Abstract
The hippocampal dentate gyrus (DG) is an area of active proliferation and neurogenesis within the adult brain. The molecular events controlling adult cell genesis in the hippocampus essentially remain unknown. It has been reported previously that adult male and female rats from the strains Sprague Dawley (SD) and spontaneously hypertensive (SHR) have a marked difference in proliferation rates of cells in the hippocampal DG. To exploit this natural variability and identify potential regulators of cell genesis in the hippocampus, hippocampal gene expression from male SHR as well as male and female SD rats was analyzed using a cDNA array strategy. Hippocampal expression of the gene-encoding glucose-dependent insulinotropic polypeptide (GIP) varied strongly in parallel with cell-proliferation rates in the adult rat DG. Moreover, robust GIP immunoreactivity could be detected in the DG. The GIP receptor is expressed by cultured adult hippocampal progenitors and throughout the granule cell layer of the DG, including progenitor cells. Thus, these cells have the ability to respond to GIP. Indeed, exogenously delivered GIP induced proliferation of adult-derived hippocampal progenitors in vivo as well as in vitro, and adult GIP receptor knock-out mice exhibit a significantly lower number of newborn cells in the hippocampal DG compared with wild-type mice. This investigation demonstrates the presence of GIP in the brain for the first time and provides evidence for a regulatory function for GIP in progenitor cell proliferation.
Keywords: GIP, hippocampus, proliferation, progenitor cell, dentate gyrus, peptide
Introduction
An area of active neurogenesis in the adult brain of several species, including humans, is the subgranular cell layer of the hippocampal dentate gyrus (DG) (Altman and Das, 1965; Stanfield and Trice, 1988; McKay, 1997; Eriksson et al., 1998). The cells that are born in the adult hippocampus can differentiate into mature neurons and glial cells (Palmer et al., 1997; Aberg et al., 2000; van Praag et al., 2002). Many conditions influence cell proliferation, fate determination, and survival of hippocampal progenitors in the adult rat, including stress (for review, see Eriksson and Wallin, 2004), aging (Kuhn et al., 1996), and brain injury (for review, see Parent, 2003).
We reported previously that spontaneously hypertensive (SHR) rats exhibit a significantly higher cell-proliferation rate in the hippocampal DG than Sprague Dawley (SD) rats and that male rats produce significantly more cells than their female counterparts (Perfilieva et al., 2001). In the present investigation, we exploited these natural differences and used a cDNA microarray strategy to identify novel genes that may influence the rate of cell proliferation in the adult hippocampus.
The gene encoding glucose-dependent insulinotropic polypeptide (GIP) (gastric inhibitory polypeptide) was robustly upregulated in association with increased rates of cell proliferation in the hippocampus. GIP is a 42 aa polypeptide initially isolated from endocrine cells in porcine small intestine (Brown et al., 1969; Buchan et al., 1978) and its gene (Gip) has been reported to be expressed in the rat small intestine, submandibular salivary gland, and lens epithelial cells (Higashimoto et al., 1992; Sharma et al., 1992; Tseng et al., 1993; Usdin et al., 1993; Nakajima et al., 2002). GIP is a member of the vasoactive intestinal peptide (VIP)/secretin/glucagon family of gastrointestinal regulatory polypeptides and is the only member of this peptide family that has not been detected previously in the brain. Interestingly, expression of the GIP receptor (GIPR) gene (Gipr) and GIP binding sites has been described in the adult brain, including the hippocampus (Usdin et al., 1993; Kaplan and Vigna, 1994), suggesting that GIP does indeed play a role in the brain. As with other family members, GIP has a variety of effects on different systems (Hauner et al., 1988; Kogire et al., 1992; Bollag et al., 2000). Foremost, it inhibits gastric secretion and enhances insulin release by pancreatic β islet cells in the presence of glucose (for review, see Yip and Wolfe, 2000).
The current study not only describes the presence of the Gip gene and GIP protein in the adult brain for the first time but also demonstrates that GIP influences proliferation of adult hippocampal progenitor cells. Thus, GIP may represent an important regulatory molecule for neural progenitor cell proliferation in the adult mammalian brain.
Materials and Methods
Experiments in vivo and on tissue samples
Animals. All experimental protocols were approved by the Animal Ethics Committee of Göteborg University. Five-week-old rats (male SHR, 84-88 g; male SD, 123-128 g; female SD, 115-117 g) were used for the cDNA array, semiquantitative reverse transcription (RT)-PCR, quantitative real-time PCR, and immunoquantification, and were obtained from Möllegaard Breeding Center (Ejby, Denmark). For all other experiments, 7- to 8-week-old male SD rats (260-280 g) were obtained from B&K Universal (Stockholm, Sweden). The background and generation of Gipr knock-out C57BL/6 mice used in this study have been described previously (Miyawaki et al., 1999). Breeding pairs of the Gipr knock-outs were kindly provided by Prof. Y. Seino (Department of Metabolism and Clinical Nutrition, Kyoto University, Kyoto, Japan). Mice were bred to obtain a colony of homozygous Gipr knock-outs (Gipr-/-). For all experiments, 10-week-old male Gipr-/- (n = 6) and C57BL/6 control (n = 8) mice were used. Mice were genotyped using PCR with a neo primer (TAA AGC GCA TGC TCC AGA CTG CCT T) or a genomic primer (AGT GTG AGA ATC CAG AGA AGAA TGG) as well as a genomic primer (CCA CGG TAT ACA TGA TCT GCA GGCG) that is common to both Gipr-/- and wild-type mice (Wt+/+). Mice were injected with bromodeoxyuridine (BrdU) (50 mg/kg body weight; Roche Diagnostics Scandinavia, Bromma, Sweden) intraperitoneally and killed 2 h later.
Atlas cDNA array. Male SHR (n = 5), male SD (n = 5), and female SD (n = 5) rats were killed at 5 weeks. The hippocampus from one-half of the brain was used for RNA isolation and the other half of the brain for immunofluorescence. Total RNA from each hippocampus was separately prepared according to the Atlas Pure Total RNA Labeling System User Manual (PT3231-1; catalog #K1038-1) and pooled. Preparations of cDNA probes, hybridization to arrays, and development of x-ray films were made according to the Atlas cDNA Expression Arrays User Manual (PT3140-1). The Atlas cDNA Expression Arrays contain 1176 genes. The arrays were aligned to the AtlasImage Grid Template, and a custom external background calculation was performed before the comparison of the arrays. Gene spots that gave a very high signal and overshadow into other gene spots were excluded. A threshold level was adjusted to filter out weak signals that were not attributable to actual gene expression and to choose a level at which a signal from a cDNA spot is considered above background level. The threshold level was set to only include genes that expressed a signal >200% of the background value, as recommended by Clontech (Cambridge, UK). Gene spots on the array that exhibited a signal that was 200% of the background value had a minimal but still detectable expression.
User-defined normalization was performed using housekeeping genes included on the array, and data analyses were performed using AtlasImage software (Clontech) according to the AtlasImage 1.5 User Manual. Array experiments were performed twice, each using a separate set of five rats. In total, six arrays and three groups of rats (male SD, female SD, and male SHR) were used, with the experiments performed twice. To reduce the number of false-positive candidate genes, we selected only genes exhibiting more than a fourfold difference in expression in both of the array experiments for additional analysis.
RT-PCR. Total RNA was isolated from the hippocampus, small intestine (positive control for GIP), and spleen (negative control for the GIP receptor) (Chomczynski and Sacchi, 1987) from 5-week-old rats. All reagents were obtained from Promega (Madison, WI), and the cDNA was cycled using a thermal cycler (PerkinElmer Life Sciences 2400; PerkinElmer Life Sciences, Norwalk, CT) for 35 cycles. PCR primers for Gip were designed by Clontech (Gip-P1, AAG AGG TTG AGT TCC GAT CCC ATG C; Gip-P2, GAT TGT CCT GCC AGC TCC AAA GCC), and the primers for Gipr have been described previously (Usdin et al., 1993) (Gipr-P1, GGG ACC CTC CAG CCC AAC TGC; Gipr-P2, TGA AGC CGC CTG AAC AAA CTT). As an internal standard, PCR primers detecting RNA for ribosomal protein L 27A (Rpl) (Wool et al., 1990) were used. The sequences for the PCR primers detecting Gip have been carefully examined, avoiding homology with other proteins by performing a basic local alignment search tool search in the public databases.
Sequencing. Sequencing was performed on PCR products using an ABI Prism BigDye Terminator Cycle Sequencing Ready kit (Applied Biosystems, Foster City, CA) with the same primers used for RT-PCR. The products were precipitated with 95% ethanol and 3 m NaAc resuspended in template suppression reagent (Applied Biosystems), and further analyzed on ABI PRISM 3100 Genetic Analyzer.
