Abstract
Acute brain injuries have been identified as a risk factor for developing Alzheimer's disease (AD). Because glutamate plays a pivotal role in these pathologies, we studied the influence of glutamate receptor activation on amyloid-β (Aβ) production in primary cultures of cortical neurons. We found that sublethal NMDA receptor activation increased the production and secretion of Aβ. This effect was preceded by an increased expression of neuronal Kunitz protease inhibitory domain (KPI) containing amyloid-β precursor protein (KPI-APP) followed by a shift from α-secretase to β-secretase-mediated APP processing. This shift is a result of the inhibition of the α-secretase candidate tumor necrosis factor-α converting enzyme (TACE) when associated with neuronal KPI-APPs. This KPI-APP/TACE interaction was also present in AD brains. Thus, our findings reveal a cellular mechanism linking NMDA receptor activation to neuronal Aβ secretion. These results suggest that even mild deregulation of the glutamatergic neurotransmission may increase Aβ production and represent a causal risk factor for developing AD.
Keywords: amyloid, APP, secretase, glutamate receptors, calmodulin, neurons
Introduction
Traumatic brain injuries (TBIs) have been identified as an environmental risk factor for Alzheimer's disease (AD) (Heyman et al., 1984; Mortimer et al., 1991; Guo et al., 2000; Plassman et al., 2000; Uryu et al., 2002; Fleminger et al., 2003), an age-related neurodegenerative disease associated with progressive decline of cognitive function. One of the pathological hallmarks of AD is the abnormal accumulation of amyloid-β (Aβ) (a 40-42 amino acid-long peptide) within vascular walls and in the cerebral parenchyma (Glenner and Wong, 1984; Masters et al., 1985). Indeed, among patients suffering from head injury, 30% display Aβ deposition within 7 d (Roberts et al., 1991; Roses and Saunders, 1995). These findings suggest that acute brain injuries, such as TBI, may trigger deposition of Aβ (Smith et al., 1998, 2003). In addition, a recent study pointed out the transient upregulation of β-secretase BACE-1 [β-site amyloid precursor protein (APP) cleaving enzyme] expression 24-72 h after TBI, accompanied by an elevation of its activity detected 48 h after onset (Blasko et al., 2004). Although the overall understanding of the conditions that govern Aβ accumulation into the brain parenchyma has greatly improved, the molecular mechanism linking acute brain injury to an increased Aβ production remains to be established (Roberts et al., 1994; Saido et al., 1994; Popa-Wagner et al., 1998).
It is now an accepted fact that, in acute brain injuries, glutamate (the predominant excitatory neurotransmitter in the mammalian nervous system) plays a pivotal role (Choi, 1988) in such a way that acute neuronal injury is thought to result from an excessive release of excitatory amino acids and the subsequent overactivation of their postsynaptic receptors. Accordingly, neuronal culture models of glutamate toxicity have been extensively used to investigate the mechanisms of neuronal injury associated with these pathologies. The first event in the glutamate induced neuronal injury is mediated by the activation of ion channel-linked glutamate receptors, especially NMDA receptors (NMDARs), probably because of their high-calcium (Ca2+) permeability (Choi, 1995). The subsequent activation of cytoplasmic calcium-dependent enzymes has been proposed to mediate the final step of this deleterious pathway leading to an “excitotoxic” necrosis. However, despite this intuitive link between acute brain injuries and necrosis, a growing body of evidence indicates that TBI and ischemic insults also trigger neuronal apoptosis (Bramlett and Dietrich, 2004).
The genesis of Aβ peptide is attributable to the processing of a transmembrane glycoprotein, the APP. APP is encoded by a single gene of 18 exons on chromosome 21. Exons 7, 8, and 15 of the APP gene can be alternatively spliced to produce multiple isoforms. In the brain, the major isoform transcripts result from the splicing of exons 7 and 8, which gives rise to APP695, APP751, and APP770 (Selkoe, 2001). APP770 and APP751 both contain a serine protease inhibitory domain encoded by exon 7 called Kunitz protease inhibitory domain (KPI). KPI-APPs are mainly expressed in the brain by astrocytes and microglia, whereas the KPI-deficient APP, APP695, is abundant in neurons (LeBlanc et al., 1991). APP is processed in a two-step cleavage by several proteases, named α-, β-, and γ-secretases (Selkoe, 2001). The amyloidogenic pathway involves a β-secretase cleavage of the Aβ N terminus and generates a soluble fragment named sAPPβ. The C-terminal membrane-bound remaining fragment undergoes γ-secretase processing, leading to Aβ40 or Aβ42 formation. An alternative pathway involves the cleavage of APP by α- and γ-secretases that generate a soluble APP derivative, sAPPα, containing the first 17 residues of Aβ and a 3 kDa peptide (p3) similar to the 17-40/42 region of Aβ.
By studying the relationship between acute brain injuries and AD, we may learn more about the deleterious pathway leading to AD. In the present study, we investigated how acute brain injury may act as a risk factor for AD by studying the link between excitotoxicity, apoptosis, and Aβ production.
Materials and Methods
PCR and reverse transcriptase system kits were purchased from Promega (Charbonnières, France). DMEM, poly-d-lysine, cytosine β-d-arabinoside (AraC), horse and fetal calf sera, proteinase K, sodium citrate, staurosporine (STP), calcimycin (A23187), 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxy-methyl) ester (BAPTA-AM), 1,4-dihydro-2,6-dimethyl-4-(3-nitro-phenyl)-3,5-pyridinedicarboxylic acid 2-meth-oxyethyl 1-methylethyl ester (nimodipine), J8, KN-93, anti-microtubule associated protein-2 (MAP-2), and anti-actin antibodies were obtained from Sigma (L'Isle d'Abeau, France). NMDA, AMPA, 6-cyano-7-nitroquin-oxaline-2,3-dione (CNQX), kainate, and (+)5-methyl-10,11-dihydro-5H-dibenzo(a,b)cyclohepten-5,10-imine maleate (MK-801) were from obtained from Tocris Cookson (Bristol, UK). Laminin was obtained from Invitrogen (Cergy Pontoise, France).
Semiquantitative reverse transcription-PCR. Total RNAs from primary cultures of cortical neurons were isolated by using RNAeasy extraction columns (Qiagen, Courtaboeuf, France). Total RNAs from brain tissue samples were prepared by a phenol/chloroform extraction method using the RNAxel extraction kit (Eurobio, Paris, France). One microgram of total RNA was transcribed into cDNA by using poly-dT oligonucleotides. For semiquantitative experiments, each PCR amplification was tested to reach half of the saturation curve, and an aliquot of cDNA libraries was amplified by PCR with specific oligonucleotides for β-actin (539 bp PCR product). Similarly, an aliquot of the same cDNA libraries was amplified with specific oligonucleotides for APP isoforms APP770, APP751, and APP695 (242, 222, and 401 bp, respectively, of PCR product). Conditions of amplification were 30 s at 95°C, 30 s at 58°C, and 1 min at 72°C for 25 or 30-35 cycles, corresponding to the 50% of the saturation curve of the PCR products obtained for β-actin and APP isoforms, respectively. APP primers were designed to flank the alternatively spliced exons (exons 7 and 8) to detect the expression of the three major APP isoforms found in the brain. Oligonucleotide sequences used are as follows: β-actin sense, 5′ GTG GGC CGC TCT AGG CAC AA 3′; gene location 25-45 bp and antisense 5′ CTC TTT GAT GTC ACG CAC GAT TTC 3′; 564-540 bp. APP695 oligonucleotides are as follows: sense 5′ GCA CTA ACT TGC ACG ACT ATG GCA TGC TGC TGC CCT G 3′; 500-536 bp and antisense 5′ GCT GGC TGC CGT CGT GGG AAC TCG GAC TAC CTC CTC CAC A 3′; 860-1104. APP751 oligonucleotides are as follows: sense 5′ CTA CCA CTG AGT CTG TGG AG 3′; 848-868 bp and antisense 5′ GCT GGC TGC CGT CGT GGG AAA CAC GCT GCC ACA CAC CGC C3′; 1028-1104. APP770 primers are as follows: sense 5′ CTA CCA CTG AGT CTG TGG AG 3′; 848-868 bp and antisense 5′ CTT GAG TAA ACT TTG GGA TGA CAC GCT GCC ACA CAC CGC C 3′; 1028-1068. These sequences were designed from the rat APP cDNA sequences corresponding to GenBank accession numbers X14066 and X07648. BDNF oligonucleotides are as follows: sense 5′ TGT GCG GAC CCA TGG GAC TC 3′; gene location 75-94 bp and antisense 5′ TGT CAC ACA CGC TCA GCT CC 3′; 381-4000 bp. These sequences were designed from the mouse BDNF cDNA sequences corresponding to GenBank accession number AY011461. Amplification was performed in an Eppendorf thermocycler (Eppendorf Scientific, Westbury, NY) with the Promega PCR kit.
