Abstract
A molecular genetic approach was exploited to directly test the hypothesis that voltage-gated K+ (Kv) channel pore-forming (α) subunits of the Kv4 subfamily encode the fast transient outward K+ current (IA) in cortical pyramidal neurons and to explore the functional role of IA in shaping action potential waveforms and in controlling repetitive firing in these cells. Using the biolistic gene gun, cDNAs encoding a mutant Kv4.2 α subunit (Kv4.2W362F), which functions as a dominant negative (Kv4.2DN), and enhanced green fluorescent protein (EGFP) were introduced in vitro into neurons isolated from postnatal rat primary visual cortex. Whole-cell voltage-clamp recordings obtained from EGFP-positive pyramidal neurons revealed that IA is selectively eliminated in cells expressing Kv4.2DN. The densities and properties of the other Kv currents are unaffected. In neurons expressing Kv4.2DN, input resistances are increased and the (current) thresholds for action potential generation are decreased. In addition, action potential durations are prolonged, the amplitudes of afterhyperpolarizations are reduced, and the responses to prolonged depolarizing inputs are altered markedly in cells expressing Kv 4.2DN. At low stimulus intensities, firing rates are increased in Kv4.2DN-expressing cells, whereas at high stimulus intensities, Kv4.2DN-expressing cells adapt strongly. Together, these results demonstrate that Kv4α subunits encode IA channels and that IA plays a pivotal role in shaping the waveforms of individual action potentials and in controlling repetitive firing in visual cortical pyramidal neurons.
Keywords: Kv channels, Kv4.2DN, gene gun, neuronal excitability, repetitive firing, remodeling
Introduction
Voltage-gated K+ (Kv) channels play fundamental roles in controlling neuronal excitability and, in most neurons, multiple Kv currents, with unique time- and voltage-dependent properties, are coexpressed. This multiplicity likely has physiological significance in that Kv currents with distinct properties should contribute differently to control resting potentials, input resistances, action potential waveforms and repetitive firing patterns. Nevertheless, it has been difficult to clarify the functional roles of individual Kv currents, primarily because of the paucity of highly specific Kv channel blockers. In addition, although molecular studies have revealed considerable diversity in Kv channel pore-forming and accessory subunits, the relationships between these subunits and functional Kv channels are poorly understood (Coetzee et al., 1999; Pongs, 1999; Song, 2002). Identification of the molecular correlates of neuronal Kv channels would facilitate efforts focused on defining the roles of individual Kv channel types in controlling neuronal firing properties and responses to synaptic inputs.
Rapidly activating and inactivating Kv currents, referred to as IA, are present in many central and peripheral neurons (Rogawski, 1985; Dodson and Forsythe, 2004; Jerng et al., 2004). Electrophysiological studies suggest that IA channels are predominantly expressed in the somal and dendritic compartments of pyramidal neurons, although transient K+ currents are also evident in axons and presynaptic terminals in some cells (Dodson and Forsythe, 2004). In addition to regulating the excitability and output properties of pyramidal neurons (Bekkers, 2000a,b; Kang et al., 2000), somatodendritic IA channels modulate the backpropagation (Stuart et al., 1997) of action potentials (into dendrites), impacting synaptic responses and plasticity (Hoffman et al., 1997; Hausser et al., 2000; Johnston et al., 2003). Patch-clamp recordings suggest that IA densities vary along the dendrites in hippocampal and cortical pyramidal neurons (Hoffman et al., 1997; Johnston et al., 2003) and that the properties of dendritic IA channels are modulated by protein kinase A- and C-dependent phosphorylation (Hoffman and Johnston, 1998; Schrader et al., 2002; Yuan et al., 2002). As in other parts of the CNS (Song et al., 1998; Tkatch et al., 2000; Song, 2002; Trimmer and Rhodes, 2004), Kv α subunits of the Kv4 subfamily are robustly expressed in the hippocampus and cortex (Sheng et al., 1992; Maletic-Savatic et al., 1995; Serodio and Rudy, 1998), and Kv4.2 has been shown to be a target of hippocampal protein kinase A (Schrader et al., 2002). These findings have been interpreted as suggesting that Kv4.2 encodes somatodendritic IA channels (Jerng et al., 2004).
The experiments here were undertaken to directly test the hypothesis that Kv4 α subunits encode IA channels in pyramidal neurons in (rat) primary visual cortex. In these studies, a mutant Kv4.2 subunit, which functions as a dominant negative, Kv4.2DN (Barry et al., 1998), was introduced with enhanced green fluorescent protein (EGFP) into cortical neurons using the biolistic “gene gun.” Whole-cell recordings from EGFP-positive pyramidal cells revealed that the IA is selectively eliminated in cells expressing Kv4.2DN. In addition, input resistances are increased, (current) thresholds for action potential generation are decreased, action potentials are prolonged, and repetitive firing patterns are altered by the elimination of IA.
Materials and Methods
Isolation and in vitro maintenance of visual cortical neurons. Visual cortical neurons were isolated from postnatal day 4 (P4) to P7 Long-Evans rat pups using previously described methods (Locke and Nerbonne, 1997a). Briefly, after anesthesia with 5% halothane, animals were decapitated, and the brains were removed. Primary visual cortices were isolated, minced, and incubated in papain-containing solution at 35°C. Isolated cortical neurons were obtained by trituration and subsequent centrifugation. Dissociated cells were resuspended in minimal Eagle's essential medium containing 5% heat-inactivated horse serum and plated at a density of 10 × 103/cm2 on monolayers of cortical astrocytes [prepared as described by Raff et al. (1979)]. Cells were maintained in a 95% O2/5% CO2 37°C incubator, and the medium was exchanged every other day.
Transfection of isolated visual cortical neurons. Isolated visual cortical neurons were transfected using the biolistics method, as described previously for sympathetic neurons (Malin and Nerbonne, 2000, 2002). In these experiments, 1.6 μm gold beads were coated with pCMV-EGFP (Clontech, Palo Alto, CA), which encodes EGFP, or with pBK-CMV-Kv4.2DN (Malin and Nerbonne, 2002) and pCMV-EGFP in a 4:1 ratio. In some experiments, cells were transfected with a pore mutant of Kv2.1 that also functions as a dominant negative, Kv2.1DN (Malin and Nerbonne, 2002). In this case, the beads were coated with pBK-CMV-Kv2.1DN together with pCMV-EGFP in a 4:1 ratio. In all experiments, coated beads were propelled (450 psi; 2 mm carrier distance) into cortical neurons at 2 d in vitro using the biolistics gene gun (Bio-Rad, Hercules, CA). After transfections, the cultures appeared healthy, and the expression of EGFP was detected under epifluorescence illumination within 24 h.
Immunohistochemistry. Immunohistochemical experiments were completed to examine the expression and distribution of Kv4.2 and Kv4.3 in isolated postnatal cortical neurons maintained for several days in vitro. Cultures were prepared as described above and were fixed after 72 h in vitro in 4% paraformaldehyde in PBS at pH 7.4 overnight at 4°C. After washing with PBS and a 1 h incubation in blocking buffer (PBS containing 5% normal goat serum, 0.2% Triton X-100, and 0.1% NaN3), cells were incubated overnight at 4°C with a monoclonal anti-Kv4.2 or a monoclonal anti-Kv4.3 antibody (Brunet et al., 2004) at 2 μg/ml in blocking buffer. After washing with PBS, cells were then exposed for 1 h at room temperature to a indocarbocyanine 3-conjugated goat-anti-mouse IgG secondary antibody (Chemicon, Temecula, CA) diluted 1:1000 in blocking buffer. Labeling was visualized under epifluorescence illumination, and individual Kv4.2- and Kv4.3-expressing cells were photographed.
Electrophysiological recordings. Whole-cell voltage-clamp and current-clamp recordings were obtained from large, pyramidal-shaped wild-type (untransfected) and EGFP-positive visual cortical neurons 48-72 h after transfection at room temperature (22-25°C). Recording pipettes, fabricated from borosilicate glass using a horizontal puller (model P-87; Sutter Instruments, Novato, CA), had resistances of 2-5 MΩ when filled with the standard recording solution (see below). Data were collected using an Axopatch 1D patch-clamp amplifier (Molecular Devices, Union City, CA). Experimental parameters were controlled with a P5-120 Gateway 2000 computer (Gateway, Irvine, CA), interfaced to the electrophysiological recording equipment through a Digidata 1200 interface, using the pClamp software (Molecular Devices). For all voltage-clamp recordings, the bath solution contained the following (in mm): 140 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 10 glucose, 0.001 tetrodotoxin (TTX), and 0.1 CdCl2, pH 7.4 (310 mOsm). The pipette solution contained the following (in mm): 135 KCl, 10 HEPES, 10 glucose, 10 EGTA, 5 Mg-ATP, and 0.3 Tris-GTP, pH 7.3 (310 mOsm). For current-clamp recordings, the TTX and CdCl2 were omitted from the bath solution, and the intracellular free-Ca2+ concentration in the pipette solution was fixed at 10-7 m by lowering the EGTA concentration to 5 mm in the presence of 1 mm CaCl2.
