Abstract
Nuclei isolated from green fluorescent protein-marked neurons in the cerebral cortex of juvenile mice (14–21 d after birth) were injected into enucleated oocytes that were allowed to develop into blastocysts. Embryonic stem (ES) cell lines were established from the inner cell mass of 76 cloned blastocysts after injecting 2026 neuronal nuclei. Some ES cells were injected individually into enucleated oocytes (nuclear transfer). Other ES cells were transferred into the blastocoeles of tetraploid blastocysts (tetraploid complementation). Two-cell embryos after nuclear transfer were transferred to the oviducts of surrogate mothers. Four (1.5%) of 272 nuclear-transferred two-cell embryos developed to term, and two (0.7%) developed into fertile adults. Nineteen (1.9%) of 992 tetraploid blastocysts receiving ES cells reached term, and 10 (1.0%) developed into adults. These findings demonstrate that some of the nuclei of differentiated neurons in the cerebral cortex of juvenile mice maintain developmental pluripotency.
Keywords: pyramidal neurons, GABAergic neurons, cloning, nuclear transfer, embryonic stem (ES) cells, developmental plasticity
Introduction
Neurons in the adult CNS are terminally differentiated. They never divide during animal's lifetime except for some neurons in the olfactory bulbs and hippocampus (Gage, 2000; Temple, 2001). This property may be associated with irreversible changes in the nuclear organization of the differentiated neurons of the CNS (Akhmanova et al., 2000) such as aneuploidy (Rehen et al., 2001) and DNA rearrangement analogous to that of the immune system (Chun and Schatz, 1999).
Eggan et al. (2004) and Li et al. (2004) recently reported the birth of mice cloned by the tetraploid complementation technique using terminally differentiated olfactory sensory neurons as the nuclear donor. This indicates that the nuclei of olfactory sensory neurons maintain developmental pluripotency. However, the peripheral neurons are distinct from the CNS neurons with regard to their origin and regeneration capacity (Graziadei and Monti Graziadei, 1983). Previous experiments using neurons of the CNS for cloning have met with limited success. Although several cloned adult mice have been obtained using the nuclei of embryonic, differentiated neural cells (Yamazaki et al., 2001), no live offspring have been cloned from the cerebral cortical neural cells of newborn animals by direct nuclear transfer to enucleated oocytes (Makino et al., 2005). To date, no live offspring have been produced from the cerebral cortical neurons of adult mice (Wakayama et al., 1998; Osada et al., 2002). Here, we report the production of fertile offspring from the cerebral cortical neurons of juvenile mice by first converting the neurons into embryonic stem (ES) cells, then injecting their nuclei into the cytoplasm of enucleated oocytes or transferring the ES cells into the blastocoeles of tetraploid embryos.
Materials and Methods
Mice. Vector plasmids carrying the Cre recombinase gene were constructed using an 8.5 kb fragment of pMM279 containing calcium–calmodulin-dependent protein kinase IIα (αCaMKII) promoter sequences (Tsien et al., 1996). Transgenic founder mice were generated by injecting the plasmids into the pronuclei of zygotes of C57BL/6 mice. Of three transgenic mouse lines generated, one (Cam–Cre) demonstrated the efficient expression of Cre protein in a forebrain-specific manner, as reported previously (Tsien et al., 1996). Transgenic mouse lines in which the Cre gene was inserted into the endogenous Nex-1 gene (Nex–Cre) (S. Goebbels, M. Schwab, and K.-A. Nave, unpublished observations) and in which the green fluorescent protein (GFP) gene was inserted into the GAD67 gene in GAD67–GFP knock-in mice (Yanagawa et al., 2001) were obtained from Drs. N. Tamamaki and Y. Yanagawa (Gunma University, Gunma, Japan), respectively. CAG–CAT–enhanced GFP (EGFP) mice with the C57BL/6 background (Kawamoto et al., 2000) were backcrossed to DBA/2 mice. The second and third generations of backcrosses were used in this study. All mice were housed and used for experiments in accordance with standard ethical guidelines for the care and use of laboratory animals [National Institutes of Health Standards for Treatment of Laboratory Animals (1985)], and this study was approved by the Animal Care and Use Committee of Osaka University.
Preparation of donor cells. Donor cells were isolated from the cerebral cortex of Cam/CAG, Nex/CAG, and GAD67–GFP mice, which harbor Cam–Cre and CAG–CAT–EGFP alleles (Cam/CAG), Nex–Cre and CAG–CAT–EGFP alleles (Nex/CAG), and the GAD67–GFP allele, respectively (supplemental data 1, available at www.jneurosci.org as supplemental material). Cerebral cortices of the transgenic mice at 14–21 d after birth were dissociated by vigorous shaking for 20 min in Neurobasal-A medium supplemented with B27 (Invitrogen, San Diego, CA) containing papain (12 mg/ml; Worthington, Lakewood, NJ) at 30°C. After several pipettings, the cell suspension was loaded onto OPTI-prep cell separation reagent (Invitrogen) and spun at 1000 × g for 15 min, according to the manufacturer's instructions. Neuron-rich fractions were collected and resuspended in Neurobasal-A medium supplemented with B27 before sorting.
Cell sorting. Resuspended cells were sorted by Epics Altra (Beckmann Coulter, Miami, FL) according to the manufacturer's instructions (supplemental data 1, available at www.jneurosci.org as supplemental material). Before each run, the accuracy of the sorting was verified with a flow-check fluorosphere (Beckmann Coulter).
