Abstract
Accumulating evidence suggests that amyloid protein aggregation is pathogenic in many diseases, including Alzheimer's disease. However, the mechanisms by which protein aggregation mediates cellular dysfunction and overt cell death are unknown. Recent reports have focused on the potential role of amyloid oligomers or protofibrils as a neurotoxic form of amyloid-β (Aβ) and related amyloid aggregates. Here we describe studies indicating that overt neuronal cell death mediated by Aβ1-40 is critically dependent on ongoing Aβ1-40 polymerization and is not mediated by a single stable species of neurotoxic aggregate. The extent and rate of neuronal cell death can be controlled by conditions that alter the rate of Aβ polymerization. The results presented here indicate that protofibrils and oligomeric forms of Aβ most likely generate neuronal cell death through a nucleation-dependent process rather than acting as direct neurotoxic ligands. These findings bring into question the use of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide formazan assay (MTT assay) as a reporter of Aβ-mediated neuronal cell death and suggest that diffusible Aβ protofibrils and oligomers more likely mediate subtle alterations of synaptic function and long-term potentiation rather than overt neuronal cell death. These results have been extended to Aβ1-42, the non-Aβ component of Alzheimer's disease amyloid plaques, and human amylin, suggesting that nucleation-dependent polymerization is a common mechanism of amyloid-mediated neuronal cell death. Our findings indicate that ongoing amyloid fibrillogenesis may be an essential mechanistic process underlying the pathogenesis associated with protein aggregation in amyloid disorders.
Keywords: Alzheimer's disease, amyloid-β, neurotoxicity, neuronal cell death, nucleation-dependent polymerization, amyloidogenic proteins
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by extracellular amyloid plaques, neurofibrillary tangles, neuronal dysfunction, and overt neuronal cell death. A major constituent of amyloid plaques is the 39-43 amino acid amyloid-β (Aβ) protein (Glenner and Wong, 1984), which is constitutively produced by normal cleavage of the membrane-bound amyloid-β precursor protein (Seubert et al., 1992). A variety of evidence suggests that Aβ accumulation is a pathogenic event in Alzheimer's disease. Amyloid-β-containing extracellular plaques are an early and invariant feature of the disease. All known familial AD mutations lead to increased production or cerebral deposition (Selkoe, 2001) or aggregate state (Nilsberth et al., 2001) of Aβ. Furthermore, the levels of soluble Aβ correlate closely with synaptic loss and markers of disease severity (Lue et al., 1999; McLean et al., 1999).
A variety of studies have demonstrated neurotoxicity associated with the treatment of neuronal cultures with aggregated Aβ (Yankner, 1996) and other amyloidogenic proteins (Caughey and Lansbury, 2003), supporting a pathogenic role for amyloid accumulation in various disease states. Amyloid-β neurotoxicity has been shown to correlate with the presence of fibrils or β-sheet structures (Simmons et al., 1994; Howlett et al., 1995; Seilheimer et al., 1997). However, the mechanisms by which Aβ aggregation mediates neuronal cell death are unknown.
If protein aggregation is a critical component of Aβ-mediated pathology, then manipulations that affect the aggregation state of Aβ should provide insight into these neurotoxic properties. Aβ aggregation has been shown to proceed by a multistep, nucleation-dependent process (Jarrett and Lansbury, 1993). Formation of the nucleation seed is rate limiting, so that, in the absence of preformed seed fibrils, there is a significant lag period for the formation of Aβ fibrils, followed by a rapid fibril elongation phase once seed fibrils have been generated. The lag time for fibril formation can be dramatically shortened by the addition of preformed fibril seeds to monomer solutions of Aβ (Jarrett and Lansbury, 1993). The rate of monomer incorporation into polymer increases with both increasing concentration of seed and increasing concentration of monomer (Naiki and Nakakuki, 1996). Therefore, the rate of Aβ fibril formation is controlled by both fibril seed concentration and monomer concentration.
The results presented here demonstrate that treatments with fibrillar Aβ or soluble Aβ alone are not toxic to primary human or rodent neurons. However, when fibrillar Aβ is used to seed the polymerization of soluble Aβ, neuronal cell death develops that is directly proportional to the extent of polymerization. It is the process of Aβ polymerization, rather than any neurotoxic oligomeric species, that correlates with neuronal cell death. Using this two-component system (fibrillar Aβ and soluble Aβ), we demonstrate precise control of both the initiation and termination of Aβ-mediated neuronal cell death. We reveal that lot-to-lot variability of Aβ neurotoxicity is attributable to variable amounts of fibrillar Aβ and soluble Aβ found in biochemically identical lots of Aβ1-40. These studies demonstrate that neuronal cell death is mediated by nucleation-dependent amyloid polymerization. These findings reveal a novel conceptual framework for studying the structure-activity relationships of amyloidogenic peptides, allow for the precise control of cellular response to Aβ and related peptides, and have implications for the therapeutic treatment of Alzheimer's disease and other amyloid disorders.
Materials and Methods
Atomic force microscopy. Samples of Aβ solutions (100-200 μl) were added to freshly cleaved mica and incubated for 5-10 min at room temperature. Samples were gently washed twice with 200 μl/wash of filtered, deionized water. All images were generated by atomic force microscopy (AFM) (Molecular Imaging, Tempe, AZ) operated in Magnetic AC Mode. Aβ samples were imaged under filtered, deionized water using tips with a spring constant of ∼0.5 N/m, with a resonance frequency generally between 20 and 50 kHz in water (depending on the particular tip or cantilever being used). An 18 × 18 μm area was typically scanned at three to five lines per second using 512 × 512 data points per line sampling. Images were collected using both height and deflection mode.
Transmission electron microscopy. Samples (10 μl) of Aβ solutions were adsorbed to 400 mesh Formvar carbon-coated copper grids (FCF400-Cu; Electron Microscopy Sciences, Fort Washington, PA). Aβ samples were left on for 1 min and then negatively stained for 1 min with 1% ammonium molybdate. The stain was wicked off, and the grids were air dried. Grids were examined on a Philips (Aachen, Germany) 400 electron microscope located at the University of California, San Francisco Electron Microscopy Facility in the Department of Biochemistry and Biophysics.
Freshly solubilized Aβ. Aβ1-40 lyophilized powder (catalog #641-10, lots MF-0641, MF-1041, MF-1141, and ME-0541; California Peptide Research, Napa, CA) was dissolved to 1 mm (1 mg of dry powder per 175 μl of water based on quantitative amino acid analysis) using glass vials, incubated at room temperature for 3 min, aliquoted into glass vials, and snap frozen on ethanol and dry ice.
Fibrillar Aβ or “seed” preparation. Aβ1-40 lyophilized powder (catalog #641-10, lots MF-0641, MF-1041, MF-1141, and ME-0541; California Peptide Research) was dissolved to 1 mm (1 mg of dry powder per 175 μl of water) using glass vials, aged for 3 d at 37°C, aliquoted into glass vials, and snap frozen on ethanol and dry ice. At least 90% of the total peptide present in these preparations was in the aggregated form, based on the observation that >90% of peptide was removed by a 100,000 × g spin (data not shown). Based on the morphology of aggregated Aβ, as determined using transmission electron microscopy (TEM) (see Fig. 1), aggregated Aβ is referred to as fibrillar Aβ in this paper. These fibrillar Aβ preparations are also likely to contain Aβ protofibrils, as described previously (Harper and Lansbury, 1997; Walsh et al., 1999). The relative proportions of soluble and fibrillar Aβ in different peptide preparations and treatments were determined by measuring the protein concentration of Aβ after centrifugation (using Aβ1-40 as a standard) and by measuring the characteristic 120 nm red shift of the excitation spectrum of the benzothiazole dye thioflavin-T (ThT) that occurs after ThT binding to fibrillar Aβ (LeVine, 1997).
Figure 1.