All PCR products for APP770, APP751, and APP695 displayed the expected size and the specificity of the PCR products amplified was confirmed by digestion with restriction enzymes (AluI for APP770 and APP695; and RsaI for APP751).
Finally, agarose gels were acquired with a CCD camera, and two-dimension densitometry analysis was determined by using the Opti-Quant software (Packard Bioscience, Meriden, CT)
Primary cell cultures. Mouse cortical cultures of neurons were prepared from 14- to 15-d-old embryos as described previously (Rose et al., 1993). After 3 d in vitro (DIV), neurons were treated with 10 μm AraC to inhibit proliferation of non-neuronal cells. All of the experiments were performed on pure neuronal cultures (> 98% of microtubule associated protein-2 immunoreactive cells) after 12-14 DIV.
Exposure of glutamatergic agonists. AMPA treatment was performed in the presence of the noncompetitive NMDA receptor antagonist MK-801 (10 μm) to inhibit secondary NMDA receptor activation. Addition of the AMPA receptor (AMPAR) antagonist CNQX (10 μm) was used to block AMPA receptor during NMDA treatment (7.5 for 24 h and 50 μm for 3 h exposure). These conditions induced <25% of neuronal cell death as estimated by lactated dehydrogenate (LDH) activity.
Serum deprivation- and staurosporine-induced apoptosis. At 7 DIV, near pure neuronal cultures were transferred during 24 h into serum-free DMEM in the presence of MK-801 (10 μm) to block secondary excitotoxic injury. Cycloheximide (1 μg/ml), a protein synthesis inhibitor, was used to confirm the features of cell death. Neuronal cell death was estimated by counting dying neurons stained with 0.4% trypan-blue dye.
STP (200 nm) was added for 6 or 12 h in pure neuronal cultures at 13-14 DIV in serum-free DMEM supplemented with 10 μm glycine. MK-801 was added to prevent secondary NMDA receptor activation.
KCl-induced depolarizations. KCl applications (50 mm) were performed for either 3 or 24 h by incubating 14-d-old (14 DIV) neuronal cultures in the presence of MK-801 (10 μm) and CNQX (10 μm) into serum-free DMEM supplemented with glycine (10 μm). The L-type Ca2+-channel antagonist, nimodipine (1 μm), was used to inhibit the depolarizations induced by KCl.
Calcium signaling. Primary cultures of neurons (14 DIV) were pretreated (1 h) with the membrane-permeant calcium chelator BAPTA-AM (10 μm) before NMDA or KCl applications. Exposures with the L-type Ca2+ channel inhibitor nimodipine (1 μm) were performed for 3 or 24 h, respectively, in the presence or absence of NMDA or KCl.
Western blotting and immunoprecipitation. At the indicated times, cells were harvested in a lysis solution containing 50 mm Tris-HCl, pH 7.6, 1% NP-40 (Sigma), 150 mm NaCl, and 2 mm EDTA, with 1 mm phenylmethylsulfonyl fluoride (PMSF) in the presence of a protease inhibitor mixture (Sigma). Cell lysates were centrifuged for 10 min at 12,000 rpm, supernatants were isolated, and corresponding pellets were resuspended with the proteases inhibitor-containing lysis buffer to extract membrane-bound proteins. Plasma membranes were solubilized in lysis buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 0.5% Triton X-100, 1 mm EGTA, 0.1% SDS, 1% deoxycholate, 1 mm PMSF) in the presence of protease inhibitors (Sigma). Once resuspended, membrane lysates were subjected to centrifugation, and the soluble fraction was removed for electrophoresis analysis. Equally, conditioned media were harvested in the presence of 1 mm PMSF and protease inhibitor mixture to be finally concentrated 10-fold using a vacuum system and desalted using Microcon columns (Amicon, Millipore, Beverly, MA). Protein amounts were determined by the Bradford protein assay (BCA Protein Assay; Pierce, Rockford, IL) and normalized to 20-250 μg of protein per sample. Electrophoreses were done on 8% SDS-polyacrylamide Tris-glycine gels or 16.5% Tris-tricine gels containing 8 m urea. Thereafter, gels were transferred to a polyvinylidene difluoride (PVDF) membrane (polyscreen membrane; PerkinElmer, Paris, France), and membranes were blocked in nonfat milk containing 3% bovine serum albumin and probed with the appropriate antiserum. Blots were finally developed with an enhanced chemiluminescence Western blotting detection system (Western Lightning Chemiluminescence Reagent Plus; PerkinElmer).
For immunoprecipitations, aliquots (500 μg) of protein extracts were diluted to 500 μl with dilution buffer and incubated with appropriate antibody. The mixture was incubated overnight at 4°C and mixed with 40 μl of protein G-Sepharose, Fast Flow (Amersham Biosciences, Little Chalfont, UK) for 1 h. The beads were washed twice in Buffer A and twice with Buffer B and then proteins were eluted in 40 μl of loading SDS-PAGE buffer by boiling. Proteins were fractioned on 6% Tris-glycine SDS-PAGE gels or 18% Tris-Tricine SDS-PAGE gels and transferred onto PVDF membrane.
Fluorescent immunocytochemistry. To detect membrane-bound KPI-APP proteins, murine cortical neurons were washed with PBS and incubated with the R7 antiserum for 1 h at 37°C in serum-free DMEM onto living cells. Cells were then gently washed and fixed with ice-cold 4% PFA and incubated for 1 h with the Alexa Fluor 488-conjugated antibody (Invitrogen, Leiden, The Netherlands).
Once KPI-APPs were revealed, neurons were incubated overnight with the MAP-2 antibody. Cells were then washed and incubated for 1 h with the appropriate secondary biotin-conjugated antibody. Antibody-antigen complexes were revealed with streptavidin Alexa Fluor 555 conjugate (Invitrogen).
Antibodies. The monoclonal mouse antibody 22C11 (1:1000; Roche Diagnostics, Meylan, France) corresponding to residues 66-81 of APP was used to identify either membrane-bound protein or soluble derivatives. The following other primary antibodies/antisera were used: 6E10 and 4G8 against Aβ1-17 and Aβ17-24, respectively (Signet Laboratories, Dedham, MA), polyclonal antiserum against APP99-126 (Chemicon, Temecula, CA), R7 (1:1000) against KPI-APP proteins, R1736 (1:1000) against sAPPα (Haass et al., 1992), 192wt (1: 500) against sAPPβ, FCA3542 (Barelli et al., 1997), APPCter-C17 (1:5000) against APP C terminus (Sergeant et al., 2002), s.c.-6416 (1: 1000) against the C terminus of tumor necrosis factor-α converting enzyme (TACE; Santa Cruz Biotechnology, Santa Cruz, CA), and antibodies raised against MAP-2 (1:200), actin (1: 250), and KPI-APP301-315 (1:100), respectively (Sigma). Rabbit polyclonal antisera R1742 (Bussiere et al., 2002), R600 (Behrouz et al., 1989), and APPCter-C17 are kind gifts from Nicolas Sergeant, Luc Buée, and André Delacourte. Both R1742 an R600 were tested against synthetic Aβ1-40 and Aβ1-42 peptides (Sigma) to determine their specificities (data not shown).
Aβ fluorometric ELISAs. Aβ peptides present in the conditioned culture media were captured with an antibody raised against the N-terminal domain of Aβ(8-17) and revealed with a secondary antibody raised against the C-terminal extremity (40 or 42-end) of Aβ (BioSource, Nivelles, Belgium). Recombinant rodent synthetic Aβs (Calbiochem, La Jolla, CA, San Diego) were used for the calibration curves for each ELISA performed.
