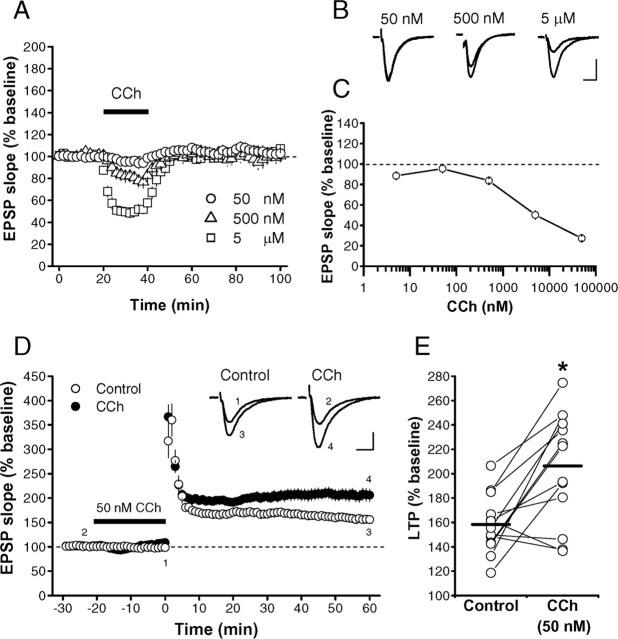
In the CA1 region of hippocampal slices of the C57BL/6J mouse, CCh at concentrations ≥500 nm caused dose-dependent inhibition of the field EPSPs evoked by stimulation of Schaffer collaterals (Auerbach and Segal, 1996) (Fig. 1A-C). The inhibition was completely blocked by atropine (1 μm), a nonselective mAChR antagonist (data not shown), indicating that it was mediated solely by mAChRs. In contrast, 50 nm CCh had only a little effect on EPSPs (Fig. 1A-C) and never induced network oscillation in our conditions (data not shown). CCh at 50 nm exhibited no long-lasting effects on EPSPs (Fig. 1A), although it caused only a slight but insignificant (p > 0.5, unpaired t test) change in EPSP slopes during and shortly after the drug application. Thus, we used 50 nm CCh to activate mAChRs in the following experiments. It is very important to use such a low concentration of CCh, because LTP induction is influenced by the change in basal synaptic transmission by itself, unrelated to mAChR activation. High-frequency stimulation (100 Hz, 1 s) of Schaffer collaterals induced normal LTP in control slices (158.3 ± 6.4% of baseline; n = 13; N = 13) (Fig. 1D,E). In contrast, LTP was enhanced (206.3 ± 12.2% of baseline; n = 13; N = 13; p < 0.003, paired t test) in the slices from the same animals to which 50 nm CCh had been applied for 20 min before the same tetanic stimulation (Fig. 1D,E).
Figure 1.
Low concentrations of CCh enhance LTP with little effect on basal synaptic transmission. A, CCh inhibited EPSPs in a dose-dependent manner. B, Representative traces of EPSPs at each concentration of CCh. C, Dose-response curve of the CCh-induced synaptic inhibition (5 nm, 88.5 ± 3.0% of baseline, n = 5, N = 3; 50 nm, 95.4 ± 4.0% of baseline, n = 8, N = 7; 500 nm, 83.7 ± 2.2% of baseline, n = 4, N = 4; 5 μm, 50.2 ± 4.0% of baseline, n = 4, N = 4; 50 μm, 27.3 ± 2.3% of baseline, n = 5, N = 4). CCh was applied to the slice from 20 to 40 min. Averaged EPSP slope values from 38 to 39 min were plotted against CCh concentrations. D, LTP induced by high-frequency stimulation (100 Hz, 1 s at time 0) in the presence (•) and absence (○) of 50 nm CCh. Washing out CCh was started just after the conditioning. Sample traces in the inset represent EPSPs recorded at the times indicated by the numbers. E, Summary of all experiments. The data points in the slices obtained from the same mouse are connected by thin lines. Horizontal bars indicate the mean values. *p < 0.003 (paired t test). Calibration: 10 ms, 0.2 mV. (The same applies also to the following figures unless otherwise noted.)