Quantitative real-time PCR assay. Total RNA from the hippocampus of 5-week-old male SHR rats and male and female SD rats was prepared using Fast Prep Kit-Green (Bio 101, Carlsbad, CA), followed by DNase treatment (DNA-Free; Ambion, Austin, TX). A primer pair and one probe were designed for Gip using Assay by Design for Gene Expression Assays (Applied Biosystems) according to the guidelines provided by the manufacturer and sequence data (GenBank accession number Z19564). The following probe and primers were used: forward primer, CAA GAC TTT GTG AAC TGG CTT CTG; reverse primer, AGC CCG GGC CTC TCT CT; probe, (FAM) TGT TTC CAG TCA TTC TT (Applied Biosystems). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene (TaqManā Rodent GAPDH Control Reagents; part number 4308313; Applied Biosystems). The reactions were run on an ABI Prism 7700 Sequence Detection system (Applied Biosystems).
In situ hybridization. Male SD rats were decapitated, and the brains were sectioned (14 μm) in a cryostat (Dittes, Heidelberg, Germany) and thaw-mounted on pretreated glass slides (ProbeOn; Fisher Scientific, Pittsburgh, PA). Using MacVector software (IBI, New Haven, CT), oligonucleotide probes were selected based on an optimum ratio of guanosine plus cytosine/total nucleotide numbers (50-65%) and minimal homology (not more than 80%) with GenBank-entered sequences. Oligonucleotide probes were reversed and complementary to GGC TTT GGA GCT GGC AGG ACA ATC TCA GAG AAA CGA GGA GAA AGA GGC (nucleotides 313-360) and TGC TGG CCC CCG ACC ACG AGG CCC AAG GTA TGC AGA GGG GAC TTT CAT (nucleotides 148-195) of rat Gip mRNA (Higashimoto et al., 1992; Sharma et al., 1992) and GTA CAG GTG AGC ACT GAC TTG GGC TGA AGC TCA AGA GTT GGT TCT GCC (nucleotides 61-108) and CCT GTT CAC GTC TTT CAT GCT GCG AGC AGG GGC CAT CCT CAC CCG AGA (nucleotides 682-729) of rat Gipr mRNA (Usdin et al., 1993) (MWG Biotech, Ebersberg, Germany). The probes were labeled with [33P]deoxy-ATP (dATP), and the specific activity of the labeled probes was 3 × 109 cpm/μg. In situ hybridization was performed essentially as described previously (Dagerlind et al., 1992). The [33P]dATP-labeled sections were exposed to β-max autoradiography film for 2 months.
Immunohistochemistry. Sectioning, staining, and detection of immunofluorescence in rat brain sections were performed as described previously (Aberg et al., 2000). Dilutions of primary antibodies used for tissue sections were as follows: primary antibodies, monoclonal mouse GIP (3.65H; 1:1000); polyclonal rabbit GIP (1:100; Chemicon, Temecula, CA), polyclonal rabbit GIP (1:100; Euro-Diagnostica, Malmö, Sweden), rabbit GIP receptor (1:500), rabbit GFAP (1:500; Dako, High Wycombe, UK), guinea pig GFAP (1:1000; Advanced ImmunoChemical, Long Beach, CA), mouse neuronal-specific nuclear protein (NeuN) (1:30; Chemicon), goat doublecortin (1:400; Santa Cruz Biotechnology, Santa Cruz, CA), and goat Sox-2 (1:200; Santa Cruz Biotechnology). Secondary antibodies for brain sections were Alexa Fluor 488- and Alexa Fluor 594-conjugated antibodies (1:400; Molecular Probes, Leiden, The Netherlands) and cyanine 5 (Cy5)-, Texas Red-, and FITC-conjugated antibodies (all 1:150; Jackson ImmunoResearch, West Grove, PA). For peroxidase detection (using 0.25 mg/ml diaminobenzidine), a biotinylated horse anti-mouse IgG secondary antibody (1:160; Vector Laboratories, Burlingame, CA) was used followed by application of an avidin-biotin-peroxidase complex. For antigen retrieval of GIP, sections were placed in a microwave four times for 2 min each (Moulinex Micro-Chef MO55; 650 W, 230 V, 50 Hz) in TBS. As negative controls, sections were incubated with corresponding concentrations of normal serum or Ig instead of primary antibody followed by the appropriate secondary antibody. Incubations with only the secondary antibody were also performed. The specificity of the GIP antibodies was also assessed by preincubation with excess of GIP antigen.
Immunoquantification. Brain sections including the hippocampus of 5-week-old male SHR and male and female SD rats were stained using a monoclonal GIP antibody (see above). Sections were anatomically compared so that equivalent locations were chosen. Two sections per rat and four rats per group were stained simultaneously and evaluated. Computer-based quantification of staining intensity was performed and aided by EasyImage (Nikon, Tokyo, Japan).
Intracerebroventricular GIP infusion. Adult male SD rats (260-280 g; B&K Universal) were intubated and ventilated with isoflurane in an O2/N2O mix (30:70). An infusion cannula connected to an osmotic pump (Alzet brain infusion kit II and 2001 osmotic pump; Alzet, Palo Alto, CA) was placed in the third cerebral ventricle (0.3 mm posterior from bregma along the midline, 5 mm below skull surface). Each rat was infused (1 μl/h) for 5 d with either GIP (1.92 nmol/d; n = 5) or vehicle (0.1 m PBS; n = 6) and killed on the last day. All animals received a single daily intraperitoneal injection of BrdU (50 mg/kg body weight; Roche Diagnostics Scandinavia) during the 5 d that GIP was infused. Infusion via the third ventricle was chosen so that both hemispheres would be equally affected by the infusion and to minimize damage to the brain, which can itself influence proliferation (Chirumamilla et al., 2002). Quantification of BrdU-positive and Ki-67-positive cells was performed in the granule cell layer (GCL) of both hemispheres.
Detection of proliferating cells and cell counting. Brains from rats given intracerebroventricular injections of PBS or GIP (see above) and brains from Gipr-/- and Wt+/+ mice were sectioned and stained for BrdU. To stain the rat brain sections, a primary mouse BrdU antibody (1:400; Roche Diagnostics Scandinavia) and biotinylated horse anti-mouse IgG (1:125) secondary antibody (Vector Laboratories) were used as described previously (Perfilieva et al., 2001). To stain the mouse brain sections, a primary rat BrdU antibody (1:400; Oxford Biotech, Oxford, UK) and a biotinylated donkey anti-rat IgG (1:500) secondary antibody (Jackson ImmunoResearch) were used. Rat brain sections were also stained for Ki-67 (1:200; Novocastra Laboratories, Newcastle upon Tyne, UK). For each animal, the total number of BrdU-positive and Ki-67-positive cells in the GCL, including the subgranular layer, and their corresponding sample volume were determined. For BrdU, 12 (rats) or 10 (mice) immunoperoxidase-stained, 40-μm-thick coronal sections taken 240 μm (rats) or 160 μm (mice) apart were analyzed. For Ki-67, 14 fluorescence-stained, 40-μm-thick coronal sections of rat brains were taken 160 μm apart and analyzed. The cross-sectional areas of GCL were obtained using a CCD camera linked to a digital imaging system (Nikon). Results are expressed as BrdU-positive or Ki-67-positive cells per cubic millimeter.
Experiments in vitro and on cell-culture samples
Cell cultures. Clonal adult hippocampal progenitor cells (AHPs) from rat (Palmer et al., 1997) were cultured in DMEM/Hams's F12 (1:1) containing N2 supplement (Invitrogen, Täby, Sweden) and 20 ng/ml human fibroblast growth factor-2 (FGF-2). (Prepro Tech EC, London, UK). Cells were used between passages 5 and 15 after cloning.
RT-PCR. Total RNA isolated from cultured AHPs and PCR was performed as described above for tissue RNA.
Immunocytochemistry. AHPs were cultured as described previously (Aberg et al., 2001, 2003). Dilutions of primary antibodies used for cell cultures were as follows: rabbit GIP receptor (1:500) (Lewis et al., 2000), mouse Nestin (1:500; PharMingen, San Diego, CA), mouse NeuN (1:30; Chemicon), mouse Map2ab (1:100; Sigma, St. Louis, MO), monoclonal mouse GIP (3.65H; 1:1000; kindly provided by Dr. Alison Buchan, University of British Columbia, Vancouver, British Columbia, Canada), rabbit GIP (1:75; Chemicon), and mouse Ki-67 (1:75; Novocastra Laboratories). Secondary antibodies were Alexa Fluor 488- and Alexa Fluor 594-conjugated antibodies (1:2500; Molecular Probes) and Cy5-, Texas Red-, and FITC-conjugated antibodies (all 1:150; Jackson ImmunoResearch). As negative controls, cells were incubated with corresponding concentrations of normal serum or Ig instead of primary antibody followed by the appropriate secondary antibody. Incubations with only the secondary antibody were also performed. The specificity of the GIP antibodies was also assessed by preincubation with excess of GIP antigen. Hoechst staining of individual nuclei was used to visualize cultured cells.
