Abstract
The GABAA receptor subtypes responsible for the anxiolytic effects of nonselective benzodiazepines (BZs) such as chlordiazepoxide (CDP) and diazepam remain controversial. Hence, molecular genetic data suggest that α2-rather than α3-containing GABAA receptors are responsible for the anxiolytic effects of diazepam, whereas the anxiogenic effects of an α3-selective inverse agonist suggest that an agonist selective for this subtype should be anxiolytic. We have extended this latter pharmacological approach to identify a compound, 4,2′-difluoro-5′-[8-fluoro-7-(1-hydroxy-1-methylethyl)imidazo[1,2-á]pyridin-3-yl]biphenyl-2-carbonitrile (TP003), that is an α3 subtype selective agonist that produced a robust anxiolytic-like effect in both rodent and non-human primate behavioral models of anxiety. Moreover, in mice containing a point mutation that renders α2-containing receptors BZ insensitive (α2H101R mice), TP003 as well as the nonselective agonist CDP retained efficacy in a stress-induced hyperthermia model. Together, these data show that potentiation of α3-containing GABAA receptors is sufficient to produce the anxiolytic effects of BZs and that α2 potentiation may not be necessary.
Keywords: GABAA, benzodiazepine, anxiety, α3 subunit, agonist, stress-induced hyperthermia
Introduction
Nonselective benzodiazepine (BZ) agonists such as diazepam produce their clinical effects via the BZ binding site of GABAA receptors containing α1, α2, α3, or α5 subunits in combination with a β and a γ2 subunit (Pritchett et al., 1989; Pritchett and Seeburg, 1990). The recent use of transgenic mice that possess either point-mutated or deleted α subunits has demonstrated a role for α1- and α5-containing GABAA receptors in mediating the sedative effects (Rudolph et al., 1999, 2001; McKernan et al., 2000) and certain cognitive effects (Collinson et al., 2002; Crestani et al., 2002) produced by nonselective agonists, respectively. However, the GABAA receptor subtype responsible for anxiolysis remains to be clearly determined, with GABAA receptors containing either α2or α3 subunits as prime candidates.
Pharmacological evidence would suggest that the GABAA α3 receptor subtype specifically is involved in anxiety. Thus, a compound with binding affinity and inverse agonist efficacy selective for α3-containing receptors was shown to be anxiogenic on the rat elevated plus maze and produced an increase in medial prefrontal cortex DOPAC concentrations comparable with that observed by immobilization stress and by the nonselective BZ inverse agonist FG7142 (Atack et al., 2005). These data suggest that inverse agonism at α3-containing GABAA receptors is sufficient to produce the behavioral and neurochemical features of anxiety. In addition, it has been shown that a compound with greater α3 versus α2 efficacy produced a stronger anxiolytic-like effect on the rat elevated plus maze compared with a compound with greater α2 versus α3 efficacy (Reynolds et al., 2002).
In contrast, analysis of mice containing a point mutation of either the α2 or α3 subunit suggests that anxiolysis may be mediated through α2-containing receptors. Diazepam-induced anxiolysis appeared absent in mice with the α2H101R point mutation but present in mice with the α3H126R point mutation compared with wild-type controls when assessed using two locomotor-dependent tests: light-dark choice and elevated plus maze (Löw et al., 2000). However, because α2H101R mice are more sensitive to the locomotor impairing effects of diazepam than wild-type controls (Wafford et al., 2004), there remains some controversy as to whether the lack of an anxiolytic-like effect in α2H101R mice might be more related to hypoactivity than anxiolysis per se (Crestani et al., 2001; Reynolds et al., 2001).
BZ site agonists and inverse agonists have opposite effects at the cellular and behavioral level. Nonselective agonists are anxiolytic, anticonvulsant, and sedating, whereas nonselective inverse agonists are anxiogenic, convulsant, or proconvulsant and increase vigilance (Facklam et al., 1992). Accordingly, because an α3-selective inverse agonist is anxiogenic (Atack et al., 2005), we hypothesized that an α3-selective agonist would be anxiolytic, and because it lacked efficacy at the α1 subtype, it should be nonsedating. Consequently, we identified a compound, 4,2′-difluoro-5′-[8-fluoro-7-(1-hydroxy-1-methylethyl)imidazo [1,2-á]pyridin-3-yl]biphenyl-2-carbonitrile (TP003), that has agonism at the α3 subtype and negligible agonist efficacy at the α1, α2, and α5 subtypes. The present study details the experiments conducted with TP003 to assess the anxiolytic potential of this compound in both rodent and primate behavioral models.
Materials and Methods
All animal experiments were conducted according to Home Office license guidelines of the United Kingdom Animals (Scientific Procedures) Act of 1986.
Drugs. GABA, chlordiazepoxide (CDP), diazepam, flunitrazepam, flumazenil (Ro 15-1788), and Ro 15-4513 were obtained from Sigma-Aldrich (Dorset, UK), and 4,2′-difluoro-5′-[8-fluoro-7-(1-hydroxy-1-methylethyl)imidazo[1,2-á]pyridin-3-yl]biphenyl-2-carbonitrile (TP003) was synthesized at Merck Sharp and Dohme Research Laboratories (Harlow, UK).
