Abstract
In the pilocarpine model of temporal lobe epilepsy, mossy fibers coexpress the inhibitory transmitter neuropeptide Y (NPY) with glutamate. The effects of endogenous and applied NPY on recurrent mossy fiber synaptic transmission were investigated with the use of whole-cell voltage-clamp and field recordings in rat hippocampal slices. Applied NPY reversibly inhibited synaptic transmission at recurrent mossy fiber synapses on dentate granule cells but not at perforant path or associational-commissural synapses. It also reduced the frequency of miniature EPSCs (mEPSCs) in granule cells from epileptic, but not control, rats and depressed granule cell epileptiform activity dependent on the recurrent mossy fiber pathway. These actions of NPY were mediated by activation of presynaptic Y2 receptors. The Y2 receptor antagonist (S)-N2-[[1-[2-[4-[(R,S)-5,11-dihydro-6(6H)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]argininamide (BIIE0246) not only blocked the effects of NPY but also enhanced recurrent mossy fiber synaptic transmission, the frequency of mEPSCs, and the magnitude of mossy fiber-evoked granule cell epileptiform activity when applied by itself. Several observations supported the selectivity of BIIE0246. These results suggest that even the spontaneous release of NPY (or an active metabolite) from recurrent mossy fibers is sufficient to depress glutamate release from this pathway. Tonic release of NPY accounts at least partially for the low probability of glutamate release from recurrent mossy fiber terminals, impedes the ability of these fibers to synchronize granule cell discharge, and may protect the hippocampus from seizures that involve the entorhinal cortex. This pathway may synchronize granule cell discharge more effectively in human brain than in rat because of its lower expression of NPY.
Keywords: epilepsy, hippocampus, neuropeptide Y, mossy fiber, presynaptic inhibition, granule cell
Introduction
A unique feature of temporal lobe epilepsy is the anatomical reorganization of the dentate gyrus (Represa et al., 1989; Sutula et al., 1989; Babb et al., 1991; Houser, 1992; Franck et al., 1995). This phenomenon is replicated in several animal models of epilepsy, including the pilocarpine-treated rat (Nadler, 2003). Dentate granule cells become interconnected through the growth of recurrent mossy fibers. These mossy fiber collaterals mediate recurrent excitation (Wuarin and Dudek, 1996; Molnár and Nadler, 1999; Okazaki et al., 1999; Feng et al., 2003), a type of innervation that is hardly present on dentate granule cells in normal brain. Formation of recurrent excitatory circuitry in the dentate gyrus could contribute to progressively enhanced excitability (Gorter et al., 2001; Zhang et al., 2002), because in nonepileptic animals, dentate granule cells have been shown to resist the propagation of seizures from the entorhinal cortex to the hippocampus (Collins et al., 1983; Stringer et al., 1989; Lothman et al., 1992). The recurrent mossy fiber pathway contributes to a reduced threshold for granule cell synchronization (Tauck and Nadler, 1985; Cronin et al., 1992; Masukawa et al., 1992; Patrylo and Dudek, 1998; Hardison et al., 2000; Okazaki and Nadler, 2001), which would presumably facilitate the participation of these cells in seizures.
Seizures dramatically alter the expression of neuropeptide Y (NPY) in the dentate gyrus (Schwarzer et al., 1995; Lurton and Cavalheiro, 1997; Makiura et al., 1999; Vezzani et al., 1999a). NPY, a 36-amino acid polypeptide, is one of the most abundant and widely distributed neuroactive peptides in the brain (Hökfelt et al., 1998). It is normally expressed by subpopulations of GABA neurons in the CNS and sympathetic fibers in the periphery. Seizures enhance the expression of NPY throughout neocortical and limbic regions, and dentate granule cells express NPY de novo. Strong NPY immunoreactivity persists in granule cells of epileptic brain (Vezzani et al., 1999a), including the brain of pilocarpine-treated epileptic rats (Scharfman et al., 2000), for months at least. NPY is transported through the mossy fibers to their terminals, from which it can be released (McCarthy et al., 1998). This is the only known instance in which NPY is released by a glutamate pathway. Seizures also alter the expression of NPY receptors. The six Gi/Go-coupled receptors for NPY may be located at both presynaptic and postsynaptic sites. The Y1 and Y2 receptors are the most prominently expressed in the hippocampus (Redrobe et al., 1999). Seizures markedly increase the density of Y2 receptors in the dentate gyrus, whereas the density of Y1 receptors declines (Gobbi et al., 1998; Schwarzer et al., 1998). Applied NPY inhibits synaptic transmission at most excitatory synapses of the rat hippocampus, including mossy fiber synapses on CA3 pyramidal cells (Klapstein and Colmers, 1993), and suppresses epileptiform activity (Klapstein and Colmers, 1997). Presynaptic Y2 receptors mediate at least some of these inhibitory actions of NPY (El Bahh et al., 2002). Thus, in epileptic brain, mossy fiber terminals release both the excitatory amino acid glutamate and the inhibitory neuropeptide NPY. Corelease of NPY may explain, in part, why stimulation of the mossy fibers in vitro (Tauck and Nadler, 1985; Cronin et al., 1992; Patrylo and Dudek, 1998; Hardison et al., 2000) or the perforant path in vivo (Buckmaster and Dudek, 1997) usually does not evoke reverberating excitation in the dentate gyrus, even in the presence of robust recurrent mossy fiber growth. This study examined the effects of endogenous and applied NPY on synaptic transmission at recurrent mossy fiber synapses and on granule cell epileptiform activity evoked by activating these synapses. Our findings suggest that even spontaneous release of endogenous NPY or an active metabolite strongly depresses the activity of this pathway.
Materials and Methods
Pilocarpine-induced status epilepticus. Male Sprague Dawley rats (175-200 g; Zivic Laboratories, Pittsburgh, PA) were given injections of pilocarpine (340-380 mg/kg, i.p.) 30 min after pretreatment with methyl-scopolamine and terbutaline (2 mg/kg, i.p., each). Most rats so treated developed status epilepticus, defined as a continuous limbic motor seizure of stage 2 or higher (Racine, 1972). Initially, status epilepticus was terminated 3.5 h after onset with a single injection of sodium phenobarbital (50 mg/kg, i.p.). These rats did not always develop a robust recurrent mossy fiber pathway (Timm score of 3-4) (see Fig. 1 A, B) (Okazaki et al., 1995), however. Because extensive mossy fiber growth was required for this study, subsequent experiments used rats whose status epilepticus had been allowed to self-terminate after ≥6 h or longer. In these instances, a fan mounted atop the cage blew a stream of cool air on the animal, to minimize seizure-induced hyperthermia (Sloviter et al., 2003). Lengthening the duration of status epilepticus ensured that all animals exhibited robust recurrent mossy fiber growth. Thus, a higher fraction of granule cells responded to mossy fiber stimulation, and compound EPSCs tended to be larger. No difference in either mossy fiber NPY expression or NPY pharmacology was apparent. There was also no increase in mortality. Although animals in the present study were not monitored systematically after recovery from status epilepticus, previous studies demonstrated all rats that develop status epilepticus exhibit spontaneous limbic motor seizures after a 1-3 week latent period (Mello et al., 1993; Lemos and Cavalheiro, 1996).
Figure 1.
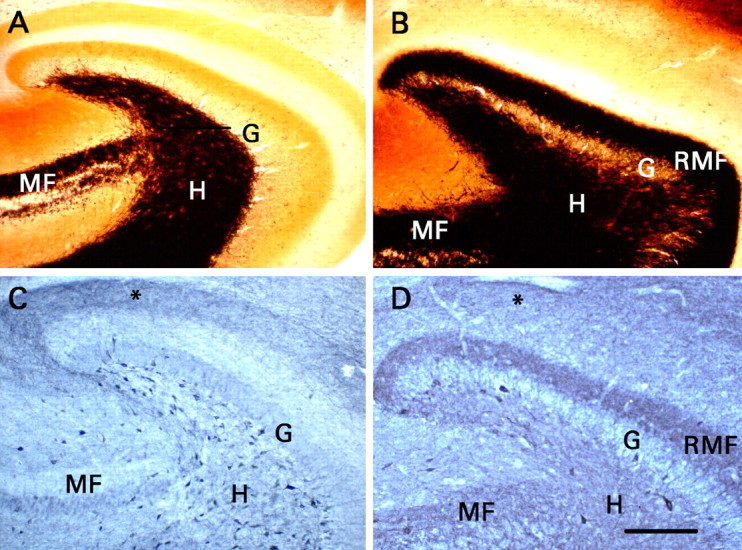
Recurrent mossy fibers that expressed NPY were present when electrophysiological studies were performed. Transverse sections were cut from the same portion of the hippocampal formation used for recording. G, Granule cell body layer; H, dentate hilus; MF, mossy fiber pathway in area CA3b-CA3c; RMF, recurrent mossy fiber pathway. A, Timm histochemistry in a section from a control rat. Black mossy fiber-like staining is present only in the mossy fiber pathway of area CA3 and the dentate hilus. B, Timm histochemistry in a section from a rat that had developed status epilepticus and did not receive phenobarbital. Mossy fiber-like Timm staining is present in the inner third of the dentate molecular layer, where recurrent mossy fibers innervate granule cell dendrites. C, NPY immunohistochemistry in a section from a control rat. Note the presence of numerous immunoreactive somata in the dentate hilus, terminal immunoreactivity in the outer part of the dentate molecular layer (asterisk), and little immunoreactivity either in the mossy fibers of area CA3 or in the inner third of the dentate molecular layer. D, NPY immunohistochemistry in a section from a rat that had developed status epilepticus and did not receive phenobarbital. Note the neoexpression of NPY in the mossy fibers of area CA3b-CA3c, in the hilus, and particularly in the recurrent mossy fibers. Status epilepticus reduced the number of hilar NPY-immunoreactive somata markedly, and terminal immunoreactivity in the outer part of the molecular layer (asterisk) was less distinct than in C. C, D, Staining of granule and pyramidal cell bodies resulted from nonspecific binding of the secondary antibody and does not signify expression of NPY. Scale bar (in D) A-D, 200 μm.
Some rats exhibited only a few brief behavioral seizures, but not status epilepticus, in response to pilocarpine. Histological tests revealed no evidence of neuronal degeneration or supragranular mossy fiber growth in these animals (Okazaki et al., 1999). We also have not found any significant difference in electrophysiological response between these animals and age-matched untreated rats (Molnár and Nadler, 1999; Okazaki et al., 1999; Hardison et al., 2000; Okazaki and Nadler, 2001). They were therefore used as controls to account for any possible action of pilocarpine not mediated by status epilepticus.