Priming effect of endogenous ACh on the induction of LTP
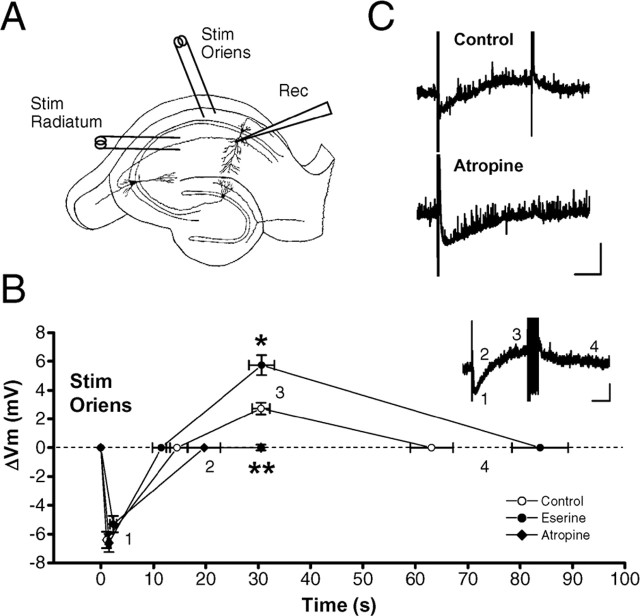
Repetitive stimulation in the stratum oriens (Fig. 2A) induced the release of ACh in the stratum radiatum from cholinergic terminals originating mainly from the septal nucleus (Cole and Nicoll, 1984): a short train of repetitive stimulation (40 Hz, 0.5 s) in the stratum oriens caused depolarization of the pyramidal cell (the peak of depolarization excluding spikes, 2.7 ± 0.4 mV; n = 22; N = 19), which was significantly increased (p < 0.0002, one-way ANOVA, Fisher's PLSD test) by eserine (5.7 ± 0.7 mV; n = 26; N = 19), a cholinesterase inhibitor, and was at the peak ∼30 s after the train (Fig. 2B,C). This slow depolarization was completely blocked by atropine (0.01 ± 0.2 mV; n = 17; N = 14; p < 0.002, one-way ANOVA, Fisher's PLSD test) (Fig. 2B,C). During the slow depolarization, some neurons generated spikes (Fig. 2B, inset) in the absence (3 of 22 neurons examined) and presence (11 of 26 neurons examined) of eserine. In the presence of atropine, no neurons showed firing (17 neurons examined).
Figure 2.
Endogenous ACh increases the excitability of pyramidal cells. A, The arrangement of electrodes in the hippocampal slice. Stim Radiatum, Stimulation electrode in the stratum radiatum; Stim Oriens, stimulation electrode in the stratum oriens; Rec, recording electrode. B, Summary of slow EPSPs in the presence (•) and absence (○) of eserine, a cholinesterase inhibitor, and in the presence of atropine (♦), a nonselective mAChR antagonist. Numbers indicate the following: 1, a peak point of hyperpolarization; 2, a point returning to the baseline level; 3, a peak point of depolarization excluding spike components; and 4, a point returning to the baseline level. Because the slow depolarization was absent in the presence of atropine, the membrane potential at the time of the peak point of depolarization in control conditions was measured as the peak point (3). Inset, A representative trace of the slow EPSP induced by a short train of stimulation (40 Hz, 0.5 s) in the stratum oriens. C, Representative traces of slow EPSPs in the absence (Control) and presence (Atropine) of atropine. Whole-cell patch-clamp recordings were made in pyramidal cells, and membrane potential was held at -60 mV in the current-clamp mode. Calibration: 10 s, 5 mV. *p < 0.0002; **p < 0.002 (one-way ANOVA, Fisher's PLSD test).
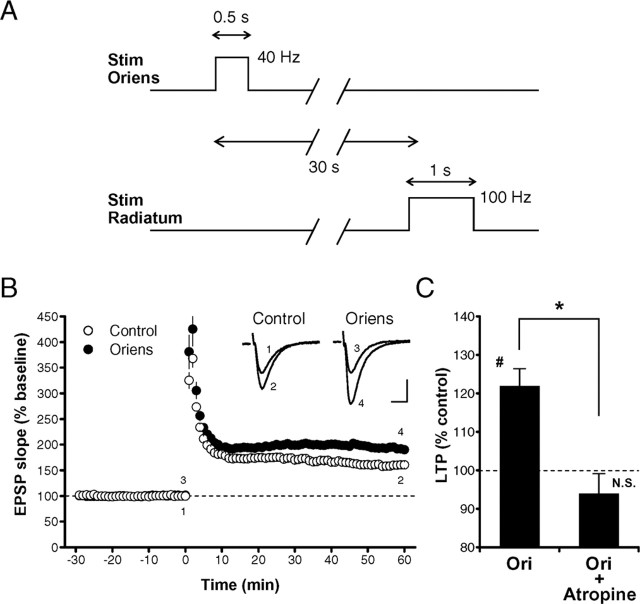
When a short train of repetitive stimulation (40 Hz, 0.5 s) in the stratum oriens was applied 30 s before the LTP-inducing tetanic stimulation (100 Hz, 1 s) in the stratum radiatum (Fig. 3A), resulting LTP was significantly enhanced (193.1 ± 7.1% of baseline; n = 16; N = 16) compared with LTP in control slices (159.9 ± 5.2% of baseline; n = 16; N = 16; p < 0.0003, paired t test) from the same mice (Fig. 3B,C). This enhancement (enhancement ratio, 121.9 ± 4.5% of control; n = 16; N = 16), which was comparable with that observed in the presence of 50 nm CCh (Fig. 1D,E), was completely abolished by atropine (enhancement ratio, 93.9 ± 5.2% of control; p < 0.003, unpaired t test) (Fig. 3C), indicating that it was mediated by mAChRs activated by endogenous ACh.
