Abstract
Obesity is a major public health problem. Palatability (i.e., the reinforcing value of food, derived from orosensory cues) is a significant factor in determining food intake and contributes to increased consumption leading to obesity. The nucleus accumbens is a ventral striatal region that is important for both appetitive and consummatory behaviors and has been implicated in modulating palatability. In this study, we investigated palatability encoding in the firing of nucleus accumbens neurons in rats. Nucleus accumbens neurons with significant changes in firing rate during consummatory behavior displayed one of two principal firing patterns. Firing in one class of nucleus accumbens neurons was correlated with the palatability of sucrose reinforcers; changes in neural activity in this class consisted primarily of excitations. Within this group of neurons, a subset was sensitive to the relative value of sucrose reinforcers, as assessed by a behavioral contrast paradigm. A second and distinct population of nucleus accumbens neurons, with changes in firing that were pre-dominantly inhibitions, was not sensitive to reinforcer palatability; rather, these inhibitions were present even during unreinforced bouts of licking. In addition, the onset of these inhibitions typically occurred before the initiation of the licking behavior itself. We propose that two primary classes of nucleus accumbens neurons contribute to neural processing immediately before and during reinforcer consumption: inhibitions related to initiation and maintenance of consummatory behaviors and excitations that encode reinforcer palatability.
Keywords: nucleus accumbens, palatability, obesity, striatum, appetitive behavior, consummatory behavior
Introduction
Obesity is a major threat to health in industrialized nations, and its prevalence is rapidly increasing in developing nations (World Health Organization, 2000). Weight gain results when caloric intake exceeds energy expenditure, and obesity results when this imbalance occurs over a sustained period of time. Food palatability (i.e., the reinforcing value of food derived from sensory qualities such as taste and texture) is one factor that potently promotes food intake (Saper et al., 2002). Because the sensory qualities associated with palatability typically signal high energy density, palatability-driven feeding provides an important safeguard against food scarcity by promoting consumption of energy-dense foods. In the present context of extraordinary food abundance and accessibility, however, the same mechanism is likely a significant cause of the rapid and recent increase in obesity (Hill and Peters, 1998). Identifying the neural mechanisms through which palatability is represented in the brain and promotes food intake is an important step in developing novel therapies to combat obesity.
The nucleus accumbens (NAcc) is a ventral striatal nucleus with a critical role in both appetitive and consummatory phases of behavior (Berridge and Robinson, 1998). In particular, elegant pharmacological experiments have shown that endogenous opioid signaling in the NAcc contributes significantly to consummatory behaviors (Kelley et al., 2002), likely through modulation of the palatability of consumed reinforcers. In support of this hypothesis, opioid microinfusion into the NAcc is particularly effective in increasing the intake of energy-dense foods (Pecina and Berridge, 2000; Zhang and Kelley, 2002). Conversely, opioid antagonists injected into the NAcc can block the intake of normally preferred reinforcers without affecting consumption of less palatable alternatives (Kelley et al., 1996).
Elucidating the mechanisms that underlie these pharmacological effects requires a better understanding of neural encoding in the NAcc. Specifically, is the palatability of natural reinforcers represented in the firing of NAcc neurons, and if so, how? Certainly, it is clear that the sensory properties of reinforcers can affect firing in the NAcc. For instance, the neural activity in the primate ventral striatum, including the NAcc, encodes the magnitude and identity of a juice reward (Williams et al., 1993; Hassani et al., 2001; Cromwell and Schultz, 2003). In contrast, studies of the rodent NAcc have emphasized the relative paucity of units that discriminate between different natural reinforcers (Carelli et al., 2000, 2002; Carelli and Wondolowski, 2003). Thus, despite strong pharmacological evidence suggesting the NAcc plays an important role in modulating palatability, the nature of palatability encoding in the firing of NAcc neurons remains poorly understood.
Here, we recorded from NAcc neurons in rats consuming sucrose reinforcers to determine whether the activity of NAcc neurons in the rodent encodes reinforcer palatability and whether firing in NAcc neurons tracks the changing relative value of a reinforcer in a behavioral contrast paradigm.
Materials and Methods
Experimental subjects. Male Long-Evans rats (n = 19; Charles River Laboratories, Wilmington, MA) were used for electrophysiological recording. Rats were food restricted to maintain 90% ad libitum body weight and water restricted, with 2 h ad libitum access to water after daily experimental sessions.
Behavioral paradigms. Behavioral paradigms were conducted in Plexiglas chambers (42 × 53 × 40 cm) equipped with a white noise generator (Med Associates, St. Albans, VT). Each chamber contained a single lick spout with attached photobeam lickometer (Colbourn Instruments, Allentown, PA), which was used to detect and timestamp individual licks. Three different solutions could be delivered from the lick spout without mixing, because three separate tubes were housed within the outer diameter of the lick spout and extended to the tip of the spout. There was no dead space associated with switching solutions. Gravity flow delivery of solutions was independently computer controlled by two-way solenoid valves (Fisher Scientific, Pittsburgh, PA) using commercial software (MedPC, Med Associates).
Two behavioral paradigms were used in this study. The sucrose discrimination task was used to test for the presence of neurons modulated by reinforcer palatability. In each trial, rats could obtain one of three solutions (0, 3, or 10% sucrose solution) by delivering licks to a single spout. The availability of the solution was signaled by a white noise cue, which was turned off with the first lick delivered to the spout. The white cue was identical for all three solutions. Thus, animals were never cued to the identity of the solution available, which was selected semirandomly (up to three successive presentations of any particular solution were allowed). After an initial lick delivered to the spout, the solution was available for an interval lasting 1.0, 1.5, or 2.0 s. Duration of reward availability was randomized to avoid expectation-driven behaviors that might influence NAcc neural activity. During the period of reward delivery only, each lick delivered to the spout triggered the delivery of ∼10 μl of solution. Animals were required to abstain from licking the spout for a period of 5 s to initiate a new trial. Rats were trained on this task until they completed ≥100 trials in a single behavioral session.
In the second behavioral task, a contrast paradigm was used to determine whether firing in NAcc neurons encodes the relative value of a 3% sucrose solution. Trial timing and initiation were identical to the first paradigm. However, only the 0 and 3% sucrose solutions were delivered for the first ∼60 trials of each session. Thereafter, only the 3 and 10% sucrose solutions were made available for consumption. This paradigm robustly decreased the reinforcing value of the 3% sucrose solution, as measured behaviorally.
Surgical techniques. After training, rats were stereotaxically implanted bilaterally with eight-wire electrode arrays (NB Labs, Denison, TX) directed at the nucleus accumbens (anteroposterior, 1.2-1.5; mediolateral, 0.7-1.2; dorsoventral, 7.0-7.5). Arrays were composed of blunt-cut stainless-steel 50 μm wires in a 2 × 4 arrangement.
Single-unit recording and discrimination. Neural signals were recorded with a unity head stage amplifier, amplified 10,000-fold, and captured digitally using commercial hardware and software (Plexon Instruments, Dallas, TX). Discrimination of individual units was performed off-line using principal component analysis of waveform shape.
Single units were identified by waveform shape, interspike interval, and firing characteristics. In many instances, single units were stable across multiple days. In these cases, only the data gathered from the first day of recording of that unit was included in analyses of firing properties to ensure that the aggregate data did not over-represent stable single units.