Western blot analysis. AHPs were plated on polyornithine/laminin-coated plates at a density of 2 × 104 cells/cm2. The cells were lysed in cold radioimmunoprecipitation assay buffer containing 1% protease inhibitor mixture (Sigma) and centrifuged at 12,000 × g for 5 min at 4°C. The supernatant was analyzed for protein concentration using the Lowry assay. Protein was also isolated from rat hippocampus, in which GIP receptor mRNA has been described previously (Usdin et al., 1993). The Western blot analysis was performed using 10% polyacrylamide gels. Samples of 15 μg of protein and a negative control using rat serum were loaded onto the gel. Protein was transferred to a polyvinylidene fluoride membrane and incubated with primary rabbit GIP receptor (1:500) antibody. Controls without primary antibody were also performed. The membranes were incubated in secondary HRP-conjugated donkey anti-rabbit (1:1000; Amersham Biosciences, Piscataway, NJ), treated with chemiluminescence substrate (Roche Diagnostics Scandinavia), and recorded on film.
Proliferation assay. AHPs were seeded at 0.2 × 104 cells/cm2 on 24-well plates in culture medium containing human FGF-2 (20 ng/ml; a well documented proliferative agent for AHPs) and grown for 48 h. After an additional 48 h of growth without FGF-2, cells were incubated with FGF-2 (20 ng/ml; four experiments), porcine GIP (Sigma) alone (eight experiments), or in combination with FGF-2 (eight experiments) for 48 h. Controls were cells grown without FGF-2. Each experiment represents the mean of at least four different culture wells. The cell-proliferation assay was performed using the CyQUANT Cell Proliferation kit (Molecular Probes) and a GENios microplate reader (TECAN Austria, Grödig, Austria) according to the instructions of the manufacturer.
Methyl-[3H]thymidine-assay. Cultured AHPs were seeded at 0.5 × 104 cells/cm2 on 48-well plates in culture medium and left to grow for 48 h before being labeled with methyl-[3H]thymidine and incubated with GIP (1 nm) or FGF-2 (20 ng/ml) for 24 h. Controls were cells grown without FGF-2. Cells were lysed in 0.4 m NaOH, transferred to scintillation vials, mixed with 0.4 m HCl, and assayed for DNA synthesis by scintillation spectrometry. To block the effects of GIP, a receptor antagonist (ANTGIP; GIP [7-30]NH2) (Tseng et al., 1996) was manufactured (Sigma-Genosys, Cambridge, UK), and 1 μm was applied to the cells 20 min before incubation with GIP. The mean for each experiment was calculated from four different culture wells, and each experiment was performed four times.
Apop Tag. Cultured AHPs were seeded at 1 × 104 cells/cm2 on glass coverslips, treated the same way as for the proliferation assay, and incubated with GIP (1 nm). Cells were fixed and stained for apoptosis according to the Apop Tag kit user manual (Apop Tag S160 direct; Intergene Company, Purchase, NY). A negative control without terminal deoxynucleotidyl transferase enzyme was included, and positive controls with addition of either H2O2 (100 μm and 1 mm) or DNase I (1 μg/ml) were included. In the last washing step, the cells were incubated with the nuclear dye bisbenzimide (Hoechst 33258; Sigma) for 30 min. Apoptotic or dead cells were identified by green fluorescence in the nuclei, and Hoechst nuclear dye was used to determine the total number of cells. Three coverslips per experiment were stained from four different experiments. Positive cells were quantified by scoring the immunoreactivity of 1000-3000 cells systematically observed in six nonoverlapping fields in each coverslip.
Lactate dehydrogenase activity. Release of lactate dehydrogenase (LDH) from dying cells was measured using a routine photometric method (Department of Clinical Chemistry, Sahlgrenska University Hospital, Göteborg, Sweden) (Aberg et al., 2003). Cell-culture medium was collected from culture wells seeded for the Apop Tag staining (see above). The mean for each experiment was calculated from three different culture wells, and each experiment was performed four times. The coefficient of variation for the assay was 1.7%, and the assay standard curve was linear for enzyme activities between 0.1 and 20 μkat/dm3.
Statistics. Comparisons between groups were made using one-way ANOVA followed by a Fisher's post hoc test, when appropriate, throughout the study. A p value <0.05 was considered statistically significant. All values are expressed as means ± SEM.
Results
In vivo studies
Expression levels of the Gip gene in the hippocampus vary in parallel with cell-proliferation rates in the DG in rat
To identify novel genes that might be associated with neural proliferation in the young adult rat hippocampus, we compared gene expression between three groups of rats known to differ with regard to neural progenitor cell proliferation in the adult DG (Perfilieva et al., 2001). Hippocampal RNA was isolated from normal prepubescent male SHR and male and female SD rats (summarized in Fig. 1A) and used to synthesize cDNA probes for hybridization to an Atlas rat 1.2 cDNA array. The hybridization results are shown in Figure 1A, in which stained spots represent each gene in the array. A comparison of hippocampal gene expression profiles from male SHR to male SD rats revealed 11 differentially expressed genes between the two groups. We subsequently performed a second comparison between male and female SD rats that revealed 30 differentially expressed genes (supplementary Table 1, available at www.jneurosci.org as supplemental material). We then chose to further investigate genes that demonstrated an expression profile in the hippocampus that varied in parallel with the in vivo proliferation level of cells in the DG in both comparisons. Gip was upregulated in SHR males compared with SD males and in SD males compared with SD females. Gip was the only gene of 1176 genes analyzed that exhibited this pattern in both comparisons.
Figure 1.
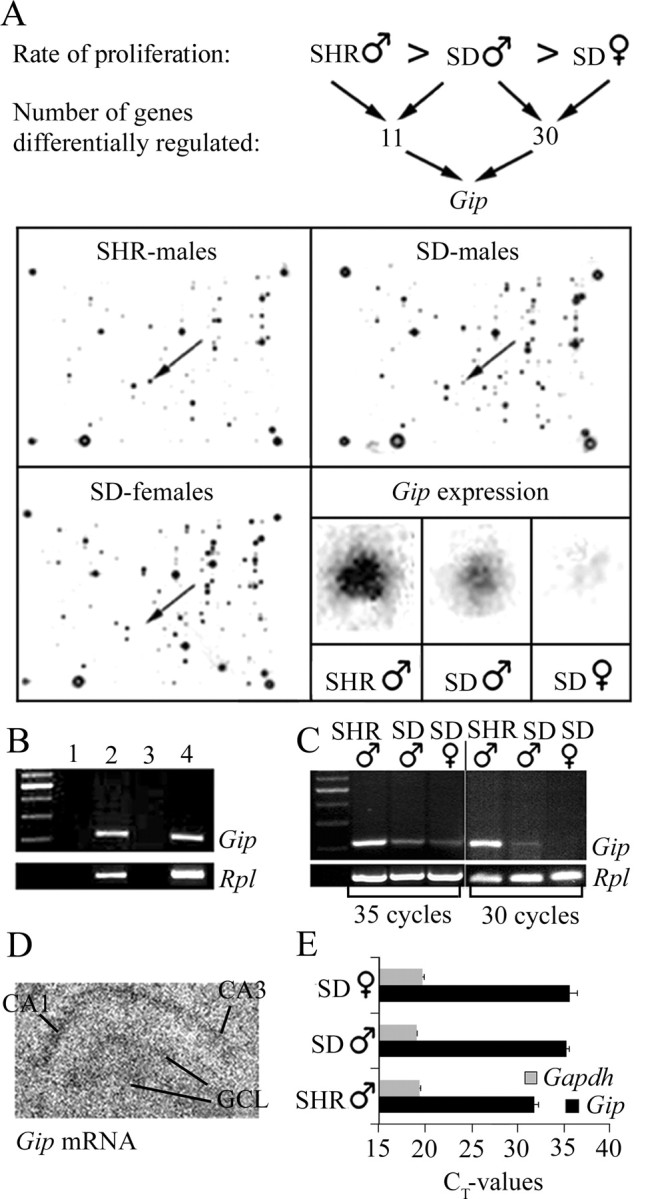
The Gip gene is expressed in adult rat hippocampus. A schematic picture of our microarray strategy is viewed in A, where the results obtained were analyzed with regard to variation in parallel with in vivo proliferation levels of cells in rat DG. Arrows indicate the gene dots representing the Gip gene on arrays from the three groups, and the corresponding gene dots are enlarged in the bottom right panel. PCR analysis confirmed the Gip gene expression (B) in the hippocampus (lane 2) and small intestine (positive control; lane 4). Negative controls are without cDNA (lane 1) or hippocampal RNA without RT (lane 3). As an internal standard, PCR primers detecting RNA for ribosomal protein L 27A (Rpl) were used. C, Semiquantitative PCR comparisons of hippocampal RNA from SHR males, SD males, and SD females. D, In situ hybridization for Gip mRNA showed a weak but specific localization in the GCL as well as the CA1-CA3 region. E, Quantitative real-time PCR using a different set of primers confirmed the expression of the Gip gene in hippocampus of the three groups. As an internal standard, PCR primers detecting RNA for GAPDH (Gapdh) were used. SHR males showed the highest expression, viewed here as exhibiting a lower CT value (threshold cycle; cycle number when the system begins to detect the increase in the signal associated with an exponential growth of PCR product during the log-linear phase) and starting the amplification at a lower cycle number. However, the expression level was similar in both male and female SD rats.