TACE activity assay. TACE activity was determined by using the α-secretase activity kit and recombinant human TACE (R&D Systems, Minneapolis, MN). The experimental procedure was performed as described by the manufacturer.
Diffuse head injury model. All procedures were performed in strict compliance with French regulations (D2001-486) and with the EC regulations 96 (Official Journal of European Community L358 12/18/1986) on animal care and use.
The model of diffuse head injury was elaborated as described previously (Hellal et al., 2003). Briefly, Swiss mice weighing 26-28 g (Janvier, France) were anesthetized under 2% halothane balanced with air and oxygen. Closed head trauma was induced by a 50 g weight dropped along a stainless-steel string. This experimental paradigm creates a pattern of diffuse degeneration into the brain accompanied with a deficit of the functional outcome (Hellal et al., 2003). At the indicated time, mice presenting a significant deficit of the functional outcome were deeply anesthetized with isoflurane and killed, and their brains were immediately removed and briefly rinsed in chilled saline. Tissue samples were taken vertically from the fresh brain at the site of lesion using a 4-mm-diameter punch and were used for Western blot analysis.
Human brain tissues. The brain tissues used in this study were provided by the Institute for Brain Aging and Dementia Tissue Repository. Neuropathological analyses were performed to determine amyloid plaque burden and tauopathology in the tissue samples. Researchers performing the biochemical analysis of cortical brain extracts were blinded.
Statistical analysis. Results are expressed as mean ± SD (SD). Statistical analyses were performed with StatView (Abacus, Berkeley, CA) by one-way ANOVA followed by Bonferroni-Dunn's test or Student's t test.
Results
NMDA exposure induces the neuronal expression of KPI-APPs
After acute brain injuries, a shift in APP mRNA expression occurs altering the balance of generated transcripts from APP695 toward KPI-containing APP (Abe et al., 1991; Kim et al., 1998). The mechanism that sustains this effect remains unresolved but could be connected to the deleterious pathways activated by such insults. To determine the cellular and molecular mechanisms triggering this upregulation of KPI-APP, we exposed primary cultures of murine cortical neurons (containing <2% of glial cells) to different experimental paradigms promoting apoptosis or necrosis.
Although acute neuronal injury is thought to result from an excessive release of excitatory amino acids and the subsequent activation of their postsynaptic receptors, various clues have emerged suggesting that apoptotis may also exert a significant role in traumatic neuronal injury (Linnik et al., 1993; MacManus et al., 1993; Schulz et al., 1999). In primary cortical cultures, both serum deprivation (7 DIV) and application of the protein kinase inhibitor staurosporine (14 DIV) induced typical features of apoptosis: a gradual shrinkage of the neuronal cell body, a nuclear condensation, and appearance of DNA fragmentation. The addition of cycloheximide (1 μg/ml), a protein synthesis inhibitor, abolished neuronal cell death. These paradigms induced ∼40-50% of neuronal cell death after 24 h. Neither serum deprivation nor staurosporine-induced apoptosis resulted in a modification in the pattern of APP mRNA expression (data not shown).
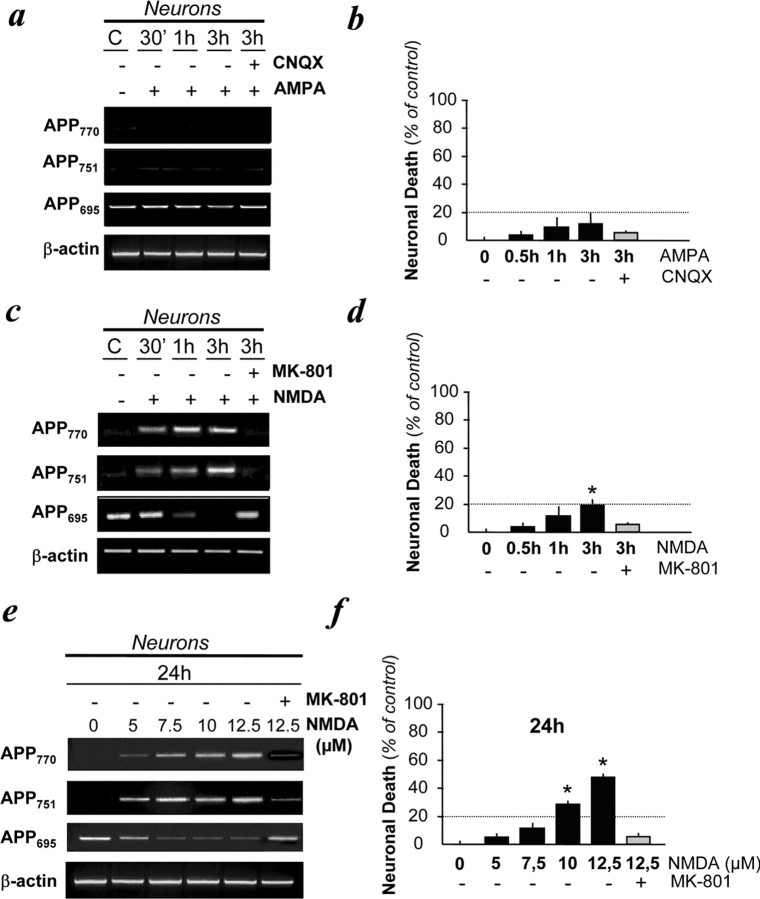
Morphologically distinct from apoptosis, excitotoxicity is a process characterized by a prominent and early cell swelling. To test the possibility that this neuronal cell death mechanism could alter the transcription of APP, cultured neurons were exposed to excitotoxic paradigms. To induce excitotoxicity, we incubated cortical neurons with glutamatergic agonists: NMDA (50 μm) and AMPA (50 μm) for 30 min, 1 h, and 3 h. These treatments resulted in <20% of neuronal cell death at 3 h (Fig. 1). Although AMPA application (in the presence of MK-801, a non competitive NMDA antagonist to avoid secondary NMDAR activation) did not modify the pattern of APP mRNA expression (Fig. 1a), NMDA exposure induced the neuronal expression of KPI-APP transcripts and simultaneously a decrease in APP695 mRNA expression as early as 30 min after NMDA exposure (Fig. 1c). This effect was abolished by the coapplication of the NMDAR antagonist, MK-801, at 10 μm. Confirming the previous findings, this increased neuronal KPI-APP/APP695 mRNA ratio was also induced by lower doses of NMDA (from 5 to 12.5 μm) applied for 24 h (Fig. 1e). Estimated posttreatment assessment of neuronal death revealed that a low concentration of NMDA (7.5 μm) for 24 h induced neuronal expression of mRNA KPI-APPs without significantly affecting neuronal survival (Fig. 1f). Consequently, double immunocytochemical analysis performed with the R7 polyclonal antiserum raised against the KPI domain of APP (Refolo et al., 1989) and with an antibody raised against the neuronal marker MAP-2 demonstrated that a 24 h exposure to 7.5 μm NMDA induced KPI-APP immunoreactivity at the neuronal plasma membrane (Fig. 2).
Figure 1.
Expression of KPI-APP mRNAs in cultured cortical neurons subjected to NMDA-induced excitotoxicity. a, Transcriptional expression of APP isoforms in neurons exposed to AMPA (50 μm) in the presence of the AMPA antagonist CNQX (10 μm) for 30 min, 1 h, and 3 h. All conditions were performed with MK-801 (10 μm). b, Estimation of neuronal cell death in paradigms presented in a by determining the activity of LDH released by dying neurons. Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 16; p < 0.001). c, Transcriptional expression of APP isoforms in neurons exposed to NMDA (50 μm) in the presence or absence of MK-801 (10 μm) for 30 min, 1 h, and 3 h. All conditions were performed with CNQX (10 μm). d, Estimation of neuronal cell death in paradigms presented in c. Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 16; p < 0.001). e, Dose-dependent increased expression of KPI-APPs mRNAs in neurons exposed to NMDA in the presence or absence of MK-801 (10 μm) for 24 h. All conditions were performed with CNQX (10 μm). f, Estimation of neuronal cell death in paradigms presented in e. Note that the 24 h application of 7.5 μm NMDA can lead to neuronal death similar to that observed by treating neurons with 50 μm NMDA for 3 h. Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 16; p < 0.001). C, Control.