Data analysis: sucrose discrimination. Lick bouts were defined to capture periods of near-continuous licking. The first lick delivered to the lick spout after trial onset defined the beginning of each lick bout. The conclusion of a lick bout was defined either by the time of the last lick delivered during the period of reinforcer delivery (i.e., the last reinforced lick) or the onset time of the first interlick interval exceeding 0.5 s, whichever yielded the longest bout. The 0.5 s threshold for interlick interval was chosen because it corresponds to a deep trough in the power spectral density of licking, below a strong peak of ∼7 Hz (data not shown), and thus served to identify discrete bouts of licking. The last reinforced lick was used as the criterion to define the minimum reinforced bout length to ensure that reinforced licks were never included in unreinforced bouts (UBs).
Often, rats engaged in licking behavior during the intertrial interval, when no liquid was ever delivered. The 0.5 s interlick interval criterion was used to define discrete bouts of licking confined entirely to this intertrial interval (designated UBs). Interlick intervals >0.5 s marked the conclusion of one lick bout and the initiation of the subsequent lick bout.
Significant changes in neural activity occurring during lick bouts were identified in two ways. First, firing during all reinforced lick bouts occurring in a single session was grouped and compared with firing during a baseline period, a 2 s interval beginning 4 s before licking bout onset. This baseline period occurred during the obligatory pretrial interval in which no licks occurred and excluded the 2 s just before the onset of lick bouts, when transient modulations apparently related to approach behaviors were common. Significant inhibitions and excitations were identified using the nonparametric Mann-Whitney rank sum test using commercial software (SigmaStat; SPSS, Chicago, IL). Second, single units with neural firing discriminating between sucrose concentrations were identified using nonparametric one-way ANOVA (Kruskal-Wallis test), comparing firing rates during lick bouts for different solutions. For all neurons showing significant results in the ANOVA, Dunn's post hoc test was used to identify significant pair-wise differences (p < 0.05).
Some NAcc neurons showed especially transient phasic excitations or inhibitions confined to bout onset. In cases in which the change in firing rate in the first second of the bout was at least threefold more than the change calculated over the entire bout, the firing rate in the first second after bout onset was used for statistical analysis.
Very brief lick bouts (≪1 s) had the potential to greatly increase the trial-by-trial variability in spike frequencies calculated over the duration of the lick bout. In NAcc neurons with typically low firing rates, spike rates calculated for these brief bouts varied between low values (usually 0) and very high values (as division by a time interval <1 s inflated calculated spike rate). To avoid this problem, all lick bouts <0.6 s were discarded.
Relative reward encoding. Intervals defining lick bouts and baseline periods used for analysis of firing rates were defined as above (Data analysis: sucrose discrimination). Single units encoding relative reinforcer value were identified using the Mann-Whitney rank sum test by comparing firing during 3% sucrose consumption during the first and second parts of the behavioral session, when that solution was paired with 0 and 10% sucrose, respectively. Firing was normalized to baseline firing rate for this comparison to correct for baseline drift over the course of the behavioral session. Single units in which spike rate was not changed by relative value were tested for modulation by sucrose concentration by comparing firing across lick bouts for 0, 3, and 10% sucrose (combining bouts of 3% sucrose consumption during the first and second parts of the session).
Correlation of bout length/firing rate with time. Satiety encoding in NAcc single units was investigated by calculating changes in normalized firing rate over the course of the behavioral session. Statistical comparison was performed using two-way ANOVA (firing rate vs time in session for each of the three sucrose reinforcers). Time in the behavioral session was binned into “early” and “late” time periods before and after the midpoint of the entire session time, respectively.
The analysis described above detects changes in firing rate occurring over the behavioral session but does not relate the magnitude of any such changes to the magnitude of satiety-related behavioral changes occurring during that time frame. To more explicitly examine potential covariation of firing rate and bout length over behavioral sessions in the entire population of palatability-modulated units, a two-part analysis was used. For each unit, the correlation coefficient relating firing rate and time elapsed in the session was calculated for each sucrose reinforcer. This calculation was repeated for bout length versus time. The correlation between these coefficients was then calculated. Correlation coefficients, rather than raw slope values, were used in this comparison, because the correlation coefficient provides a bounded measure of the strength of the association between the correlated variables. Spearman's rank order correlation was used to test for correlations between bout length/firing rate and time.
Onset/offset of changes in neural activity. To examine the timing of changes in NAcc neural firing relative to the initiation of licking behavior, onset and offset times of significant changes in neural firing rate were calculated from all lick bouts lasting 2 s or longer. This allowed analysis of neural firing during well defined, prolonged periods of consummatory behavior. The mean firing rate during the time window of 2-4 s before the onset of licking served as the baseline firing rate. For each neuron, a smoothed (boxcar filter with 0.3 s kernel) perievent histogram (100 ms bins) was constructed around the beginning of the lick bout. The onset time of the change in neural activity was defined as the timestamp of the first bin after the baseline period, of eight or more successive bins, with firing rates more than three SDs from the baseline firing rate. This algorithm ensured that only sustained periods of inhibition or excitation were considered in defining onset times. Units that did not meet this requirement (those with noisy histograms or very transient changes in firing rate) were not included in this analysis. Of the total number of units recorded in the sucrose discrimination task, 36 NAcc inhibitions and 24 excitations met the criterion for inclusion in this analysis. A similar procedure was used for calculating offset times, using the timestamp of the last bin of eight or more successive bins with firing rates more than three SDs from the baseline firing.
Calculation of firing rate during unreinforced licking. For NAcc neurons inhibited before and during lick bouts, we determined how neural firing rate varied as a function of unreinforced licking behavior, which occurred between periods of reinforcer delivery. To do so, we first defined the time interval in each trial that included only bouts of unreinforced licks (unreinforced interval); this period began with the last lick of a reinforced lick bout and ended with the first lick of the subsequent reinforced lick bout. For each neuron, we calculated both firing rate and lick rate in a 4 s time window beginning at the onset of this unreinforced interval. We then advanced this 4 s window in 1 s increments across the entire unreinforced interval, calculating firing rate and lick rate at each point. This analysis was repeated for each trial occurring in the behavioral session.
Histology. Rats were deeply anesthetized with pentobarbital, and recording sites were marked by passing 20 μA current for 20 s through each electrode. Rats were perfused with a solution of 10% formaldehyde and 3% potassium ferricyanide to mark sites of iron deposition. Brains were cryoprotected in 25% sucrose, 40 μm coronal sections were cut on a freezing microtome, and sections were mounted, dried, and coverslipped. Recording sites were located under a light microscope and recorded on atlas figures adapted from Paxinos and Watson (1997).
Results
Behavior in the sucrose discrimination task
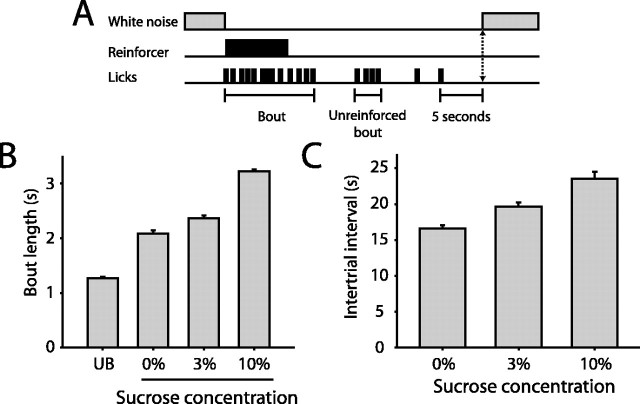
Multiple measures of behavior in the sucrose discrimination task (Fig. 1A) confirmed that the palatability of sucrose reinforcers increases monotonically with increasing sucrose concentration. For reinforced lick bouts, mean bout time averaged 3.2 ± 0.03 s for 10% sucrose (mean ± SEM), decreased to 2.4 ± 0.05 s for 3% sucrose, and decreased further to 2.1 ± 0.07 s for 0% sucrose (Fig. 1B). Bouts of unreinforced licking (termed unreinforced bouts) were shortest, averaging 1.3 ± 0.8 s. All differences were significant (F(3,4081) = 1358.1, p < 0.001, one-way ANOVA; p < 0.05 for all post hoc comparisons). The sucrose discrimination task required an obligatory 5 s period without licking to initiate each new trial; thus, the intertrial interval, measured from the end of a reinforced lick bout to the onset of a new trial, was determined by the animals' ability to withhold licking for this period. Intertrial intervals varied significantly as a function of the palatability of the reinforcer delivered (F(2, 4790) = 304.6; p < 0.001). Intertrial intervals were longest after 10% sucrose delivery (23.6 ± 1.0 s) and significantly shorter for 3 and 0% sucrose (19.6 ± 0.6 and 16.6 ± 0.5 s, respectively; p < 0.05 for all comparisons).