All protocols were approved in advance by the Duke University Institutional Animal Care and Use Committee
Preparation and incubation of hippocampal slices. Animals were decapitated under deep ether anesthesia 10-20 weeks after pilocarpine administration. Transverse 400-μm-thick slices of the caudal hippocampal formation were prepared with a vibratome and incubated in a high-Mg2+ artificial CSF (ACSF) that consisted of (in mm) 122 NaCl, 25 NaHCO3, 3.1 KCl, 1.8 CaCl2, 12 MgSO4, 0.4 KH2PO4, and 10 d-glucose, pH 7.4, by gassing with 95% O2/5% CO2. Before whole-cell patch-clamp recording, the slices were incubated for 45-60 min at 34°C and then at room temperature. Before field-potential recording, slices were incubated at room temperature continuously. Experimentation began after at least 1 h of incubation. Slices used for electrophysiological recording corresponded to horizontal plates 98-100, as described by Paxinos and Watson (1986).
Whole-cell patch-clamp recording. A slice was transferred to a submersion-type recording chamber mounted on a microscope stage, barely submerged in standard ACSF (1.2 mm MgSO4) at room temperature (22-24°C), and superfused at 2-3 ml/min. Whole-cell patch-clamp recordings were obtained from dentate granule cells with the assistance of infrared-differential interference contrast optics or by the “blind” approach (Blanton et al., 1989). Patch electrodes fashioned from borosilicate glass (1.5 mm outer diameter; Sutter Instruments, Novato, CA) had a tip resistance of 6-7.5 MΩ. The tip was filled with a solution that contained (in mm) 140 cesium gluconate, 15 HEPES, 3.1 MgCl2, 1 CaCl2, and 11 EGTA, pH 7.2 and 276 mOsm. The electrode was then backfilled with an internal solution that contained 120 mm cesium gluconate, 10 mm HEPES, 2 mm MgATP, 1 mm EGTA, 5 mm creatine phosphate, 20 U/ml creatine phosphokinase, and 10 mm N-ethyllidocaine chloride (QX-314), pH 7.2 and 276 mOsm. Whole-cell access was achieved in current-clamp mode; only cells with resting potential (Vm) values greater than -70 mV were accepted for study. Recordings were made with an Axopatch 200B or 200A amplifier (Axon Instruments, Foster City, CA) beginning ∼20 min after break-in. Series resistances of 11-16 MΩ were compensated 70-75%. Recordings were rejected if the series resistance varied by >20%. Input resistance (RN) was calculated from the current deflection produced by a 200 ms, 5 mV hyperpolarization from a holding potential of -80 mV. Constant-current rectangular electrical stimuli (0.1 ms duration) were delivered through a monopolar electrode fashioned from 25-μm-diameter nichrome wire insulated to the tip with Formvar. Despite the seemingly high currents used in some experiments, stimuli appeared to be quite localized. For example, moving the electrode only 50 μm laterally from the center of stratum lucidum abolished both antidromic and orthodromic responses of granule cells to stimulation of the mossy fibers. Signals were filtered below 2 kHz, sampled at 10 kHz, and stored for analysis off-line with pClamp8 software (Axon Instruments). The effects of NPY and NPY receptor ligands were evaluated between 5 and 8 min after the compound was added to the superfusion medium. By this time, the effect of the compound was maximal.
NMDA receptor-mediated EPSC. The NMDA receptor-mediated component of the compound EPSC was isolated pharmacologically by addition to the superfusion medium of 30 μm bicuculline, to block GABAA receptors, and 10 μm 2,3-dihydroxy-6-nitro-7-sulfonyl-benzo[f] quinoxaline (NBQX), to block AMPA and kainate receptors. Recordings were made at a holding potential of -20 or -30 mV. d-2-Amino-5-phosphonopentanoate (d-AP-5; 50 μm) abolished the evoked current when added to the superfusion medium at the end of the experiment, confirming that the current was mediated entirely by NMDA receptors. Pathways were stimulated under each condition with a train of 10 pulses at a frequency of 0.1 Hz and intensity of 100-600 μA. The effects of test compounds were computed from the average of the 10 evoked responses. Responses to mossy fiber and perforant path stimulation were studied in slices prepared from rats that had developed status epilepticus, whereas responses to stimulation of the associational-commissural fibers were studied in slices from control rats. See the supplemental figure (available at www.jneurosci.org as supplemental material) for placement of the stimulating electrode in each instance.
Minimal electrically evoked recurrent mossy fiber EPSC. These responses were recorded in the presence of 30 μm bicuculline at a holding potential of -80 mV. The stimulating electrode was placed in the granule cell body layer ∼200 μm from the recorded cell. The stimulus intensity was set initially at 200 μA and then reduced until some stimuli evoked no response in the recorded cell. Response failures appeared at stimulus intensities between 10 and 150 μA. The same intensity was used for all recordings from that cell. Responses were accepted as minimal EPSCs if their peak amplitude was at least twice the baseline noise and they appeared at a constant latency <5 ms. All such responses were abolished by adding 10 μm NBQX to the superfusion medium. A train of 50 stimuli was presented at a frequency of 0.2 Hz. Properties of the minimal recurrent mossy fiber EPSC were computed after electronically subtracting the average of all traces without a minimal EPSC from the average of all traces with a minimal EPSC. Latency to onset, peak amplitude, 10-90% rise time, decay time constant (τ), and charge transfer per response were determined with functions incorporated in pClamp8. Occasionally, a spontaneous event overlapped a portion of the evoked response. In these instances, the trial was counted as a success but was excluded from the computation of response properties.
Miniature EPSC. Spontaneous synaptic currents were recorded in the presence of 30 μm bicuculline and 1 μm tetrodotoxin (TTX) at a holding potential of -80 mV. Events were recorded for 3 min under each experimental condition with the use of pClamp8. Miniature EPSC (mEPSC) frequency and the properties of mEPSCs were analyzed with the use of MiniAnalysis (Synaptosoft, Decatur, GA). The amplitude threshold for detecting mEPSCs was the same as for minimal electrically evoked EPSCs. This conservative criterion presumably eliminated many of the mEPSCs that originated from the distal portions of the dendrites, thus biasing the analysis toward responses that originated close to the soma. After MiniAnalysis had identified the putative mEPSCs automatically, each record was examined manually to exclude false positives. Peak amplitude, 10-90% rise time, τ, and charge transfer per response were determined for each verified mEPSC and averaged to obtain a single set of values for each recording. Spontaneous events that overlapped were not used for the computation of mEPSC properties.
Field-potential recording. Only hippocampal slices from rats that had developed status epilepticus were used in these studies. Individual slices were transferred to a small experimental chamber maintained at 35°C, barely submerged in standard ACSF, and superfused at 2 ml/min. A cut was placed between areas CA1 and CA3 to prevent reverberating excitation in the hippocampal-entorhinocortical circuit (see supplemental figure, available at www.jneurosci.org as supplemental material). The recurrent mossy fibers were activated as described above at an intensity that evoked an antidromic population spike ≥85% of the maximal amplitude (mean intensity, 450 μA; range, 260-620 μA). The perforant path was activated with an electrode placed in the subiculum. An extracellular glass recording electrode filled with 1 m NaCl (resistance, 5-7 MΩ) was placed in the granule cell body layer where the antidromic population spike was of the greatest amplitude (Okazaki et al., 1999). Responses were amplified with AC coupling, filtered below 3 kHz, digitized at 10 kHz, and stored to disk with the use of pClamp8.
The superfusion medium in these experiments contained 6 mm K+ and 30 μm bicuculline to maximize the probability of evoking granule cell epileptiform activity (Okazaki and Nadler, 2001). Mossy fibers were stimulated, and extracellular recordings were acquired every 30 s for 70 min. Mossy fiber stimulation evoked a relatively stable epileptiform response by this time. This response was identified as spiking activity (sharp waves at least three times larger in amplitude than background activity and <7 ms in duration) on the trace after the antidromic population spike. The magnitude of epileptiform activity was quantified by measurement of the coastline index (Dingledine et al., 1986), as described previously (Hardison et al., 2000; Okazaki and Nadler, 2001). The coastline index allows for statistical comparisons among treatment groups regardless of the mechanism responsible for those differences. It was computed for the 387 ms period beginning at the end of the antidromic population spike. To account for baseline electrical noise, the coastline index was also determined for the 108 ms period immediately before the stimulus. This value was extrapolated to 387 ms and subtracted from the poststimulus coastline index.
Mossy fiber stimulation failed to evoke granule cell epileptiform activity in some slices. In these experiments, the stimulating electrode was moved into the subiculum to evoke perforant path (medial plus lateral) responses. The perforant path was stimulated every 30 s with pulses of the same duration, shape, and intensity as those used for mossy fiber stimulation in the same slice. The coastline index was computed as described for responses to mossy fiber stimulation for the first 70 ms of the trace that followed the end of the stimulus artifact.
Test compounds were added to the superfusion medium 70 min after the switch to bicuculline/6 K+ medium. The effects were maximal within 20 min. They were quantitated by averaging the coastline indices for the 10 traces recorded between 20 and 24.5 min after the addition of test compound and comparing that to the average coastline index obtained during the 4.5 min period before its addition.
NPY immunohistochemistry. Rats were deeply anesthetized with pentobarbital and perfused transcardially with PBS, followed by 4% (w/v) phosphate-buffered paraformaldehyde. The brain was cut into 50-μm-thick horizontal sections with a vibratome, and the sections were exposed sequentially to PBS (three times for 10 min), 0.5% (v/v) H2O2 (1 h), 0.1% (v/v) Triton X-100 in PBS (PBS-T; three times for 10 min), 10% (v/v) normal goat serum in PBS-T (90 min), rabbit anti-NPY antiserum [1:1000 or 1:2000 dilution with 5% (v/v) normal goat serum/PBS-T; Peninsula Laboratories, San Carlos, CA] (overnight at 4°C), PBS-T (four times for 10 min), biotinylated goat anti-rabbit IgG [1:200 dilution with 2% (v/v) normal goat serum/PBS-T; Vector Laboratories, Burlingame, CA] (1 h), PBS (three times for 10 min), avidin-biotin-horseradish peroxidase (Vectastain Elite ABC kit; Vector Laboratories) (1 h), PBS (three times for 10 min), and 3,3′-diaminobenzidine/H2O2/nickel ammonium sulfate (DAB substrate kit for peroxidase; Vector Laboratories) (4 min). All steps were performed at room temperature unless indicated otherwise.
Mossy fiber terminals were visualized with the use of the Timm stain for heavy metals after transcardial perfusion with 0.1% (w/v) Na2S in 0.15 m sodium phosphate buffer, pH 7.4, followed by 10% formalin in PBS, pH 7.4 (Danscher, 1981).