The presence of fibrillar Aβ is essential, but not sufficient, for Aβ-mediated neuronal cell death. A, Synthetic lots of Aβ1-40 (MF-0641, MF-1141, and ME-0541) were dissolved to a nominal 1 mm in water. They were then diluted to 30 μm in basal media (MEM) and either imaged by AFM or added at 100 μl/well (n = 3) to freshly aspirated human cortical neurons. Neuronal survival was determined using alamarBlue after 3 d of treatment. Note that lot ME-0541 has a significant number of fibrils present but was not toxic to the cells. B, Stocks (1 mm) of Aβ1-40 (lot MF-0641) were diluted to 30 μm in basal media and spun at room temperature for 20 min at 20,000 × g or for 1 h at 100,000 × g. The unspun sample was kept at room temperature for 1 h. Aliquots were imaged by AFM or added to primary human cortical neuron cultures. Neurons were incubated for 3 d at 37°C and then assayed for neuronal viability using alamarBlue. All treatments were in triplicate wells. Error bars indicate ± SD. supe, Supernatant. C, DMSO stock (7.5 mm) of Aβ1-40 (soluble Aβ) and 3d aged 1 mm water stock (fibrillar Aβ), both lot MF-0641, were diluted to 30 μm in basal media (MEM) and either imaged by AFM or TEM or added at 100 μl/well to primary human cortical neurons. All AFM images shown are deflection mode. Neuronal survival was determined using alamarBlue after 2 d of continuous treatment with either soluble Aβ or fibrillar Aβ. In additional experiments, continuous treatment with fibrillar Aβ for up to 7 d also did not affect neuronal survival (data not shown). Note that the 3 d aged stock was difficult to image by AFM. Some fields would not produce clean images, perhaps attributable to an excessive amount of aggregated material in those fields. Furthermore, large aggregates that were visible by inspection of the mica plate after adsorption were lost after washing of the AFM samples. The TEM image (in which excess liquid is wicked off) is likely to be more representative of the aggregate state of the samples compared with the AFM images (in which the sample is washed with water).
Soluble Aβ preparation. Disaggregated Aβ1-40 (catalog #641-10, lots MF-0641, MF-1041, MF-1141, and ME-0541; California Peptide Research) was prepared by dissolving lyophilized powder to 7.5 mm in dimethylsulfoxide (DMSO) (23.3 μl of DMSO per 1 mg of dry powder) using glass vials and sonicating for 30 min in a bath sonicator. After dilution in basal media, DMSO-disaggregated Aβ shows no fibrillar or other large aggregates when examined by AFM (see Fig. 1). Based on previous findings that DMSO dissolution of Aβ produces monomeric solutions containing variable amounts of Aβ dimers or other small oligomers (Garzon-Rodriguez et al., 1997; Harper et al., 1999; Tseng et al., 1999), DMSO-disaggregated Aβ is referred to as soluble Aβ in this paper. Stock solutions of soluble Aβ were snap frozen in small aliquots using glass vials and stored frozen at -30°C.
I125-Aβ preparation. I125-Aβ1-40 [2000 Ci/mmol, with lactose, no BSA present; catalog #IM294-25UCi (special order from Amersham Biosciences, Arlington Heights, IL)] was dissolved in DMSO at 100 μCi/ml, transferred to polypropylene tubes, and sonicated in a water-bath sonicator for 30 min. Aliquots of Aβ were snap frozen.
Unsheared and sheared Aβ preparation. Aβ1-40 lyophilized powder (catalog #641-10, lot MF-1141; California Peptide Research) was dissolved to 1 mm (1 mg of dry powder per 175 μl of water) and snap frozen on ethanol and dry ice. A frozen 1 mm stock was diluted to 30 μm in 10 mm 2-(N-cyclohexylamino)ethanesulfonic acid, pH 9.0, and 150 mm NaCl in a final volume of 1 ml. The 30 μm stock was incubated for 2 h in a 37°C circulating water bath to allow for fibril growth; this solution was “unsheared fibrils.” Sheared fibrils were produced by passing the solution 20 times through a 27 gauge needle. Shearing increased ThT signal ∼2.5-fold. By both ThT signal and filtration, ∼50% of total peptide was in the fibrillar form (data not shown).
Tissue culture. Human cortical cultures were established using dissociated human cerebral tissue at 13-16 weeks gestation. Cortical tissue was provide by Advanced Bioscience Resources (Alameda, CA), and the protocol for obtaining fetal brain tissue complied with federal guidelines for fetal research and with the Uniformed Anatomical Gift Act. Cortical tissue was washed twice in Ca2+/Mg2+-free HBSS (H-9394; Sigma, St. Louis, MO) and then dissociated by repeated pipetting in 10 ml of cold HBSS with 1 ml of DNase (D-4513; Sigma), and the solution was passed through a 100 μm nylon cell strainer (21008-950; VWR Scientific, West Chester, PA). The cells obtained were then centrifuged for 5 min at 200 × g, resuspended in a trypsin/EDTA solution (0.05% trypsin and 0.53 mm EDTA in HBSS; T-3924; Sigma), and incubated for 20 min at 37°C. After centrifugation, cells were resuspended in neuronal medium [MEM supplemented with B27 (17504-044; Invitrogen, Gaithersburg, MD), 1% glucose, 1 mm sodium pyruvate, and 1 mm glutamine] and plated onto polyethyleneimine-coated 96-well plates at a density of 125,000 cells per well. Cultures were maintained for 3 weeks before use, and culture media was replaced twice per week. By immunostaining, the cultures are >90% neurons (positive for neurofilament or microtubule-associated protein-2). The contaminating cells are glial fibrillary acidic protein positive (astrocytes) and complement receptor 3 (MAC-1) positive (microglia) (data not shown). These cells are likely present at the time of plating but do not appear to divide while in the defined medium.
Neuronal viability measurements. Neuronal viability was determined using several techniques: (1) cellular metabolic activity [determined using alamarBlue sodium 3′-[l-phenylaminocarbonyl-3,4-tetrazolium]-bis (4-methoxy-6-nitro) benzene sulfonic acid hydrate (XTT), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)]; (2) membrane integrity [determined (a) by measuring the release of cytosolic enzyme lactate dehydrogenase (LDH) into the culture supernatant and (b) by staining with a membrane-permeable dye (Syto-13; S-7575; Molecular Probes, Eugene, OR) and a membrane-impermeable dye (propidium iodide; 1-348-639; Boehringer Mannheim, Indianapolis, IN)]; and (3) neuronal morphology (determined using phase contrast microscopy). Neuronal viability as determined using alamarBlue (catalog #DAL1100; Biosource, Camarillo, CA), XTT (catalog #1465015; Roche, Indianapolis IN), and LDH release were performed as described previously (Estus et al., 1997). Syto-13 and propidium iodide were used according to the instructions of the manufacturer. The MTT assay was obtained from Sigma (catalog #TOX-1) and was used per the instructions of the manufacturer.
Aβ1-42, human amylin, and the non-Aβ component of Alzheimer's disease amyloid plaques preparation. Seed or aggregated preparations of Aβ1-42, human amylin, and the non-Aβ component of Alzheimer's disease amyloid plaques (NAC) were made by dissolving lyophilized powder at 250 μm in PBS (Aβ1-42), 1 mm in H20 (human amylin), or 1.3 mm in H20 (NAC) and aging for 3 d at 37°C. (All three peptides were synthesized by California Peptide Research: Aβ1-42, catalog #641-15, lot MF-0639; human amylin, catalog #641-50, lot NG-0213; and NAC, catalog #641-80, lot ME-1125.) All seed preparations were aliquoted and frozen. Because Aβ1-42 is not readily soluble in PBS, the cloudy suspension that resulted from adding PBS to Aβ1-42 was aged and then sonicated for 30 min before aliquoting and freezing. Soluble fractions were made by dissolving lyophilized powder in DMSO at 5 mm, sonicating for 30 min, and snap freezing aliquots. Aβ1-42 and human amylin solutions were diluted to 20 μm in media and then filtered through 30 kDa cutoff filters before treatment (filters were rinsed in 70% EtOH in H2O, aspirated, and dried before use; centriprep #4306; Amicon, Beverly, MA).
Results
Structural requirements for Aβ-mediated neuronal cell death
To understand the morphological features required for Aβ-mediated neurotoxicity, we used AFM to identify the aggregate forms of Aβ present in three different lots of Aβ1-40 that varied significantly in their neurotoxic properties when freshly solubilized at 1 mm in water and diluted to 30 μm in tissue culture medium (Fig. 1A). Although all three freshly solubilized peptide lots contained fibrillar Aβ of comparable morphology, only lots MF-0641 and MF-1141 were neurotoxic to primary human cortical neurons. Lot ME-0541, which clearly contained fibrillar Aβ of comparable size and morphology as lots MF-0641 and MF-1141, was not neurotoxic when freshly solubilized and added to primary human cortical neurons. Lot ME-0541 had a ThT signal that was fivefold higher than that of either lot MF-0641 or lot MF-1141, indicating that lot ME-0541 contained a greater proportion of aggregated or fibrillar Aβ (data not shown.) These studies demonstrate that, although fibrillar Aβ was present in neurotoxic lots of Aβ, it was not sufficient to generate neuronal cell death.