Figure 1.
Sucrose discrimination paradigm and behavioral performance. A, Schematic of task structure. The first lick delivered after trial initiation, signaled by white noise onset, resulted in a brief period of reinforcer availability. Subjects were required to withhold licking for 5 s to initiate a new trial. Bouts designate licking episodes during reinforcer delivery, and unreinforced bouts indicate licking episodes during which no reinforcer was delivered. B, Mean bout length increased monotonically with increasing sucrose concentration (p < 0.05 for all post hoc comparisons) for 0, 3, and 10% sucrose reinforcers. C, Mean intertrial interval increased with increasing sucrose concentration (p < 0.05 for all post hoc comparisons; data taken from 22 behavioral sessions in 14 rats for B and C).
Overview of neural firing patterns in the sucrose discrimination task
We recorded a total of 220 single units in 19 rats performing the sucrose discrimination paradigm (Table 1, sucrose discrimination paradigm). Of the units with significant changes in neural activity occurring during consumption, inhibitions (62 units) were more common than excitations (27 units).
Table 1.
Summary of firing patterns
|
|
Palatability modulated |
Not palatability modulated |
Total |
|
|---|---|---|---|---|
| Sucrose discrimination paradigm (n = 220 units)a | ||||
| Excitation | 20 | 7 | 27 | |
| Inhibition | 5 | 57 | 62 | |
| None | 2 | 2 | ||
| Total | 27 | 64 | 91 | |
| Contrast paradigm (n = 237 units)b | ||||
| Relative value | 8 | |||
| Palatability | 16 | |||
| Total |
|
|
24 |
|
Modulations recorded in the sucrose discrimination paradigm. The first column shows type of modulation relative to baseline firing.
Modulations recorded in the contrast paradigm.
NAcc neurons encode reinforcer palatability
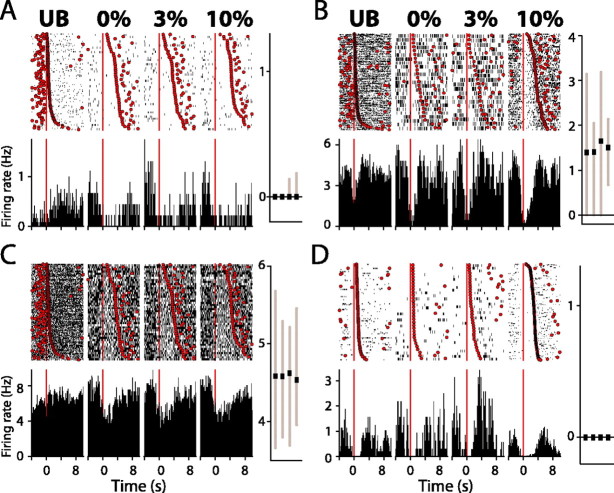
Of the 220 neurons recorded in the sucrose discrimination paradigm, a total of 27 were modulated by reinforcer palatability: firing rate in these neurons varied as a function of the reinforcer consumed. Somewhat surprisingly, given the relative rarity of excitations in the total population, a majority of these firing patterns were excitations (20 of 27; 74%) (Fig. 2A-E). Inhibitions encoding reinforcer palatability were less common (Fig. 2F), comprising 19% (5 of 27) of the units sensitive to palatability. Firing in two units was sensitive to sucrose concentration but not significantly different from baseline levels overall.
Figure 2.
Nucleus accumbens neurons are modulated by reinforcer palatability. A-F, Example units. Each panel shows a raster plot (top) and histogram (bottom) constructed around the onset of licking bouts (t = 0; red line) for (left to right) UB, 0, 3, and 10% sucrose reinforcers. Rasters are rank ordered by bout length, with the terminal lick of each bout shown (red circles). Bar charts show 25th, median, and 75th percentile spike rates for (left to right) UB, 0, 3, and 10% sucrose. Significant differences (p < 0.05; post hoc comparisons) in firing are indicated in the top portion of each bar chart (horizontal bars). A-E illustrate excitatory responses typical of NAcc neurons modulated by reinforcer palatability. F illustrates an example unit inhibited during sucrose consumption.
Palatability-driven changes in neural activity typically began at short latency after bout onset (onset time, 150 ± 176 ms; mean ± SEM) and subsided before the termination of the bout (offset, 644 ± 234 ms before bout termination). There was considerable diversity in the nature of the response occurring during lick bouts. The example unit shown in Figure 2A responded to all sucrose reinforcers at very short latency after bout onset, with the excitatory response peaking ∼350 ms after bout onset. In contrast, the example unit shown in Figure 2D responded more selectively, with significantly elevated firing rates occurring only during 10% sucrose consumption, and at longer latency, with peak response ∼1.7 s after bout onset. Almost all units (25 of 27; 93%) showed increased firing with increasing sucrose concentration. In the remaining two units, both of which were inhibited during lick bouts, inhibition increased with increasing sucrose concentration and was maximal during 10% sucrose consumption.
Rats frequently licked the lick spout during intertrial intervals. These unreinforced bouts allowed us to dissociate the impact of sensory and motor variables on NAcc firing rates, because sucrose reinforcers were never available during these licking episodes. Changes in neural activity occurring during unreinforced bouts of licking were uncommon, occurring in only two of the total 27 units. Thus, for the large majority of palatability-encoding units, changes in neural activity were exclusively driven by sensory information and not by the act of licking itself.
Satiety effects on NAcc neurons encoding palatability
Food intake is tightly regulated by antagonistic neural pathways promoting and suppressing eating (Smith, 1996). The latter include feedback pathways originating in the periphery that signal gastric distention after food ingestion (Berthoud, 2002). Such satiety signals are important modulators of palatability (Foster et al., 1996; Yeomans et al., 2001). Thus, we sought to determine whether firing in NAcc neurons encoding reinforcer palatability was also influenced by satiety developing over the course of experimental sessions.