Production of cloned offspring. Large cells (∼8–10 μm), cells with neurites, or both were selected as donors from the sorted cells. Apical dendrites were observed in some GFP-expressing cells, as shown in Figure 2 B. All selected cells were found to be EGFP+ and neuronal-specific nuclear protein positive (NeuN+) by immunohistochemical examination [Cam/CAG: 320 NeuN+ cells, 320 EGFP+ cells of 320 cells selected (100%); Nex/CAG: 186 NeuN+ cells, 186 EGFP+ cells of 186 cells selected (100%); GAD67–GFP: 192 NeuN+, 192 EGFP+ cells of 192 cells selected (100%) (supplemental data 1, available at www.jneurosci.org as supplemental material)]. Nuclear injection into enucleated oocytes and in vitro culture of reconstructed oocytes were performed according to Wakayama et al. (1998). ES cells were established as described by Hochedlinger and Jaenisch (2002), with minor modifications. Briefly, blastocysts that developed from the neuronal-nucleus-injected oocytes were grown on mitomycin C-treated embryonic BALB/cA fibroblasts in DMEM supplemented with 17.5% FBS and 1000 U ml–1 leukemia inhibitory factor (LIF). After ∼7 d of culture, the expanded inner cell mass was trypsinized and cultured in DMEM with 17.5% KnockOut Serum Replacement (Invitrogen) and 1000 U ml–1 LIF. After several passages, cell lines with stable expansion were used for further analyses. The generation of mice by tetraploid complementation was performed as described by Nagy et al. (1993).
Figure 2.
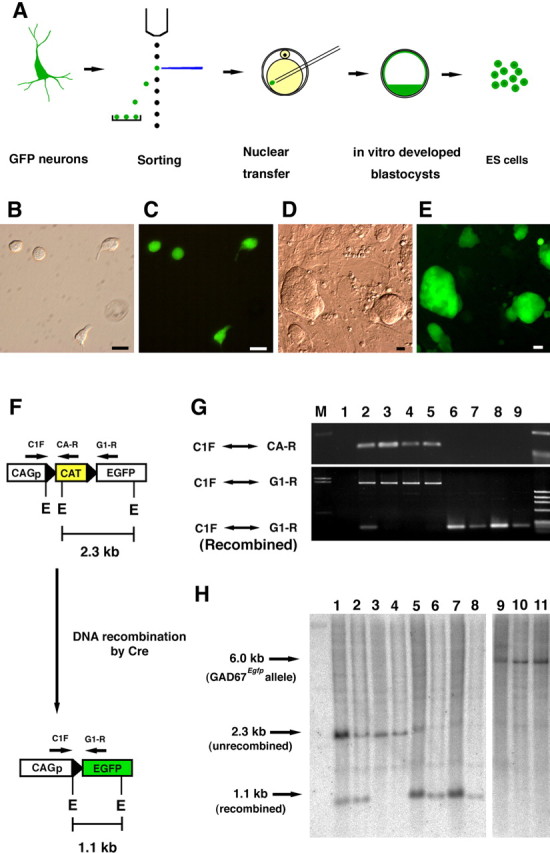
Production of ES cells from GFP-marked neurons by nuclear transfer. A, The procedure for generating ES cells from a blastocyst developed from an oocyte injected with a GFP-expressing neuronal nucleus. B–E, Donor cells from CAM/CAG mice collected by cell sorting (B, C) and ES cells derived from neurons with GFP expression (D, E). Photographs are in bright field (B, D) and fluorescence (C, E). Scale bar, 10 μm. E, Recognition sites of the EcoRI endonuclease. F, Schematic representation of Cre-mediated DNA recombination in the CAG–CAT–EGFP transgene. CAGp, CAG promoter. G, PCR analyses of DNA from BDF 1 wild-type mice (lane 1), the cortex (lane 2), heart (lane 3), liver (lane 4), and tail (lane 5) of Cam/CAG, and from the Cam-1 (lane 6), Cam-2 (lane 7), Nex-1 (lane 8), and Nex-2 (lane 9) transgenic mice. Each DNA sample was amplified using the primer sets designated in F. M, Marker. H, Southernblot analysis of EcoRI-digested DNA of the Cam/CAG mouse (neocortex, lane1;liver, lane 3), the Nex/CAG (neocortex, lane 2; liver, lane 4), Cam-1 (lane 5), Cam-2 (lane 6), Nex-1 (lane 7), Nex-2 (lane 8), GAD67 knock-in mice (tail; lane 9), Gad67-1 (lane 10), and Gad67-2 (lane 11). A0.7 kb cDNA fragment of EGFP was used as the probe. Recombined transgenes (1.1 kb) were detected in lanes 1, 2, and 5–8.
