Abstract
Dopaminergic modulation of glutamate neurotransmission in prefrontal cortex (PFC) microcircuits is commonly perceived as a basis for cognitive operations. Yet it appears that although the control of recurrent excitation between deep-layer prefrontal pyramids may involve presynaptic and postsynaptic D1 receptor (D1R) mechanisms, pyramid-to-interneuron communication will engage a postsynaptic D1R component. The substrate underlying such target-specific neuromodulatory patterns was investigated in the infragranular PFC with immunoelectron microscopy for D1R and parvalbumin, a marker for fast-spiking interneurons. In addition to their proverbial postsynaptic expression, gold-labeled D1Rs were distinctly distributed on perisynaptic/extrasynaptic membranes and the axoplasm of 13% of excitatory-like, presumably glutamatergic varicosities. Most importantly, presynaptic D1Rs were highly specific with regard to the cellular compartment and neurochemical identity of the postsynaptic neuron, being present in spine-targeting varicosities but distinctly absent from those synapsing with parvalbumin profiles often coexpressing D1Rs. We define therein an axonal D1 heteroreceptor component, apparently mediating volume neurotransmission, yet strategically positioned to convey target cell-specific modulation of the glutamatergic drive. We also indicate that presynaptic D1R mechanisms may indeed be associated with recurrent excitation in prefrontal microcircuits, consistent with physiological evidence for a role of these receptors in modulating the persistent activity-profile of neurons essential for working memory.
Keywords: dopamine D1 receptor, glutamate, parvalbumin, presynaptic, prefrontal cortex, recurrent excitation, persistent activity, working memory
Introduction
The primate prefrontal cortex (PFC) receives a wealth of mesocortical dopaminergic (DAergic) input, critically implicated in “executive” cognitive functions, planning and reward, whereas dysregulation of DA signaling is manifest in a variety of neuropsychiatric disorders (Goldman-Rakic, 1987, 1995; Lidow et al., 1998; Carlsson et al., 2001; Frankle et al., 2003). It is also well appreciated that cortical DA may exert “diffuse,” so-called volumetric signaling via nonsynaptic DA receptors (DARs) on pyramidal principal cells and nonpyramidal interneurons (for review, see Goldman-Rakic et al., 1999; Zoli et al., 1999; Pickel, 2000). It is agreed, however, that if DAergic effects on the cortical circuitry were not pervasive but rather compartmentalized in a modular manner, segregation of extrasynaptic signaling would be fundamental in governing DA physiology. It would constrain volume communication, ensuring an exquisite degree of target specificity in modulating interactions within and between neural ensembles, and, ultimately, cellular fractionation of cognitive processes (Goldman-Rakic, 1996).
Along these lines, studies in behaving nonhuman primates have established that members of the D1-like receptor (D1-LR) subfamily (i.e., D1R and D5R subtypes) (Missale et al., 1998) modulate excitatory transmission in prefrontal microcircuits that generate stimulus-independent persistent activity, a property essential for working memory (Sawaguchi and Goldman-Rakic, 1991; Williams and Goldman-Rakic, 1995). Although the cellular basis for the persistent activity recorded in the PFC is essentially unknown, it is thought to involve D1-LR modulation of pyramidal neuron excitability and presynaptic depression of excitatory transmission in a layer- and input-specific manner (Henze et al., 2000; Gao et al., 2001; Seamans et al., 2001; Urban et al., 2002; Gonzalez-Islas and Hablitz, 2003; for review, see Seamans and Yang, 2004). Moreover, in vitro paired and triple whole-cell recordings in deep PFC layers demonstrate that presynaptic effects can be observed only at synapses between pyramids and not between pyramidal neurons and fast-spiking (FS) interneurons (Gao and Goldman-Rakic, 2003), implying target-cell specificity via compartmentalization of extrasynaptic (i.e., axo-axonic) DA signaling.
It is remarkable that despite such vital direct or indirect presynaptic actions, with an emerging potential for pharmacological manipulation of individual synapses, a comprehensive analysis of axonal D1R expression in the prefrontal circuitry is still needed (Goldman-Rakic et al., 2004). We report that in the infragranular PFC, a D1 heteroreceptor is distinctly localized on perisynaptic and extrasynaptic membranes of excitatory-like (e-l) varicosities, whereas its presynaptic expression can be specific with regard to the cell type, subcellular compartment, and neurochemical identity of the postsynaptic neuron.