Figure 3.
Enhancement of LTP by endogenous ACh released from cholinergic fibers. A, The experimental protocol for examining the effect of stimulation in the stratum oriens on LTP. Stim Radiatum, Stimulation in the stratum radiatum; Stim Oriens, stimulation in the stratum oriens. B, LTP was enhanced by endogenous ACh released by stimulation in the stratum oriens (•) compared with LTP in control slices without the stimulation (○). C, Summary of the LTP enhancement induced by stratum oriens (Ori) stimulation in the absence and presence (Atropine, 191.8 ± 11.1% of baseline, n = 7, N = 7; Ori + Atropine, 178.5 ± 10.6% of baseline, n = 7, N = 7) of atropine. The magnitude of LTP (50-60 min after the tetanus) with stratum oriens stimulation was divided by that without the stimulation in each pair of slices from the same mouse, and the ratio was averaged for each condition (Ori, 121.9 ± 4.5% of control; Ori + Atropine, 93.9 ± 5.2% of atropine alone). *p < 0.003 (unpaired t test); #p < 0.0003 (paired t test); N.S., not significant.
Muscarinic regulation of synaptic transmission is mediated by M1 mAChRs
Because LTP in the CA1 region is induced and expressed postsynaptically (Malinow and Malenka, 2002), the enhancement of LTP by mAChR activation is likely mediated by the postsynaptic mAChRs. In the hippocampus, M1 and M3 receptors are abundantly expressed in the postsynaptic site, and the M1 receptor shows the strongest expression in the CA1 region among all of the subtypes (Levey et al., 1995; Fisahn et al., 2002). Because pharmacological identification of mAChR subtypes involved in physiological phenomena used to be a difficult task, which is primarily attributable to the lack of subtype selectivity of mAChR ligands, we used KO mice lacking a subtype(s) of mAChRs in the following experiments.
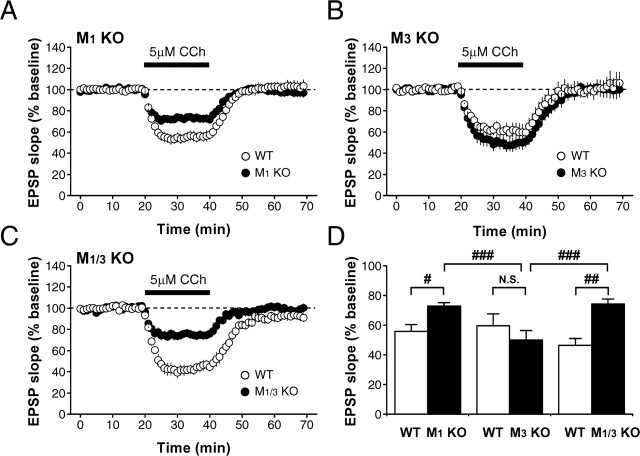
We found that synaptic inhibition induced by higher concentrations of CCh (5 μm) was partially but significantly (p < 0.007, one-way ANOVA, Fisher's PLSD test) reduced in mice lacking M1 mAChRs (wild type, to 55.8 ± 4.5% of baseline, n = 7, N = 4; mutant, to 72.7 ± 2.5% of baseline, n = 10, N = 6) (Fig. 4A,D), whereas it was intact (p > 0.2, one-way ANOVA, Fisher's PLSD test) in mice lacking M3 mAChRs (wild type, to 59.7 ± 8.0% of baseline, n = 3, N = 3; mutant, to 50.0 ± 6.4% of baseline, n = 5, N = 5) (Fig. 4B,D). A similar degree of synaptic inhibition to that in mice lacking M1 mAChRs (p > 0.7, one-way ANOVA, Fisher's PLSD test) was observed in mice lacking M1/M3 mAChRs (wild type, to 46.4 ± 4.6% of baseline, n = 5, N = 4; mutant, to 74.3 ± 3.2% of baseline, n = 6, N = 3; p < 0.0005, one-way ANOVA, Fisher's PLSD test) (Fig. 4C,D). The synaptic inhibition in M1/M3 mutant mice was significantly smaller (p < 0.002, one-way ANOVA, Fisher's PLSD test) than that in M3 mutant mice. These results rule out the possibility that the residual M1 mAChRs in mice lacking M3 mAChRs functionally compensated the missing M3 mAChRs and indicate that M3 mAChRs are not involved in synaptic inhibition evoked by CCh (Fig. 4C,D). Thus, it is strongly suggested that M1 mAChRs play a major role in the CA1 region, contrary to the previous report (Auerbach and Segal, 1996) showing pharmacologically that M3 mAChRs are exclusively involved in this inhibition.