Confirmation of Gip gene expression in the hippocampus was performed using RT-PCR and in situ hybridization (Fig. 1B-D). In total RNA from both the hippocampus and small intestine, a PCR product corresponding to 220 bp was obtained (Fig. 1B). Sequencing of the PCR product confirmed the expression of the Gip gene in the rat hippocampus. Consistent with the cDNA array results, Gip expression was highest for male SHR rats and lowest for female SD rats when analyzed using semiquantitative RT-PCR for 35 cycles (Fig. 1C). Indeed, the 220 bp band could not be detected in female SD rats when only 30 PCR cycles were performed. To localize expression of the Gip gene in rat hippocampal sections, we performed in situ hybridization, using two different oligonucleotide probes, on sections from male SD rats. Weak Gip mRNA expression was demonstrated in the CA1-CA3 region and the DG, including the GCL (Fig. 1D). We further confirmed expression of the Gip gene in these three groups using quantitative real-time PCR and a different set of primers (Fig. 1E). Again, SHR males had the highest Gip expression using quantitative real-time PCR, but in this case, the expression level was similar in both male and female SD rats.
Immunohistochemistry reveals the presence of GIP protein in the adult hippocampus
The presence of GIP protein in the adult rat hippocampus was examined immunohistochemically using both a monoclonal and a polyclonal antibody against GIP. GIP immunoreactivity could be observed in CA1, CA2, and CA3 and in scattered larger cells with neuronal morphology in the hilus (Fig. 2A). Quantification of GIP immunoreactivity in the GCL of all groups (Fig. 2B) demonstrated that GIP protein content is greater in animals exhibiting higher rates of cell proliferation, in accordance with the pattern described in the present study for Gip RNA. Of particular interest to the current investigation, the GCL in the hippocampus contained a large amount of GIP immunoreactivity with a characteristic cytoplasmic staining pattern (Fig. 2C). Colocalization of GIP immunoreactivity with the neuronal marker NeuN, but not the astrocytic cell marker GFAP, was observed in rat brain sections (Fig. 2C-F). Cells in the GCL that express doublecortin, a marker of developing neuroblasts, also expressed GIP (Fig. 2G-I). Moreover, GIP immunoreactivity could also be observed in cells expressing Sox-2, a potential progenitor cell marker (D'Amour and Gage, 2003; Komitova and Eriksson, 2004). However, Sox-2 expression was also observed in cells that did not exhibit any GIP immunoreactivity (data not shown), suggesting that GIP is present only in a subpopulation of progenitor cells (Fig. 2J,K).
Figure 2.
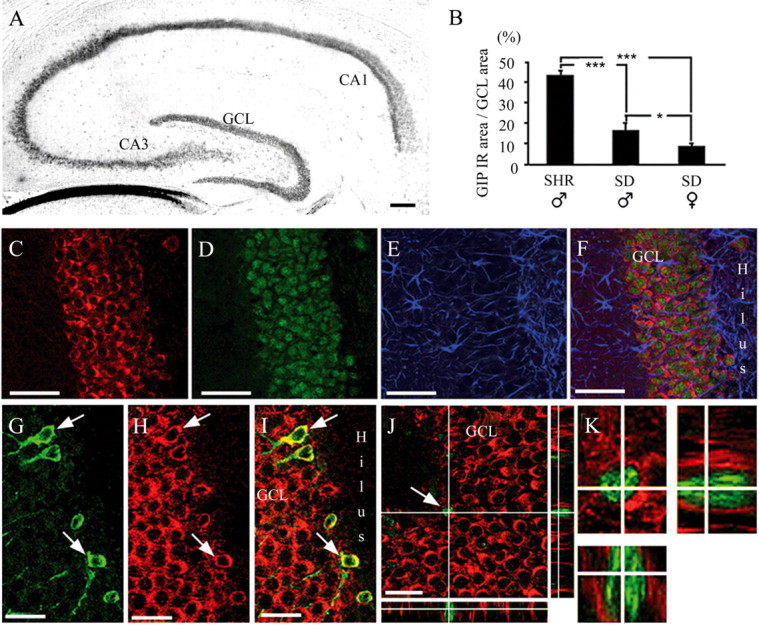
GIP immunoreactivity is present in the adult rat hippocampus. A, Apart from the GCL, GIP immunoreactivity was also observed in the CA1-CA3 region and in scattered cells in the hilus. GIP immunoreactivity (IR) in brain slices from SHR males, SD males, and SD females (B) was measured, and the immunoreactive area of the GCL was compared with the total GCL area; *p < 0.05; ***p < 0.001. GIP immunoreactivity (C; red) was colocalized with NeuN (D; green) but not with GFAP (E; blue) in the hippocampal GCL (F; merged). Developing neuroblasts in the GCL expressing doublecortin (G; green) and GIP (H; red) are shown as merged in I. Arrows in G-I indicate a doublecortin-positive neuroblast also expressing GIP. The GCL contains Sox-2-immunoreactive progenitor cells (J, K; green) also exhibiting GIP immunoreactivity (J, K; red). The arrow in J indicates the enlarged cell shown in K. Scale bars: A, 100 μm; C-F, 50 μm; G-J, 20 μm.
The adult hippocampus expresses the Gipr gene and GIPR protein
Gipr (GIP receptor) mRNA in the GCL was demonstrated via in situ hybridization using two different oligonucleotide probes (Fig. 3A). Moreover, we observed expression of the Gipr gene in hippocampal tissue using RT-PCR in accordance with another study (Usdin et al., 1993) (Fig. 6A). Immunohistochemical detection of the GIPR revealed expression throughout the hippocampal GCL (Fig. 3B). GIPR immunoreactivity could be observed in progenitor cells expressing Sox-2 (Fig. 3B) and in neuroblasts expressing doublecortin (Fig. 3C-E). Moreover, GIPR immunoreactivity was also present in mature granule cells and was colocalized with expression of NeuN (Fig. 3F-H), demonstrating that the GIPR is expressed in both mature neurons and undifferentiated cells.
Figure 3.

The GIP receptor is present in both mature neurons and progenitor cells in the adult rat DG. In situ hybridization for the Gipr gene showed weak but specific expression in the DG, particularly in the GCL (A). GIPR immunoreactivity (B; red) was observed in progenitor cells also expressing Sox-2 (B; green). GFAP-immunopositive cells (B; blue) also expressing Sox-2 did not express the GIPR. The arrow in B indicates a GIPR-expressing progenitor cell without GFAP expression. GIPR immunoreactivity (C, E; red) was also observed in developing neuroblasts expressing doublecortin (D, E; green). The arrows in C-E indicate a doublecortin/GIPR-immunopositive cell. In addition to being produced by progenitor cells, GIPR (F, H; red) was also present in mature granule cells in the GCL together with NeuN (G, H; green). Scale bars: B, 20 μm; C-E, 8 μm; F-H, 20 μm.
Figure 6.