Materials and Methods
Tissue processing. Coronal brain sections through the dorsolateral PFC (Walker's area 46) were obtained from two adult rhesus monkeys (Macaca mulatta) as described previously (Paspalas and Goldman-Rakic, 2004a). Briefly, after transcardial perfusion with phosphate-buffered 4% paraformaldehyde and 0.1% glutaraldehyde, brains were vibrosliced at 60 μm, cryoprotected with 30% buffered sucrose in glass vials, and immersed in liquid nitrogen for storage at -80°C. Corresponding brain sections from two macaques killed for another study (Paspalas and Goldman-Rakic, 2004a) with 4% paraformaldehyde and 0.08% glutaraldehyde were used in parallel experiments as positive controls (see below, Methodological considerations). All experimental procedures were conducted in accordance with federal and institutional guidelines.
To block nonspecific reactivity, tissue was preincubated for 1 h in 50 mm Tris-buffered saline (TBS) supplemented with 50 mm glycine (Sigma, St. Louis, MO), 2% bovine serum albumin (Vector Laboratories, Burlingame, CA), and 10% normal goat serum (NGS; Jackson ImmunoResearch, West Grove, PA). Some sections were also treated for 10 min with 0.5% sodium borohydride (Sigma) in TBS before protein blocking (glycine was omitted) and transferred to the primary antibodies.
Antibodies. The D1R monoclonal antibody (isotype rat IgG2a; Sigma) is derived from the 1-1-F11 s.E6 mouse/rat hybridoma (Harlan, India-napolis, IN) against a C-terminal 97 aa sequence of the human D1R. It shows no cross-reactivity with the known DAR subtypes, including the homologous D5R, and has been thoroughly characterized in humans and nonhuman primates (Levey et al., 1993; Smiley et al., 1994; Bergson et al., 1995). When gold conjugates are used for probing the C-terminal anti-D1R, they label the cytoplasmic face of neuronal membranes reflecting the predicted transmembrane configuration of the D1R (see Fig. 1C-E). The anti-D1R protein is recognized by both anti-rat and anti-mouse sera because of the extensive homology of rodent IgGs.
Figure 1.
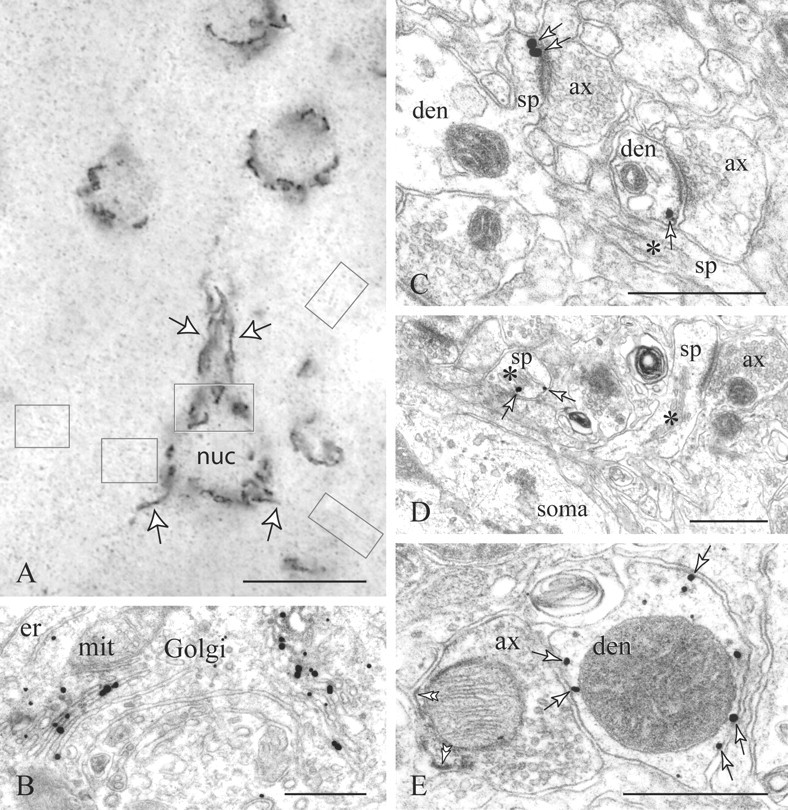
D1Rs in the PFC visualized with silver-enhanced nanogold. A, B, Correlated light and electron microscopy reveals immunoreactive Golgi complexes arranged at the perinuclear cytoplasm (soma frame in A, B) and proximal primary dendrites (A, arrows). The faint puncta (neuropil frames) correspond to dendritic and axonal immunoreactivity. In distal dendrites (den), D1Rs (arrows) appear on perisynaptic (C) and overtly extrasynaptic membranes (E). E, One such dendrite lies next to a DAB-labeled PV varicosity with a pleomorphic vesicle cluster; the PV axon is D1R-. Deposition of the immunoprecipitate on patches of the axolemma (double arrowheads) and the mitochondrial outer membrane is a common diffusion artifact. In spines (sp; asterisks mark spine apparata), D1Rs are expressed on perisynaptic and extrasynaptic sites and on endomembranes including the spine apparatus (arrows in C, D). ax, Axon; er, endoplasmic reticulum; mit, mitochondrion; nuc, nucleus. Scale bars: A, 10 μm; B-E, 400 nm.
