In the article “Differential Maturation of GABA Action and Anion Reversal Potential in Spinal Lamina I Neurons: Impact of Chloride Extrusion Capacity,” by Matilde Cordero-Erausquin, Jeffrey A. M. Coull, Dominic Boudreau, Matthias Rolland, and Yves De Koninck, which appeared on pages 9613-9623 of the October 19, 2005 issue, the most recent versions of Figures 6 and 7 were not used because of a printer's error. The correct versions of both figures, as well as each corresponding legend, are printed here.
Figure 6.
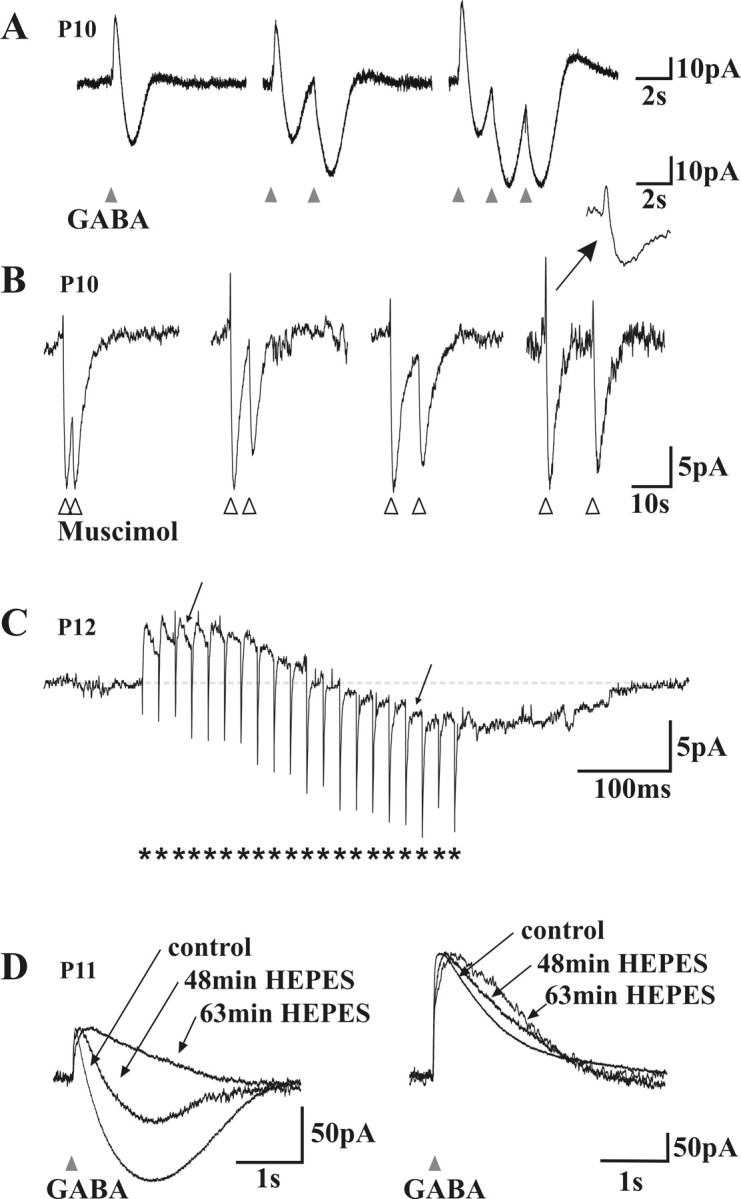
Mechanism of GABAA receptor-mediated biphasic responses. A, Voltage-clamp recordings of the response of a neuron to different patterns of brief (20 ms) GABA applications The cell displayed a biphasic (outward/inward) response to GABA (left). Note that the second (middle) and third (right) application of GABA with 2 s intervals produce a monophasic inward current. Note also the small outward tail response after repetitive applications. B, Voltage-clamp recordings of the response of a neuron to pairs of brief (20 ms) muscimol applications (5, 10, 15, or 25 s interval; in the presence of CGP 54523). The second response was also monophasic when superimposed on the inward phase of the first response. Note that repetitive applications of muscimol do not induce the small outward tail. Inset, An enlargement on a faster timescale (same as in A). Traces are normalized to the amplitude of the first response to help comparisons. C, Voltage-clamp trace of a neuron responding to a train of focal electrical stimulations (short burst of 20 pulses, indicated by asterisks, at 25 ms intervals). Note the slowly developing inward phase and how individual synaptic currents have their polarity inverted (arrows) during this late inward phase. D, Effect of the replacement of the bicarbonate buffer by a HEPES buffer in the ACSF. Left, The inward phase of the biphasic responses observed near resting potential faded with time in HEPES. Right, At depolarized holding potentials (-39 mV), in which outward only responses were recorded, the decay phase of the responses was prolonged in HEPES. Traces are normalized to the amplitude of the first response to allow for comparison of the kinetics.