In all experiments, tip potentials were zeroed before membrane-pipette seals were formed. Depolarization-activated outward K+ currents were routinely recorded in response to 300 ms or 6 s depolarizing voltage steps to test potentials between -30 and +70 mV from a holding potential of -70 mV. Inwardly rectifying K+ currents were recorded in response to 500 ms hyperpolarizing voltage steps to test potentials between -70 and -140 mV from a holding potential of -50 mV. Currents were low-pass filtered at 5 kHz and digitized at 0.2-10 kHz. To examine the kinetics of recovery of the voltage-gated K+ currents from steady-state inactivation, a three-pulse protocol was used. Each cell was first depolarized to +50 mV for 10 s to activate and inactivate the outward K+ currents. Cells were then hyperpolarized to -70 mV (or -90 mV) for varying times, ranging from 10 ms to 9.5 s, before the test depolarization to +50 mV, presented to assess the extent of recovery. Single action potentials and action potential trains were recorded in response to brief (5 ms) and prolonged (500 ms), respectively, depolarizing current injections of variable amplitudes.
Data analysis. Data were compiled and analyzed using Clampfit (Molecular Devices) and Excel (Microsoft, Redmond, WA). The spatial control of the membrane voltage in each cell was assessed by analyzing the decays of the capacitative transients evoked during ±10 mV voltage steps from the holding potential of -70 mV; only cells with capacitative transients well described by single exponentials were analyzed further. Whole-cell membrane capacitances (Cm) were determined by integration of the capacitative transients evoked during brief (25 ms) subthreshold (±10 mV) voltage steps from the holding potential (-70 mV). Input resistances (Rin) were calculated from the steady-state currents recorded in response to the -10 mV hyperpolarizing voltage steps. Leak currents at -70 mV were always ≤20 pA and were not corrected. In each cell, the series resistance (Rs) was calculated by dividing the time constant of decay of the capacitive transient by the Cm (determined in the same cell). The mean ± SEM Rs was ≤9.0 ± 1.5 MΩ, and, in all cells, Rs was compensated electronically by ∼90%. Because current amplitudes were ≤10 nA, voltage errors resulting from the uncompensated Rs were always ≤10 mV and were not corrected. Current amplitudes Rin and Rs were monitored continuously during all recordings. Only cells in which these parameters remained constant were analyzed further.
Peak outward K+ currents in individual cells were defined as the maximum value of the outward K+ currents evoked during (300 ms or 6 s) depolarizing voltage steps. Plateau (steady-state) outward K+ currents were defined as the currents remaining at the end of the 6 s voltage steps. To determine the amplitudes of the individual components of the total Kv currents, the decay phases of the currents were fitted by the sum of two or three exponentials; all parameters were free. Current amplitudes, measured in individual cells, were normalized for differences in cell size (whole-cell Cm), and current densities are reported (in picoamperes per picofarads). The kinetic analyses also provided the time constants of inactivation of the Kv current components in wild-type, EGFP-expressing, and Kv4.2DN plus EGFP-expressing visual cortical pyramidal neurons.
All current-clamp recordings were obtained from cells with over-shooting action potentials and stable resting membrane potentials less than or equal to -50 mV. The (current) threshold for action potential generation in each cell was defined as the minimal current, applied for 5 ms, required to evoke a single action potential. The latency to firing in action potential was defined as the time after the onset of depolarizing current injection required to bring the membrane to the (voltage) threshold for action potential generation. Action potential durations at 50% (and 90%) repolarization were determined by measuring the widths of the action potentials when the membrane voltage had returned halfway (or 90% of the way) back to the resting potential; values are reported in milliseconds. The amplitudes and durations of afterhyperpolarizations were determined by measuring the maximal deflections from, and the time required to return to, the resting membrane potential, respectively. To examine the effects of Kv4.2DN on repetitive firing, the initial firing frequency (the inverse of the time between the first and second action potentials of the train) was measured in individual cells at different stimulus intensities.
All averaged and normalized data are presented as means ± SEM. The statistical significance of apparent differences between wild-type, EGFP-expressing, and Kv4.2DN plus EGFP-expressing cells was examined using the Student's t test, and, where appropriate, p values are presented in the text.
Results
Outward K+ currents in rat visual cortical pyramidal neurons
In the experiments here, cortical neurons were isolated from the primary visual cortices of P4-P7 rats, plated on glial monolayers, and maintained in vitro. Large, pyramidal-shaped neurons were selected for recordings (Desai et al., 1999), and whole-cell depolarization-activated outward K+ currents were recorded in the presence of 1 μm TTX and 0.1 mm CdCl2 to block voltage-gated Na+ and Ca2+ currents, respectively. Representative outward currents recorded from the majority (16 of 20) of isolated visual cortical pyramidal neurons (see also below) in response to brief (300 ms) and prolonged (6 s) membrane depolarizations to varying test potentials (-30 to + 70 mV) from a holding potential of -70 mV are presented in Figure 1A. The rates of rise and the amplitudes of the currents increase with increasing membrane depolarization; the largest and most rapidly activating current in Figure 1A was evoked at +70 mV. No outward currents were recorded when the K+ in the recording pipettes was replaced with Cs+ (n = 7). The depolarization-activated outward currents recorded and analyzed here, therefore, reflect only the currents through Ca2+-independent Kv channels.
Figure 1.
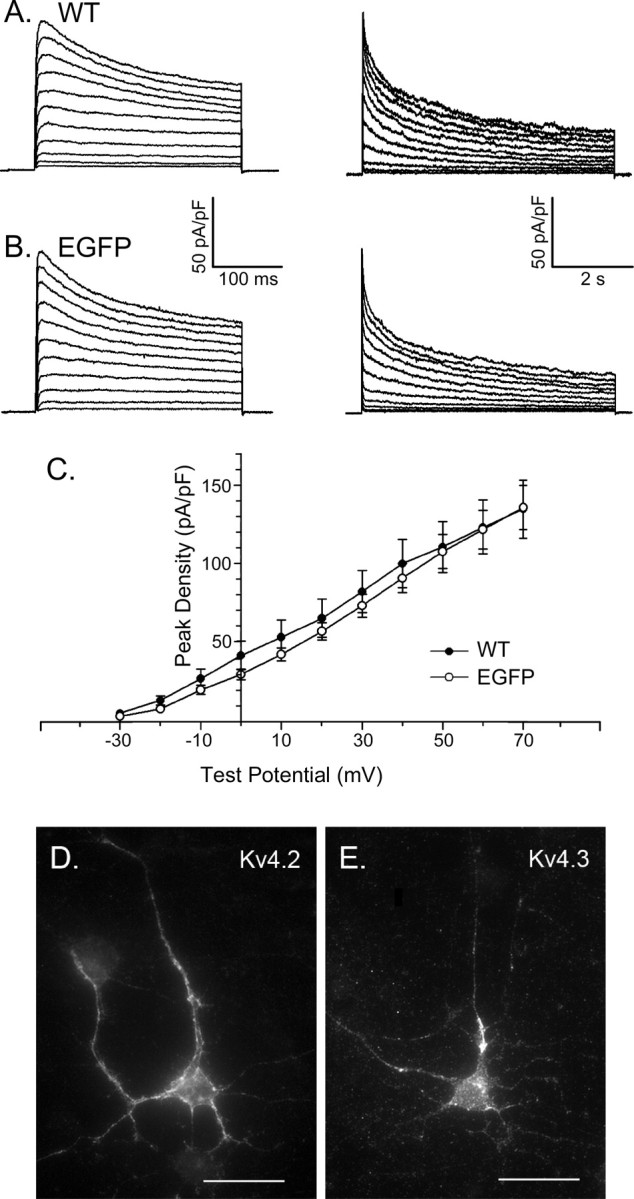
Voltage-gated outward K+ current waveforms and Kv4 α subunit expression in cortical pyramidal neurons. A, B, Whole-cell voltage-gated outward K+ (Kv) currents were recorded from isolated (rat) visual cortical pyramidal neurons in response to 300 ms (left panels) and 6 s (right panels) depolarizing voltage steps to test potentials between -30 and + 70 mV from a holding potential of -70 mV. Experiments were conducted as described in Materials and Methods with 1 μm TTX and 0.1 mm CdCl2 in the bath solution to block voltage-gated inward Na+ and Ca2+ currents, respectively. Representative recordings from nontransfected, wild-type and transfected EGFP-expressing cells are illustrated in A and B, respectively. C, Mean ± SEM normalized peak outward K+ current densities are plotted as a function of test potential. Outward K+ current waveforms, peak outward K+ current densities, and the current-voltage relation of the peak outward K+ currents are indistinguishable in wild-type (WT) and EGFP-expressing (rat) visual cortical pyramidal neurons. D, E, Immunohistochemical experiments (see Materials and Methods) reveal the expression of Kv4.2 (D) and Kv4.3 (E) in the soma and dendrites of isolated cortical neurons. Error bars represent SEM.
