Abstract
Endocannabinoids are important mediators of short- and long-term synaptic plasticity, but the mechanisms of endocannabinoid release have not been studied extensively outside the hippocampus and cerebellum. Here, we examined the mechanisms of endocannabinoid-mediated long-term depression (eCB-LTD) in the dorsal striatum, a brain region critical for motor control and reinforcement learning. Unlike other cell types, strong depolarization of medium spiny neurons was not sufficient to yield detectable endocannabinoid release. However, when paired with postsynaptic depolarization sufficient to activate L-type calcium channels, activation of postsynaptic metabotropic glutamate receptors (mGluRs), either by high-frequency tetanic stimulation or an agonist, induced eCB-LTD. Pairing bursts of afferent stimulation with brief subthreshold membrane depolarizations that mimicked down-state to up-state transitions also induced eCB-LTD, which not only required activation of mGluRs and L-type calcium channels but also was bidirectionally modulated by dopamine D2 receptors. Consistent with network models, these results demonstrate that dopamine regulates the induction of a Hebbian form of long-term synaptic plasticity in the striatum. However, this gating of plasticity by dopamine is accomplished via an unexpected mechanism involving the regulation of mGluR-dependent endocannabinoid release.
Keywords: striatum, endocannabinoid, mGluR, dopamine, L-type calcium channel, medium spiny neuron
Introduction
Postsynaptic endocannabinoid release provides a mechanism for rapid retrograde regulation of synaptic strength on both short and long time scales (Alger, 2002; Kreitzer and Regehr, 2002; Wilson and Nicoll, 2002). In the cerebellum, hippocampus, neocortex, substantia nigra, and ventral tegmental area (VTA), strong postsynaptic depolarization elicits an endocannabinoid-dependent short-term presynaptic inhibition known as depolarization-induced suppression of excitation (DSE) or inhibition (DSI) (Kreitzer and Regehr, 2001a,b; Ohno-Shosaku et al., 2001, 2002b; Wilson and Nicoll, 2001; Trettel and Levine, 2003; Yanovsky et al., 2003; Melis et al., 2004). In the hippocampus, neocortex, amygdala, and striatum, endocannabinoids also mediate a presynaptic form of long-term depression (eCB-LTD) (Gerdeman et al., 2002; Marsicano et al., 2002; Robbe et al., 2002b; Chevaleyre and Castillo, 2003; Sjostrom et al., 2003). The postsynaptic mechanisms responsible for triggering eCB-LTD are not well understood, although activation of metabotropic glutamate receptors (mGluRs) is required in the hippocampus, striatum, and amygdala (Sung et al., 2001; Robbe et al., 2002b; Chevaleyre and Castillo, 2003; Azad et al., 2004).
Here, we further investigate the mechanisms underlying the endocannabinoid release that triggers eCB-LTD at excitatory synapses on medium spiny neurons in the dorsal striatum, a brain region important for motor planning and reward-dependent learning. We specifically focus on the role of two important properties of medium spiny neurons that distinguish them from other cell types: their down-state to up-state transitions and their rich innervation by midbrain dopaminergic afferents (Wilson, 1998). In vivo, medium spiny neurons rest in the down state, at resting membrane potentials ranging between -60 and -90 mV. Synchronous excitatory input from cortex shifts the membrane potential of medium spiny neurons cells by 20-30 mV to the up state, which ranges between -40 and -70 mV (Wilson and Kawaguchi, 1996). These state transitions affect both the source and magnitude of postsynaptic calcium signaling (Carter and Sabatini, 2004), but the role of state transitions in synaptic plasticity is not known. Similarly, although the effects of dopamine (DA) on medium spiny neuron physiology, including its role in synaptic plasticity, have been examined extensively, the literature on this topic remains confusing (Nicola et al., 2000). The control of striatal synaptic plasticity by DA is particularly important, given that influential theories of reinforcement learning require such DA-dependent modulation (Montague et al., 1996, 2004; Schultz et al., 1997; Schultz, 2002).
Our results suggest that mechanisms controlling endocannabinoid release from medium spiny neurons differ from other cell types. Unlike neurons in other brain regions (Alger, 2002; Kreitzer and Regehr, 2002; Wilson and Nicoll, 2002), strong depolarization alone is not sufficient to trigger endocannabinoid release. Instead, mGluR activation combined with L-type calcium channel activation is required and triggers eCB-LTD, which can be generated by pairing moderate-frequency afferent stimulation with brief depolarizations that mimic up-state transitions. This eCB-LTD was prevented by blocking DA D2 receptors and enhanced by their activation. Thus, the endocannabinoid release from medium spiny neurons that triggers LTD in the dorsal striatum requires both DA release and up-state-dependent calcium signaling.
Materials and Methods
Electrophysiology. Coronal slices (300 μm thick) containing the dorsal striatum were prepared from the brains of 3- to 4-week-old Sprague Dawley rats. Transverse slices (300 μm thick) of cerebellum were prepared from 14-d-old rats. Slices were superfused with an external saline solution containing the following (in mm): 125 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.25 NaH2PO4, and 16.6 glucose, bubbled with 95% O2/5% CO2. Picrotoxin (50 μm) was added to the external solution to suppress synaptic currents mediated by GABAA receptors. All drugs were purchased from Tocris Cookson (Ballwin, MO).
Whole-cell voltage-clamp recordings from medium spiny neurons or cerebellar Purkinje neurons were obtained using infrared-differential interference contrast video microscopy. Medium spiny neurons were identified using morphological (flat appearance, medium size, large initial axon segment) and electrophysiological characteristics (biexponential decay of capacitive transients, EPSC decay time constant of 4-5 ms, no rectification of synaptic currents measured at -60 and +40 mV shortly after break-in). In contrast, striatal interneurons display a rounded appearance, rapid single-exponential decay of capacitive transients, rapid EPSC decay kinetics of 1-2 ms, and strong rectification of synaptic currents measured at -60 and +40 mV shortly after break-in; we did not record any further from these interneurons. Glass electrodes (2-3 MΩ) were filled with a solution containing the following (in mm): 120 Cs-MeSO3, 15 CsCl, 8 NaCl, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, 10 tetraethylammonium, and 5 N-(2,6-dimethylphenylcarbamoyl-methyl)triethylammonium bromide, adjusted to pH 7.3 with CsOH. Synaptic currents were monitored at a holding potential of -70 mV (mimicking the down state) or -50 mV (mimicking the up state). Access resistance and leak currents were monitored continuously, and experiments were rejected if these parameters changed significantly during recording.
In the striatum, excitatory afferents, the majority of which arise from cortex, were stimulated with a glass electrode filled with external saline and placed between the recorded medium spiny neuron and cortex, typically ∼100 μm from the cell body. In the cerebellum, the stimulus electrode was placed in the molecular layer to stimulate parallel fibers. Stimulus intensity was adjusted to yield EPSC amplitudes between 200 and 400 pA. The interstimulus interval was 20 s (for paired stimuli) or 10 s (for one stimulus). During LTD induction, the stimulus intensity was increased to mimic the large synchronous cortical input received in vivo.
Data acquisition and analysis. EPSCs were acquired with an Axopatch 200B (Molecular Devices, Union City, CA), filtered at 2 kHz, and digitized at 10 kHz. Acquisition and analysis were performed using custom Igor Pro (WaveMetrics, Lake Oswego, OR) software routines. The magnitude of LTD was calculated by averaging EPSC values from 20 to 30 min after the induction protocol and comparing this to the average EPSC during the 10 min baseline. Paired-pulse ratios (PPRs) were defined as EPSC 2/EPSC 1, with an interstimulus interval of 50 ms. PPR was calculated as the ratio of the mean of the second response over the mean of the first response (Kim and Alger, 2001). Summary data are reported as mean ± SEM. For each set of manipulations that are presented in each figure, control experiments were interleaved with the experimental manipulation and then combined to generate the control summary graphs. Statistical significance was evaluated using Student's t test, and correlation was calculated using Pearson's product moment correlation.