Figure 7.
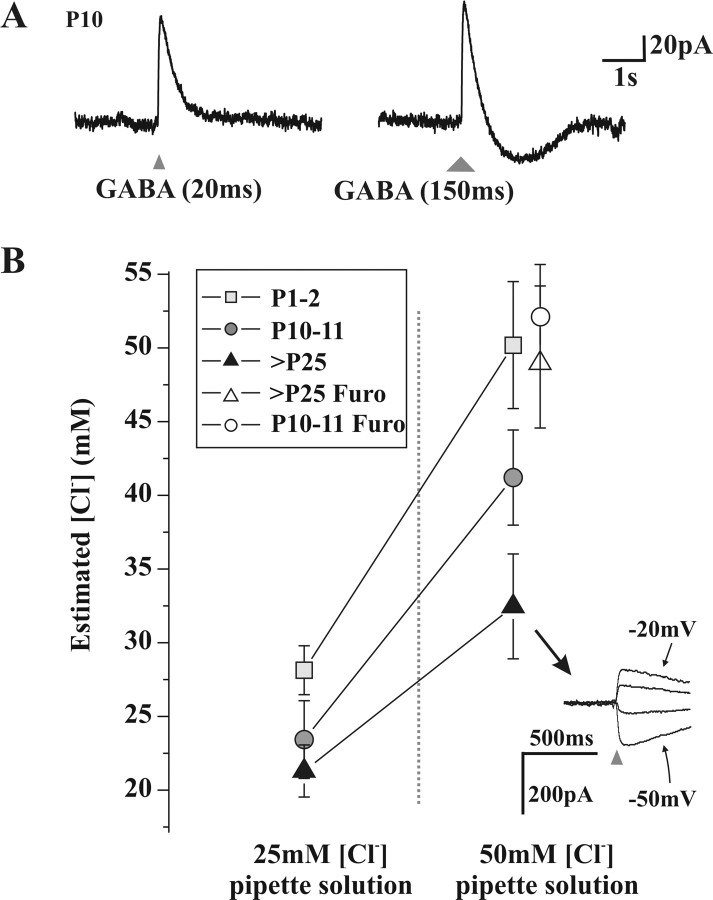
Measurement of Cl- extrusion capacity at different developmental stages. A, Voltage-clamp recordings of a neuron presenting a monophasic outward current in response to a 20 ms application of GABA (triangle, left), whereas a 150 ms application (larger triangle, right) produces a biphasic current response. B, Estimated [Cl-]i based on Eanion measurements (see Materials and Methods) in whole-cell recorded neurons, with two high [Cl-] pipette solutions, at three developmental stages. There is a significant effect of age (p < 0.001) and pipette [Cl-](p < 0.001) on the resulting [Cl-]i (two-way ANOVA). In the presence of the KCC2 antagonist furosemide (Furo), the estimated [Cl-]i of P10-P11 and adult neurons was not different from that of the pipette solution (50 mm). Inset, Example of responses to 20 ms applications of GABA (triangles) obtained at different holding potentials (10 mV apart) of an adult neuron with 50 mm [Cl-] in the pipette.