Figure 2.
Expression of KPI-APP isoforms in cultured cortical neurons subjected to NMDA receptor activation. KPI-APP expression in neurons after a sublethal exposure of 7.5 μm NMDA for 24 h. Neurons were stained with an antibody raised against the neuronal marker MAP-2 (red), and KPI-APP protein expression was determined by using the polyclonal R7 antiserum against the KPI domain of APP (green). KPI-APPs appeared colocalized (yellow) to neuronal plasma membrane when images were overlaid.
Based on these findings, NMDA-mediated excitotoxic but not apoptotic experimental paradigms were able to mimic the alteration of APP transcripts previously reported after acute brain injuries.
Calcium influx through NMDA receptors induces membrane expression of KPI-APP
Because massive calcium (Ca2+) influx through the NMDARs has been shown extensively to be a critical event in excitotoxic neuronal death (MacDermott et al., 1986), we tested the possibility that the increase in the KPI-APP/APP695 ratio observed in neurons exposed to NMDA was mediated by an increase in intracellular Ca2+ concentration.
First, we pretreated neuronal cultures with the membrane-permeant Ca2+ chelator BAPTA-AM (10 μm) 1 h before NMDA treatment. In these conditions, the NMDA-dependent increase in neuronal APP770 and APP751 mRNA expression (Fig. 3a) was fully blocked by intracellular Ca2+ chelation, implying a calcium-dependent control of KPI-APPs mRNA expression in neurons.
Figure 3.
Calcium-dependent KPI-APP expression in neurons after NMDA receptor activation. a, Reverse transcription-PCR analysis of APP mRNAs expression after NMDA receptor stimulation in the presence or absence of the membrane-permeant calcium chelator BAPTA-AM (10 μm). b, Relative expression of APP770, APP751, APP695, and BDNF mRNAs compared with β-actin in neurons subjected to application of either 7.5 μm NMDA or 50 mm KCl for 24 h estimated by densitometry (n = 12). Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 12; p < 0.001). Dark bars, Sham-wash control; gray bars, NMDA-treated neurons; light gray bars, KCl-induced depolarized neurons; hatched bars, (co-)application of nimodipine (1 μm). c, Western blot analyses of KPI-APP proteins (top) and full-length APP (bottom) in murine-cultured neurons after NMDA or KCl applications for 24 h. Blots were rehybridized with an actin antibody to estimate the total amount of proteins loaded. d, Relative expression of total APP compared with actin from experiments presented in c estimated by densitometry. Results are the mean of three experiments (n = 3). Dark bars, Sham-wash control; dark gray bars, NMDA-treated neurons; light gray bars, MK-801-treated neurons; bold-hatched bars, KCl-exposed neurons; hatched bars, coapplication of nimodipine (1 μm). C, Control; MW, molecular weight; Nimo., nimodipine.
To determine whether this response was selectively triggered by NMDAR activation, we studied whether calcium entry through voltage-sensitive calcium channels (VSCCs) activated by KCl-induced depolarizations in the presence of NMDAR and AMPAR antagonists would result in a similar modification of APP transcription. As demonstrated previously (Tao et al., 1998; West et al., 2001), KCl exposure promoted the neuronal expression of mRNA encoding for BDNF. This upregulation of BDNF transcripts was abolished by the coapplication of an L-type VSCC blocker (nimodipine at 1 μm), whereas the application of NMDA did not affect BDNF mRNA neuronal expression (Fig. 3b). In contrast, KCl-induced depolarizations failed to modify the neuronal pattern of APP mRNA expression (Fig. 3b).
At a protein level, immunoblotting analysis with the R7 antiserum raised against the KPI domain of APP confirmed that neurons do not express a detectable amount of KPI-APPs in control conditions (Fig. 3c). However, 7.5 μm NMDA exposure resulted in a marked increase in KPI-APP, whereas treatment with KCl did not modify neuronal KPI-APP expression. Accordingly, expression of full-length APPs was determined by using the antibody 22C11, raised against the N terminus of APP ectodomain (residues 66-81) and confirmed the upregulation of APP higher molecular weight isoforms containing the KPI domain (Fig. 3c). Densitometric quantification of the blots revealed no overall change of total APP levels expressed by neurons but indicated that after NMDAR activation, ∼30% of the total neuronal pool of APPs is a KPI-containing isoform (Fig. 3d).
Overall, these results indicate that the influx of Ca2+ resulting from a sublethal activation of the NMDA receptor controls the induction of the neuronal expression of KPI-APPs.
Neuronal KPI-APP expression enhances Aβ production and reduces α-secretase end products
Because KPI-containing APPs have been shown to be more amyloidogenic than APP695 (Ho et al., 1996), we investigated the influence of NMDA-induced KPI-APP expression in neurons on the APP processing. To this end, we performed immunoprecipitation/Western blot analysis from the conditioned media of cultured neurons to estimate the respective levels of APP derivatives secreted by neurons after NMDA application.
As reported previously (Haass et al., 1993), sAPPα is predominant in the conditioned media of control neuronal cultures. A 24 h NMDA exposure dramatically reduced sAPPα immunoreactivity (Fig. 4a). The coapplication of MK-801 restored the production of sAPPα to basal levels. This observed lowering of sAPPα immunoreactivity after NMDAR stimulation is accompanied by an elevation of sAPPβ, whereas the total amount of soluble APP molecules (sAPPx) remained unchanged (Fig. 4a). Similarly to sAPPα, cotreatment with MK-801 prevented the shift toward sAPPβ genesis induced by NMDA receptor activation. These data were quantified by densitometry analysis for both sAPPα/APP and sAPPβ/APP ratios (Fig. 4a).
Figure 4.
NMDA receptor stimulation leads to an inhibition of sAPPα release and an increased Aβ production. a, Immunoprecipitations of sAPPα or sAPPβ and total sAPPs in conditioned media of cultured neurons treated with NMDA (7.5 μm) for 24 h. sAPPα was revealed with the R1736 antiserum, sAPPβ with the 192 antiserum, and sAPPs with the 22C11 antibody after capturing all APP isoforms with the 22C11 antibody. sAPPx/total sAPP ratios were estimated by densitometry. Open bars, sAPPα; filled bars, sAPPβ. Results are the mean of four experiments performed in triplicate. Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 12; *p < 0.001 to control; #p < 0.02 to NMDA). b, Western blot analysis of extracellular Aβ in neurons after NMDA application for 24 h by using antisera raised against either the C-terminal extremity of Aβ42 (R1742) (top) or Aβ1-10 (R600) (bottom). p3 levels and Aβ levels were estimated by densitometry. Open bars, p3; filled bars, Aβ. Results are the mean of four experiments performed in triplicate. Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 12; *p < 0.001 to control; #p < 0.001 to NMDA). c, Quantitative determination of Aβ production by ELISA from the same extracts used in b. d, Western blot analysis of intracellular Aβ in neurons after NMDA application for 24 h by using R600 antiserum. Actin levels are shown in the bottom blot. IP22C11, Immunoprecipitated with 22C11; MW, molecular weight.
Because β-secretase activity is necessary to Aβ genesis, the amounts of Aβ and p3 peptides present in the neuronal conditioned culture media were evaluated by using an antiserum raised against the C-terminal extremity of Aβ42 termed R1742 to immunoprecipitate both Aβ and p3 (Fig. 4b). In control conditions, p3 was predominant in the extracellular medium. Incubation with 7.5 μm NMDA for 24 h resulted in an elevation of secreted Aβ42 that was abolished by the coapplication of MK-801 (Fig. 4b).
To confirm that the peptide identified in the extracellular media after NMDA stimulation was Aβ, an additional antiserum, termed R600, raised against Aβ1-10 was used to detect Aβ under the same experimental conditions. This experiment strengthened our previous observations that NMDA exposure led to an elevation of soluble Aβ after 24 h (Fig. 4b).