Figure 4.
M1 mAChRs are associated with synaptic inhibition by a higher concentration of CCh. A-C, A relatively high concentration of CCh (5 μm) caused depression of EPSPs, which was partially diminished in M1 (A) and M1/M3 (C) mutant mice but intact in M3 mutant mice (B). CCh was applied to the slice from 20 to 40 min. D, Summary of the synaptic inhibition caused by 5 μm CCh in wild-type (WT), M1 (M1 KO), M3 (M3 KO), and M1/M3 (M1/M3 KO) mutant mice. Averaged EPSP slope values from 38 to 39 min were used for the statistical analysis. #p < 0.007; ##p < 0.0005; ###p < 0.002 (one-way ANOVA, Fisher's PLSD test); N.S., not significant.
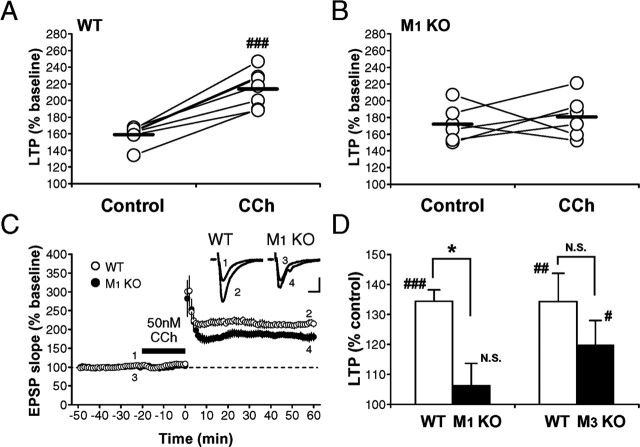
Mediation of the LTP enhancement by M1 mAChRs
As had been reported previously (Anagnostaras et al., 2003), LTP in the hippocampal CA1 region was indistinguishable (p > 0.1, unpaired t test) between wild-type (159.2 ± 4.0% of baseline; n = 7; N = 7) and M1 mutant (172.2 ± 8.0% of baseline; n = 6; N = 6) mice (Fig. 5A). However, cholinergic fibers are severed in slice preparations, and ACh may not be released by tetanic stimulation of Schaffer collaterals, likely explaining the lack of differences in LTP between the genotypes. Thus, we compared the priming effect of mAChR activation on LTP induction between wild-type and mutant mice. As already shown in C57BL/6J mice (Fig. 1), LTP was enhanced (p < 0.0002, paired t test) in the presence of 50 nm CCh in wild-type mice (control, 159.2 ± 4.0% of baseline; CCh, 213.8 ± 7.7% of baseline; n = 7; N = 7) (Fig. 6A,C,D); however, the enhancing effect of CCh on LTP was absent (enhancement ratio: wild type, 134.4 ± 3.8% of control; mutant, 106.4 ± 7.3% of control; p < 0.008, unpaired t test) in M1 mutant mice (control, 172.2 ± 8.0% of baseline; CCh, 180.8 ± 9.4% of baseline; n = 6; N = 6; p > 0.7, paired t test) (Fig. 6B-D). The magnitude of LTP in M1 mutant mice in the presence of CCh was not significantly different (p > 0.06, unpaired t test) from that in wild-type mice in the absence of CCh. In contrast, LTP was unaltered in M3 mutant mice (wild type, 159.2 ± 6.3% of baseline, n = 9, N = 9; mutant, 166.0 ± 7.6% of baseline, n = 9, N = 9; p > 0.5, unpaired t test) (Fig. 5B), but a significant priming effect (control, 166.0 ± 7.6% of baseline, n = 9, N = 9; CCh, 195.3 ± 10.3% of baseline, n = 9, N = 9; p < 0.05, paired t test) was observed, which was statistically indistinguishable (enhancement ratio: wild type, 134.3 ± 9.4% of control; mutant, 119.8 ± 8.2% of control; p > 0.2, unpaired t test) from that in wild-type mice (Fig. 6D). These results suggest that the priming effect of CCh on LTP was mediated primarily by M1 mAChRs.