Binding affinity. The affinity of TP003 at the BZ binding site of human recombinant GABAA receptors was determined as described in detail previously (Hadingham et al., 1993, 1996; Wafford et al., 1996). In brief, GABAA receptors containing β3 and γ2 subunits plus an α1, α2, α3, α4, α5, or α6 subunit were stably expressed in mouse fibroblast L(tk-) cells. Cells were grown and harvested, and cell membranes were prepared and incubated with various concentrations of TP003 and either 1.8 nm [3H]Ro 15-1788 (for α1-, α2-, α3-, or α5-containing receptors) or 8.0 nm [3H]Ro 15-4513 (for α4- or α6-containing receptors) (both radioligands obtained from PerkinElmer, Boston, MA). Nonspecific binding was defined using either 10 μm flunitrazepam or 10 μm Ro 15-4513 for [3H]Ro 15-1788 and [3H]Ro 15-4513, respectively.
Electrophysiology. Whole-cell patch-clamp experiments were performed on either L(tk-) cells stably expressing human α1β3γ2, α2β3γ2, α3β3γ2, and α5β3γ2 GABAA receptors or human embryonic kidney (HEK) cells transiently transfected with rat α2H101Rβ3γ2 GABAA, receptors as described previously (Brown et al., 2002). In these experiments, the membrane potential was voltage clamped at -20 mV. Curves were fitted using a nonlinear square-fitting program to the equation f(x) = Bmax/[1 + (EC50/x)n], where x is the drug concentration, EC50 is the concentration of drug eliciting a half-maximal response, and n is the Hill coefficient.
Rat elevated plus maze. The elevated plus maze was constructed of four arms measuring 10 × 50 cm. Two open arms were orientated in the north-south direction, with two closed arms lying in the east-west direction enclosed by 40-cm-high walls. The plus maze was lit by plane-polarized light from four fluorescent strip lights that provided even illumination of all areas of the maze. Male Sprague Dawley rats (240-280 g; n = 17-18 per treatment group; Charles River, Manston, UK) were placed in the center of the elevated plus maze 30 min after administration of either vehicle [0.5% methylcellulose, orally (p.o.)] or one of three doses of TP003 (experiment 1: 0.03, 0.1, or 0.3 mg/kg, p.o.; experiment 2: 0.3, 1, or 3 mg/kg, p.o.) or the full BZ agonist CDP (5 mg/kg, i.p.) and allowed to explore the maze for 5 min. Behavior was monitored by a video camera, which was connected via a VP118 tracking unit (HVS Image, Buckingham, UK) to a personal computer. Flexible maze software (HVS Image) was then used to calculate the percentage of time spent in the open arms and the number of entries in open arms during the 5 min trial period. These measurements were then analyzed using an ANOVA and the Student-Newman-Keuls post hoc test. Finally, after their 5 min trial on the elevated plus maze, rats were taken, and the occupancy of brain BZ binding sites was measured using an in vivo [3H]Ro 15-1788 binding assay, essentially as described previously (Atack et al., 1999, 2005).
Mouse rotarod. Male Swiss Webster mice (20-26 g; n = 8 per treatment group; Bantin and Kingman, Hull, UK) were first trained on a rotarod (model 7600; Ugo Basile, Comerio, Italy) with a diameter of 4 cm, at 16 rpm. Mice were considered trained when they completed three consecutive 120 s trials. Four hours later, animals were dosed with either vehicle (0.5% methylcellulose, p.o.) or one of four doses of TP003 (0.01, 0.1, 1, or 10 mg/kg, p.o.) or diazepam (10 mg/kg, p.o.) 30 min before being tested. The duration that a mouse could remain on the rotarod was recorded up to a maximum of 120 s. The data were analyzed using an ANOVA and the Student-Newman-Keuls post hoc test.
Squirrel monkey conditioned emotional response. Adult male squirrel monkeys (Saimiri sciureus; 0.7-1.4 kg) were placed in a standard Plexiglas primate chair situated inside a sound-attenuated operant box. A panel positioned in front of the monkey was fitted with a retractable response lever, a house light, a red cue light, and a faucet into which a fruit-flavored liquid reinforcer could be delivered. While in the chair, the distal portion of the monkey's tail was placed in a tail stock. A white-noise generator provided background masking noise throughout the test session.
Monkeys were pretrained under an escalating fixed ratio schedule to press the retractable response lever to obtain 0.25 ml of fruit-flavored liquid reinforcer. The reinforcer was removed, if not consumed, after 0.5 s, to prevent caching. Only animals with a stable response rate >10 per min were used in the study presented. Test sessions, controlled by an Acorn A5000/Arachnid microcomputer, were initiated by the extension of the response lever into the operant chamber and the illumination of the house light. At a time point, selected randomly between 5 and 35 min, the red cue light that served as the conditioned stimulus (CS), was illuminated for a period of 60 s. Just before the red light was extinguished, the monkeys could receive a small shock (1-4 mA; 0.5 s duration). During initial conditioning the frequency and intensity of the shock was titrated manually until the monkeys demonstrated a suppression in lever-pressing rates during the illumination of the CS >50% of the lever-pressing rate when the CS was not illuminated. Subsequently, the frequency of the shock was pseudo-randomized so that, on average, one shock was received for every 10 test sessions completed. The 40 min test session was concluded by turning off the house light and the retraction of the response lever. On days that the monkeys received drug, the test session differed from that described above only in that the CS period was fixed at 10 min and the dosed monkeys did not receive shock.