Affinity-purified antibodies to parvalbumin (PV) (Swant, Bellinzona, Switzerland) were raised in rabbits against rat muscle PV and recognize both the rodent and human protein (Kägi et al., 1987) [see Gabbott and Bacon (1996) for the macaque neocortex]. Fluid-phase adsorption of the PV antibody (working dilution) with recombinant purified PV (1-5 μg of protein; Swant) for 12 h at 4°C abolished immunoreactivity (data not shown). The antibody produced an immunolabeling pattern identical to that described in the PFC with polyclonal and monoclonal antibodies raised against mammalian or amphibian PV (see Results).
Single gold-based D1R immunocytochemistry. Primary D1R antibodies diluted 1:500 in TBS plus 2% NGS (N-TBS) were applied for 36 h at 4°C. Sections were washed in N-TBS supplemented with 0.07% Tween 20 and incubated for another 2 h in 1:200 goat anti-rat Fab′ fragments conjugated to a 1.4 nm gold cluster (nanogold; Nanoprobes, Yaphank, NY). The two-layer immunocomplex was fixed for 5 min in 1% phosphate-buffered glutaraldehyde and, after a thorough wash in ultrapure water, nanogold was enhanced for 8-10 min on ice with a silver autometallographic developer (HQ Silver; Nanoprobes).
Dual gold-peroxidase-based D1R-PV immunocytochemistry. Sections were incubated in antibodies against D1R (1:300) and PV (1:3000-5000) in N-TBS for 36 h at 4°C. Secondary immunoprobes were applied for 3 h as a mixture of 1:200 anti-rat or anti-mouse nanogold-Fab′ fragments (Nanoprobes) and 1:500 human-adsorbed anti-rabbit biotinylated F(ab′)2 fragments (Jackson ImmunoResearch), both raised in goats. A human/rat/mouse-adsorbed biotinylated F(ab′)2 fragment raised in donkeys against rabbit IgG was used in parallel experiments to control for potential cross-reactivities between the anti-rabbit secondary antibody and the rodent D1R primary antibody. After glutaraldehyde fixation, the D1R-bound nanogold was silver-enhanced as described for single immunocytochemistry. Sections were finally transferred to 1:200 avidin-biotinylated peroxidase complex (Vectastain ABC; Vector Laboratories) for 2 h, and the PV-bound peroxidase was visualized in 0.025% diaminobenzidine (DAB) in TBS with the addition of 0.005% hydrogen peroxide for 8-12 min. To eliminate traces of endogenous biotin, part of the tissue was also treated with the avidin-biotin blocker from Vector Laboratories before the dual immunoprocedure. These sections and another group labeled for PV with peroxidase-antiperoxidase complexes and DAB as a chromogen (Paspalas and Goldman-Rakic, 2004a), as well as identically immunolabeled brain sections from two other macaques (see above, Tissue processing), were used as positive controls for reagent and method selectivity. Negative controls included omission/substitution of primary and/or secondary antibodies followed by silver autometallography for 12 min at room temperature or the DAB enzymatic reaction for 20 min.
Electron microscopy. Sections were postfixed for 15 min in 1% buffered osmium tetroxide, treated with ethanolic uranyl acetate en bloc, and finally embedded in Durcupan epoxy resin (Fluka, Steinheim, Switzerland). Layers V and VI were sampled for thin sectioning and ultrastructural analysis under a JEM-1010 (Jeol, Tokyo, Japan) transmission electron microscope at 80 kV.
To prevent penetration artifacts (see below, Methodological considerations), only 10-14 ultrathin sections spanning ≈1 μm from the tissue/plastic interface were used for dual immunoelectron microscopy and quantification. Images were captured on film (EM 4489; Eastman Kodak, Rochester, NY) or digitally (BioScan 792; Gatan, Pleasanton, CA), and individual panels were adjusted for brightness and contrast with Photoshop 7.0 image-editing software (Adobe Systems, San Jose, CA).