Results
Effects of moderate-frequency afferent stimulation
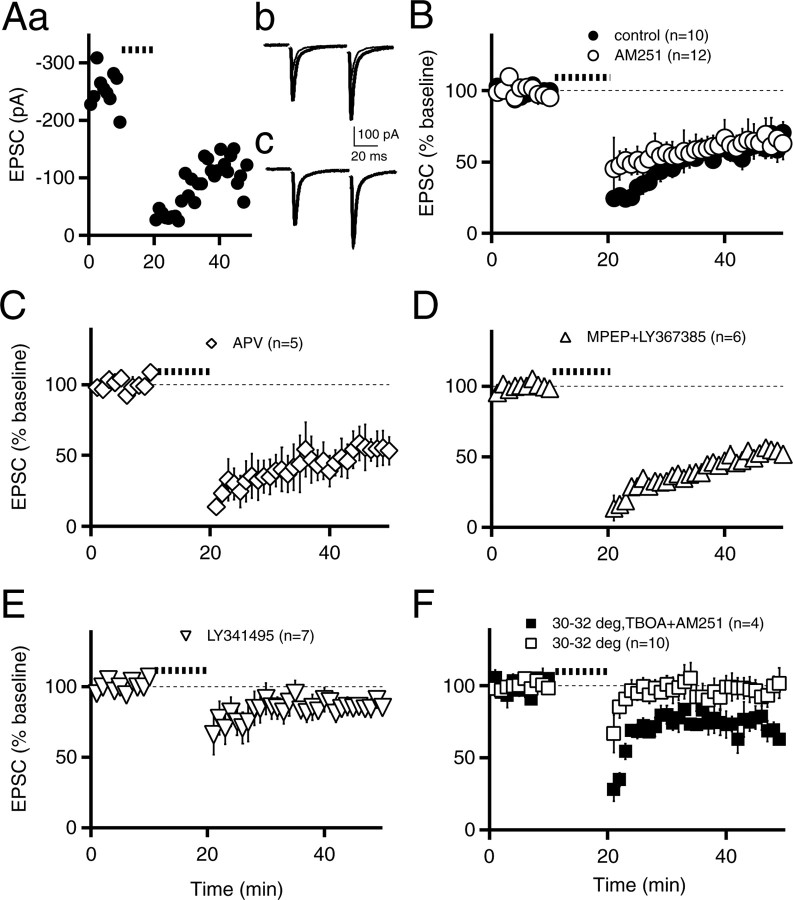
Moderate-frequency stimulation (10-13 Hz for 5 or 10 min) of excitatory cortical afferents has been reported to induce an eCB-LTD in both the dorsal and ventral striatum (Robbe et al., 2002b; Hoffman et al., 2003; Ronesi and Lovinger, 2005). Although in our experiments, a similar induction protocol (10 Hz stimulation for 10 min at room temperature) elicited a reliable LTD (60 ± 7% of control baseline; n = 10; p < 0.001) (Fig. 1A,B), surprisingly, this LTD was not accompanied by a significant increase in the paired-pulse ratio (106 ± 9% of control; n = 10; p > 0.05) and was not prevented by the cannabinoid receptor 1 (CB1) antagonist N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1 H-pyrazole-3-carboxamide (AM251) (64 ± 10% of control, n = 12, p < 0.001, LTD in AM251 vs baseline in AM251; p > 0.05, LTD control vs LTD in AM251) (Fig. 1B). Therefore, we next tested whether, as in the hippocampus, NMDA receptors or postsynaptic mGluRs were required. LTD was still present in d-APV (51 ± 12% of control, n = 5, p < 0.05, LTD in APV vs baseline in APV; p > 0.05, LTD control vs LTD in APV) (Fig. 1C) or (S)-(+)-α-amino-4-carboxy-2-methyl-benzeneacetic acid (LY367385) and 2-methyl-6-(phenylethynyl)-pyridine (MPEP) (blockers of mGluR1 and mGluR5, respectively) (51 ± 4% of control, n = 6, p < 0.001, LTD in LY367385/MPEP vs baseline in LY367385/MPEP; p > 0.05, LTD control vs LTD in LY367385/MPEP) (Fig. 1D). However, the broad-spectrum mGluR antagonist 2S-2-amino-2-(1S,2S-2-carboxycyclopropyl-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495; 100 μm) significantly reduced the magnitude of LTD (86 ± 6% of control; n = 7; p < 0.05; LTD control vs LTD in LY341495) (Fig. 1E), suggesting that presynaptic mGluRs are involved.
Figure 1.
Moderate-frequency stimulation can elicit presynaptic mGluR-dependent LTD. A, EPSC amplitudes are plotted for an individual experiment (Aa). In this graph, and in subsequent panels, the thick dotted line indicates 10 min of 10 Hz afferent stimulation. Average EPSC traces are shown in Ab for 0-10 min (thick trace) and for 40-50 min (thin trace). In Ac, the initial EPSC from the average at 40-50 min (thin trace) has been scaled to the initial EPSC of the average at 0-10 min (thick trace). B, EPSC amplitudes are plotted in control conditions (n = 10; filled circles) and in the presence of 1 μm AM251 (n = 12; open circles). C, EPSCs in the presence of 50 μm d-APV (n = 5; open diamonds). D, EPSCs in the presence of 100 μm LY367385 and 10 μm MPEP (n = 6; open triangles). E, EPSCs in the presence of 100 μm LY341495 (n = 7; open inverted triangles). F, EPSCs recorded at 30-32°C (open squares) and at 30-32°C in the presence of 10 μm TBOA and 1 μm AM251 (n = 4; filled squares). deg, Degrees. In all panels and subsequent figures, each point represents an average of three consecutive stimuli. EPSC amplitudes have been normalized to the initial 10 min baseline where indicated. Data are mean ± SEM. Experiments were performed at room temperature and at 30-32°C where noted.
A previous report has demonstrated that activation of presynaptic mGluRs can lead to a form of LTD in the striatum (Robbe et al., 2002a). In our experimental conditions, such LTD may occur during prolonged moderate-frequency stimulation because there is sufficient glutamate accumulation to strongly activate these receptors, particularly at lower temperatures at which glutamate uptake is significantly reduced. To test this hypothesis, we next asked whether this form of LTD could still be elicited at higher temperatures, which enhance glutamate uptake (Asztely et al., 1997). Consistent with this prediction, at 30-32°C, 10 Hz stimulation for 10 min did not induce LTD (98 ± 8% of control; n = 10; p > 0.05) (Fig. 1F). To specifically test whether increased glutamate uptake was responsible for the lack of LTD at higher temperatures, we repeated this experiment in the presence of the glutamate reuptake inhibitor dl-threo-β-benzyloxyaspartate (TBOA; 10 μm) and found that LTD was restored (72 ± 8% of control, n = 4, p < 0.05, LTD in TBOA vs baseline in TBOA; p < 0.05, LTD 30-32 vs LTD 30-32°C in TBOA) (Fig. 1F). This was not caused by any action of endocannabinoids, because AM251 (1 μm) was also present throughout the experiment. We conclude that at temperatures closer to the physiological condition, prolonged moderate-frequency afferent stimulation induces neither eCB-LTD nor LTD triggered by presynaptic mGluRs, although the latter can be elicited under conditions of reduced glutamate uptake.