Before the test sessions, conducted Monday through Friday, monkeys had restricted access to water and were fed at least 30 min after completion of testing with a calorie-controlled diet, supplemented by fresh fruit. CS suppressions, lever-pressing rates during the illumination of the red light (CS) expressed as a percentage of the lever-pressing rates in the 60 s before its illumination, served as the measurement of anxiolytic-like behavior. Monkeys with stable CS suppressions, ≥50%, were dosed with vehicle, or diazepam, or TP003 30 min before test sessions using a within-subject pseudo-Latin-square design (experiment 1: diazepam at 0.1, 0.3, and 1 mg/kg, p.o., in 0.5% (w/v) methylcellulose; experiment 2: TP003 at 0.03, 0.3, and 3 mg/kg, p.o., in 100% polyethyleneglycol). Data were analyzed using a one-way ANOVA with repeated measures and a priori paired Student's t tests.
Generation of α2H101R mice. The GABAA receptor α2H101R knock-in mice were generated similar to the α1H101R mice (McKernan et al., 2000). In brief, an α2 subunit-specific cDNA probe was used to screen a bacterial artificial chromosome (BAC) library containing genomic mouse DNA (Research Genetics, Huntsville, AL). The targeting vector was generated from overlapping BAC-derived subclones in pBlue-script covering 12 kb of genomic DNA containing exons 4 and 5 of the α2 gene. The His101 to Arg101 codon change was introduced by site-directed mutagenesis and was labeled with a novel BspE1 restriction site. The targeting vector containing an 8.1 kb NdeI-SacI long arm including the H101R mutation, the 1kb NdeI short arm, the neomycin resistance gene, and the HSV-TK gene was introduced into AB2.2 embryonic stem (ES) cells in several independent experiments. Targeting frequency, which was confirmed by PCR and Southern blot analysis, was 1:100, but only 1:250 ES cell clones retained the mutation. Only one of several highly chimeric males gave rise to germ-line transmission. Chimeric mice were bred with the deleter mice (Schwenk et al., 1995) to eliminate the neomycin resistance gene in the genome. Homozygous and wild-type littermate controls were established after additional breeding. Animals used in the experiment were offspring of heterozygous pairings, were in a mixed 50% C57BL6J-50% 129SvEv genetic background, on average, and were all male.
Stress-induced hyperthermia. Under aseptic conditions, α2H101R male mice were anesthetized with isoflurane in oxygen and implanted with a radiotelemetry transmitter (TA10TA-F20; Datasciences, Minne-apolis, MN) intraperitoneally through a midline abdominal incision. Mice were housed singly during recovery and testing procedures. Mice were certified fit to continue on procedure by a veterinary surgeon. Samples of core temperature and locomotor activity were recorded for 10 s every minute over the period of the experiment. After acclimatization to the testing room, animals were administered vehicle (0.5% methylcellulose, i.p.), 15 mg/kg CDP, or 1 mg/kg TP003 and transferred 60 min later into a novel cage inhabited previously by another male mouse for 1 week to allow bedding to become soiled. After an additional 90 min, animals were returned to their home cages. Changes in temperature were calculated as difference from baseline and expressed as mean ± SEM. Area under the curve (AUC) was calculated for “before stress” and “after stress,” and statistical comparisons between AUCs were made using an ANOVA with repeated measures [AUC for before stress = sum of mean temperature change (T1:T60) - 0.5 × (T1 + T60)].