Methodological considerations. We exploited the resolution power of a particulate and, unlike DAB (see Fig. 1 E) (Novikoff, 1980), nondiffusible gold immunoprobe to reveal perisynaptic and extrasynaptic axonal D1Rs. The possibility of additional presynaptic expression within the main body of the excitatory synapse, thus eluding immunodetection, should be also considered. Pre-embedding methods are indeed notorious for failing to visualize certain receptors within protein-dense matrices (Baude et al., 1995). However, such methodological constraints seem unlikely here, considering that D1R detection was not hindered at the peripheral active zone (see Fig. 2C) and pre-embedding nanogold has effectively been used for labeling G-protein-coupled receptors at the presynaptic grid of other synapses [e.g., metabotropic glutamate receptor 7 (mGluR7) (Shigemoto et al., 1996), mGluR4 (Corti et al., 2002), and D2R (Paspalas and Goldman-Rakic, 2004b)].
Figure 2.
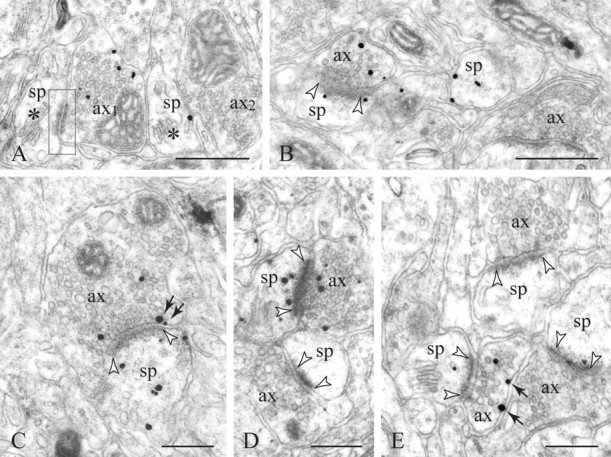
D1Rs in axons and adjoining structures. A, Two varicosities [axon 1 (ax1) and ax2] and two dendritic spines (sp) with well defined spine apparata (asterisks) are juxtaposed but show no synaptic interaction in this plain of section; the junctional specialization in frame is an adherens junction. Ax1 and one spine display D1R-nanogold labeling. B-E, Typically, the D1R+ boutons establish a symmetric synaptic contacts (between arrowheads) with D1R+ or D1R-spines. In D, note the D1R+ synaptic pair bordering an immunonegative pair. Presynaptic D1Rs are distributed on extrasynaptic membranes (arrows in E) or perisynaptically often within the peripheral active zone of the excitatory synapse (arrows in C). This is generally accompanied by axoplasmic labeling. Scale bars: A, B, 400 nm; C-E, 200 nm.
We also combined D1R gold labeling with an enzymatic immunoreaction to identify PV interneurons. However, given that individual epitopes are not equally accessible (physically or stereologically) and that antibodies differ in their kinetics and other qualities, including the nature of conjugated markers, simultaneous antigen visualization is inherently subject to bias. Hence, false-negative results will occur if certain regions displaying reactive PV do not present equally accessible D1Rs, and vice versa. To minimize bias, we limited our analysis to those samples that showed, apart from the commonly strong PV immunoreactivity, distinct D1R labeling within a field captured at 20,000× magnification (14.6 μm2) and, optimally, in neighboring cellular profiles and/or within the PV structures per se (see Figs. 3, 4).
Figure 3.