Effects of high-frequency afferent stimulation
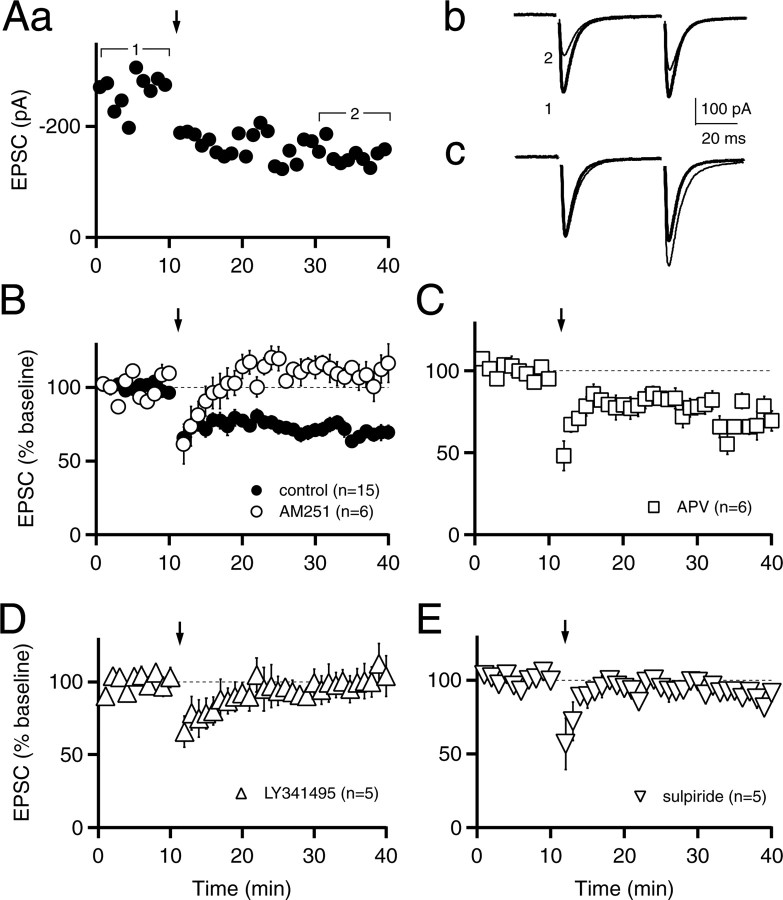
High-frequency stimulation paired with postsynaptic depolarization has also been reported to induce eCB-LTD in the dorsal striatum (Gerdeman et al., 2002), and therefore, we next tested this induction protocol. At room temperature, 100 Hz stimulation for 1 s paired with depolarization to 0 mV (repeated four times at 10 s intervals) did not elicit reliable LTD (89 ± 7% of control; n = 5; p > 0.05; data not shown). However, when we increased the temperature to 30-32°C, a more robust LTD was observed (70 ± 4% of control; n = 15; p < 0.001) (Fig. 2A,B) and was accompanied by an increase in the paired-pulse ratio (112 ± 4% of control; n = 15; p < 0.01). Consistent with intracellular and field recording experiments (Calabresi et al., 1992; Lovinger et al., 1993), this form of LTD was still present in d-APV (72 ± 6% of control, n = 6, p < 0.01, LTD in APV vs baseline in APV; p > 0.05, LTD control vs LTD in APV) (Fig. 2C). Also consistent with previous results (Gerdeman et al., 2002), LTD was not present in AM251 (110 ± 8% of control, n = 6, p > 0.05, LTD in AM251 vs baseline in AM251; p < 0.001, LTD control vs LTD in AM251) (Fig. 2B), indicating a role for endocannabinoids. Furthermore, this eCB-LTD was also absent with the mGluR antagonist LY341495 (100 μm) in the external saline (100 ± 10% of control, n = 5, p > 0.05, LTD in LY341495 vs baseline in LY341495; p < 0.001, LTD control vs LTD in LY341495) (Fig. 2D) and was significantly reduced by the DA D2 receptor antagonist sulpiride (91 ± 5% of control; n = 5; p < 0.05; LTD control vs LTD in sulpiride) (Fig. 2E), suggesting that endocannabinoid release in the striatum requires mGluR activation and can be modulated by DA.
Figure 2.
High-frequency stimulation paired with postsynaptic depolarization induces endocannabinoid-mediated LTD. A, EPSC amplitudes are plotted for an individual experiment (Aa). The arrow in this and subsequent panels indicates 1 s of 100 Hz stimulation paired with postsynaptic depolarization from -70 to 0 mV, repeated four times at 10 s intervals. Average EPSC traces collected from 0-10 min (1; thick trace) and 30-40 min (2; thin trace) are shown in Ab. In Ac, the initial EPSC from the average at 30-40 min (thin trace) has been scaled to the initial EPSC of the average at 0-10 min (thick trace). B, EPSC amplitudes are plotted in control conditions (n = 15; filled circles) and in the presence of 1 μm AM251 (n = 6; open circles). C, EPSCs in 50 μm d-APV (n = 6; open squares). D, EPSCs in 100 μm LY341495 (n = 5; open triangles). E, EPSCs in 10 μm sulpiride (n = 5; open inverted triangles). Experiments were performed at 30-32°C. Data are mean ± SEM.
Mechanisms controlling endocannabinoid release
The results thus far indicate that endocannabinoids can be released and trigger LTD using an induction protocol that pairs brief high-frequency stimulation with strong postsynaptic depolarization. In the cerebellum, hippocampus, VTA, and cortex, strong postsynaptic depolarization alone can elicit release of endocannabinoids, which transiently inhibit neurotransmitter release for tens of seconds in a process known as DSE or DSI (Kreitzer and Regehr, 2001a,b; Ohno-Shosaku et al., 2001, 2002b; Wilson and Nicoll, 2001; Trettel and Levine, 2003; Yanovsky et al., 2003; Melis et al., 2004). Therefore, we examined whether depolarization alone could induce endocannabinoid release from medium spiny neurons in the dorsal striatum. Surprisingly, when medium spiny neurons were depolarized from -70 to 0 mV for 1 or 5 s at room temperature, minimal or no DSE was detected after 10 s (1 s depolarization, 104 ± 4% of control, n = 4, p > 0.05; 5 s depolarization, 88 ± 3% of control, n = 3, p > 0.05). Similar results were found when IPSCs were monitored or when experiments were performed at 30-32°C (data not shown). In contrast, in the cerebellum and hippocampus, EPSCs are inhibited by >30-50% at 10 s after similar depolarizations (Kreitzer and Regehr, 2001a; Ohno-Shosaku et al., 2002b).
DA D2 agonists have been reported to elicit endocannabinoid release in the striatum in vivo, and in the VTA, the DA D2 agonist quinpirole enhances DSE (Giuffrida et al., 1999; Melis et al., 2004). We therefore tested whether quinpirole (10 μm) might enhance the release of endocannabinoids in response to strong depolarization of medium spiny neurons. However, DSE was still not detected when the depolarizations were applied in the presence of quinpirole (1 s depolarization, 102 ± 4%, n = 4, p > 0.05; 5 s depolarization, 100 ± 3%, n = 4, p > 0.05). Therefore, in contrast to the many other cell types that have been examined, depolarization alone is not an effective means of releasing endocannabinoids from medium spiny neurons.