Results
In vitro properties of TP003
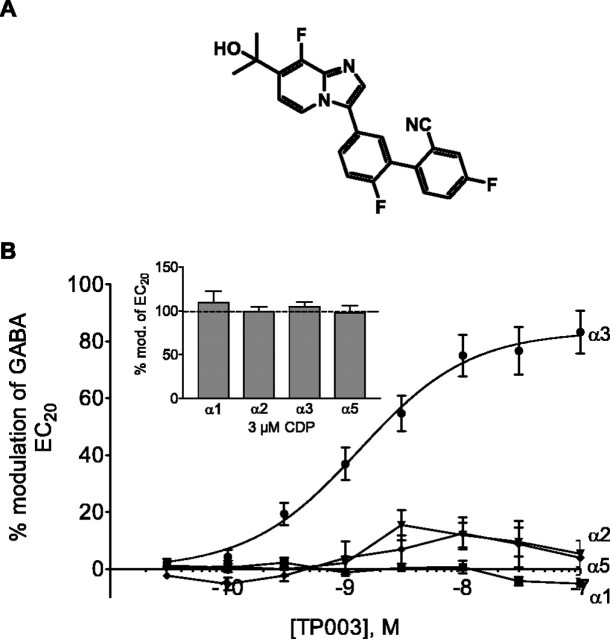
TP003 (Fig. 1A) is an α3-selective BZ agonist that has subnanomolar affinity for the BZ site on recombinant human GABAA receptors containing β3 and γ2 subunits along with an α1, α2, α3, or α5 subunit (Table 1) but very low affinity (Ki >1 μm) for the corresponding receptors containing either an α4 or α6 subunit. The efficacy of TP003 at the BZ site was measured by whole-cell patch-clamp recording from Ltk-cells expressing the above combinations of GABAA receptor subunits. The degree of potentiation of a submaximal, EC20 GABA response (a GABA concentration that elicits a current 20% of the maximal GABA response) demonstrated that TP003 has no efficacy at the α1 subtype (i.e., behaves as a BZ site antagonist), is a high-efficacy agonist at the α3 subtype (maximal potentiation, 83%; cf. potentiation of the BZ full agonist CDP, 105%), and has very low efficacy (essentially at antagonist levels) at the α2 and α5 subtypes (<15% potentiation of that produced by a full agonist BZ such as diazepam or CDP) (Fig. 1B). In addition, at concentrations up to 1 μm, TP003 had no efficacy at α2H101Rβ3γ2 receptors expressed in HEK cells (data not shown). Consequently, the in vivo effects of TP003 will be mediated primarily by GABAA receptors containing an α3 subunit. The compound was not evaluated at γ1- or γ3-containing receptors, but these are very low in abundance and generally have a lower affinity for BZ ligands. In addition, TP003 showed little effect in a wide-ranging binding screen at other receptors.
Figure 1.
Properties of TP003. A, Structure of TP003. B, Concentration-response curves representing potentiation by TP003 of whole-cell GABA currents evoked by an EC20 concentration on cells expressing human recombinant GABAA α1β3γ2 (n = 6), α2β3γ2 (n = 5), α3β3γ2 (n = 8), and α5β3γ2 (n = 7) receptors. The maximum potentiation produced by TP003 at the α3 subtype was 83%. The inset histogram shows the extent of potentiation of an EC20-equivalent concentration of GABA by 3 μm CDP at the α1, α2, α3, and α5 subtypes. Error bars indicate SEM. mod, Modulation.
Table 1.
Binding affinity of TP003 to recombinant human GABAA receptors expressed in Ltk—cells using [3H]Ro 15-1788 ([3H]flumazenil) as a radioligand for α1-, α2-, α3-, and α5-containing receptors and [3H]Ro 15-4513 for α4- and α6-containing receptors
|
Receptor combination |
Binding Ki (nm) |
|---|---|
| α1β3γ2 | 0.32 ± 0.21 |
| α2β3γ2 | 0.54 ± 0.17 |
| α3β3γ2 | 0.50 ± 0.18 |
| α4β3γ2 | 2435 ± 1041 |
| α5β3γ2 | 0.26 ± 0.08 |
| α6β3γ2
|
1815 ± 767 |
Data represent means ± SEM of five to eight determinations.
In vivo properties of TP003
Rat elevated plus maze
TP003 induced anxiolytic-like activity in the rat elevated plus maze (Fig. 2), increasing the time spent on the open arms and the number of open-arm entries with a minimum effective dose of 0.3 mg/kg (orally; low-dose experiment: F(4,84) > 4.56, p < 0.005; high-dose experiment: F(4,84) > 7.12, p < 0.0001; ANOVA). The extent of the maximum increase in percentage of time spent in the open arms or entries into the open arms produced by TP003 was comparable with that produced by the positive control CDP. Finally, at the minimal effective dose of TP003 in the elevated plus maze (0.3 mg/kg), the level of receptor occupancy was 75%, whereas that for CDP was 25%.
Figure 2.
A, B, The effects of low doses (0.03, 0.1, and 0.3 mg/kg, p.o.) (A) and high doses (0.3, 1, and 3 mg/kg, p.o.) (B) of TP003 on the rat elevated plusmaze. The percentage of time spent on the open arms and the number of entries into the open arms are shown (mean ± SEM). CDP (5 mg/kg, i.p.) was included as the positive control; n = 17-18 per treatment group. *p < 0.05 compared with vehicle (0.5% methylcellulose). veh, Vehicle.
Mouse rotarod
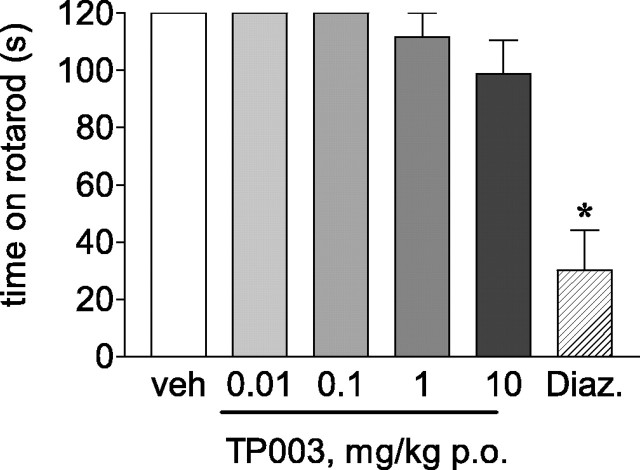
Sedation/ataxia studies showed that in contrast to diazepam (10 mg/kg, p.o.), which impaired rotarod performance compared with vehicle-treated mice (F(5,42) = 18.3; p < 0.0001; ANOVA), TP003 (0.01-10 mg/kg, p.o.) was without effect (Fig. 3) even at a dose (10 mg/kg) corresponding to essentially 100% occupancy of mouse brain BZ binding sites (data not shown).
