Abstract
α-Latrotoxin, a potent stimulator of exocytosis from neurons and neuroendocrine cells, has been studied intensively, but the mechanisms of its actions are poorly understood. Here, we developed a new method to generate active recombinant α-latrotoxin and conducted a structure/function analysis of the toxin in stimulating Ca2+-dependent exocytosis. α-Latrotoxin consists of a conserved N-terminal domain and C-terminal ankyrin-like repeats. After cleavage of an N-terminally fused purification tag of glutathione S-transferase (GST), the recombinant toxin strongly stimulated exocytosis, whereas the GST-fused toxin was much less potent. The GST-fused toxin bound to the receptors [neurexin 1α; CL1 (CIRL/latrophilin 1)] as efficiently as did the GST-cleaved toxin but was much less effective in inserting into the plasma membrane and inducing cation conductance. The toxin with deletion of the last two ankyrin-like repeats still bound the receptors but could neither stimulate exocytosis nor induce cation conductance efficiently. The abilities of the mutated toxins to stimulate exocytosis correlated well with their abilities to induce cation conductance, but not their binding to the receptors. Our results indicate that (1) C-terminal ankyrin-like repeats and a free (unfused) N terminus are both required for the toxin to form pores, which is essential for Ca2+-dependent exocytosis, and (2) receptor binding alone is not sufficient to stimulate Ca2+-dependent exocytosis.
Keywords: α-latrotoxin, exocytosis, neurexin 1α, CL1, cation conductance, PC12 cell
Introduction
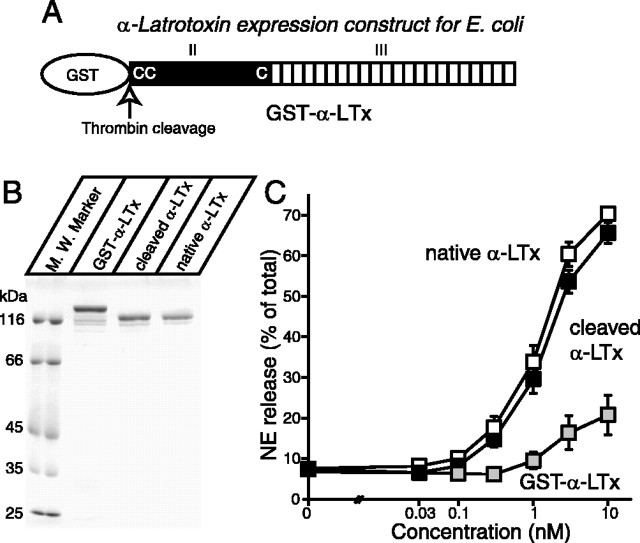
α-Latrotoxin, a member of latrotoxins isolated from black widow spider venom, has been extensively used to study the mechanisms of neurotransmitter exocytosis (Südhof, 2001; Nicholson and Graudins, 2002; Ushkaryov et al., 2004). Similar to the other members of latrotoxins, α-latrotoxin is a large protein (130 kDa) composed of four domains: (1) a cleaved signal peptide (domain I), (2) a conserved N-terminal domain with three invariant cysteine residues (domain II), (3) a domain composed of 15-22 ankyrin-like repeats (domain III), and (4) a C-terminal domain (domain IV) that is cleaved during venom maturation (Kiyatkin et al., 1990, 1993; Dulubova et al., 1996; Volynski et al., 1999). Mature latrotoxins are composed of domains II and III (see Fig. 1A).
Figure 1.
Analysis of recombinant α-latrotoxins. A, Schematic representation of mature α-latrotoxin and recombinant α-latrotoxins. Mature α-latrotoxin is composed of two domains (domains II and III). The conserved N-terminal domain is shown (II). Locations of the only three cysteine residues that are conserved in all latrotoxins are shown as C's. The C-terminal region, composed of 22 ankyrin-like repeats, is shown (III). B, Analysis of purified recombinant α-latrotoxins. The recombinant α-latrotoxins (GST-α-LTx, GST-cleaved α-LTx; ∼1-2 μg) along with native α-latrotoxin were analyzed by SDS-PAGE and Coomassie staining. C, GST-cleaved recombinant α-latrotoxin stimulates Ca2+-dependent exocytosis with a similar potency to native α-latrotoxin. A stimulation of NE secretion from PC12 cells by indicated concentrations of native α-LTx (white squares; n = 12), cleaved recombinant α-LTx (black squares; n = 10), and GST-α-LTx (gray squares; n = 3) is shown. Error bars indicate SEM.
α-Latrotoxin stimulates both Ca2+-independent exocytosis and extracellular Ca2+-dependent exocytosis. Its exocytotic effect requires binding to neuronal receptors (Grasso et al., 1980). Three classes of receptors have been identified: (1) neurexin 1α, a neuronal protein with a single transmembrane domain (Ushkaryov et al., 1992), (2) CIRL/latrophilin 1 (CL1) (Davletov et al., 1996; Krasnoperov et al., 1997), a member of the CL family, which are G-protein-coupled receptors (Sugita et al., 1998; Ichtchenko et al., 1999), and (3) protein tyrosine phosphatase σ (Krasnoperov et al., 2002a), a recently found receptor for the toxin. In either neurexin 1α or CL1 knock-out mice (Geppert et al., 1998; Tobaben et al., 2002), the neuronal sensitivity to α-latrotoxin was reduced, indicating that these two receptors constitute the major receptors for α-latrotoxin.
Interacting with its receptors, α-latrotoxin forms pores in the plasma membrane, evidenced by direct visualization of the toxin tetramers inserted into the membrane of liposomes using cryo-EM (Orlova et al., 2000). In cells that are normally insensitive to α-latrotoxin expressing exogenous toxin receptors (neurexin 1α or CL1), the toxin formed pores when bound to its receptor (Van Renterghem et al., 2000). Signaling-deficient mutants of the receptors are capable of facilitating pore formation and are sufficient for α-latrotoxin to induce Ca2+-dependent exocytosis (Sugita et al., 1998, 1999). In contrast, a recent report suggested that a specific mutant of α-latrotoxin (α-LTxN4C) stimulated Ca2+-dependent exocytosis without forming pores (Volynski et al., 2003). Thus, it remains unclear whether the pore formation is essential for the stimulation of Ca2+-dependent exocytosis or the activation G-protein-coupled CL1 receptor is sufficient for this process.
Mutational analysis using recombinant α-latrotoxin has identified the essential cysteine residues in domain II for binding to neurexin 1α and CL1 (Ichtchenko et al., 1998). Active recombinant toxins have been made using a baculovirus expression system, but the yield was relatively low. Thus, systematic structure/function analysis of α-latrotoxin has been a challenge because of a lack of the sufficient tool. Here, we generated active α-latrotoxin using a novel strain of Escherichia coli and created a series of truncated α-latrotoxins. Using these toxins, we investigated the structural requirements of α-latrotoxin in stimulating Ca2+-dependent exocytosis, specifically the critical roles of N-terminal insertion and C-terminal ankyrin-like repeats. We also demonstrate that the ability of the mutated toxins to stimulate exocytosis correlate with their abilities to induce cation conductance.
Materials and Methods
Expression constructs
α-Latrotoxin constructs. A 6-histidine (6His) tag sequence was introduced at the SalI-HindIII site of pGex-KG (Guan and Dixon, 1991) and pGex-KG-His was generated. This plasmid was used as a parental expression plasmid for the expression of all of the recombinant α-latrotoxins. We first subcloned a full-length cDNA of mature α-latrotoxin gene (residues 1-1177), without a stop codon, into the EcoRI-XbaI site of pGex-KG-His (pGex α-LTx-1). However, the C-terminal 6His tag turned out to be ineffective for the purification of the expressed protein. Therefore, the stop codon was introduced before the 6His sequence for pGex α-LTx. A series of C-terminal truncated α-latrotoxins (pGex α-LTxΔ1, Δ2, Δ3, and Δ8) were generated by PCR. pGex α-LTxΔ1 encodes residues 1-1149, pGex α-LTxΔ2 encodes residues 1-1116, pGex α-LTxΔ3 encodes residues 1-1080, and pGex α-LTxΔ8 encodes residues 1-907. We named these truncated constructs to indicate the number of deleted ankyrin-like repeats (i.e., α-LTxΔn, n = the number of deleted repeats). The N4C mutant (pGex α-LTxN4C) was generated according to Ichtchenko et al. (1998). We also introduced N-terminal myc tag for some of the recombinant α-latrotoxin constructs. In these constructs, the myc tag sequence was introduced at the BamHI site of pGexKG-His.
Neurexin 1α constructs. pCMVIG-N1α-31 and control pCMVIG-C have been described previously (Sugita et al., 2001).
CL1 construct. A 2.4 kb EcoRI/SalI PCR fragment encoding residues 1-766 of CL1 was subcloned into the EcoRI/SalI site of pCMV-IG9 (Sugita et al., 2001), generating pCMVIG-CL1-1.