To finally quantify our findings, we performed an Aβ enzyme-linked immunosorbent assay (ELISA) on harvested conditioned media. After 24 h, a sublethal NMDA exposure induced a moderate elevation of Aβ40 production and an approximate fivefold increase in the amount of Aβ42 present in the conditioned media (Fig. 4c). Of note, a similar increase in intraneuronal Aβ was observed (Fig. 4d), suggesting an elevation of Aβ production.
To resume, the NMDA-dependent expression of KPI-APP in neurons is accompanied by an enhanced formation of components of the amyloidogenic pathway (i.e., sAPPβ and Aβ).
The calcium/calmodulin-dependent kinase axis is required for the induction of KPI-APPs and Aβ42
To identify the intracellular mediators involved in the NMDA-induced increase in KPI-APP/APP695 ratio in neurons, we evaluated the implication of two Ca2+-activated enzymes associated with the NMDAR: the calmodulin (CaM) and the CaM-dependent kinase II (CaMKII) (Husi et al., 2000) by using selective inhibitors of either CaM (J8) or CaMKs (KN-93) (Fig. 5). A 1 h pretreatment with each inhibitor blocked both the neuronal KPI-APPs mRNA appearance induced by NMDA (at 50 μm for 3 h) (Fig. 5a) and the membrane expression of the KPI-APP proteins induced by a lower dose of NMDA (at 7.5 μm for 24 h) (Fig. 5b). These results demonstrate the involvement of the CaM/CaMK axis in the shift expression toward KPI-APP expression induced by NMDAR activation in cortical neurons. Subsequently, inhibitors of the CaM/CaMK axis restored the production of sAPPα (Fig. 5c) and abolished the increase in Aβ in the neuronal conditioned bathing media after NMDA incubation (Fig. 5d).
Figure 5.
NMDA-dependent Aβ production in neurons is mediated by a calmodulin/calmodulin kinase axis. a, Reverse transcription-PCR analysis of APP transcripts in neurons after NMDA receptor stimulation (50 μm) for 3 h in the presence of 10 μm CNQX plus 1 μm nimodipine and with pretreatment for 1 h with an inhibitor of either 1 μm calmodulin (J8) or 10 μm calmodulin kinase-II(KN-93).b, Immunoprecipitation of membrane-bound KPI-APP proteins in neurons after NMDA receptor stimulation (7.5 μm) for 24 h in the presence of 10 μm CNQX plus 1 μm nimodipine and with or without the pretreatment for 1 h of an inhibitor of either 1 μm calmodulin (J8) or 10 μm calmodulin kinase-II (KN-93). No Ab, No antibody. c, Immunoblotting analysis of sAPPα generation in conditioned media of cultured neurons incubated in the same conditions indicated in b. d, Western blot analysis of Aβ generation in murine-cultured neurons incubated in the same conditions indicated in b. IP 22C11, Immunoprecipitated with 22C11; WB, Western blot; MW, molecular weight.
Incubation with anti-KPI antibodies prevents NMDA-induced Aβ production
To validate the idea that KPI-APP expression at the neuronal membrane played a pivotal role in the increased Aβ production after NMDAR activation, we exposed cortical neurons to the R7 antiserum in the presence of NMDA (at 7.5 μm). Incubation with the R7 antiserum abolished both the NMDA-induced decrease in sAPPα (Fig. 6a) and Aβ42 production (Fig. 6b). To validate the effect observed with the R7 antiserum, a commercially available polyclonal antibody developed to detect the KPI domain of APP770 (residues 301-315) was used under similar conditions and confirmed our previous findings (data not shown). Because the blockade of NMDA-induced Aβ production observed after R7 antiserum incubation could be attributable to a nonspecific alteration of APP processing by the antiserum, we performed parallel experiments either with the 22C11 monoclonal antibody raised against the N terminus of APP ectodomain (APP) and with a polyclonal antiserum raised against APP99-126 (data not shown) in the presence of NMDA. In contrast to R7, both antibodies did not reverse NMDA-induced Aβ42 elevation (Fig. 6a,b).
Figure 6.
KPI-dependent Aβ42 production in cortical neurons is reversed by the coincubation of a blocking antibody raised against the KPI domain. a, Immunoprecipitation of sAPPα generation in conditioned media of cultured neurons treated with NMDA (7.5 μm) in the presence of an antibody raised against either the KPI domain of APP or the N terminus of APP (residues 66-81) for 6 h. sAPPα levels were estimated by densitometry. Results are the mean of three experiments performed in triplicate. Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 9; *p < 0.01 to control). b, Western blot analysis of Aβ42 generation in neurons in the same protein samples used in a. Note that Aβ was immunoprecipitated and revealed with the FCA3542 antiserum against Aβ42. Levels of Aβ were estimated by densitometry. Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 9; *p < 0.01 to control). IP 22C11, Immunoprecipitated with 22C11; WB, Western blot.
Thus, our findings indicate that the expression of KPI-APP proteins coincides with a shift from a dominant α-secretase to a β-secretase processing of APP after NMDAR activation.
The KPI domain of APP interacts with the α-secretase candidate TACE
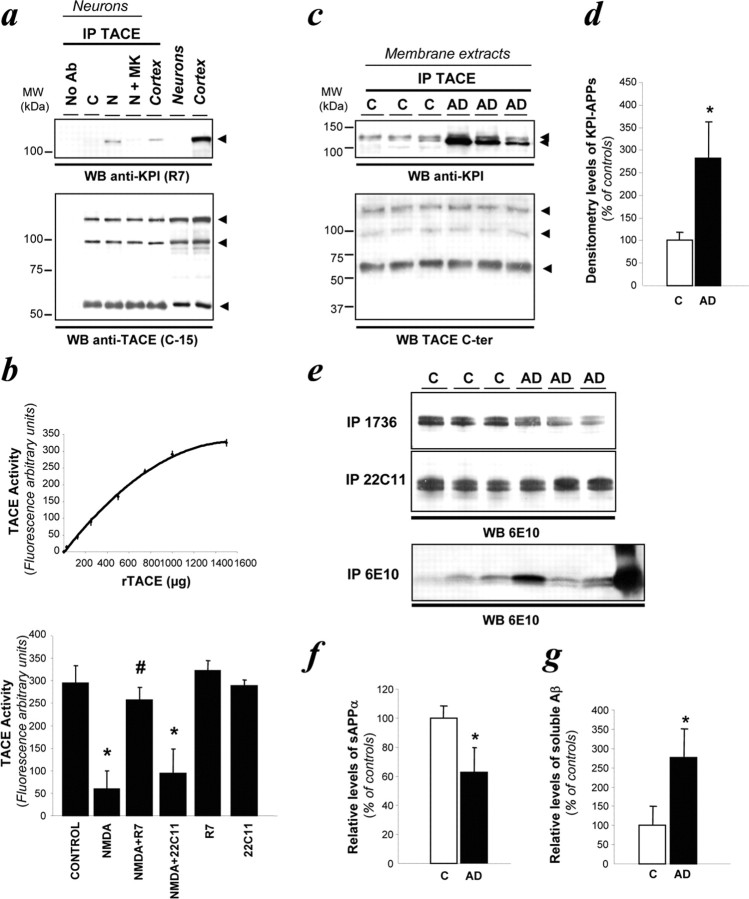
To further understand how the neuronal expression of KPI-APP molecules could alter α-secretase activity, we determined whether the α-secretase candidate, TACE, interacted correctly with KPI-APP (Buxbaum et al., 1998; Skovronsky et al., 2000).
To detect TACE in neurons, we used an antibody raised against its C terminus that allowed us to identify the 120 kDa full-length precursor (containing a prodomain), the 100 kDa mature form and the 60 kDa truncated form of TACE (Schlondorff et al., 2000; Skovronsky et al., 2000) (Fig. 7a). When TACE was immunoprecipitated from neuronal lysates, pro-TACE, mature TACE, and cleaved TACE were detected. Neither global nor respective expression levels of TACE isoforms were modified by NMDA application.
Figure 7.