Depolarization-activated outward K+ currents activate rapidly in wild-type visual cortical pyramidal neurons (Albert and Nerbonne, 1995; Locke and Nerbonne, 1997a), although there is some variability among cells in peak outward Kv current amplitudes/densities (Fig. 1C) and in the decay phases of the currents. Similar results were obtained in cells isolated from animals at P4-P7, as well as in cells maintained for various times (2-5 d) in vitro. In the vast majority (∼80%) of cells, outward K+ current waveforms similar to those in Figure 1A were recorded. As reported previously (Albert and Nerbonne, 1995; Locke and Nerbonne, 1997a), the decay phases of the outward K+ currents in these cells were well described by the sum of three exponentials with mean ± SEM (n = 16) inactivation time constants (τdecay;at +60 mV) of 62 ± 7, 529 ± 72, and 2874 ± 235 ms (Fig. 2D). The rapid component of current decay is attributed to the presence of a fast transient outward “A” current, typically referred to as IA (Kang et al., 2000; Shibata et al., 2000; Song, 2002) or ISA (Hoffman et al., 1997; Johnston et al., 2003; Jerng et al., 2004). The more slowly inactivating (tdecay ≈ 500 ms), transient outward K+ current that is also evident in rat visual cortical projection neurons (Albert and Nerbonne, 1995; Locke and Nerbonne, 1997a) is referred to as ID (Storm, 1988). The very slowly decaying outward K+ current component (τdecay ≈ 3000 ms) is referred to as IK, and the steady-state, noninactivating component of the total depolarization-activated outward K+ current is referred to as Iss. In a subset (4 of 20; 20%) of cortical pyramidal neurons, only two exponentials were required to describe the decay phases of the outward currents. The decay time constants derived from these fits were 535 ± 67 and 2527 ± 327 ms, consistent with the expression of only ID and IK (and Iss) in these cells. In a small subset of cortical pyramidal neurons, therefore, IA is not evident.
Figure 2.
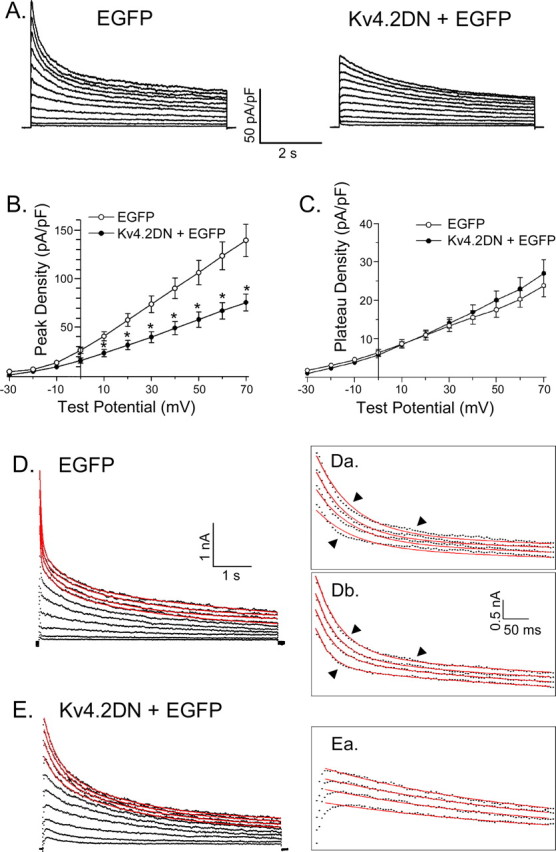
Expression of Kv4.2DN reduces peak outward K+ currents and eliminates the rapid component of current decay (IA) in cortical pyramidal neurons. A, Whole-cell Kv currents were recorded (as described in the legend to Fig. 1) from cortical pyramidal neurons expressing EGFP alone or EGFP and Kv4.2DN. As is evident, the waveforms of the currents recorded from cells expressing Kv4.2DN plus EGFP (right panel) are distinct from those recorded from cells expressing EGFP alone (left panel). B, Mean ± SEM (n = 25) peak outward K+ current densities are reduced significantly (p < 0.001) at all test potentials in Kv4.2DN plus EGFP-expressing cells. C, In contrast, mean ± SEM plateau outward K+ current densities in cells expressing Kv4.2DN plus EGFP and cells expressing EGFP alone are not significantly different. D, E, Exponential fits (plotted as lines in red) to the decay phases of the outward K+ currents (plotted as points in black) in EGFP-expressing (D) and Kv4.2DN plus EGFP-expressing (E) cells are displayed. D, E, Insets, The currents (plotted as points in black) at the more positive potentials are displayed on an expanded time scale with the fits (plotted as lines in red) superimposed. The decay phases of the outward currents in cells expressing EGFP alone (D) are not well described by the sum of two exponentials (Da), and three components (Db) are required to fit the experimental data; the arrows are placed to illustrate the differences in the quality of the fits with two (Da) and three (Db) exponential components. In cells expressing Kv4.2DN and EGFP (E), in contrast, the rapid component of current decay is not evident, and the decay phases of the currents are well described (Ea) by the sum of two exponentials. Error bars represent SEM.
Selective elimination of IA in Kv4.2DN-expressing cortical pyramidal neurons
As noted in the Introduction, considerable evidence has accumulated suggesting that Kv4 α subunits encode IA channels in the somatodendritic compartments of cortical and hippocampal pyramidal neurons. Importantly, immunohistochemical experiments revealed that both Kv4.2 (Fig. 1D) and Kv4.3 (Fig. 1E) are readily detected in the cell soma and in the dendrites of cortical pyramidal neurons isolated at postnatal day 5 and maintained for 72 h in vitro. To test directly the hypothesis that IA in these cells reflects the expression of Kv4 α subunits, isolated cortical neurons were transfected with the mutant Kv4.2 dominant-negative (Kv4.2DN) construct (Barry et al., 1998). In these experiments, gold particles were coated either with cDNA constructs encoding Kv4.2DN and EGFP or with the EGFP cDNA construct alone, and cells were transfected using the biolistics gene gun (see Materials and Methods). Within ∼24 h of transfection, EGFP expression was readily detected under epifluorescence illumination. Control experiments revealed that the waveforms of the outward K+ currents in the vast majority (∼80%) of cells expressing EGFP alone (Fig. 1B) are similar to those recorded in most (∼80%) wild-type cells (Fig. 1A). The densities and the current-voltage relationships of the peak outward K+ currents in EGFP-expressing and wild-type cortical pyramidal neurons are indistinguishable (Fig. 1C). The decay phases of the outward K+ currents in most cells are best described by the sum of three exponentials (Table 1), and the relative densities of IA, ID, IK, and Iss in EGFP-expressing and wild-type visual cortical pyramidal neurons are not significantly different (Table 1). In addition, similar to wild-type cells, in ∼20% (5 of 26) of the EGFP-expressing cells, the rapidly inactivating current component, IA, was absent (Table 1). The expression of EGFP, therefore, does not appear to measurably alter the properties or the functioning of depolarization-activated outward K+ currents in cortical pyramidal neurons.
Table 1.