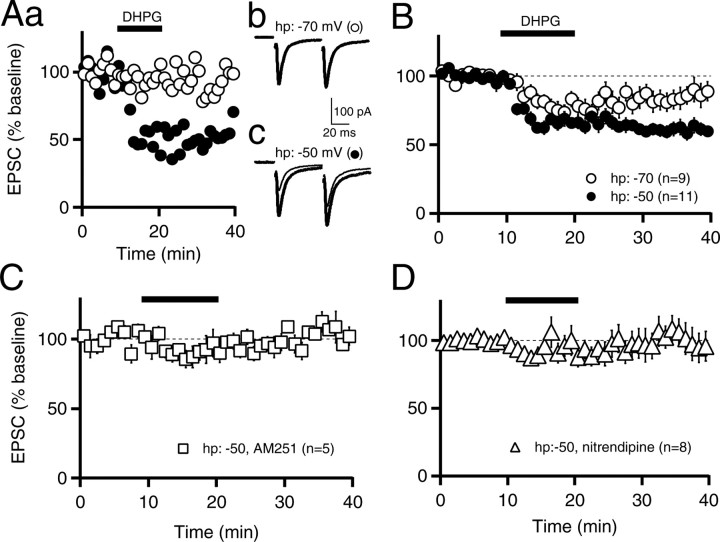
We next tested whether activation of postsynaptic mGluRs alone could induce endocannabinoid release. In the cerebellum, hippocampus, ventral striatum, and auditory brain stem, the type I mGluR agonist 3,4-dihydroxyphenylglycine (DHPG) elicits endocannabinoid release and inhibition of synaptic currents (Maejima et al., 2001; Varma et al., 2001; Ohno-Shosaku et al., 2002a; Robbe et al., 2002b; Chevaleyre and Castillo, 2003; Rouach and Nicoll, 2003; Galante and Diana, 2004; Kushmerick et al., 2004). With medium spiny neurons voltage clamped at -70 mV at room temperature, application of DHPG (100 μm) for 10 min caused only a small suppression of EPSCs when measured 20 min after start of wash-out (85 ± 7% of control; n = 9; p < 0.05) (Fig. 3A,B). However, when medium spiny neurons were voltage clamped at -50 mV, a membrane potential similar to that which occurs during the up state in vivo, DHPG application led to a large and stable LTD of EPSCs (61 ± 4% of control, n = 11, p < 0.001, DHPG-LTD at -50 mV vs baseline at -50 mV; p < 0.01, DHPG-LTD at -50 mV vs DHPG-LTD at -70 mV) (Fig. 3A,B), which was accompanied by an increase in the paired-pulse ratio (125 ± 6% of control; n = 11; p < 0.01). Therefore, all subsequent experiments were performed at a holding potential of -50 mV.
Figure 3.
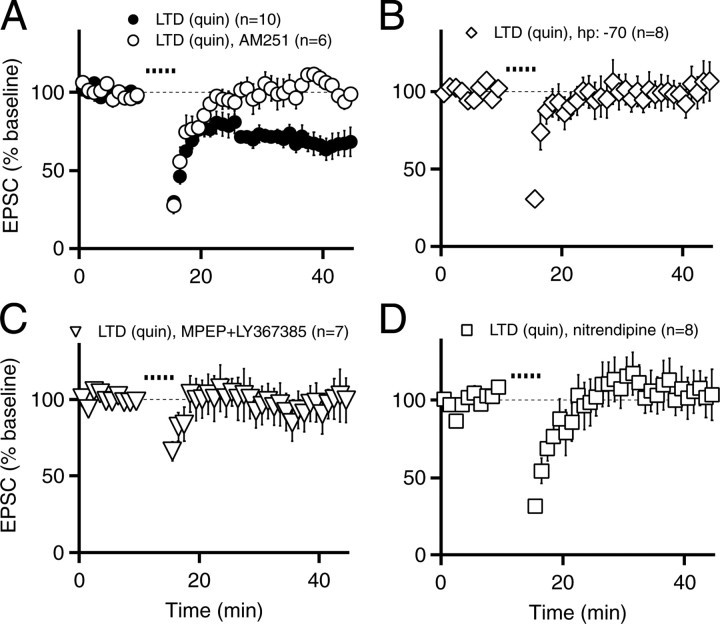
Mechanisms of mGluR-mediated endocannabinoid release from medium spiny neurons. A, Postsynaptic neurons were voltage clamped at either -70 mV (open circles) or -50 mV (filled circles), and EPSC amplitudes are plotted over time for two individual experiments (Aa). In this and subsequent panels, 100 μm DHPG was applied from 10-20 min, as indicated by the black bar above the graph. Average EPSC traces are shown overlaid for 0-10 min (thick traces) and 30-40 min (thin traces) for holding potentials of -70 mV (Ab) and -50 mV (Ac). B, EPSCs recorded at a holding potential of -70 mV (n = 9; open circles) or -50 mV (n = 11; filled circles). C, EPSCs recorded at a holding potential of -50 mV in the presence of 1 μm AM251 (n = 5; open squares). D, EPSCs recorded at -50 mV in 10 μm nitrendipine (n = 8; open triangles). Experiments were performed at room temperature. Data are mean ± SEM. hp, Holding potential.
To examine whether DHPG-induced LTD is mediated by endocannabinoids, we applied DHPG in the presence of AM251 (1 μm) and found that DHPG-LTD was abolished completely (103 ± 5% of control, n = 5, p > 0.05, DHPG-LTD in AM251 vs baseline in AM251; p < 0.001, DHPG-LTD control vs DHPG-LTD in AM251) (Fig. 3C), indicating that endocannabinoid signaling was required. Because receptor-driven endocannabinoid release is enhanced greatly by elevated intracellular calcium (Brenowitz and Regehr, 2005; Hashimotodani et al., 2005), we asked whether calcium entry was necessary for DHPG-mediated endocannabinoid release in medium spiny neurons. We specifically focused on L-type calcium channels, because in cultured medium spiny neurons, mGluR activation has been reported to induce calcium influx through L-type calcium channels (Mao and Wang, 2003). Furthermore, recent work suggests that in the up state, L-type calcium channel signaling is particularly prominent (Carter and Sabatini, 2004). To test whether L-type calcium channels are also involved in mGluR-triggered endocannabinoid release, we applied DHPG in the presence of nitrendipine (10 μm) and found that DHPG-LTD was blocked completely (100 ± 11% of control, n = 8, p > 0.05, DHPG-LTD in nitrendipine vs baseline in nitrendipine; p < 0.01, DHPG-LTD control vs DHPG-LTD in nitrendipine) (Fig. 3D). Thus, activation of mGluRs leads to endocannabinoid release that requires L-type calcium channels. This explains why holding cells at -70 mV, a membrane potential at which L-type channels are mostly inactivated, reduced the effect of DHPG, with the small remaining depression (∼15%) (Fig. 3B) caused by either incomplete inactivation of L-type calcium channels or spillover from neighboring cells in response to the robust activation of postsynaptic mGluRs elicited by DHPG application.
Dopamine modulation of state-dependent eCB-LTD
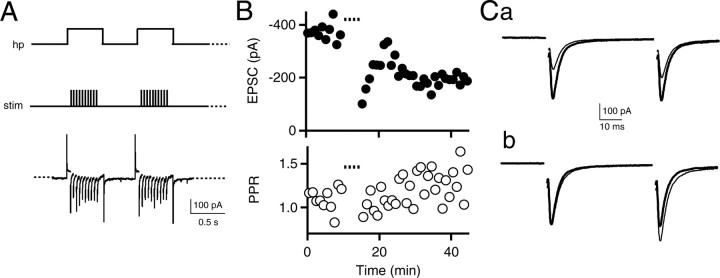
The enhancement of mGluR-triggered endocannabinoid release by subthreshold depolarization suggested that the up state of medium spiny neurons might be critical for induction of eCB-LTD under physiological conditions. To test this hypothesis, we examined whether a more physiologically realistic induction protocol, in which we paired 0.5 s postsynaptic depolarizations from -70 to -50 mV (mimicking the down- to up-state transition) with 10 stimuli delivered at 25 Hz (mimicking synchronous cortical excitation), could elicit eCB-LTD. This pairing was repeated every second for 5 min at 30-32°C (Fig. 4A) and successfully elicited LTD (84 ± 6% of control baseline; n = 11; p < 0.05) (Figs. 4B, 5A). LTD was not present in AM251 (101 ± 9% of control, n = 4, p > 0.05, LTD in AM251 vs baseline in AM251; p < 0.1, LTD control vs LTD in AM251) (Fig. 5A), suggesting a role for endocannabinoids.