Materials. NPY, N2-(diphenylacetyl)-N-([4-hydroxyphenyl]methyl)-d-arginine amide (BIBP3226), d-gluconic acid lactone, cesium hydroxide (99.9%; 50% by weight), HEPES, EGTA, creatine phosphate, creatine phosphokinase, pilocarpine hydrochloride, (-)scopolamine methyl bromide, and terbutaline hemisulfate were purchased from Sigma (St. Louis, MO); d-AP-5, NBQX, l-2-amino-4-phosphonobutyric acid (l-AP-4), and TTX were purchased from Tocris Cookson (Ellisville, MO); bicuculline methiodide and NPY (3-36) were purchased from Research Biochemicals (Natick, MA); and QX-314 was purchased from Alomone Laboratories (Jerusalem, Israel). (S)-N2-[[1-[2-[4-[(R, S)-5,11-dihydro-6(6H)-oxodibenz[b,e]azepin-11-yl]-1-piperazinyl]-2-oxoethyl]cyclopentyl]acetyl]-N-[2-[1,2-dihydro-3,5(4H)-dioxo-1,2-diphenyl-3H-1,2,4-triazol-4-yl]ethyl]-argininamide (BIIE0246) was a gift from Boehringer-Ingelheim (Biberach an der Riss, Germany).
Results
Long-lasting expression of NPY in mossy fibers after pilocarpine-induced status epilepticus
In the dentate gyrus of control rats, somata immunoreactive for NPY were mainly confined to the hilus (Fig. 1C). Hilar NPY-immunoreactive neurons were presumably HIPP cells (soma located in the hilus, axonal projection to the perforant path zone), which are known to coexpress NPY, somatostatin, and GABA (Freund and Buzsáki, 1998). Immunostaining was also present in the outer part of the molecular layer, where axons of the HIPP cells terminate. Three changes were evident in sections from rats that had developed status epilepticus (status epilepticus group) and were analyzed 10-20 weeks after pilocarpine administration (Fig. 1D). First, NPY immunoreactivity appeared de novo in the mossy fiber pathway, including the recurrent mossy fibers. Mossy fiber immunoreactivity was strongest in the inner portion of the dentate molecular layer, the locus of recurrent mossy fibers. Second, there were markedly fewer NPY-immunoreactive somata in the dentate hilus. Third, immunostaining in the outer part of the molecular layer was less distinct, consistent with the loss of HIPP cells. These findings replicate previous reports (Lurton and Cavalheiro, 1997; Vezzani et al., 1999a; Scharfman et al., 2000) and confirm that recurrent mossy fibers expressed NPY at the time our electrophysiological studies were performed.
NPY inhibited transmission at recurrent mossy fiber synapses selectively and reversibly
Stimulation of the mossy fibers in the presence of bicuculline evoked a response in the recorded granule cell that usually consisted of both monosynaptic and polysynaptic components. Isolating the NMDA receptor-mediated component of the response by adding NBQX to the superfusion medium and recording at a holding potential of -20 or -30 mV eliminated the polysynaptic components (Salin et al., 1996; Feng et al., 2003). Thus, our initial electrophysiological studies used the NMDA receptor-mediated compound EPSC, because this response is likely to be purely monosynaptic. In hippocampal slices from the status epilepticus group, NPY (1 μm) inhibited transmission at recurrent mossy fiber synapses (Fig. 2). The NMDA component of the compound EPSC was reduced by an average of 70% (p < 0.01; paired t test). The effect of NPY reversed slowly after washout; the response only regained its initial amplitude after ≥60 min.
Figure 2.
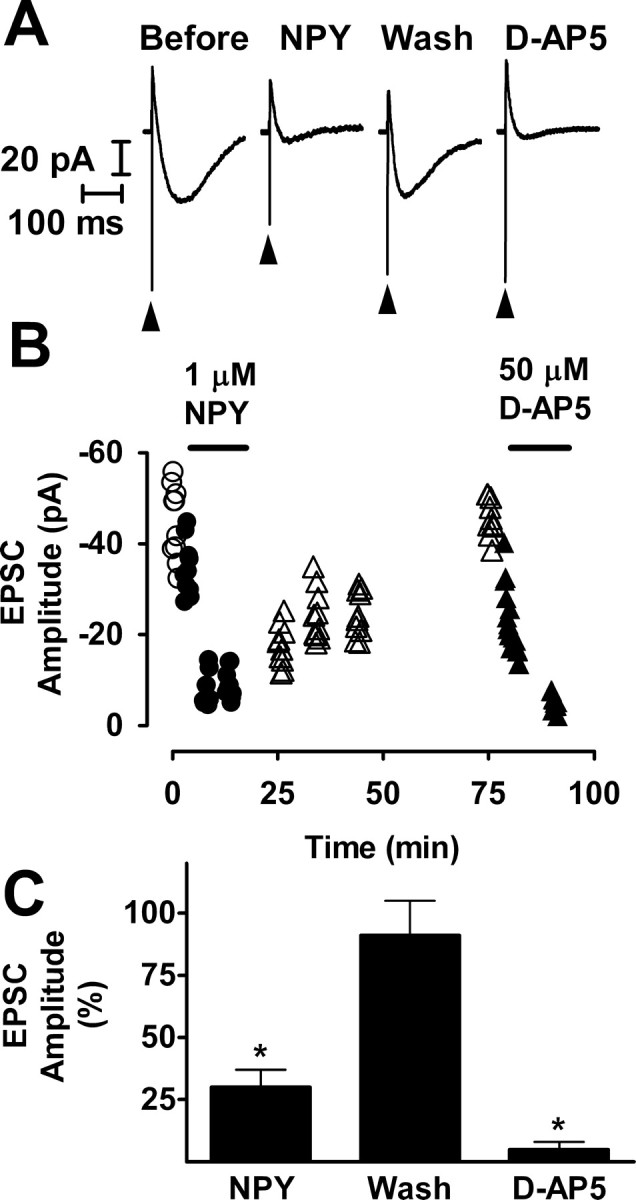
NPY inhibited recurrent mossy fiber synaptic transmission reversibly. A, Recordings taken from the experiment illustrated in B are NMDA receptor-mediated compound recurrent mossy fiber EPSCs and are the averages of 10 traces. These responses were recorded in the presence of 10 μm NBQX and 30 μm bicuculline at a holding potential of -20 mV. NPY (1 μm) reduced the size of the response markedly and reversibly. The arrowheads indicate stimulus artifacts. Wash, Washout. B, At various times during this representative experiment, a train of 10 stimuli was applied to the mossy fibers at a frequency of 0.1 Hz. Stimuli were presented, and responses were recorded just before the addition of NPY to the superfusion medium (○), during exposure to NPY (•), during washout of NPY from the tissue (▵), and during exposure to d-AP-5 (▴). For each response, peak amplitude is plotted against the time during the experiment at which it was recorded. C, Mean values ± SEM for seven cells to which NPY was applied, three cells that were washed free of NPY (Wash), and five cells to which d-AP-5 was applied after complete or nearly complete recovery from the effect of NPY. *p < 0.01 by paired t test with respect to responses recorded just before exposure to NPY.
Dentate granule cells normally receive excitatory innervation from the perforant path, which originates in the entorhinal cortex, and the associational-commissural pathway, which arises from mossy cells of the hilus. NPY (1 μm) did not significantly affect the NMDA component of the compound perforant path EPSC in slices from the status epilepticus group (Fig. 3). Associational-commissural synaptic transmission was studied in slices from control rats, because pilocarpine-induced status epilepticus essentially destroys this pathway (Okazaki et al., 1999; Nadler and Jiao, 2004). Stimuli were applied to the inner third of the molecular layer, and the NMDA component of the evoked response was studied. In seven cells, 50 μm l-AP-4 reduced the response by 45 ± 7% (mean ± SEM; p < 0.005; paired t test). This finding suggests that the stimulus activated predominantly associational-commissural fibers because terminals in this pathway express the inhibitory metabotropic receptor mGluR4, which is highly sensitive to l-AP-4 (Shigemoto et al., 1997). In contrast, the adjacent medial perforant path does not express l-AP-4-sensitive receptors. NPY did not affect the response significantly. Thus, NPY inhibited recurrent mossy fiber transmission selectively.
Figure 3.
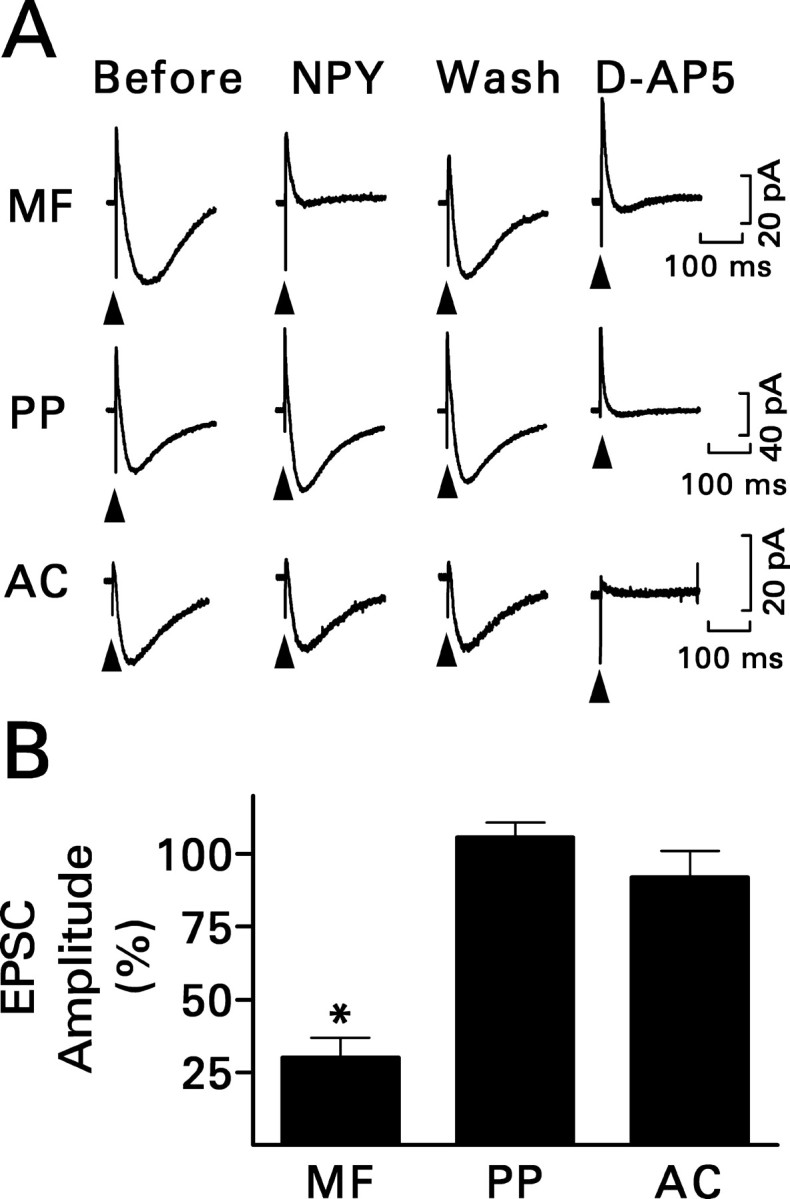
NPY (1 μm) inhibited recurrent mossy fiber transmission selectively. A, Recordings are representative NMDA receptor-mediated compound EPSCs evoked by stimulating the indicated pathway and are the averages of 10 traces. Only responses to mossy fiber (MF) stimulation were reduced by NPY. The response mostly recovered during washout (Wash) of NPY and was eliminated by d-AP-5. See Figure 2 A for other details. PP, Perforant path; AC, associational-commissural pathway. B, Grouped data for the effect of NPY obtained from six (perforant path) or seven (mossy fiber and associational-commissural) granule cells. Values are means ± SEM; *p < 0.01 by paired t test with respect to responses recorded just before exposure to NPY.