Figure 3.
The effects of TP003 on the mouse rotarod (mean ± SEM time in seconds on rotarod during a 2 min trial). Diazepam (Diaz; 10 mg/kg, p.o.) was included as the positive control; n = 8 per treatment group. *p < 0.05 compared with vehicle (veh).
Squirrel monkey conditioned emotional response
TP003 was also assessed in the squirrel monkey conditioned emotional response (CER) paradigm, which is sensitive to the anxiolytic effects induced by BZs, whereby administration of diazepam induces an increase in lever-pressing rates normally suppressed by a CS associated with shock (Fig. 4B). TP003 (0.3 mg/kg, p.o.) also induced anxiolytic-like activity (Fig. 4A), causing a release in responding normally suppressed by the CS stimulus (F(3,24) = 50.10; p < 0.05; ANOVA), of approximately the same magnitude as that observed with diazepam. This anxiolytic-like effect of TP003 was not attributable to nonspecific (e.g., sedative) reductions in response rates because there was no difference between the groups before the CS was presented (vehicle, 114 ± 19 presses/min; TP003: 0.03 mg/kg, 133 ± 19 presses/min; 0.3 mg/kg, 124 ± 20 presses/min; 3 mg/kg, 106 ± 19 presses/min). These data indicate that in both rodents and a non-human primate, a high-efficacy α3-selective compound is anxiolytic like at doses that are not sedative. It should also be noted that the increase in responding during the CS period is not related to analgesia because during the drug test period, no shock was administered during presentation of the CS.
Figure 4.
A, B, The effects of diazepam (Diaz; n = 10; B) and TP003 (n = 9; A) on response rates during the CS period of the conditioned emotional response paradigm (mean ± SEM percentage of pre-CS responding). *p < 0.05 compared with vehicle (veh).
Mouse stress-induced hyperthermia
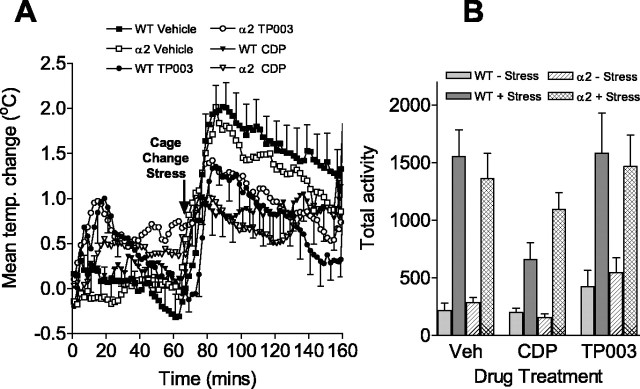
Initially, we investigated the role of the α2 subtype in anxiety using the stress-induced hyperthermia (SIH) model in α2H101R mutant mice implanted with telemetry devices that measured the response to an unconditioned/contextual stress of being placed into a cage previously inhabited by another male mouse. The resting body temperature of wild-type and α2H101R mice was not significantly different (mean ± SEM: WT, 36.4 ± 0.3°C; α2, 37.0 ± 0.2°C). Exposure to the cage-change stress caused an increase in body temperature of ∼2°C in both genotypes (Fig. 5A). Pilot experiments showed that diazepam produced nonspecific reductions in body temperature before exposure to the stress (data not shown), thus making interpretation of the data difficult. CDP does not produce such effects and so was used as the reference BZ in this assay. Administration of CDP (15 mg/kg, i.p.) had no effect on body temperature 60 min before the cage-change stress but markedly attenuated the SIH. These data were analyzed using a repeated-measures ANOVA using drug [vehicle, CDP, or TP003 (1 mg/kg, i.p.)] and genotype (wild type or α2H101R) as independent factors and stress (before and after cage change) as the repeated measure. As expected, there was a highly significant effect of stress (F(2,22) = 110; p < 1 × 10-6), but there was no main effect of genotype (F(1,11) = 0.83; p = 0.38) or drug (F(2,22) = 0.77; p = 0.47). However, there was a significant interaction of drug by stress (F(2,22) = 8.12; p < 0.005), but none of the other interactions were significant (drug by genotype, genotype by stress, or genotype by drug by stress; F < 0.6 in all cases). Planned linear comparisons confirmed that each drug treatment was significantly different from vehicle (vehicle vs CDP: F(1,12) = 9.69, p < 0.01; vehicle vs TP003: F(1,12) = 17.6, p < 0.002) and not different from each other (CDP vs TP003: F(1,12) = 0.044, p = 0.84). These results indicate that both wild-type and α2H101R mice respond in the same way to CDP treatment (i.e., the anxiolytic effect of CDP does not require drug action at the α2 subtype).
Figure 5.