Immunohistochemistry. Histological examination was performed as described previously (Osada et al., 1999). Under anesthesia with avertin, mice were perfused transcardially with ice-cold 4% paraformaldehyde in PBS. Isolated tissues were then postfixed for 2–6 h in the same fixative. After equilibration in 25–30% sucrose in PBS, the tissues were embedded in OTC compound and frozen on dry ice. Sections were cut 5–12 μm thick and mounted on glass slides. Some were cut 30–50 μm thick for the free-floating method. After blocking with 5% normal goat serum or serum of the species used for producing the secondary antibody, the sections were incubated with each of the following antibodies at 4°C overnight: rabbit anti-GFP (1:10–200; Clontech, Cambridge, UK), mouse anti-Cre (1:4000; Chemicon, Temecula, CA), mouse anti-αCaMKII (clone 3E11; a gift from Dr. T. Yamauchi, Ehime University, Matsuyama, Japan), mouse anti-NeuN (immunohistochemistry, 1:400; immunocytochemistry,1:100; Chemicon), mouse anti-GAD67 (1: 400; Chemicon), mouse anti-CNP (1:500; Chemicon), mouse anti-GFAP (1:200; Sigma, St. Louis, MO), mouse anti-microtubule-associated protein 2 (anti-MAP-2; 1:100; Sigma), rabbit anti-neuron-specific class-IIIβ-tubulin [1:400; a gift from Dr. Y. Arimatsu, Mitsubishi Kagaku Institute of Life Sciences (Takiguchi-Hayashi et al., 1998)], and rabbit anti-Spot35/Calbindin-D28K [a gift from Dr. T. Yamakuni, Tohoku University, Sendai, Japan (Yamakuni et al., 1984)]. Immunoreactivity was visualized with cyanine 3 (Cy3)-conjugated anti-rabbit IgG, Cy3-conjugated anti-mouse IgG, or biotinylated anti-mouse IgG donkey antibody (Jackson ImmunoResearch, West Grove, PA) with Alexa594-conjugated streptavidin (Molecular Probes, Eugene, OR). Nuclei were stained with TO-PRO-3 iodide (Molecular Probes). Immunofluorescence was examined using a confocal laser-scanning microscope (LSM Pascal; Zeiss, Oberkochen, Germany). Klüver–Barrera staining and the detection of acetylcholinesterase activity were conducted by conventional methods using paraffin-embedded sections.
Southern blotting and PCR typing. The EcoRI–NotI fragment containing EGFP cDNA from pEGP-N1 (Clontech) was used as a probe for Southern blotting (Kawamoto et al., 2000). The PCR analyses were conducted according to the manufacturer's instructions (TaKaRa, Tokyo, Japan). The primer sets specific for the sequences of the CAG promoter, CAT gene, and EGFP gene were used as described previously (Kawamoto et al., 2000). The simple sequence length polymorphism primer sets for PCR typing were prepared as described by Dietrich et al. (1992).
Results
Visualization of differentiated cortical neurons by fluorescence marking
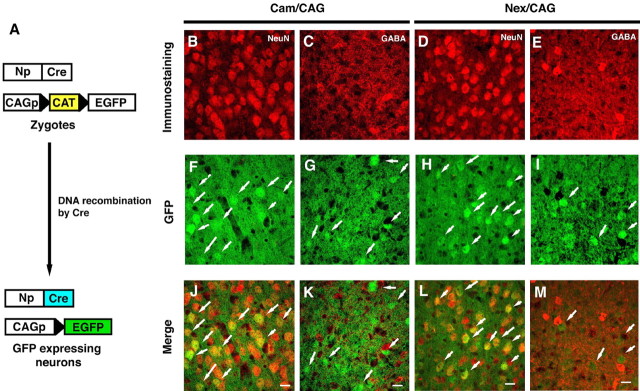
To track neocortical neurons, we used the Cre/loxP-site-specific recombination system in vivo (Fig. 1A) (Hoess et al., 1982). In Cam/CAG mice harboring the Cam–Cre allele and CAG–CAT–EGFP allele, the majority of the EGFP-expressing cells were pyramidal neurons (Fig. 1B–L). Immunohistochemistry revealed that the GFP-expressing cells were predominantly positive for NeuN and αCaMKII and not for GAD67, GFAP, or CNPase (data not shown). The cell counts showed that almost all the EGFP+ cells were positive for NeuN immunoreactivity [535 NeuN+ EGFP+ of 552 EGFP+ (96.9%)]. After cell sorting (supplemental data 1, available at www.jneurosci.org as supplemental material), the vast majority of cells in the fractions enriched for living, relatively large, and EGFP-positive cells were found to be neuronal [1562 NeuN+ cells of 1575 sorted cells (99.2%)].
Figure 1.
Characteristics of the genetically marked cells in the cerebral cortex of Cam/CAG and Nex/CAG transgenic mice. A, Schematic representation of Cre-mediated recombination in the Cam/CAG and Nex/CAG transgenic mice. Cre expression driven by the neuronal gene promoter (Np) excises the CAT/stop cassette flanked by the loxP sequences. CAGp, CAG promoter. B–M, Confocal microscopic analyses of GFP-expressing cells in the neocortex of the genetically manipulated Cam/CAG (B, C, F, G, J, K) and Nex/CAG (D, E, H, I, L, M) transgenic mice. Red, NeuN immunoreactivity (B, D) and GABA immunoreactivity (C, E); green, GFP fluorescence (F–I). J–M, Merged images. Scale bars, 10 μm.