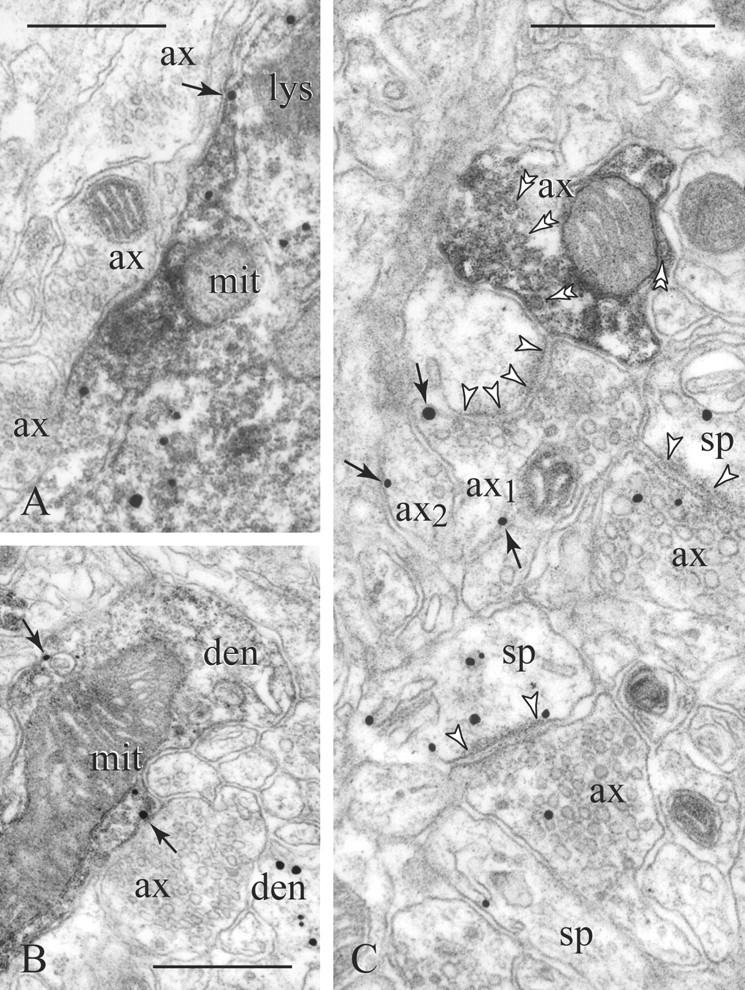
D1Rs in PV (diffuse precipitate) cellular profiles. A, PV perikarya display D1Rs in association with endomembranes, whereas only a fraction is expressed on the plasmalemma (arrow). B, Similarly, in PV dendrites (den), D1Rs may appear intracellularly and on nonsynaptic membranes (arrows). Note a second D1R-immunoreactive dendrite shown to the bottom right in B. C, A PV varicosity (double arrowheads point to synaptic vesicles) lies next to an e-l bouton [axon 1 (ax1)] forming a synapse with perforated postsynaptic density (between arrowheads). Unlike the PV axon, ax1 and neighboring ax2 show perisynaptic and extrasynaptic D1R labeling (arrows). Two other synaptic pairs also express D1Rs. mit, Mitochondrion; lys, lysosome; sp, spine. Scale bars, 400 nm.
Figure 4.
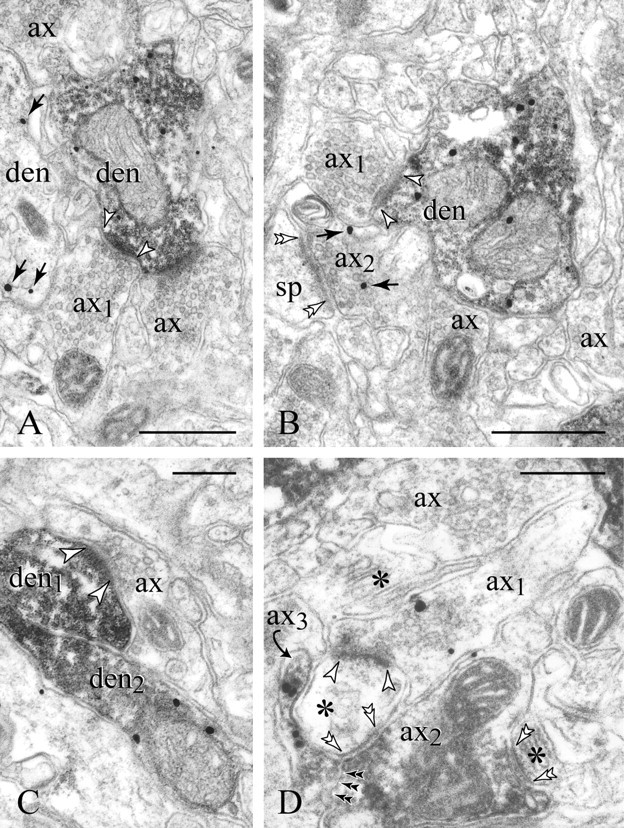
Target-specific expression of presynaptic D1Rs. A, B, Axon 1 (ax1) synapses with a PV dendrite expressing D1R. Note the widening of the intercellular space to a 20 nm synaptic cleft (arrowheads) and the cluster of 40 nm clear synaptic vesicles, typical of e-l varicosities. The postsynaptic specialization is partially obscured by the immunoprecipitate. Other varicosities of similar ultrastructure (ax), all D1R-, surround the PV dendrites. Ax2, which forms an axospinous synapse (double arrowheads in B), is the only profile expressing presynaptic D1Rs (arrows). A second dendrite (den) in A also displays immunolabeling. In C, the postsynaptic PV dendrite (den1) is D1R- (compare with the immunopositive den2). D, In this rather uncommon configuration, the e-l D1R+ bouton ax1 synapses with a dendritic spine (between arrowheads); the same spine receives a paired symmetric synaptic contact (double arrowheads) from a PV axon (ax2) that also furnishes another axospinous synapse, shown to the bottom right in D (asterisks mark spine apparata). A D1R+ PV varicosity (ax3, curved arrow) enwraps the synaptic triad. Double black arrowheads point to the ax2-ax3 interface. sp, Spine. Scale bars: A, B, 400 nm; C, D, 200 nm.