A, B, The effects of CDP (15 mg/kg, i.p.) and TP003 (1 mg/kg, i.p.) in the mouse SIH model on the mean temperature change (A) and activity (B); n = 6-8 per group. *p < 0.05 compared with vehicle of the same genotype before stress. Error bars indicate SEM. WT, Wild type; temp., temperature; Veh, vehicle.
The telemetry devices used to measure body temperature also recorded activity as the mice explored their home cage. These data (Fig. 5B) showed that the baseline home-cage activity of wild-type and α2H101R mice were similar. When the cage-change stress was applied, activity increased for both genotypes under vehicle and both drug treatments (p < 0.05, ANOVA), as would be expected because the mouse was exposed to a novel, potentially threatening environment. CDP did not affect activity before the stress but did blunt the increase in activity during the cage-change stress with a trend toward less reduction (p = 0.071) in the α2H101R mice compared with wild type. TP003 induced a modest, nonsignificant increase in activity before the stress but did not alter the marked increase caused by the stress compared with vehicle-treated mice.
Discussion
The use of molecular genetic and pharmacological approaches has begun to delineate which α subunit-containing GABAA receptors are associated with particular aspects of the diverse pharmacology of nonselective BZs such as diazepam. However, although there is general agreement that the α1 subtype mediates the sedative effects of BZs (Rudolph et al., 1999; McKernan et al., 2000) and the α5 subtype plays a role in aspects of cognition (Collinson et al., 2002; Crestani et al., 2002), there remains controversy over which of the subtypes, α2 versus α3, is associated with anxiolysis (Low et al., 2000; Atack et al., 2005). Thus, using a molecular genetic approach, the anxiolytic effects of diazepam were absent in α2H101R mice but retained in α3H126R mice (Low et al., 2000), suggesting a role for the α2 subtype in mediating the anxiolytic effects of diazepam. Although this conclusion is apparently supported by the fact that a compound described as possessing agonist efficacy selective for the α2 subtype (Johnstone et al., 2004) is anxiolytic, it should be pointed out that the efficacy of this compound at α3- and α5-containing receptors has not been described. In contrast, the fact that an α3-selective inverse agonist was anxiogenic (Atack et al., 2005) suggests that the α3 subtype plays a role in the anxiolytic effects of nonselective BZs. In the present study, we identified the compound TP003 and characterized its in vivo properties to test the hypothesis that an α3-selective agonist should be anxiolytic, thus supporting a critical role for the α3 subtype specifically in anxiety.
TP003 is an imidazopyridine that is structurally related to the benzimidazole NS2710 (Jensen et al., 2002) and possesses equivalent high (subnanomolar) affinity for GABAA receptors containing either an α1, α2, α3, or α5 subunit but much lower affinity (Ki >1 μm) for α4- and α6-containing receptors. The compound was not evaluated at γ1- or γ3-containing receptors, but these are very low in abundance and generally have lower affinity for BZ ligands. Furthermore, TP003 showed no affinity for either the agonist (GABA) or the convulsant (open-channel) binding sites of either recombinant human or native rat brain GABAA receptors as measured using [3H]muscimol and [3H]TBOB binding assays, respectively. In addition, TPA003 had much lower (>100-fold less) affinity for a wide range (∼170) of other receptors, transporters, and enzymes (MDS Pharma Services, Phoenix, AZ; data not shown).
TP003 is distinct from the triazolopyridazine L-838417 (McKernan et al., 2000), because although both compounds have comparable and equivalent nanomolar affinity for α1-, α2-, α3-, and α5-containing GABAA receptors and have different efficacies at the various subtypes, the nature of this subtype-selective efficacy differs between compounds. Hence, both TP003 and L-838417 are functionally inert at the α1 subtype, which presumably accounts for the reduced sedation liability for both compounds. However, whereas L-838417 possesses partial agonist efficacy at the α2, α3, and α5 subtypes, TP003 possesses appreciable agonist efficacy only at the α3 subtype. Consequently, although L-838417 is anxiolytic like in animal models (McKernan et al., 2000), it is not possible to ascribe this anxiolysis to a particular GABAA receptor subtype. In contrast, the in vivo effects of TP003 are presumably related solely to the α3 subtype.
TP003 clearly produced an anxiolytic-like effect in the rat elevated plus maze, with a maximal effect (i.e., increase in time spent on the open arms) equivalent to that seen with the nonselective full agonist CDP. Similarly, in the primate model TP003 produced a marked, dose-dependent increase in responding during the CS period with a maximal effect comparable with the nonselective full agonist diazepam. Thus, in the absence of significant efficacy at the α2 subtype, TP003 was able to produce marked anxiolytic-like effects in both a rat unconditioned model and a non-human primate conditioned model of anxiety. Moreover, TP003 did not produce sedation as measured using the mouse rotarod, consistent with the lack of efficacy of TP003 at the α1 subtype and similar to the nonsedating properties of L-838417, which is also devoid of α1 efficacy (McKernan et al., 2000).