Expression and purification of recombinant α-latrotoxins
pGex-α-LTx was transformed into Origami B (DE3) cells and the bacteria were grown at 37°C until confluent (OD, ∼1.0), and the expression of the recombinant protein was induced by isopropyl-β-d-thiogalactopyranoside (50 μm) at 18°C for 20 h. The culture was centrifuged, and the resulting pellet was resuspended in PBS containing Complete tablets for protease inhibition (Roche, Laval, Quebec, Canada). The resuspended culture was passed twice through a French cell press, and insoluble materials were cleared by centrifugation, and the recombinant α-latrotoxin in the supernatant was purified by glutathione agarose (GE Healthcare, Montreal, Quebec, Canada). To remove the glutathione S-transferase (GST) tag, we used thrombin (GE Healthcare) to digest the fusion protein attached onto the glutathione-agarose for 2 h at room temperature. After cleavage of GST with thrombin, the recombinant toxin contains an extra 13 residues (GSPGISGGGGGIL) derived from the linker sequence of pGex-KG at the N terminus, in addition to the native α-latrotoxin sequence. Thrombin was then irreversibly inactivated by PMSF. The purified recombinant toxins were dialyzed with physiological saline solutions (PSSs) containing the following (in mm): 145 NaCl, 5.6 KCl, 2.2 CaCl2, 0.5 MgCl2, 5.6 glucose, and 15 HEPES, pH 7.4, and stored in PSS containing 10% glycerol at -80°C. Under this condition, we found that the recombinant α-latrotoxin retains its stimulatory activity of norepinephrine (NE) release from pheochromocytoma 12 (PC12) cells for at least several months. Other truncated toxins were generated similarly.
PC12 secretion assay
PC12 cells are maintained in 10 cm dishes containing DMEM (Invitrogen, San Diego, CA) with 5% horse serum, 5% calf serum (HyClone, Logan, UT), and 100 U/ml penicillin/streptomycin (Sugita et al., 1999; Wang et al., 2004, 2005). For secretion assays, PC12 cells were plated in 24-well plates and labeled with 0.5 μCi/ml [3H]NE (GE Healthcare) in the presence of 0.5 mm ascorbic acid for 12-16 h. The labeled PC12 cells were incubated with the fresh complete DMEM for 1-5 h to remove unincorporated [3H]NE. The cells were washed once with PSS, and NE secretion was stimulated with 200 μl of PSS containing various concentrations (0.03-10 nm) of native α-latrotoxin, the recombinant toxins. Secretion was terminated after a 15 min incubation by chilling to 0°C, and samples were centrifuged at 4°C for 3 min. Supernatants were removed, and the pellets were solubilized in 0.1% Triton X-100 for liquid scintillation counting.
Expression and purification of Ig-fusion protein
COS-7 cells were transfected with pCMVIG-C, pCMVIG-N1α-31, or pCMVIG-CL1-1 by electroporation. Briefly, COS-7 cells in 10 cm dishes were digested with 1 ml of trypsin/EDTA for 2 min, 7 ml of DMEM growth medium (containing 10% fetal bovine serum and 100 U/ml penicillin/streptomycin) was added, and the cells were harvested. After centrifugation, COS-7 cells were washed with 6 ml of cytomix buffer containing the following (in mm): 120 KCl, 0.15 CaCl2, 10 K2HPO4, 10 KH2PO4, 2 EGTA, 5 MgCl2, and 25 HEPES, pH 7.6, and centrifuged. The resultant COS-7 cell pellets were resuspended in 500 μl of the cytomix buffer, containing 2 mm ATP and 5 mm reduced glutathione with ∼10 μg of the plasmid, and electroporated. Electroporated COS-7 cells were plated on 10 cm dishes with DMEM growth medium, which was replaced the next day. Three days after transfection, the medium of the transfected COS-7 cells was harvested, adjusted to 10 mm Tris-HCl, pH 8.0, and 1 mm EDTA, and cleared by centrifugation. The recombinant proteins in the supernatant were purified by protein A-agarose (Sigma, Oakville, Ontario, Canada).
Binding experiments between recombinant α-latrotoxins and neurexin 1α/CL1
Approximately 2 μg of α-LTx or GST-fused α-latrotoxin (GST-α-LTx) was mixed with protein A-agarose that attaches ∼3 μg of IG-C, IG-N1α-31, or IG-CL1-1 in 500 μl of PSS containing 0.1% Triton X-100, and incubated overnight at 4°C. Protein A-agarose was washed five times with 500 μl of PSS containing 0.1% Triton X-100. After removing the washing solution, the agarose was resuspended in 40 μl of 2 × SDS-PAGE sample buffer and boiled. The supernatant was subjected to SDS-PAGE and Coomassie staining.
In the experiments that used GST-myc proteins for binding, the agarose was resuspended in 120 μl of 2× sample buffer, and 20 μl of the supernatant after boiling was subjected to SDS-PAGE and the proteins were transferred to nitrocellulose. Myc-fusion proteins were probed by monoclonal antibody against myc (9E10; Covance, Denver, PA) using enhanced chemiluminescent detection.
Cell culture and transfection
Human embryonic kidney tSA-201 cells were maintained in a 37°C CO2 incubator in standard DMEM supplemented with 10% fetal bovine serum, 200 U/ml penicillin, and 0.2 mg/ml streptomycin (Feng et al., 2001). The cells were transiently transfected with cDNAs encoding for neurexin 1α in pCMV5, and gWIZ-green fluorescent protein (gWIZ-GFP) at a 4:1 molar ratio, using a standard calcium phosphate protocol. The cells were subsequently removed to 29°C and CO2 for 1-2 d before patch-clamp experiments.
Electrophysiological recordings
Whole-cell (ruptured) recordings were performed as described previously (Feng et al., 2001). Specifically, an Axopatch 700A amplifier (Molecular Devices, Foster City, CA) was linked to a personal computer equipped with pClamp, version 9. Patch pipettes (Sutter borosilicate glass; BF 150-86-15) were pulled using a Sutter P-87 microelectrode puller and subsequently fire-polished using a Narishige microforge. Pipettes (in the range of 2-4 MΩ) were filled with internal solution containing the following (in mm): 140 cesium methanesulfonate, 1 MgCl2, 10 EGTA, and 10 HEPES, pH 7.2, adjusted with CsOH. The cells (membrane capacitance, 18-25 pF) were used for the recordings in standard bath recording solution containing the following (in mm): 140 NaCl, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.4, adjusted with NaOH. Whole-cell currents were elicited by stimulation protocols stepping from a holding potential of -60 mV to various test potentials using Clampex software. A ramp protocol following various voltage steps was used to estimate the current-voltage relationship (see Fig. 5). The external recording solution in the absence or presence of 5 nm α-latrotoxin was perfused onto the cells using a gravity-driven perfusion system. Data were filtered at 1 kHz (-3 dB) using a four-pole Bessel filter and digitized at a sampling frequency of 2 kHz. Data were analyzed using Clampfit (Molecular Devices) and SigmaPlot 4.0 (Jandel Scientific, San Rafael, CA).
Figure 5.
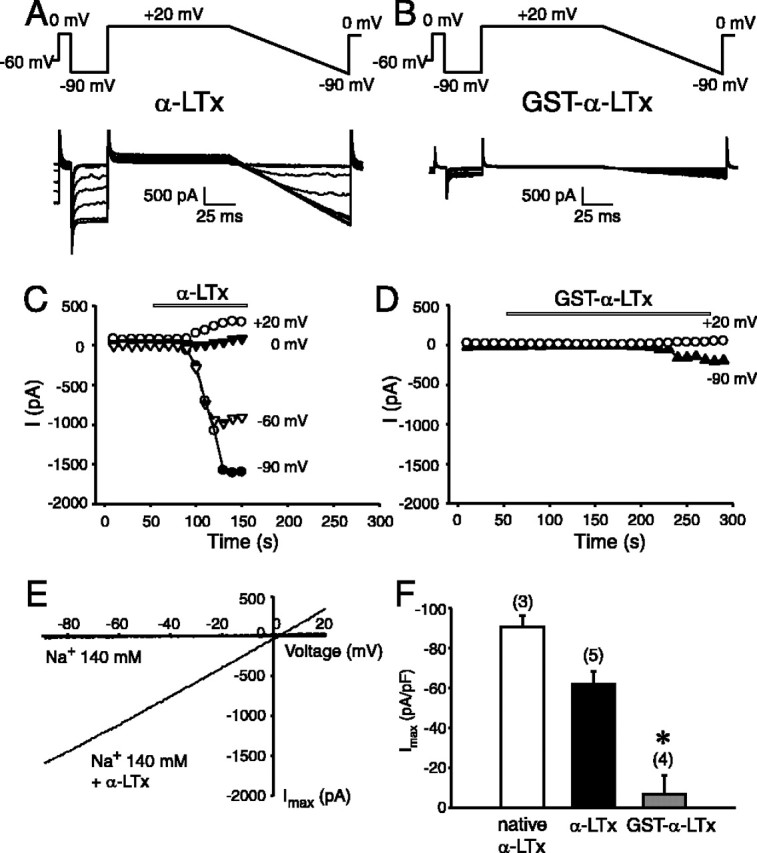
The abilities of recombinant α-latrotoxins (α-LTx, GST-α-LTx) to induce cation current on cells expressing neurexin 1α. A, B, Typical representatives of superimposed current trances recorded during episodes of stimulation before and during application of 5 nm α-LTx (A) and 5 nm GST-α-LTx (B). The standard stimulation command protocol is indicated on the top panel. The cell at a hold potential of -60 mV was depolarized to 0 mV, hyperpolarized to -90 mV, and then +20 mV followed by a ramp from +20 to -90 mV. Calibration: 500 pA, 25 ms. C, D, Time courses of development of toxin effect in the same cells as in A and B. The current amplitudes were measured at the end of the pulses at +20, 0, -60, and -90 mV, as indicated. The recordings were performed in the standard extracellular solution containing 140 mm NaCl. Applications of the recombinant α-LTx (C) or GST-α-LTx (D) are indicated by the bar. E, Current-voltage relationship of αLTx-induced cation current recorded during the voltage ramps before and during α-LTx effect. Notice the large increase in slope in the presence of αLTx. F, Comparison of the potency of recombinant α-LTx with native α-LTx. The maximal current amplitudes were measured at -90 mV and normalized with the cell size. The data are presented as mean ± SEM. The number of experiments is indicated on the top of the bars. The difference between the effect of GST-α-LTx and the other forms is statistically significant (*p < 0.05).