NPY action mediated by the Y2 receptor
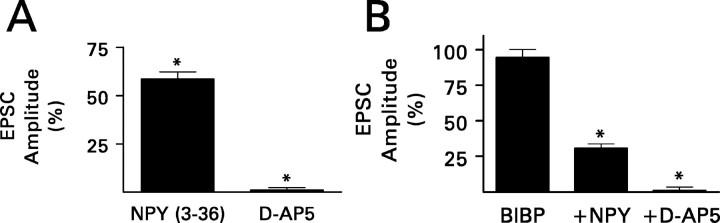
NPY inhibits transmission at Schaffer collateral-commissural synapses in hippocampal area CA1 (Weiser et al., 2000; El Bahh et al., 2002) and in the thalamic reticular nucleus (Sun et al., 2001) by activating presynaptic Y2 receptors. NPY inhibited recurrent mossy fiber transmission by a similar mechanism. NPY (3-36) (1 μm), a Y2 receptor agonist (Grandt et al., 1996), replicated the action of NPY. It reduced the NMDA component of the compound recurrent mossy fiber EPSC by an average of 41% (p < 0.01; paired t test) (Fig. 4A). Furthermore, application of the Y2 receptor antagonist BIIE0246 (100 nm) (Doods et al., 1999; Dumont et al., 2000; Weiser et al., 2000) prevented the action of NPY (Fig. 5). However, BIBP3226 (1 μm), a specific Y1 receptor antagonist (Doods et al., 1996), had no effect (Fig. 4B).
Figure 4.
A, The Y2 receptor agonist NPY (3-36) (1 μm) replicated the inhibition by NPY of recurrent mossy fiber synaptic transmission, as assessed from its effect on the peak amplitude of the NMDA receptor-mediated compound EPSC. Data were obtained from six granule cells exposed to NPY (3-36) and then to d-AP-5 (50 μm) in the continued presence of NPY (3-36). B, The Y1 receptor antagonist BIBP3226 (BIBP; 1 μm) did not change the peak amplitude of the NMDA receptor-mediated compound recurrent mossy fiber EPSC significantly when applied by itself, nor did it prevent inhibition by NPY. Data were obtained from six granule cells exposed to BIBP3226, then to NPY (1 μm) in the continued presence of BIBP3226, and finally to d-AP-5 (50 μm) in the continued presence of both BIBP3226 and NPY. Values are means ± SEM; *p < 0.01 by paired t test with respect to responses recorded just before the application of NPY receptor ligand.
Figure 5.
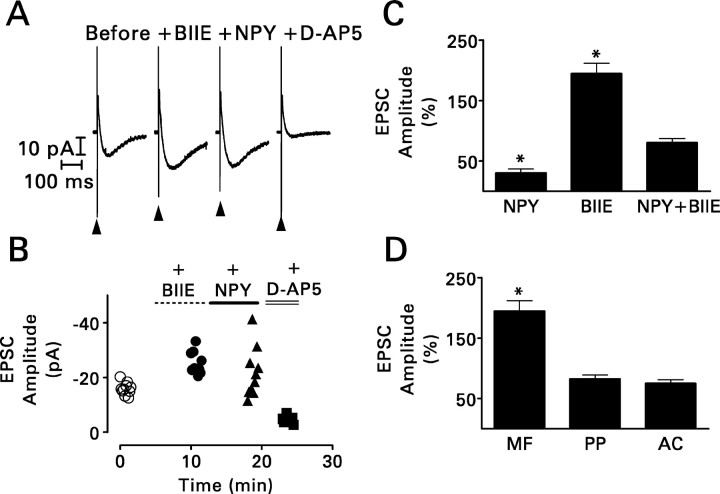
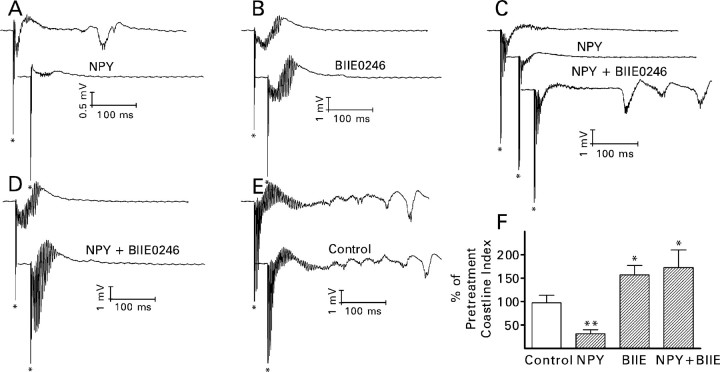
BIIE0246 (BIIE; 100 nm) essentially blocked the effect of NPY (1 μm) and enhanced recurrent mossy fiber synaptic transmission when applied by itself. Its effects were restricted to recurrent mossy fiber synapses. A, Recordings from the experiment shown in B are NMDA receptor-mediated compound recurrent mossy fiber EPSCs and are the averages of 10 traces. BIIE0246 increased response amplitude when applied by itself. The addition of NPY to the superfusion medium in the continued presence of BIIE0246 hardly affected the size of the response. See Figure 3A for other details. B, During this representative experiment, a hippocampal slice was superfused sequentially with BIIE0246 (•), BIIE0246 plus NPY (▴), and finally BIIE0246 plus NPY plus 50 μm d-AP-5 (▪). A 0.1 Hz train of 10 stimuli was applied to the mossy fibers just before the addition of BIIE0246 to the superfusion medium (○) and after complete equilibration of the slice with each modified superfusion medium. For each response, peak amplitude is plotted against the time during the experiment at which it was recorded. Note that in the presence of BIIE0246 alone, the amplitude of the response to the first stimulus in the train was approximately the same as the amplitude of the succeeding responses. Thus, BIIE0246 enhanced response amplitude in the absence of a prepulse. BIIE0246 also attenuated the effect of NPY. This experiment was replicated three times with similar results. C, Grouped data (means ± SEM) obtained from seven cells exposed to NPY alone, six cells exposed to BIIE0246 alone, and five cells exposed simultaneously to NPY and BIIE0246. *p < 0.01 by paired t test with respect to responses recorded just before the application of the test compound(s). D, BIIE0246 increased the peak amplitude of the NMDA receptor-mediated compound recurrent mossy fiber (MF) EPSC but not that of similar responses evoked by stimulation of perforant path (PP) or associational-commissural (AC) fibers. Values are means ± SEM for six (mossy fiber), four (perforant path), or three (associational-commissural) granule cells. *p < 0.01 by paired t test with respect to responses recorded just before exposure to BIIE0246.
BIIE0246 enhanced transmission at recurrent mossy fiber synapses selectively
BIIE0246 (100 nm) not only blocked the inhibitory action of NPY but also nearly doubled the size of the mossy fiber-evoked EPSC when applied by itself (p < 0.01; paired t test) (Fig. 5A-C). The full effect of the antagonist was evident with the first stimulus in a 0.1 Hz train (Fig. 5B); neither a previous stimulus nor repetitive presynaptic activity was required. BIIE0246 had no such effect on transmission at perforant path or associational-commissural synapses (Fig. 5D).
In recordings from six granule cells in slices prepared from control rats, mossy fiber stimulation evoked no synaptic current in the absence or presence of BIIE0246 nor did we observe any BIIE0246-induced current. In recordings from granule cells of the status epilepticus group, neither BIIE0246 nor NPY had any consistent effect on the leak current. Furthermore, neither compound altered RN measured at a holding potential of -80 mV. The values for BIIE0246 were as follows: control, 580 ± 50 MΩ; BIIE0246, 540 ± 40 MΩ (mean ± SEM; n = 24). The values for NPY were as follows: control, 550 ± 60 MΩ; NPY, 540 ± 70 MΩ (mean ± SEM; n = 10).
NPY and BIIE0246 had opposite effects on transmission rate of the minimal recurrent mossy fiber EPSC
Minimal electrical stimulation in the granule cell body layer activated an “all-or-none” synaptic response in the recorded granule cell (Feng et al., 2003). These responses were evoked only in granule cells from the status epilepticus group and are attributable to activation of one or a few recurrent mossy fiber synapses. Minimal stimulation at a frequency of 0.2 Hz evoked a synaptic response in only 32 ± 2% of trials (mean ± SEM; n = 18), a finding consistent with previous reports (Molnár and Nadler, 1999; Feng et al., 2003; Scharfman et al., 2003). Thus, 68% of minimal stimuli normally result in response failure when applied at low frequency.
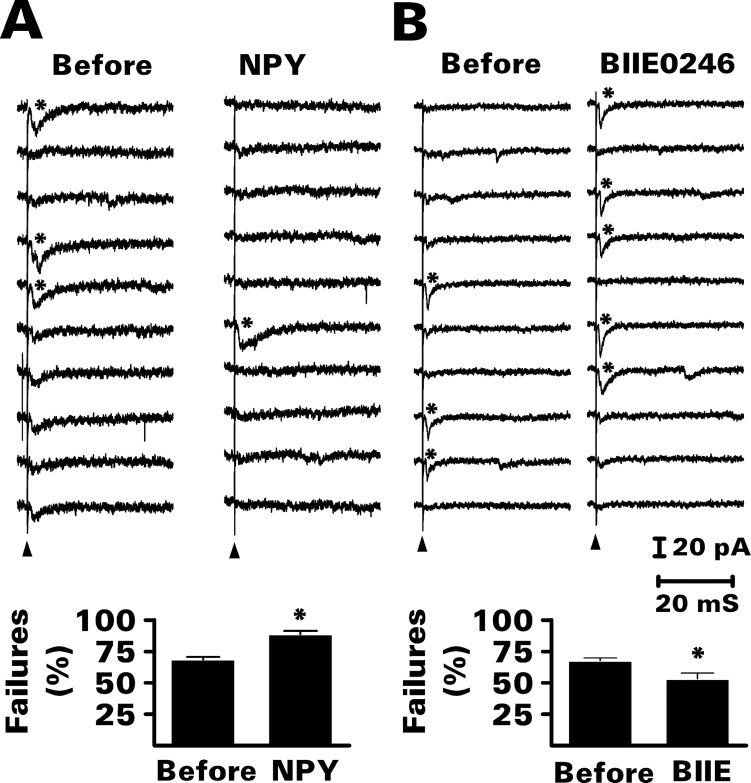
NPY increased the percentage of response failures by an average of 23%, whereas BIIE0246 reduced the failure rate by an average of 20% (p < 0.01 for both; paired t test) (Fig. 6). These changes were not accompanied by any significant effect on the latency, mean peak amplitude, or response kinetics of the minimal EPSC (Table 1), consistent with a presynaptic site of action.
