Abstract
Herbal cannabis, smoked in the form of marihuana or hashish, is the most common illicit drug consumed in the Western world. In the brain, cannabinoids interact with neuronal CB1 receptors, thereby producing a marked reduction of motor activity. Here, we report that the motor depressant effect produced by the cannabinoid receptor agonist (-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]trans-4-(3-hydroxypropyl)cyclohexanol (CP55,940) is attenuated by genetic inactivation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32), which is abundantly expressed in the medium spiny neurons of the striatum. Point mutation of Thr34, the protein kinase A (PKA) phosphorylation site of DARPP-32, produces a similar reduction in the effect of the CB1 agonist. In contrast, point mutation of Thr75, a site on DARPP-32 specifically phosphorylated by cyclin-dependent kinase 5, does not affect the behavioral response to CP55,940. Activation of CB1 receptors, either by an agonist or by inhibition of reuptake of endogenous cannabinoids, stimulates phosphorylation at Thr34, thereby converting DARPP-32 into an inhibitor of protein phosphatase-1. Genetic inactivation either of dopamine D2 receptors or of adenosine A2A receptors reduces the phosphorylation of DARPP-32 at Thr34 and the motor depression produced by CP55,940. Our data indicate that a considerable proportion of the psychomotor effect of cannabinoids can be accounted for by a signaling cascade in striatal projection neurons involving PKA-dependent phosphorylation of DARPP-32, achieved via modulation of dopamine D2 and adenosine A2A transmission.
Keywords: basal ganglia, movement, CB1 receptor, adenosine, D2, knock-out
Introduction
Cannabinoids, such as Δ9-tetrahydrocannabinol, the major psychoactive ingredient of marihuana and hashish, are known to produce a marked depression of motor activity. This effect, which is characterized by rigid immobility, or catalepsy, and decreased locomotion, occurs via activation of neuronal cannabinoid receptors belonging to the CB1 subtype (Ledent et al., 1999).
CB1 receptors are abundantly expressed in the basal ganglia (Herkenham et al., 1990), a group of subcortical structures that regulate motor behavior. In the striatum, the major component of the basal ganglia, CB1 receptors regulate neurotransmission in various manners. For instance, WIN-55,212–2 (R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate), a CB1 receptor agonist, inhibits the release of glutamate from corticostriatal synapses, thereby reducing excitatory postsynaptic currents evoked on medium-sized spiny neurons (Gerdeman and Lovinger, 2001). CB1 receptor agonists are also able to decrease GABA release from recurrent axon collaterals of medium spiny neurons, thereby reducing inhibitory synaptic transmission intrinsic to the striatum (Szabo et al., 1998). The endocannabinoid system has been shown to exert both negative and positive control on striatal dopamine D2 receptor transmission. Activation of dopamine D2 receptors results in increased levels of the endocannabinoid anandamide, which acts as a negative feedback by reducing the motor stimulant effect of quinpirole, a dopamine D2 receptor agonist (Giuffrida et al., 1999; Beltramo et al., 2000). Recently, however, anandamide has been proposed to act as a downstream effector that mediates the inhibition produced by dopamine D2 receptors on GABA transmission (Centonze et al., 2004). These various and contrasting actions of cannabinoids led us to analyze their overall effects on striatal medium spiny neurons in an attempt to clarify specific molecular changes involved in the generation of CB1 receptor-mediated motor depression.
The dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) (Walaas et al., 1983) is highly and specifically expressed in striatal projection neurons (Walaas and Greengard, 1984; Ouimet et al., 1998), in which it acts as a modulator of the cAMP pathway. Phosphorylation catalyzed by protein kinase A (PKA) at Thr34 converts DARPP-32 into an inhibitor of protein phosphatase-1 (Hemmings et al., 1984). This, in turn, prevents dephosphorylation of downstream target proteins, thereby amplifying the effects produced by activation of the cAMP/PKA cascade (Greengard, 2001). DARPP-32 is implicated in the generation of motor responses produced by various classes of drugs that activate or inhibit the cAMP/PKA pathway in striatal medium spiny neurons. Thus, changes in DARPP-32 phosphorylation are involved in the psychomotor effects produced by amphetamine (Fienberg et al., 1998), caffeine (Lindskog et al., 2002), and antipsychotics (Fienberg et al., 1998).
In this study, we examined the effects produced by activation of CB1 receptors on DARPP-32 phosphorylation and the possible involvement of DARPP-32 in the psychomotor effects of a CB1 receptor agonist. Our results indicate that phosphorylation of DARPP-32 on Thr34 is necessary for a full behavioral response to cannabinoids. They also suggest a molecular mechanism accounting for the motor depressant effect of cannabinoids, which involves suppression of dopamine D2 receptor-mediated inhibition of DARPP-32 phosphorylation in striatal projection neurons.