Figure 5.
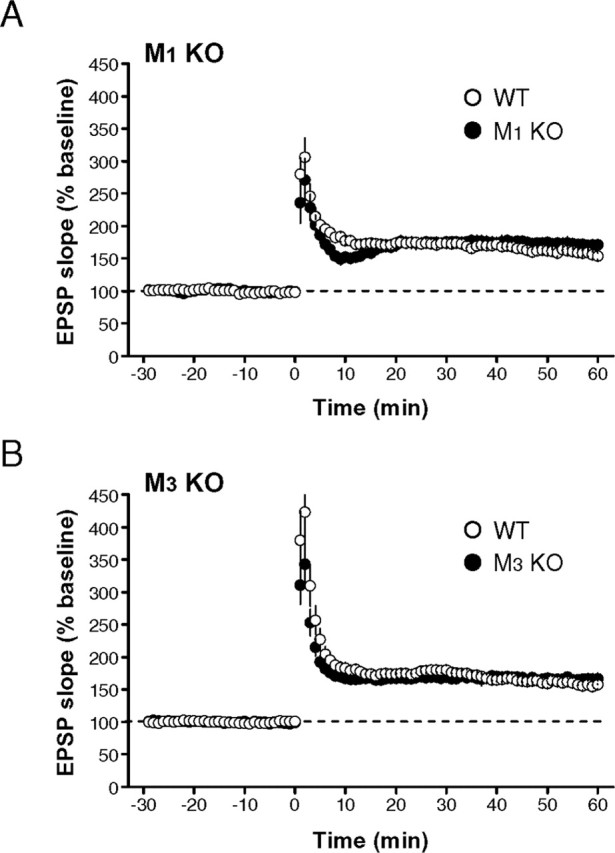
LTP is not impaired in either M1 or M3 mutant mice. A, B, LTP induced by 100 Hz, 1 s stimulation was intact in both M1 (A) and M3 (B) mutant mice.
Figure 6.
CCh-induced enhancement of LTP is mediated by M1, but not M3, mAChRs. A-C, LTP was enhanced by a low concentration of CCh (50 nm) in wild-type mice (A, C), but the enhancement was absent in M1 mutant mice (B, C). Washing out CCh was started just after the conditioning. D, Summary of the LTP enhancement in wild-type, M1, and M3 mutant mice. The magnitude of LTP (50-60 min after the tetanus) in the presence of CCh was divided by that in the absence of CCh in each pair of slices from the same mouse, and the ratio was averaged for each genotype. *p < 0.008 (unpaired t test); #p < 0.05; ##p < 0.002; ###p < 0.0002 (paired t test); N.S., not significant.
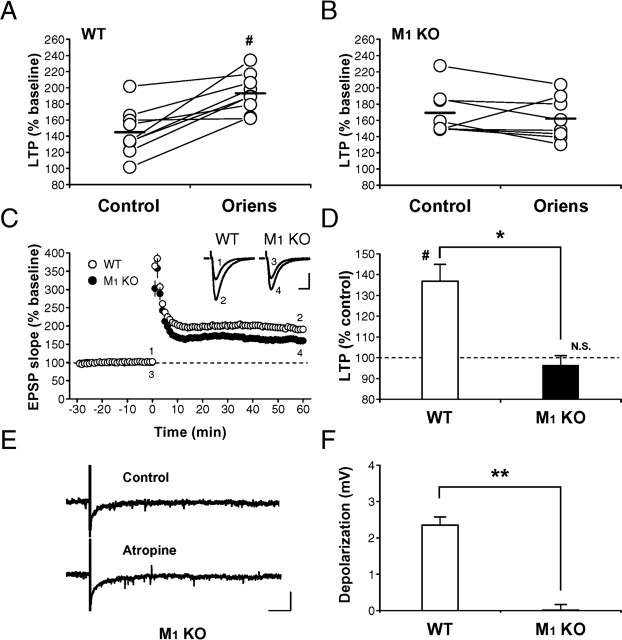
Finally, we conducted the experiments using M1 mutant mice to investigate whether the priming effect induced by endogenous ACh is mediated by M1 mAChRs. When a short train of repetitive stimulation (40 Hz, 0.5 s) in the stratum oriens was applied 30 s before the tetanic stimulation (100 Hz, 1 s) in the stratum radiatum in the same way as applied in the experiments of Figure 3, LTP was enhanced in wild-type mice (control, 145.0 ± 9.2% of baseline; oriens, 193.1 ± 7.5% of baseline; n = 9; N = 9; p < 0.002, paired t test) (Fig. 7A,C), whereas the enhancing effect on LTP was absent in M1 mutant mice (control, 169.3 ± 9.3% of baseline; oriens, 161.9 ± 8.8% of baseline; n = 8; N = 8; p > 0.3, paired t test) (Fig. 7B,C). Thus, the LTP enhancement induced by the stratum oriens stimulation was completely abolished in M1 mutant mice (enhancement ratio: wild type, 136.9 ± 8.1% of control, n = 9, N = 9; mutant, 96.4 ± 4.6% of control, n = 8, N = 8; p < 0.002, unpaired t test) (Fig. 7D). The involvement of M1 mAChRs in the LTP enhancement by the stratum oriens stimulation was further confirmed by the absence of the slow depolarization in M1 mutant mice (wild type, 2.4 ± 0.2 mV, n = 5, N = 4; mutant, 0.02 ± 0.2 mV, n = 7, N = 5; p < 0.0001, unpaired t test) (Fig. 7E,F). These results strongly suggest that the priming effect of endogenous ACh on LTP was mediated by M1 mAChRs.