Results
D1R immunocytochemistry revealed highly reactive Golgi complexes in pyramidal perikarya and their proximal primary dendrites (Fig. 1A,B). In the neuropil (Fig. 1C-E), dendritic spines were the most prevalent immunoreactive profiles, with label also present in various caliber dendrites and, notably, the axonal compartment (see following sections). For a general account of prefrontal D1Rs, see the original descriptions by Levey et al. (1993) and Smiley et al. (1994) as well as subsequent reports (Bergson et al., 1995; Muly et al., 1998). Here, we shall present a quantitative high-resolution analysis of D1Rs in the axon and adjoining structures, with emphasis on presynaptic and postsynaptic expression patterns at individual synapses. Note that reference to presynaptic and postsynaptic D1Rs is not intended to imply their positioning in the synapse per se, and is solely to indicate their status relevant to the flow of information from one neuron to another.
A presynaptic D1 heteroreceptor component
Presynaptic D1Rs were typically visualized in axonal varicosities (called D1R+ here-after), measuring 0.24-0.46 μm (minor axis) when cross-sectioned through an established synapse and containing clustered 40 nm round agranular vesicles and occasionally larger clear vesicles (Fig. 2). Dense-cored vesicles of the type that typically accompanies monoaminergic and peptidergic (i.e., GABAergic) boutons were generally not present. The D1R+ boutons established asymmetric (Gray type I) synaptic contacts featuring wide active zones with a 20 nm synaptic cleft (Fig. 2C,E) and often perforated postsynaptic densities (Fig. 3C). Varicosities of this typical ultrastructure and synaptology are presumed to use glutamate as an excitatory neurotransmitter [see Peters et al. (1991) for correlation between form and synaptic function]. Hence, we refer to these axonal profiles as e-l varicosities.
Consistent with the e-l morphology was the involvement of D1R+ boutons in axospinous synapses. Virtually every postsynaptic target was categorized as dendritic spine on the basis of form, size, organelle content (including a spine apparatus), and/or continuity with the parent dendrite (Peters et al., 1991). In only 151 of 1290 D1R+ boutons examined (n = 660 and 630 in 10 blocks from brains I and II, respectively; original magnification, 25,000×) did the postsynaptic element resemble a fine dendrite (49 and 28 cases, respectively; data not shown) or was this postsynaptic element not able to be positively identified (74 total cases); cellular profiles of poor ultrastructure were excluded.
To quantify the prevalence of axonal D1R expression, we examined in single sections 1200 varicosities (600 per brain) of distinct e-l morphology (see Materials and Methods for details on tissue sampling). In total, 13.4% (±3.8% SD) (brain I, 15%, 90 boutons; brain II, 11.8%, 71 boutons) of e-l varicosities displayed immunolabeling in association with the plasma membrane and the axoplasm. Note that these values would tend to underestimate the D1R+ axonal fraction, because they derive from single sections and because we could not control for presynaptic expression below the immunodetection threshold.
Axolemmal and axoplasmic D1Rs
Axolemmal D1Rs, defined as particles associated with the cytoplasmic face of the axonal membrane (see Materials and Methods, Antibodies), were distributed on perisynaptic and extrasynaptic sites. It is noteworthy that perisynaptic D1Rs were often embedded within the active zone proper at the margins of the synapse (Fig. 2C), in addition to being located at a distance of 50 nm along the lateral axolemma (Fig. 3C). Extrasynaptic D1Rs were variously expressed on membranes distal (>50 nm) to the synapse (Figs. 2E, 3C) and, occasionally, in intervaricose segments identified by their continuity with an immunoreactive varicosity. Axolemmal expression was clearly not in response to juxtaposition with putative DAergic-like varicosities, because almost invariably, the dopaminoceptive membranes could be seen facing dendrites and particularly other e-l varicosities (Figs. 2E, 3C, 4B). It is also worth noting that spines postsynaptic to D1R+ boutons were typically devoid of convergent synaptic input (i.e., synaptic triads) (Goldman-Rakic et al., 1999), a proposed basis for fine-tuning of individual glutamatergic synapses by DA and possibly other neuromodulators. In addition to membrane immunolabeling, almost every D1R+ bouton displayed axoplasmic immunoparticles intermingled with the vesicle cluster (Figs. 2, 3C).