Materials and Methods
Animals. Male C57BL/6 mice (20–30 g) were obtained from M&B (Ry, Denmark). Wild-type and DARPP-32 knock-out mice (Fienberg et al., 1998) were generated from the offspring of DARPP-32+/+ × DARPP-32+/+ and DARPP-32–/– × DARPP-32–/– mating pairs. These mating pairs were obtained from heterozygous mice, which were backcrossed for at least 20 generations on a C57BL/6 background. DARPP-32+/+ × DARPP-32+/+ and DARPP-32–/– × DARPP-32–/– mating was performed separately for no more than two generations. Mice bearing a mutation in which Thr34 or Thr75 were replaced by a nonphosphorylatable Ala (Thr34 → Ala and Thr75 → Ala mutant mice, respectively) (Svenningsson et al., 2003) were obtained from heterozygous animals generated from C57BL/6 × 129SV hybrids bred for one generation on a C57BL/6 background. Dopamine D2 receptor knock-out and wild-type mice (Baik et al., 1995) were generated from heterozygous animals, bred for five generations on a C57BL/6 background. Adenosine A2A receptor knock-out mice and wild-type mice (Ledent et al., 1997) were generated from heterozygous animals and bred for at least 10 generations on a CD1 background. Mice were age matched, and only male offspring were used. All experiments were approved by the Swedish Animal Welfare Agency.
Drugs. (-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]trans-4-(3-hydroxypropyl)cyclohexanol (CP55,940; Tocris Cookson, Avonmouth, UK), N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-pyrazole-3-carboxamide (SR141716A; a gift from Sanofi-Synthelabo, Montpellier, France), N-(4-hydroxyphenyl)-arachidonamide (AM404; a gift from Dr. Daniele Piomelli, University of California at Irvine, Irvine, CA), and (E)-1,3-diethyl-8-(3,4-dimethoxystyryl)-7-methyl-3,7-dihydro-1H-purine-2,6-dione (KW6002) (a gift from Hoffmann-La Roche, Basel, Switzerland) were suspended by sonication in a solution of 8% Tween 80 in saline and injected intraperitoneally.
Measurement of catalepsy. Mice were treated with drug or vehicle (8% Tween 80 in saline), and catalepsy was determined at various intervals after injection. Briefly, the animals were placed on a grid tilted 60° with their snouts pointing upward and were first allowed to habituate for 30 s. At the end of this period, the time during which the mice remained immobile on the grid was measured by an observer blind to mouse genotype and drug treatment. The session was terminated when the animal moved a paw or if it remained immobile for >120 s.
Determination of phosphorylated DARPP-32. Mice were injected intraperitoneally with vehicle or drug and killed by decapitation after various periods of time. The heads of the animals were immediately immersed in liquid nitrogen for 6 s. The brains were then removed, and the striata were rapidly (20 s) dissected out on an ice-cold surface, sonicated in 750 μl of 1% sodium dodecylsulfate, and boiled for 10 min (Svenningsson et al., 2000). Aliquots (3 μl) of the homogenate were used for protein determination. Equal amounts of protein (30 μg; containing equal amounts of DARPP-32) from each sample were loaded onto 10% polyacrylamide gels. The proteins were separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Towbin et al., 1979). PhosphoThr34-DARPP-32 and phosphoThr75-DARPP-32 were detected using a monoclonal (Snyder et al., 1992) and a polyclonal (Bibb et al., 1999) antibody, respectively. A monoclonal antibody generated against DARPP-32, which is not phosphorylation state specific, was used to estimate the total amount of DARPP-32 (Hemmings and Greengard, 1986). Antibody binding was revealed using goat anti-mouse HRP-linked IgG (diluted 1:10,000) and the ECL immunoblotting detection system. Chemiluminescence was detected by autoradiography. Quantification of the phospho-DARPP-32 bands was done by densitometry using NIH Image (version 1.52) software.
Results
Activation of CB1 receptors stimulates phosphorylation of DARPP-32 at Thr34
In a first series of experiments, we examined the ability of CB1 receptors to regulate DARPP-32. The activity of DARPP-32 is controlled by phosphorylation at multiple sites, including two critical threonyl residues located in positions 34 and 75. Phosphorylation of Thr34 is catalyzed by PKA and converts DARPP-32 into an inhibitor of protein phosphatase-1 (Hemmings et al., 1984); in contrast, phosphorylation at Thr75 is catalyzed by cyclin-dependent kinase 5 and converts DARPP-32 into an inhibitor of PKA (Bibb et al., 1999). Systemic administration of CP55,940 increased DARPP-32 phosphorylation at the PKA site, Thr34 (Fig. 1a–c), without producing any consistent modification of Thr75 phosphorylation (data not shown). Half-maximal stimulation of Thr34 phosphorylation was observed at 0.3 mg/kg CP55,940, and maximal stimulation was observed at 0.5 mg/kg (Fig. 1a). The level of phosphoThr34-DARPP-32 reached a peak 1 h after CP55,940 administration and returned to near baseline levels after 2 h (Fig. 1b). The selective CB1 receptor antagonist, SR141716A, prevented the increase in phosphoThr34-DARPP-32 induced by CP55,940, confirming the specific involvement of CB1 receptors in the regulation of DARPP-32 phosphorylation (Fig. 1c). SR141716A did not produce any effect on DARPP-32 phosphorylation when administered alone. This observation is in agreement with previous work showing that SR141716A does not affect motor activity (Rinaldi-Carmona et al., 1994) and supports the idea that, under normal conditions, the level of activation of CB1 receptors is negligible.