Figure 6.
NPY (1 μm) increased and BIIE0246 (100 nm) reduced the percentage of transmission failures during minimal electrical stimulation of recurrent mossy fibers. Additional data from these experiments are presented in Table 1. A, Effect of NPY. Top, The 10 consecutive traces (from a total of 50) shown are from a representative experiment. In this example, only 3 of the first 10 stimuli presented at a frequency of 0.2 Hz evoked a minimal EPSC (asterisk) before exposure to NPY. Bath application of NPY reduced the transmission rate to 1 of 10. Bottom, Grouped data (means ± SEM) from 10 granule cells demonstrated a significant increase in the percentage of response failures. *p < 0.01 by paired t test with respect to recordings made just before exposure to NPY. B, Effect of BIIE0246. Top, See A for details. In this example, BIIE0246 increased the transmission rate of minimal EPSCs from 3 of 10 to 5 of 10. Bottom, Grouped data (means ± SEM) from eight granule cells demonstrated a significant reduction in the percentage of response failures. *p < 0.01 by paired t test with respect to recordings made just before exposure to BIIE0246 (BIIE).
Table 1.
Neither NPY (1 μm) nor BIIE0246 (100 nm) altered properties of minimal electrically evoked recurrent mossy fiber EPSCs
|
|
Latency (ms) |
Amplitude (pA) |
10-90% rise time (ms) |
τ(ms) |
Charge transfer (fC) |
|---|---|---|---|---|---|
| Before NPY | 2.7 ± 0.3 | 17.0 ± 1.8 | 2.9 ± 0.8 | 29.7 ± 3.4 | 285 ± 34 |
| Plus NPY | 2.8 ± 0.4 | 14.9 ± 1.4 | 3.9 ± 1.0 | 28.2 ± 3.7 | 279 ± 39 |
| Before BIIE0246 | 3.5 ± 0.2 | 14.8 ± 3.9 | 3.2 ± 0.6 | 29.0 ± 4.6 | 299 ± 79 |
| Plus BIIE0246 |
3.5 ± 0.2 |
16.1 ± 4.2 |
3.7 ± 0.7 |
35.4 ± 4.6 |
329 ± 82 |
Properties of averaged minimal EPSCs were determined before and during exposure to the test compound. Values are means ± SEM for 10 (NPY) or 8 (BIIE0246) granule cells per group. For all comparisons between test compound and pretreatment values, p ≫ 0.05 (paired t test).
NPY and BIIE0246 had opposite effects on mEPSC frequency in granule cells from the status epilepticus group
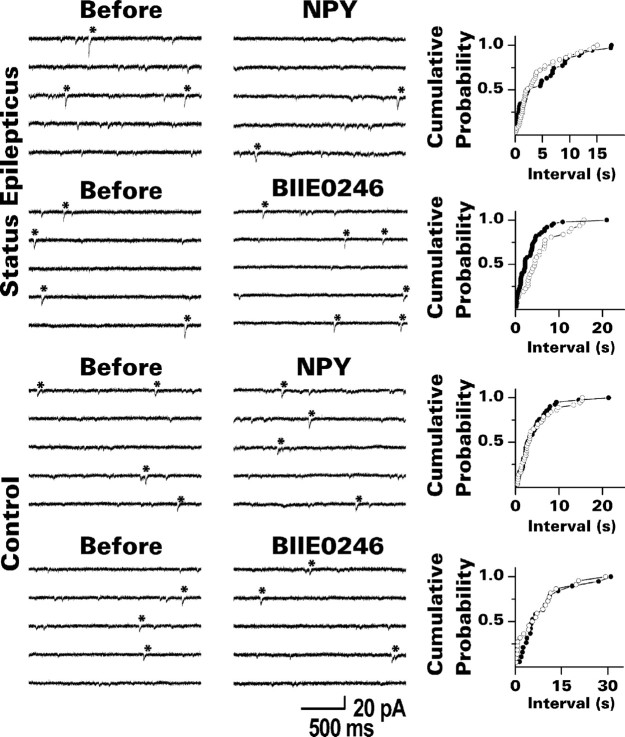
Spontaneous EPSCs were recorded from dentate granule cells in the presence of bicuculline and TTX at a holding potential of -80 mV. One would expect spontaneous glutamate release from recurrent mossy fiber synapses to account for many of the mEPSCs recorded in granule cells from the status epilepticus group, because these synapses are located close (≤130 μm) to the soma (Molnár and Nadler, 1999). In addition, the conservative criteria used to identify mEPSCs biased the analysis in favor of events that originated from the proximal portion of the dendrites. NPY reduced the frequency of mEPSCs identified in this manner by an average of 17% (p < 0.01; paired t test) (Fig. 7, Table 2). BIIE0246 applied by itself had the opposite effect; mEPSC frequency increased by an average of 27% (p < 0.01; paired t test). Analysis of interevent intervals (Fig. 7) supported the finding of more frequent mEPSCs in the presence of BIIE0246 (p < 0.01; Kolmogorov-Smirnov test). Exposure to NPY revealed a trend toward longer interevent intervals. This effect did not reach statistical significance, however, probably because most recurrent mossy fiber mEPSCs were already suppressed by endogenously released NPY. Neither compound changed the mean peak amplitude or response kinetics of mEPSCs, whether mean values (Table 2) or cumulative probability plots (data not shown) were analyzed. This result is again consistent with a presynaptic action of these compounds. In addition, neither NPY nor BIIE0246 altered mEPSC frequency in granule cells from control rats (Fig. 7, Table 2).
Figure 7.
NPY (1 μm) reduced and BIIE0246 (100 nm) increased the frequency of mEPSCs in granule cells from the status epilepticus group but not in granule cells from control rats. The traces shown are from representative experiments. Individual mEPSCs that met our criterion for analysis are indicated by asterisks. To the right of each set of traces is a cumulative probability plot for the time interval between identified mEPSCs. ○, Just before exposure to the test compound; •, during exposure to the test compound. Note that BIIE0246 shifted the plot to the left in the experiment from the status epilepticus group, consistent with a higher mEPSC frequency. NPY produced only a small change in the opposite direction. Neither compound affected the interevent interval in slices from control rats. Grouped data from these experiments are presented in Table 2.
Table 2.
NPY reduced and BIIE0246 increased only the frequency of mEPSCs recorded from dentate granule cells and only in granule cells from the status epilepticus group
|
Group |
|
Frequency (Hz) |
Amplitude (pA) |
10-90% rise time (ms) |
τ (ms) |
Charge transfer (fC) |
|---|---|---|---|---|---|---|
| Status epilepticus | Before NPY | 0.29 ± 0.07 | 12.4 ± 0.8 | 2.9 ± 0.3 | 24.5 ± 1.5 | 163 ± 12 |
| Plus NPY | 0.24 ± 0.07* | 12.3 ± 1.0 | 3.2 ± 0.3 | 26.6 ± 2.5 | 183 ± 16 | |
| Before BIIE0246 | 0.33 ± 0.08 | 11.6 ± 0.3 | 3.6 ± 0.3 | 22.0 ± 2.0 | 142 ± 10 | |
| Plus BIIE0246 | 0.42 ± 0.09* | 11.3 ± 0.3 | 3.9 ± 0.2 | 21.7 ± 1.7 | 148 ± 11 | |
| Control | Before NPY | 0.33 ± 0.14 | 10.6 ± 0.3 | 4.7 ± 0.6 | 27.0 ± 2.2 | 178 ± 9 |
| Plus NPY | 0.34 ± 0.15 | 10.7 ± 0.3 | 4.5 ± 0.6 | 26.7 ± 1.9 | 181 ± 11 | |
| Before BIIE0246 | 0.30 ± 0.08 | 11.1 ± 0.3 | 4.5 ± 0.4 | 28.1 ± 1.7 | 178 ± 13 | |
|
|
Plus BIIE0246 |
0.32 ± 0.07 |
11.1 ± 0.3 |
4.7 ± 0.3 |
29.7 ± 3.3 |
189 ± 21 |
The number of mEPSCs recorded during a 3 min period and the properties of averaged mEPSCs were determined before and during exposure to the test compound. Values are means ± SEM for eight granule cells per group. *p < 0.01 compared with values obtained just before exposure to the test compound (paired t test). All other comparisons between test compound and pretreatment values yielded p » 0.05.
NPY and BIIE0246 had opposite effects on granule cell epileptiform activity
Whole-cell patch-clamp recordings suggested that both endogenously released and applied NPY inhibited glutamate release from recurrent mossy fiber terminals. Because recurrent mossy fibers interconnect dentate granule cells and could potentially synchronize their firing, we investigated the effects of NPY and BIIE0246 on granule cell epileptiform activity in hippocampal slices from the status epilepticus group. These slices were superfused at 35°C with medium that contained 30 μm bicuculline and 6 mm [K+]o. Under these conditions, mossy fiber stimulation evokes granule cell epileptiform activity that depends on the formation of recurrent mossy fiber connections (Patrylo and Dudek, 1998; Hardison et al., 2000; Okazaki and Nadler, 2001).
Within 70 min of superfusion with bicuculline/6 K+ medium, stimulation of the mossy fibers evoked in the granule cell body layer a biphasic (negative-positive) slow wave on which multiple population spikes were superimposed (Fig. 8). In some experiments, delayed bursts were also observed. The magnitude of granule cell epileptiform activity remained relatively stable during the next 24.5 min, if the superfusion medium was not changed (Fig. 8E,F). Epileptiform activity was attenuated strongly by the addition of NPY (1 μm) to the medium, consistent with its inhibition of recurrent mossy fiber synaptic transmission. NPY reduced the magnitude of granule cell epileptiform activity by an average of 69% (p < 0.025; paired t test) (Fig. 8A,F). In five of the seven slices, delayed bursts were present before the addition of NPY. NPY suppressed the bursts completely in four instances and reduced their magnitude dramatically in the other. This result suggests that the delayed bursts resulted from polysynaptic activation of recurrent mossy fibers. BIIE0246 (300-500 nm) enhanced epileptiform activity by an average of 57% when applied by itself (p < 0.05; paired t test) (Fig. 8B,F). It also blocked the action of NPY completely, whether applied after the effect of NPY had developed fully (Fig. 8C) or concomitantly with NPY (Fig. 8D,F). In one experiment, the addition of BIIE0246 to the superfusion medium caused the appearance of delayed bursts, although NPY was also present (Fig. 8C). Thus, the effects of NPY and BIIE0246 on mossy fiber-evoked granule cell epileptiform activity closely resembled their effects on synaptic activity in the recurrent mossy fiber pathway.