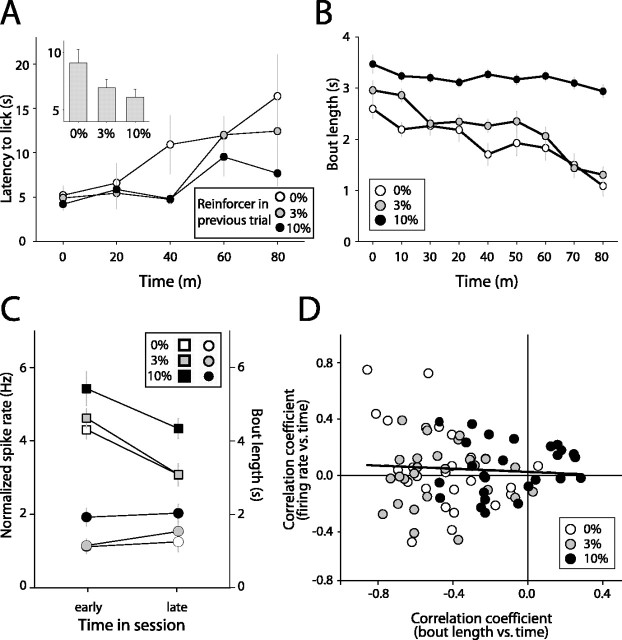
Two behavioral measures suggested that the reinforcing value of sucrose solutions declined over the course of the experimental session, consistent with increasing satiety. Mean latency to begin licking after trial initiation increased significantly over the course of the behavioral session (Fig. 3A) (main effect of time, two-way ANOVA, F(4, 2529) = 5.886; p < 0.001). In addition, mean bout length decreased significantly over the course of the behavioral session (Fig. 3B) (main effect of time, two-way ANOVA, F(8, 2580) = 8.659; p < 0.001). The decline in bout length was significant for each of the three sucrose concentrations (p < 0.05, within factors comparison).
Figure 3.
Satiety signaling in nucleus accumbens neurons. A, Mean latency to lick after trial initiation increased over the course of the behavioral session as a function of the reinforcer received in the previous trial. Inset shows mean latency for each of the three reinforcers. B, Mean bout length devoted to consumption of each of the three sucrose reinforcers declined significantly over the behavioral session (all p < 0.05). C, In a typical palatability-modulated neuron, firing rate remained constant over the course of the behavioral session, even as mean lick bout length diminished significantly (all p < 0.05 for bout lengths; all p > 0.05 for spike rates; squares indicated bout lengths; circles indicate firing rates). D, In the entire population of palatability-modulated units, firing rate and bout length did not covary over the course of the behavioral session (r = 0.07; p > 0.05).
In the majority of palatability-encoding units (24 of 27), firing rates remained stable over the course of the behavioral session. This was true even in cases in which lick bout lengths devoted to sucrose consumption declined substantially over the same period (Fig. 3C). In a single neuron, firing decreased significantly, in parallel with declining lick bout lengths; spike rates increased in two units, even as lick bout lengths declined significantly (data not shown). In the population of palatability-encoding neurons, there was no tendency for firing rate and bout length to covary during behavioral sessions (Fig. 3D) (p > 0.05). The absence of consistent correlations in NAcc firing with behavioral measures of satiety suggests that satiety-related inputs are mostly absent from NAcc palatability-encoding units.
Palatability encoding in the nucleus accumbens
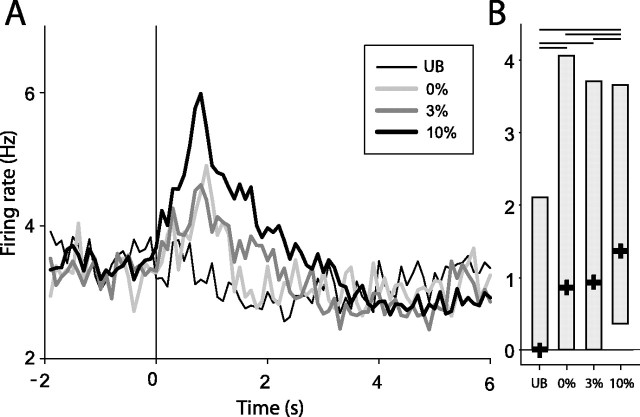
The mean response of all 27 NAcc units modulated by reinforcer palatability is shown in Figure 4. Analysis of the mean change in neural activity confirmed a significant main effect of the reinforcer consumed on neural firing in this population of neurons (F(3, 5328) = 278.52; p < 0.001). All pair-wise comparisons were significant (p < 0.05), except for firing during 0 versus 3% sucrose.
Figure 4.
A, Histogram of mean firing for all units modulated by reinforcer palatability (n = 27) constructed around bout onset (t = 0). B, Median firing for units modulated by reinforcer palatability. Box plots show 25th, median, and 75th percentile spike rates for each bout type, with significant differences shown with horizontal bars (p < 0.05; post hoc comparisons).
It is interesting to note that the similar level of neural activity evoked by 0 and 3% sucrose was mirrored by behavioral measures showing small differences in preference for these two reinforcers. Overall, bout length was significantly longer during consumption of 3% sucrose (Fig. 1B). However, preference for 3% sucrose relative to 0% sucrose was small (averaging 14% longer lick bouts; compared with 36% longer bouts for 10 vs 3%, and 55% longer bouts for 10 vs 0% sucrose). It was also variable over behavioral sessions, because bout lengths devoted to 3% sucrose consumption were significantly longer than those for 0% sucrose in only 7 of 22 behavioral sessions (compare with 18 of 22 sessions in which 10% bouts were significantly longer than 3% bouts). These findings raised the possibility that NAcc firing is closely aligned with preference and not with the sensory properties of the reinforcer per se. To explore this issue explicitly, we tested NAcc firing using a behavioral contrast paradigm.
Behavior in the contrast paradigm
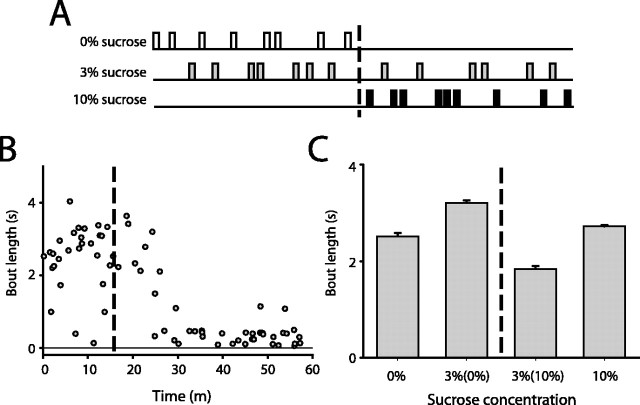
Contrast paradigms provide a simple experimental means of manipulating the relative value of a reinforcer (Flaherty et al., 1994). To probe NAcc encoding of relative reinforcer value, we adapted our initial sucrose discrimination task, presenting only 0 and 3% sucrose during the initial period of each behavioral session and 3 and 10% sucrose subsequently (Fig. 5A). This contrast paradigm significantly decreased the reinforcing value of the 3% sucrose solution, as assessed by the significant decrease in bout lengths devoted to 3% sucrose consumption across these periods (Fig. 5C) (main effect of solution, F(3,3016) = 322.2, p < 0.001; p < 0.05 for all post hoc comparisons).
Figure 5.
Schematic of contrast paradigm. A, Zero or 3% sucrose was delivered in the first portion of the behavioral session and 3 or 10% sucrose in the second part of the session. B, Bout lengths devoted to 3% sucrose consumption in an example session declined rapidly when the paired solution was switched from 0 to 10% sucrose (broken line). C, Mean bout length devoted to consumption of 3% sucrose declined significantly when paired with 10% sucrose, relative to pairing with 0% sucrose (p < 0.05 for all post hoc comparisons, one-way ANOVA; 19 behavioral sessions in 10 rats).