Figure 1.
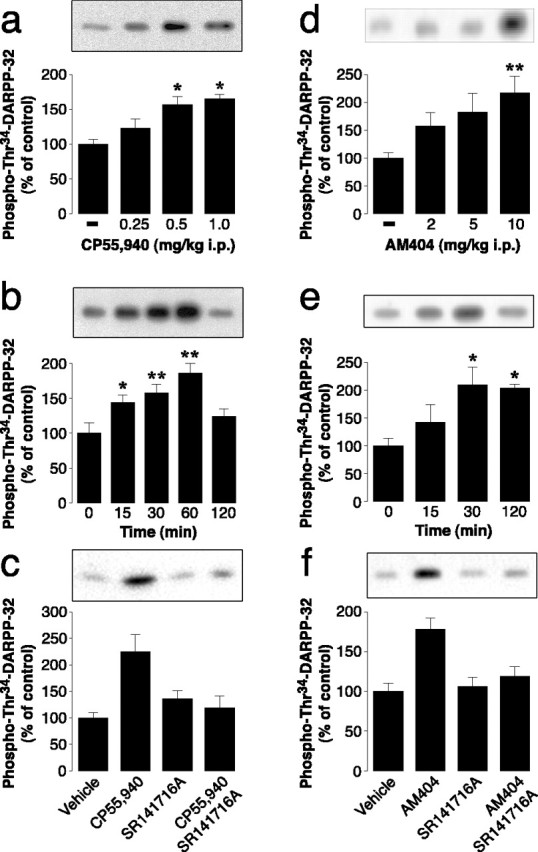
Activation of CB1 receptors or blockade of endogenous cannabinoid reuptake stimulates DARPP-32 phosphorylation. a, d, Mice were treated intraperitoneally with CP55,940 (a) or AM404 (d) and decapitated 15 min (a) or 30 min(d) after injection. b, e, Mice were treated intraperitoneally with 0.5 mg/kg CP55,940 (b) or with 10 mg/kg AM404 (e) and decapitated at various times (15–120 min) after injection. c, f, Mice were treated intraperitoneally with 5.0 mg/kg SR141716A (specific CB1 inhibitor) 15 min before administration of 0.5 mg/kg CP55,940 (c) or 10 mg/kg AM404 (f) and decapitated 45 min later. The striatal levels of phosphoThr34-DARPP-32 were determined as described in Materials and Methods. The top panels show representative gels; the bottom panels show summaries of each group of experiments. The amount of phosphorylated DARPP-32 is expressed as a percentage of that determined after vehicle administration. Data represent means ± SEM (n = 7–12). *p < 0.01 and **p < 0.001 versus vehicle-treated group; one-way ANOVA followed by Dunnett's test. Treatment with SR141716A significantly reduced the effects of CP55,940 (F(1,31) = 10.39; p < 0.01) and AM404 (F(1,33) = 6.62; p < 0.05); two-way ANOVA.
We also examined the ability of endogenous cannabinoids (anandamide and 2-arachidonylglycerol) to regulate DARPP-32 phosphorylation. We found that the effect of CP55,940 on DARPP-32 phosphorylation was mimicked by AM404, an inhibitor of anandamide and 2-arachidonylglycerol reuptake (Beltramo and Piomelli, 2000) (Fig. 1d,e). The AM404-induced increase in phosphoThr34-DARPP-32 was blocked by SR141716A (Fig. 1f). Thus, activation of CB1 receptors, achieved either by administering a synthetic agonist or by elevating the levels of endogenous cannabinoids, resulted in increased phosphorylation of DARPP-32 at Thr34, the PKA phosphorylation site.
PhosphoThr34-DARPP-32 is involved in CB1 receptor-mediated catalepsy
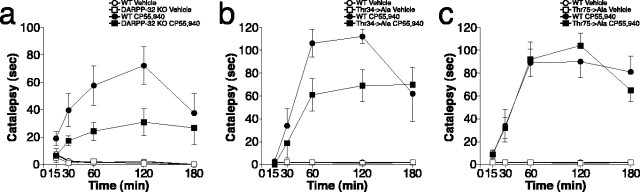
Based on the results from biochemical studies, we investigated whether DARPP-32 is required for the generation of the behavioral response to activation of CB1 receptors. As shown in Figure 2a, CP55,940 (a cannabinoid receptor agonist) induced a cataleptic effect in wild-type mice, which was maximal 2 h after systemic administration. We found that the motor depressant effect of CP55,940 was strongly attenuated in DARPP-32 null mice (Fig. 2a). To evaluate the involvement of PKA-catalyzed phosphorylation of DARPP-32 at Thr34 in the psychomotor effects produced by activation of CB1 receptors, we tested the ability of CP55,940 to elicit catalepsy in Thr34 → Ala mutant mice. The results indicated that, in these animals, CP55,940-induced catalepsy was significantly attenuated (Fig. 2b). Moreover, we found that the cataleptic response produced by the CB1 receptor agonist in Thr75 → Ala mutant mice was indistinguishable from that elicited in wild-type littermates (Fig. 2c).
Figure 2.