To determine whether the presence of fibrillar Aβ was required for mediating neuronal cell death, neurotoxic lot MF-0641 was freshly solubilized at 1 mm in water, diluted to 30 μm in tissue culture medium, and then centrifuged at 20,000 × g for 20 min or 100,000 × g for 60 min before addition of the supernatant to neuronal cultures. Centrifugation at 20,000 × g did not remove the shorter Aβ fibrils, nor did it diminish the neuronal cell death associated with lot MF-0641 (Fig. 1B). However, centrifugation at 100,000 × g removed all fibrillar Aβ and associated neuronal cell death. Although centrifugation at 100,000 × g did remove fibrillar Aβ as measured by AFM (Fig. 1B), it did not have a measurable affect on the protein concentration of lot MF-0641 before or after centrifugation (data not shown). These findings suggest that neurotoxic lot MF-0641 contained very small quantities of fibrillar Aβ. In fact, the ThT signal of neurotoxic lot MF-0641 shown in Figure 1A was comparable with the ThT signal obtained with DMSO-disaggregated lot MF-0641 shown in Figure 1C. In contrast, the ThT signal of neurotoxic lot MF-0641 increased 20-fold after aging for 3 d at 37°C (Fig. 1C and data not shown). This study clearly demonstrates that small quantities of fibrillar Aβ are essential, but not sufficient, for Aβ-mediated neuronal cell death.
Similar results were obtained when neurotoxic lot MF-0641 was prepared as a DMSO stock solution before dilution into tissue culture medium. DMSO-solubilized lot MF-0641 did not contain fibrillar Aβ as demonstrated by AFM and was not toxic to cortical neurons (Fig. 1C). Based on the lack of fibrillar Aβ in DMSO-solubilized Aβ1-40 and a similar appearance of DMSO-solubilized Aβ to preparations after centrifugation at 100,000 × g for 60 min, we will operationally refer to these preparations as “soluble Aβ.” Previous publications have demonstrated that similar preparations of Aβ1-40 contain monomer Aβ as well as Aβ dimers and other low n-mer aggregates (Garzon-Rodriguez et al., 1997; Harper et al., 1999; Tseng et al., 1999). In addition to the lack of neuronal cell death associated with the removal of fibrillar Aβ from peptide solutions, we found that neurotoxic activity was also abolished if peptide stocks were allowed to aggregate for extended periods of time (overaging). Aβ lot MF-0641 was toxic when freshly prepared at 1 mm in water and then diluted into tissue culture medium (Fig. 1A). However, its neurotoxic properties were abolished when 1 mm stocks were allowed to aggregate for an additional 3 d at 37°C (Fig. 1C). Thus, both centrifugation experiments and solubilization/aging experiments demonstrate the lack of neuronal cell death associated with soluble Aβ alone or fibrillar Aβ alone.
Nucleation-dependent Aβ polymerization is an essential component of Aβ-mediated neuronal cell death
Previous studies using an all-D-enantiomer of Aβ suggested that the neurotoxic actions of Aβ are not attributable to a stereoisomer-specific ligand-receptor interaction but are more likely attributable to amyloid-mediated cellular interactions that are critically dependent on fibril formation (Cribbs et al., 1997). The lack of neuronal cell death found after neuronal cultures were treated with either fibrillar Aβ alone or soluble Aβ alone further indicated that Aβ-mediated neuronal cell death might be a dynamic process associated with protein aggregation rather than a more conventional ligand-receptor interaction. Because Aβ fibril elongation is a nucleation-dependent polymerization process, we sought to determine whether nucleation-dependent polymerization of Aβ fibrils along the neuronal plasma membrane would elicit Aβ-mediated neurotoxicity. To direct Aβ polymerization to the neuronal cell surface, fibrillar Aβ1-40 was incubated in cultures of primary human cortical neurons for 1 h, and then the culture wells were washed to remove unbound fibrils. Whereas treatment with 1 μm fibrillar Aβ for up to 7 d was not toxic to human cortical neurons, the addition of 20 μm soluble Aβ to cultures previously treated with fibrillar Aβ resulted in robust neuronal cell death after 2-3 d (Fig. 2). Interestingly, neuronal cultures pretreated for 1 h with fibrillar Aβ and then maintained for 7 d in culture demonstrated robust neuronal cell death 2-3 d after the addition of soluble Aβ, suggesting that fibrillar Aβ forms stable interactions with the neuronal cell membrane (data not shown). The fibrillar Aβ used in these studies were prepared from solutions containing highly aggregated, short, bundled fibrils, as determined by TEM (Fig. 1C). Similar results have been obtained using fibrillar Aβ and soluble Aβ prepared from a number of different lots of Aβ1-40. In addition, we found that freshly solubilized lots of Aβ1-40 that contain fibrils, as determined by AFM, can also be used as a source of fibrillar Aβ (data not shown). Although we have not extensively characterized the biochemical properties of the fibrillar Aβ used in these studies, its appearance by AFM and TEM (fibril lengths >1 μm and height/width of 7-10 nm) is similar to the structural properties reported previously for Aβ fibrils (Walsh et al., 1997).
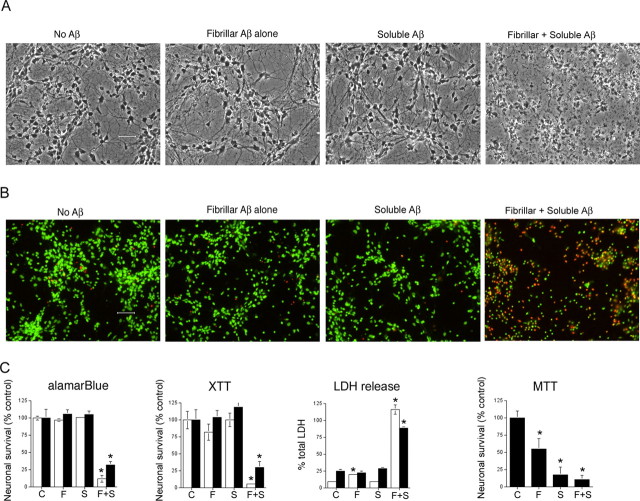
Figure 2.
The presence of both soluble Aβ and fibrillar Aβ are required for Aβ-mediated neuronal cell death. Cultures of human cortical neurons were treated with media alone or media containing 1 μm fibrillar Aβ. After 1 h, culture medium was aspirated and replaced with media alone or media containing 20 μm soluble Aβ. In total, four treatment conditions were used in this study: pretreatment with media alone followed by media-alone treatment, which was used as the control condition (“C”); pretreatment with fibrillar Aβ, followed by media-alone treatment (“F”); pretreatment with media alone, followed by soluble Aβ treatment (“S”); and pretreatment with fibrillar Aβ, followed by soluble Aβ treatment (“F + S”). Primary neuronal cultures were incubated for 3 d and then assayed for neuronal viability by phase contrast microscopy (A), live cell (green) and dead cell (red) staining (B), and by measuring cellular redox activity using alamarBlue, the tetrazolium redox dye XTT that forms a soluble formazan product, and the tetrazolium redox dye MTT that forms an insoluble formazan product (C). C, Third panel, The integrity of the neuronal cell membrane was quantitatively determined by measuring the release of the cytoplasmic enzyme LDH into the culture medium. Total LDH in cells was determined by lysing cells with 1% Triton X-100. This value was used to calculate percentage of total LDH released. The white and black bars represent data obtained using independent culture preparations. The modest effect of fibrillar Aβ on LDH release obtained in one experiment (white bars), although significant, was not repeated in additional studies using fibrillar Aβ. Error bars indicate ± SD. *p < 0.001 versus control cultures. Statistical analysis was based on the means ± SD (n = 6) using ANOVA, followed by Fisher's PLSD test using StatView software (version 5.0; SAS Institute, Cary, NC).
The neuronal cell death associated with nucleation-dependent Aβ polymerization was confirmed using methods that assess both the metabolic integrity of the neuronal cultures and the presence of an intact cell surface membrane (Fig. 2). Treatment with both fibrillar Aβ and soluble Aβ was required to demonstrate significant effects on neuronal viability as assessed by cell morphology (Fig. 2A), disruption of plasma membrane as determined by live/dead cell staining (Fig. 2B) and the release of the cytoplasmic enzyme LDH (Fig. 2C), and loss of mitochondrial potential as measured by two different redox dyes, XTT and alamarBlue (Fig. 2C). In addition to measuring overt neuronal cell death after treatment with fibrillar Aβ and/or soluble Aβ, biochemical responses associated with Aβ-mediated neuronal cell death were also evaluated using this two-component experimental paradigm. In particular, activation and phosphorylation of Jun kinase and c-Jun, critical components of the cellular signaling pathway mediating Aβ neurotoxicity (Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001), were also found to require treatment with both fibrillar Aβ and soluble Aβ (data not shown), indicating that activation of this cell death signaling pathway was tightly coupled to nucleation-dependent Aβ polymerization. Therefore, a variety of independent measurements of neuronal viability clearly demonstrated that ongoing Aβ polymerization appeared to be an essential component of Aβ-mediated neuronal cell death.