Measurement of α-latrotoxin insertion into PC12 membrane
Eighty percent confluent PC12 cells in 10 cm dishes were washed once with PSS and incubated with 4 ml of PSS containing 5 nm recombinant α-latrotoxins (α-LTx, GST-α-LTx, or α-LTxΔ3) for an indicated time (2 or 15 min). The supernatant was removed, and an aliquot (500 μl) of the supernatant was centrifuged at 21,910 × g for 3 min at 4°C. The cleared supernatant was mixed with an equal volume of 2× SDS-PAGE sample buffer. The PC12 cells were washed twice with 8 ml of ice-cold PSS and harvested in 8 ml of 0.1 m Na2CO3, pH 11.5. The harvested cells were washed three times with 0.1 m Na2CO3. Each washing step consisted of vigorous homogenization of pelleted membranes in 8 ml of 0.1 m Na2CO3, incubation at 0°C for 30 min with occasional mixing, and centrifugation at 100,000 × g for 45 min at 4°C. Washed membranes were resuspended in 200 μl of SDS-PAGE sample buffer. Forty microliters of the supernatant and 20 μl of the PC12 membrane in the sample buffer were analyzed by SDS-PAGE gel and immunoblotting using polyclonal antibody against α-latrotoxin (Alomone Labs, Jerusalem, Israel) and enhanced chemiluminescent detection.
Statistics
The data were presented as mean ± SEM. Statistical analysis was performed using SigmaStat 2.0 (Jandel Scientific). Differences between mean values from each experimental group were tested using a Student's t test for two groups and one-way ANOVA for multiple comparisons. Differences were considered significant if p < 0.05.
Results
Generation of active α-latrotoxin using a novel strain of E. coli
To pursue structure/function analysis of α-latrotoxin, production of an active recombinant toxin is essential. Previously, α-latrotoxin expressed in bacteria has lacked biological activity, apparently because of the strong reductase activities in E. coli. Such reductase activities would prevent the formation of disulfide bonds within α-latrotoxin, which are essential for binding to its surface receptors (Ichtchenko et al., 1998). Thus, we hypothesized that the use of a recently developed strain of E. coli, Origami (commercially available from Novagen, Madison, WI), in which both thioredoxin reductase and glutathione reductase are inactivated, would improve the production of active α-latrotoxin. We expressed the full-length α-latrotoxin as a GST fusion protein (Fig. 1A). Figure 1B showed an SDS-PAGE analysis of GST fusion full-length α-latrotoxin (GST-α-LTx) that was eluted with reduced glutathione. To remove the GST tag, we used thrombin to digest the fusion protein attached onto the glutathione-agarose. Cleaved α-LTx was similar in size to native α-latrotoxin (Fig. 1B). The recombinant α-LTx contains an extra 13 residues (GSPGISGGGGGIL) derived from the linker sequence of pGex-KG, in addition to the native α-latrotoxin sequence.
We examined the abilities of the recombinant full-length α-latrotoxins (GST-α-LTx, α-LTx) to stimulate NE release from PC12 cells. As shown in Figure 1C, α-LTx potently stimulated NE release, and the dose-dependent stimulation of NE release by α-LTx was similar to that of native α-latrotoxin. In contrast, the stimulatory action of GST-α-LTx was much less potent (Fig. 1C). These data demonstrated that we succeeded in the generation of active α-latrotoxin using the strain of E. coli, but GST fusion to the toxin suppresses the toxin activity.
GST-fused α-latrotoxin binds to cell surface receptors as efficiently as GST-cleaved α-latrotoxin
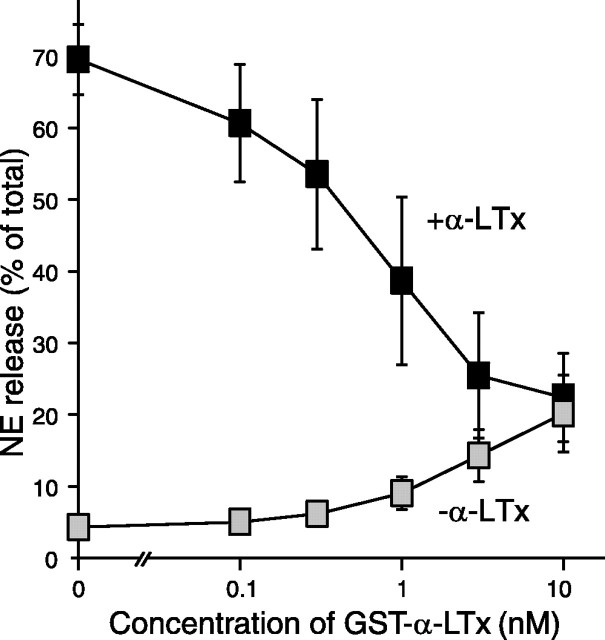
The poor stimulatory effect of GST-α-LTx could be attributable to impaired binding to the surface receptors in PC12 cells. Alternatively, it could be caused by its inability to activate downstream signaling pathways, including the pore formation. Khvotchev and Südhof (2000) suggested that the N terminus of α-latrotoxin is inserted in the membrane during its stimulating actions. We hypothesized that the presence of N-terminal GST may prevent the membrane insertion of the N terminus of α-latrotoxin without affecting its binding to the receptor. If this were the case, we would expect that preincubation with GST-α-LTx would inhibit the action of α-LTx by competing for the binding sites on the PC12 cells. We found that NE release stimulated by 3 nm α-LTx was strongly suppressed with preincubation (for 3 min) with increasing concentrations (0.1-10 nm) of GST-α-LTx in a concentration-dependent manner (Fig. 2). This result indicates that GST-α-LTx competes with α-LTx for binding to the cell surface receptors. Thus, the GST tag does not appear to affect the ability of the recombinant α-latrotoxin to bind to the receptor.
Figure 2.
Suppression of α-LTx-stimulated NE release with preincubation of GST-α-LTx. PC12 cells were preincubated with the indicated concentration of GST-α-LTx for 3 min and stimulated for NE release with or without 3 nm α-LTx. Note that GST-α-LTx alone (gray squares) weakly stimulated NE release. Error bars indicate SEM (n = 4).
We confirmed this finding with biochemical binding experiments. Two major cell surface receptors for α-latrotoxin have been identified: neurexin 1α and CL1 (Ushkaryov et al., 1992; Davletov et al., 1996; Krasnoperov et al., 1997; Lelianova et al., 1997). Using transfected COS-7 cells, we produced Ig fusion proteins in which sequences from neurexin 1α and CL1 were linked to the human Ig Fc fragment. For neurexin 1α, we expressed the fifth and sixth laminin-neurexin-sex hormone-binding globulin (LNS) domains and the expressed protein (IG-N1α-31) was purified and immobilized on protein A-agarose (Fig. 3A,C) (Sugita et al., 2001). As a control, we expressed the signal peptide and 18 N-terminal amino acids of neurexin 1α (IG-C) (Fig. 3A,C). In addition to the expressed proteins, protein A-agarose copurified both IgG heavy and light chains that are contained in the culture medium of COS-7 cells (Fig. 3C). For CL1, we expressed the entire extracellular domain of CL1 (IG-CL1-1). CL1 is cleaved at the cysteine-rich domain before the transmembrane region, resulting in two subunits [subunit 1 (p120) and subunit 2 (p85)] (Fig. 3B) (Krasnoperov et al., 1997, 2002b). In our construct, a portion of subunit 2 is directly fused to Ig and subunit 1 is copurified through the tight binding to subunit 2. On the SDS-PAGE gel, we found, in addition to the ∼120 kDa band (mature subunit 1), a more intense ∼90 kDa band, which is likely to be an incompletely glycosylated subunit 1 (Fig. 3D).
Figure 3.