For additional experiments and to confirm the accuracy of this experimental strategy, we used another transgenic mouse line with neuronal EGFP expression driven by the endogenous promoter of the Nex-1 gene (Bartholoma and Nave, 1994). Cell counts from tissue sections and cell sorting revealed that almost all of the EGFP-expressing cells from the Nex/CAG mice were pyramidal neurons [648 NeuN+ cells of 650 EGFP+ cells (99.7%) in tissue sections; 309 NeuN+ cells of 310 EGFP+ cells (99.7%) after cell sorting]. GABAergic neurons were isolated from GAD67 GFP knock-in mice (supplemental data 1, available at www.jneurosci.org as supplemental material) by the strategy described above (Fig. 2A). Cells isolated based on their GFP expression (supplemental data, available at www.jneurosci. org as supplemental material) were identified as GABAergic neurons [1052 NeuN+ cells of 1057 EGFP+ cells (99.5%); 112 GAD67+ cells of 112 EGFP+ cells (100%)].
Generation of ES cell lines from the nuclei of cerebral cortical neurons by nuclear transfer
We generated ES cell lines from the blastocysts that developed from enucleated oocytes receiving the nuclei of GFP-marked (some were genetically marked) neurons (Fig. 2A). A minority (10–15%) of the reconstructed oocytes that received the nuclei of pyramidal or GABAergic neurons developed into blastocysts in vitro (Table 1). Six ES cell lines were established from 76 blastocysts derived from genetically marked neuronal nuclei (Table 1). Of the six ES cell lines, four (Cam-1, Cam-2, Nex-1, and Nex-2) were marked permanently by EGFP driven by the CAG promoter (Fig. 2D,E). Cre-mediated recombination was confirmed in the ES cells derived from EGFP-expressing donor neurons by PCR and Southern blot analyses (Fig. 2F–H). Genotyping by PCR showed the excision of the loxP-flanked CAT gene in the established ES cells (Fig. 2G). Southern blot analyses confirmed the presence of recombined alleles in all four ES cell lines derived from donor neurons in which the Cre-mediated DNA recombination had occurred (Fig. 2H). PCR typing showed the differential distribution of chromosomal segments of C57BL/6 and DBA/2 inbred strains in the ES cells, because CAG–CAT–EGFP mice were backcrossed to DBA/2 mice before their use in this study (data not shown).
Table 1.
Production of ES cell lines from cerebral cortical neurons by nuclear transfer
|
|
Enucleated oocytes |
Embryos developed to |
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Neuronal marker used |
Injected with cerebral neuronal nuclei |
Survived |
With pseudo pronuclei (%)a
|
Two cell (%)b
|
Blastocysts (%)b
|
ES cell lines (%) establishedb
|
|||
| αCaMKII | 587 | 275 | 162 (28) | 117 (72) | 25 (15) | 2 (1) | |||
| Nex | 769 | 419 | 263 (34) | 153 (58) | 31 (12) | 2 (1) | |||
| GAD67 | 670 | 373 | 210 (31) | 133 (63) | 20 (10) | 2 (1) | |||
| Total |
2026 |
1067 |
635 (31) |
403 (63) |
76 (12) |
6 (1) |
|||
The percentage of injected oocytes, determined 5 h after Sr2+ activation.
The percentage of oocytes with pseudo pronuclei.
Generation of cloned mice using ES cells derived from nuclear transferred oocytes
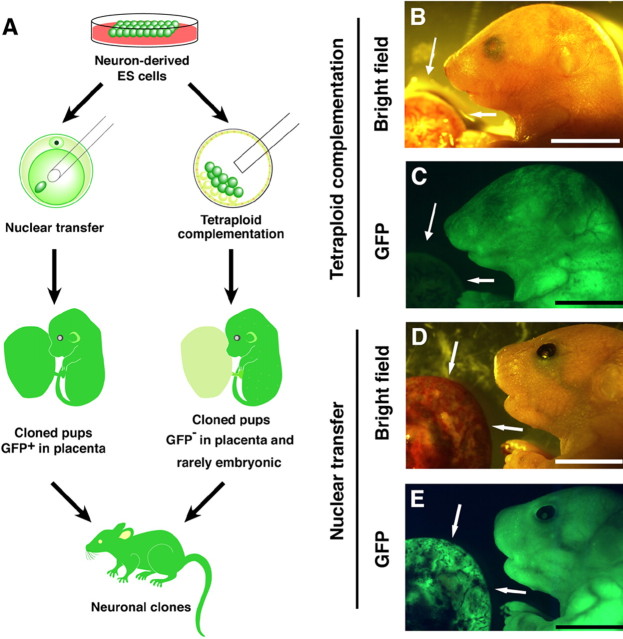
To generate mice from neuron-derived ES cells, we first transferred these ES cells into the blastocoeles of tetraploid blastocysts, a process called tetraploid complementation (Fig. 3A). Using these ES cell lines (Cam-1, Cam-2, and GAD67-2), we obtained 19 pups (Table 2). All the embryos from the Cam-1 and Cam-2 ES cell lines expressed EGFP fluorescence in their bodies but not in their placentas (Fig. 3B,C). Embryos from the GAD67-2 ES cells expressed EGFP according to the endogenous Gad67 gene expression pattern (Tamamaki et al., 2003). Of the 19 live-born pups we obtained, 10 grew into adults (Table 2). We next injected the nuclei of the neuron-derived ES cells into enucleated oocytes. A total of four live-born pups were obtained, two from Cam-2 ES cells and one each from the Nex-1 and GAD67-2 ES cells (Table 2). The Cam-2 and Nex-1 ES cell-derived embryos showed EGFP fluorescence in both the placenta and embryonic body (Fig. 3D,E). Of the four pups, one from the Cam-2 ES cells (Cremarked) and one from the GAD67-2 ES cells (GFP-marked without Cre) reached adulthood.