The reduction of the cellular MTT to MTT formazan is not a reliable early indicator of Aβ-mediated neuronal cell death
Tetrazolium salts, such as MTT, are routinely used as colorimetric agents for measuring cell viability in cytotoxicity assays (Hansen et al., 1989). Cellular metabolic activity, or more precisely cellular redox activity, results in the reduction of soluble MTT to the insoluble MTT formazan. The MTT assay has been extensively used in studies addressing the neurotoxic properties of Aβ. However, it is likely that use of the MTT assay in Aβ neuronal cell death studies has confounded the interpretation of experiments using various aggregate forms of Aβ. It has been reported by several laboratories that treatment with Aβ can lead to a decrease in MTT formazan production in the absence of overt cell death (Shearman et al., 1994; Hertel et al., 1996; Liu et al., 1997; Soriano et al., 2003). A number of different explanations have been proposed for this observation, including Aβ effects on membrane properties, MTT formazan exocytosis, and unknown intracellular targets whose interaction with Aβ is mediated by endosomal/lysosomal acidification (Hertel et al., 1996; Liu et al., 1997; Kane et al., 1999). Although the mechanisms by which Aβ can reduce insoluble MTT formazan formation in the absence of overt cell death are in dispute, the MTT formazan assay has been reported to be a specific early indicator of Aβ-mediated cell death (Shearman et al., 1994).
We and others have found that amyloidogenic proteins such as Aβ uniquely alter cellular MTT formazan crystal formation such that needle-shaped MTT formazan crystals are formed that puncture the cell plasma membrane (Shearman et al., 1995; Hertel et al., 1996) (data not shown). As reported previously (Shearman et al., 1995; Hertel et al., 1996), this effect of Aβ on MTT formazan crystal formation is not shared with other structurally related tetrazolium redox dyes. Overall, these findings suggest that the MTT assay might not be a reliable indicator of overt Aβ-mediated neuronal cell death.
To directly test the reliability of the insoluble MTT formazan assay as an early indicator of Aβ-mediated neuronal cell death, we treated sister neuronal cultures with fibrillar Aβ alone, soluble Aβ alone, or both fibrillar Aβ and soluble Aβ and compared the neuronal viability determined using the MTT assay with that obtained by measurement of plasma membrane integrity using LDH release and cellular redox activity using two different soluble redox dyes, XTT and alamarBlue. As shown by the black bars in Figure 2C, treatment of primary human cortical neurons with either fibrillar Aβ alone or soluble Aβ alone had no effect on LDH release or cellular redox activity when assayed using soluble redox dyes. In contrast, treatment with either fibrillar Aβ alone or soluble Aβ alone had a significant affect on neuronal viability only when cellular redox activity was determined using the insoluble MTT formazan assay. We repeated these studies a number of times using primary mouse, rat, and human cortical neurons and have consistently found the MTT formazan assay to indicate Aβ neurotoxicity under conditions in which there is no detectable Aβ effect on neuronal viability as determined by phase contrast microscopy, live/dead cell staining, LDH release, and soluble redox dyes (data not shown). Because Aβ-mediated neuronal cell death, as determined using a variety of methods other than the insoluble MTT formazan assay, requires treatment with both soluble Aβ and fibrillar Aβ, the reduction of insoluble MTT formazan found after treatment with either soluble Aβ alone or fibrillar Aβ alone cannot be interpreted as an early indicator of neuronal cell death.
Our observation that treatment of human cortical neurons with soluble Aβ can lead to a reduction in MTT formazan formation in the absence of overt neuronal cell death is contrary to previous studies that did not show an effect of soluble Aβ on MTT formazan formation using cultured rat primary cortical neurons (Walsh et al., 1999) or rat primary hippocampal neurons (Ward et al., 2000). However, these previous studies only evaluated the effects of soluble Aβ on MTT formazan formation after relatively short treatment periods (0.5-2 h), whereas the studies reported here used treatment periods of 2-3 d. The results presented here demonstrate that, with longer incubation time, soluble Aβ can lead to a reduction in the MTT assay in the absence of overt neuronal cell death.
Based on these and previous findings (Patel et al., 1996; Liu et al., 1997; Soriano et al., 2003), the studies reported here strongly indicate that the MTT formazan assay is an unreliable indicator of Aβ-mediated neuronal cell death. Furthermore, these findings suggest that previous results on the neurotoxic properties of Aβ fibrils, protofibrils, or other oligomeric species should be interpreted with caution if neuronal cell death was inferred using the MTT formazan assay.
The extent and rate of neuronal cell death can be precisely controlled by conditions that alter the rate of nucleation-dependent Aβ polymerization
To further characterize the role of nucleation-dependent polymerization in Aβ-mediated neurotoxicity, we evaluated the effect of fibrillar Aβ pretreatment on the interaction of soluble Aβ with primary human cortical neurons. Using trace amounts of soluble 125I-Aβ1-40, we found that pretreatment of cultured cortical neurons with fibrillar Aβ dramatically enhanced the incorporation of soluble Aβ onto the neuronal monolayer (Fig. 3A). Under the conditions used (1 μm fibrillar Aβ and 20 μm soluble Aβ), the incorporation of soluble Aβ was linear over 2 d of treatment. Overt neuronal cell death was only associated with the incorporation of soluble Aβ along the neuronal cell surface (Fig. 3B), further suggesting that ongoing fibril polymerization might be critical for mediating Aβ neurotoxicity.
Figure 3.
Soluble Aβ incorporates onto Aβ seeded neurons. A, Human neurons were treated with 1 μm fibrillar Aβ or vehicle for 1 h, followed by aspiration. A solution of 20 μm soluble Aβ containing 125I-Aβ1-40 (20,000 cpm/100 μl) was then added to the cultures. At various time points, cells were washed twice with PBS and solubilized for gamma counting. Solubilization was done by adding 150 μl/well Solvable (catalog #6NE9100; Packard, Meridian, CT), mixing, and transferring to a tube. Radioactivity from these samples was then measured on a gamma counter. Wells were then washed twice with PBS at 150 μl/wash. These washes were also collected and gamma counted. Counts from the two final washes were added to counts from Solvable-harvested wells to get total counts incorporated on cells. B, Sister neuronal cultures were assayed for viability using alamarBlue. All treatments were in triplicate wells. Error bars indicate ± SD.
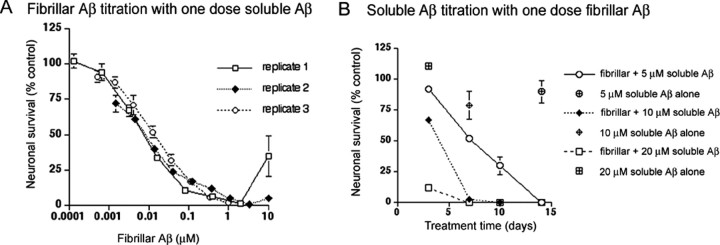
If nucleation-dependent amyloid polymerization is a critical component of Aβ-mediated neurotoxicity, then conditions that enhance or diminish the rate of Aβ polymerization should have similar effects on the rate and extent of Aβ-mediated neuronal cell death. Because the rate of nucleation-dependent polymerization is proportional to both seed concentration and monomer concentration, we evaluated the effects of fibrillar Aβ concentration and soluble Aβ concentration on Aβ-mediated neuronal cell death. Human cortical neurons were pretreated with various concentrations of fibrillar Aβ for 1 h, the culture medium was aspirated, and the neurons were then maintained for 3 d in the presence of 20 μm soluble Aβ. As expected, increasing concentrations of fibrillar Aβ from 0.1 nm to 1 μm dramatically enhanced the extent of Aβ-mediated neuronal cell death (Fig. 4A). Similar results were obtained when fibrillar Aβ concentration was kept constant and soluble Aβ concentration was varied from 5 to 20 μm. As shown in Figure 4B, increasing the concentration of soluble Aβ enhanced both the rate and extent of Aβ-mediated neuronal cell death after 3, 7, 10, and 14 d of treatment. These studies clearly demonstrate a close relationship between the rate of Aβ polymerization and both the rate and extent of Aβ-mediated neuronal cell death.
Figure 4.