Figure 7.
Enhancement of LTP by endogenous ACh released from cholinergic fibers is mediated by M1 mAChRs. A, B, LTP enhancement induced by stratum oriens stimulation in wild-type (A) and M1 mutant (B) mice. C, Averaged time course of LTP in wild-type (○) and M1 mutant (•) mice with the stratum oriens stimulation. D, Summary of the LTP enhancement in wild-type (WT) and M1 mutant (M1 KO) mice. The magnitude of LTP (50-60 min after the tetanus) with stratum oriens stimulation was divided by that without the stimulation in each pair of slices from the same mouse, and the ratio was averaged for each genotype. E, The slow EPSP was absent in M1 mutant mice (Control), and atropine had no effect on membrane potential responses (Atropine). Calibration: 10 s, 5 mV. F, Summary of the amplitude of slow EPSPs in wild-type (WT) and M1 mutant (M1 KO) mice. *p < 0.002; **p < 0.0001 (unpaired t test); #p < 0.002 (paired t test); N.S., not significant.
Discussion
We have demonstrated in this study that endogenous ACh released from cholinergic terminals by physiological stimulation in hippocampal slices can modify synaptic plasticity. It has been shown that activation of the septohippocampal cholinergic pathway raises the excitability of hippocampal pyramidal neurons (for review, see Nicoll, 1985) and that this pathway plays a critical role in memory functions (for review, see Blokland, 1996). Furthermore, sensory stimuli such as visual, auditory, and tactile ones can increase the release of ACh in the hippocampus in freely-behaving rats (Inglis and Fibiger, 1995), and these stimuli produce arousal and attention, which play a critical role in memory formation. It has also been reported that ACh release in the hippocampus correlates with spatial learning performance in freely-moving rats (Fadda et al., 2000). Thus, the physiological modulation of LTP by ACh discovered in this study is likely to underlie the facilitation of memory formation in the hippocampus by the cholinergic activity, and the M1 mAChR is a key player in this process. Identification of the responsible mAChR subtype for the enhancement of synaptic plasticity should facilitate the development of new drugs improving memory disorders.
Our previous studies have shown that hippocampal LTP is closely associated with the ability of hippocampus-dependent learning (Kiyama et al., 1998; Manabe et al., 1998). As for M1 mutant mice, there are a few papers reporting different results. In one report, M1 mutant mice show an increased locomotor activity as well as elevated dopaminergic transmission in the striatum (Gerber et al., 2001). Another study also reports an increased locomotor activity but almost intact hippocampus-dependent learning in M1 mutant mice (Miyakawa et al., 2001). Recently, another group has found that LTP induced by 100 Hz stimulation is intact in M1 mutant mice, but they exhibit impairment in nonmatching-to-sample working memory, suggesting that the M1 mAChR is involved in memory formation during which the cortex and hippocampus interact (Anagnostaras et al., 2003). These apparently inconsistent results on behavioral analyses were obtained in rather complicated tasks (Miyakawa et al., 2001; Anagnostaras et al., 2003). A variety of behavioral phenotypes may result from variable degrees of contribution of ACh in complicated tasks used in those studies.
Higher concentrations of cholinergic agonists have been used in previous studies to examine the effects of mAChR activation on synaptic transmission (Huerta and Lisman, 1993; Auerbach and Segal, 1996; Shimoshige et al., 1997). We also observed in the present study that a high concentration of CCh (5 μm) causes large depression of excitatory synaptic transmission in the hippocampus, which is partially but significantly reduced in M1 and M1/M3 mutant mice (Fig. 4). Although we do not know which subtypes of mAChRs are responsible for the residual depression in M1 and M1/M3 mutant mice at present, it might be caused by presynaptic mechanisms through M2 and/or M4 mAChRs, as shown in our previous paper (Fukudome et al., 2004) reporting the involvement of M2 mAChRs in the inhibition at the hippocampal inhibitory synapse. However, because CCh is resistant to hydrolysis by cholinesterase enzymes and ACh is far more susceptible to the enzymes, it is unclear whether this possible presynaptic inhibition occurs physiologically in the hippocampus. Additional studies are necessary to clarify the mechanism of synaptic depression caused by the mAChR activation.