Because efficacy at the α2 subtype did not appear to be required for anxiolysis, we hypothesized that TP003 would retain its anxiolytic-like efficacy in α2H101R point-mutated mice. However, the differential effects of pharmacologically induced changes in activity in wild-type and transgenic mice can confound the interpretation of locomotor-based experiments (Reynolds et al., 2001; Crestani et al., 2001). Because this differential locomotor effect is also observed with α2H101R mice (Wafford et al., 2004), we examined the effects of TP003 and the nonselective agonist CDP using the physiological SIH model. The SIH is an integral part of an individual's response to the perception of a distressing situation (Marazziti et al., 1992) and, as a manifestation of autonomic hyperactivity, is one of the diagnostic items of generalized anxiety mentioned in DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition). Furthermore, it has also been shown that compounds that are all known to be effective anxiolytics in the clinic, including BZ and 5-HT1A receptor agonists, are effective in SIH (for review, see Olivier et al., 2003).
The data from α2H101R mutant mice clearly show that the efficacy of CDP at the α2 subtype is not critical for the blockade of SIH, and, consequently, the α1, α3, and/or α5 subtypes must mediate this effect. These data, combined with the reduction of SIH by TP003 in both genotypes, demonstrate that α2 efficacy is not required and potentiation of the α3 subtype alone is sufficient to produce anxiolytic-like effects.
Moreover, although other behavioral assays may be confounded through sedation because of test compounds, the activity data from the SIH test indicates that hypoactivity as a result of sedation cannot explain the anxiolytic-like reduction of body temperature in response to the stress. Although there was a trend for CDP to reduce the increase in activity caused by the stress compared with vehicle-treated mice, the overall activity was still higher than before the animals were stressed. Also, CDP did not reduce activity before the stress was applied, indicating that it was not sedative in its own right; rather, it was reducing the response to stress.
Although experiments with TP003 suggest that α3-containing GABAA receptors play a significant role in mediating the anxiolysis produced by BZs, an anxiolytic function for α2 receptors cannot be excluded. Hence, at the minimal effective dose of TP003 in the elevated plus maze (0.3 mg/kg), the receptor occupancy required for anxiolysis was 75%, and given the fairly similar α3 efficacies of TP003 and CDP (83 and 105% potentiation of a GABA EC20, respectively), this is appreciably higher than the 25% occupancy required for anxiolysis with CDP. This suggests that the α3 efficacy of CDP is not solely responsible for anxiolysis (otherwise TP003 anxiolytic occupancy would be more like the 25% required for CDP) and indicates that other GABAA receptor subtypes, most probably the α2 subtype, may contribute to anxiolysis or may potentiate the effects mediated via the α3-containing receptors. It is interesting to note that TP003 produces an anxiolytic-like effect of equivalent magnitude to the nonselective BZ (either CDP or diazepam) in both the plus maze and CER tests, perhaps suggesting that if α2 does have a role in anxiolysis, its effects are not simply additive to those of α3.
Finally, studies performed over many years in rodents and primates have identified the amygdala and the bed nucleus of the stria terminalis as important in producing responses associated with fear and anxiety (Pesold and Treit, 1994; Fendt and Fanslow, 1999; Davis, 2000; LeDoux, 2000; Kalin et al., 2001; Walker et al., 2003). However, these two regions are involved in different types of response. Hence, the amygdala is known to be important in mediating conditioned fear (Fendt and Fanslow, 1999; Davis, 2000; LeDoux, 2000), whereas the bed nucleus of the stria terminalis is more important in unconditioned/contextual fear responses (Pesold and Treit, 1994; Kalin et al., 2001; Walker et al., 2003). The expression of the α3 subunit in both the amygdala and the bed nucleus (Pirker et al., 2000) is consistent with (but clearly not proof of) TP003 exerting its anxiolytic-like efficacy via these brain regions in both the primate conditioned (CER) and rodent unconditioned (elevated plus maze and SIH) assays. Furthermore, the localization of the α2 subunit within the amygdala suggests that this subtype may also play a role in amygdala-mediated (i.e., conditioned) anxiety (Marowsky et al., 2004).
In conclusion, TP003 was clearly anxiolytic like in rodent ethological and physiological models of anxiety (elevated plus maze and SIH) as well as in a conditioned non-human primate anxiety model (CER) and retained its anxiolytic-like effects in α2H101R point-mutated mice. Moreover, TP003 showed no overt signs of sedation. The present findings provide compelling evidence to support a critical role of the GABAA α3 receptor subtype in mediating anxiety-related processes in both rodents and non-human primates and highlight GABAA subtype-selective drugs as potential nonsedating anxiolytics (Atack, 2005). If α3-selective BZ agonists are subsequently shown to be effective in human anxiety disorders, as they are in animal models of anxiety, then they would potentially have fewer side effects than the existing nonselective BZs.
Footnotes
We gratefully acknowledge A. Butler for assistance in compiling the figures and pictorial editing of this manuscript.
Correspondence should be addressed to Dr. Rebecca Dias, The Neuroscience Research Centre, Merck Sharp and Dohme Research Laboratories, Terlings Park, Eastwick Road, Harlow, Essex CM20 2QR, UK. E-mail: rebecca_dias@merck.com.