Thr34 of DARPP-32 is required for the motor depressant effect of CP55,940. Catalepsy was determined 15, 30, 60, 120, and 180 min after administration of CP55,940 (0.5 mg/kg) to wild-type (WT) mice (a–c), DARPP-32 knock-out mice (a), Thr34 mutant (Thr34 → Ala) mice (b), or Thr75 mutant (Thr75 → Ala) mice (c). Data represent means ± SEM (n = 6–10). The effect of CP55,940 was significantly reduced in DARPP-32 knock-out mice (F(1,29) = 4.40; p < 0.05) and Thr34 → Ala mice (F(1,24) = 4.42; p < 0.05; repeated-measures ANOVA).
Intact dopamine D2 and adenosine A2A receptor transmission are required for full cannabinoid action
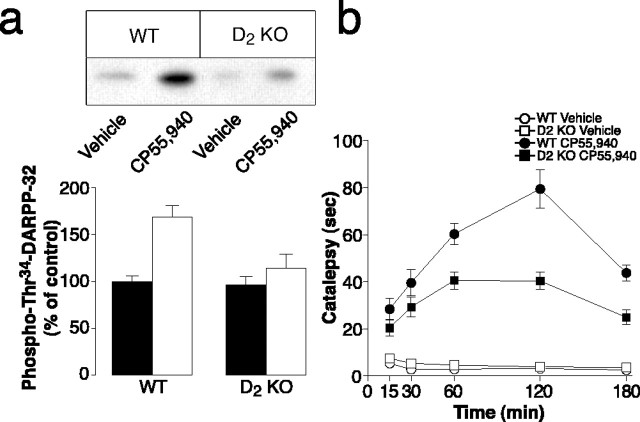
Previous evidence indicates that, in the striatum, blockade of dopamine D2 receptors increases PKA-dependent phosphorylation of DARPP-32 (Svenningsson et al., 2000). In view of the ability of CB1 receptors to antagonize D2 receptor-mediated responses (Giuffrida et al., 1999; Beltramo et al., 2000), we tested the possibility that the stimulation of Thr34 phosphorylation and the motor depression caused by activation of CB1 receptors require intact dopamine D2 receptor transmission. We found that, in D2 receptor-deficient mice, the increase in phosphoThr34-DARPP-32 produced by CP55,940 was markedly reduced (Fig. 3a). Neither genetic inactivation of dopamine D2 receptors nor treatment with CP55,940 modified the immunoreactivity corresponding to total (i.e., phosphorylated plus dephosphorylated) DARPP-32 (data not shown). In agreement with the biochemical data and further supporting the idea of an involvement of phosphorylation of Thr34 of DARPP-32 in the psychomotor effect of cannabinoids, we also found that, in D2 receptor knock-out mice, the cataleptic response to CP55,940 was diminished (Fig. 3b).
Figure 3.
Dopamine D2 receptors are required for the biochemical and behavioral effects of CP55,940. a, Wild-type (WT) or dopamine D2 receptor knock-out (D2 KO) mice were treated intraperitoneally with vehicle (filled bars) or CP55,940 (0.5 mg/kg, i.p.; open bars) and killed 60 min later. The striatal levels of phosphoThr34-DARPP-32 were determined as described in Materials and Methods. The top panel shows representative gels; the bottom panel shows summaries of experiments. The amount of phosphorylated DARPP-32 is expressed as a percentage of that determined in wild-type mice after vehicle administration. Data represent means ± SEM(n=6–8). The effect of CP55,940 was significantly reduced in D2 receptor knock-out mice (F(1,24) = 5.76; p < 0.05); two-way ANOVA. b, Catalepsy was determined 15, 30, 60, 120, and 180 min after administration of CP55,940 (0.5 mg/kg) to wild-type or D2 knock-out mice. Data represent means ± SEM (n = 6). The effect of CP55,940 was significantly reduced in D2 knock-out mice (F(1,20) = 4.44; p < 0.05; repeated-measures ANOVA).
Dopamine D2 receptors are coexpressed with adenosine A2A receptors in a large population of striatal medium spiny neurons. In these cells, the state of phosphorylation of DARPP-32 at Thr34 reflects a balance between activation of adenosine A2A receptors, which stimulate PKA by increasing the production of cAMP, and activation of dopamine D2 receptors, which reduce PKA activity via inhibition of the production of cAMP. Thus, increases in Thr34 phosphorylation produced by blockade of dopamine D2 receptors are dependent on intact adenosine A2A receptor transmission (Svenningsson et al., 2000). Based on these considerations and taking into account the involvement of dopamine D2 receptors in cannabinoid-mediated responses (compare Fig. 3), we examined the possible role of A2A receptors in the effects of CP55,940. We found that the ability of the CB1 receptor agonist to stimulate DARPP-32 phosphorylation at Thr34 was strongly reduced in A2A receptor-null mice (Fig. 4a). Moreover, in the same strain of mice, the cataleptic response to CP55,940 was also attenuated (Fig. 4b). We also examined the effect of pharmacological blockade of adenosine A2A receptors on CP55,940-induced phosphorylation of DARPP-32 and motor depression. We found that administration of the selective A2A receptor antagonist, KW6002 (3 mg/kg), reduced the ability of CP55,940 to increase Thr34 phosphorylation (Fig. 4c). KW6002 was also able to attenuate the catalepsy produced by administration of the CB1 receptor agonist (Fig. 4d), thereby confirming the importance of intact adenosine A2A receptor transmission for a full behavioral action of cannabinoids.