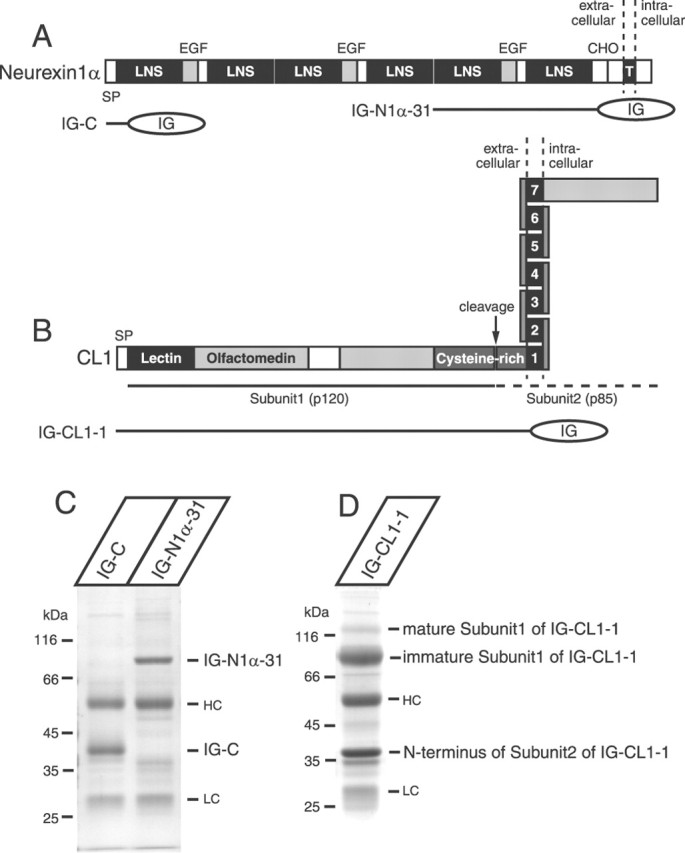
Analysis of purified recombinant neurexin 1 α and CL 1 proteins expressed in COS-7 cells. A, Domain structure of neurexin 1α and location of Ig fusion proteins used for binding to α-latrotoxin. Principal features of neurexin 1α are indicated on the top as follows: SP, signal peptide; LNS, LNS domains; EGF, epidermal growth factor-like sequences; CHO, carbohydrate attachment site; T, transmembrane region. B, Domain structure of CL1 and location of Ig fusion protein used for binding to α-latrotoxin. SP, signal peptide; lectin, lectin-like domain; olfactomedin, a domain homologous to olfactomedins and myocilin. Close to the membrane is a cysteine-rich domain that contains a cleaving site. C, Purified recombinant neurexin 1α (the fifth and the sixth LNS domains; IG-N1α-31) was analyzed with the control protein (IG-C) by SDS-PAGE and Coomassie staining. D, Analysis of the recombinant, entire extracellular domain of CL1 (IG-CL1-1) by SDS-PAGE and Coomassie staining. HC, Heavy chain; LC, light chain.
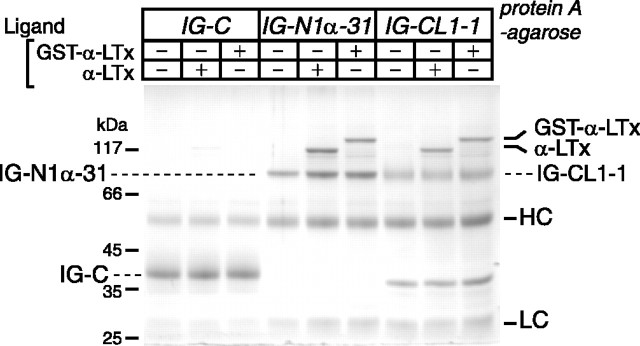
We examined whether α-LTx and GST-α-LTx can bind to these immobilized recombinant receptors. We found that both IG-N1α-31 and IG-CL1-1 captured α-LTx and GST-α-LTx (Fig. 4). In contrast, control protein (IG-C) captured little or no recombinant toxins (Fig. 4). Thus, we conclude that GST-α-LTx can bind to the receptors as efficiently as α-LTx and that the poor stimulatory action of GST-α-LTx is not a result of its inability to bind to the cell surface receptors.
Figure 4.
α-LTx and GST-α-LTx bind to recombinant neurexin 1α and CL1. Affinity chromatography of α-LTx and GST-α-LTx on the control protein (IG-C), the neurexin 1α protein (IG-N1α-31), or the CL1 protein (IG-CL1-1) immobilized onto protein A-agarose is shown. After washing, the bound proteins on protein A-agarose were analyzed by SDS-PAGE and Coomassie staining. HC, Heavy chain; LC, light chain.
GST-fused α-latrotoxin forms Ca2+ ionophores less efficiently than GST-cleaved α-latrotoxin
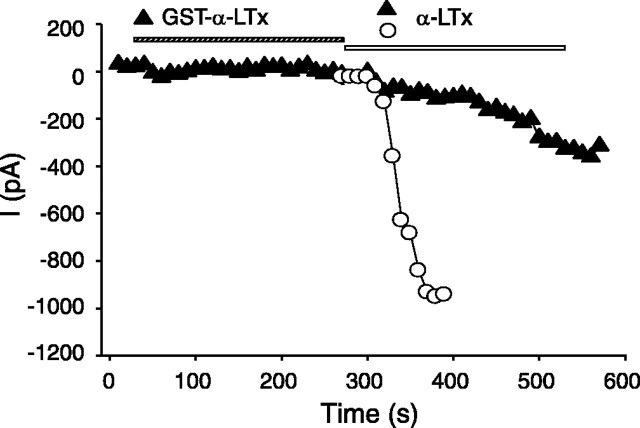
Previous studies demonstrated that native α-latrotoxin induces cation conductance in the presence of Ca2+ by interacting with neurexin 1α (Hlubek et al., 2000; Van Renterghem et al., 2000; Volynski et al., 2000). To evaluate the ionophore property of the recombinant α-latrotoxin, we performed whole-cell patch-clamp recordings to test whether the recombinant α-latrotoxin induces cation conductance in neurexin 1α-expressing cells. Shown in Figure 5, we found that cleaved α-LTx (5 nm) induced a large cation current in the cells expressing neurexin 1α, using a recording command protocol adopted from Van Renterghem et al. (2000). Representative recordings are shown in Figure 5A. As shown in Figure 5C, the current amplitude measured at difference steady-state voltage steps increased and reached maximum within a couple of minutes after toxin application. No current conductance was observed in either the absence of the toxin (Fig. 5E) or in the mock cells that do not express neurexin 1α (data not shown). The reversal potential of the cation current is between 0 and +3 mV (Fig. 5E) in the recording condition of high external Na+ and internal Cs+ solutions. Our findings are consistent with the previous report that examined the effects of native α-latrotoxin (Van Renterghem et al., 2000). The maximal current density induced by the recombinant toxins is shown in Figure 5F. The currents were recorded at a voltage step of -90 mV and normalized with the cell size.
We hypothesized that the poor stimulatory action of GST-α-LTx may be attributable to interference of the GST tag in the formation of cation conductance. To address this issue, we compared the pore-forming effect of GST-α-LTx to the cleaved α-LTx. The inward current induced by GST-α-LTx (5 nm) was 10-fold smaller than cleaved α-LTx (5 nm) (6.65 ± 9.14 pA/pF, n = 4, vs 62.02 ± 6.34 pA/pF, n = 5; p < 0.05) (Fig. 5B,F), and its action of inducing cation current was much slower (Fig. 5D). Because the GST tag did not affect the binding affinity of the toxin to its receptors (Fig. 4), our findings indicate that the GST tag likely prevents the pore-forming effect of the toxin. To further test whether GST-α-LTx binding to the receptor (neurexin 1α) would affect α-LTx effect, we compared the cation conductance induced by α-LTx with that observed after the cells were preincubated with GST-fused toxin. As shown in the examples in Figure 6, a preapplication of GST-α-LTx (5 nm) substantially suppressed the ion conductance induced by α-LTx (5 nm). The maximal current density induced by 5 nm α-LTx was reduced when the cells expressed the GST-fused toxin, and this effect was statistically significant (p < 0.05). Our results indicate that, although both α-LTx and GST-α-LTx bind to neurexin 1α, only α-LTx efficiently induces cation conductance. We suggest that the N terminus of α-latrotoxin has to be free to form pores and that the N-terminally fused GST prevents the efficient pore formation.
Figure 6.
Preapplication of GST-α-LTx attenuates α-LTx effect. Representative time courses of development of the recombinant α-LTx effect on neurexin 1α-expressing cells are shown. The application of the toxins is indicated by the bar (top). The current amplitudes were measured at the end of the pulses at -90 mV. The recordings were performed in the standard extracellular solution containing 140 mm NaCl. A same concentration of cleaved α-LTx (5 nm) elicited smaller current when it was given after the application of the GST-bound form (5 nm).
We also noticed that current conductance (62.02 ± 6.34 pA/pF; n = 5) evoked by the cleaved form of the recombinant α-LTx was still relatively less when compared with its native form (native α-LTx; 90.69 ± 5.24 pA/pF; n = 3) at the same concentration (Fig. 5F). We suspected that this difference may come from the fact that the recombinant α-LTx contains an extra 13 residues derived from the linker sequence of pGex-KG (Guan and Dixon, 1991) at the N terminus in addition to the native α-latrotoxin sequence. We attempted to generate the recombinant α-latrotoxin with less extra residues by subcloning the α-latrotoxin sequence at the BamHI site instead of the EcoRI site of pGex-KG but found that thrombin did not cleave GST from this short-linker GST-fusion α-latrotoxin. As a result, the potential inhibitory effect of the 13 residues has not been addressed.