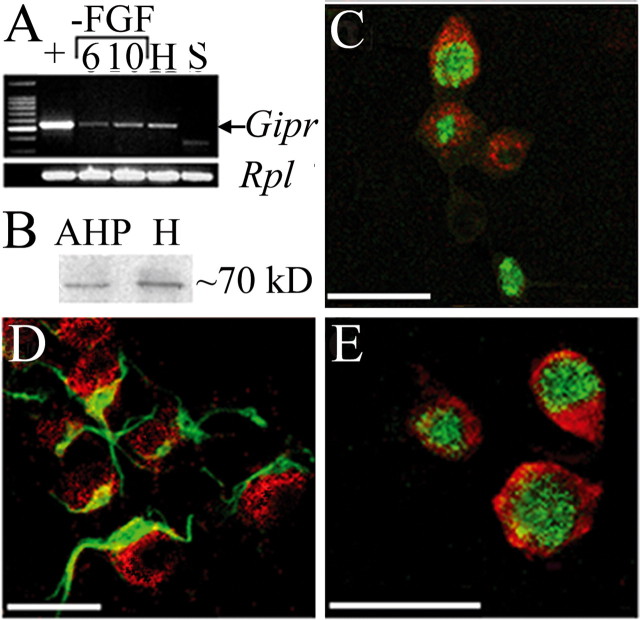
The GIP receptor is present in cultured AHPs. A, PCR analysis demonstrated expression of the Gipr gene in cultured AHPs. Lane 1 (+) shows RNA from proliferating cell cultures (+FGF-2). Cells were also allowed to differentiate for 6 and 10 d (lanes 2 and 3). Lane H, Hippocampus (positive control); lane S, spleen (negative control). B, Western blot analysis demonstrated the presence of the GIPR in protein from cultured AHPs and the hippocampus. The GIPR (C-E; red) was also observed in cultured mitotic AHPs expressing Ki-67 (C; green) and in cells expressing Nestin (D; green) as well as cells expressing NeuN (E; green). Scale bars: C, D, 20 μm; E, 25 μm.
GIP increases cell proliferation in the adult GCL
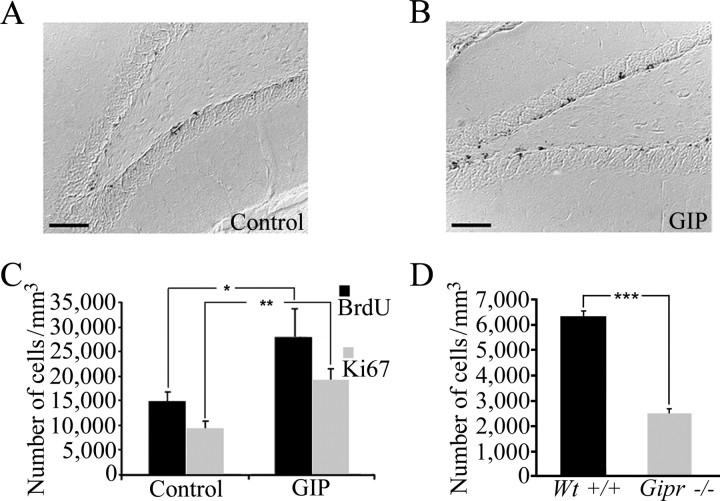
To investigate whether GIP influences proliferation of cells in the adult GCL in vivo, we analyzed the number of newly formed cells in the rat GCL after chronic intracerebroventricular infusion of GIP (Fig. 4A,B). In animals that underwent GIP treatment, the number of BrdU-positive cells in the GCL was 86% higher than controls (Fig. 4C). Moreover, to verify that the observed difference in proliferation was not an effect on survival of the newborn cells, the number of Ki-67-positive cells in the GCL was quantified in both groups. Results demonstrated that the animals that had been given GIP had 105% more Ki-67-positive cells compared with PBS-treated animals (Fig. 4C). To verify the effect of GIP on cell proliferation, we also analyzed the number of BrdU-positive cells in mice lacking the GIP receptor. The Gipr-/- mice did not show any obvious abnormalities in phenotype, behavior, or breeding abilities compared with wild-type mice. The effects on the entero-insular axis and obesity in the Gipr-/- mice have been described previously (Miyawaki et al., 1999, 2002). When comparing the number of newborn cells in the GCL of Gipr-/- and Wt+/+ mice 2 h after BrdU injection, the GCL of Gipr-/- mice contained less than one-half the number of BrdU-positive cells observed in Wt-/- mice (Fig. 4D). Thus, GIP does indeed influence cell proliferation in the adult hippocampus.
Figure 4.
GIP increases cell proliferation in the adult GCL. The density of BrdU-positive cells in the GCL was determined in rats that had been given intracerebroventricular PBS (A) or GIP (B). Quantification of BrdU-positive and Ki-67-positive cells (C) showed that GIP-treated animals exhibited 86% more BrdU-positive cells and 105% more Ki-67-positive cells than animals treated with PBS (control). Quantification of BrdU-positive cells in the GCL of Gipr-/- and wild-type (Wt+/+) mice (D) demonstrated that Gipr-/- mice only have ∼40% of the number of newborn cells compared with Wt+/+ mice. Means ± SEM are given. *p < 0.05; **p < 0.01; ***p < 0.001. Scale bars: A, B, 100 μm.
In vitro studies
Cultured AHPs express both the Gip gene and GIP protein
To investigate whether GIP was also produced by hippocampal progenitor cells in vitro, cultured AHPs were analyzed with regard to Gip gene expression and GIP immunoreactivity. RNA isolated from cultured AHPs demonstrated expression of the Gip gene when analyzed with RT-PCR. The same band was observed in cells cultured in the presence or absence of FGF-2 (Fig. 5A), suggesting that the Gip gene is expressed in both immature cells and more differentiated cells. Moreover, GIP immunoreactivity was also observed in cultured AHPs. Colabeling of GIP with both a neuronal marker, Map2ab (Fig. 5B-E), and a marker of cell division, Ki-67 (Fig. 5F-I), was observed. No colocalization of GIP and GFAP was observed in the present study.
Figure 5.
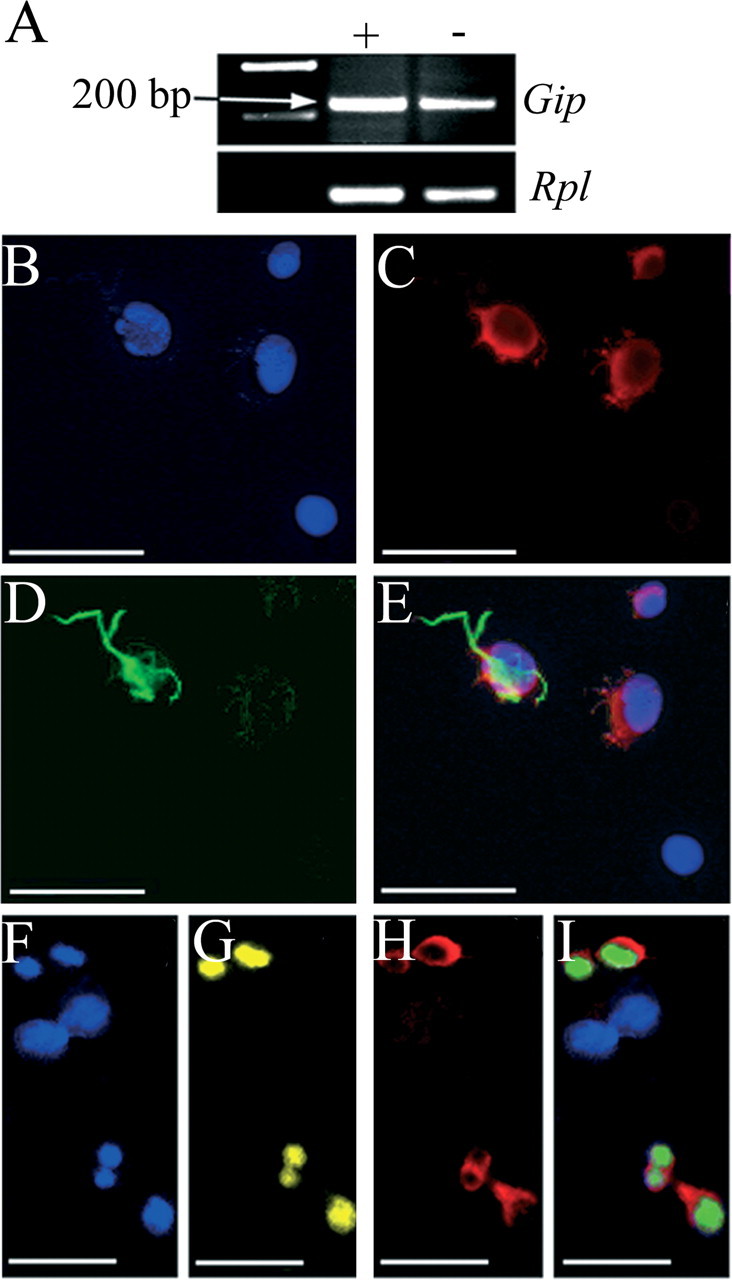
GIP is present in cultured AHPs. A, Cultured AHPs express the Gip gene, as demonstrated by PCR. As an internal standard, PCR primers detecting Rpl were used. The lanes marked + and - represent RNA from cells cultured with and without FGF-2, respectively. The progenitor cells in culture were also immunoreactive for GIP (C; red) in combination with Map2ab (D; green), as shown merged in E. Mitotic cells expressing Ki-67 (G; yellow) also expressed GIP (H; red), shown merged in I. Nuclei in B and F are marked with Hoechst (blue). Scale bars, 25 μm.