Figure 4.
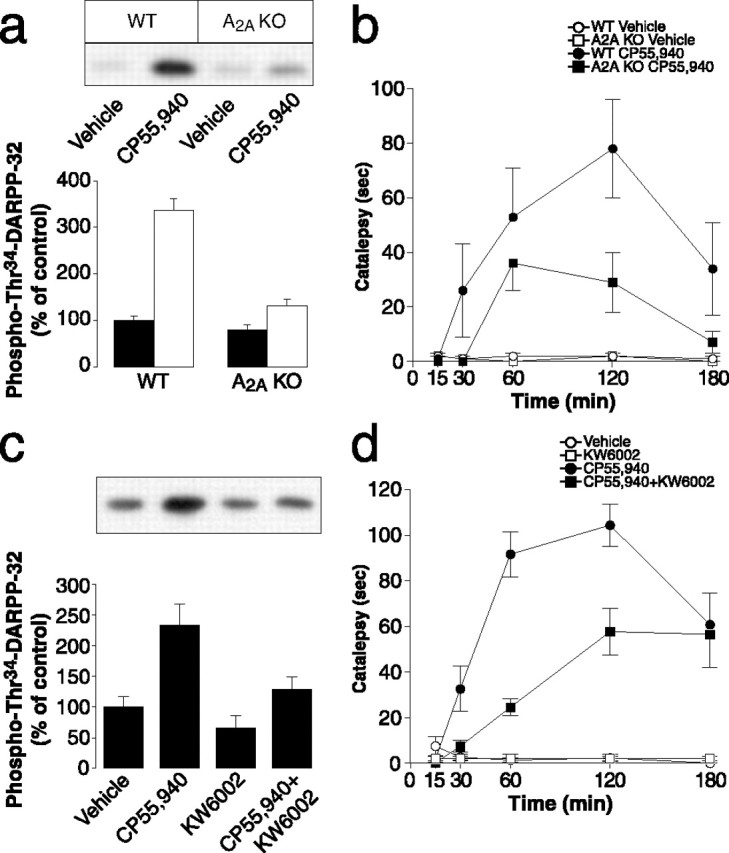
Adenosine A2A receptor activation is required for the biochemical and behavioral effects of CP55,940. a, b, Wild-type (WT; CD1 background) or adenosine A2A receptor knock-out (A2A KO) mice were treated intraperitoneally with vehicle (filled bars) or CP55,940 (0.5 mg/kg, i.p.; open bars). c, d, C57BL/6 mice were treated intraperitoneally with vehicle, CP55,940, KW6002, or a combination of the two drugs (injected at the same time). Mice were killed 60 min after vehicle or drug administration, and the striatal levels of phosphoThr34-DARPP-32 were determined as described in Materials and Methods. The top panels show representative gels; the bottom panels show summaries of experiments. The amount of phosphorylated DARPP-32 is expressed as a percentage of that determined in wild-type mice after vehicle administration. Data represent means ± SEM (n = 8–11). The effect of CP55,940 was significantly reduced in A2A receptor knock-out mice (F(1,36) = 51.45; p < 0.01), and treatment with KW6002 significantly reduced the effect of CP55,940 (F(1,33) = 5.006; p < 0.05; two-way ANOVA). b, d, Catalepsy was determined 15, 30, 60, 120, and 180 min after administration of CP55,940 (0.5 mg/kg) to wild-type or A2A knock-out mice (b) or administration of CP55,940, KW6002, or a combination of the two drugs to C57BL/6 mice (d). Data represent means ± SEM (n = 6–15). The effect of CP55,940 was significantly reduced in A2A knock-out mice (F(1,26) = 4.60; p < 0.05); treatment with KW6002 significantly reduced the effect of CP55,940 (F(1,96) = 9.015; p < 0.05; repeated-measures ANOVA).
Discussion
One finding of this study is that activation of CB1 receptors, achieved by systemic administration of CB1 receptor agonists or by enhancing the levels of endogenous cannabinoids, resulted in increased phosphorylation of DARPP-32 at the PKA site, Thr34, and that this phosphorylation is required for the generation of a full behavioral response to cannabinoids. Another conclusion stemming from this study is that the reduction in motor activity produced by stimulation of CB1 receptors appears to be mediated by activation of the cAMP/PKA pathway in a specific group of striatal projection neurons, which express dopamine D2 and adenosine A2A receptors.