Figure 4.
State-dependent LTD in the dorsal striatum. A, State-dependent LTD induction protocol. The membrane holding potential (hp) was varied between -70 and -50 mV every 0.5 s for a total of 5 min. Steps to -50 mV were paired with 10 presynaptic stimuli at 25 Hz (stim). Medium spiny neuron responses to this stimulus protocol are shown below for an individual experiment. Capacitance transients have been truncated, and stimulus artifacts have been blanked for clarity. B, EPSC amplitude (top graph; filled circles) is plotted for an individual experiment. The dotted line above the graph represents the time of LTD induction. The PPR (bottom graph; open circles) is shown below. Ca, Average EPSC trace from 0-10 min (thick trace) overlaid with average EPSC trace from 30-40 min (thin trace) from the experiment shown in A and B. Cb, Average EPSC trace from 0-10 min (thick trace) overlaid with the scaled average EPSC trace from 30-40 min (thin trace). Each point represents an average of three EPSCs. Experiments were performed at 30-32°C.
Figure 5.
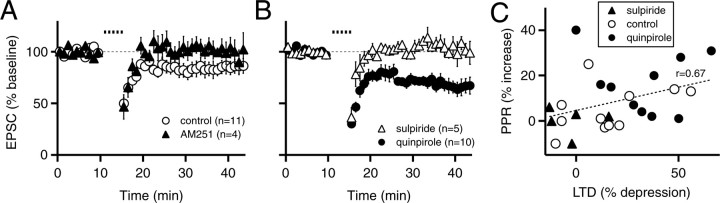
Dopamine modulation of state-dependent LTD. A, EPSC amplitudes before and after state-dependent LTD induction in control conditions (n = 12; open circles) and in the presence of 1 μm AM251 (n = 4; filled triangles). B, EPSC amplitudes before and after state-dependent LTD induction in the presence of 10 μm quinpirole during LTD induction (n = 10; filled circles) or in 10 μm sulpiride during LTD induction (n = 5; open triangles). C, The increase in the PPR is plotted as a function of the amount of the LTD present at 35-45 min. The dotted line represents a linear fit of the data, and r is the Pearson's correlation coefficient. In A and B, the time of LTD induction is indicated by the dotted line. Experiments were performed at 30-32°C. Data are mean ± SEM.
These results indicate that this induction protocol, which was designed to mimic up-state transitions, may elicit a form of eCB-LTD. However, the average magnitude of this eCB-LTD was modest (16% depression) (Fig. 5A). Because dopamine D2 activation has been linked to endocannabinoid release as well as LTD in the striatum (Calabresi et al., 1997; Giuffrida et al., 1999; Tang et al., 2001), we next examined whether D2 receptors could modulate the magnitude of this eCB-LTD. First, we confirmed previous work that application of the D2 agonist quinpirole (10 μm) alone has minimal effect on basal synaptic transmission (Nicola and Malenka, 1998) (96 ± 9% of control; n = 9; p > 0.05; data not shown). When quinpirole was applied during the 5 min induction protocol, however, the magnitude of eCB-LTD was significantly larger than in control conditions (67 ± 6% of control; n = 10; p < 0.05; LTD control vs LTD in quinpirole) (Fig. 5B) and was accompanied by an increase in the paired-pulse ratio (116 ± 6% of control; n = 10; p < 0.05). Conversely, the dopamine D2 antagonist sulpiride (10 μm) blocked this form of eCB-LTD (103 ± 8% of control, n = 5, p < 0.05, LTD control vs LTD in sulpiride; p > 0.05, LTD in sulpiride vs baseline in sulpiride) (Fig. 5B). Sulpiride had only a small effect on the eCB-LTD elicited by DHPG application (72 ± 4% of control; n = 8; p < 0.05; DHPG-LTD control vs DHPG-LTD in sulpiride; data not shown), suggesting that D2 receptors modulate, but do not control, mGluR-triggered endocannabinoid release. The magnitude of the increase in the paired-pulse ratio was correlated with the magnitude of state-dependent eCB-LTD (Pearson's correlation coefficient, r = 0.67) (Fig. 5C), a result that strongly suggests the involvement of a presynaptic expression mechanism. Interestingly, the change in the paired-pulse ratio often was delayed compared with the initial large decrease in the EPSC (Fig. 4B). This suggests that the induction protocol may have elicited additional short-term changes that do not directly modulate transmitter release such as transient failures of axonal conduction or postsynaptic modifications.
We proceeded to further characterize this form of eCB-LTD, always performing experiments in the presence of quinpirole (10 μm) to enhance its magnitude. First, we confirmed that in the presence of quinpirole, this induction protocol still elicited a form of LTD that required endocannabinoid signaling as evidenced by its block by AM251 (103 ± 4%, n = 6, p > 0.05, LTD in AM251 vs baseline in AM251; p < 0.05, LTD control vs LTD in AM251) (Fig. 6A). Second, to test whether the subthreshold depolarizations, which were designed to mimic up-state transitions, were required to induce this eCB-LTD, we used the same induction protocol, except cells were held continuously at -70 mV. This prevented the generation of eCB-LTD (100 ± 10% of control, n = 8, p > 0.05, LTD at -70 mV vs baseline at -70 mV; p < 0.01, LTD control vs LTD at -70 mV) (Fig. 6B). Third, to test whether, like the eCB-LTD elicited by DHPG application or high-frequency tetanic stimulation, this state-dependent eCB-LTD required postsynaptic mGluR activation, we applied the induction protocol in the presence of LY367385 and MPEP. This manipulation prevented the eCB-LTD (95 ± 13% of control, n = 7, p > 0.05, LTD in LY367385/MPEP vs baseline in LY367385/MPEP; p < 0.05, LTD control vs LTD in LY367385/MPEP) (Fig. 6C). Finally, eCB-LTD was also blocked in the presence of the L-type calcium channel blocker nitrendipine (105 ± 13% of control, n = 8, p > 0.05, LTD in nitrendipine vs baseline in nitrendipine; p < 0.01, LTD control vs LTD in nitrendipine) (Fig. 6D). Together, these results indicate that the release of endocannabinoids (and subsequent eCB-LTD) caused by this induction protocol requires mechanisms similar to those underlying the LTD elicited by the mGluR agonist DHPG. Importantly, both forms of eCB-LTD depend on subthreshold depolarizations, which mimic the up state and the consequent calcium influx through L-type channels.
Figure 6.
Mechanisms of state-dependent LTD. A, EPSC amplitudes before and after state-dependent LTD induction in control conditions (n = 10; filled circles) and in the presence of 1 μm AM251 (n = 6; open circles). B, EPSC amplitudes before and after state-dependent LTD induction when the postsynaptic membrane potential was kept at -70 mV throughout the induction protocol (n = 8; open diamonds). C, EPSCs amplitudes before and after LTD in the presence of LY367385 (100 μm) and MPEP (10 μm) (n = 7; open inverted triangles). D, EPSC amplitudes before and after LTD in 10 μm nitrendipine (n = 8; open squares). All experiments included 10 μm quinpirole during the 5 min LTD induction protocol. The dotted line above the graphs indicates the time of LTD induction. Experiments were performed at 30-32°C. Data are mean ± SEM. quin, Quinpirole; hp, holding potential.