The extent of Aβ-mediated neuronal cell death is dependent on both fibrillar Aβ concentration and on soluble Aβ concentration. A, Human cortical neurons were pretreated for 1 h with the indicated concentrations of fibrillar Aβ. Nonadhering fibrillar Aβ was removed by aspiration, and 20 μm soluble Aβ was added to the cultures. Neuronal cultures were incubated for 3 d and then assayed for viability using alamarBlue. Three independent neuronal culture preparations were assayed on different days using frozen aliquots of the same fibrillar Aβ preparation. We presume that the variability seen at the highest concentration of fibrillar Aβ is attributable to variations in the amount of fibrillar Aβ left behind after aspiration of the fibrillar Aβ pretreatment. Sufficiently high concentrations of fibrillar Aβ can inhibit toxicity, as seen in Figure 6, and this variability was eliminated when the fibrillar Aβ pretreatment was washed with media alone before adding soluble Aβ (data not shown). B, Human cortical neurons were pretreated for 1 h with 1 μm fibrillar Aβ. Pretreatment was aspirated, and 5 μm (circles), 10 μm (diamonds), or 20 μm (squares) soluble Aβ was added to the cultures. Neuronal viability was determined by alamarBlue after 3, 7, 10, and 14 d of treatment. All treatments were in triplicate wells. Error bars indicate ± SD.
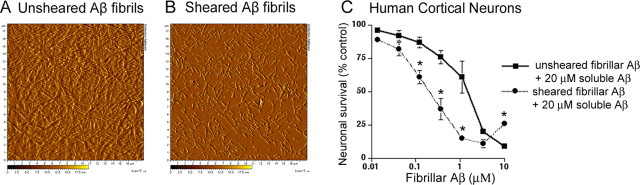
Studies on nucleation-dependent protein polymerization have demonstrated that the rate of polymerization is dependent on both the concentration of fibril seeds and the number of free fibril ends (Harper and Lansbury, 1997). Specifically, conditions that enhance the number of free fibril ends will enhance the rate of protein polymerization, even if the concentrations of fibrils and monomers are kept constant. Based on this relationship, we would expect treatments that increase the number of free Aβ fibril ends, without changing the concentrations of fibrillar or soluble Aβ, would also enhance the rate of Aβ-mediated neuronal cell death. To address this relationship experimentally, we sheared Aβ fibrils by repeated passage through a 27 gauge needle. Using AFM (Fig. 5A,B) and TEM (data not shown), we found that the sheared fibrils were significantly shorter than unsheared Aβ fibrils. Pretreatment with sheared Aβ fibrils, followed by treatment with soluble Aβ, resulted in significantly greater neuronal cell death after 3 d of treatment compared with pretreatment with unsheared Aβ fibrils. For example, similar effects on neuronal survival were found after 1 h pretreatment with either 1.2 μm unsheared Aβ fibrils or 0.3 μm sheared Aβ fibrils, followed by 3 d of treatment with 20 μm soluble Aβ (Fig. 5C). Neuronal cultures treated with unsheared or sheared Aβ fibrils alone did not exhibit cell death (data not shown), suggesting that both unsheared and sheared fibrils require soluble Aβ to mediate overt neuronal cell death. Thus, the neurotoxic potency of Aβ increases as the number of fibril ends increases, as would be expected for a nucleation-dependent polymerization process.
Figure 5.
Increasing the number of fibril ends by shearing leads to increased potency of Aβ fibril seeds. Deflection mode AFM images of unsheared (A) and sheared (B) Aβ fibrils generated at pH 9.0, 150 mm NaCl. These conditions were found to produce long, nonlaterally associated fibrils as determined by AFM. Samples (30 μm) were incubated for ∼5 min at room temperature on mica either immediately before (for unsheared samples) or immediately after (for sheared samples) shearing. The shorter, sheared fibrils do not stick well to mica at pH 9.0. NaCl was spiked in to a final concentration of 250 mm in the sheared fibrils immediately before adding to mica to enhance the adsorption of sheared fibrils to the mica surface. Although this treatment did enhance fibril adsorption, it is important to note that fibril adsorption to mica surface is not quantitative, and thus the apparent reduction in fibril content after shearing is likely attributable to differences in adsorption rather than loss of fibrils caused by shearing. Thioflavin-T signal was increased 2.5-fold in sheared samples compared with the unsheared samples (data not shown). C, Human cortical neurons were treated with the indicated concentration of sheared and unsheared fibrils (at pH 9.0, 150 mm NaCl) for 1 h. Dilutions were performed with 10 μm soluble Aβ present to ensure that the fibrils did not disaggregate by loss of monomer from fibril ends. Nonadhering Aβ fibrils were removed by aspiration, and 20 μm soluble Aβ was added to the cultures. Cultures were maintained for 3 d, and neuronal viability was determined by alamarBlue. Statistical analysis was based on the means ± SD (n = 6). *p = 0.005 versus cultures treated with unsheared Aβ fibrils based on ANOVA, followed by Fisher's PLSD test using StatView software (version 5.0; SAS Institute). ANOVA indicated that there was a group effect of cultures treated with unsheared Aβ fibrils versus cultures treated with sheared Aβ fibrils (p = 0.002), as well as an interaction effect between dose and treatment (p < 0.0001).
The neurotoxic dependency on both fibrillar Aβ concentration and soluble Aβ concentration was reproduced using four different lots of Aβ1-40 peptide, demonstrating that these findings were not unique to individual lots of Aβ peptide. However, the neurotoxic potency of fibrillar Aβ did vary from lot to lot, suggesting that the number of free fibril ends was peptide lot and preparation dependent (data not shown). Nonetheless, the neurotoxic potency of individual preparations of fibrillar Aβ was highly reproducible, and frozen aliquots of fibrillar Aβ showed consistent neurotoxic activity across multiple experiments (Fig. 4A). These findings provide a highly reproducible method by which to control the rate of Aβ-mediated neuronal cell death and associated signaling pathways and should allow a greater understanding of the molecular events associated with amyloid-mediated pathology. Previous studies using human cerebrovascular smooth muscle cells have demonstrated that cell surface Aβ fibril formation plays an important role in causing pathologic responses in this cell type (Van Nostrand et al., 1998). Confocal microscopy studies using anti-Aβ and anti-neural cell adhesion molecule antibodies also indicates that Aβ polymerization is closely associated with the neuronal cell surface under conditions that promote overt neuronal cell death (data not shown). Together, these series of studies strongly suggest that the nucleation-dependent polymerization of Aβ1-40, most likely along the neuronal cell surface, is an essential component of Aβ-mediated neuronal cell death. However, our interpretation of these studies did not take into consideration the potential for stable soluble Aβ oligomers to be generated by the treatment conditions used in these studies. Such stable and toxic Aβ oligomers could be generated by the process of Aβ polymerization and then diffuse onto the neuronal cell surface. We therefore conducted a series of studies in which fibrillar Aβ and soluble Aβ were mixed in solution for various lengths of time before treatment of primary neuronal cultures to determine whether there was a soluble component of Aβ-mediated neuronal cell death or whether Aβ-mediated neuronal cell death was more tightly associated with Aβ polymerization and overt fibril formation. In particular, we reasoned that, if Aβ neuronal cell death was dependent on fibril elongation along the neuronal cell surface, then solutions of fibrillar Aβ and soluble Aβ would be toxic to neurons only if the solution conditions maintained ongoing Aβ polymerization. However, if stable neurotoxic oligomers of Aβ were generated in solution by the process of Aβ polymerization, then solutions of fibrillar Aβ and soluble Aβ would be toxic to neurons even if solution conditions did not maintain ongoing Aβ polymerization. As shown in Figure 6A, solutions containing 25 μm soluble Aβ and 62, 125, or 250 nm fibrillar Aβ were preincubated up to 6 h at 37°C before addition to neuronal cultures. After 30 min of preincubation, all three fibril concentrations generated solutions with very similar neurotoxic potencies. The neuronal cell death found in these treatments was comparable with that obtained using freshly solubilized neurotoxic lots of Aβ (Fig. 1). The relatively small amount of fibrillar Aβ required to induce neuronal cell death is consistent with our observation that neurotoxic lots of Aβ1-40 contain very small amounts of fibrillar Aβ (<5% of total peptide concentration of neurotoxic lots MF-0641 and MF-1141 were in fibrillar form based on ThT and centrifugation experiments; data not shown). However, with longer preincubation times up to 6 h, solutions containing the higher concentration of fibrillar Aβ showed dramatic loss of neurotoxic potency. One potential interpretation of these studies was that the solutions containing higher concentrations of fibrillar Aβ had consumed all of the soluble Aβ and could no longer maintain ongoing Aβ polymerization when added to neuronal cultures. To directly address this possibility, we repeated these studies using 62 nm fibrillar Aβ and either 20 or 40 μm soluble Aβ. Whereas the solution containing 20 μm soluble Aβ lost its neurotoxic activity when preincubated for 24 h, the solution containing 40 μm soluble Aβ maintained its ability to cause neuronal cell death (Fig. 6B). Thus, increasing the soluble Aβ concentration directly extended the duration of neurotoxic activity associated with a fixed concentration of fibrillar Aβ. Conversely, as predicted for a nucleation-dependent polymerization process, the addition of fibrillar Aβ should inhibit neuronal cell death when amounts sufficient to consume all available soluble Aβ are added to neuronal cultures. Cortical cultures were pretreated for 1 h with 1 μm fibrillar Aβ, washed, and then treated with 20 μm soluble Aβ, plus either 0 or 5 μm fibrillar Aβ. After 3 d of treatment, neuronal viability was determined using the alamarBlue assay. As shown in Figure 6C, adding an additional 5 μm fibrillar Aβ inhibited neuronal cell death by 85% compared with the cultures pretreated with 1 μm fibrillar Aβ and 20 μm soluble Aβ alone. Thus, through a variety of treatment conditions that altered the rate and extent of Aβ polymerization, we have been able to demonstrate a strong correlation of the rate and extent of Aβ polymerization with the rate and extent of Aβ-mediated neuronal cell death.