The ability of CP55,940 and AM404 to stimulate DARPP-32 phosphorylation at Thr34 is somewhat unexpected, because CB1 receptors are known to inhibit the activity of adenylyl cyclase and reduce the levels of cAMP via coupling to Gi/o-proteins (Howlett et al., 1986; Bidaut-Russell et al., 1990) (but see Maneuf and Brotchie, 1997). This notion, however, rests on studies performed in isolated systems such as cultured cells, brain membranes, or tissue slices, which do not take into account the effects of systemic activation of CB1 receptors under normal physiological circumstances. In this regard, it should be noted that stimulation of cAMP accumulation has been shown to occur in striatal neurons after coactivation of CB1 and dopamine D2 receptors (Glass and Felder, 1997). The mechanism underlying this phenomenon is not clear, but recent evidence shows that heterodimerization with dopamine D2 receptors is associated with a shift of CB1 receptor signaling from inhibition to stimulation of adenylyl cyclase (Kearn et al., 2005). It is therefore possible that the increase in PKA-catalyzed phosphorylation of DARPP-32 produced by cannabinoids in intact animals is a result of stimulation of postsynaptic CB1 receptors in the presence of tonic activation of dopamine D2 receptors. This explanation is in line with the diminished effect of CP55,940 on DARPP-32 phosphorylation observed in dopamine D2 receptor knock-out mice and is supported by studies reporting the presence of CB1 receptors on the dendrites of striatal medium spiny neurons (Rodríguez et al., 2001).
Several studies have demonstrated that activation of glutamate receptors results in dephosphorylation of DARPP-32 (Svenningsson et al., 2004). Thus, another possible mechanism responsible for the increase in phosphoThr34-DARPP-32 produced by cannabinoid receptor agonists could involve reduction of glutamate release (Gerdeman and Lovinger, 2001) and suppression of DARPP-32 dephosphorylation. Indeed, CB1 receptors are, in large part, expressed presynaptically, and cannabinoids have been proposed to act as inhibitory retrograde messengers at glutamatergic corticostriatal synapses (Gerdeman et al., 2002).
Our data show a comparable attenuation of the ability of CP55,940 to induce catalepsy in DARPP-32 knock-out mice and Thr34 → Ala DARPP-32 mutant mice. However, the data presented in Figure 2 also show a different sensitivity to the CB1 receptor agonist among the groups of wild-type animals used in the three sets of experiments. Thus, the catalepsy induced by CP55,940 in the wild-type group used to evaluate the response of DARPP-32 knock-out mice (compare Fig. 2a) is lower than that observed in the wild-type groups used to determine the effects of mutation of Thr34 or Thr75 (compare Fig. 2b,c). This difference is most likely attributable to the genetic background of the strains of mice used in these studies. DARPP-32 knock-out and corresponding wild-type mice were backcrossed for several generations on a C57BL/6 background. In contrast, Thr34 → Ala DARPP-32 mutant mice, Thr75 → Ala DARPP-32 mutant mice, and wild-type littermates were generated from mating pairs bred only for one generation on a C57BL/6 background.
In this study, we propose that phosphorylation of DARPP-32 at Thr34 is involved in the motor depression produced by activation of CB1 receptors. Phosphorylation of Thr34, however, has been implicated previously in the hyperlocomotor effect produced by psychostimulants such as amphetamine and cocaine (Fienberg et al., 1998). This apparent discrepancy can be explained by considering the specific neuronal populations affected by psychostimulants and by CB1 receptor agonists. Striatal medium spiny neurons project either directly or indirectly via globus pallidus and subthalamic nucleus to the output stations of the basal ganglia (i.e., substantia nigra pars reticulata/entopeduncular nucleus). Because of these differences in connectivity, it is generally assumed that activation of the striatonigral neurons of the direct pathway promotes locomotor activity, whereas activation of the striatopallidal neurons of the indirect pathway reduces locomotion (Gerfen, 1992).
The increase in DARPP-32 phosphorylation at Thr34 produced by cocaine and amphetamine is mediated via activation of dopamine D1 receptors, which are positively coupled to cAMP production and preferentially expressed in striatonigral neurons (Gerfen, 1992). Indeed, administration of the dopamine D1 receptor antagonist SCH23390 [8-chloro-2,3,4,5-tetrahydro-3–5-1h-3-benzazepin-7-ol] prevents the increase in Thr34 phosphorylation produced by cocaine (Svenningsson et al., 2000; Valjent et al., 2004). In contrast, the effect of cocaine on DARPP-32 phosphorylation is essentially unaltered by blockade of adenosine A2A receptors (Svenningsson et al., 2000), which are selectively expressed in striatopallidal neurons (Fink et al., 1992; Schiffmann and Vanderhaeghen, 1993). The present findings, which show that the ability of CP55,940 to stimulate Thr34 phosphorylation requires intact adenosine A2A receptor transmission, indicate that CB1 receptor agonists act on striatopallidal neurons. Therefore, it appears that stimulation of the cAMP pathway and phosphorylation of DARPP-32 at Thr34 promotes motor activation or motor depression depending on whether it occurs at the level of striatonigral or striatopallidal neurons, respectively. It should be noted that our results do not rule out the possibility that CB1 receptors increase DARPP-32 phosphorylation at Thr34 in striatonigral neurons. However, our results suggest that the ability of CP55,940 to regulate DARPP-32 in striatopallidal projection neurons exerts a preponderant effect on CB1 receptor-mediated control of motor activity.