Truncations of C-terminal ankyrin-like repeats led to losses in stimulating Ca2+-dependent exocytosis
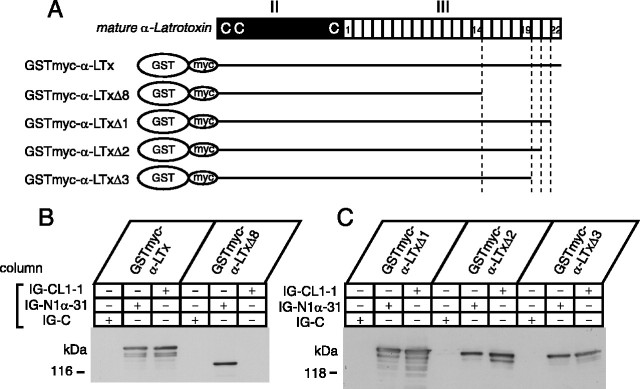
Two-thirds of α-latrotoxin consists of 22 ankyrin-like repeats (Fig. 1), the role of which has not been investigated. To determine the functional role of the ankyrin-like repeats, we generated four different α-latrotoxin expression constructs whose C-terminal ankyrin-like repeat sequences were serially truncated: pGex α-LTxΔ1, Δ2, Δ3, and Δ8 (schematically shown in Fig. 7A). The number (Δn) in the name of these truncated constructs indicates the number of deleted C-terminal ankyrin-like repeats. Similar to α-LTx, these truncated toxins were expressed as GST fusion proteins and GST was cleaved with thrombin.
Figure 7.
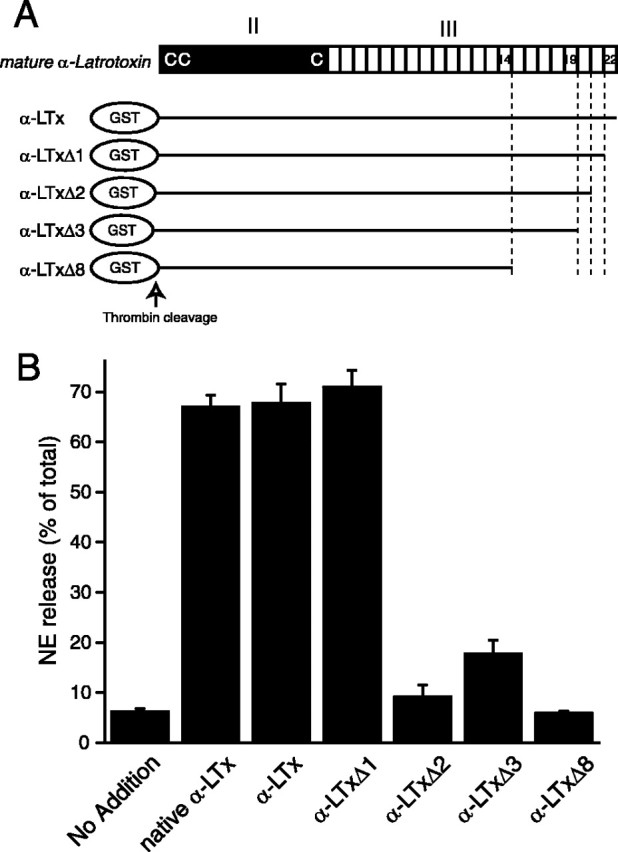
Truncation of C-terminal ankyrin-like repeats abolishes the abilities of recombinant α-latrotoxins to stimulate Ca2+-dependent exocytosis. A, Structure of recombinant α-latrotoxins, the effects of which were tested on Ca2+-dependent exocytosis. C's indicate the three cysteine residues that are conserved in all latrotoxins. B, NE secretion was triggered by 10 nm (each) recombinant α-latrotoxins. Error bar indicates SEM (n = 4-8).
We examined the ability of these C-terminally truncated versions of α-latrotoxin to stimulate Ca2+-dependent NE release from PC12 cells (Fig. 7B). We found that α-LTxΔ1 stimulates NE release from PC12 cells as potently as α-LTx and native α-latrotoxin. In fact, the stimulating action of α-LTxΔ1 is sometimes larger than that of α-LTx. Thus, the deletion of the last (22nd) ankyrin-like repeat did not reduce the ability of α-latrotoxin to trigger Ca2+-dependent NE release. In contrast, the removal of the additional one ankyrin-like repeat (α-LTxΔ2) or two ankyrin-like repeats (α-LTxΔ3) drastically reduced the ability of the recombinant α-latrotoxin to trigger NE release from PC12 cells. Furthermore, after the removal of eight ankyrin-like repeats, the recombinant α-latrotoxin (α-LTxΔ8) completely lost its stimulating activity (Fig. 7B).
C-terminal ankyrin-like repeats are required for binding to CL1 but not to neurexin 1α
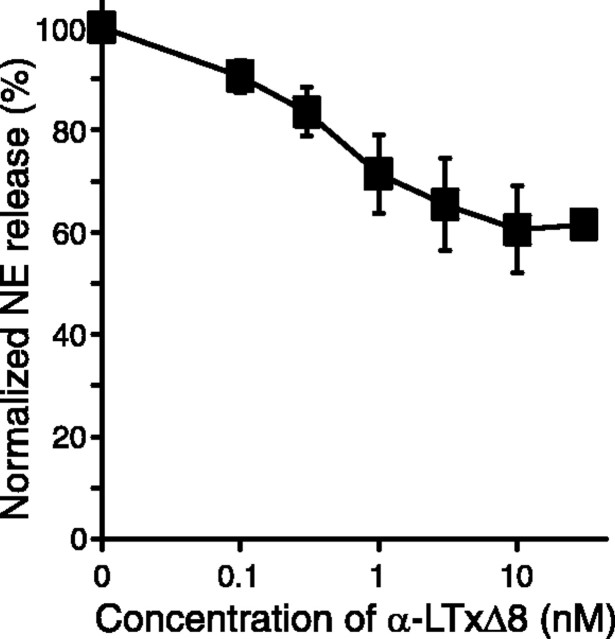
The results from the release assays in PC12 cells suggest that C-terminal ankyrin-like repeats are critical for either the binding to the receptors or for the pore formation. To distinguish between these two possibilities, we examined whether α-LTxΔ8 is able to suppress the action of α-LTx. If α-LTxΔ8 is able to bind to the receptors of α-latrotoxin, α-LTxΔ8 should inhibit the stimulatory action of α-LTx. If α-LTxΔ8 is no longer able to bind to the receptors, it should have no effects on the action of α-LTx. NE release by 3 nm α-LTx was suppressed with increasing concentrations (0.1-10 nm) of α-LTxΔ8 (Fig. 8). This result suggests that α-LTxΔ8 competes with α-LTx for the receptor binding. However, the inhibition by α-LTxΔ8 appears to be partial.
Figure 8.
Partial suppression of α-LTx-stimulated NE release with preincubation of α-LTxΔ8. PC12 cells were preincubated with the concentration of α-LTxΔ8 for 3 min and stimulated for NE release with or without 3 nm α-LTx. The stimulatory actions of α-LTx were partially suppressed with increasing concentrations of α-LTxΔ8 (n = 4-7). Error bars indicate SEM.
This partial inhibition could be attributable to the reduced affinity of α-LTxΔ8 to both of the cell surface receptors (neurexin 1α and CL1). Alternatively, α-LTxΔ8 selectively loses the affinity to either neurexin 1α or CL1. Because the inhibitory action of α-LTxΔ8 is saturated at the higher concentration (>10 nm) (Fig. 8), we reasoned that the weak inhibition of α-LTxΔ8 is caused by its selective binding to only one of the two major receptors. We tested the binding ability of α-LTxΔ8 to neurexin 1α and CL1. Because the expression level of α-LTxΔ8 is lower, we inserted a myc tag between GST and the N terminus of α-latrotoxin for facilitating the detection of bound α-LTxΔ8 as well as α-LTx (Fig. 9A). We found that only neurexin 1α efficiently captured GST-myc-α-LTxΔ8, whereas both neurexin 1α and CL1 captured GST-myc-α-LTx (Fig. 9B). Thus, C-terminal ankyrin-like repeats are selectively required for binding to CL1. Furthermore, the results suggest that partial suppression of the stimulatory action of α-LTx by preincubated α-LTxΔ8 is probably caused by its competitive binding to neurexin 1α.
Figure 9.
Effects of the deletion of ankyrin-like repeats from recombinant α-latrotoxin on binding to neurexin 1α and CL1. A, Schematic representation of recombinant α-latrotoxins, the binding of which to neurexin 1α and CL1 was examined. C's indicate the three cysteine residues that are conserved in all latrotoxins. B, C, Immunoblot analysis of the bound recombinant α-latrotoxins on immobilized control proteins (IG-C), neurexin 1α (IG-N1α-31), and CL1 (IG-CL1-1). Bound α-latrotoxins were probed by monoclonal antibody against c-myc.
We next examined whether other (intermediate) C-terminal truncated α-latrotoxins (α-LTxΔ1, Δ2, Δ3) bind to CL1 and neurexin 1α (Fig. 9A). As expected, all of them bound to neurexin 1α. In addition, they also bound to CL1 (Fig. 9C). Thus, profound loss of stimulation of exocytosis by α-LTxΔ2 and α-LTxΔ3 is not attributable to the loss of binding to CL1. Therefore, the abilities of these C-terminal truncated toxins to stimulate exocytosis do not correlate well with their abilities to bind to CL1 or neurexin 1α.