Figure 8.
NPY (1 μm) reduced and BIIE0246 (300-500 nm) increased the magnitude of mossy fiber-evoked granule cell epileptiform activity. A-E, Traces from representative experiments. The top response in each pair was recorded after superfusion for 65.5-70 min with medium that contained 30 μm bicuculline and 6 mm K+. The lower response was recorded 20-24.5 min after the addition of the test compound(s) to the superfusion medium. The asterisk indicates the stimulus artifact. A, NPY reduced the magnitude of evoked short-latency epileptiform activity and the amplitude of the underlying negative wave. It also abolished the delayed burst. B, BIIE0246 increased the magnitude of evoked short-latency epileptiform activity and the amplitude of the underlying negative wave. C, The addition of BIIE0246 to the superfusion medium not only reversed the effect of previously applied NPY but also enhanced evoked short-latency epileptiform activity compared with pretreatment recordings and caused the appearance of delayed bursts. D, BIIE0246 increased the magnitude of evoked short-latency epileptiform activity despite coapplication of NPY. E, Simply continuing superfusion with bicuculline/6 K+ medium for 20-24.5 min did not consistently change either the evoked short-latency epileptiform activity or the number and amplitude of any delayed bursts. F, Grouped data from seven (control and NPY), five [BIIE0246 (BIIE)], or eight (NPY plus BIIE0246 applied simultaneously) hippocampal slices. Control, Continued superfusion without the addition of test compound. Values are means ± SEM; *p < 0.05; **p < 0.025 by paired t test with respect to recordings made just before exposure to the test compound(s).
In 13 slices (32%), mossy fiber stimulation failed to evoke granule cell epileptiform activity during superfusion with bicuculline/6 K+ medium. In seven of these slices, stimulation of the perforant path evoked a short-latency negative wave on which multiple population spikes were superimposed (Fig. 9A). The negative wave indicated the presence of a current sink close to the granule cell body layer, consistent with disynaptic activation of recurrent mossy fibers. Delayed bursts were also observed in three instances. In contrast to mossy fiber-evoked bursts, short-latency bursts evoked by perforant path stimulation seldom stabilized within the time frame of our recordings. The number and size of superimposed population spikes grew continuously. NPY (1 μm) reduced the magnitude of the short-latency activity in these seven slices by 37 ± 6% (mean ± SEM; p < 0.025; paired t test) and abolished the delayed bursts. In six additional slices, NPY (3-36) (1 μm) reduced the magnitude of the short-latency activity by 23 ± 7% (mean ± SEM; p < 0.025; paired t test). In contrast, during the 20-24.5 min period when NPY would normally have been applied, the magnitude of the evoked burst grew by 47 ± 16% (mean ± SEM; n = 7) if the superfusion medium was not changed.
Figure 9.
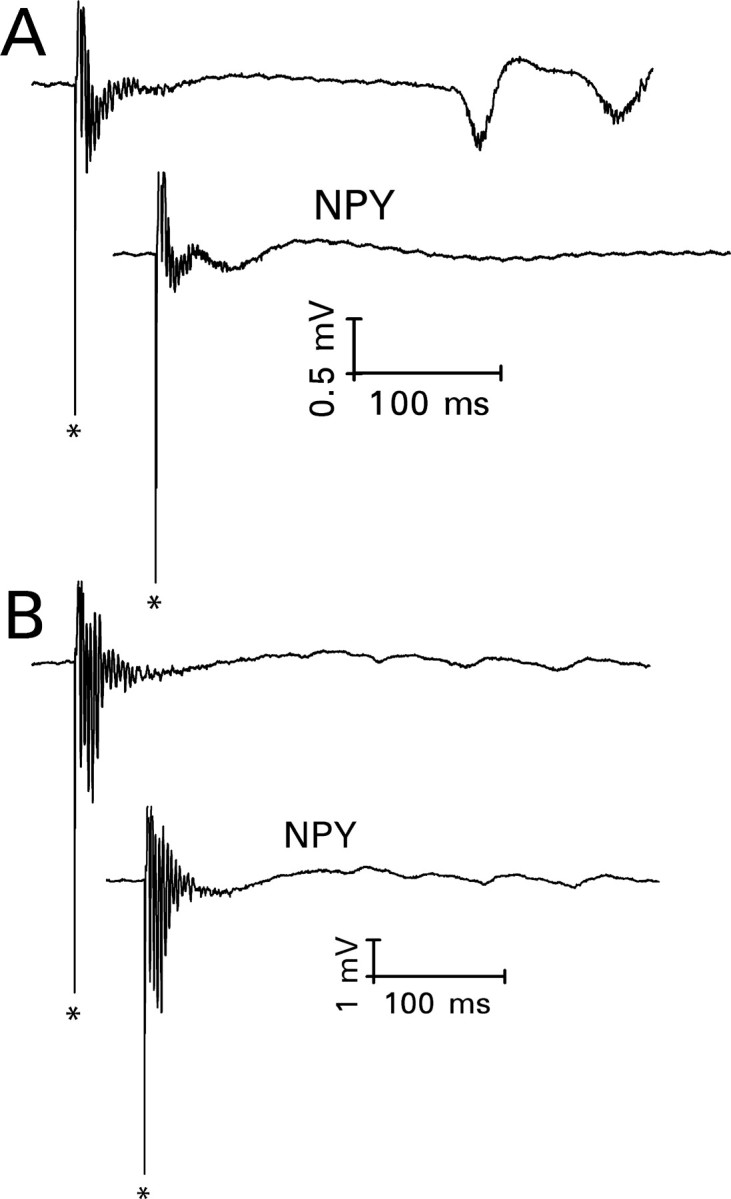
NPY (1 μm) reduced the magnitude of perforant path-evoked granule cell epileptiform activity only when there was evidence of a disynaptically activated recurrent mossy fiber component. The top response in each pair was recorded after superfusion for 65.5-70 min with medium that contained 30 μm bicuculline and 6 mm K+. The bottom response was recorded 20-24.5 min after the addition of NPY to the superfusion medium. A, Perforant path stimulation evoked a negatively directed wave on which multiple population spikes were superimposed, followed by delayed bursts. The negative wave suggests the activation of recurrent mossy fiber synapses, which are located close to the granule cell body layer. NPY reduced the magnitude of evoked short-latency epileptiform activity and the amplitude of the underlying negative wave. It also abolished the delayed bursts. B, In a hippocampal slice from a different rat, perforant path stimulation evoked a positively directed wave on which multiple population spikes were superimposed. Similar responses were obtained in hippocampal slices from untreated rats, which have only a rudimentary recurrent mossy fiber pathway. NPY had no consistent effect on responses of this type.
In the remaining six slices, perforant path stimulation evoked a response similar to that recorded in slices from control rats superfused with bicuculline/6 K+ medium: namely, multiple discrete population spikes superimposed on a positively directed wave (Fig. 9B). This “graded” activity also increased continuously; in 20-24.5 min, the coastline index increased by 24 ± 13% (mean ± SEM; n = 8) during superfusion with unmodified bicuculline/6 K+ medium. The coastline index increased by 17 ± 6% (mean ± SEM) during exposure to NPY for this period of time, just slightly less than in the absence of NPY. Similar results were obtained with NPY (3-36) in two additional slices. Thus, NPY little affected a type of hyperexcitable perforant path response that can be evoked in the absence of recurrent mossy fiber growth.
Discussion
Our results demonstrate that NPY inhibits transmission at recurrent mossy fiber synapses of epileptic brain and reduces their ability to synchronize granule cell discharge. NPY acts primarily through Y2 receptors and has no such effect at synapses on dentate granule cells made by other excitatory pathways. No postsynaptic effect was evident under the present recording conditions. Thus, NPY and Y2 receptor ligands may be used as tools to distinguish effects of recurrent mossy fiber sprouting from those of other changes that take place in this region during epileptogenesis. Importantly, the Y2 receptor antagonist BIIE0246 enhanced recurrent mossy fiber transmission, as well as granule cell epileptiform discharge evoked by mossy fiber stimulation, when applied by itself. BIIE0246 increased the amplitude of the compound NMDA receptor-mediated recurrent mossy fiber EPSC and the transmission rate of minimal electrically evoked EPSCs in the absence of a prepulse. It also increased the frequency of mEPSCs. These findings imply that even spontaneous release of NPY (or an active metabolite of NPY) from the recurrent mossy fiber pathway is sufficient to regulate synaptic transmission.
Selectivity and mechanism of NPY
NPY inhibited transmission at recurrent mossy fiber synapses but not at other excitatory synapses on dentate granule cells. This finding is consistent with a previous report that NPY inhibits transmission at mossy fiber synapses on CA3 pyramidal cells but not at perforant path synapses on dentate granule cells (Klapstein and Colmers, 1993). Thus, some glutamate inputs are sensitive to NPY, whereas others are not. However, NPY inhibited granule cell responses to perforant path stimulation in hippocampal slices from human tissue resected for medically intractable temporal lobe epilepsy (Patrylo et al., 1999a). It was possible that perforant path fibers express some NPY receptors in epileptic brain but not in normal brain. Our results suggest that this is not necessarily the case, at least in the rat. The depression of perforant path-evoked responses in human material may reflect a species difference in receptor expression. Alternatively, stimuli used in the human studies may have activated the recurrent mossy fiber pathway disynaptically, and it may have been this component of the response that was reduced by NPY. Our results support the latter hypothesis. Stimulation of the perforant path in most of the disinhibited hippocampal slices from the status epilepticus group evoked a negative wave on which multiple population spikes were superimposed (Patrylo et al., 1999b). Epileptiform responses of this type are observed only in the presence of recurrent mossy fiber growth, and they were attenuated by NPY. Conversely, the graded responses evoked by perforant path stimulation in other slices, which can also be evoked in slices from control rats, were unaffected. We therefore suggest that in the human studies, NPY reduced a polysynaptic component of the perforant path-evoked response that involved the activation of recurrent mossy fiber synapses.