One of the critical findings in this study is that LTP enhancement is induced by physiological activation of mAChRs. The hippocampus is heavily innervated by cholinergic inputs originating from neurons in the septal nucleus, which have been reported to fire at ∼50 Hz or less frequently in freely-moving animals (Petsche et al., 1962), suggesting that the stimulus frequency (40 Hz) used in this study is similar to that observed in the intact septum of living animals. Furthermore, in slice preparations, stimulation of cholinergic fibers in the stratum oriens at 20-50 Hz for 0.5 s has been shown to be optimal for evoking slow EPSPs (Cole and Nicoll, 1984). Thus, the phenomenon discovered in this research is likely to occur in living animals, and activation of this process may improve memory impairment observed in some mental disorders.
A previous study has reported that the threshold of LTP induction in the CA1 region of hippocampal slices is lowered by a relatively low concentration (100 nm) of CCh (Auerbach and Segal, 1994), which seems to be mediated by mAChRs. Although the molecular mechanism is unclear, this phenomenon may also be mediated by M1 mAChRs. However, in in vivo experiments, there are contradictory reports concerning the involvement of mAChRs in the modulation of LTP in the hippocampal CA1 region. One study has shown that the threshold for LTP induction is reduced by the preconditioning medial septum tetanus (Ovsepian et al., 2004), although another study has reported that LTP is suppressed by high-frequency stimulation of the medial septum (Newlon et al., 1991). In addition to the reports concerning the role of M1 mAChRs in LTP modulation, the involvement of M2 mAChRs has also been reported. Shimoshige et al. (1997) have reported the involvement of M2 mAChRs in the enhancement of LTP in the CA1 region of guinea pig hippocampal slices in pharmacological experiments in vitro. Using knock-out mice, Seeger et al. (2004) have shown that LTP in the CA1 region of hippocampal slices is suppressed in M2 mutant mice, which is restored by a GABAA receptor antagonist. Thus, in some conditions, M2 mAChRs are associated with the modulation of LTP induction through GABAergic inhibitory synaptic transmission. Although we cannot exclude the involvement of M2 mAChRs in the modulation of LTP in our present study, the contribution of inhibitory interneurons to the modulation may not be sufficiently large in our conditions. Therefore, it still remains to be elucidated more precisely how mAChR activation regulates the mechanism of LTP induction.
In the present study, using hippocampal slice preparations, we have demonstrated that endogenous ACh released from cholinergic terminals can enhance synaptic plasticity and that this enhancement can be induced even ∼30 s after the stratum oriens stimulation. Although we have not performed systematic analyses on the time course of LTP enhancement by endogenous ACh, we have selected the time lag of 30 s, based on the results shown in Figure 2, and, in fact, we can observe significant enhancement of LTP with this time lag. These results suggest that the release of ACh from cholinergic fibers in the hippocampus before LTP-inducing conditioning is critical for the modulation of LTP and presumably for better memory formation.
Footnotes
This research was supported by Grants-in-Aid for Scientific Research (T.M.), by Center for Brain Medical Science, 21st Century Center of Excellence Program (T.S. and T.M.), by Special Coordination Funds for Promoting Science and Technology (T.M.), by a Grant-in-Aid for Scientific Research on Priority Areas 16067101 (M.M.) from the Ministry of Education, Science, Sports, Culture and Technology of Japan, by Uehara Memorial Foundation (T.M.), by Terumo Life Science Foundation (T.M.), by Sumitomo Foundation (T.M.), by Naito Foundation (T.M.), by Takeda Foundation (T.M.), and by an Industrial Technology Research Grant Programme from the New Energy and Industrial Technology Development Organization of Japan (M.M). T.S. was a recipient of Research Fellowship of the Japan Society for the Promotion of Science for Young Scientists. We thank Yuji Kiyama and Shinji Kusakawa for their participation in preliminary experiments, Itone Nishizaki for her technical assistance, and Ayako M. Watabe for her helpful comments on this manuscript.
Correspondence should be addressed to Dr. Toshiya Manabe, Division of Neuronal Network, Department of Basic Medical Sciences, Institute of Medical Science, University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo, 108-8639, Japan. E-mail: tmanabe-tky@umin.ac.jp.