CB1 receptor activation is sufficient to induce LTD
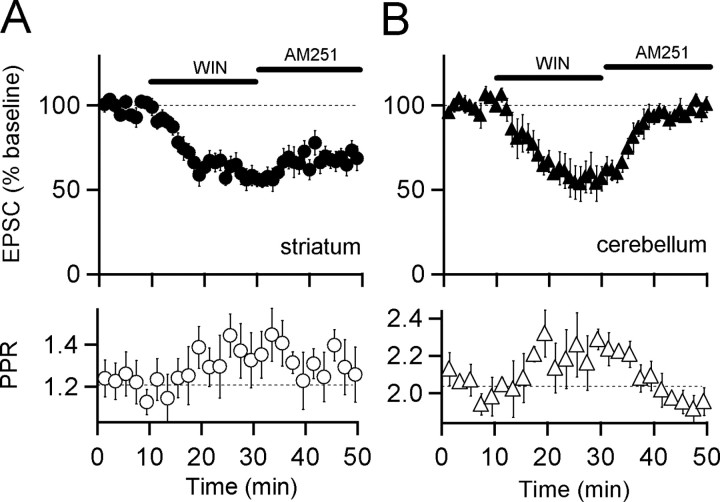
A key issue with regard to the mechanisms of eCB-LTD is whether activation of CB1 receptors alone is sufficient to elicit LTD or whether some other secondary signals are also required. Two studies in the dorsal striatum report that the actions of CB1 agonists can be reversed fully by subsequent application of CB1 antagonists when assayed by extracellular field potential recordings (Huang et al., 2001; Ronesi et al., 2005), suggesting that CB1 activation alone is not sufficient to induce LTD. However, a study in the ventral striatum reported that EPSC inhibition by CB1 receptors is not fully reversed by a CB1 antagonist (Robbe et al., 2001). To address this issue, we performed similar experiments and found that application of the CB1 agonist R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2; 1 μm) reduced EPSCs (60 ± 6% of control; n = 9; p < 0.01) (Fig. 7A) and increased the paired-pulse ratio (115 ± 8% of control; n = 9; p < 0.1). Importantly, this CB1-mediated depression of EPSCs was not reversed by washing out WIN55,212-2 and applying the selective high-affinity CB1 receptor antagonist AM251 (5 μm) (69 ± 6% of control; n = 9; p < 0.05 baseline vs AM251).
Figure 7.
A CB1 receptor agonist is sufficient to induce LTD in the striatum but not in the cerebellum. A, Top, Normalized EPSC amplitudes recorded from striatal medium spiny neurons are plotted over time (n = 9; filled circles). The time of WIN55,212-2 (1 μm) and AM251 (5 μm) application is marked by black bars in both panels. Bottom, Paired-pulse ratios are plotted over time for the same data shown in the top panel (open circles). B, Top, Normalized EPSC amplitudes recorded from cerebellar Purkinje neurons are plotted over time (n = 3; filled triangles). Bottom, Paired-pulse ratios are plotted for the same data shown in the top panel (open triangles). Experiments were performed at room temperature. Data are mean ± SEM. WIN, WIN55,212-2.
As a control for the efficacy of AM251 in blocking the activation of CB1 receptors during the wash-out of WIN55,212-2, we performed identical experiments while recording EPSCs generated at cerebellar parallel fiber to Purkinje cell synapses, where short-term inhibition by endocannabinoids is well documented (Kreitzer and Regehr, 2001a). In contrast to the striatum, we found that in cerebellar slices, presynaptic inhibition of EPSCs by the CB1 receptor agonist WIN55,212-2 was completely reversible. Bath application of WIN55,212-2 (1 μm) reduced parallel fiber EPSCs recorded from Purkinje cells to the same extent as that observed in medium spiny neurons (57 ± 9% of control; n = 3; p < 0.05) (Fig. 7B), while also increasing the paired-pulse ratio (111 ± 3% of control; n = 3; p < 0.05). However, this inhibition by WIN55,212-2 (1 μm) was reversed fully by subsequent application of AM251 (5 μm) (96 ± 4% of control; n = 3; p > 0.05; baseline vs AM251).
To further test whether the actions of CB1 agonists in the striatum are presynaptic, we applied WIN55,212-2 (1 μm) while recording with GDP-β-S (0.6 mm) in the recording pipette to disrupt postsynaptic G-protein-coupled-receptor signaling and found a similar magnitude of EPSC inhibition (58 ± 7% of control with GDP-β-S; n = 3; p > 0.05; WIN55,212-2 control vs WIN55,212-2 with postsynaptic GDP-β-S). We also applied WIN55,212-2 (1 μm) in the presence of a broad-spectrum mGluR antagonist LY341495 (100 μm), an adenosine A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (1 μm), and a GABAB antagonist (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid (2 μm) to block any possible nondirect cannabinoid actions (Brown et al., 2003) and found a similar inhibition (64 ± 7% of control; n = 4; p > 0.05; WIN55,212-2 control vs WIN55,212-2 with metabotropic blockers). Furthermore, WIN55,212-2 (1 μm) also inhibited EPSCs in the presence of the dopamine D1 antagonist SCH23390 [(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (5 μm) and the dopamine D2 antagonist sulpiride (10 μm) (62 ± 6% of control; n = 10; p > 0.05; WIN55,212-2 control vs WIN55,212-2 in dopamine receptor antagonists). Together, these data suggest that in the striatum, CB1 agonists act directly on presynaptic CB1 receptors and that activation of these receptors alone is sufficient to induce LTD.
Discussion
Striatal medium spiny neurons serve critical roles in adaptive behavior and neuropsychiatric disease. Thus, understanding the detailed mechanisms of plasticity at corticostriatal synapses has important functional implications. Based on previous work (Choi and Lovinger, 1997; Hoffman et al., 2003; Ronesi and Lovinger, 2005), we used whole-cell voltage-clamp recordings to test whether moderate- (10-25 Hz) and high- (100 Hz) frequency stimulation yields LTD mediated by endocannabinoids. We elicited eCB-LTD with high-frequency stimulation, paired with strong postsynaptic depolarization. However, detectable endocannabinoid release did not occur in response to strong depolarization alone. We also found that membrane potential greatly influenced the eCB-LTD elicited by the mGluR agonist DHPG, attributable to an important role for L-type calcium channels. Based on these results, we developed a novel protocol to induce eCB-LTD, in which we paired moderate frequency stimulation (25 Hz) with subthreshold membrane depolarization to mimic the down- to up-state transitions observed in vivo. This protocol yielded eCB-LTD, which was modulated by dopamine D2 receptors and was dependent on postsynaptic mGluR activation and L-type calcium channels (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Induction of eCB-LTD in the striatum
Early studies of synaptic plasticity in the striatum used brief high-frequency stimulation and recording techniques that allowed postsynaptic cells to fire action potentials (Calabresi et al., 1992; Lovinger et al., 1993; Walsh, 1993). In these conditions, LTD was the predominant form of plasticity and appeared to require both dopamine D1- and D2-type receptors (Calabresi et al., 1992, 1997; Tang et al., 2001), type I mGluRs (Gubellini et al., 2001; Sung et al., 2001), L-type calcium channels (Choi and Lovinger, 1997), and a host of intracellular signaling pathways (Centonze et al., 2003). More recent studies have presented compelling evidence that a form of LTD, which is presynaptically expressed and caused by postsynaptic release of endocannabinoids, can be elicited (Gerdeman et al., 2002; Robbe et al., 2002b). However, the specific roles of mGluRs, L-type calcium channels, and DA receptors in this process remained unclear.