NAcc neurons encode relative reinforcer value
We recorded from a total of 237 single units in the NAcc of 19 rats during performance in the contrast paradigm (Table 1, contrast paradigm). Twenty-four units (10%) showed firing rates that varied significantly as a function of sucrose concentration. Of these, 8 units (30%) showed changes in firing rates, which varied as a function of the relative value of the 3% sucrose solution. In all of these units, responses during 3% sucrose consumption were greatest in the first part of the behavioral session (when paired with 0% sucrose) and diminished during consumption of 3% sucrose paired with 10% (Fig. 6). As for palatability-encoding units recorded in the sucrose discrimination task, responses began at short latency after the onset of licking. Spike rates in the majority of units were not significantly different from baseline during unreinforced licking; a single unit was excited during unreinforced bouts. Analysis of the population response of all units encoding relative reinforcer value (n = 8) confirmed that the mean excitatory response was significantly smaller during consumption of the devalued 3% sucrose solution (paired with 10% sucrose), relative to 3% paired with 0% sucrose (Fig. 6F) (p < 0.05 for post hoc comparison). Because we found few correlates of satiety encoding in palatability-encoding neurons (Fig. 3C,D), this decrease in the mean firing rate is unlikely to have been driven by the development of satiety. Furthermore, although the mean neural activation occurring during 3% sucrose consumption declined when 10% sucrose was made available, responses occurring during 10% sucrose were robust during this period, consistent with NAcc encoding of relative value.
Figure 6.
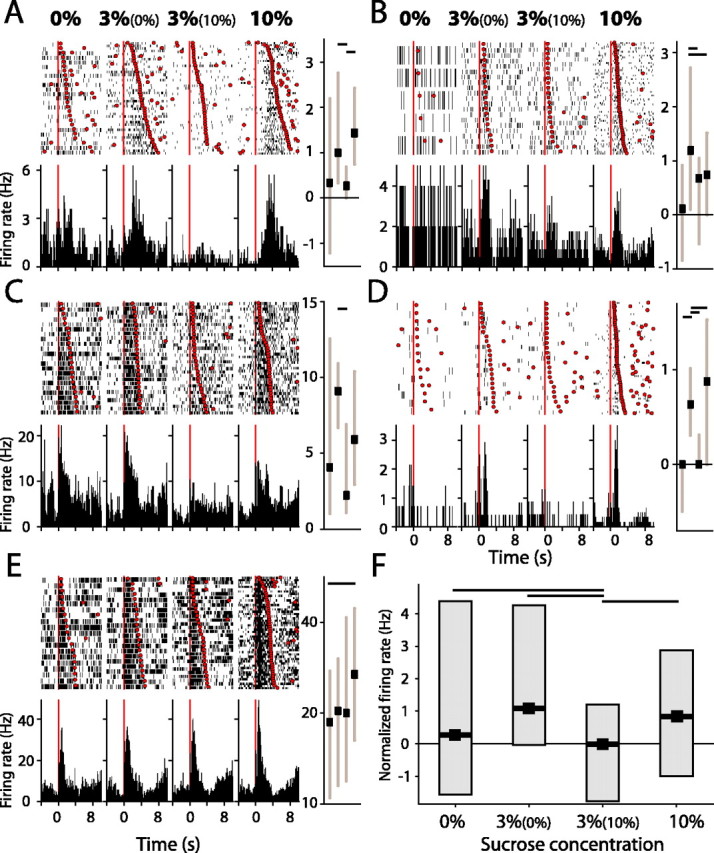
Nucleus accumbens neurons are modulated by relative reinforcer value. A-D, Example units show a significant change in firing during 3% sucrose consumption as a function of the paired sucrose reinforcer. Responses to 0% sucrose, 3% (paired with 0%), 3% (10%), and 10% sucrose are shown in each panel; all other conventions are identical to those in Figure 2. Spike rates in bar charts are normalized to baseline firing. E, Example unit in which firing is not altered by contrast paradigm. F, Median firing for all units modulated by the relative value of 3% sucrose solution (n = 8). Horizontal bars show significant differences in firing rate (p < 0.05, post hoc comparisons, one-way ANOVA).
Firing in the remainder of the palatability-encoding units recorded in the contrast paradigm was driven by the sensory properties of sucrose reinforcers rather than changes in the relative value of sucrose reinforcers (Fig. 6E). These data suggest that NAcc neurons represent sensory information at multiple hierarchal levels of sensory processing. Although encoding in a subset of neurons reflects the sensory properties of a given reinforcer, firing in a distinct subset is related specifically to the relative value of that reinforcer, information that might be used to more directly guide behavior.
Onset of NAcc inhibitions precedes initiation of consummatory behavior
Of all firing patterns identified during the sucrose discrimination task, inhibitions occurring during lick bouts were by far the most common (62 of 91; 68%). Two features of this class of NAcc neurons were particularly distinctive. First, inhibitions were much less likely to be modulated by palatability than excitations. Second, in contrast to excitations, the onset of inhibitions typically preceded the initiation of licking.
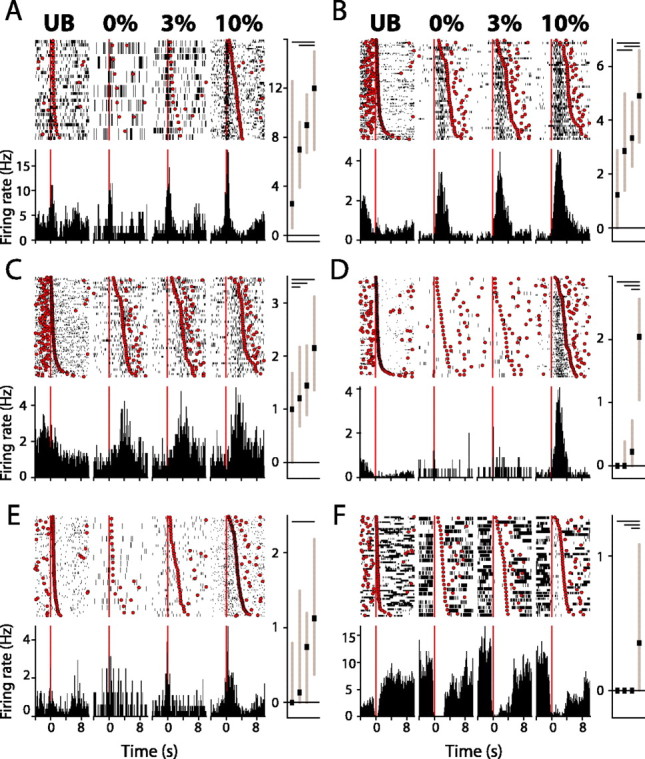
Firing in most inhibited NAcc neurons did not vary with reinforcer palatability (Fig. 7). Only five of 62 units (8%) were sensitive to reinforcer palatability (compare with 20 of 27 excitations encoding palatability, 74%, a significantly higher proportion; p < 0.001, χ2 test). As a class, inhibitions did not discriminate between sucrose reinforcers differing in palatability (Fig. 8A,B) (F(3,11163) = 6.45; p > 0.05), nor did the magnitude of inhibitions occurring during unreinforced licking differ from that occurring during reinforced licking (Fig. 8B) (p > 0.05). These units were inhibited during licking behavior, independent of the receipt of any reinforcer.
Figure 7.
Inhibition of nucleus accumbens units before and during consummatory behaviors. A-D, Examples of nucleus accumbens units inhibited during consummatory behavior. All graph conventions are identical to those in Figure 2. In the typical examples shown, inhibitions were not differentially modulated by sucrose reinforcers or even the presence of any reinforcer (compare UB firing to that occurring during sucrose consumption). In addition, inhibition onset preceded licking bout onset in each example shown.
Figure 8.