Figure 3.
Mice cloned from neuronal nuclei-derived ES cells. A, Scheme of the production of cloned fetuses and adults. B, C, Pups derived from Cam-1 ES cells by tetraploid complementation. D, E, Pups derived from the Cam-2 ES cells by nuclear transfer. Note that the embryo generated by tetraploid complementation expressed GFP fluorescence in embryonic tissues but not in the placenta (arrows). In contrast, the embryo generated by nuclear transfer showed GFP fluorescence in both the embryo and placenta (arrows). B, D, Bright field; C, E, UV illumination. Scale bar, 1 cm.
Table 2.
Production of mouse clones by tetraploid complementation (TC) and nuclear transfer (NT)
|
Age of cell donor (day after birth) |
Identification of ES cells |
Cloning methods |
4n blastocysts receiving ES cells |
Enucleated oocytes |
Embryos transferred to |
Number of mice live born |
Number of mice developed to adults |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injected with ES cell nuclei |
Survived (%) |
With pseudo pronuclei (%) |
Uterus |
Oviducta
|
TC (%)b
|
NT (%)c
|
TC (%) |
NT(%) |
|||||||||
| 14 | Cam-1 | TC | 295 | 295 | 3 (1.0) | 0 (0) | |||||||||||
| 15 | Cam-2 | 194 | 194 | 10 (5.2) | 7 (3.6) | ||||||||||||
| 21 | Nex-1 | 345 | 345 | 0 (0) | 0 (0) | ||||||||||||
| 15 | GAD67-2 | 158 | 158 | 6 (3.8) | 3 (1.0) | ||||||||||||
| 14 | Cam-1 | NT | 707 | 438 (62) | 282 | 80 | 0 (0) | 0 (0) | |||||||||
| 15 | Cam-2 | 565 | 317 (56) | 247 | 77 | 2 (2.6) | 1 (1.3) | ||||||||||
| 21 | Nex-1 | 641 | 377 (59) | 289 | 83 | 1 (1.2) | 0 (0) | ||||||||||
| 15 | GAD67-2 | 266 | 169 (64) | 84 | 32 | 1 (3.2) | 1 (3.2) | ||||||||||
| Total |
992 |
2179 |
1301 (60) |
902 (41.4) |
992 |
272 |
19 (1.9) |
4 (1.5) |
10 (1.0) |
2 (0.7) |
|||||||
All embryos that reached two-cell stage were transferred into oviducts of surrogate mothers.
The percentage of chimeric blastocysts transferred to the uterus.
The percentage of two-cell embryos transferred to the oviducts.
Phenotypes of mice cloned from neuronal nuclei
Of the pups generated from neuron-derived ES cells, six had open eyelids at birth (one from the Cam-1 ES cell line, one from Cam-2, and three from GAD67-2 by tetraploid complementation and one from Cam-2 by nuclear transfer) (Fig. 3D). This unusual feature of the newborn pups was not transmitted to the next generation, when the cloned mice born with open eyelids matured and were naturally mated. No other unusual or abnormal phenotypes were noted in the animals cloned from cerebral neurons. Southern blotting analyses confirmed that the cloned mice possessed recombinant alleles identical with the genotypes of the donor ES cells (data not shown). Gross morphology of the brain was normal in all pups examined on the day of birth. Pups cloned from Cam-2 ES cells by tetraploid complementation showed GFP fluorescence in almost all their tissues, including the brain (Fig. 4B). Histological examination of the brain revealed no obvious defects in the laminar structure of the cerebral cortex in the cloned pups at birth. Immunohistochemical examinations showed that the neurons positive for the NeuN and GAD67 neuronal markers were normally distributed among the cerebral cortical neurons of mice cloned with the nuclei of Cam-2 and GAD67-2 ES cells (Fig. 4C–H). The brains of three adult clones (two mice cloned from pyramidal neuronal nuclei and one from a GABAergic neuronal nucleus) were processed with Nissl with Luxol fast blue (Fig. 5A–C) and acetylcholinesterase (Fig. 5D–F) staining. No obvious defects in the cytoarchitecture or cholinergic neural circuits were noted, although a slightly decreased acetylcholinesterase activity and weaker myelin staining were noted in one of the mice cloned from GAD67-2 ES cells by tetraploid complementation. Immunostaining using antibodies against MAP-2 (Fig. 5G–L), GAD67 (Fig. 5M–R), and βIII-tubulin (data not shown) to assess the dendritic formation, GABAergic neuronal distribution, and axonal projections revealed no obvious major abnormalities in the brains of the cloned mice.
Figure 4.