Consistent with previous results, we found that high-frequency stimulation (100 Hz) paired with strong postsynaptic depolarization evoked eCB-LTD at 30-32°C (Gerdeman et al., 2002) but not at room temperature. These results indicate that endocannabinoid biosynthesis may be enhanced at higher temperatures, consistent with the increased magnitude of DSE observed in the cerebellum at 34°C (Kreitzer and Regehr, 2001a). However, inconsistent with previous findings (Ronesi and Lovinger, 2005), prolonged (5-10 min) 10 Hz stimulation while voltage clamping at -70 mV did not elicit eCB-LTD but induced instead, at room temperature only, a form of LTD that required presynaptic, but not postsynaptic, mGluRs. We were able to induce eCB-LTD when short bursts of 25 Hz stimulation were paired with subthreshold depolarization to -50 mV. Based on these results, we suggest that induction of eCB-LTD in the striatum requires moderate (25 Hz) to high-frequency (100 Hz) stimulation during membrane depolarization sufficient to activate L-type calcium channels. However, when postsynaptic cells are not (or inadequately) voltage clamped, moderate frequency afferent stimulation would likely depolarize cells enough to activate L-type calcium channels and thus sufficient endocannabinoid release to generate eCB-LTD. Importantly, we found agonist activation of CB1 receptors alone was sufficient to induce LTD. This suggests that presynaptic CB1 receptors may modulate transmitter release in the striatum via mechanisms different from those in other structures such as the cerebellum.
Mechanisms of endocannabinoid release
Endocannabinoids are a class of lipophilic membrane-derived signaling molecules, the most prominent of which are anandamide and 2-arachidonoylglycerol (2-AG). Both depolarization and metabotropic receptor activation can elicit endocannabinoid release (Alger, 2002; Kreitzer and Regehr, 2002; Wilson and Nicoll, 2002), although the precise steps required for endocannabinoid biosynthesis are still being elucidated (Di Marzo, 1999). Strong depolarization of voltage-clamped neurons results in large increases in intracellular calcium (10-50 μm) (Brenowitz and Regehr, 2003), which have been suggested to activate phospholipase D and anandamide biosynthesis. In contrast, metabotropic receptor activation appears to activate phopholipase C and DAG lipase in a process modulated by modest elevations in intracellular calcium, yielding 2-AG (Hashimotodani et al., 2005).
In both dorsal and ventral striatum, eCB-LTD requires postsynaptic mGluR activation (Sung et al., 2001; Robbe et al., 2002b). However, in contrast to results obtained from hippocampus and cerebellum (Maejima et al., 2001; Chevaleyre and Castillo, 2003), we found that L-type calcium channel antagonists block mGluR-triggered release of endocannabinoids, as measured by the generation of eCB-LTD. Furthermore, in the striatum, depolarization alone did not elicit any detectable endocannabinoid release and activation of postsynaptic mGluRs (either with DHPG or moderate-frequency stimulation) was only effective when postsynaptic neurons were voltage clamped at -50 mV. These results suggest that in medium spiny neurons, the rise in calcium caused by activation of voltage-dependent calcium channels is insufficient to generate physiologically significant amounts of endocannabinoids. Furthermore, release of endocannabinoids caused by postsynaptic mGluR activation either requires or is greatly facilitated by the rise in calcium triggered by L-type calcium channel activation.
Medium spiny neurons in vivo exhibit distinct membrane fluctuations between a hyperpolarized down state and a depolarized up state. Synchronous synaptic excitation from cortical afferents drives medium spiny neurons from the down state into the up state, from which they fire action potentials (Wilson and Kawaguchi, 1996). A recent study indicates that this 20-30 mV subthreshold depolarization shifts the predominant source for dendritic calcium influx, decreasing the contribution of T-type calcium channels while enhancing the contribution of L-type channels (Carter and Sabatini, 2004). Our findings that membrane depolarization and L-type calcium channels are required for mGluR-triggered endocannabinoid release and thereby eCB-LTD are entirely consistent with this observation and provide one important physiological role for this membrane potential-dependent change in the source of calcium.
Dopamine modulation of eCB-LTD
Despite its importance, the electrophysiological effects of DA on medium spiny neurons have been difficult to elucidate. Both D1 and D2 receptors can modulate multiple voltage-dependent conductances in medium spiny neurons in complex ways (Nicola et al., 2000). With regard to synaptic effects, even the simple issue of whether DA significantly affects basal transmission at excitatory synapses onto medium spiny neurons remains controversial (Nicola et al., 2000; Bamford et al., 2004). The role of DA in regulating striatal synaptic plasticity has also been studied, with studies suggesting that both D1 and D2 receptors may be important for LTD, whereas D1 receptors may be required for long-term potentiation (Centonze et al., 2001; Wickens et al., 2003).
Here, we define a specific role for D2 receptors in the triggering of eCB-LTD. In our experiments, both glutamatergic and dopaminergic afferents were likely activated, yielding the eCB-LTD observed in control conditions (Fig. 5A). Sulpiride, a D2 receptor antagonist, completely blocked state-dependent eCB-LTD, although it had only a modest effect on DHPG-induced LTD. These results indicate that endogenous DA importantly contributes to the generation of eCB-LTD by synaptic activity and confirms previous work implicating D2 receptors in LTD in the striatum (Calabresi et al., 1997; Tang et al., 2001). Conversely, the D2 receptor agonist quinpirole enhanced the magnitude of state-dependent eCB-LTD, a finding consistent with previous work reporting that D2 receptor activation enhances endocannabinoid release in the striatum in vivo as well as the VTA in vitro (Giuffrida et al., 1999; Melis et al., 2004). Based on these results, it might be expected that state-dependent eCB-LTD should only be observed in the subpopulation of striatal cell that express D2 receptors (Wilson, 1998). Although we did not observe an obvious dichotomy in the ability to induce LTD, this is likely because of the variability inherent in studying synaptic plasticity phenomena. Thus, additional studies in which it is possible to specifically record from D2-containing versus D2-lacking striatal cells will be required to address this issue.
Functionally, DA cell firing in the substantia nigra and VTA has been suggested to encode reward prediction errors, which contribute to reward-dependent learning (Schultz, 1998, 2002). Release of DA in the striatum has specifically been suggested to gate long-term synaptic plasticity, thus providing a mechanism by which learning-induced circuit adaptations are only instantiated when the appropriate “reward/teaching” signal occurs (Montague et al., 1996, 2004; Schultz et al., 1997; Schultz, 2002). Consistent with this idea, DA receptor activation has been reported to be required for various forms of synaptic plasticity in the striatum (Calabresi et al., 1992, 1997; Dos Santos Villar and Walsh, 1999; Tang et al., 2001). Here, we demonstrate that enhancement of endocannabinoid release by DA is critical for eliciting eCB-LTD (Gerdeman et al., 2003). Although this form of synaptic plasticity does not involve NMDA receptors, it is “Hebbian” in that it requires synaptic activity (to activate mGluRs) as well as membrane depolarization (to activate L-type calcium channels). Thus, DA-dependent gating of this form of LTD in the striatum via its effects on endocannabinoid release fulfills the theoretical requirements of computational models of reinforcement learning. Indeed, it will be of great interest to test the functional importance of eCB-LTD in the dorsal striatum by examining reward-dependent learning in animals in which CB1 receptors in this structure have been blocked by genetic or pharmacological manipulations.