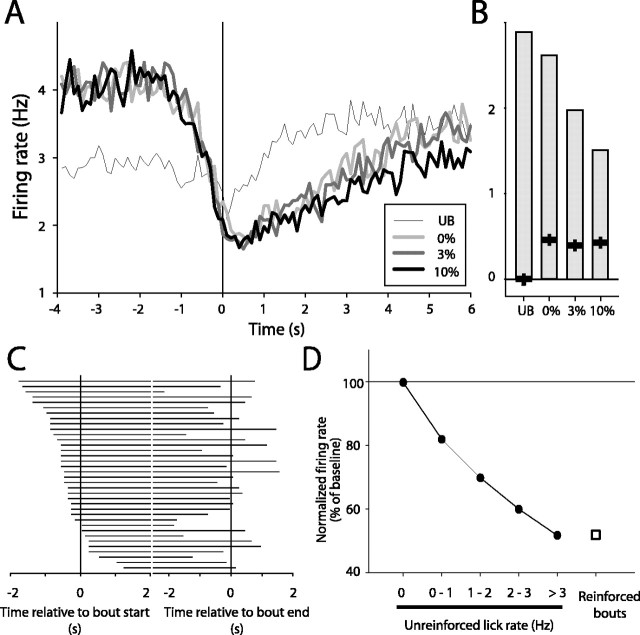
Mean response of nucleus accumbens units inhibited before and during lick bouts. A, Histogram of mean firing for units inhibited during consummatory behavior, constructed around bout onset (t = 0; n = 57). Note that the mean onset of inhibition preceded licking onset. B, Median firing rates; firing rates did not differ as a function of bout type (p > 0.05; one-way ANOVA). All lick bout modulations were significantly inhibited relative to baseline firing rate (median baseline firing, 1.5 Hz; p < 0.05 for all post hoc comparisons). C, Onset of inhibition preceded bout onset in a significant majority of units (27 of 36 units; p < 0.05). D, During unreinforced licking, mean firing rate was inversely proportional to lick rate (p < 0.001; one-way ANOVA; circles; firing isnormalized to spike rate during a baseline period without licking). At high lick rates, the amplitude of firing during unreinforced licking (51.7% of maximal firing) was very similar to that observed during reinforced licking (51.9% of baseline firing; square).
Although the amplitude of inhibitions occurring during reinforced and unreinforced lick bouts did not differ, mean activity in the period before unreinforced bouts was substantially lower than that occurring during the baseline period before reinforced lick bouts (Fig. 8 A). We reasoned that this difference in firing might reflect differences in licking behavior occurring during this period. Although licking before unreinforced bouts was common, the design of the behavioral paradigm precluded licking during the baseline period preceding reinforced bouts. We examined this issue explicitly by calculating the firing rate in these neurons during periods of unreinforced licking only (see Materials and Methods). Indeed, the mean firing rate during this period showed a strong inverse relationship to the lick rate (Fig. 8D) (F(4,109133) = 2048.6; p < 0.001 for one-way ANOVA, comparing all groups). When unreinforced lick rates were high (>3 Hz), the amplitude of the inhibition in these neurons (firing rate, 51.7 ± 0.5% of baseline firing) was very similar to that occurring during reinforced lick bouts (firing rate, 51.9 ± 1.4% of baseline firing). Thus, the low mean firing rate that occurred before unreinforced bouts reflects licking occurring during this period. More importantly, this analysis underscores the absence of sensory modulation in this class of neurons; changes in licking behavior alone fully account for changes in the neural activity of this group of neurons.
The onset of NAcc inhibitions typically occurred before initiation of licking behavior. This was true for a significant majority of all inhibited neurons (75%; 27 of 36 units meeting analysis criteria; p < 0.05, χ2 test) (see Materials and Methods) (Fig. 8C). The mean time of inhibition onset across inhibited neurons preceded the initiation of licking by 425 ± 100 ms and recovered to baseline firing levels just before bout termination (-47.3 ± 161 ms, relative to bout offset). The timing of NAcc inhibitions was generally similar for all lick bouts, including unreinforced bouts (see example neurons in Fig. 7).
Recording sites
Histological analysis was used to confirm placement of electrodes in the NAcc (Fig. 9A). Recording sites included both the core and the shell of the NAcc. All of the firing patterns reported here were encountered in both the core and the shell regions; there was no statistical tendency for preferential localization to either of these compartments (all p > 0.05; χ2 analysis comparing shell vs core recording sites), nor did firing properties, such as baseline firing rate and peak evoked firing rate, differ significantly between core and shell recording sites (all p > 0.05).
Figure 9.
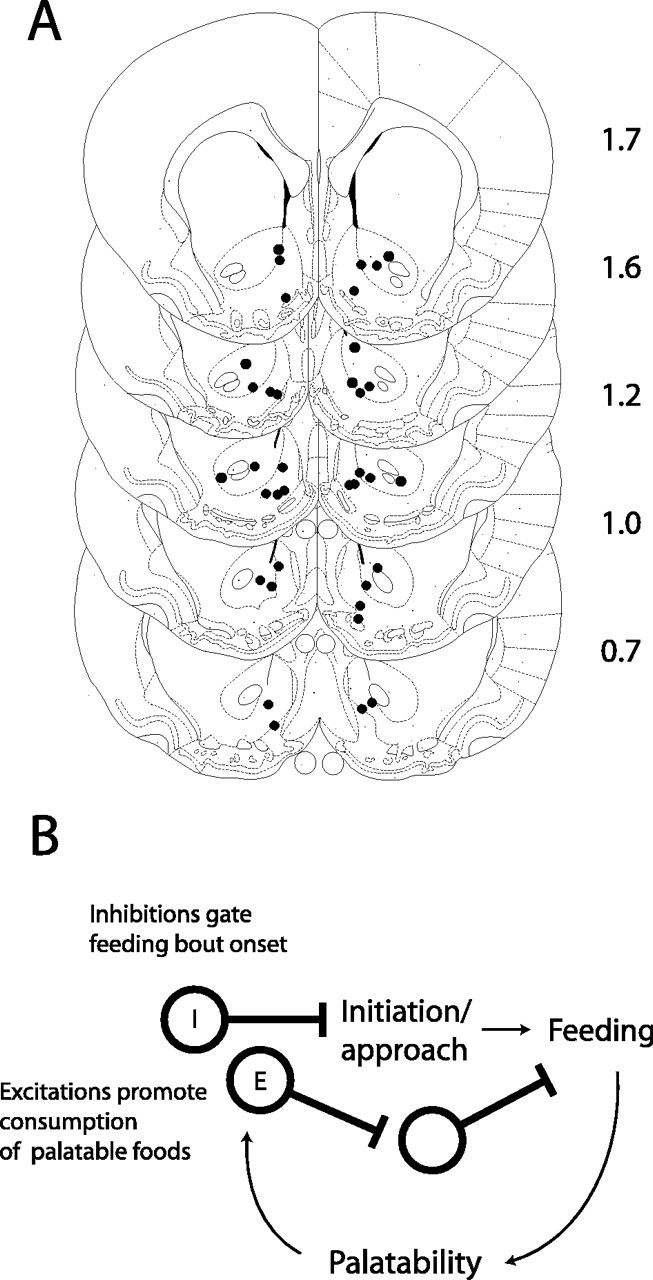
A, Location of recording sites. Anteroposterior coordinates are shown to the right of each coronal section. B, Model for modulation of feeding by the nucleus accumbens. Inhibition (I) onset typically precedes the onset of feeding and thus may gate initiation of consummatory behaviors through disinhibition of downstream areas controlling feeding. Excitations (E) modulated by reinforcer palatability may promote or maintain consumption for especially palatable reinforcers.
Discussion
In this study, we describe a population of NAcc neurons that encode the palatability of sucrose reinforcers. Furthermore, neural activity in a subset of these neurons is not a simple function of the orosensory properties of the reinforcers (i.e., sucrose concentration) but closely tracks the animals' preference for a given reinforcer, as assessed by a behavioral contrast paradigm. In the majority of NAcc units, excitations observed during consumption of sucrose were not related to the motor act of consumption, because they failed to respond during periods of unreinforced licking.