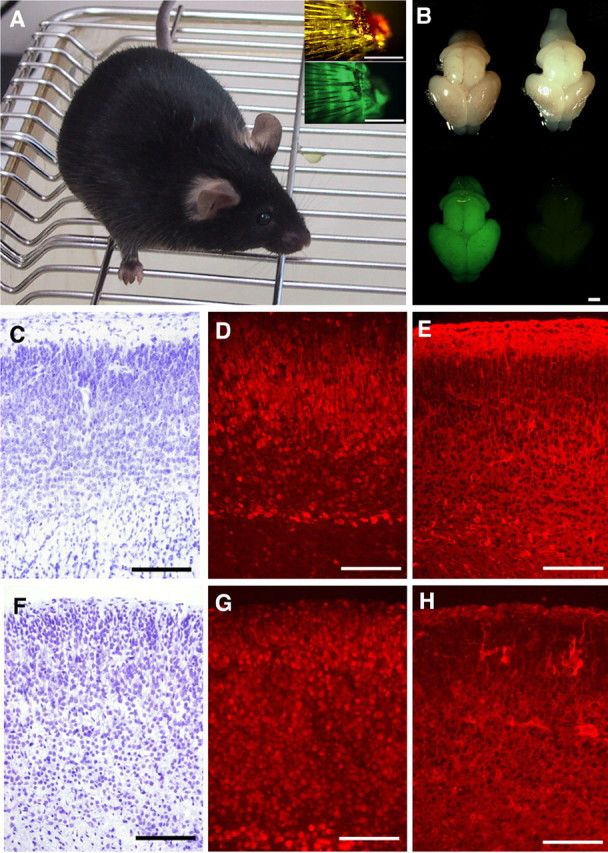
A mouse cloned from a Cam-2 ES cell, at 6 weeks of age. A, Insets show images of the tail tips photographed using bright-field (top) and fluorescence (bottom) microscopy. B, Gross morphology (top) and GFP-fluorescence (bottom) of the brains of pups on the day of birth. The brains of a pup cloned from Cam-2 ES cells by tetraploid complementation (left) and that of an age-matched normal C57BL/6 mouse (right) are shown. C–H, Sections of the cerebral cortex of a mouse clone from Cam-2ES cells (C–E) and Gad 67-2ES cells (F–H). Nisslstaining (C, F) and indirect immunofluorescence using an antibody to NeuN (D, G) and anti-GAD67 (E, H). No obvious cytoarchitectural deformities were detected. Scale bars: A, B, 1 mm; C–H, 50 μm.
Figure 5.
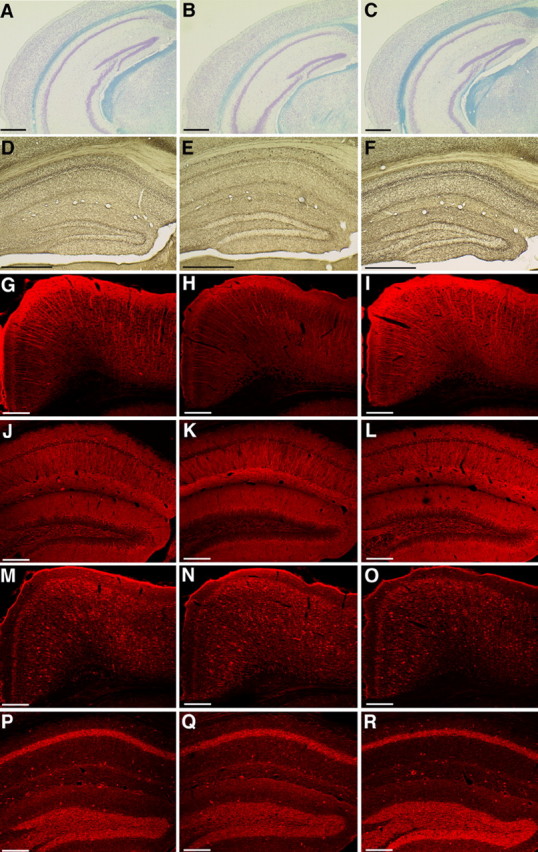
Sections of the cerebral hemisphere of adult mice cloned from the nucleus of a pyramidal neuron (A, D, G, J, M, P) and a GABAergic neuron (B, E, H, K, N, Q) and of an age-matched normal C57BL/6 mouse (C, F, I, L, O, R). A–C, Comparison of the laminar formation and myelination by Klüver–Barrera staining. D–F, Cholinergic neurons with acetylcholinesterase activity are seen to form neural circuits. Immunohistochemistry of integrated dendritic maturation and axonal formation of the cerebral cortex (G–I, M–O) and hippocampus (J–L, P–R) using antibodies against MAP-2 (G–L) and Gad67 (M–R). Scale bar, A–F, 500μm; G–R, 200 μm.
Discussion
The successful cloning of various species of animals has been achieved using a variety of somatic cells from embryonic, fetal, and adult individuals (Wilmut et al., 2002). The most common method of cloning thus far is the introduction of the nucleus of a single somatic cell into an enucleated oocyte, either by electrofusion or mechanical injection, followed by stimulation of the oocyte to develop. Developing embryos at either the cleavage stage or morula/blastocyst stage are transferred to the oviduct or uterus of surrogate dams. Although surprisingly high success rates of cloning have been reported from time to time, the overall success rates (the proportion of live offspring developed from nuclear transferred oocytes) are a few percent or less in most cases, regardless of the animal species or cell type used for cloning (Wilmut et al., 2002). In another cloning method, a cloned blastocyst is prepared first, then ES cells are prepared from this blastocyst, and several ES cells are transplanted into the blastocoele of a tetraploid blastocyst. Chimeric blastocysts thus prepared are then transferred into the uterus of surrogate dams. This is called tetraploid complementation. Interestingly, the tetraploid cells in a chimeric embryo disappear one by one, and by the time of birth, all or virtually all of the embryonic cells are of ES cell origin (Nagy et al., 1993). The tetraploid cells contribute only to the extra-embryonic tissues (placenta) in term fetuses. If a normal offspring is born after direct nuclear transfer (the injection of a particular somatic cell into an enucleated oocyte), we can say that the nucleus in question has developmental totipotency. When an offspring is born after tetraploid complementation, we can say only that the nucleus of the cell in question has pluripotency. In this study, we report that we could obtain normal live offspring by tetraploid complementation using the neurons of juvenile mice, indicating that these cells have developmental pluripotency. Because we also obtained two normal offspring from enucleated oocytes that received the nuclei of neuron-derived ES cells (one from a Cre-marked neuron, one from a GFP-marked but not Cre-marked neuron) (Table 2), we can additionally say that some of the neurons we used had developmental totipotency.