Truncations of C-terminal ankyrin-like repeats affect the ability of α-latrotoxin to form Ca2+ ionophores
We hypothesized that, in addition to regulating the binding to CL1, C-terminal ankyrin-like repeats are involved in the pore formation and that their stimulatory actions are correlated with their pore-forming abilities. To test this hypothesis, we examined the abilities of C-terminal truncated α-latrotoxins to induce cation conductance via neurexin 1α-dependent mechanisms using whole-cell patch-clamp recording. Using the same stimulation command protocol described in Figure 5A, we tested whether truncated α-latrotoxins have similar capability to the full-length toxin in inducing the cation conductance. Should the ankyrin-like repeats be involved in the pore formation, we would expect to observe an alteration in current amplitudes in response to the truncated toxins. As predicted, the cation conductance of the cells is largely dependent on the number of ankyrin-like repeats of the toxin. Figure 10A shows example recordings obtained in the presence of 5 nm α-LTxΔ1, α-LTxΔ2, α-LTxΔ3, or α-LTxΔ8. Surprisingly, deletion of the last ankyrin-like repeat (α-LTxΔ1) dramatically enhanced the current conductance, whereas deletions of an additional one or two ankyrin-like repeats (α-LTxΔ2 and α-LTxΔ3) caused a significant reduction (p < 0.05). Furthermore, a deletion of eight ankyrin-like repeats (α-LTxΔ8) resulted in no detectable current conductance. Thus, the abilities of recombinant α-latrotoxins to induce cation conductance are precisely correlated with their abilities to stimulate Ca2+-dependent exocytosis. The time courses of toxin effects are shown in Figure 10B. α-LTxΔ1 caused a progressive induction of the current, which reached a magnitude approximately three to four times that of the α-LTx or native α-latrotoxin. This suggests that the 22nd ankyrin-like repeat is involved in stabilization of the pore-forming property of the toxin. By comparison, additional deletions of the ankyrin-like repeats reduced or prevented the ion conductance, even at prolonged exposures to the truncated toxins, suggesting that the 15th to 21st repeats are essential for the pore-forming effect of the toxin. The data are summarized in Figure 10C. Finally, preincubation of α-LTxΔ8 interfered with the pore-forming action of α-LTxΔ1, supporting our finding that α-LTxΔ8 still binds to neurexin 1α (data not shown).
Figure 10.
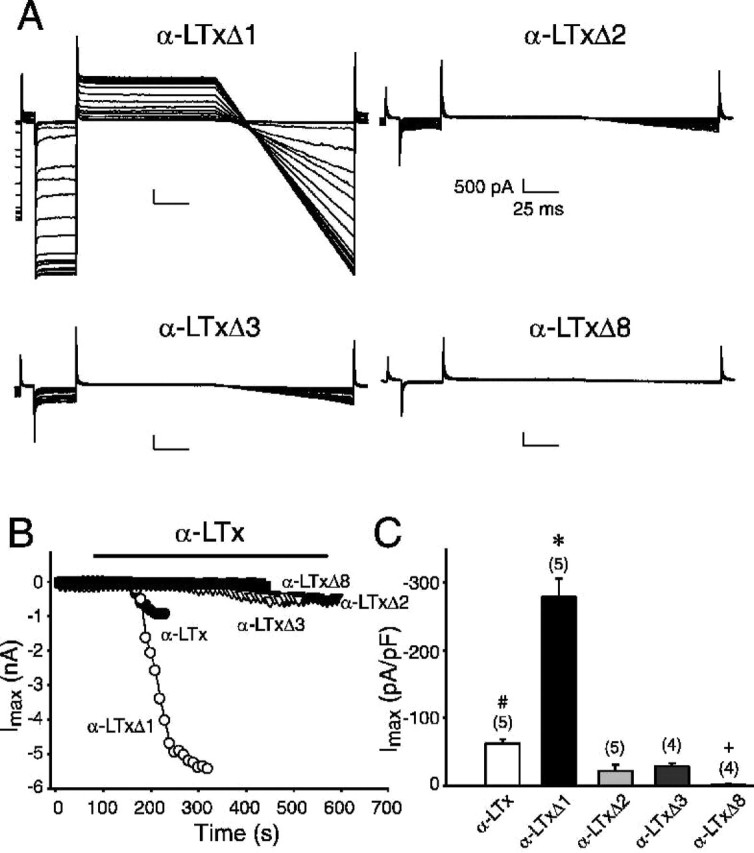
The C-terminal ankyrin-like repeats are critical for the pore-forming effect of recombinant α-latrotoxin. A, Typical representatives of superimposed current trances recorded during episodes of stimulation before and during application of recombinant α-latrotoxins (5 nm) with different numbers of ankyrin-like repeats, using the standard stimulation command protocol indicated in Figure 5. Calibration: 500 pA (current amplitude), 25 ms (recording time). The recordings were performed in the standard extracellular solution containing 140 mm NaCl. B, Representative time courses of development of pore-forming effect of truncated toxins in the same cells as in A (except for the full-length toxin, α-LTx). The current amplitudes were measured at the end of the pulses at -90 mV. Applications of the toxins are indicated by the bar. C, Comparison of the potency of the full-length and truncated α-LTxs. The maximal current amplitudes were measured either at the end of the observed stable effect for 30 s or at 4 min for those showing to be less effective. The values are averages of three consecutive points from each experiment and normalized with the cell size. The data are presented as mean ± SEM. The number of experiments is indicated on the top of the bars. The effect of α-LTxΔ1 is significantly (*p < 0.05) greater than that of the full-length and all other truncated α-LTxs. The full-length toxin is significantly (#p < 0.05) greater that α-LTxΔ2, Δ3, and Δ8. +α-LTxΔ8 is significantly (p < 0.05) less than α-LTxΔ2 and Δ3. No significant difference was found between α-LTxΔ2 and Δ3.
Molecular mechanisms underlying the inability of GST-fused and C-terminal truncated mutants to induce cation conductance
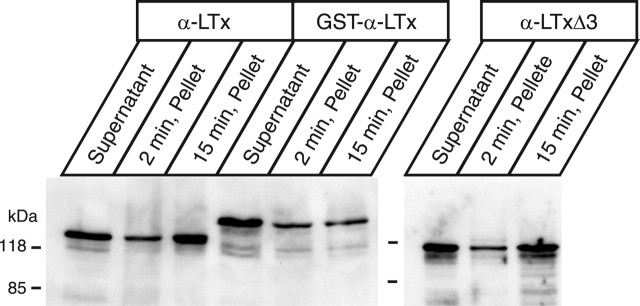
As shown above, we found that the GST-fused or C-terminally truncated α-latrotoxin mutants do not induce cation conductance efficiently. However, the molecular mechanisms underlying their inabilities to form pores remain unclear. In the case of GST-fused α-LTx, we suggested a possibility that GST may interfere with the ability of the toxin to insert the membrane, because the N-terminal region of α-LTx was shown previously to be inserted into the membrane (Khvotchev and Südhof, 2000). In this study, native α-latrotoxin was found to become an operational integral membrane protein and resistant to washing with 0.1 m Na2CO3 when the toxin was incubated with synaptosomes or neurexin 1α-transfected COS-7 cells for 30 min. We conducted similar experiments using our recombinant α-latrotoxins and PC12 cells. We found that, indeed, GST-cleaved, full-length α-LTx showed time-dependent membrane insertion with PC12 cells, that is, a larger amount of α-LTx attached to the PC12 membrane became resistant to washing with 0.1 m Na2CO3 after 15 min incubation than 2 min incubation (Fig. 11). Such a marked time-dependent increase in the membrane integration was not observed for GST-fused α-LTx (Fig. 11). This result directly suggests that the presence of GST prevents the effective membrane insertion of the toxin with PC12 membranes. In contrast, time-dependent membrane insertion was observed for α-LTxΔ3 (Fig. 11). Thus, C-terminal ankyrin repeats appear not to be critically involved in membrane insertion.
Figure 11.
Time-dependent insertion of recombinant α-latrotoxins with PC12 cells. PC12 cells were incubated with 5 nm recombinant α-latrotoxins (α-LTx, GST-α-LTx, or α-LTxΔ3) for an indicated time and washed three times with 0.1 m Na2CO3, pH 11.5. The recombinant toxins that did not bind to PC12 cells (supernatant), and the toxins that attached to the PC12 membranes after washing with Na2CO3 (pellet) were probed with polyclonal antibody against α-latrotoxin.
Another possibility for the inability of the toxin mutants to induce cation conductance is their inability to form tetramers. The formation of the tetramer was beautifully demonstrated by Dr. Y. Ushkaryov's group (Orlova et al., 2000; Volynski et al., 2003) using cryo-EM, native electrophoresis, and cross-linking. We conducted cross-linking experiments with 30 μm bis(sulfosuccinimidyl)suberate (BS3), which was used to capture α-latrotoxin tetramers previously (Orlova et al., 2000). We confirmed the formation of dimers of GST-cleaved α-LTx by this cross-linker but unfortunately did not observe efficient formation of tetramers in response to divalent cations (i.e., Ca2+) (data not shown). At present, we do not know the reason for our less efficient capturing of tetramers by BS3. In the cross-linking experiment, we also found that GST-α-LTx seems to form nonspecific multimers (dimers, trimers, tetramers, quadromers, etc). It is known that GST forms dimers. Thus, it is conceivable that the presence of GST induces nonspecific multimers and prevents the efficient formation of tetramers of GST-α-LTx, which may underlie its reduced membrane insertion and/or pore formation.