Figure 6.
Aβ-mediated neuronal cell death requires ongoing nucleation-dependent polymerization. A, Fibrillar Aβ (62 nm) (open circles), 125 nm fibrillar Aβ (filled squares), or 250 nm fibrillar Aβ (filled diamonds) was added to 25 μm soluble Aβ and left to incubate at 37°C for the indicated lengths of time before adding to primary human cortical neurons. Cells were treated for 3 d, and then neuronal survival was determined by alamarBlue. Note that increasing concentrations of fibrillar Aβ led to a loss of neuronal cell death, presumably because of depletion of soluble Aβ. B, Fibrillar Aβ (62 nm) was added to either 20 or 40 μm soluble Aβ and left to age at 37°C for the indicated times before adding to primary human cortical neurons. Note that the loss of neuronal cell death found using 62 nm fibrillar Aβ at 24 h of aging is prevented by using a higher soluble Aβ concentration. C, Human cortical neurons were pretreated with 1 μm fibrillar Aβ for 1 h. Nonadhering fibrillar Aβ was removed by aspiration, and cultures were treated with 20 μm soluble Aβ with or without 5 μm fibrillar Aβ. Cultures were incubated for 3 d, and then neuronal survival was determined by alamarBlue. The addition of 5 μm fibrillar Aβ significantly inhibited the neuronal cell death normally found using 20 μm soluble Aβ, presumably caused by depletion of soluble Aβ. All treatments were intriplicate wells. Error bars indicate ± SD.
To further investigate the possibility that a stable, soluble species of Aβ could be mediating neurotoxicity under these conditions, we evaluated the conditioned media from neuronal cultures treated with fibrillar Aβ and soluble Aβ. Cortical cultures were pretreated for 1 h with fibrillar Aβ and washed extensively, and then soluble Aβ was added to the cultures. After 1, 2, or 3 d of treatment, the conditioned media from these neuronal cultures was transferred to cortical cultures that had been pretreated with fibrillar Aβ or media alone. Donor cultures were assayed for neuronal viability at the time of media transfer, whereas recipient cultures were assayed for neuronal viability 3 d after treatment with conditioned media. As shown in Figure 7A, donor cultures displayed progressive neuronal cell death over the 3 d of treatment with fibrillar Aβ and soluble Aβ. Interestingly, recipient neuronal cultures that were pretreated with media alone did not demonstrate neurotoxicity after treatment with conditioned media, even when the conditioned media came from donor neuronal cultures displaying >80% neuronal cell death (Fig. 7). However, recipient neuronal cultures that were pretreated with fibrillar Aβ displayed dramatic neuronal cell death under all treatment conditions (Fig. 7B). The failure of donor culture conditioned media to generate Aβ neurotoxicity in the absence of pretreatment with fibrillar Aβ strongly indicates that neuronal cell death was not caused by the formation of stable, soluble neurotoxic Aβ species. If stable, neurotoxic Aβ oligomers mediated this neuronal cell death, then there would not be an absolute requirement for fibrillar Aβ pretreatment of recipient cultures. These studies provide additional support that nucleation-dependent polymerization is an essential component of Aβ-mediated neuronal cell death.
Figure 7.
Aβ-mediated neuronal cell death generated by nucleation-dependent polymerization cannot be transferred to naive cells. A, Human cortical neurons were pretreated with 1 μm fibrillar Aβ for 1 h. Nonadhering fibrillar Aβ was removed by aspiration, followed by three washes with media alone. Soluble Aβ (40 μm) was then added to the cultures. After 1, 2, or 3 d of treatment, the media was removed and transferred to sister cultures. The “donor” cultures were then assayed for neuronal viability (alamarBlue) to assess the extent of neuronal cell death at the time of transfer. B, Recipient cultures were pretreated for 1 h with either 1 μm fibrillar Aβ or media alone (“naive” cells). Nonadhering fibrillar Aβ was removed by aspiration, and cultures were treated with conditioned media from donor cultures in A. Neuronal viability in “recipient” cultures was then determined using alamarBlue 3 d after treatment with donor conditioned media. All treatments were in triplicate wells. Error bars indicate ± SD.
Previous studies have demonstrated that microglia and astrocytes can modulate Aβ-mediated neuronal cell death (Combs et al., 1999; Malchiodi-Albedi et al., 2001; Paradisi et al., 2004). This suggests the possibility that the neuronal cell death observed here is caused by soluble toxins released from contaminating glial cells rather than from a direct action of Aβ polymerization on neurons. Two lines of evidence suggest that the results reported here are not attributable to soluble toxins released by contaminating glial cells. First, the use of B27 as a serum supplement dramatically reduces the number of contaminating microglial cells (<1%) and contaminating astrocytes (<10%). Second, we were unable to demonstrate neurotoxic activity in the conditioned media obtained in these experiments, strongly suggesting that the neurotoxic effects seen here are not attributable to soluble toxic factors released by contaminating glial cells.
Nucleation-dependent polymerization as a common component of amyloid-mediated neurotoxicity
Because protein aggregation is associated with a variety of neurodegenerative disorders, it was of interest to determine whether nucleation-dependent polymerization was a common component of the neurotoxic properties associated with other amyloidogenic proteins. In particular, we evaluated whether Aβ1-42, human amylin, and NAC also require both fibrils and soluble peptide to be present to generate neuronal cell death. As shown in Figure 8, the neuronal cell death associated with all three peptides required the presence of both fibrillar and soluble peptide. Although the concentrations of fibrillar and soluble peptide required for neuronal cell death, as well as the method of generating fibrils, varies for different amyloidogenic peptides, a common feature of amyloid-mediated neuronal cell death is the presence of two components, fibrillar and soluble peptide. Thus, nucleation-dependent polymerization appears to be a common mechanistic feature of amyloid-mediated neuronal cell death.
Figure 8.
Nucleation-dependent polymerization is a common feature of amyloid-mediated neuronal cell death. Human neuronal cells were treated in basal medium with fibril seed (5 μm for Aβ1-42, 1.25 μm for human amylin, and 10 μm for NAC) or media (for the treatments with soluble peptide alone) for 1 h, nonadhering fibrils were removed by aspiration, and the cells were washed and then treated with soluble peptide (Aβ1-42 at 20 μm, human amylin at 5 μm, and NAC at 12.5 μm) or media (for the fibril alone treatments). Neuronal viability was determined using alamarBlue after 3 d of treatment with Aβ1-42 and human amylin and after 6 d of treatment with NAC. All treatments were in triplicate wells. Error bars indicate ± SD.
Discussion
Over the last two decades, accumulating evidence has implicated abnormal protein aggregation as a mechanistic feature of a variety of amyloidogenic disorders, including Alzheimer's disease (Selkoe, 2003). Although significant progress has been made in understanding the structural requirement for Aβ fibrillogenesis (Harper and Lansbury, 1997), there still remains tremendous uncertainty in regards to the structural requirements for Aβ-mediated neuronal cell death (Caughey and Lansbury, 2003). Studies on the neurotoxic properties of Aβ have proven difficult to interpret because of significant lot-to-lot variability found in biochemically identical synthetic preparations of Aβ peptides (May et al., 1992) and the use of a variety of culture systems and functional endpoints when assessing the cellular toxicity associated with Aβ treatment. In this regard, the majority of recent studies on the neurotoxic properties of Aβ have used the MTT assay, in part because of the high sensitivity and reproducibility of MTT reduction in response to Aβ treatment (Shearman et al., 1994). Based on findings obtained with the MTT assay, several groups have reported neurotoxicity associated with soluble oligomeric forms of Aβ (Walsh et al., 1999; Dahlgren et al., 2002; Chromy et al., 2003). However, previous studies and the results reported here demonstrate that both fibrillar Aβ treatment alone and soluble Aβ treatment alone can cause MTT reduction, without causing overt cell death as measured using the tetrazolium redox dye XTT, alamarBlue, or cellular LDH release (Fig. 2). In contrast, overt neuronal cell death in response to Aβ treatment requires the presence of both fibrillar Aβ and soluble Aβ and is tightly correlated with experimental conditions that are known to maintain ongoing Aβ polymerization.