Cultured AHPs express both the Gipr gene and GIPR protein
Cultured AHPs were analyzed for the presence of the Gipr gene and GIPR protein to investigate whether these cells have the ability to respond to GIP. Expression of the Gipr gene was strongest in proliferating cell cultures (plus FGF-2) and decreased after removal of FGF-2 (Fig. 6A). Western blot analysis also revealed the presence of the GIPR protein in cultured AHPs (Fig. 6B). Colocalization of the GIPR with markers of cell division and of progenitor cells (Ki-67 and Nestin, respectively) as well as NeuN was observed, again demonstrating that the receptor is present in both undifferentiated progenitor cells and mature neurons (Fig. 6C-E).
GIP increases proliferation in cultured AHPs
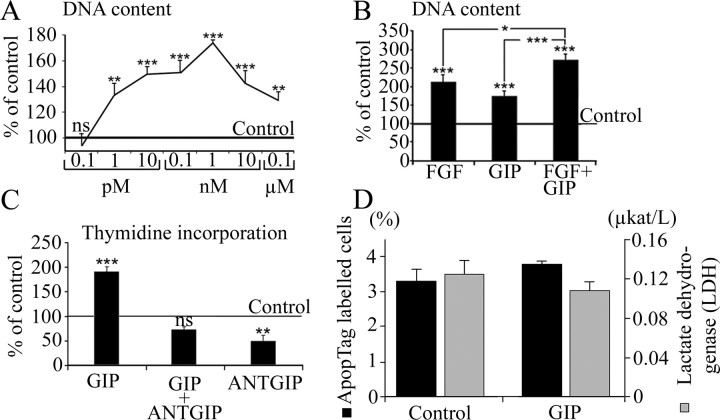
Subsequent studies were designed to investigate whether GIP might influence proliferation of neural progenitor cells in vitro. Using a commercial proliferation assay, GIP increased the rate of cell proliferation in a dose-dependent manner, with significant increases between concentrations of 1 pm and 0.1 μm (Fig. 7A). The greatest effect was achieved at a GIP concentration of 1 nm, resulting in a 175% increase in DNA content compared with controls. At 0.1 pm, the proliferative effect was absent. FGF-2 alone resulted in an increase in DNA content to 212% of controls (Fig. 7B). When incubating cells with 1 nm GIP in addition to FGF-2, a greater effect on proliferation was observed, with an increase in cell growth to 272% of controls (Fig. 7B). At the beginning of the experiment, ∼6 × 103 cells were seeded, and at the end of the experiment, there were ∼1.5 × 105 cells in control wells without FGF-2, 3 × 105 cells in wells with FGF-2, and 2.5 × 105 cells in wells with GIP. These results were confirmed using a methyl-[3H]thymidine incorporation assay, in which 1 nm GIP significantly increased thymidine incorporation to 188% of controls (Fig. 7C). To block the effect of GIP, the cells were treated with ANTGIP (GIP [7-30]NH2). ANTGIP has been described previously as a specific GIP receptor antagonist with no agonistic properties (Tseng et al., 1996). When treated with ANTGIP before GIP incubation, thymidine incorporation was not significantly different from control levels. Most interestingly, ANTGIP alone, in the absence of GIP, significantly reduced thymidine incorporation to 48% of controls, presumably by blocking the influence of endogenously produced GIP on cell proliferation.
Figure 7.
GIP induces proliferation of cultured AHPs. A, GIP increased proliferation in a dose-dependent manner. B, Treatment of the cells with a combination of FGF-2 and GIP (1 nm) resulted in a greater DNA content than treatment with the two substances on their own. Values were calculated as a percentage of DNA content obtained from cells grown without FGF-2 and are shown as means ± SEM. C, The proliferative effect of GIP was confirmed using a methyl-[3H]thymidine incorporation assay, in which 1 nm GIP significantly increased thymidine incorporation. The proliferative effect of GIP was abolished when the cells were pretreated with 1 μm ANTGIP. ANTGIP also decreased cell proliferation to ∼50% when used alone. Cell-death analyses, including Apop Tag labeling of cells (black bars) and lactate dehydrogenase measurements (gray bars) of cultured AHPs after GIP treatment, showed no difference from control (D; AHPs cultured without FGF-2 or GIP). *p < 0.05; **p < 0.01; ***p < 0.001.
GIP does not influence cell death in cultured AHPs
To determine whether the increase in cell number observed in the in vitro proliferation assays was caused by a survival, rather than a mitogenic effect of GIP, we measured cell death with two different methods (Fig. 7D). Using Apop Tag, cell death in the control experiments without FGF-2 (20 ng/ml) or GIP (1 nm) was extremely low and not statistically different from cell death in cells treated with GIP. Additionally, LDH release into the culture medium was not significantly different between controls and cells treated with GIP, verifying the assumption that GIP does not influence survival of these cells but most likely acts to stimulate proliferation.
Discussion
In the present investigation, we describe the presence of GIP in the mammalian brain for the first time and also give a detailed description of the pattern of expression in the hippocampal GCL. GIP is the last remaining member of the VIP/secretin/glucagon family of gastrointestinal regulatory polypeptides to be described in the brain. Other family members such as VIP, pituitary adenylate cyclase activating polypeptide (PACAP), and glucagonlike peptide 1 (GLP-1) are all classified as neurotransmitters and are widely distributed throughout the brain (Sims et al., 1980; Jin et al., 1988; Masuo et al., 1993; Hannibal, 2002). Moreover, they have been demonstrated to be involved in a variety of brain functions such as neuromodulation, brain development, cell-cycle regulation, differentiation, and cell death as well as regulation of food intake and body temperature (for review, see Sherwood et al., 2000). Expression of the Gip gene and GIP immunoreactivity is widespread throughout the brain (J. Nyberg, C. Jacobsson, and P. S. Eriksson, unpublished data), suggesting that GIP also might be involved in several functions of the brain. In the present investigation, we provide insight into one of its potential biological roles in the brain: hippocampal progenitor cell proliferation.
Although the expression of the other members of the secretin-glucagon family of gastrointestinal regulatory polypeptides has been described previously in the brain (Sherwood et al., 2000), previous efforts to detect Gip mRNA in the brain have been un-successful (Higashimoto et al., 1992; Usdin et al., 1993). We are confident that both our PCR primers and antibodies have not simply detected a related peptide, because sequence analysis of the PCR product demonstrated that we had indeed detected the gene encoding Gip. Moreover, it is also highly unlikely that our antibody detected another member of the VIP/secretin/glucagon family of peptides, because the specificities and sensitivities of different GIP antibodies have already been thoroughly investigated, and these studies concluded that the monoclonal C-terminal-specific antibody used in the present study is the most specific (Buchan et al., 1982; Sjolund et al., 1983). Our antibody has also been tested previously for its specificity through preincubation with VIP, secretin, glucagon, and somatostatin (Buchan et al., 1982; Damholt et al., 1999). Therefore, we reasonably conclude that the adult hippocampus produces GIP.
In the present investigation, we have shown that the regulation of progenitor cell proliferation is one potential function of GIP in the brain. Because some other members of the VIP/secretin/glucagon family of neuropeptides have demonstrated neuroprotective effects (Pincus et al., 1994; Waschek, 1995; Vaudry et al., 1999), it may be argued that the observed GIP-induced increase in BrdU-positive cells may result as much from increased survival as it does from stimulated proliferation. However, no difference was observed when we investigated the rate of cell death in cell-cultured AHPs treated with and without GIP. Moreover, in contrast to BrdU incorporation, Ki-67 antibodies only identify cells that are proliferating at the time the animal was killed, and thus such analysis is not affected by treatments that increase cell survival. We also observed that Gipr-/- mice produce significantly less cells in the adult GCL compared with Wt+/+ mice, providing the loss-of-function data needed to implicate endogenous GIP in the regulation of cell proliferation in the adult GCL. A proliferative role for GIP in the hippocampus is perhaps not surprising, because GIP is mitogenic in several other cell types of different origins, for example quiescent adrenal tumor cells (Chabre et al., 1998), vascular endothelial cells (Ding et al., 2003), and the osteoblastic-like cell line MG-63 (Zhong et al., 2003), and acts as a growth factor for β cells (Trumper et al., 2001). Furthermore, other members of this family of neuropeptides, such as PACAP, VIP, growth hormone-releasing hormone, and GLP-2, have been demonstrated to have growth-stimulating qualities (Lin et al., 1992; Pincus et al., 1994; Waschek, 1995; Lu et al., 1998; Vaudry et al., 1999; Sherwood et al., 2000; Suh et al., 2001).