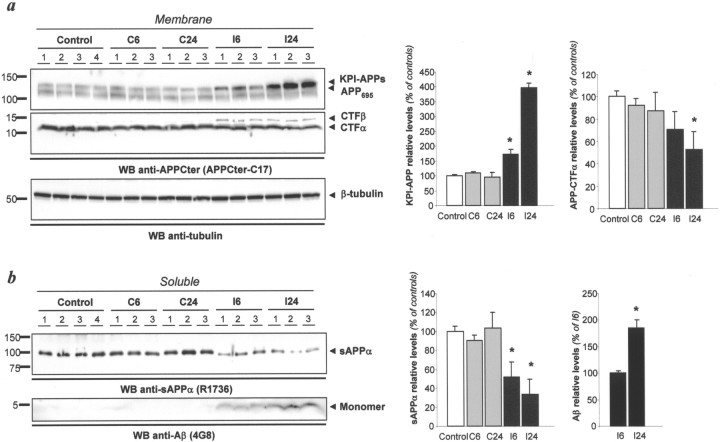
TBI enhances Aβ production. a, Using membrane preparations, total APP and APP-CTFs levels were determined by Western blot using APPCter-C17 in three to four animals per group. KPI-APP expression was slightly increased 6 h after TBI and was clearer at 24 h after trauma. In parallel, amounts of CTF-α were reduced, whereas amounts of CTF-β were increased above the threshold of the detection limit. β-Tubulin was used as loading control (bottom box). Densitometric analyses of presented immunoblots are displayed to the right (ANOVA; n = 3; *p < 0.01 to control). b, Estimation of the amount of sAPPα (top blot) and Aβ (bottom blot) after TBI. Densitometric analyses of presented immunoblots are displayed to the right (ANOVA; n = 3; *p < 0.05 to control or to the ipsilateral signal at 6 h after trauma for Aβ). C, Control; I, tissue samples ipsilateral to the impact; 6, 6 h after trauma; 24, 24 h after trauma. WB, Western blot.
Although no interaction was observed between KPI-APP and TACE in control conditions, immunoprecipitation with the TACE antibody revealed with R7 revealed a direct interaction between TACE and KPI-APP in neurons exposed to a sublethal NMDA treatment (Fig. 7a, lane 3). Coapplication of MK-801 inhibited this association between KPI-APPs and TACE (Fig. 7a, lane 4). Because neuronal TACE interacts with KPI-APP, we tested whether this association affects TACE activity. To this end, we monitored neuronal TACE activity under these experimental conditions by using a TACE activity assay (Fig. 7b). NMDA receptor activation reduced neuronal TACE activity by ∼80% compared with controls [59 ± 40 vs 294 ± 39 arbitrary units (AU)]. The coincubation of R7 antibody with NMDA restored the neuronal α-secretase activity to basal level (257 ± 27 AU), whereas the monoclonal antibody 22C11 or the polyclonal antiserum against APP99-126 failed to restore normal TACE activity.
To resume, after a sublethal NMDA exposure, neurons express KPI-APPs that interact with neuronal α-secretase candidate TACE and inhibit TACE function.
Aβ production is increased after traumatic brain injury
To validate the relevance of our in vitro findings, we examine the influence of TBI on APP expression and processing. The model of TBI used creates a pattern of diffuse degeneration triggered by increased concentration of extracellular glutamate (Arundine and Tymianski, 2004). First, we examined the time course of cortical APP expression in tissue samples of mice subjected to TBI by immunoblot. Specific labeling was densitometrically measured and normalized to controls. In membrane extracts, KPI-APP expression increased in ipsilateral cortices as early as 6 h after TBI (172 ± 33%; p < 0.05) and persisted 24 h later (396 ± 92%; p < 0.01) (Fig. 7a). This increase in KPI-containing isoforms was accompanied with the accumulation of C-terminal fragment-β (CTF-β) and a concomitant reduction of CTF-α (52 ± 9%; p < 0.01), suggesting a shift from a predominant α-secretase to β-secretase processing of APP. To confirm these findings, sAPPα and Aβ levels were estimated in soluble protein extracts (Fig. 7b). Six hours after TBI, sAPPα production was downregulated by ∼50% (51 ± 16), whereas 4G8-immunoreactive Aβ levels were increased above the detection limit of our assay. Eighteen hours later, sAPPα levels were reduced to ∼35% (34 ± 16) of naive brain levels and soluble Aβ amounts elevated to nearly twofold (187 ± 15%) when compared with the 6 h post-TBI time point. Overall, these in vivo data demonstrate that TBI induced a modification of APP and Aβ expression pattern similar to the modification observed in cortical neurons exposed to NMDA, suggesting that NMDAR activation governs APP expression and processing in the brain.
Formation of KPI-APP/TACE complex is associated with increased soluble Aβ levels in AD brains
To further validate these experimental findings obtained in rodents, we examined the presence of the TACE/KPI-APP complex in brain tissues of AD patients and age-matched controls. In membrane preparations from AD patients, the KPI-APPs/TACE complex was increased by ∼2.8-fold in AD brains when compared with controls (2.86 ± 0.71; n = 3) (Fig. 8c,d). In addition to the presence of the KPI-APP/TACE complex, sAPPα levels were also reduced in AD brains by 20% compared with controls (Fig. 8e,f). Finally, the levels of soluble Aβ were higher in AD brain extracts than in age-matched controls (Fig. 8e,g). Of note, the soluble Aβ levels obtained from AD brains were not directly correlated to the plaque loads measured previously. Overall, these results confirm that the KPI-APP/TACE complex is related to a reduced sAPPα levels and to an enhanced production of soluble Aβ peptides in AD brains.
Figure 8.
KPI-APP immunoprecipitates with α-secretase candidate TACE in neurons stimulated by NMDA and inhibits TACE function. a, KPI-APPs were immunoprecipitated (IP) with an anti-TACE polyclonal antibody and revealed with either R7 (top blot) or anti-TACE antibody (bottom blot). Anti-TACE antibody recognizes all forms of TACE (pro-, mature, and cleaved). No Ab, No antibody. b, TACE activity assay was validated by using an increasing concentration of recombinant TACE (rTACE) (top). TACE activity was determined in cultured cortical neurons in the same conditions described in Figure 6 (bottom). Statistical analysis was realized by ANOVA followed by Bonferroni-Dunn's test (n = 24; *p < 0.001 to control; #p < 0.01 to NMDA). c, KPI-APPs were immunoprecipitated with an anti-TACE polyclonal antibody and revealed with either the rabbit polyclonal antibody against KPI-APP301-315 (top blot) or anti-TACE antibody (bottom blot). Anti-TACE antibody recognizes all forms of TACE (pro-, mature, and cleaved). d, Densitometry analysis of KPI-APP relative levels expressed as percentage of controls. Results are the mean of three experiments performed in triplicate. Statistical analysis consisted of a t test analysis (n = 3; *p < 0.01 to control). e, Immunoblotting analysis of sAPPα, APP, and Aβ in human brain soluble extracts. sAPPα, APP, and Aβ were immunoprecipitated using R1736, 22C11, or 6E10, respectively, and detected with 6E10. R1736 recognizes N+O- and N-glycosylated sAPPα, and 22C11 detects both APP695 and KPI-APPs, whereas 6E10 is raised against Aβ1-17 (i.e., Aβ1-x and Aβ11-x). The last lane of the Western blot for Aβ (bottom gel) corresponds to synthetic Aβ42 used as a standard. f, g, Densitometric analysis of sAPPα and total Aβ relative levels expressed as a percentage of controls. Results are the mean of three experiments performed in triplicate. Statistical analysis consisted of a t test analysis (n = 3; *p < 0.01 to control). C, Age-matched controls; AD, Alzheimer's disease brain extracts; WB, Western blot; MW, molecular weight.