Outward Kv currents in EGFP- and Kv4.2DN plus EGFP-expressing cortical neurons a
|
|
EGFP |
Kv4.2DN plus EGFP |
Kv2.1DN plus EGFP |
||
|---|---|---|---|---|---|
| Peak (pA/pF) | 132 ± 13 | 120 ± 23 | 90 ± 10* | 99 ± 10 | |
| IA (pA/pF) | 50 ± 4 | 48 ± 4 | |||
| IA τdecay (ms) | 67 ± 9 | 59 ± 8 | |||
| ID (pA/pF) | 25 ± 3 | 46 ± 14 | 28 ± 4 | 18 ± 3 | |
| ID τdecay (ms) | 553 ± 81 | 538 ± 69 | 516 ± 76 | 540 ± 53 | |
| IK (pA/pF) | 36 ± 4 | 44 ± 4 | 38 ± 4 | 20 ± 3** | |
| IK τdecay (ms) | 2994 ± 213 | 1762 ± 222 | 2529 ± 259 | 3026 ± 270 | |
| Iss (pA/pF) | 23 ± 2 | 30 ± 9 | 25 ± 2 | 16 ± 3 | |
|
n
|
21 |
5 |
25 |
15 |
|
*Peak current densities are significantly (p < 0.01) lower in Kv4.2DN plus EGFP-expressing cells than in cells expressing EGFP alone. **The value is significantly (p < 0.01) lower in Kv2.1DN plus EGFP-expressing cells than in EGFP- or Kv4.2DN plus EGFP-expressing cells.
All values are means ± SEM; current densities were determined at +60 mV (holding potential, −70 mV); n = number of cells.
The waveforms of the outward K+ currents in cells expressing Kv4.2DN (+EGFP) (Fig. 2A), in contrast, are distinct from those recorded from wild-type (Fig. 1A) and EGFP-expressing cells (Fig. 1B). Specifically, the rapid component of current decay, IA, which is prominent in most wild-type (Fig. 1A) and EGFP-expressing (Fig. 1B) cortical pyramidal neurons, appears to be missing in cells expressing Kv4.2DN (+EGFP) (Fig. 2A). Peak outward K+ current densities at all test potentials are significantly (p < 0.001) lower in Kv4.2DN plus EGFP-expressing cells than in cells expressing EGFP alone (Fig. 2B). The mean ± SEM density of the (plateau) currents remaining at the end of the 6 s depolarizations in EGFP- and Kv4.2DN plus EGFP-expressing cells, in contrast, are not significantly different (Fig. 2C).
The decay phases of the outward currents in EGFP-expressing cells were best described by the sum of three exponentials (Fig. 2D), with mean ± SEM (n = 21) τdecay values of 67 ± 9, 553 ± 81, and 2994 ± 213 ms (Table 1), consistent with the expression of IA, ID, IK, and Iss. In contrast, the decay phases of the outward currents in all (n = 25) Kv4.2DN-expressing cells were best described by the sum of two exponentials (Fig. 2E), with mean ± SEM time constants of 516 ± 76 and 2529 ± 259 ms (Table 1). The faster decay time constant (τdecay = 516 ± 76 ms) obtained from these fits is significantly (p < 0.001) larger than the τdecay for IA (Table 1) but is very similar to the τdecay for ID in wild-type and EGFP-expressing (Table 1) cells. The simplest interpretation of these observations is that IA is selectively eliminated in cells expressing Kv4.2DN and that ID is unaffected. The mean ± SEM τdecay value for the very slowly decaying current component, IK, in Kv4.2DN plus EGFP-expressing cells is also not significantly different from that determined in wild-type and EGFP-expressing cells (Table 1). Similar to ID, therefore, the expression of Kv4.2DN does not measurably affect the properties (or the densities) of IK (Table 1). In addition, the selective elimination of IA observed (Fig. 2) is specific for Kv4.2DN expression. In cells expressing a mutant Kv2.1 dominant-negative construct (Kv2.1DN) that has been shown to selectively block delayed rectifier K+ currents in peripheral neurons (Malin and Nerbonne, 2002), for example, IA densities are indistinguishable from control (wild-type) cells (Table 1). In pyramidal cells expressing Kv2.1DN, however, IK density is selectively attenuated (Table 1).
The whole-cell membrane capacitances (Cm) of Kv4.2DN plus EGFP-expressing cells (mean ± SEM, 22 ± 2 pF; n = 25) and in cells expressing EGFP alone (mean ± SEM, 23 ± 2 pF; n = 30) were not significantly different. In contrast, and as discussed further below, the mean ± SEM input resistance of Kv4.2DN plus EGFP-expressing cells (1.61 ± 0.33 GΩ; n = 25) was significantly (p < 0.001) higher than in cells expressing EGFP alone (mean ± SEM, 1.11 ± 0.21 GΩ; n = 30) (Table 2).
Table 2.
Passive and active membrane properties of EGFP-, Kv4.2DN plus EGFP-, and Kv2.1DN plus EGFP-expressing cortical neurons a
|
|
Cm |
Rin |
RMP (mV) |
APthresh (pA) |
APA (mV) |
APD50 (ms) |
APD90 (ms) |
AHP (mV) |
|---|---|---|---|---|---|---|---|---|
| EGFP (n = 30) | 23 ± 2 | 1.1 ± 0.2 | −55 ± 1 | 40 ± 5 | 104 ± 3 | 3.8 ± 0.2 | 6.7 ± 0.4 | −7.8 ± 0.4 |
| Kv4.2DN plus EGFP (n = 25) | 22 ± 2 | 1.6 ± 0.3* | −56 ± 4 | 20 ± 5** | 96 ± 6 | 8.4 ± 0.7** | 17.1 ± 1.3** | −1.9 ± 0.3** |
| Kv2.1DN plus EGFP (n = 10) |
23 ± 2 |
1.2 ± 0.3 |
−55 ± 2 |
39 ± 6 |
99 ± 6 |
3.8 ± 0.2 |
13.0 ± 0.7*
|
−3.4 ± 1.7*
|
RMP, Resting membrane potential; AHP, afterhyperpolarization; APthresh, current threshold to evolve a single action potential in response to a 5 ms depolarizing input; APA, action potential amplitude; ADP50 and ADP90, action potential durations at 50 and 90% repolarization.
aAll values are means ± SEM. Values are significantly different (*p < 0.05; **p < 0.01) from those determined in cells expressing EGFP alone.
The fast component of recovery is eliminated in Kv4.2DN-expressing cortical pyramidal neurons
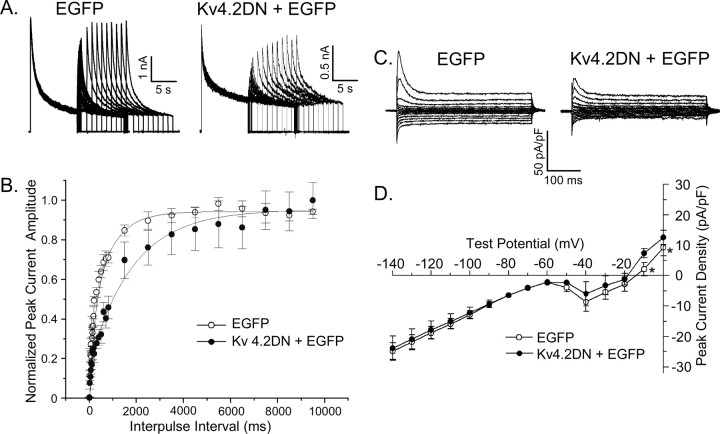
In addition to rapid inactivation, IA in most neurons is characterized by rapid recovery from inactivation (Jerng et al., 2004). Subsequent experiments were focused, therefore, on examining the time course of outward K+ current recovery from steady-state inactivation. In these experiments, EGFP- and Kv4.2DN plus EGFP-expressing cells were first depolarized to +50 mV for 10 s (prepulse) to inactivate the currents and were subsequently hyperpolarized to -70 mV for varying times ranging from 10 to 9500 ms (to allow recovery from inactivation). Test depolarizations to +50 mV were then presented to assess the extent of recovery. Typical current waveforms recorded from EGFP-expressing and Kv4.2DN plus EGFP-expressing neurons using this paradigm are illustrated in Figure 3A. The amplitudes of the peak outward K+ currents evoked at +50 mV after each recovery period in each cell were determined and normalized to the peak current amplitudes recorded during the 10 s prepulses (in the same cell). Mean ± SEM normalized current amplitudes were then determined and plotted as a function of recovery time (Fig. 3B). The recovery data for the EGFP-expressing cells (n = 14) were best described by the sum of two exponentials with mean ± SEM recovery time constants (τrec) (at -70 mV) of 100 ± 20 and 950 ± 75 ms. The relative contributions of the fast and slow components of recovery of the peak currents were ∼45 and ∼35%, respectively; ∼20% of the peak outward current in wild-type cells is contributed by Iss, which is noninactivating.