A comparison of the biochemical and behavioral data presented in this study indicates that the motor response to CP55,940 outlasts the effect on DARPP-32 phosphorylation. It is therefore likely that the increase in phosphoThr34-DARPP-32 induced by the CB1 agonist produces effects on downstream target proteins that persist even when the phosphorylation/activation of DARPP-32 has subsided. In fact, changes in the state of phosphorylation of ligand- and/or voltage-gated ion channels, which are modulated via DARPP-32 phosphorylation (Greengard, 2001), may result in secondary changes in signaling involved in the late phase of the motor response to CP55,940. Such secondary changes would still be dependent on DARPP-32 phosphorylation, without necessarily requiring the presence of the phosphorylated protein.
This study shows that the ability of CP55,940 to phosphorylate DARPP-32 and produce catalepsy requires dopamine D2 receptors. Based on previous data indicating the existence of a negative control exerted by cannabinoids on dopamine D2 receptor transmission (Giuffrida et al., 1999; Beltramo et al., 2000), we propose that activation of CB1 receptors increases Thr34 phosphorylation by antagonizing the inhibition exerted by dopamine D2 receptors on the cAMP/PKA cascade. Considerable evidence indicates the existence of a complex interaction between dopamine D2 receptors and adenosine A2A receptors. In particular, it has been found that biochemical and behavioral responses produced by blockade of dopamine D2 receptors are strongly attenuated in the absence of adenosine A2A receptors (Svenningsson et al., 2000; Chen et al., 2001), which maintain a normal level of cAMP production in striatopallidal neurons. In light of these observations, our findings that biochemical and behavioral effects of CP55,940 are reduced in A2A receptor knock-out mice further strengthens the idea that CB1 receptors act by counteracting dopamine D2 receptor transmission. Such a counteraction would result in disinhibition/facilitation of adenosine A2A receptor-dependent activation of the cAMP/PKA/DARPP-32 cascade in the striatal projection neurons of the indirect pathway. Thus, we propose that CB1 receptors act on striatopallidal neurons as follows: (1) by reducing dopamine D2 receptor transmission, (2) by disinhibiting A2A receptor-dependent activation of PKA, and (3) by phosphorylating DARPP-32 at Thr34.
Dopamine D2 receptors and adenosine A2A receptors exert opposite effects on striatopallidal neurons and on motor function. However, our results show that their genetic inactivation results in a similar attenuation of the biochemical and behavioral effects of CP55,940. This paradox can be explained by assuming that CB1 receptors act by antagonizing D2 receptor transmission and promoting A2A receptor transmission. Such opposite regulations are equally prevented in knock-out mice. Indeed, dopamine D2 and adenosine A2A receptor-null mice show reduced responses to both agonists and antagonists (Boulay et al., 1999, 2000; Ledent et al., 1997).
Previous studies indicated that the psychostimulant effect of caffeine, an adenosine A2A receptor antagonist, involves phosphorylation of DARPP-32 at Thr75 (Lindskog et al., 2002). This change in DARPP-32 phosphorylation resulted in reduced PKA activity and suppression of protein phosphorylation in striatopallidal neurons. Remarkably, the present results show that cannabinoids produce their motor depressant effect by an opposite action on protein phosphorylation in the same population of projection neurons. Thus, activation of CB1 receptors leads to stimulation of DARPP-32 phosphorylation at Thr34, reduction of PP-1 activity, and increased phosphorylation of downstream target proteins involved in the regulation of the state of excitability of striatopallidal neurons. These downstream target phosphoproteins remain to be identified.
Footnotes
This work was supported by Swedish Research Council Grants 13482 (G.F.) and 2553 (B.B.F.), the Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, Hôpitaux Universitaires de Strasbourg, the Mission Interministérielle à la Lutte contre la Drogue et la Toxicomanie, Association pour la Recherche sur le Cancer, European Union Grant LSHM-CT-2004-005166 (E.B.), The Peter Jay Sharp Foundation, and National Institutes of Health Grants MH40899 and DA10044 (P.G.). A.U. was a recipient of a fellowship from the Wenner-Gren Foundations. L.P. was a recipient of a fellowship from the Foundation Blanceflor Ludovisi-Boncompagni née Bildt.
Correspondence should be addressed to Gilberto Fisone, Department of Neuroscience, Karolinska Institutet, Retzius väg 8, 17177 Stockholm, Sweden. E-mail: gilberto.fisone@neuro.ki.se.
A. Usiello's present address: Centro di Ingegneria Genetica, Biotecnologie avanzate, 80131 Naples, Italy.
L. Pozzi's present address: Department of Neuroscience, “Mario Negri” Institute for Pharmacological Research, 20157 Milan, Italy.