Our results suggest that NPY inhibited recurrent mossy fiber synaptic transmission predominantly by activating presynaptic Y2 receptors. NPY (3-36) replicated its action, and BIIE0246 blocked it. Y2 receptors also mediate the NPY-induced inhibition of Schaffer collateral-commissural synaptic transmission onto CA1 pyramidal cells in the rat (Colmers et al., 1991; Greber et al., 1994; Weiser et al., 2000; El Bahh et al., 2002). However, Guo et al. (2002) reported that Y5 receptors mediate the NPY-induced inhibition of responses to mossy fiber stimulation in the mouse. This discrepancy may be explained by a difference in receptor expression/function between rat and mouse, between epileptic and normal brain, or between mossy fiber synapses on granule cells and pyramidal cells. It should be noted that our results do not exclude some participation of Y5 receptors in the action of NPY on recurrent mossy fiber synapses.
Previous studies indicated that NPY inhibits synaptic transmission by a presynaptic mechanism (Colmers et al., 1987, 1988; Klapstein and Colmers, 1993; Greber et al., 1994; McQuiston and Colmers, 1996; Qian et al., 1997; Sun et al., 2001). Our results are entirely consistent with this view. NPY reduced the transmission rate of minimal electrically evoked recurrent mossy fiber EPSCs and the frequency of mEPSCs in dentate granule cells without affecting response amplitude or kinetics significantly. It did not affect mEPSC frequency in granule cells from control rats, nor did it change membrane current or resistance in granule cells from either the status epilepticus or control groups. The mechanism by which NPY inhibits transmitter release appears to differ in different pathways. NPY depresses transmission at Schaffer collateral-commissural synapses on CA1 pyramidal cells, at least in part by inactivation of voltage-dependent Ca2+ channels (Qian et al., 1997). Similarly, the ability of NPY to reduce the frequency of spontaneous (-TTX), but not of miniature (+TTX), EPSCs in CA3 pyramidal cells (McQuiston and Colmers, 1996; Guo et al., 2002) is consistent with Ca2+ channel inactivation. Conversely, NPY reduces mEPSC frequency in neurons of the suprachiasmatic nucleus (van den Pol et al., 1996) and hypothalamus (Fu et al., 2004), as it did in dentate granule cells from the status epilepticus group. Evidently, NPY can inhibit either action potential-dependent or action potential-independent glutamate release. In recurrent mossy fibers, NPY appears to act on a process that is either downstream of or independent of Ca2+ influx. An additional action on Ca2+ channels is not excluded, however.
Tonic inhibition of recurrent mossy fiber transmission
Our conclusion that spontaneous release of endogenous NPY (or an active metabolite) inhibits recurrent mossy fiber transmission relies on the specificity of BIIE0246. Previous studies suggested that BIIE0246 is a highly specific antagonist. It had no action on NPY receptors other than Y2 nor on receptors for many other substances (Doods et al., 1999; Dumont et al., 2000). It blocked the inhibitory effect of NPY at Schaffer collateral-commissural synapses while having no effect, either presynaptic or postsynaptic, when applied by itself (El Bahh et al., 2002). There was no indication of inverse agonist activity. The present study is consistent with high specificity for BIIE0246 on the Y2 receptor. The action of this compound was selective for responses evoked by mossy fiber stimulation and for mEPSCs in granule cells from rats with a recurrent mossy fiber pathway. It did not change membrane current or resistance in the absence of mossy fiber stimulation, and no effects were observed in hippocampal slices from control rats. Thus, actions of BIIE0246 used alone reveal effects of endogenous NPY on Y2 receptors.
In neurons that normally release NPY, the peptide is released in significant amounts only during high-frequency activity (Kennedy et al., 1997; Sun et al., 2003). Our results suggest a different relationship between presynaptic activity and release of NPY in mossy fibers. In this location, spontaneous or tonic release appears sufficient to disrupt synaptic transmission. Recently, Fu et al. (2004) reported an inhibitory postsynaptic effect of spontaneous NPY release in the hypothalamus. These effects might occur through spontaneous vesicle fusion or a nonexocytotic leak of neuropeptide from terminals. The latter possibility is in accord with findings of McCarthy et al. (1998) on NPY expressed in mossy fibers after a pentylenetetrazole-induced seizure. They reported that NPY was present mainly in its oxidized form. Methionine sulfoxide NPY was released from mossy fiber synaptosomes by a Ca2+-independent process and bound to Y2 receptors with about one-sixth the affinity of native NPY. Thus, tonic nonexocytotic efflux of this metabolite may account, at least in part, for the depression of glutamate release we observed through feedback activation of presynaptic Y2 receptors. Additional studies are needed to test this hypothesis as well as to determine whether evoked release of native NPY enhances the inhibitory effect.
Implications for temporal lobe epilepsy
The ability of recurrent mossy fibers to synchronize granule cell discharge is limited by a low probability of glutamate release (Molnár and Nadler, 1999; Feng et al., 2003; Scharfman et al., 2003). Our results suggest that tonic release of NPY or an active metabolite explains this low release probability, at least in part. Blocking this type of presynaptic inhibition with BIIE0246 enhanced granule cell epileptiform activity driven by the recurrent mossy fiber pathway. Thus, de novo expression of NPY in the mossy fiber pathway may protect the hippocampus from seizures that originate in or involve the entorhinal cortex. Because NPY is expressed throughout the mossy fiber pathway, it seems likely that its release and/or that of its active metabolite would inhibit transmission at all mossy fiber synapses, including those on CA3 pyramidal cells and inhibitory interneurons. The net effect of these actions could be quite complex and requires further study. However, one would expect global depression of mossy fiber synapses to disrupt hippocampal function. With respect to the recurrent branch of the mossy fiber pathway, tonic feedback inhibition of glutamate release by NPY effectively restrains its activity under basal conditions. It is only when the pathway is driven at moderate frequencies (1-2 Hz) (Feng et al., 2003) and/or when [K+]o is increased modestly (Hardison et al., 2000) that synaptic facilitation overcomes this inhibitory influence and allows reverberating excitation to emerge.
The anticonvulsant/antiepileptogenic properties of NPY have drawn considerable interest (Vezzani et al., 1999b; Colmers and El Bahh, 2003; Vezzani and Sperk, 2004). Importantly, the same changes in NPY receptors observed in rat models of epilepsy have been reported in hippocampi surgically resected for medically intractable temporal lobe epilepsy (Furtinger et al., 2001). Immunohistochemical studies have not detected in human material the robust expression of mossy fiber NPY reported in animal models. However, there is an increased density of NPY-immunoreactive fibers in the terminal lamina of the recurrent mossy fiber projection (de Lanerolle et al., 1989; Mathern et al., 1995; Furtinger et al., 2001). It has not been determined whether this finding signifies expression of NPY by some recurrent mossy fibers or the growth of NPY-immunoreactive interneuron processes. In either case, the recurrent mossy fiber pathway would be expected to synchronize granule cell discharge more effectively in epileptic human brain than in the rat, because of its lower expression of NPY.
Footnotes
This work was supported by National Institutes of Health Grants NS 17771 and NS 38108. We thank K. Gorham for secretarial assistance.
Correspondence should be addressed to Dr. J. Victor Nadler, Department of Pharmacology and Cancer Biology, Box 3813, Duke University Medical Center, Durham, NC 27710. E-mail: nadle002@acpub.duke.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251718-12$15.00/0
References
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF (1991) Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience 42: 351-363. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco J, Kriegstein AR (1989) Whole-cell recording from neurons in slices of reptilian and mammalian cortex. J Neurosci Methods 30: 203-210. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE (1997) Network properties of the dentate gyrus in epileptic rats with hilar neuron loss and granule cell axon reorganization. J Neurophysiol 77: 2685-2696. [DOI] [PubMed] [Google Scholar]
- Collins RC, Tearse RG, Lothman EW (1983) Functional anatomy of limbic seizures: focal discharges from medial entorhinal cortex in rats. Brain Res 280: 25-40. [DOI] [PubMed] [Google Scholar]
- Colmers WF, El Bahh B (2003) Neuropeptide Y and epilepsy. Epilepsy Curr 3: 53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ (1987) Presynaptic action of neuropeptide Y in area CA1 of the rat hippocampal slice. J Physiol (Lond) 383: 285-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Lukowiak K, Pittman QJ (1988) Neuropeptide Y action in the rat hippocampal slice: site and mechanism of presynaptic action. J Neurosci 8: 3827-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmers WF, Klapstein GJ, Fournier A, St-Pierre S, Treherne KA (1991) Presynaptic inhibition by neuropeptide Y in rat hippocampal slice in vitro is mediated by a Y2 receptor. Br J Pharmacol 102: 41-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J, Obenaus A, Houser CR, Dudek FE (1992) Electrophysiology of dentate granule cells after kainate-induced synaptic reorganization of the mossy fibers. Brain Res 573: 305-310. [DOI] [PubMed] [Google Scholar]
- Danscher G (1981) Histochemical demonstration of heavy metals. A revised version of the sulphide silver method suitable for both light and electron microscopy. Histochemistry 71: 1-16. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD (1989) Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res 495: 387-395. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Hynes MA, King GL (1986) Involvement of N-methyl-d-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol (Lond) 380: 175-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doods H, Gaida W, Wieland HA, Dollinger H, Schnorrenberg G, Esser F, Engel W, Eberlein W, Rudolf K (1999) BIIE0246: a selective and high affinity neuropeptide Y Y2 receptor antagonist. Eur J Pharmacol 384: R3-R5. [DOI] [PubMed] [Google Scholar]
- Doods HN, Wieland HA, Engel W, Eberlein W, Willim KD, Entzeroth M, Wienen W, Rudolf K (1996) BIBP 3226, the first selective neuropeptide Y1 receptor antagonist: a review of its pharmacological properties. Regul Pept 65: 71-77. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Cadieux A, Doods H, Pheng LH, Abounader R, Hamel E, Jacques D, Regoli D, Quirion R (2000) BIIE0246, a potent and highly selective non-peptide neuropeptide Y Y2 receptor antagonist. Br J Pharmacol 129: 1075-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Bahh B, Cao JQ, Beck-Sickinger AG, Colmers WF (2002) Blockade of neuropeptide Y2 receptors and suppression of NPY's anti-epileptic actions in the rat hippocampal slice by BIIE0246. Br J Pharmacol 136: 502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Molnár P, Nadler JV (2003) Short-term frequency-dependent plasticity at recurrent mossy fiber synapses of the epileptic brain. J Neurosci 23: 5381-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck JE, Pokorny J, Kunkel DD, Schwartzkroin PA (1995) Physiologic and morphologic characteristics of granule cell circuitry in human epileptic hippocampus. Epilepsia 36: 543-558. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G (1998) Interneurons of the hippocampus. Hippocampus 6: 347-470. [DOI] [PubMed] [Google Scholar]