Figure 3.
The rapidly recovering outward K+ current (IA) is eliminated, and inward rectifier K+ currents are unaffected, in Kv4.2DN plus EGFP-expressing cortical pyramidal neurons. To examine the kinetics of recovery of the outward K+ currents from inactivation, a three-pulse protocol was used. After inactivating the currents during 10 s prepulses to +50 mV, EGFP- and Kv4.2 plus EGFP-expressing cortical pyramidal neurons were hyperpolarized to -70 mV for varying times (ranging from 10 to 9500 ms) before test depolarizations to +50 mV were presented to assess the extent of recovery. A, Representative current waveforms recorded from cells expressing EGFP alone (left panel) or Kv4.2DN plus EGFP (right panel) are displayed. Peak outward current amplitudes evoked at +50 mV after each recovery period in each (EGFP- or Kv4.2DN plus EGFP-expressing) cell were measured and normalized to the peak outward current amplitude recorded from the same cell after the 9.5 s recovery period. B, Mean ± SEM normalized peak K+ currents are plotted as a function of recovery time. The rapid component of peak current recovery, clearly evident in EGFP-expressing cells (n = 14), is not evident in Kv4.2DN plus EGFP-expressing cells (n = 9), consistent with the elimination of IA. C, Representative K+ currents recorded from EGFP-expressing (left panel) and Kv4.2DN plus EGFP-expressing (right panel) cells in response to 300 ms voltage steps to test potentials, ranging from -140 to 0 mV from a holding potential of -70 mV, are illustrated. D, Mean ± SEM peak K+ current densities in EGFP-expressing (n = 10) and Kv4.2DN plus EGFP-expressing (n = 12) cells are plotted as a function of test potential. Over the voltage range of -140 to -20 mV, K+ current densities in EGFP- and Kv4.2DN plus EGFP-expressing cells are not significantly different. Only at the more depolarized test potentials (-10 to 0 mV) are peak K+ currents significantly (*p < 0.01) lower in Kv4.2 plus EGFP-expressing cells than in EGFP-expressing cells. Error bars represent SEM.
Analyses of the current waveforms after each recovery period (in individual records, such as those illustrated in Fig. 3A) provided similar estimates of the relative amplitudes of the fast (IA) and slow (ID plus IK) components of the total (peak) outward K+ current, as well as of Iss. In cells expressing Kv4.2DN plus EGFP (n = 9), the recovery data were best described by a single exponential with a τrec (at -70 mV) of 1075 ± 125 ms (Fig. 3B). The fast component of peak outward recovery is eliminated in cells expressing Kv4.2DN plus EGFP (Fig. 3C), consistent with the loss of IA (Table 1). In addition, in Kv4.2DN plus EGFP-expressing cells, the relative contribution of Iss to the peak current (∼35%) is increased because of the elimination of IA. In contrast to the marked attenuation of depolarization-activated outward K+ currents, no effects of Kv4.2DN expression on inwardly rectifying, IK1, currents in cortical pyramidal neurons (Fig. 3C,D) were observed.
Action potentials are prolonged in Kv4.2DN-expressing cortical pyramidal neurons
Previous studies have suggested that IA plays an important role in shaping action potential waveforms, in determining interspike intervals, and in regulating repetitive firing in central neurons (Jerng et al., 2004). Because the time- and voltage-dependent properties of IA vary in different cell types, the functioning of IA is expected to be variable as well as, perhaps, cell-type specific. Although understanding the functional role(s) of IA has long been of interest, previous studies focused on exploring IA functioning directly have been complicated by the paucity of specific IA blockers. Nevertheless, several approaches have been exploited, and novel insights have been provided. Combined action potential and voltage-clamp recordings, for example, suggest that IA channels likely play an important role in action potential repolarization in cortical pyramidal neurons (Kang et al., 2000). It has also been demonstrated that the input resistances of visual cortical neurons are increased in the presence of an often used IA-selective blocker, 4-aminopyridine (4-AP) (Locke and Nerbonne, 1997b). In addition, the current thresholds for action potential generation and the latency to firing are reduced, and action potentials are reportedly prolonged significantly (p < 0.001) in the presence of millimolar concentrations of 4-AP (Locke and Nerbonne, 1997b). In visual cortical neurons, however, ID is also blocked effectively by millimolar 4-AP (Locke and Nerbonne, 1997a). To determine which of the effects of 4 mm 4-AP can be attributable to the loss of IA, subsequent experiments were aimed at evaluating directly the role of IA in shaping the waveforms of individual action potentials and in controlling the repetitive firing properties in visual cortical pyramidal neurons expressing Kv4.2DN.
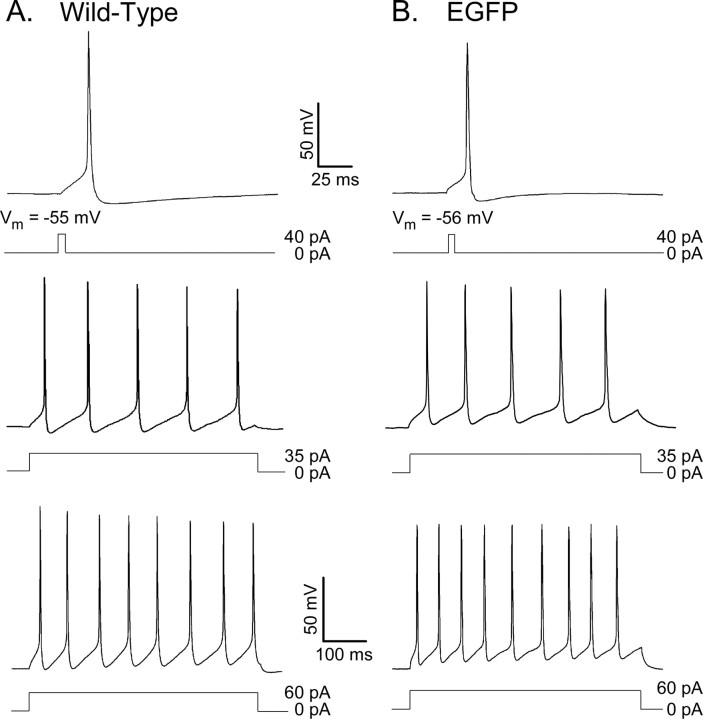
Action potentials in isolated visual cortical pyramidal neurons are brief, and, in response to prolonged current injections, these cells fire repetitively (Fig. 4A). In all cells, action potentials rise rapidly to a maximal potential of approximately +50 mV, and repolarization is rapid (Fig. 4A). The waveforms of individual action potentials and repetitive firing patterns evoked in EGFP-expressing neurons (Fig. 4B) are indistinguishable from those recorded in wild-type cells (Fig. 4A). As in wild-type cells, single action potentials are routinely evoked by brief (5 ms) depolarizing current injections; the mean ± SEM current threshold for firing was 40 ± 5 pA (Table 2). In addition, resting membrane potentials (mean ± SEM, -55 + 1 mV; n = 30) and action potential amplitudes (mean ± SEM, 104 + 3 mV) measured in EGFP-expressing neurons are indistinguishable from those measured in wild-type cells. Repetitive firing patterns and firing frequencies, in response to prolonged depolarizing current injections in wild-type (Fig. 4A) and EGFP-expressing (Fig. 4B) cortical pyramidal neurons, are indistinguishable. By all criteria, therefore, the presence of EGFP does not appear to measurably affect the passive or the active membrane properties of cortical pyramidal neurons.
Figure 4.
EGFP-expressing (wild-type) visual cortical pyramidal neurons are regular spiking. A, B, Single action potentials, evoked by brief (5 ms) depolarizing current injections, and repetitive firing patterns, evoked by prolonged (500 ms) depolarizing current injections, were recorded from isolated wild-type (A) and EGFP-expressing (B) cortical pyramidal neurons, as described in Materials and Methods. The amplitudes and durations of the injected currents are illustrated below the voltage records. As is evident, action potentials in these cells are brief, and afterhyperpolarizations are pronounced. In response to prolonged current injections, the number of action potentials evoked and the rate of firing are increased as the amplitude of the depolarizing current injection is increased.