A distinct population of NAcc neurons exhibited inhibitions during bouts of consummatory behavior. Firing rates in most NAcc inhibitions were not differentially modulated by reinforcers varying in palatability; indeed, the magnitude of these inhibitions remained unchanged during unreinforced bouts of licking. In addition, in sharp contrast to the excitations, the onset of NAcc inhibitions most often occurred before the initiation of the licking behavior itself. Thus, we propose that two primary classes of NAcc neurons make distinct contributions to neural processing immediately before and during reinforcer consumption: excitations that encode reinforcer palatability and inhibitions related to the initiation and maintenance of consummatory motor behaviors.
Findings from some previous studies suggest that the sensory properties of natural reinforcers play a minimal role in modulating NAcc neural activity. For instance, a comparison of the NAcc neural correlates of water and ∼20% sucrose consumption concluded that most NAcc units do not discriminate between these natural reinforcers (Roop et al., 2002). In a different study, firing patterns related to reward receptacle entry occurred independent of the delivery of the reinforcer itself (Nicola et al., 2004b). These results agree well with the functional properties that we described in the class of NAcc neurons inhibited during consummatory behavior. We have shown that the amplitude of inhibition in this class of neurons is independent of the sensory properties of the reinforcer. Instead, the amplitude of inhibition is tightly correlated with licking behavior, and furthermore, the onset time of these inhibitions precedes licking behavior itself (Fig. 8). These characteristics converge in suggesting that these neurons encode information related to the motor act of initiating and sustaining a consummatory bout rather than the sensory properties of consumed items.
Pharmacological studies are consistent with a role for these neurons in guiding the initiation of consummatory behavior. Inhibition of activity in the NAcc shell, through blockade of glutamatergic signaling or application of GABA receptor agonists, induces feeding reminiscent of lateral hypothalamic stimulation (Maldonado-Irizarry et al., 1995; Stratford and Kelley, 1997). These pharmacological results, together with the present electrophysiological findings, suggest that the class of NAcc neurons with inhibitions beginning before licking bouts exerts tonic inhibition on downstream areas controlling food intake; under normal conditions, this inhibition must be relieved to permit initiation of a feeding bout. Pharmacological inhibition of these neurons, then, would drive feeding through sustained disinhibition of neural loci controlling food intake. Evidence suggests these target areas include the ventral tegmental area, lateral hypothalamus, and nucleus of the solitary tract (Will et al., 2003). Intriguingly, two recent reports suggest that this class of NAcc inhibitions interface with hypothalamic brain regions signaling hunger and satiety. Infusion of muscimol into the NAcc shell results in an increase in c-fos labeling in lateral hypothalamic neurons containing orexin (Baldo et al., 2004) and a concomitant decrease in c-fos labeling in proopiomelanocortin-containing neurons in the arcuate nucleus, which comprise a critical node in the neural pathways signaling satiety (Zheng et al., 2003).
Schultz and collaborators have shown that excitations in neurons of the ventral striatum of primates can be finely tuned to the sensory properties of reinforcers, because they are correlated with both the magnitude and identity of a juice reward (Apicella et al., 1991; Hollerman et al., 1998; Hassani et al., 2001; Cromwell and Schultz, 2003). Findings from Hassani et al. (2001) are particularly intriguing in suggesting ventral striatal encoding of preference, because preferred juice reinforcers often elicited more firing than nonpreferred counterparts (Hassani et al., 2001). Our results show that when reward preference is explicitly varied using a contrast paradigm, a subset of NAcc excitations is sensitive to the relative value of a sucrose reinforcer. Representation of preference in the NAcc suggests this nucleus may closely guide behavior, consistent with the well known proposal that the NAcc serves as a limbicmotor interface (Mogenson et al., 1980).
NAcc units encoding relative reinforcer value comprise a relatively small fraction of the total sample of units we isolated. These findings are consistent with previous studies of striatal encoding, in that only a small fraction of all neurons is modulated by any given task element (Cromwell and Schultz, 2003; Nicola et al., 2004a).
It is possible that changes in the periphery, such as adaptation of gustatory receptor signaling, could contribute to the changes in NAcc firing that we observed in the contrast paradigm. We cannot rule out this possibility, but we think it unlikely for two reasons. First, adaptation in the neural response of taste cells to the second of two successive sweet stimuli is much reduced by including a control rinse between deliveries of the stimuli (Cummings et al., 1993). However, contrast effects are not diminished when a water rinse is included between alternating trials in a simultaneous contrast paradigm (Grigson and Norgren, 1995). Second, simultaneous contrast effects can be obtained using tastants (e.g., sucrose and polycose) that show little peripheral cross-adaptation (Cummings et al., 1993; Rehnberg et al., 1996).
We did not find evidence of satiety-driven changes in the firing rate of palatability-encoding neurons (Fig. 3C,D). This result argues that satiety effects do not contribute to the decrement in excitatory responses during 3% sucrose consumption in the contrast paradigm (Fig. 6). However, our results do not rule out the possibility that satiety signaling may drive firing in NAcc neurons. Rats in our experimental paradigm consumed a relatively small caloric load (mean, 3.8 ± 0.4 kcal) over experimental sessions lasting up to 2 h. Studies manipulating palatability through preloading typically use a more caloric load infused in a single bolus (Zhao and Cabanac, 1994; Hajnal et al., 1999). Studies of the effects of similar large, acutely administered preloads on NAcc firing are needed to further explore a potential role for satiety signals in modulating palatability encoding in this brain region.
NAcc units encoding palatability represent a potential electrophysiological substrate for the pharmacological effects of opioids on feeding behavior. Both systemic and intra-NAcc infusion of μ-opioid receptor agonists specifically increase intake of especially palatable reinforcers and increase positive taste-reactivity displays (Gosnell et al., 1990; Pecina and Berridge, 2000; Kelley et al., 2002). Endogenous opioids in the NAcc modulate palatability under normal conditions, because the nonspecific opioid antagonist naloxone reduces sucrose (but not chow) intake when infused directly into the NAcc (Kelley et al., 1996). We hypothesize that opioids exert their behavioral effects by acting on the class of NAcc neurons modulated by reinforcer palatability, presumably by increasing neural activity in this class of excitations. Although the direct effects of opioid ligands are characteristically inhibitory, net excitatory effects could result through disinhibitory circuit mechanisms, as has been demonstrated in the ventral tegmental area (Johnson and North, 1992) and the nucleus raphe magnus (Pan et al., 1990).
In our experiments, palatability-encoding excitations were not present during bouts of unreinforced licking, in contrast to recent findings of reward-independent excitations occurring during reward receptacle entry in a discriminative stimulus task (Nicola et al., 2004b). We speculate that cue-driven reward expectation may account for the difference between these findings. The auditory cue signaling reinforcer availability in our sucrose discrimination task was never predictive of reinforcer identity, in contrast to the tone predictive of reward used in the discriminative stimulus task. Thus, cue-driven expectation is likely to play an important role in NAcc firing patterns occurring during the time of expected reinforcer delivery. This proposal is consistent with a recent report of novel NAcc reward receptacle-related excitations that appear during the extinction phase of a reinstatement paradigm (Janak et al., 2004).
NAcc neurons that are inhibited before and during feeding bouts may, in addition to controlling feeding bouts through disinhibition, promote competing behaviors, as proposed by Nicola et al. (2004). There is experimental evidence supporting this hypothesis, as in rats performing an operant task for sucrose reward, ∼50% of the NAcc neurons possessing operant-related phasic excitations were also inhibited during reinforcer consumption (Nicola et al., 2004b).