Copyright © 2005 Society for Neuroscience 0270-6474/05/2511194-07$15.00/0
References
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6: 51-58. [DOI] [PubMed] [Google Scholar]
- Auerbach JM, Segal M (1994) A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol 72: 2034-2040. [DOI] [PubMed] [Google Scholar]
- Auerbach JM, Segal M (1996) Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J Physiol (Lond) 492: 479-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A (1996) Acetylcholine: a neurotransmitter for learning and memory? Brain Res Rev 21: 285-300. [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJM (1998) Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50: 279-290. [PubMed] [Google Scholar]
- Cole AE, Nicoll RA (1984) Characterization of a slow cholinergic postsynaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol (Lond) 352: 173-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadda F, Cocco S, Stancampiano R (2000) Hippocampal acetylcholine release correlates with spatial learning performance in freely moving rats. NeuroReport 11: 2265-2269. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O (1998) Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature 394: 186-189. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Yamada M, Duttaroy A, Gan JW, Deng CX, MacBain CJ, Wess J (2002) Muscarinic induction of hippocampal gamma oscillations requires coupling of the M1 receptor to two mixed cation currents. Neuron 33: 615-624. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Taketo MM, Watanabe M, Manabe T, Kano M (2004) Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signaling. Eur J Neurosci 19: 2682-2692. [DOI] [PubMed] [Google Scholar]
- Gerber DJ, Sotnikova TD, Gainetdinov RR, Huang SY, Caron MG, Tonegawa S (2001) Hyperactivity, elevated dopaminergic transmission, and response to amphetamine in M1 muscarinic acetylcholine receptor-deficient mice. Proc Natl Acad Sci USA 98: 15312-15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE (1993) Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature 364: 723-725. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Fibiger HC (1995) Increases in hippocampal and frontal cortical acetylcholine release associated with presentation of sensory stimuli. Neuroscience 66: 81-86. [DOI] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M (1998) Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor ϵ1 subunit. J Neurosci 18: 6704-6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ (1995) Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15: 4077-4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84: 87-136. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103-126. [DOI] [PubMed] [Google Scholar]
- Manabe T, Noda Y, Mamiya T, Katagiri H, Houtani T, Nishi M, Noda T, Takahashi T, Sugimoto T, Nabeshima T, Takeshima H (1998) Facilitation of long-term potentiation and memory in mice lacking nociceptin receptors. Nature 394: 577-581. [DOI] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM (2000) Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA 97: 9579-9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER (1998) Cognitive neuroscience and the study of memory. Neuron 20: 445-468. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 21: 5239-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlon PG, Goldberg SJ, Hayes RL (1991) High-frequency septal stimulation suppresses long-term potentiation (LTP) in the CA1 region of rat hippocampus. Brain Res 544: 320-324. [DOI] [PubMed] [Google Scholar]
- Nicoll RA (1985) The septo-hippocampal projection: a model cholinergic pathway. Trends Neurosci 8: 533-536. [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA (1990) Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev 70: 513-565. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M (2003) Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci 18: 109-116. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Anwyl R, Rowan MJ (2004) Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: in vivo study. Eur J Neurosci 20: 1267-1275. [DOI] [PubMed] [Google Scholar]
- Petsche H, Stumpf C, Gogolak G (1962) The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol 14: 202-211. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Puertas R, Pascual J, Vilaró T, Pazos Á (1997) Autoradiographic distribution of M1, M2, M3, and M4 muscarinic receptor subtypes in Alzheimer's disease. Synapse 26: 341-350. [DOI] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J (2004) M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24: 10117-10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoshige Y, Maeda T, Kaneko S, Akaike A, Satoh M (1997) Involvement of M2 receptor in an enhancement of long-term potentiation by carbachol in Schaffer collateral-CA1 synapses of hippocampal slices. Neurosci Res 27: 175-180. [DOI] [PubMed] [Google Scholar]
- Shimuta M, Yoshikawa M, Fukaya M, Watanabe M, Takeshima H, Manabe T (2001) Postsynaptic modulation of AMPA receptor-mediated synaptic responses and LTP by the type 3 ryanodine receptor. Mol Cell Neurosci 17: 921-930. [DOI] [PubMed] [Google Scholar]
- Tayebati SK, Amenta F, El-Assouad D, Zaccheo D (2002) Muscarinic cholinergic receptor subtypes in the hippocampus of aged rats. Mech Ageing Dev 123: 521-528. [DOI] [PubMed] [Google Scholar]
- Williams S, Johnston D (1988) Muscarinic depression of long-term potentiation in CA3 hippocampal neurons. Science 242: 84-87. [DOI] [PubMed] [Google Scholar]
- Winson J (1978) Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science 201: 160-163. [DOI] [PubMed] [Google Scholar]