Target cell-specific expression of presynaptic D1Rs
The prime synaptic target of the e-l D1R+ bouton is apparently the spine-laden pyramidal dendrite (Feldman, 1984; DeFelipe and Farinas, 1992). In fact, with few possible exceptions, nonpyramidal cells (including FS interneurons) bear smooth or sparsely spinous dendrites and receive major excitatory input on dendritic shafts (Houser et al., 1984). Nonetheless, to rule out the possibility that FS interneurons might receive synapses from D1R+ boutons, we combined D1R and PV immunocytochemistry. The expression of the calcium-binding protein PV is a distinctive neurochemical marker for FS cells, as long established from correlated studies of their firing and axon ramification patterns (Hendry et al., 1989; DeFelipe, 1997; Kawaguchi and Kubota, 1998).
In accord with previous descriptions (for review, see DeFelipe, 1997) [see Williams et al. (1992), Condé et al. (1994), and Gabbott and Bacon (1996) for the macaque PFC], the PV-immunoreactive profiles included nonpyramidal perikarya, smooth dendrites, and a plethora of varicosities featuring pleomorphic synaptic vesicles (Fig. 1E, compare with e-l boutons in Fig. 2) and forming symmetric (Gray type II) synaptic contacts. The PV neurons received numerous axodendritic synapses from e-l varicosities often covering a significant portion of the dendritic circumference (Fig. 4A,B). These boutons generally displayed ultrastructure and synaptic features (Williams et al., 1992) identical to the D1R+ varicosity forming axospinous synapses (Fig. 4B, compare ax1 with ax2); however, they did not express D1R immunoreactivity in the axoplasm or the axolemma (Fig. 4). Although immunodetection limitations may also apply here, it is very unlikely that there would be a biased “loss” of D1R immunoreactivity in the PV-targeting bouton in particular (see Materials and Methods, sampling criteria in Methodological considerations); thus the e-l varicosity synapsing with PV neurons either lacks D1Rs or specifically features minimal, almost undetectable D1R levels.
D1Rs in postsynaptic and adjoining structures
Dendritic spines postsynaptic to D1R+ boutons usually expressed D1R immunoreactivity on perisynaptic and extrasynaptic sites and on endomembranes, including the spine apparatus (Fig. 3C). However, nearly one-fifth of such axospinous contacts (17 and 21% for brains I and II, respectively; n = 200 per brain) involved immunonegative (D1R-) spines (Figs. 3C, 4D). From this and our data of presynaptic D1R expression in general, we can extrapolate that almost 11% of e-l varicosities in the infragranular PFC would express D1R and synapse with D1R+ spines. Accordingly, many more e-l but D1R-boutons will be targeting D1R-immunopositive (Fig. 1C) or -immunonegative spines, as exemplified in Figure 2D, in which an immunonegative synaptic pair lies subjacent to a highly reactive pair (both the bouton and spine are D1R+).
D1Rs have been localized previously with fluorescence and DAB immunocytochemistry to subsets of PFC interneurons, notably the PV cells (Muly et al., 1998). Indeed, certain PV dendrites receiving numerous synapses from e-l varicosities (i.e., D1R-) displayed plasmalemmal and, particularly, cytoplasmic D1R immunoparticles (Fig. 3B). D1R immunoreactivity was nearly absent from PV axons (Fig. 3C). The rare coexpression of D1Rs and PV in axonal profiles was observed in small-sized boutons participating in multipartite arrangements with e-l varicosities (Fig. 4D). Although there is irrefutable evidence for a PV-D1R cellular element in the frontal cortex (Le Moine and Gaspar, 1998; Gorelova et al., 2002; Gao and Goldman-Rakic, 2003), the originally reported colocalization of PV with D1Rs (close to 100%) (Muly et al., 1998) could not be confirmed here, which may be attributable to species-specificity characteristics of the immunoprobes combined in the previous report.