Footnotes
This work was supported by a Ruth L. Kirchenstein National Research Service Award (F32 DA016879-01 to A.C.K.) and by National Institutes of Health Grant DA009264 (R.C.M.). We thank members of the Malenka laboratory and Jeremy Dittman for helpful discussions and Bernardo Sabatini for providing custom Igor acquisition software.
Correspondence should be addressed to Robert C. Malenka, Department of Psychiatry and Behavioral Sciences, Stanford University Medical School, Medical School Lab Surge Building, Room P104, 1201 Welch Road, Palo Alto, CA 94305. E-mail: malenka@stanford.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/2510537-09$15.00/0
References
- Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68: 247-286. [DOI] [PubMed] [Google Scholar]
- Asztely F, Erdemli G, Kullmann DM (1997) Extrasynaptic glutamate spillover in the hippocampus: dependence on temperature and the role of active glutamate uptake. Neuron 18: 281-293. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G (2004) Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci 24: 9953-9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Zhang H, Schmitz Y, Wu NP, Cepeda C, Levine MS, Schmauss C, Zakharenko SS, Zablow L, Sulzer D (2004) Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42: 653-663. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG (2003) Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci 23: 6373-6384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG (2005) Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron 45: 419-431. [DOI] [PubMed] [Google Scholar]
- Brown TM, Brotchie JM, Fitzjohn SM (2003) Cannabinoids decrease corticostriatal synaptic transmission via an effect on glutamate uptake. J Neurosci 23: 11073-11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G (1992) Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci 12: 4224-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E (1997) Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci 17: 4536-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL (2004) State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron 44: 483-493. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P (2001) Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci 13: 1071-1077. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Pisani A, Bernardi G, Calabresi P (2003) Dopamine, acetylcholine and nitric oxide systems interact to induce corticostriatal synaptic plasticity. Rev Neurosci 14: 207-216. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE (2003) Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron 38: 461-472. [DOI] [PubMed] [Google Scholar]
- Choi S, Lovinger DM (1997) Decreased probability of neurotransmitter release underlies striatal long-term depression and postnatal development of corticostriatal synapses. Proc Natl Acad Sci USA 94: 2665-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V (1999) Biosynthesis and inactivation of endocannabinoids: relevance to their proposed role as neuromodulators. Life Sci 65: 645-655. [DOI] [PubMed] [Google Scholar]
- Dos Santos Villar F, Walsh JP (1999) Modulation of long-term synaptic plasticity at excitatory striatal synapses. Neuroscience 90: 1031-1041. [DOI] [PubMed] [Google Scholar]
- Galante M, Diana MA (2004) Group I metabotropic glutamate receptors inhibit GABA release at interneuron-Purkinje cell synapses through endocannabinoid production. J Neurosci 24: 4865-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM (2002) Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci 5: 446-451. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci 26: 184-192. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D (1999) Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci 2: 358-363. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Saulle E, Centonze D, Bonsi P, Pisani A, Bernardi G, Conquet F, Calabresi P (2001) Selective involvement of mGlu1 receptors in corticostriatal LTD. Neuropharmacology 40: 839-846. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin H-S, Kano M (2005) Phospholipase Cβ serves as a coincidence detector through its Ca dependency for triggering retrograde endocannabinoid signal. Neuron 45: 257-268. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Oz M, Caulder T, Lupica CR (2003) Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J Neurosci 23: 4815-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS (2001) Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. J Physiol (Lond) 532 3: 731-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE (2001) Random response fluctuations lead to spurious paired-pulse facilitation. J Neurosci 21: 9608-9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2001a) Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717-727. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2001b) Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 21: RC174(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG (2002) Retrograde signaling by endocannabinoids. Curr Opin Neurobiol 12: 324-330. [DOI] [PubMed] [Google Scholar]
- Kushmerick C, Price GD, Taschenberger H, Puente N, Renden R, Wadiche JI, Duvoisin RM, Grandes P, von Gersdorff H (2004) Retroinhibition of presynaptic calcium currents by endocannabinoids released via postsynaptic mGluR activation at a calyx synapse. J Neurosci 24: 5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A (1993) Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol 70: 1937-1949. [DOI] [PubMed] [Google Scholar]
- Maejima T, Ohno-Shosaku T, Kano M (2001) Endogenous cannabinoid as a retrograde messenger from depolarized postsynaptic neurons to presynaptic terminals. Neurosci Res 40: 205-210. [DOI] [PubMed] [Google Scholar]
- Mao L, Wang JQ (2003) Group I metabotropic glutamate receptor-mediated calcium signalling and immediate early gene expression in cultured rat striatal neurons. Eur J Neurosci 17: 741-750. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530-534. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL (2004) Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci 24: 53-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ (1996) A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 16: 1936-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD (2004) Computational roles for dopamine in behavioural control. Nature 431: 760-767. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Malenka RC (1998) Modulation of synaptic transmission by dopamine and norepinephrine in ventral but not dorsal striatum. J Neurophysiol 79: 1768-1776. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC (2000) Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci 23: 185-215. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M (2001) Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29: 729-738. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M (2002a) Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci 15: 953-961. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M (2002b) Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci 22: 3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ (2001) Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci 21: 109-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Alonso G, Chaumont S, Bockaert J, Manzoni OJ (2002a) Role of P/Q-Ca2+ channels in metabotropic glutamate receptor 2/3-dependent presynaptic long-term depression at nucleus accumbens synapses. J Neurosci 22: 4346-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ (2002b) Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA 99: 8384-8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi J, Lovinger DM (2005) Induction of striatal long-term depression by moderate frequency activation of cortical afferents in rat. J Physiol (Lond) 562: 245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Nicoll RA (2003) Endocannabinoids contribute to short-term but not long-term mGluR-induced depression in the hippocampus. Eur J Neurosci 18: 1017-1020. [DOI] [PubMed] [Google Scholar]
- Schultz W (1998) Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1-27. [DOI] [PubMed] [Google Scholar]
- Schultz W (2002) Getting formal with dopamine and reward. Neuron 36: 241-263. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science 275: 1593-1599. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB (2003) Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 39: 641-654. [DOI] [PubMed] [Google Scholar]
- Sung KW, Choi S, Lovinger DM (2001) Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol 86: 2405-2412. [DOI] [PubMed] [Google Scholar]
- Tang K, Low MJ, Grandy DK, Lovinger DM (2001) Dopamine-dependent synaptic plasticity in striatum during in vivo development. Proc Natl Acad Sci USA 98: 1255-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trettel J, Levine ES (2003) Endocannabinoids mediate rapid retrograde signaling at interneuron right-arrow pyramidal neuron synapses of the neocortex. J Neurophysiol 89: 2334-2338. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE (2001) Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 21: RC188(1-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JP (1993) Depression of excitatory synaptic input in rat striatal neurons. Brain Res 608: 123-128. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI (2003) Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol 13: 685-690. [DOI] [PubMed] [Google Scholar]
- Wilson CJ (1998) Basal ganglia. In: The synaptic organization of the brain (Shepherd GM, ed), pp 329-375. New York: Oxford UP.
- Wilson CJ, Kawaguchi Y (1996) The origins of two-state spontaneous membrane potential fluctuations of neostriatal spiny neurons. J Neurosci 16: 2397-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588-592. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA (2002) Endocannabinoid signaling in the brain. Science 296: 678-682. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Mades S, Misgeld U (2003) Retrograde signaling changes the venue of postsynaptic inhibition in rat substantia nigra. Neuroscience 122: 317-328. [DOI] [PubMed] [Google Scholar]