- Fu L-Y, Acuna-Goycolea C, van den Pol AN (2004) Neuropeptide Y inhibits hypocretin/orexin neurons by multiple presynaptic and postsynaptic mechanisms: tonic depression of the hypothalamic arousal system. J Neurosci 24: 8741-8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G (2001) Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci 21: 5804-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi M, Gariboldi M, Piwko C, Hoyer D, Sperk G, Vezzani A (1998) Distinct changes in peptide YY binding to, and mRNA levels of, Y1 and Y2 receptors in the rat hippocampus associated with kindling epileptogenesis. J Neurochem 70: 1615-1622. [DOI] [PubMed] [Google Scholar]
- Gorter JA, van Vliet EA, Aronica E, Lopes da Silva FH (2001) Progression of spontaneous seizures after status epilepticus is associated with mossy fibre sprouting and extensive bilateral loss of hilar parvalbumin and somatostatin-immunoreactive neurons. Eur J Neurosci 13: 657-669. [DOI] [PubMed] [Google Scholar]
- Grandt D, Schimiczek M, Rascher W, Feth F, Shiveley J, Lee TD, Davis MT, Reeve JR, Michel MC (1996) Neuropeptide Y 3-36 is an endogenous ligand selective for Y2 receptors. Regul Pept 67: 33-37. [DOI] [PubMed] [Google Scholar]
- Greber S, Schwarzer C, Sperk G (1994) Neuropeptide Y inhibits potassium-stimulated glutamate release through Y2 receptors in rat hippocampal slices in vitro. Br J Pharmacol 113: 737-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Castro PA, Palmiter RD, Baraban SC (2002) Y5 receptors mediate neuropeptide Y actions at excitatory synapses in area CA3 of the mouse hippocampus. J Neurophysiol 87: 558-566. [DOI] [PubMed] [Google Scholar]
- Hardison JL, Okazaki MM, Nadler JV (2000) Modest increase in extracellular potassium unmasks effect of recurrent mossy fiber growth. J Neurophysiol 84: 2380-2389. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z-Q, Landry M, Bao L, Schalling M, Koistinaho J, DeArmond SJ, Pruisiner S, Gong J, Walsh JH (1998) Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev 26: 154-166. [DOI] [PubMed] [Google Scholar]
- Houser CR (1992) Morphological changes in the dentate gyrus in human temporal lobe epilepsy. Epilepsy Res [Suppl] 7: 223-234. [PubMed] [Google Scholar]
- Kennedy B, Shen GH, Ziegler MG (1997) Neuropeptide Y-mediated pressor responses following high-frequency stimulation of the rat sympathetic nervous system. J Pharmacol Exp Ther 281: 291-296. [PubMed] [Google Scholar]
- Klapstein GJ, Colmers WF (1993) On the sites of presynaptic inhibition by neuropeptide Y in rat hippocampus in vitro Hippocampus 3: 103-112. [DOI] [PubMed] [Google Scholar]
- Klapstein GJ, Colmers WF (1997) Neuropeptide Y suppresses epileptiform activity in rat hippocampus in vitro. J Neurophysiol 78: 1651-1661. [DOI] [PubMed] [Google Scholar]
- Lemos T, Cavalheiro EA (1996) Status epilepticus and the late development of spontaneous seizures in the pilocarpine model of epilepsy. Epilepsy Res [Suppl] 12: 137-144. [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH (1992) The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res [Suppl] 7: 301-313. [PubMed] [Google Scholar]
- Lurton D, Cavalheiro EA (1997) Neuropeptide-Y immunoreactivity in the pilocarpine model of temporal lobe epilepsy. Exp Brain Res 116: 186-190. [DOI] [PubMed] [Google Scholar]
- Makiura Y, Suzuki F, Chevalier E, Onténiente B (1999) Excitatory granule cells of the dentate gyrus exhibit a double inhibitory neurochemical content after intrahippocampal administration of kainate in adult mice. Exp Neurol 159: 73-83. [DOI] [PubMed] [Google Scholar]
- Masukawa LM, Uruno K, Sperling M, O'Connor MJ, Burdette LJ (1992) The functional relationship between antidromically evoked field responses of the dentate gyrus and mossy fiber reorganization in temporal lobe epileptic patients. Brain Res 579: 119-127. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Pretorius JK, Leite JP (1995) Reactive synaptogenesis and neuron densities for neuropeptide Y, somatostatin, and glutamate decarboxylase immunoreactivity in the epileptogenic human fascia dentata. J Neurosci 15: 3990-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JB, Walker M, Pierce J, Camp P, White JD (1998) Biosynthesis and metabolism of native and oxidized neuropeptide Y in the hippocampal mossy fiber system. J Neurochem 70: 1950-1963. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Colmers WF (1996) Neuropeptide Y2 receptors inhibit the frequency of spontaneous but not miniature EPSCs in CA3 pyramidal cells of rat hippocampus. J Neurophysiol 76: 3159-3168. [DOI] [PubMed] [Google Scholar]
- Mello LEAM, Cavalheiro EA, Tan AM, Kupfer WR, Pretorius JK, Babb TL, Finch DM (1993) Circuit mechanisms of seizures in the pilocarpine model of chronic epilepsy: cell loss and mossy fiber sprouting. Epilepsia 34: 985-995. [DOI] [PubMed] [Google Scholar]
- Molnár P, Nadler JV (1999) Mossy fiber-granule cell synapses in the normal and epileptic rat dentate gyrus studied with minimal laser photostimulation. J Neurophysiol 82: 1883-1894. [DOI] [PubMed] [Google Scholar]
- Nadler JV (2003) The recurrent mossy fiber pathway of the epileptic brain. Neurochem Res 28: 1649-1658. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Jiao Y (2004) Mossy fiber sprouting correlates with loss of GluR2-immunoreactive hilar neurons. Soc Neurosci Abstr 30: 566.21. [Google Scholar]
- Okazaki MM, Nadler JV (2001) Glutamate receptor involvement in dentate granule cell epileptiform activity evoked by mossy fiber stimulation. Brain Res 915: 58-69. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Evenson DA, Nadler JV (1995) Hippocampal mossy fiber sprouting and synapse formation after status epilepticus in rats: visualization after retrograde transport of biocytin. J Comp Neurol 352: 515-534. [DOI] [PubMed] [Google Scholar]
- Okazaki MM, Molnár P, Nadler JV (1999) Recurrent mossy fiber pathway in rat dentate gyrus: synaptic currents evoked in presence and absence of seizure-induced growth. J Neurophysiol 81: 1645-1660. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Dudek FE (1998) Physiological unmasking of new glutamatergic pathways in the dentate gyrus of hippocampal slices from kainate-induced epileptic rats. J Neurophysiol 79: 418-429. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, van den Pol AN, Spencer DD, Williamson A (1999a) NPY inhibits glutamatergic excitation in the epileptic human dentate gyrus. J Neurophysiol 82: 478-483. [DOI] [PubMed] [Google Scholar]
- Patrylo PR, Schweitzer JS, Dudek FE (1999b) Abnormal responses to perforant path stimulation in the dentate gyrus of slices from rats with kainate-induced epilepsy and mossy fiber reorganization. Epilepsy Res 36: 31-42. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, Ed 2. Orlando, FL: Academic.
- Qian J, Colmers WF, Saggau P (1997) Inhibition of synaptic transmission by neuropeptide Y in rat hippocampal area CA1: modulation of presynaptic Ca2+ entry. J Neurosci 17: 8169-8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32: 281-284. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Dumont Y, St-Pierre J-A, Quirion R (1999) Multiple receptors for neuropeptide Y in the hippocampus: putative roles in seizures and cognition. Brain Res 848: 153-166. [DOI] [PubMed] [Google Scholar]
- Represa A, Robain O, Tremblay E, Ben-Ari Y (1989) Hippocampal plasticity in childhood epilepsy. Neurosci Lett 99: 351-355. [DOI] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA (1996) Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci USA 93: 13304-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL (2000) Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci 20: 6144-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Berger RE, Goodman JH (2003) Electrophysiological evidence of monosynaptic excitatory transmission between granule cells after seizure-induced mossy fiber sprouting. J Neurophysiol 90: 2536-2547. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Williamson JM, Lothman EW, Vezzani A, Sperk G (1995) Somatostatin, neuropeptide Y, neurokinin B and cholecystokinin immunoreactivity in two chronic models of temporal lobe epilepsy. Neuroscience 69: 831-845. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Kofler N, Sperk G (1998) Up-regulation of neuropeptide Y-Y2 receptors in an animal model of temporal lobe epilepsy. Mol Pharmacol 53: 6-13. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N (1997) Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci 17: 7503-7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M (2003) “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol 459: 44-76. [DOI] [PubMed] [Google Scholar]
- Stringer JL, Williamson JM, Lothman EW (1989) Induction of paroxysmal discharges in the dentate gyrus: frequency dependence and relationship to afterdischarge production. J Neurophysiol 62: 126-135. [DOI] [PubMed] [Google Scholar]
- Sun Q-Q, Akk G, Huguenard JR, Prince DA (2001) Differential regulation of GABA release and neuronal excitability mediated by neuropeptide Y1 and Y2 receptors in rat thalamic neurons. J Physiol (Lond) 531: 81-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q-Q, Baraban SC, Prince DA, Huguenard JR (2003) Target-specific neuropeptide Y-ergic synaptic inhibition and its network consequences within the mammalian thalamus. J Neurosci 23: 9639-9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L (1989) Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol 26: 321-330. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV (1985) Evidence of functional mossy fiber sprouting in the hippocampal formation of kainic acid-treated rats. J Neurosci 5: 1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Obrietan K, Chen G, Belousov AB (1996) Neuropeptide Y-mediated long-term depression of excitatory activity in suprachiasmatic nucleus neurons. J Neurosci 16: 5883-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Sperk G (2004) Overexpression of NPY and Y2 receptors in epileptic brain tissue: an endogenous neuroprotective mechanism in temporal lobe epilepsy? Neuropeptides 38: 245-252. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Ravizza T, Moneta D, Conti M, Borroni A, Rizzi M, Samanin R, Maj R (1999a) Brain-derived neurotrophic factor immunoreactivity in the limbic system of rats after acute seizures and during spontaneous convulsions: temporal evolution of changes as compared to neuropeptide Y. Neuroscience 90: 1445-1461. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Sperk G, Colmers WF (1999b) Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci 22: 25-30. [DOI] [PubMed] [Google Scholar]
- Weiser T, Wieland HA, Doods HN (2000) Effects of the neuropeptide Y Y2 receptor antagonist BIIE0246 on presynaptic inhibition by neuropeptide Y in rat hippocampal slices. Eur J Pharmacol 404: 133-136. [DOI] [PubMed] [Google Scholar]
- Wuarin J-P, Dudek FE (1996) Electrographic seizures and new recurrent excitatory circuits in the dentate gyrus of hippocampal slices from kainate-treated epileptic rat. J Neurosci 16: 4438-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cui S-S, Wallace AE, Hannesson DK, Schmued LC, Saucier DM, Honer WG, Corcoran ME (2002) Relations between brain pathology and temporal lobe epilepsy. J Neurosci 22: 6052-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]