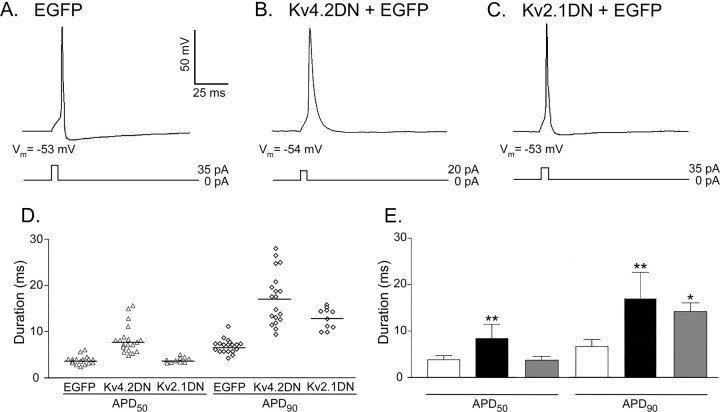
The resting membrane potentials and the amplitudes of evoked action potentials in EGFP- and Kv4.2DN plus EGFP-expressing cortical pyramidal neurons are not significantly different (Table 2). The current thresholds to evoke (single) action potentials in Kv4.2DN plus EGFP-expressing neurons, however, are significantly (p < 0.001) lower than in cells expressing EGFP alone (Table 2). The latency to action potential firing in response to brief, low-amplitude current injections is shorter in cells expressing Kv4.2DN plus EGFP (Fig. 5B) compared with wild-type or EGFP-expressing (Fig. 5A) cells. In addition, the waveforms of evoked (single) action potentials in EGFP-expressing (Fig. 5A) and Kv4.2DN plus EGFP-expressing (Fig. 5B) cortical pyramidal neurons are distinct. In EGFP-expressing cells, action potential durations, measured at 50% repolarization, ranged from 2.4 to 5.6 ms, with mean ± SEM (n = 30) of 3.8 ± 0.2 ms (Fig. 5D). Measured at 90% repolarization, action potential durations in these (EGFP-expressing) cells ranged from 4.0 to 11 ms, with a mean of 6.7 ± 0.4 ms (Fig. 5D). In Kv4.2DN plus EGFP-expressing cells, however, action potential durations at both 50 and 90% repolarization are considerably more variable (Fig. 5D). In addition to the increased heterogeneity in action potential durations in individual cells (Fig. 5D), mean (± SEM) action potential durations at 50 and 90% repolarization are significantly (p < 0.001) longer (Fig. 5E) in Kv4.2DN plus EGFP-expressing neurons than in cells expressing EGFP alone (Table 2).
Figure 5.
Action potentials are prolonged markedly in Kv4.2DN plus EGFP-expressing visual cortical pyramidal neurons. A-C, Representative action potentials recorded from EGFP- (A), Kv4.2DN plus EGFP- (B) and Kv2.1DN plus EGFP- (C) expressing cells, as described in the legend to Figure 4, are illustrated. The amplitudes and durations of the current injections are illustrated below the records. Action potentials were elicited in response to lower-amplitude current injections in Kv4.2DN plus EGFP-expressing cells (B) compared with cells expressing EGFP alone (A) or EGFP plus Kv2.1DN (C). In addition, action potential waveforms are distinct. Action potential durations, measured at 50% (APD50) and 90% (APD90) repolarization, in individual cells, for example, are more heterogeneous (D) in Kv4.2DN plus EGFP-expressing cells than in cells expressing EGFP or EGFP plus Kv2.1DN (mean ± SD). E, APD50 and APD90 values are significantly (**p < 0.001) longer in Kv4.2DN plus EGFP-expressing cells than in the cells expressing EGFP alone. In Kv2.1DN plus EGFP-expressing cells, in contrast, only mean ± SD APD90 values are significantly (*p < 0.005) longer than in wild-type cortical pyramidal neurons; APD50 values are not measurably affected by the expression of Kv2.1DN. Error bars represent SEM.
The functional effects observed (Fig. 5, Table 2) are specific for the expression of Kv4.2DN and the elimination of IA, as demonstrated by the distinct effects of expression of the mutant Kv2.1 dominant-negative construct (Kv2.1DN) that selectively attenuates IK (Table 1). As illustrated in Figure 5C, expression of Kv2.1DN has rather modest affects on action potential waveforms in cortical pyramidal neurons. Action potential durations at 90% repolarization are increased with expression of Kv2.1DN (Fig. 5E), whereas action potential durations at 50% repolarization are unaffected. In addition, the expression of Kv2.1DN does not affect the input resistances or the currents required to evoke action potentials in cortical pyramidal neurons (Table 2).
There are also marked reductions in the amplitudes and the durations of afterhyperpolarizations in Kv4.2DN plus EGFP-expressing neurons (Fig. 5B) compared with EGFP-expressing (Fig. 5A) and wild-type (Fig. 4A) cells. The mean ± SEM amplitudes of the afterhyperpolarizations in EGFP-expressing and Kv4.2DN plus EGFP-expressing neurons were -7.8 ± 0.4 and -1.9 ± 0.3 mV, respectively (Table 2). The expression of Kv2.1DN also affects the amplitudes of afterhyperpolarizations (Table 2), although the magnitudes of the reductions in amplitudes are considerably smaller than observed with Kv4.2DN.
Repetitive-firing patterns are altered in Kv4.2DN-expressing cortical pyramidal neurons
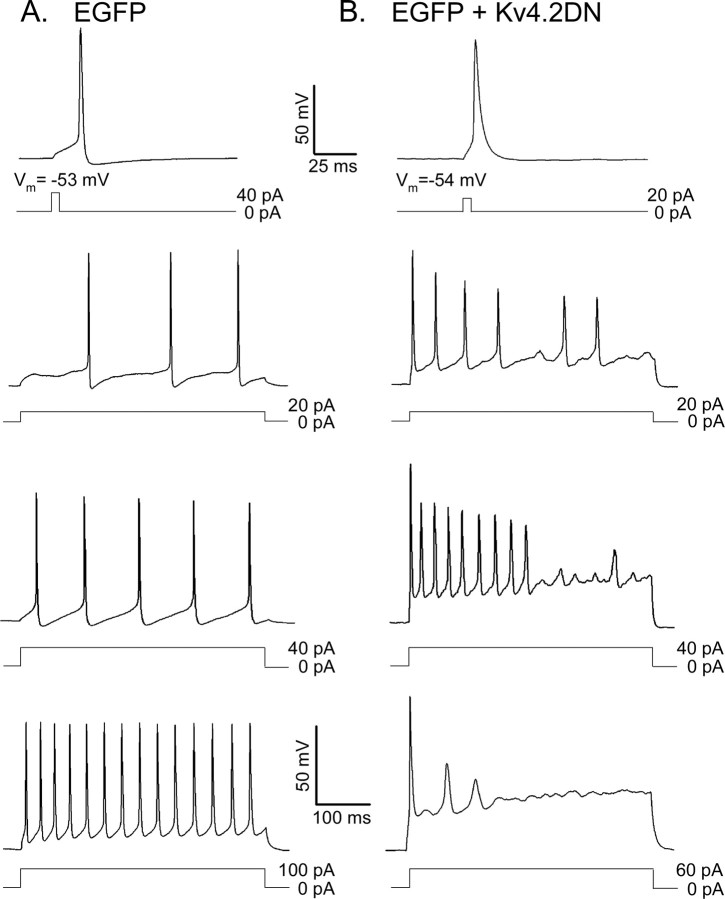
The effects of Kv4.2DN expression on the repetitive firing properties of cortical pyramidal neurons were examined by measuring the responses of Kv4.2DN plus EGFP-expressing cells to 500 ms depolarizing current injections of varying amplitudes (from 5 to 100 pA). As in wild-type cells (Locke and Nerbonne 1997b), all EGFP-expressing cortical pyramidal neurons display regular-spiking behavior. Firing frequencies increase with stimulus strength (Fig. 6A), with maximal firing frequencies observed in the range of 20-30 Hz under the recording conditions used here. Although there are some differences among cells in the extent of spike-frequency adaptation, the vast majority (>80%) of EGFP-expressing cortical pyramidal neurons fire repetitively through the duration of the 500 ms current injections, and firing rates increase with increasing depolarizing injections (Fig. 6A).
Figure 6.