The reason we could obtain cloned offspring using neuron-derived ES cells, and not by conventional nuclear transfer, is not clear, but it could be that the nucleus (genome) of donor cells has more chances to be reprogrammed from the differentiated state to the embryonic state while it is in the cytoplasm of preimplantation embryos and ES cells than when the nucleus of interest is transferred directly and only once into the cytoplasm of enucleated oocytes. In tetraploid complementation, several neuron-derived ES cells are placed in the blastocoele of tetraploid blastocysts. Even if reactivation of genes contributing to placental development is defective in these ES cells, functional placenta will develop from the tetraploid cells. This is probably why tetraploid complementation yielded a higher rate of embryo development (1.0%) than did direct nuclear transfer (0.7%) (Table 2). In direct nuclear transfer, the cell nucleus to be injected into an enucleated oocyte must be completely normal (genetically and epigenetically) with respect to the genes involved in the development of both embryonic and extra-embryonic tissue. It is conceivable that the embryonic genes in the donor neurons are more likely to be reactivated while the neuronal nuclei are within multiplying cloned ES cells than when they are in the inner cell-mass cells.
The low efficiency of the development of neuron-derived cloned embryos raises the possibility that the cloned mice are derived from the nuclei of the relatively rare non-neuronal cells. Genetic marking has been used to characterize donor cells by GFP expression and retrospective genotyping (Eggan et al., 2004; Li et al., 2004). However, the marking system used here cannot rule out the possibility that some of the postnatal day 14 (P14) to P21 GFP-expressing cerebral cortical neurons used as donors may not have survived to adulthood, that the Cam–Cre transgenic mice could produce misexpressed Cre protein, that the Nex/CAG or GAD67–GFP knock-in mice could show an unexpected expression of GFP in non-neuronal cells, or that EGFP diffusion into these cells may have resulted in a false-positive cell-count rate. The use of GAD67–GFP knock-in mice does not give conclusive evidence on the origin of ES cells established from this study (Li et al., 2004). However, the characterization of the GFP-expressing cells with several neuronal markers revealed that the experimental strategy in this study allowed a more accurate assessment of the developmental potential of differentiated neurons than has ever been achieved for nuclear transfer analyses.
The variable differentiation potentials of individual neurons may affect the cloning success rate or even select for the few cells that can be reprogrammed. Recent observations revealed the epigenetic regulation of stochastic gene expression among individual neurons, which could help to explain the specification of neuronal circuit formation (Serizawa et al., 2003; Neve et al., 2004; Esumi et al., 2005). Although the molecular mechanisms of the epigenetic reprogramming of somatic cell nuclei is primarily unknown, it is speculated that the variable epigenetic state of the nucleus of each neuron may affect the epigenetic reprogramming machinery, resulting in a distinction between the few neurons that can be reprogrammed and those that cannot.
This study showed the transformation of differentiated neuronal nuclei from juvenile mice into nuclei with developmental pluripotency. It is still unclear whether the nuclei of all the neurons in the CNS are reprogrammable and whether the nuclei of the more advanced neurons in adults can retain developmental totipotency. A better understanding of nuclear reprogramming will shed light on why neuronal cloning shows such low efficiency and may elucidate the biological causes of the restriction. The derivation of ES cells from a single neuronal nucleus will provide a biological tool with which to assess the relative contribution of epigenetic and genetic regulation as a novel approach to understand neuronal differentiation and physiology.
Footnotes
This work was supported by a grant-in-aid from the Ministry of Education, Science, Sports, and Culture of Japan (T.Y.), the Core Research for Evolutional Science and Technology of Japan Science and Technology Agency (T.Y.), and the Center of Excellence funding program of Osaka University (T.O.). We thank Dr. Stefan Moisyadi for critical review and editing of this manuscript; Dr. Jun-ichi Miyazaki for providing the CAG–CAT–EGFP mice; Dr. Yuchio Yanagawa for the GAD67–EGFP mice; Dr. Kou-ichi Jishage, Dr. Hiroshi Suzuki, and Makoto Sambo for the production of the Cam–Cre mice; Dr. Yamauchi for providing the antibody to αCaMKII; and Yoshimi Kawamura, Hiroyuki Katoh, and Harumi Masuda for technical assistance.
Correspondence should be addressed to either of the following: Tomoharu Osada, Department of Regenerative and Developmental Biology, Mitsubishi Kagaku Institute of Life Sciences, 11 Minamiooya, Machida, Tokyo 194-8511, Japan, E-mail: osada@libra.ls.m-kagakia.co.jp; or Takeshi Yagi, KOKRO Biology Group, Department of Integrated Biology, Graduate School of Frontier Biosciences, Osaka University, Suita, Osaka 565-0871, Japan, E-mail: yagi@fbs.osaka-u.ac.jp.