Copyright © 2005 Society for Neuroscience 0270-6474/05/2510682-07$15.00/0
References
- Atack JR (2005) The benzodiazepine binding site of GABAA receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs 14: 601-618. [DOI] [PubMed] [Google Scholar]
- Atack JR, Smith AJ, Emms F, McKernan RM (1999) Regional differences in the inhibition of mouse in vivo [3H]Ro 15-1788 binding reflect selectivity for α1 versus α2 and α3 subunit-containing GABAA receptors. Neuro-psychopharmacology 20: 255-262. [DOI] [PubMed] [Google Scholar]
- Atack JR, Hutson PH, Collinson N, Marshall G, Bentley G, Moyes C, Cook SM, Collins I, Wafford KA, McKernan RM, Dawson GR (2005) Anxiogenic properties of an inverse agonist selective for α3 subunit-containing GABAA receptors. Br J Pharmacol 144: 357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002) Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136: 965-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW (2002) Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci 22: 5572-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Mohler H, Rudolph U (2001) Anxiolytic-like action of diazepam: GABAA receptors containing the α2-subunit. Trends Pharmacol Sci 22: 403. [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U (2002) Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA 99: 8980-8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M (2000) The role of the amygdala in conditioned and unconditioned fear and anxiety. In: The amygdala (Aggleton JP, ed), pp 213-287. New York: Oxford UP.
- Facklam M, Schoch P, Bonetti EP, Jenck F, Martin JR, Moreau JL, Haefely WE (1992) Relationship between benzodiazepine receptor occupancy and functional effects in vivo of four ligands of differing intrinsic efficacies. J Pharmacol Exp Ther 261: 1113-1121. [PubMed] [Google Scholar]
- Fendt M, Fanslow MS (1999) The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev 23: 743-760. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ (1993) Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acid A receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3, and α5-containing human γ-aminobutyric acidA receptors. Mol Pharmacol 43: 970-975. [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ (1996) Cloning of cDNAs encoding the human γ-aminobutyric acid type A receptor α6 subunit and characterization of the pharmacology of α6-containing receptors. Mol Pharmacol 49: 253-259. [PubMed] [Google Scholar]
- Jensen ML, Timmermann DB, Varming T, Johansen TH, Ahring PK (2002) Two distinct actions of the novel anxiolytic compound NS 2710 on GABAA receptors. Soc Neurosci Abstr 28: 491.8. [Google Scholar]
- Johnstone TB, Hogenkamp DJ, Coyne L, Su J, Halliwell RF, Tran MB, Yo-shimura RF, Li WY, Wang J, Gee KW (2004) Modifying quinolone antibiotics yields new anxiolytics. Nat Med 10: 31-32. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE (2001) The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J Neurosci 21: 2067-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000) The amygdala and emotion. In: The amygdala (Aggleton JP, ed), pp 289-310. New York: Oxford UP.
- Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U (2000) Molecular and neuronal substrate for the selective attenuation of anxiety. Science 290: 131-134. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Di Muro A, Castrogiovanni P (1992) Psychological stress and body temperature changes in humans. Physiol Behav 52: 393-395. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Fritschy JM, Vogt KE (2004) Functional mapping of GABAA receptor subtypes in the amygdala. Eur J Neurosci 20: 1281-1289. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, et al. (2000) Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci 3: 587-591. [DOI] [PubMed] [Google Scholar]
- Olivier B, Zethof T, Pattij T, van Boogaert M, van Oorschot R, Leahy C, Oosting R, Bouwknecht A, Veening J, van der Gugten J, Groenink L (2003) Stress-induced hyperthermia and anxiety: pharmacological validation. Eur J Pharmacol 463: 117-132. [DOI] [PubMed] [Google Scholar]
- Pesold C, Treit D (1994) The septum and amygdala differentially mediate the anxiolytic effects of benzodiazepines. Brain Res 638: 295-301. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000) GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101: 815-850. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Seeburg PH (1990) Gamma-aminobutyric acid A receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem 54: 1802-1804. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Luddens H, Seeburg PH (1989) Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science 245: 1389-1392. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, McKernan RM, Dawson GR (2001) Anxiolytic-like action of diazepam: which GABAA receptor subtype is involved? Trends Pharmacol Sci 22: 402-403. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, Ferris P, Lincoln RJ, Stanley JL, cook SM, Alder L, Guiblin A, Chambers M, Atack JR, Street LJ, Dawson GR (2002) Which GABAA receptor subtype mediates the anxiolytic effects of benzodiazepines: is it alpha2 or alpha3? FENS Forum Abstr 1: 154.13. [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H (1999) Benzodiazepine actions mediated by specific γ-aminobutyric acid A receptor subtypes. Nature 401: 796-800. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Mohler H (2001) GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci 22: 188-194. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky KA (1995) Cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23: 5080-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford K, Thompson S, Thomas D, Sikela J, Wilcox A, Whiting P (1996) Functional characterization of human gamma-aminobutyric acid A receptors containing the α4 subunit. Mol Pharmacol 50: 670-678. [PubMed] [Google Scholar]
- Wafford KA, Macaulay AJ, Fradley R, O'Meara GF, Reynolds DS, Rosahl TW (2004) Differentiating the role of gamma-aminobutyric acid type A (GABAA) receptor subtypes. Biochem Soc Trans 32: 553-556. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M (2003) Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199-216. [DOI] [PubMed] [Google Scholar]