In addition to an absolute requirement for the presence of both fibrillar Aβ and soluble Aβ, Aβ-mediated neuronal cell death shares in a variety of other properties associated with nucleation-dependent polymerization. Previous studies have demonstrated that the rate and extent of Aβ polymerization is dependent on fibril concentration and monomer concentration (Naiki and Nakakuki, 1996). As demonstrated here, Aβ-mediated neuronal cell death is associated with the polymerization of soluble Aβ, most likely along the neuronal cell surface (Fig. 3 and data not shown), and, like nucleation-dependent polymerization, the rate and extent of Aβ-mediated neuronal cell death is directly proportional to the concentration of fibrillar Aβ and soluble Aβ (Fig. 4).
Another important line of evidence supporting a critical role for Aβ polymerization as a mechanism for Aβ-mediated neuronal cell death is the observation that shearing fibrils (and thereby increasing the number of free fibril ends) enhances the neurotoxic potency of a given concentration of fibrillar Aβ (Fig. 5). This observation is consistent with Aβ-mediated neuronal cell death being attributable to growth of fibrils by addition of monomer to the fibril ends, as has previously been demonstrated for nucleation-dependent Aβ polymerization (Harper and Lansbury, 1997).
An alternative mechanistic explanation for Aβ-mediated neuronal cell death is based on findings that stable or metastable oligomeric forms of Aβ are generated during the process of Aβ fibrillogenesis, and that these oligomers or protofibrils are directly toxic to neurons (Hartley et al., 1999; Dahlgren et al., 2002; Chromy et al., 2003). However, we have been unable to demonstrate that neurotoxic soluble or diffusible oligomeric forms of Aβ are generated during the active process of Aβ-mediated neuronal cell death (Fig. 7). Furthermore, we found that neurotoxic solutions of Aβ require the presence of both fibrillar Aβ and soluble Aβ (Figs. 1, 6) suggesting that oligomeric forms of Aβ are not sufficient to generate overt neuronal cell death.
Our review of the published studies on the neurotoxic properties of Aβ oligomers indicate that most studies have relied on the MTT assay as an indication of neurotoxicity rather than overt neuronal cell death and therefore are not inconsistent with out results. Nonetheless, both protofibrils (Hartley et al., 1999) and amyloid-derived diffusible ligands (Longo et al., 2000) have been reported to cause cell death using methods that do not rely on the MTT assay. However, it is important to note that these studies used experimental conditions that could promote Aβ polymerization (solutions of Aβ protofibrils or oligomers containing sufficient soluble Aβ to promote polymerization), suggesting that the findings reported here are not inconsistent with these studies. Based on the issues discussed here on the use of the MTT assay as a reporter of Aβ-mediated neuronal cell death, it is important that future studies do not rely solely on the MTT assay as a reporter of overt neuronal cell death. Recent findings indicate that oligomeric forms of Aβ can directly inhibit hippocampal long-term potentiation (Walsh et al., 2002; Wang et al., 2002, 2004), suggesting that diffusible oligomeric forms of Aβ may be involved in subtle alterations of synaptic function and long-term potentiation. Together with our findings that nucleation-dependent Aβ polymerization appears to be essential for mediating overt neuronal cell death, it is likely that different oligomeric forms and processes associated with Aβ fibrillogenesis may differentially affect synaptic activity, neuronal function, and neuronal cell death in Alzheimer's disease.
The finding that Aβ polymerization is an essential component of Aβ-mediated neuronal cell death has several practical advantages for studies on the neurotoxic properties of amyloidogenic proteins. The studies reported here provide an explanation for why biochemically identical lots of Aβ have such profound differences in neurotoxic properties. For any given solution of Aβ to be toxic, it must contain quantities and ratios of fibrillar Aβ and soluble Aβ that are sufficient to maintain ongoing polymerization. Stock solutions of Aβ that contain small amounts of fibrillar Aβ will appear nontoxic or weakly toxic (Fig. 4B) and require aging to generate sufficient fibril seeds to promote more robust nucleation-dependent polymerization. In contrast, stock solutions of Aβ that contain large amount of fibrillar Aβ will rapidly incorporate free Aβ monomer/oligomers and will lose toxicity once polymerization has terminated as a result of depletion of free Aβ monomer/oligomers (Fig. 6).
Nucleation-dependent polymerization provides a robust and highly reproducible method for studying Aβ-mediated neuronal cell death (Fig. 4A). In addition, our findings that both fibrillar and soluble peptide are required for neuronal cell death in response to Aβ1-42, NAC, and human amylin (Fig. 8) suggest that nucleation-dependent polymerization may be a common mechanism of amyloid-mediated cell death. Varying the amount and ratio of fibrillar and soluble peptide can precisely control the rate and extent of polymerization and allow an analysis of cellular responses to amyloidogenic peptides under conditions in which the rate and extent of cell death is also precisely controlled. Furthermore, the use of this two-component system allows cellular responses to fibrillar peptide alone or soluble peptide alone to be studied in the absence of overt cell death and thereby provides a method to study more subtle cellular response to amyloidogenic peptides.
The finding that nucleation-dependent polymerization is a critical component of Aβ-mediated neuronal cell death provides a mechanistic framework for evaluating both experimental and clinical studies on the role of Aβ in Alzheimer's disease. In particular, our findings on Aβ polymerization provide a simple explanation for the experimental findings that familial AD mutations lead to increased production or steady-state levels of Aβ (Selkoe, 2001) or enhanced Aβ protofibril formation (Nilsberth et al., 2001). Elevated levels of soluble Aβ would enhance the rate of Aβ polymerization and associated neurodegeneration and is consistent with the findings the many of the familial AD mutations lead to early-onset Alzheimer's disease. A role for Aβ polymerization is also consistent with the finding that the levels of soluble Aβ correlate most closely with synaptic loss and markers of disease severity in AD brain (Lue et al., 1999; McLean et al., 1999). Higher levels of either fibrillar seed or soluble Aβ in brain parenchyma should result in higher rates of Aβ polymerization and associated neurodegeneration.
The findings reported here have implications for therapeutic approaches to Alzheimer's disease. If Aβ polymerization is required for neuronal cell death in AD, then any approach that reduces the amount of soluble Aβ in the brain may be therapeutically valuable, whether or not such treatment actually reverses existing plaque burden. As such, reduction of the amount of soluble Aβ present in the brain parenchyma could dramatically reduce the rate of Aβ polymerization and thereby the onset and rate of associated neurodegeneration. Similarly, treatments that enhance the clearance of amyloid plaques should also retard the rate of Aβ polymerization by removing a source of fibrillar Aβ and thereby protect against nucleation-dependent neuronal cell death. In fact, recent clinical data indicates that antibodies against Aβ plaques slow cognitive decline in patients with AD (Hock et al., 2003). Our data indicate that nucleation-dependent polymerization may be a common mechanistic feature of the cellular pathology associated with amyloidogenic proteins. Hopefully, a fuller understanding of the cellular responses to nucleation-dependent polymerization will provide novel therapeutic insight into AD and associated amyloidogenic diseases.
Footnotes
We thank our many colleagues at Elan Pharmaceuticals for helpful discussions and support throughout the execution of these studies. In particular, we extend our appreciation to Drs. Karen Chen, Stephen Freedman, and Ivan Lieberburg for insightful discussions and their valuable comments on the studies presented here.
Correspondence should be addressed to Dr. Russell E. Rydel, Elan Pharmaceuticals, 800 Gateway Boulevard, South San Francisco, CA 94080. E-mail: russell.rydel@elan.com.
M. Wogulis' present address: Section of Molecular and Cellular Biology, University of California at Davis, One Shields Avenue, Davis, CA 95616.