DOI:10.1523/JNEUROSCI.1591-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/258368-07$15.00/0
References
- Akhmanova A, Verkerk T, Langeld A, Grosveld F, Galjart N (2000) Characterization of transcriptionally active and inactive chromatin domain in neurons. J Cell Sci 113: 4463–4474. [DOI] [PubMed] [Google Scholar]
- Bartholoma A, Nave KA (1994) NEX-1: a novel brain-specific helix-loop-helix protein with autoregulation and sustained expression in mature cortical neurons. Mech Dev 48: 217–228. [DOI] [PubMed] [Google Scholar]
- Chun J, Schatz DG (1999) Rearranging views on neurogenesis: neuronal death in the absence of DNA end-joining proteins. Neuron 22: 7–10. [DOI] [PubMed] [Google Scholar]
- Dietrich W, Katz H, Lincoln SE, Shin HS, Friedman J, Dracopoli NC, Lander E (1992) A genetic map of the mouse suitable for typing intraspecific crosses. Genetics 131: 423–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan K, Baldwin K, Tackett M, Osborne J, Gogos J, Chess A, Axel R, Jaenisch R (2004) Mice cloned from olfactory sensory neurons. Nature 428: 44–49. [DOI] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T (2005) Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet 37: 171–176. [DOI] [PubMed] [Google Scholar]
- Gage FH (2000) Mammalian neural stem cells. Science 287: 1433–1438. [DOI] [PubMed] [Google Scholar]
- Graziadei PP, Monti Graziadei AG (1983) Regeneration in the olfactory system of vertebrates. Am J Otolaryngol 4: 228–233. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Jaenisch R (2002) Monoclonal mice generated by nuclear transfer from mature B and T donor cells. Nature 415: 1035–1038. [DOI] [PubMed] [Google Scholar]
- Hoess RH, Ziese M, Sternberg N (1982) P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc Natl Acad Sci USA 79: 3398–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto S, Niwa H, Tashiro F, Sano S, Kondoh G, Takeda J, Tabayashi K, Miyazaki J (2000) A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett 470: 263–268. [DOI] [PubMed] [Google Scholar]
- Li J, Ishii T, Feinstein P, Mombaerts P (2004) Odorant receptor gene choice is reset by nuclear transfer from mouse olfactory sensory neurons. Nature 428: 393–399. [DOI] [PubMed] [Google Scholar]
- Makino H, Yamazaki Y, Hirabayashi T, Kaneko R, Hamada S, Kawamura Y, Osada T, Yanagimachi R, Yagi T (2005) Mouse embryos and chimera cloned from neural cells in the postnatal cerebral cortex. Cloning Stem Cells 7: 45–61. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC (1993) Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA 90: 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve G, Zucker J, Daly M, Chess A (2004) Stochastic yet biased expression of multiple Dscam splice variants by individual cells. Nat Genet 36: 240–246. [DOI] [PubMed] [Google Scholar]
- Osada T, Ikegami S, Hayashi-Takiguchi K, Fukui-Kato Y, Yamazaki Y, Higashinakagawa T, Sakaki Y, Takeuchi T (1999) Increased anxiety and impaired pain response in puromycin-sensitive aminopeptidase gene-deficient mice obtained by a mouse gene-trap method. J Neurosci 19: 6068–6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Kusakabe H, Akutsu H, Yagi T, Yanagimachi R (2002) Adult murine neurons: their chromatin and chromosome changes and failure to support embryonic development as revealed by nuclear transfer. Cytogenet Genome Res 97: 7–12. [DOI] [PubMed] [Google Scholar]
- Rehen SK, McConnell MJ, Kaushal D, Kingsbury MA, Yang AH, Chun J (2001) Chromosomal variation in neurons of the developing and adult mammalian nervous system. Proc Natl Acad Sci USA 98: 13361–13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H (2003) Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science 302: 2088–2094. [DOI] [PubMed] [Google Scholar]
- Takiguchi-Hayashi K, Sato M, Sugo N, Ishida M, Sato K, Uratani Y, Arimatsu Y (1998) Latexin expression in smaller diameter primary sensory neurons in the rat. Brain Res 801: 9–20. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T (2003) Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurobiol 467: 60–79. [DOI] [PubMed] [Google Scholar]
- Temple S (2001) The development of neural stem cells. Nature 414: 112–117. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S (1996) Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87: 1317–1322. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Perry ACF, Zuccotti M, Johnson KR, Yanagimachi R (1998) Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature 394: 369–374. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, Wells DN, Young LE (2002) Somatic cell nuclear transfer. Nature 419: 583–586. [DOI] [PubMed] [Google Scholar]
- Yamakuni T, Usui H, Iwanaga T, Kondo H, Odani S, Takahashi Y (1984) Isolation immunohistochemical localization of a cerebellar protein. Neurosci Lett 45: 235–240. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Makino H, Hamaguchi-Hamada K, Hamada S, Sugino H, Kawase E, Miyata T, Ogawa M, Yanagimachi R, Yagi T (2001) Assessment of the developmental totipotency of neural cells in the cerebral cortex of mouse embryo by nuclear transfer. Proc Natl Acad Sci USA 98: 14022–14026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa Y, Kaneko K, Kanbara N, Totsuka M, Yagi T, Obata K (2001) Development of mouse expressing GFP in GABAergic neurons. Neurosci Res Suppl 25: S77. [Google Scholar]