In the adult rat DG, GIP was observed both in proliferating progenitor cells as well as in more mature neuronal cells. Interestingly, a subpopulation of Sox-2-positive progenitor cells did not express GIP, suggesting that the presence or absence of GIP expression may distinguish between two different populations of progenitor cells. We have demonstrated previously two separate populations of Sox-2-positive cells in the adult hippocampus, separated by the absence or presence of GFAP expression (Komitova and Eriksson, 2004). The Sox-2-positive/GFAP-positive cells were mostly quiescent, and the Sox-2-positive/GFAP-negative cells were proliferating and corresponded to transit-amplifying neuronal precursor cells. In the present study, we could not find cells expressing both GIP and GFAP. Therefore, it is likely that the Sox-2-positive, GIP-negative cells observed correspond to the quiescent cells and the Sox-2-positive, GIP-positive cells are proliferating neuronal precursor cells. In support of this, cells in the rat DG expressing doublecortin, a transient marker of neuronal-committed progenitor cells (Brown et al., 2003), also expressed GIP. This suggests that GIP may act as a marker of hippocampal cells that are restricted to a neuronal cell lineage, from early neuronal commitment until the final maturation of the neuron. The same pattern of expression has also been observed for presenilin-1, an unrelated protein expressed in mature neurons as well as ∼70% of the progenitor cells in the adult mouse hippocampus (Wen et al., 2002). Accordingly, these authors also concluded that presenilin-1 might be a marker of distinct lineages of neural progenitors.
In cultured AHPs, GIP and FGF-2 together resulted in a greater effect on cell proliferation than the two peptides on their own, suggesting the possibility that GIP and FGF-2 work through different intracellular signaling mechanisms to increase the level of proliferation.
Alternatively, FGF-2 may upregulate the Gipr gene, thereby increasing the cell responsiveness toward GIP, similar to the effect of FGF-2 on the insulin-like growth factor-I receptor (Aberg et al., 2003). However, most interestingly was the finding that the specific GIP receptor antagonist, ANTGIP, decreased cell proliferation to control levels when applied just before GIP in culture and decreased the proliferation rate to 50% in the absence of GIP. The latter result is probably attributable to the blockage of endogenously produced GIP in vitro. Indeed, we have demonstrated that GIP is produced by the cultured AHPs, and it is possible that GIP also is secreted out to the culture medium.
Because of the insulinotropic action of GIP, there has been considerable interest in studying the role of GIP and GIPR in type 2 diabetes. Type 2 diabetes results in decreased responsiveness of the pancreas to GIP, because of decreased expression of Gipr mRNA and GIPR protein in pancreatic islets (Lynn et al., 2001). Deficits in spatial learning (Biessels et al., 1996) as well as lower cell-proliferation rates in the DG (Jackson-Guilford et al., 2000) that can be improved by treadmill exercise (Kim et al., 2003) have been demonstrated in streptozotocin-induced diabetic rats, suggesting a link between adult neurogenesis and diabetes. Gipr-/- mice also exhibit glucose intolerance and impaired insulin secretion after orally administered glucose (Miyawaki et al., 1999). Because the present investigation demonstrates less proliferation in the DG of Gipr-/- mice, one may hypothesize that the deficits in spatial learning and decreased hippocampal cell genesis observed in experimental animal models of diabetes may be attributable to impaired GIPR activation in the brain. Although additional studies are needed to clarify the exact role of GIP in the hippocampus and in other brain regions, the demonstrated importance of the other members of the VIP/secretin/glucagon family of peptides in regulating brain function indicates that we have opened up a new and potentially exciting area of investigation.
Footnotes
This work was supported by grants from the Swedish Medical Research Council Projects 12X-1253 (P.S.E.) and K2002X-72X-10358-10A (B.M.), Swedish Cancer Research Foundation Project 3911 (O.N.), the Göteborg University Faculty of Medicine, the John and Brit Wennerströms Foundation for Neurological Research, the Rune and Ulla Amlövs Foundation for Neurological and Rheumatological Research, Stiftelsen Göteborgs MS förenings forskning och byggnadsfond, Stiftelsen Handlanden Hjalmar Svenssons Forskningsfond, and the Edit Jacobssons Foundation. We thank Alison Buchan (University of British Columbia, Vancouver, British Columbia, Canada) for providing the monoclonal GIP antibody and Genanalys (Sahlgrenska University Hospital, Göteborg University, Göteborg, Sweden) for the sequencing and expert advice on RT-PCR. We thank Georg H. Kuhn and Michael Nilsson for advice and comments on this manuscript. We also thank Annika Dahl and Malin Palmer for technical assistance. We are grateful to Dr. F. H. Gage for the kind gift of adult-derived neuronal progenitor cells.
Correspondence should be addressed to Jenny Nyberg, The Arvid Carlsson Institute for Neuroscience at the Institute of Clinical Neuroscience, Göteborg University, Sahlgrenska University Hospital, 413 45 Göteborg, Sweden. E-mail: jenny.nyberg@neuro.gu.se.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251816-10$15.00/0
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS (2000) Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci 20: 2896-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg MA, Ryttsen F, Hellgren G, Lindell K, Rosengren LE, MacLennan AJ, Carlsson B, Orwar O, Eriksson PS (2001) Selective introduction of antisense oligonucleotides into single adult CNS progenitor cells using electroporation demonstrates the requirement of STAT3 activation for CNTF-induced gliogenesis. Mol Cell Neurosci 17: 426-443. [DOI] [PubMed] [Google Scholar]
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS (2003) IGF-I has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci 24: 23-40. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319-335. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Kamal A, Ramakers GM, Urban IJ, Spruijt BM, Erkelens DW, Gispen WH (1996) Place learning and hippocampal synaptic plasticity in streptozotocin-induced diabetic rats. Diabetes 45: 1259-1266. [DOI] [PubMed] [Google Scholar]
- Bollag RJ, Zhong Q, Phillips P, Min L, Zhong L, Cameron R, Mulloy AL, Rasmussen H, Qin F, Ding KH, Isales CM (2000) Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology 141: 1228-1235. [DOI] [PubMed] [Google Scholar]
- Brown JC, Pederson RA, Jorpes E, Mutt V (1969) Preparation of highly active enterogastrone. Can J Physiol Pharmacol 47: 113-114. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467: 1-10. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Polak JM, Capella C, Solcia E, Pearse AG (1978) Electronimmunocytochemical evidence for the K cell localization of gastric inhibitory polypeptide (GIP) in man. Histochemistry 56: 37-44. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Ingman-Baker J, Levy J, Brown JC (1982) A comparison of the ability of serum and monoclonal antibodies to gastric inhibitory polypeptide to detect immunoreactive cells in the gastroenteropancreatic system of mammals and reptiles. Histochemistry 76: 341-349. [DOI] [PubMed] [Google Scholar]
- Chabre O, Liakos P, Vivier J, Bottari S, Bachelot I, Chambaz EM, Feige JJ, Defaye G (1998) Gastric inhibitory polypeptide (GIP) stimulates cortisol secretion, cAMP production and DNA synthesis in an adrenal adenoma responsible for food-dependent Cushing's syndrome. Endocr Res 24: 851-856. [DOI] [PubMed] [Google Scholar]
- Chirumamilla S, Sun D, Bullock MR, Colello RJ (2002) Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma 19: 693-703. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156-159. [DOI] [PubMed] [Google Scholar]
- Dagerlind A, Friberg K, Bean AJ, Hokfelt T (1992) Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry 98: 39-49. [DOI] [PubMed] [Google Scholar]