Discussion
Because patients who previously suffered from brain trauma or stroke have an increased probability to develop amyloid plaques (Nagy et al., 1997; Snowdon et al., 1997), acute brain disorders have been identified as an important environmental risk for developing AD (Cotman et al., 1996). However, the molecular mechanism that mediates this effect remains unclear. To address this question, we studied the expression pattern of APP and the production of Aβ in primary neuronal cultures subjected to necrosis and apoptosis, two different biochemical pathways leading to neuronal cell death that have been identified in acute brain injury (Lee et al., 1999). Our major findings are as follows. First, in primary cortical neuronal cultures, sublethal NMDAR activation triggered a shift in APP expression toward KPI-APPs, an effect mediated by Ca2+ entry through the NMDAR and a subsequent CaM/CaMK activation. Second, the neuronal expression of KPI-APPs after NMDAR activation favor β-secretase processing of APP instead of normally dominant α-secretase cleavage and consequently increased Aβ production and secretion in the culture media. Third, TBI produces a similar modification of the pattern of APP expression and Aβ production in the injured tissue. Fourth, this effect resulted from a marked inhibition of TACE activity after neuronal expression of KPI-APPs. Finally, the formation of KPI-APP/TACE complexes is associated with reduced sAPPα levels and enhanced soluble Aβ levels in AD brains.
Murine cortical cell cultures have been used extensively to explore the molecular mechanisms involved in the perturbation of cellular homeostasis induced by acute brain injuries. We studied the expression pattern of APP in primary neuronal cultures (Rose et al., 1993) exposed to different paradigms of cell death identified during cerebral ischemia: apoptosis and excitotoxic necrosis. Although neither apoptosis nor AMPA exposure altered the pattern of expression of APP mRNA, application of NMDA increased the neuronal transcription of KPI-APP proteins and decreased the amount of mRNA encoding for the normally dominant APP695. The modification of APP transcripts described in the present study is in agreement with a previous report indicating that glutamate exposure induced the expression of KPI-APPs in neurons (Willoughby et al., 1995). In addition, we demonstrate that this modification of APP expression is a result of an NMDA-mediated Ca2+ influx and subsequent CaM/CaMK axis activation. Because proteomic analysis of the NMDAR-adhesion protein-signaling complexes has revealed that both CaM and CaMKII are linked to the C terminus of the NMDAR subunits, these enzymes have been identified as a preferential enzymatic system involved in converting NMDA-mediated Ca2+ influx into kinase activation (Husi et al., 2000; Husi and Grant, 2001). Thus, after NMDAR activation, it can be hypothesized that CaMKII modifies the neuronal profile of APP mRNA transcripts. This hypothesis is strengthened by a recent report that demonstrated the implication of CaM/CaMK axis in a Ca2+-mediated alternative splicing of mRNA in neurons (Xie and Black, 2001).
The neuronal expression of KPI-APP proteins induced by NMDAR activation leads to a predominant Aβ secretion instead of peptide p3, revealing a shift from an α-secretase toward β-secretase driven proteolysis of APP. This shift seems to be mediated by the KPI region of APP. Indeed, although the exact nature of the interaction between KPI-APP molecules and α-secretase candidate TACE remains to be further analyzed, the incubation of blocking antibodies raised against the KPI domain reversed the amyloidogenic effect of NMDA-induced expression of APP770 and APP751 without affecting the overall APP expression level or the shedding of its ectodomain. The influence of KPI-APP expression on secretase activities has been approached previously. Ho et al. (1996) have demonstrated that the transfection of KPI-APP in murine neuroblastoma cell lines induced a more drastic reduction of sAPPα than the transfection of APP695, suggesting that KPI-APP is more amyloidogenic than APP695. Moreover, the murine transgenic model of AD expressing KPI-containing APP elicit amyloid plaques at 6 months of age compared with 10-12 months for models using APP lacking the KPI domain (Hsiao et al., 1996; Sturchler-Pierrat et al., 1997; Hsiao, 1998). These findings are consistent with the idea of a pivotal role of KPI isoforms in Aβ amyloidosis (Moir et al., 1998). If the neuronal increase in KPI-APP does promote amyloidosis, the most likely mechanism would be by either altering Aβ catabolism or by driving the processing of APPs from α-secretase to β-secretase. In the former scenario, it could be speculated that KPI-APP proteins could inhibit serine proteases that normally degrade Aβ. One caveat for this postulate would be that sAPPα levels should also be altered following the modulation of Aβ. In our studies, it was not the case, because sAPPα levels were decreased and Aβ levels increased, suggesting that the potential inhibition of Aβ degradation by serine proteases is not modified by the neuronal expression of KPI-APP molecules. However, we evidenced the formation of a complex between α-secretase candidate TACE and neuronal KPI-APPs after NMDA exposure that resulted in an inhibition of TACE activity. Accordingly, this effect led to a shift from a predominant α-secretase to β-secretase cleavage of APP molecules.
In addition to the enhanced formation of Aβ resulting from this shift and its well known deleterious effects on neuronal survival, the lowering of sAPPα in the extracellular compartment might have important consequences for stressed neuronal cells, because sAPPα molecules have been shown to display neuroprotective effects (Mattson et al., 1993). However, the precise functional mechanism of how the KPI domain of APPs, initially described for its serine protease inhibitor properties, inhibits the metalloproteinase TACE [i.e., ADAM (a disintegrin and metalloprotease) protein family] remains undetermined but will be part of our future analyses.
In summary, the present study reveals a direct linkage between sustained but sublethal NMDAR activation and Aβ production in neurons. Although accumulation of Aβ after acute brain injuries may involve multiple mechanisms (Cotman et al., 1996), it can be suggested that even modest deregulation of the glutamatergic neurotransmission in the parenchyma surrounding the lesion may have an important impact on the probability to develop amyloid plaques. Thus, the pharmacological modulation of NMDAR function could represent a promising therapeutic intervention to reduce Aβ accumulation after acute brain injuries. A better understanding of the relationship between glutamatergic neurotransmission deregulation and the amyloidogenic pathway may improve our knowledge about AD itself and determine a better way to treat it.
Footnotes
This work was supported by the Centre National de la Recherche Scientifique, the University of Caen, and the Institut Paul Hamel. S. L. was supported by a doctoral fellowship from the Conseil Régional de Basse Normandie. We thank Dennis Selkoe, Luc Buée, André Delacourte, Nicolas Sergeant, Frédéric Checler, Peter Seubert, and Nikolaos Robakis for providing the R1736, R1742, R600, APPCter-C17, FCA3542, 192wt, and R7 antisera. Also, we thank Patrick Cleary and Hae-Sun Park for their technical help and Karen H. Ashe for her support.
Correspondence should be addressed to Dr. Alain Buisson, Unité Mixte de Recherche, Centre National de la Recherche Scientifique 6185, Centre Cyceron, Boulevard H. Becquerel, BP5229, 14074 Caen Cedex, France. E-mail: buisson@cyceron.fr.