Repetitive firing is altered in Kv4.2DN plus EGFP-expressing cortical pyramidal neurons. Representative action potential waveforms and repetitive firing patterns recorded from EGFP-expressing (A) and Kv4.2DN plus EGFP-expressing (B) cells. Individual action potentials and action potential trains were evoked by 5 and 500 ms depolarizing current injections, as described in the legend to Figure 4. In cells in which IA has been eliminated, the current threshold to evoke an action potential is reduced significantly (p < 0.001) compared with wild-type cells. At low stimulus intensities, cells expressing Kv4.2DN plus EGFP (B) are more excitable than cells expressing EGFP alone (A), as evidenced by the higher firing rates observed in cells in response to low-amplitude current injections. Although the initial firing rate is increased in both EGFP- and Kv4.2DN plus EGFP-expressing cells with higher stimulus intensities, the effects of increasing amplitude current injections are distinct. In EGFP-expressing cells, the firing frequency increases monotonically with the amplitude of the injected current. In Kv4.2 plus EGFP-expressing cells, however, the frequency of firing is increased in response to low-amplitude current injections compared with cells expressing EGFP alone, although firing is often not maintained. In response to large-amplitude current injections, cells expressing Kv4.2 DN plus EGFP actually cease firing.
In cortical neurons expressing Kv4.2DN plus EGFP, the current threshold required to evoke repetitive firing is decreased, and the responses to prolonged current injections are altered markedly (Fig. 6). In response to low-amplitude current injections (5 and 30 pA), Kv4.2DN plus EGFP-expressing cells fire repetitively, and the instantaneous firing frequency is higher (Fig. 6B) than in cells expressing EGFP alone (Fig. 6A). At higher stimulus intensities, however, the increased input resistances of Kv4.2DN plus EGFP-expressing neurons, combined with the loss of IA, results in substantial steady-state membrane depolarization during prolonged current injections (Fig. 6B). This steady-state depolarization counters repolarization and slows repetitive firing. In response to 500 ms current injections of larger magnitude (≥40 pA), Kv4.2DN-expressing cells adapt strongly and actually stop firing (Fig. 6B). The elimination of IA, therefore, increases the excitability of cortical pyramidal neurons at low stimulus intensities and reduces excitability of these cells at high stimulus intensities.
Discussion
Kv4 α subunits encode IA in cortical pyramidal neurons
A molecular genetic approach, exploiting a mutant Kv4.2 subunit, Kv4.2DN, that functions as a dominant negative (Barry et al., 1998), was used to test the hypothesis that Kv4 α subunits encode the rapidly activating and rapidly inactivating transient outward K+ current, IA, in cortical pyramidal neurons and to explore the functional role of IA in shaping action potentials and controlling repetitive firing in these cells. Previous studies demonstrated that Kv4.2DN is specific for Kv4-encoded channels and that currents encoded by individual Kv4 α subunits, e.g., Kv4.2 and Kv4.3 (Tsaur et al., 1997), are similarly affected. The results presented reveal that IA is eliminated in cells expressing Kv4.2DN, thereby demonstrating directly that Kv4 α subunits encode IA in these cells. Additional studies will be needed to define the specific role(s) of Kv4.2 and other Kv4 subunits, Kv4.3 and Kv4.1, in the generation of IA in cortical pyramidal neurons.
Neither the amplitudes nor the properties of the other Kv currents, ID, IK, or Iss, which are expressed in cortical pyramidal neurons, are affected measurably by the expression of Kv4.2DN and the resulting elimination of IA. The simplest interpretation of these combined observations is that ID, IK, and Iss reflect the expression of distinct molecular entities and likely are encoded by distinct Kv α subunit subfamilies. The selective attenuation of IK in cells expressing Kv2.1DN is consistent with a role for Kv2.1 in the generation of IK. Additional experiments will be necessary to explore this hypothesis further and to define directly the molecular correlates of ID, IK, and Iss. In addition, however, the observations here that ID, IK, and Iss are unaffected by the expression of Kv4.2DN and the elimination of IA suggest that electrical remodeling does not occur in cortical projection neurons in vitro with the removal of IA.
Functional consequences of the elimination of IA
The simplest interpretation of the observation that the input resistances of cortical pyramidal neurons expressing Kv4.2DN are higher than in cells expressing EGFP alone is that some IA channels in these cells are open at the resting membrane potential. This finding is supported by voltage-clamp data documenting an IA “window current” in the -70 to -50 mV range (Stuart et al., 1997). Elimination of the channels (open at rest) with the expression of Kv4.2DN might be expected to result in membrane depolarization. Interestingly, however, the elimination of IA does not measurably affect the resting membrane potentials of cortical pyramidal neurons, suggesting that channels other than IA channels are the primary determinants of resting potentials in these cells. The impact of the increased input resistances of cells expressing Kv4.2DN, however, is evident in increased membrane excitability. The mean (±SEM) current threshold for action potential generation is reduced significantly (p < 0.001) in Kv4.2DN-expressing cells, compared with wild-type cells. These results suggest that IA regulates excitability in cortical projection neurons by opposing depolarizing inputs and influencing the current required to reach the threshold for action potential generation. Because of the increased input resistances of Kv4.2DN-expressing cells, the latency to firing action potentials in response to a low-amplitude current injection is increased significantly (p < 0.001).
In addition to increasing excitability, the functional “knock-out” of IA in vitro also results in markedly increased action potential durations. These observations are consistent with previous studies suggesting an important role for IA channels in controlling action potential durations in cortical pyramidal neurons (Locke and Nerbonne, 1997b; Kang et al., 2000). Indeed, all phases of action potential repolarization are increased significantly (p < 0.001) in Kv4.2DN plus EGFP-expressing cells. In addition, the effects of Kv4.2DN expression (and the elimination of IA) on input resistances and action potential durations are specific. Attenuation of IK in cells expressing Kv2.1DN, for example, has no effect on input resistances, the currents required to evoke action potentials, or action potential durations at 50% repolarization. Only action potential durations at 90% repolarization and the amplitudes of afterhyperpolarizations are measurably affected by the expression of Kv2.1DN. The amplitudes and durations of afterhyperpolarizations are also reduced in cells expressing Kv4.2DN.
Repetitive firing in cortical pyramidal neurons is also affected by the loss of IA. Because of increased input resistances, smaller currents are required to reach the threshold for action potential generation in Kv4.2DN plus EGFP-expressing cells than in cells expressing EGFP alone or in cells expressing Kv2.1DN plus EGFP. In addition, although cells expressing Kv4.2DN fire at higher frequencies in response to smaller current injections than do wild-type cells, excitability is reduced, and firing rates are decreased at high stimulus intensities.
Relationship to previous studies
The results presented here demonstrate directly a role for Kv4 α subunits in the generation of depolarization-activated K+ channels in (rat) visual cortical pyramidal neurons. These observations are consistent with previous suggestions that Kv4 subunits (and specifically Kv4.2) encode IA channels in pyramidal neurons in the hippocampus (Sheng et al., 1992; Serodio and Rudy, 1998; Johnston et al., 2003; Jerng et al., 2004; Trimmer and Rhodes, 2004). Using the same dominant-negative strategy as exploited here, however, it has been demonstrated directly that Kv4 α subunits encode IA channels in (peripheral) neurons isolated from the (rat) superior cervical (sympathetic) ganglia (Malin and Nerbonne, 2000). A similar strategy was exploited to demonstrate a role for Kv4 α subunits in the generation of IA in cerebellar granule cells (Johns et al., 1997; Shibata et al., 2000). It has also been reported recently that IA is attenuated in CA1 hippocampal neurons expressing Kv4.2DN (Cai et al., 2004). To our knowledge, however, this study is the first to demonstrate directly that Kv4 α subunits encode IA channels in cortical pyramidal neurons and to explore the role of IA in regulating the excitability of these cells.
Paradoxically, IA plays a dual role in the regulation of membrane excitability. The expression of IA decreases the input resistance of cortical pyramidal neurons and increases the current (threshold) required to fire an action potential. The presence of IA lowers the excitability of pyramidal neurons at low stimulus intensities because of the fact that some channels are open at rest and function to shunt the impact of depolarizing inputs. The elimination of IA, therefore, increases excitability, at least at low stimulus intensities. It is also clear, however, that IA channels, which activate, inactivate, and perhaps most importantly, recover rapidly from inactivation, function to allow cells to respond to prolonged depolarizing inputs of highly variable amplitudes and durations. When IA is eliminated, action potential repolarization is slowed, afterhyperpolarizations are reduced in amplitude and time course, and repetitive firing is altered. The elimination of IA markedly reduces the response to high-intensity stimuli, and cortical neurons lacking IA actually cease firing during prolonged large-amplitude current injections. The net effect of losing or attenuating IA, therefore, will depend on the stimulus intensity. Given that cortical neurons typically function in a voltage range near rest (or the threshold for action potential generation) and that individual stimuli are low in amplitude and brief, it seems likely that the loss of IA will be evident as an increase (rather than a decrease) in the excitability of cortical pyramidal neurons. Consistent with this view, the attenuation of IA in acquired temporal lobe epilepsy is associated with increased dendritic excitability in CA1 hippocampal neurons (Bernard et al., 2004).