D. Cunningham's present address: Biogen Idec, 3010 Science Park Road, San Diego, CA 92191.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251071-10$15.00/0
References
- Bozyczko-Coyne D, O'Kane TM, Wu ZL, Dobrzanski P, Murthy S, Vaught JL, Scott RW (2001) CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Aβ-induced cortical neuron apoptosis. J Neurochem 77: 849-863. [DOI] [PubMed] [Google Scholar]
- Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26: 267-298. [DOI] [PubMed] [Google Scholar]
- Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, Finch CE, Krafft GA, Klein WL (2003) Self-assembly of Aβ(1-42) into globular neurotoxins. Biochemistry 42: 12749-12760. [DOI] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE (1999) Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of β-amyloid and prion proteins. J Neurosci 19: 928-939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs DH, Pike CJ, Weinstein SL, Velazquez P, Cotman CW (1997) All-D-enantiomers of β-amyloid exhibit similar biological properties to all-L-β-amyloids. J Biol Chem 272: 7431-7436. [DOI] [PubMed] [Google Scholar]
- Dahlgren KN, Manelli AM, Stine Jr WB, Baker LK, Krafft GA, LaDu MJ (2002) Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem 277: 32046-32053. [DOI] [PubMed] [Google Scholar]
- Estus S, Tucker HM, van Rooyen C, Wright S, Brigham EF, Wogulis M, Rydel RE (1997) Aggregated amyloid-β protein induces cortical neuronal apoptosis and concomitant “apoptotic” pattern of gene induction. J Neurosci 17: 7736-7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon-Rodriguez W, Sepulveda-Becerra M, Milton S, Glabe CG (1997) Soluble amyloid Aβ-(1-40) exists as a stable dimer at low concentrations. J Biol Chem 272: 21037-21044. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885-890. [DOI] [PubMed] [Google Scholar]
- Hansen MB, Nielsen SE, Berg K (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119: 203-210. [DOI] [PubMed] [Google Scholar]
- Harper JD, Lansbury Jr PT (1997) Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu Rev Biochem 66: 385-407. [DOI] [PubMed] [Google Scholar]
- Harper JD, Wong SS, Lieber CM, Lansbury Jr PT (1999) Assembly of Aβ amyloid protofibrils: an in vitro model for a possible early event in Alzheimer's disease. Biochemistry 38: 8972-8980. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ (1999) Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19: 8876-8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel C, Hauser N, Schubenel R, Seilheimer B, Kemp JA (1996) β-amyloid-induced cell toxicity: enhancement of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide-dependent cell death. J Neurochem 67: 272-276. [DOI] [PubMed] [Google Scholar]
- Hock C, Konietzko U, Streffer JR, Tracy J, Signorell A, Muller-Tillmanns B, Lemke U, Henke K, Moritz E, Garcia E, Wollmer MA, Umbricht D, de Quervain DJ, Hofmann M, Maddalena A, Papassotiropoulos A, Nitsch RM (2003) Antibodies against β-amyloid slow cognitive decline in Alzheimer's disease. Neuron 38: 547-554. [DOI] [PubMed] [Google Scholar]
- Howlett DR, Jennings KH, Lee DC, Clark MS, Brown F, Wetzel R, Wood SJ, Camilleri P, Roberts GW (1995) Aggregation state and neurotoxic properties of Alzheimer β-amyloid peptide. Neurodegeneration 4: 23-32. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Lansbury Jr PT (1993) Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73: 1055-1058. [DOI] [PubMed] [Google Scholar]
- Kane MD, Schwarz RD, St Pierre L, Watson MD, Emmerling MR, Boxer PA, Walker GK (1999) Inhibitors of V-type ATPases, bafilomycin A1 and concanamycin A, protect against beta-amyloid-mediated effects on 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 72: 1939-1947. [DOI] [PubMed] [Google Scholar]
- LeVine III H (1997) Stopped-flow kinetics reveal multiple phases of thioflavin T binding to Alzheimer beta (1-40) amyloid fibrils. Arch Biochem Biophys 342: 306-316. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peterson DA, Kimura H, Schubert D (1997) Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69: 581-593. [DOI] [PubMed] [Google Scholar]
- Longo VD, Viola KL, Klein WL, Finch CE (2000) Reversible inactivation of superoxide-sensitive aconitase in Abeta1-42-treated neuronal cell lines. J Neurochem 75: 1977-1985. [DOI] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J (1999) Soluble amyloid-β peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol 155: 853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchiodi-Albedi F, Domenici MR, Paradisi S, Bernardo A, Ajmone-Cat MA, Minghetti L (2001) Astrocytes contribute to neuronal impairment in beta A toxicity increasing apoptosis in rat hippocampal neurons. Glia 34: 68-72. [DOI] [PubMed] [Google Scholar]
- May PC, Gitter BD, Waters DC, Simmons LK, Becker GW, Small JS, Robison PM (1992) β-Amyloid peptide in vitro toxicity: lot-to-lot variability. Neurobiol Aging 13: 605-607. [DOI] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL (1999) Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol 46: 860-866. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME (2001) β-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci 21: 7551-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiki H, Nakakuki K (1996) First-order kinetic model of Alzheimer's β-amyloid fibril extension in vitro. Lab Invest 74: 374-383. [PubMed] [Google Scholar]
- Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, Stenh C, Luthman J, Teplow DB, Younkin SG, Naslund J, Lannfelt L (2001) The “Arctic” APP mutation (E693G) causes Alzheimer's disease by enhanced Aβ protofibril formation. Nat Neurosci 4: 887-893. [DOI] [PubMed] [Google Scholar]
- Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi-Albedi F (2004) Astrocyte modulation of in vitro beta-amyloid neurotoxicity. Glia 46: 252-260. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Gunasekera S, Jen A, Rohan de Silva HA (1996) β-Amyloid-mediated inhibition of redox activity (MTT reduction) is not an indicator of astroglial degeneration. NeuroReport 7: 2026-2030. [DOI] [PubMed] [Google Scholar]
- Seilheimer B, Bohrmann B, Bondolfi L, Muller F, Stuber D, Dobeli H (1997) The toxicity of the Alzheimer's β-amyloid peptide correlates with a distinct fiber morphology. J Struct Biol 119: 59-71. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741-766. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2003) Folding proteins in fatal ways. Nature 426: 900-904. [DOI] [PubMed] [Google Scholar]
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schloss-macher M, Whaley J, Swindlehurst C, McCormack R, Wolfart R, Selkoe D, Lieberburg I, Schenk D (1992) Isolation and quantification of soluble Alzheimer's β-peptide from biological fluids. Nature 359: 325-327. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Ragan CI, Iversen LL (1994) Inhibition of PC12 cell redox activity is a specific, early indicator of the mechanism of beta-amyloid-mediated cell death. Proc Natl Acad Sci USA 91: 1470-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman MS, Hawtin SR, Tailor VJ (1995) The intracellular component of cellular 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reduction is specifically inhibited by β-amyloid peptides. J Neurochem 65: 218-227. [DOI] [PubMed] [Google Scholar]
- Simmons LK, May PC, Tomaselli KJ, Rydel RE, Fuson KS, Brigham EF, Wright S, Lieberburg I, Becker GW, Brems DN, Li WY (1994) Secondary structure of amyloid-β peptide correlates with neurotoxic activity in vitro. Mol Pharmacol 45: 373-379. [PubMed] [Google Scholar]
- Soriano FX, Galbete JL, Forloni G (2003) Effect of β-amyloid on endothelial cells: lack of direct toxicity, enhancement of MTT-induced cell death and intracellular accumulation. Neurochem Int 43: 251-261. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA (2001) β-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem 77: 157-164. [DOI] [PubMed] [Google Scholar]
- Tseng BP, Esler WP, Clish CB, Stimson ER, Ghilardi JR, Vinters HV, Mantyh PW, Lee JP, Maggio JE (1999) Deposition of monomeric, not oligomeric, Aβ mediates growth of Alzheimer's disease amyloid plaques in human brain preparations. Biochemistry 38: 10424-10431. [DOI] [PubMed] [Google Scholar]
- Van Nostrand WE, Melchor JP, Ruffini L (1998) Pathologic amyloid β-protein cell surface fibril assembly on cultured human cerebrovascular smooth muscle cells. J Neurochem 70: 216-223. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB (1997) Amyloid beta-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem 272: 22364-22372. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB (1999) Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 274: 25945-25952. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ (2002) Naturally secreted oligomers of amyloid-β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416: 535-539. [DOI] [PubMed] [Google Scholar]
- Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, Viola KL, Klein WL, Stine WB, Krafft GA, Trommer BL (2002) Soluble oligomers of β-amyloid (1-42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res 924: 133-140. [DOI] [PubMed] [Google Scholar]
- Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R (2004) Block of long-term potentiation by naturally secreted and synthetic amyloid β-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci 24: 3370-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RV, Jennings KH, Jepras R, Neville W, Owen DE, Hawkins J, Christie G, Davis JB, George A, Karran EH, Howlett DR (2000) Fractionation and characterization of oligomeric, protofibrillar and fibrillar forms of beta-amyloid peptide. Biochem J 348: 137-144. [PMC free article] [PubMed] [Google Scholar]
- Yankner BA (1996) Mechanisms of neuronal degeneration in Alzheimer's disease. Neuron 16: 921-932. [DOI] [PubMed] [Google Scholar]