Copyright © 2005 Society for Neuroscience 0270-6474/05/259367-11$15.00/0
References
- Abe K, Tanzi RE, Kogure K (1991) Selective induction of Kunitz-type protease inhibitor domain-containing amyloid precursor protein mRNA after persistent focal ischemia in rat cerebral cortex. Neurosci Lett 125: 172-174. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M (2004) Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci 61: 657-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Buxbaum JD, Khorkova O, Heroux J, Sahasrabudhe S, Martinez J, Warter JM, Mohr M, Checler F (1997) Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer's disease and cerebral amyloid angiopathy cases. Mol Med 3: 695-707. [PMC free article] [PubMed] [Google Scholar]
- Behrouz N, Defossez A, Delacourte A, Hublau P, Mazzuca M (1989) Alzheimer's disease: glycolytic pretreatment dramatically enhances immunolabeling of senile plaques and cerebrovascular amyloid substance. Lab Invest 61: 576-583. [PubMed] [Google Scholar]
- Blasko I, Beer R, Bigl M, Apelt J, Franz G, Rudzki D, Ransmayr G, Kampfl A, Schliebs R (2004) Experimental traumatic brain injury in rats stimulates the expression, production and activity of Alzheimer's disease beta-secretase (BACE-1). J Neural Transm 111: 523-536. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD (2004) Pathophysiology of cerebral ischemia and brain trauma: similarities and differences. J Cereb Blood Flow Metab 24: 133-150. [DOI] [PubMed] [Google Scholar]
- Bussiere T, Friend PD, Sadeghi N, Wicinski B, Lin GI, Bouras C, Giannakopoulos P, Robakis NK, Morrison JH, Perl DP, Hof PR (2002) Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer's disease. Neuroscience 112: 75-91. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA (1998) Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem 273: 27765-27767. [DOI] [PubMed] [Google Scholar]
- Choi DW (1988) Glutamate neurotoxicity and diseases of the nervous system. Neuron 1: 623-634. [DOI] [PubMed] [Google Scholar]
- Choi DW (1995) Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends Neurosci 18: 58-60. [PubMed] [Google Scholar]
- Cotman CW, Tenner AJ, Cummings BJ (1996) Beta-amyloid converts an acute phase injury response to chronic injury responses. Neurobiol Aging 17: 723-731. [DOI] [PubMed] [Google Scholar]
- Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A (2003) Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 74: 857-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885-890. [DOI] [PubMed] [Google Scholar]
- Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA (2000) Head injury and the risk of AD in the MIRAGE study. Neurology 54: 1316-1323. [DOI] [PubMed] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, Selkoe DJ (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359: 322-325. [DOI] [PubMed] [Google Scholar]
- Haass C, Hung AY, Schlossmacher MG, Teplow DB, Selkoe DJ (1993) Beta-amyloid peptide and a 3-kDa fragment are derived by distinct cellular mechanisms. J Biol Chem 268: 3021-3024. [PubMed] [Google Scholar]
- Hellal F, Pruneau D, Palmier B, Faye P, Croci N, Plotkine M, Marchand-Verrecchia C (2003) Detrimental role of bradykinin B2 receptor in a murine model of diffuse brain injury. J Neurotrauma 20: 841-851. [DOI] [PubMed] [Google Scholar]
- Heyman A, Wilkinson WE, Stafford JA, Helms MJ, Sigmon AH, Weinberg T (1984) Alzheimer's disease: a study of epidemiological aspects. Ann Neurol 15: 335-341. [DOI] [PubMed] [Google Scholar]
- Ho L, Fukuchi K, Younkin SG (1996) The alternatively spliced Kunitz protease inhibitor domain alters amyloid beta protein precursor processing and amyloid beta protein production in cultured cells. J Biol Chem 271: 30929-30934. [DOI] [PubMed] [Google Scholar]
- Hsiao K (1998) Transgenic mice expressing Alzheimer amyloid precursor proteins. Exp Gerontol 33: 883-889. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274: 99-102. [DOI] [PubMed] [Google Scholar]
- Husi H, Grant SG (2001) Proteomics of the nervous system. Trends Neurosci 24: 259-266. [DOI] [PubMed] [Google Scholar]
- Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000) Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci 3: 661-669. [DOI] [PubMed] [Google Scholar]
- Kim HS, Lee SH, Kim SS, Kim YK, Jeong SJ, Ma J, Han DH, Cho BK, Suh YH (1998) Post-ischemic changes in the expression of Alzheimer's APP isoforms in rat cerebral cortex. NeuroReport 9: 533-537. [PubMed] [Google Scholar]
- LeBlanc AC, Chen HY, Autilio-Gambetti L, Gambetti P (1991) Differential APP gene expression in rat cerebral cortex, meninges, and primary astroglial, microglial and neuronal cultures. FEBS Lett 292: 171-178. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW (1999) The changing landscape of ischaemic brain injury mechanisms. Nature 399: A7-14. [DOI] [PubMed] [Google Scholar]
- Linnik MD, Zobrist RH, Hatfield MD (1993) Evidence supporting a role for programmed cell death in focal cerebral ischemia in rats. Stroke 24: 2002-2009. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ, Barker JL (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321: 519-522. [DOI] [PubMed] [Google Scholar]
- MacManus JP, Buchan AM, Hill IE, Rasquinha I, Preston E (1993) Global ischemia can cause DNA fragmentation indicative of apoptosis in rat brain. Neurosci Lett 164: 89-92. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82: 4245-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Cheng B, Culwell AR, Esch FS, Lieberburg I, Rydel RE (1993) Evidence for excitoprotective and intraneuronal calcium-regulating roles for secreted forms of the beta-amyloid precursor protein. Neuron 10: 243-254. [DOI] [PubMed] [Google Scholar]
- Moir RD, Lynch T, Bush AI, Whyte S, Henry A, Portbury S, Multhaup G, Small DH, Tanzi RE, Beyreuther K, Masters CL (1998) Relative increase in Alzheimer's disease of soluble forms of cerebral Abeta amyloid protein precursor containing the Kunitz protease inhibitory domain. J Biol Chem 273: 5013-5019. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA (1991) Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 20 [Suppl 2]: S28-S35. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD (1997) The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 56: 165-170. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC (2000) Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55: 1158-1166. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Schroder E, Walker LC, Kessler C (1998) Beta-amyloid precursor protein and ss-amyloid peptide immunoreactivity in the rat brain after middle cerebral artery occlusion: effect of age. Stroke 29: 2196-2202. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Salton SR, Anderson JP, Mehta P, Robakis NK (1989) Nerve and epidermal growth factors induce the release of the Alzheimer amyloid precursor from PC 12 cell cultures. Biochem Biophys Res Commun 164: 664-670. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Graham DI (1991) Beta A4 amyloid protein deposition in brain after head trauma. Lancet 338: 1422-1423. [DOI] [PubMed] [Google Scholar]
- Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI (1994) Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry 57: 419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K, Goldberg MP, Choi DW (1993) Cytotoxicity in murine neocortical cell culture. In: In vitro biological methods. Methods in toxicology (Tyson CA, Frazier JM, eds), pp 46-60. San Diego: Academic.
- Roses AD, Saunders A (1995) Head injury, amyloid beta and Alzheimer's disease. Nat Med 1: 603-604. [DOI] [PubMed] [Google Scholar]
- Saido TC, Yokota M, Maruyama K, Yamao-Harigaya W, Tani E, Ihara Y, Kawashima S (1994) Spatial resolution of the primary beta-amyloidogenic process induced in postischemic hippocampus. J Biol Chem 269: 15253-15257. [PubMed] [Google Scholar]
- Schlondorff J, Becherer JD, Blobel CP (2000) Intracellular maturation and localization of the tumour necrosis factor alpha convertase (TACE). Biochem J 347: 131-138. [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Weller M, Moskowitz MA (1999) Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol 45: 421-429. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741-766. [DOI] [PubMed] [Google Scholar]
- Sergeant N, David JP, Champain D, Ghestem A, Wattez A, Delacourte A (2002) Progressive decrease of amyloid precursor protein carboxy terminal fragments (APP-CTFs), associated with tau pathology stages, in Alzheimer's disease. J Neurochem 81: 663-672. [DOI] [PubMed] [Google Scholar]
- Skovronsky DM, Moore DB, Milla ME, Doms RW, Lee VM (2000) Protein kinase C-dependent alpha-secretase competes with beta-secretase for cleavage of amyloid-beta precursor protein in the trans-golgi network. J Biol Chem 275: 2568-2575. [DOI] [PubMed] [Google Scholar]
- Smith DH, Nakamura M, McIntosh TK, Wang J, Rodriguez A, Chen XH, Raghupathi R, Saatman KE, Clemens J, Schmidt ML, Lee VM, Trojanowski JQ (1998) Brain trauma induces massive hippocampal neuron death linked to a surge in beta-amyloid levels in mice overexpressing mutant amyloid precursor protein. Am J Pathol 153: 1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Iwata A, Graham DI (2003) Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg 98: 1072-1077. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR (1997) Brain infarction and the clinical expression of Alzheimer disease. The nun study. JAMA 277: 813-817. [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Burki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B (1997) Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci USA 94: 13287-13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME (1998) Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 20: 709-726. [DOI] [PubMed] [Google Scholar]
- Uryu K, Laurer H, McIntosh T, Pratico D, Martinez D, Leight S, Lee VM, Trojanowski JQ (2002) Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J Neurosci 22: 446-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME (2001) Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA 98: 11024-11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby DA, Rozovsky I, Lo AC, Finch CE (1995) Beta-amyloid precursor protein (APP) and APP-RNA are rapidly affected by glutamate in cultured neurons: selective increase of mRNAs encoding a Kunitz protease inhibitor domain. J Mol Neurosci 6: 257-276. [DOI] [PubMed] [Google Scholar]
- Xie J, Black DL (2001) A CaMK IV responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature 410: 936-939. [DOI] [PubMed] [Google Scholar]