- Damholt AB, Kofod H, Buchan AM (1999) Immunocytochemical evidence for a paracrine interaction between GIP and GLP-1-producing cells in canine small intestine. Cell Tissue Res 298: 287-293. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Gage FH (2003) Genetic and functional differences between multipotent neural and pluripotent embryonic stem cells. Proc Natl Acad Sci USA 100 [Suppl 1]: 11866-11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding KH, Zhong Q, Isales CM (2003) Glucose-dependent insulinotropic peptide stimulates thymidine incorporation in endothelial cells: role of endothelin-1. Am J Physiol Endocrinol Metab 285: E390-E396. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Wallin L (2004) Functional consequences of stress-related suppression of adult hippocampal neurogenesis-a novel hypothesis on the neurobiology of burnout. Acta Neurol Scand 110: 275-280. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH (1998) Neurogenesis in the adult human hippocampus. Nat Med 4: 1313-1317. [DOI] [PubMed] [Google Scholar]
- Hannibal J (2002) Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol 453: 389-417. [DOI] [PubMed] [Google Scholar]
- Hauner H, Glatting G, Kaminska D, Pfeiffer EF (1988) Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Ann Nutr Metab 32: 282-288. [DOI] [PubMed] [Google Scholar]
- Higashimoto Y, Simchock J, Liddle RA (1992) Molecular cloning of rat glucose-dependent insulinotropic peptide (GIP). Biochim Biophys Acta 1132: 72-74. [DOI] [PubMed] [Google Scholar]
- Jackson-Guilford J, Leander JD, Nisenbaum LK (2000) The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci Lett 293: 91-94. [DOI] [PubMed] [Google Scholar]
- Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK (1988) Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol 271: 519-532. [DOI] [PubMed] [Google Scholar]
- Kaplan AM, Vigna SR (1994) Gastric inhibitory polypeptide (GIP) binding sites in rat brain. Peptides 15: 297-302. [DOI] [PubMed] [Google Scholar]
- Kim HB, Jang MH, Shin MC, Lim BV, Kim YP, Kim KJ, Kim EH, Kim CJ (2003) Treadmill exercise increases cell proliferation in dentate gyrus of rats with streptozotocin-induced diabetes. J Diabetes Complications 17: 29-33. [DOI] [PubMed] [Google Scholar]
- Kogire M, Inoue K, Sumi S, Doi R, Yun M, Kaji H, Tobe T (1992) Effects of gastric inhibitory polypeptide and glucagon on portal venous and hepatic arterial flow in conscious dogs. Dig Dis Sci 37: 1666-1670. [DOI] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS (2004) Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neurosci Lett 369: 24-27. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16: 2027-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JT, Dayanandan B, Habener JF, Kieffer TJ (2000) Glucose-dependent insulinotropic polypeptide confers early phase insulin release to oral glucose in rats: demonstration by a receptor antagonist. Endocrinology 141: 3710-3716. [DOI] [PubMed] [Google Scholar]
- Lin C, Lin SC, Chang CP, Rosenfeld MG (1992) Pit-1-dependent expression of the receptor for growth hormone releasing factor mediates pituitary cell growth. Nature 360: 765-768. [DOI] [PubMed] [Google Scholar]
- Lu N, Zhou R, DiCicco-Bloom E (1998) Opposing mitogenic regulation by PACAP in sympathetic and cerebral cortical precursors correlates with differential expression of PACAP receptor (PAC1-R) isoforms. J Neurosci Res 53: 651-662. [DOI] [PubMed] [Google Scholar]
- Lynn FC, Pamir N, Ng EH, McIntosh CH, Kieffer TJ, Pederson RA (2001) Defective glucose-dependent insulinotropic polypeptide receptor expression in diabetic fatty Zucker rats. Diabetes 50: 1004-1011. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, Fujino M (1993) Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res 602: 57-63. [DOI] [PubMed] [Google Scholar]
- McKay R (1997) Stem cells in the central nervous system. Science 276: 66-71. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Yano H, Niwa H, Ban N, Ihara Y, Kubota A, Fujimoto S, Kajikawa M, Kuroe A, Tsuda K, Hashimoto H, Yamashita T, Jomori T, Tashiro F, Miyazaki J, Seino Y (1999) Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc Natl Acad Sci USA 96: 14843-14847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Yamada Y, Ban N, Ihara Y, Tsukiyama K, Zhou H, Fujimoto S, Oku A, Tsuda K, Toyokuni S, Hiai H, Mizunoya W, Fushiki T, Holst JJ, Makino M, Tashita A, Kobara Y, Tsubamoto Y, Jinnouchi T, Jomori T, et al. (2002) Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med 8: 738-742. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Nakajima E, Fukiage C, Azuma M, Shearer TR (2002) Differential gene expression in the lens epithelial cells from selenite injected rats. Exp Eye Res 74: 231-236. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH (1997) The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci 8: 389-404. [DOI] [PubMed] [Google Scholar]
- Parent JM (2003) Injury-induced neurogenesis in the adult mammalian brain. The Neuroscientist 9: 261-272. [DOI] [PubMed] [Google Scholar]
- Perfilieva E, Risedal A, Nyberg J, Johansson BB, Eriksson PS (2001) Gender and strain influence on neurogenesis in dentate gyrus of young rats. J Cereb Blood Flow Metab 21: 211-217. [DOI] [PubMed] [Google Scholar]
- Pincus DW, DiCicco-Bloom E, Black IB (1994) Trophic mechanisms regulate mitotic neuronal precursors: role of vasoactive intestinal peptide (VIP). Brain Res 663: 51-60. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Austin C, Howard A, Lo G, Nicholl CG, Legon S (1992) Characterization of rat gastric inhibitory peptide cDNA. J Mol Endocrinol 9: 265-272. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Krueckl SL, McRory JE (2000) The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr Rev 21: 619-670. [DOI] [PubMed] [Google Scholar]
- Sims KB, Hoffman DL, Said SI, Zimmerman EA (1980) Vasoactive intestinal polypeptide (VIP) in mouse and rat brain: an immunocytochemical study. Brain Res 186: 165-183. [DOI] [PubMed] [Google Scholar]
- Sjolund K, Ekelund M, Hakanson R, Moody AJ, Sundler F (1983) Gastric inhibitory peptide-like immunoreactivity in glucagon and glicentin cells: properties and origin. An immunocytochemical study using several anti-sera. J Histochem Cytochem 31: 811-817. [DOI] [PubMed] [Google Scholar]
- Stanfield BB, Trice JE (1988) Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp Brain Res 72: 399-406. [DOI] [PubMed] [Google Scholar]
- Suh J, Lu N, Nicot A, Tatsuno I, DiCicco-Bloom E (2001) PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat Neurosci 4: 123-124. [DOI] [PubMed] [Google Scholar]
- Trumper A, Trumper K, Trusheim H, Arnold R, Goke B, Horsch D (2001) Glucose-dependent insulinotropic polypeptide is a growth factor for beta (INS-1) cells by pleiotropic signaling. Mol Endocrinol 15: 1559-1570. [DOI] [PubMed] [Google Scholar]
- Tseng CC, Jarboe LA, Landau SB, Williams EK, Wolfe MM (1993) Glucose-dependent insulinotropic peptide: structure of the precursor and tissue-specific expression in rat. Proc Natl Acad Sci USA 90: 1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CC, Kieffer TJ, Jarboe LA, Usdin TB, Wolfe MM (1996) Postprandial stimulation of insulin release by glucose-dependent insulinotropic polypeptide (GIP). Effect of a specific glucose-dependent insulinotropic polypeptide receptor antagonist in the rat. J Clin Invest 98: 2440-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin TB, Mezey E, Button DC, Brownstein MJ, Bonner TI (1993) Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 133: 2861-2870. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH (2002) Functional neurogenesis in the adult hippocampus. Nature 415: 1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschek JA (1995) Vasoactive intestinal peptide: an important trophic factor and developmental regulator? Dev Neurosci 17: 1-7. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Fournier A, Vaudry H (1999) Neurotrophic activity of pituitary adenylate cyclase-activating polypeptide on rat cerebellar cortex during development. Proc Natl Acad Sci USA 96: 9415-9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen PH, Friedrich Jr VL, Shioi J, Robakis NK, Elder GA (2002) Presenilin-1 is expressed in neural progenitor cells in the hippocampus of adult mice. Neurosci Lett 318: 53-56. [DOI] [PubMed] [Google Scholar]
- Wool IG, Chan YL, Paz V, Olvera J (1990) The primary structure of rat ribosomal proteins: the amino acid sequences of L27a and L28 and corrections in the sequences of S4 and S12. Biochim Biophys Acta 1050: 69-73. [DOI] [PubMed] [Google Scholar]
- Yip RG, Wolfe MM (2000) GIP biology and fat metabolism. Life Sci 66: 91-103. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Ding KH, Mulloy AL, Bollag RJ, Isales CM (2003) Glucose-dependent insulinotropic peptide stimulates proliferation and TGF-β release from MG-63 cells. Peptides 24: 611-616. [DOI] [PubMed] [Google Scholar]