The results we present here, along with many recent findings from other investigators (Kelley et al., 2002; Zheng et al., 2003; MacDonald et al., 2004), highlight the critical role of the ventral striatum in guiding food intake, particularly that which is driven by palatability. Our data, together with previous behavioral work, are consistent with a simple model positing a dual role for the accumbens in modulating food intake (Fig. 9B). NAcc neurons that are inhibited before and during consummatory behaviors could serve to gate the initiation of feeding bouts by disinhibition of target areas controlling food intake. Consequently, NAcc excitations could serve to maintain feeding when especially palatable foods are detected. Medium spiny projection neurons in the NAcc are GABAergic; thus, the presumptive excitatory effects of these palatability-encoding units on target areas are hypothesized to occur through a disynaptic circuit involving an additional inhibitory synapse.
Palatability-driven food intake is one factor importantly linked to obesity. Our results underscore the value of additional study of the role of the ventral striatum in controlling consummatory behavior, because elucidation of function in this brain region could provide novel targets for obesity treatments.
Footnotes
This work was supported by a grant from the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco; the Wheeler Center for the Neurobiology of Addiction; the Ernest Gallo Clinic and Research Center; and National Institute on Drug Abuse Grants DA01949 and NS21445 (H.L.F.). We thank R. Van and V. Kharazia for histology assistance and J. Woolley, P. Newton, S. Nicola, and P. Janak for helpful discussions.
Correspondence should be addressed to Sharif A. Taha, Ernest Gallo Clinic and Research Center, 5858 Horton Street, Suite 200, Emeryville, CA 94608. E-mail: staha@phy.ucsf.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251193-10$15.00/0
References
- Apicella P, Ljungberg T, Scarnati E, Schultz W (1991) Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res 85: 491-500. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE (2004) Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci 19: 376-386. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28: 309-369. [DOI] [PubMed] [Google Scholar]
- Berthoud HR (2002) Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev 26: 393-428. [DOI] [PubMed] [Google Scholar]
- Carelli RM (2002) Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. “natural” reinforcement. Physiol Behav 76: 379-387. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wondolowski J (2003) Selective encoding of cocaine versus natural rewards by nucleus accumbens neurons is not related to chronic drug exposure. J Neurosci 23: 11214-11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG, Crumling AJ (2000) Evidence that separates neural circuits in the nucleus accumbens encode cocaine versus “natural” (water and food) reward. J Neurosci 20: 4255-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell HC, Schultz W (2003) Effects of expectations for different reward magnitudes on neuronal activity in primate striatum. J Neurophysiol 89: 2823-2838. [DOI] [PubMed] [Google Scholar]
- Cummings TA, Powell J, Kinnamon SC (1993) Sweet taste transduction in hamster taste cells: evidence for the role of cyclic nucleotides. J Neurophysiol 70: 2326-2336. [DOI] [PubMed] [Google Scholar]
- Flaherty CF, Turovsky J, Krauss KL (1994) Relative hedonic value modulates anticipatory contrast. Physiol Behav 55: 1047-1054. [DOI] [PubMed] [Google Scholar]
- Foster LA, Nakamura K, Greenberg D, Norgren R (1996) Intestinal fat differentially suppresses sham feeding of different gustatory stimuli. Am J Physiol 270: R1122-R1125. [DOI] [PubMed] [Google Scholar]
- Gosnell BA, Krahn DD, Majchrzak MJ (1990) The effects of morphine on diet selection are dependent upon baseline diet preferences. Pharmacol Biochem Behav 37: 207-212. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Norgren R (1995) Comparison processes contribute to behavioral and electrophysiological responsiveness to gustatory stimuli. In: Looking at ingestive consumption and kinetics symposium (Frankmann SP, Haupt TA, Moran TH, Cooper SJ, eds). Pueblo, CO: University of Southern Colorado.
- Hajnal A, Takenouchi K, Norgren R (1999) Effect of intraduodenal lipid on parabrachial gustatory coding in awake rats. J Neurosci 19: 7182-7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Cromwell HC, Schultz W (2001) Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol 85: 2477-2489. [DOI] [PubMed] [Google Scholar]
- Hill JO, Peters JC (1998) Environmental contributions to the obesity epidemic. Science 280: 1371-1374. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W (1998) Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol 80: 947-963. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chen MT, Caulder T (2004) Dynamics of neural coding in the accumbens during extinction and reinstatement of rewarded behavior. Behav Brain Res 154: 125-135. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12: 483-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ (1996) Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther 278: 1499-1507. [PubMed] [Google Scholar]
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M (2002) Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav 76: 365-377. [DOI] [PubMed] [Google Scholar]
- MacDonald AF, Billington CJ, Levine AS (2004) Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res 1018: 78-85. [DOI] [PubMed] [Google Scholar]
- Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15: 6779-6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69-97. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL (2004a) Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol 91: 1840-1865. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Yun IA, Wakabayashi KT, Fields HL (2004b) Firing of nucleus accumbens neurons during the consummatory phase of a discriminative stimulus task depends on previous reward predictive cues. J Neurophysiol 91: 1866-1882. [DOI] [PubMed] [Google Scholar]
- Pan ZZ, Williams JT, Osborne PB (1990) Opioid actions on single nucleus raphe magnus neurons from rat and guinea-pig in vitro J Physiol (Lond) 427: 519-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates. New York: Academic.
- Pecina S, Berridge KC (2000) Opioid site in nucleus accumbens shell mediates eating and hedonic “liking” for food: map based on microinjection Fos plumes. Brain Res 863: 71-86. [DOI] [PubMed] [Google Scholar]
- Rehnberg BG, MacKinnon BI, Hettinger TP, Frank ME (1996) Analysis of polysaccharide taste in hamsters: behavioral and neural studies. Physiol Behav 59: 505-516. [DOI] [PubMed] [Google Scholar]
- Roop RG, Hollander JA, Carelli RM (2002) Accumbens activity during a multiple schedule for water and sucrose reinforcement in rats. Synapse 43: 223-226. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK (2002) The need to feed: homeostatic and hedonic control of eating. Neuron 36: 199-211. [DOI] [PubMed] [Google Scholar]
- Smith GP (1996) The direct and indirect controls of meal size. Neurosci Biobehav Rev 20: 41-46. [DOI] [PubMed] [Google Scholar]
- Stratford TR, Kelley AE (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17: 4434-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Franzblau EB, Kelley AE (2003) Nucleus accumbens μ-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J Neurosci 23: 2882-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Rolls ET, Leonard CM, Stern C (1993) Neuronal responses in the ventral striatum of the behaving macaque. Behav Brain Res 55: 243-252. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2000) Obesity: preventing and managing the global epidemic. Geneva: World Health Organization. [PubMed]
- Yeomans MR, Lee MD, Gray RW, French SJ (2001) Effects of test-meal palatability on compensatory eating following disguised fat and carbohydrate preloads. Int J Obes Relat Metab Disord 25: 1215-1224. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE (2002) Intake of saccharin, salt, and ethanol solutions is increased by infusion of a μ opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 159: 415-423. [DOI] [PubMed] [Google Scholar]
- Zhao C, Cabanac M (1994) Experimental study of the internal signal of alliesthesia induced by sweet molecules in rats. Physiol Behav 55: 169-173. [DOI] [PubMed] [Google Scholar]
- Zheng H, Corkern M, Stoyanova I, Patterson LM, Tian R, Berthoud HR (2003) Peptides that regulate food intake: appetite-inducing accumbens manipulation activates hypothalamic orexin neurons and inhibits POMC neurons. Am J Physiol Regul Integr Comp Physiol 284: R1436-R1444. [DOI] [PubMed] [Google Scholar]