α-LatrotoxinN4C is almost inactive in stimulating NE release from PC12 cells
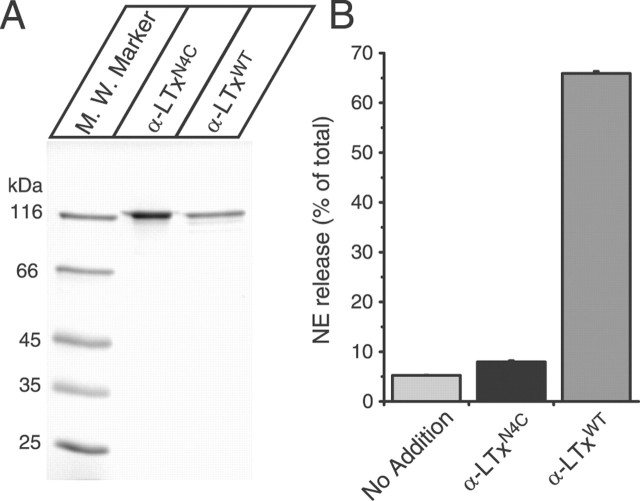
Our results showed a strong correlation between Ca2+-dependent exocytosis and the induction of cation conductance. This conclusion is not in agreement with the study of the specific mutant (α-LTxN4C) of α-latrotoxin (Capogna et al., 2003; Volynski et al., 2003). This mutant has four extra amino acids (VPRG) that were inserted between domain II and domain III (Ichtchenko et al., 1998). Remarkably, it was reported that this mutant stimulated Ca2+-dependent exocytosis without forming pores (Capogna et al., 2003; Volynski et al., 2003), although this mutant is unable to stimulate Ca2+-independent exocytosis. However, this mutant was originally found to be inactive in stimulating both Ca2+-dependent and Ca2+-independent exocytosis (Ichtchenko et al., 1998). To clarify this dispute, we expressed this insertion mutant (α-LTxN4C) in our new expression system and tested its ability to stimulate Ca2+-dependent NE release from PC12 cells. α-LTxN4C was expressed well, with purities similar to the wild-type recombinant α-LTx (α-LTxWT) (Fig. 12A). However, we found that 10 nm α-LTxN4C is largely inactive in stimulating NE release from the PC12 cells (Fig. 12B). Thus, our result is consistent with the original finding of Ichtchenko et al. (1998) that the insertion of four extra residues between domains II and III impairs the stimulatory action of α-latrotoxin. The result also supports our conclusion that the pore-forming activity of α-latrotoxin is essential for stimulating Ca2+-dependent exocytosis.
Figure 12.
α-LTxN4C is almost inactive in stimulating NE release. A, Analysis of purified recombinant α-latrotoxins. The recombinant α-latrotoxins (GST-cleaved α-LTxN4C and α-LTxWT; ∼1-2 μg) were analyzed by SDS-PAGE and Coomassie staining. B, Stimulation of NE secretion from PC12 cells with 10 nm α-LTxN4C and α-LTxWT. Error bars indicate SEM (n = 6-12).
Discussion
In this study, we established, for the first time, the method to generate active α-latrotoxin using E. coli. Our success in generating active recombinant α-latrotoxin using E. coli will facilitate the production of many mutated toxins, and the examinations of the abilities of these recombinant toxins to stimulate neurotransmitter exocytosis will shed new light for understanding the mechanisms of actions of α-latrotoxin.
Using this new expression system, we addressed structural requirements of α-latrotoxin in stimulating Ca2+-dependent exocytosis. We expressed the recombinant proteins as GST fusion proteins, which provided us with an opportunity to test the role of N-terminal insertion. Because GST is a highly soluble protein, it is reasonable to speculate that the GST tag prevents the N terminus from inserting into the membrane. Indeed, we found that the GST tag impairs the ability of the recombinant toxin to become operational integral membrane proteins (Fig. 11). We also found that the GST tag inhibits Ca2+-dependent exocytosis (Fig. 1), as well as the induction of cation conductance (Fig. 5). In contrast, the GST tag did not inhibit the binding to the receptors (Fig. 4). Our results strongly suggest that the insertion of the N terminus into the plasma membrane is critical for the pore formation, which is consistent with the previous findings (Khvotchev and Südhof, 2000).
We exploited truncated toxin mutants to examine the role of C-terminal ankyrin-like repeats for Ca2+-dependent exocytosis and pore formation. We found a direct correlation between the abilities of the truncated mutants to stimulate Ca2+-dependent exocytosis and their abilities to induce cation conductance (Figs. 7, 10). The deletion of the last two (α-LTxΔ2) or three (α-LTxΔ3) ankyrin-like repeats drastically reduced the stimulation of Ca2+-dependent exocytosis and the induction of cation conductance. The deletion of the last eight ankyrin-like repeats (α-LTxΔ8) resulted in a complete loss in Ca2+-dependent exocytosis and cation conductance induction. Because α-LTxΔ2 and α-LTxΔ3 both bound to CL1 as well as neurexin 1α (Fig. 9C), the strong reduction in stimulation of Ca2+-dependent exocytosis is not attributable to their reduced ability to bind the receptor. Because α-LTxΔ3 showed time-dependent insertion into PC12 membranes similar to the full-length α-LTx (Fig. 11), the mechanism underlying the inability of C-terminal truncated α-latrotoxin to form ion conductance remains unclear. One of the possibilities includes the critical role of the C-terminal ankyrin-like repeats in forming the tetramers. Interestingly, the deletion of the last ankyrin-like repeat (α-LTxΔ1) even enhanced the cation current (Fig. 10). α-LTxΔ1 caused a progressive induction of the current, indicating that the 22nd ankyrin-like repeat is involved in stabilization of the pore-forming property of the toxin.
Our results also revealed the requirement of C-terminal ankyrin-like repeats for binding to CL1. Previous work suggests the essential function of the conserved N-terminal domain II for binding to neurexin 1α and CL1 (Ichtchenko et al., 1998), but it was not clear whether C-terminal ankyrin-like repeats are involved for the binding to the receptors. We found that the recombinant toxin lacking the three ankyrin-like repeats (α-LTxΔ3) still binds both neurexin 1α and CL1. However, the deletion of the last eight ankyrin-like repeats (α-LTxΔ8) prevents binding to CL1 but not to neurexin 1α (Fig. 9). These results suggest that the 15th through 19th ankyrin-like repeats are selectively involved for binding to CL1.
Direct correlation between Ca2+-dependent exocytosis and the induction of cation conductance suggests that the pore formation is the major mechanism of α-latrotoxin-induced stimulation of Ca2+-dependent exocytosis. This finding corresponds with the fact that intracellularly truncated receptors (neurexin 1α and CL1) are able to contribute α-latrotoxin-mediated Ca2+-dependent exocytosis (Sugita et al., 1998, 1999). Our results also strongly suggest that binding to the cell surface receptors is not sufficient to stimulate exocytosis, because three of the recombinant mutants of α-latrotoxin, GST-α-LTx, α-LTxΔ2, and α-LTxΔ3, bound to G-protein-coupled CL1 but exhibited poor abilities to stimulate Ca2+-dependent exocytosis.
Our results did not seem to agree with the study that reported that a specific α-LTxN4C mutant stimulated Ca2+-dependent exocytosis from neuroendocrine cells and neurons without forming pores (Capogna et al., 2003; Volynski et al., 2003). However, α-LTxN4C mutant was originally reported to be largely inactive both in Ca2+-dependent and -independent exocytosis (Ichtchenko et al., 1998). We expressed α-LTxN4C in our new expression system and examined the ability of the purified toxin to stimulate NE release from PC12 cells. We found that, although this toxin was expressed well with a good purity (Fig. 12A), it did not stimulate NE release from PC12 cells (Fig. 12B). These findings are consistent with the original finding of Ichtchenko et al. (1998), and α-LTxN4C mutation does not stimulate Ca2+-dependent exocytosis. Thus, the result of α-LTxN4C further supported our conclusion that the pore formation is the major mechanism of α-latrotoxin-induced stimulation of Ca2+-dependent exocytosis.
An important feature of α-latrotoxin is its ability to stimulate exocytosis from neurons without extracellular Ca2+ (Capogna et al., 1996, 2003). However, in neuroendocrine chromaffin cells or PC12 cells, stimulation of secretion by α-latrotoxin requires extracellular Ca2+; thus, this assay system has not allowed us to investigate the mechanisms of Ca2+-independent stimulation using our recombinant α-latrotoxins. However, using hippocampal slices, we found that our recombinant α-LTx is able to stimulate spontaneous neurotransmitter release at the CA1 synapse in the absence of extracellular Ca2+ (E. Kang, P. Carlen, and S. Sugita, unpublished observation). Thus, the availability of various truncated mutants will provide us with unique opportunities to study the structural basis of α-latrotoxin to stimulate Ca2+-independent exocytosis. It is unknown why α-latrotoxin can stimulate exocytosis from neurons without extracellular Ca2+, but not from neuroendocrine cells. It has been proposed that a downstream target of α-latrotoxin exists that binds to the toxin after it has been recruited to the membrane by CL1 or neurexin 1α (Sugita et al., 1998). This downstream target is believed to be synapse-specific and not present in neuroendocrine cells that do not have synapses. This model would explain the confusing differences in the Ca2+ dependence of action of α-latrotoxin between synapses and neuroendocrine cells. We expect that availability of various truncated mutants will also contribute to identification of such a downstream target of α-latrotoxin.