DOI:10.1523/JNEUROSCI.1289-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/258432-07$15.00/0
References
- Baik J-H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377: 424–428. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Piomelli D (2000) Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid, 2-arachidonylglycerol. NeuroReport 11: 1231–1235. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Rodriguez de Fonseca F, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G, Sadile AG, Giuffrida A, Piomelli D (2000) Reversal of dopamine D2 receptor responses by an anandamide transport inhibitor. J Neurosci 20: 3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai L-H, Kwon YT, Girault J-A, Czernik AJ, Huganir R, Hemmings HC, Nairn AC, Greengard P (1999) Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature 402: 669–671. [DOI] [PubMed] [Google Scholar]
- Bidaut-Russell M, Devane WA, Howlett AC (1990) Cannabinoid receptors and regulation of cyclic AMP accumulation in the rat brain. J Neurochem 55: 21–26. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Perrault G, Borrelli E, Sanger DJ (1999) Dopamine D2 receptor knock-out mice are insensitive to the hypolocomotor and hypothermic effects of dopamine D2/D3 receptor agonists. Neuropharmacology 38: 1389–1396. [DOI] [PubMed] [Google Scholar]
- Boulay D, Depoortere R, Oblin A, Sanger DJ, Schoemaker H, Perrault G (2000) Haloperidol-induced catalepsy is absent in dopamine D(2), but maintained in dopamine D(3) receptor knock-out mice. Eur J Pharmacol 391: 63–73. [DOI] [PubMed] [Google Scholar]
- Centonze D, Battista N, Rossi S, Mercuri NB, Finazzi-Agró A, Bernardi G, Calabresi P, Maccarrone M (2004) A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology 29: 1488–1497. [DOI] [PubMed] [Google Scholar]
- Chen J-F, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hackett E, Fink SJ, Low MJ, Ongini E, Schwarzschild MA (2001) The role of the D2 dopamine receptor (D2R) in A2A adenosine receptor (A2A R)-mediated behavioral and cellular responses as revealed by A2A and D2 receptor knockout mice. Proc Natl Acad Sci USA 98: 1970–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelsten P, Song W-J, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault JA, Nestler EJ, Greengard P (1998) DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science 281: 838–842. [DOI] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM (1992) Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptor in rat striatum. Mol Brain Res 14: 186–195. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Lovinger DM (2001) CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol 85: 468–471. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM (2002) Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5: 446–451. [DOI] [PubMed] [Google Scholar]
- Gerfen CR (1992) The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci 15: 285–320. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D (1999) Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2: 358–363. [DOI] [PubMed] [Google Scholar]
- Glass M, Felder CC (1997) Concurrent activation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J Neurosci 17: 5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P (2001) The neurobiology of slow synaptic transmission. Science 294: 1024–1030. [DOI] [PubMed] [Google Scholar]
- Hemmings HCJ, Greengard P (1986) DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci 6: 1469–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings JHC, Greengard P, Tung HYL, Cohen P (1984) DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature 310: 503–505. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC (1990) Cannabinoid receptor localization in the brain. Proc Natl Acad Sci USA 87: 1932–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Qualy JM, Khachatrian LL (1986) Involvement of Gi in the inhibition of adenylate cyclase by cannabimimetic drugs. Mol Pharmacol 29: 161–165. [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M (2005) Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor crosstalk? Mol Pharmacol 67: 1697–1704. [DOI] [PubMed] [Google Scholar]
- Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaegen JJ, Costentin J, Heath JK, Vassart G, Parmentier M (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature 388: 674–678. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert J-F, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M (1999) Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283: 401–404. [DOI] [PubMed] [Google Scholar]
- Lindskog M, Svenningsson P, Pozzi L, Kim Y, Fienberg AA, Bibb JA, Fredholm BB, Nairn AC, Greengard P, Fisone G (2002) Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature 418: 774–778. [DOI] [PubMed] [Google Scholar]
- Maneuf YP, Brotchie JM (1997) Paradoxical action of the cannabinoid WIN 55,212–2 in stimulated and basal cyclic AMP accumulation in rat globus pallidus slices. Br J Pharmacol 120: 1397–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, Langley-Guillion K-C, Greengard P (1998) Quantitative immunochemistry of DARPP-32-expressing neurons in the rat caudatoputamen. Brain Res 808: 8–12. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Néliat G, Caput D, Ferrara P, Soubrié P, Brelière JC, Le Fur G (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350: 240–244. [DOI] [PubMed] [Google Scholar]
- Rodríguez JJ, Mackie K, Pickel VM (2001) Ultrastructural localization of the CB1 cannabinoid receptor in μ-opioid receptor patches of the rat caudate putamen nucleus. J Neurosci 21: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen J-J (1993) Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci 13: 1080–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder GL, Girault J-A, Chen J, Czernik AJ, Kebabian JW, Nathanson JA, Greengard P (1992) Phosphorylation of DARPP-32 and inhibitor-1 in rat choroid plexus: regulation by factors other than dopamine. J Neurosci 12: 3071–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm B, Fisone G (2000) Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci USA 97: 1856–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P (2003) Diverse psychotomimetics act through a common signaling pathway. Science 302: 1412–1415. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault J-P, Nairn AC, Greengard P (2004) DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44: 269–296. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dörner L, Pfreundtner C, Nörenberg W, Starke K (1998) Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience 85: 395–403. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76: 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol J-C, Stipanovich A, Caboche J, Lombroso P, Nairn AC, Greengard P, Hervé D, Girault J-P- (2004) Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA [DOI] [PMC free article] [PubMed]
- Walaas SI, Greengard P (1984) DARPP-32, a dopamine- and adenosine 3′: 5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular distribution in rat brain. J Neurosci 4: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas SI, Aswad DW, Greengard P (1983) DARPP-32, a dopamine- and cAMP-regulated phosphoprotein enriched in dopamine-innervated brain regions. Nature 301: 69–71. [DOI] [PubMed] [Google Scholar]