In the present study, no alterations in the properties or densities of K+ (or other) conductance pathways were evident in cells lacking IA, suggesting that, at least over the time course of the experiments here (2-3 d), channel remodeling or plasticity does not occur. These observations are in marked contrast with previous findings in the (lobster) stomatogastric ganglion neurons in which alterations in the expression of wild-type or mutant Shal (invertebrate Kv4) were shown to result in the remodeling of other (specific) ionic conductances (MacLean et al., 2003). The results presented here are somewhat surprising in light of previous reports suggesting that the intrinsic membrane properties of rat cortical neurons in vitro are quite plastic, changing as a function of previous neuronal activity (Desai et al., 1999). It has been demonstrated, for example, that voltage-gated Na+ and K+ current densities are altered in isolated cortical neurons exposed in vitro (for 7 d) to TTX (Desai et al., 1999). It is possible that these seemingly disparate observations reflect the fact that the specific experimental paradigms used in a previous study (Desai et al., 1999) and the present study were quite different. Clearly, additional studies focused on detailing the specific molecular mechanisms involved in regulating and remodeling of the intrinsic cortical neuron membrane properties are warranted. In addition, additional studies will be needed to define directly the role(s) of individual Kv4.x α subunits, as well as the role(s) of putative Kv4 subfamily accessory subunits (An et al., 2000; Nakamura et al., 2001; Nadal et al., 2003) in the generation of functional IA channels in cortical (and other) cells.
Footnotes
This work was supported by National Institutes of Health Grants R01-NS-30676 and R01-NS-41417. We thank Drs. Weinong Guo, Huilin Li, Sacha Malin, and Haodong Xu for many helpful comments and discussions during the course of this work. We also thank Amy Coleman for expert technical assistance in the preparation and maintenance of glial and neuronal cultures and Rick Wilson and Benjamin Gerber for assistance with the figures.
Correspondence should be addressed to Jeanne M. Nerbonne, Department of Molecular Biology and Pharmacology, Box 8103, Washington University Medical School, 660 South Euclid Avenue, St. Louis, MO 63110-1093. E-mail: jnerbonne@msnotes.wustl.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/259185-10$15.00/0
References
- Albert JA, Nerbonne JM (1995) Calcium-independent depolarization-activated potassium currents in superior colliculus-projecting rat visual cortical neurons. J Neurophysiol 73: 2163-2178. [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ (2000) Modulation of A-type potassium channels by a family of calcium sensors. Nature 403: 553-556. [DOI] [PubMed] [Google Scholar]
- Barry DM, Xu H, Schuessler RB, Nerbonne JM (1998) Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 α-subunit. Circ Res 83: 560-567. [DOI] [PubMed] [Google Scholar]
- Bekkers JM (2000a) Properties of voltage-gated potassium currents in nucleated patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol (Lond) 525: 593-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekkers JM (2000b) Distribution and activation of voltage-gated potassium channels in cell-attached and outside-out patches from large layer 5 cortical pyramidal neurons of the rat. J Physiol (Lond) 525: 611-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Anderson A, Becker A, Poolos NP, Beck H, Johnston D (2004) Acquired dendritic channelopathy in temporal lobe epilepsy. Science 305: 532-535. [DOI] [PubMed] [Google Scholar]
- Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM (2004) Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol (Lond) 559: 103-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao J, Tang CM, Thompson SM (2004) Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44: 351-364. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Meira E, Rudy B (1999) Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233-285. [DOI] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG (1999) Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515-520. [DOI] [PubMed] [Google Scholar]
- Dodson PD, Forsythe ID (2004) Presynaptic K+ channels: electrifying regulators of synaptic terminal excitability. Trends Neurosci 27: 210-217. [DOI] [PubMed] [Google Scholar]
- Hausser M, Spruston N, Stuart GJ (2000) Diversity and dynamics of dendritic signaling. Science 20: 739-744. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D (1998) Downregulation of transient K+ channels in dendrites of hippocampal CA1 pyramidal neurons by activation of PKA and PKC. J Neurosci 18: 3521-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D (1997) K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature 38: 869-875. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M (2004) Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci 27: 343-369. [DOI] [PubMed] [Google Scholar]
- Johns DC, Nuss HB, Marban E (1997) Suppression of neuronal and cardiac transient outward currents by viral gene transfer of dominant-negative Kv4.2 constructs. J Biol Chem 272: 31598-31603. [DOI] [PubMed] [Google Scholar]
- Johnston D, Christie BR, Frick A, Gray R, Hoffman DA, Schexnayder LK, Watanabe S, Yuan LL (2003) Active dendrites, potassium channels and synaptic plasticity. Philos Trans R Soc Lond B Biol Sci 358: 667-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA (2000) Voltage-gated potassium channels activated during action potentials in layer V neocortical neurons. J Neurophysiol 83: 70-80. [DOI] [PubMed] [Google Scholar]
- Locke RE, Nerbonne JM (1997a) Three kinetically distinct Ca2+-independent depolarization-activated K+ currents in callosal-projecting rat visual cortical neurons. J Neurophysiol 78: 2309-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke RE, Nerbonne JM (1997b) Role of voltage-gated K+ currents in mediating the regular-spiking phenotype callosal-projecting rat visual cortical neurons. J Neurophysiol 78: 2321-2335. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM (2003) Activity-independent homeostasis in rhythmically active neurons. Neuron 37: 109-120. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Lenn NJ, Trimmer JS (1995) Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro J Neurosci 15: 3840-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM (2000) Elimination of the fast transient in superior cervical ganglion neurons with expression of Kv4.2W362F: molecular dissection of IA J Neurosci 20: 5191-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Nerbonne JM (2002) Delayed rectifier K+ currents, IK, are encoded by Kv2 α-subunits and regulate tonic firing in mammalian sympathetic neurons. J Neurosci 22: 10094-10105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W, Goldberg EM, Misumi Y, Ikehara Y, Neubert TA, Rudy B (2003) The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron 37: 449-461. [DOI] [PubMed] [Google Scholar]
- Nakamura TY, Nandi S, Pountney DJ, Artman M, Rudy B, Coetzee WA (2001) Different effects of the Ca2+-binding protein, KChIP1, on two Kv4 subfamily members, Kv4.1 and Kv4.2. FEBS Lett 499: 205-209. [DOI] [PubMed] [Google Scholar]
- Pongs O (1999) Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett 452: 31-35. [DOI] [PubMed] [Google Scholar]
- Raff MC, Fields KL, Hakomori S, Mirsky R, Pruss RM, Winter J (1979) Cell-type specific markers for distinguishing and studying neurons and the major classes of glial cells in culture. Brain Res 174: 283-308. [DOI] [PubMed] [Google Scholar]
- Rogawski MA (1985) The A-current: how ubiquitous a feature of excitable cells is it? Trends Neurosci 8: 214-219. [Google Scholar]
- Schrader LA, Anderson AE, Mayne A, Pfaffinger PJ, Sweatt JD (2002) PKA modulation of Kv4.2-encoded A-type potassium channels requires formation of a supramolecular complex. J Neurosci 22: 10123-10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serodio P, Rudy B (1998) Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol 79: 1081-1091. [DOI] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY (1992) Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron 9: 271-284. [DOI] [PubMed] [Google Scholar]
- Shibata R, Nakahira K, Shibasaki K, Wakazono Y, Imoto K, Ikenaka K (2000) A-type K+ current mediated by the Kv4 channel regulates the generation of action potential in developing cerebellar granule cells. J Neurosci 20: 4145-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WJ (2002) Genes responsible for native depolarization-activated K+ currents in neurons. Neurosci Res 42: 7-14. [DOI] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ (1998) Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv. 2 and Kv4.1 subunits. J Neurosci 18: 3124-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M (1997) Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci 20: 125-131. [DOI] [PubMed] [Google Scholar]
- Tkatch T, Baranauskas G, Surmeier DJ (2000) Kv4.2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci 20: 579-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ (2004) Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol 66: 477-519. [DOI] [PubMed] [Google Scholar]
- Tsaur ML, Chou CC, Shih YH, Wang HL (1997) Cloning, expression and CNS distribution of Kv 4.3, an A-type K+ channel α subunit. FEBS Lett 400: 215-220. [DOI] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D (2002) Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci 22: 4860-4868. [DOI] [PMC free article] [PubMed] [Google Scholar]