Footnotes
This work was supported by Canada Research Chair program and Canadian Institutes of Health Research (CIHR) Grants MOP57825 (S.S.) and MOP106356 (Z.-P.F.).Z.-P.F. holds a New Investigator Award from CIHR and a Premier's Research Excellence Award of Ontario. We thank Drs. Thomas Südhof (University of Texas Southwestern, Dallas, TX) and Milton Charlton (University of Toronto, Toronto, Ontario, Canada) for helpful comments on preparing this manuscript.
Correspondence should be addressed to Dr. Shuzo Sugita, Toronto Western Research Institute, MC11-432, University Health Network, 399 Bathurst Street, Toronto, Ontario, Canada M5T 2S8. E-mail: ssugita@uhnres.utoronto.ca.
Copyright © 2005 Society for Neuroscience 0270-6474/05/2510188-•$15.00/0
References
- Capogna M, Gahwiler BH, Thompson SM (1996) Calcium-independent actions of α-latrotoxin on spontaneous and evoked synaptic transmission in the hippocampus. J Neurophysiol 76: 3149-3158. [DOI] [PubMed] [Google Scholar]
- Capogna M, Volynski KE, Emptage NJ, Ushkaryov YA (2003) The α-latrotoxin mutant LTXN4C enhances spontaneous and evoked transmitter release in CA3 pyramidal neurons. J Neurosci 23: 4044-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Shamotienko OG, Lelianova VG, Grishin EV, Ushkaryov YA (1996) Isolation and biochemical characterization of a Ca2+ independent α-latrotoxin-binding protein. J Biol Chem 271: 23239-23245. [DOI] [PubMed] [Google Scholar]
- Dulubova IE, Krasnoperov VG, Khvotchev MV, Pluzhnikov KA, Volkova TM, Grishin EV, Vais H, Bell DR, Usherwood PN (1996) Cloning and structure of δ-latroinsectotoxin, a novel insect-specific member of the latrotoxin family: functional expression requires C-terminal truncation. J Biol Chem 271: 7535-7543. [DOI] [PubMed] [Google Scholar]
- Feng ZP, Hamid J, Doering C, Bosey GM, Snutch TP, Zamponi GW (2001) Residue Gly1326 of the N-type calcium channel α1B subunit controls reversibility of ω-conotoxin GVIA and MVIIA block. J Biol Chem 276: 15728-15735. [DOI] [PubMed] [Google Scholar]
- Geppert M, Khvotchev M, Krasnoperov V, Goda Y, Missler M, Hammer RE, Ichtchenko K, Petrenko AG, Südhof TC (1998) Neurexin Iα is a major α-latrotoxin receptor that cooperates in α-latrotoxin action. J Biol Chem 273: 1705-1710. [DOI] [PubMed] [Google Scholar]
- Grasso A, Alema S, Rufini S, Senni MI (1980) Black widow spider toxin-induced calcium fluxes and transmitter release in a neurosecretory cell line. Nature 283: 774-776. [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE (1991) Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem 192: 262-267. [DOI] [PubMed] [Google Scholar]
- Hlubek MD, Stuenkel EL, Krasnoperov VG, Petrenko AG, Holz RW (2000) Calcium-independent receptor for α-latrotoxin and neurexin 1α facilitate toxin-induced channel formation: evidence that channel formation results from tethering of toxin to membrane. Mol Pharmacol 57: 519-528. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Khvotchev M, Kiyatkin N, Simpson L, Sugita S, Südhof TC (1998) α-Latrotoxin action probed with recombinant toxins: receptors recruit α-latrotoxin but do not transduce an exocytotic signal. EMBO J 17: 6188-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Bittner MA, Krasnoperov V, Little AR, Chepurny O, Holz RW, Petrenko AG (1999) A novel ubiquitously expressed α-latrotoxin receptor is a member of the CIRL family of G-protein-coupled receptors. J Biol Chem 274: 5491-5498. [DOI] [PubMed] [Google Scholar]
- Khvotchev M, Südhof TC (2000) α-Latrotoxin triggers transmitter release via direct insertion into the presynaptic plasma membrane. EMBO J 19: 3250-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyatkin N, Dulubova I, Grishin E (1993) Cloning and structural analysis of α-latroinsectotoxin cDNA. Abundance of ankyrin-like repeats. Eur J Biochem 213: 121-127. [DOI] [PubMed] [Google Scholar]
- Kiyatkin NI, Dulubova IE, Chekhovskaya IA, Grishin EV (1990) Cloning and structure of cDNA encoding α-latrotoxin from black widow spider venom. FEBS Lett 17: 127-131. [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Bittner MA, Mo W, Buryanovsky L, Neubert TA, Holz RW, Ichtchenko K, Petrenko AG (2002a) Protein-tyrosine phosphatase-sigma is a novel member of the functional family of α-latrotoxin receptors. J Biol Chem 277: 35887-35895. [DOI] [PubMed] [Google Scholar]
- Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG (2002b) Post-translational proteolytic processing of the calcium-independent receptor of α-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem 277: 46518-46526. [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, Petrenko AG (1997) α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 18: 925-937. [DOI] [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA (1997) α-Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem 272: 21504-21508. [DOI] [PubMed] [Google Scholar]
- Nicholson GM, Graudins A (2002) Spiders of medical importance in the Asia-Pacific: atracotoxin, latrotoxin and related spider neurotoxins. Clin Exp Pharmacol Physiol 29: 785-794. [DOI] [PubMed] [Google Scholar]
- Orlova EV, Rahman MA, Gowen B, Volynski KE, Ashton AC, Manser C, van Heel M, Ushkaryov YA (2000) Structure of α-latrotoxin oligomers reveals that divalent cation-dependent tetramers form membrane pores. Nat Struct Biol 7: 48-53. [DOI] [PubMed] [Google Scholar]
- Südhof TC (2001) α-Latrotoxin and its receptors: neurexins and CIRL/latrophilins. Annu Rev Neurosci 24: 933-962. [DOI] [PubMed] [Google Scholar]
- Sugita S, Ichtchenko K, Khvotchev M, Südhof TC (1998) α-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors: G-protein coupling not required for triggering exocytosis. J Biol Chem 273: 32715-32724. [DOI] [PubMed] [Google Scholar]
- Sugita S, Khvotchev M, Südhof TC (1999) Neurexins are functional α-latrotoxin receptors. Neuron 22: 489-496. [DOI] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Südhof TC (2001) A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol 154: 435-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobaben S, Südhof TC, Stahl B (2002) Genetic analysis of α-latrotoxin receptors reveals functional interdependence of CIRL/latrophilin 1 and neurexin 1α. J Biol Chem 277: 6359-6365. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC (1992) Neurexins: synaptic cell surface proteins related to the α-latrotoxin receptor and laminin. Science 257: 50-56. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Volynski KE, Ashton AC (2004) The multiple actions of black widow spider toxins and their selective use in neurosecretion studies. Toxicon 43: 527-542. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C, Iborra C, Martin-Moutot N, Lelianova V, Ushkaryov Y, Seagar M (2000) α-Latrotoxin forms calcium-permeable membrane pores via interactions with latrophilin or neurexin. Eur J Neurosci 12: 3953-3962. [DOI] [PubMed] [Google Scholar]
- Volynski KE, Volkova TM, Galkina TG, Krasnoperov VG, Pluzhnikov KA, Khvoshchev MV, Grishin EV (1999) Molecular cloning and primary structure of cDNA fragment for α-latrocrustatoxin from black widow spider venom. Bioorg Khim 25: 25-30. [PubMed] [Google Scholar]
- Volynski KE, Meunier FA, Lelianova VG, Dudina EE, Volkova TM, Rahman M, Manser C, Grishin EV, Dolly JO, Ashley RH, Ushkaryov YA (2000) Latrophilin, neurexin, and their signaling-deficient mutants facilitate α-latrotoxin insertion into membranes but are not involved in pore formation. J Biol Chem 275: 41175-41183. [DOI] [PubMed] [Google Scholar]
- Volynski KE, Capogna M, Ashton AC, Thomson D, Orlova EV, Manser CF, Ribchester RR, Ushkaryov YA (2003) Mutant α-latrotoxin (LTXN4C) does not form pores and causes secretion by receptor stimulation: this action does not require neurexins. J Biol Chem 278: 31058-31066. [DOI] [PubMed] [Google Scholar]
- Wang L, Li G, Sugita S (2004) RalA-exocyst interaction mediates GTP-dependent exocytosis. J Biol Chem 279: 19875-19881. [DOI] [PubMed] [Google Scholar]
- Wang L, Li G, Sugita S (2005) A central kinase domain of type I phosphatidylinositol phosphate kinases is sufficient to prime exocytosis: isoform specificity and its underlying mechanism. J Biol Chem 280: 16522-16527. [DOI] [PubMed] [Google Scholar]