Discussion
D1Rs in excitatory circuits
The PFC encompasses e-l boutons from diverse sources, including intrinsic, associational, and callosal pyramidal axons as well as thalamocortical afferents (Fig. 5). Pyramids are the main D1R-expressing cortical cells (Goldman-Rakic et al., 1999), and although elegant tracing studies have helped to elucidate many of their interhemispheric and intrahemispheric projections in the macaque PFC (Levitt et al., 1993; Kritzer and Goldman-Rakic, 1995; Pucak et al., 1996; Melchitzky et al., 1998), virtually nothing is known with regard to D1R expression in any of these categories of axons. In the deep PFC, physiological evidence for a D1-LR presynaptic mechanism favors locally interconnected pyramids organized in excitatory microcircuits (Gao et al., 2001; Seamans et al., 2001). This is different from the superficial PFC, in which Urban et al. (2002) identified a D1-LR-modulated excitatory input other than local collaterals of layer III pyramidal neurons. Apparently, small sample is a limiting factor, and distal monosynaptic connections would be almost impractical to test either in vitro or in vivo. Hence, at least some of these presynaptic effects appear to be layer- and input-specific and possibly species-specific (González-Burgos et al., 2002; Seamans and Yang, 2004).
Figure 5.
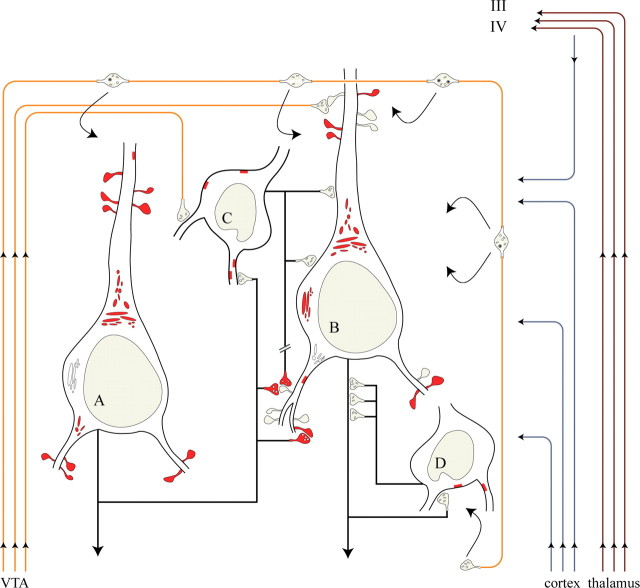
Synopsis of the various relationships between pyramidal neurons and PV interneurons, as well as D1R expression patterns in the infragranular PFC. It should be explicitly stated that the schema depicts a simplistic view of complex and certainly not fully understood principles of neocortical organization. For clarity, two pyramids (A, B) and two PV cells (C, D) are illustrated. D1R-immunoreactive spines, axon varicosities, and perinuclear Golgi complexes appear in red, and, likewise, red patches below the cell surface represent somatic and dendritic D1Rs. Synaptic and extrasynaptic (curved arrows) DAergic input from the ventral tegmental area (VTA) is shown in orange. Thalamic afferents, in brown, cross the infragranular cortex and terminate in layers IV/deep III. Blue lines represent intrinsic, associational, and callosal corticocortical afferents; they may or may not furnish D1R+ e-l varicosities (see Discussion) and thus should be potentially regarded as an integral part of the circuitry (only local axon collaterals are illustrated here). Neuron A establishes axospinous and axodendritic synapses with cells B and C, respectively, which exemplify the pattern of target-specific expression of presynaptic D1 heteroreceptors. Almost 80% of such axospinous contacts may express both presynaptic and postsynaptic D1Rs. PV interneurons provide synaptic input to the perisomatic region (C-to-B synapses) or the axon initial segment (D-to-B synapses) of pyramidal neurons. The lower discontinuous portion of one PV axon (that could originate from a neuron other than C) participates in a rare multipartite arrangement with a pyramidal axon where both express D1Rs (Fig. 4 D). In effect, DA would modulate all four neurons postsynaptically, via direct synapses (to neurons B and C) or via volume neurotransmission.
The expression of D1Rs in the thalamus is far less understood. Recently, D1Rs have been localized in neurons expressing calbindin or PV, including those in the mediodorsal thalamus that furnish the main projections to the PFC (Rodriguez-Moral and Cavada, 2003), yet it is unclear whether their axons incorporate the receptor. Nonetheless, the latter terminate in layers deep III and IV, with only few nonvaricose fibers present below this zone (Giguere and Goldman-Rakic, 1988; Erickson and Lewis, 2004). Moreover, the midlayer projections express PV and establish type I axospinous synapses (Melchitzky et al., 1999), in agreement with the classic descriptions on sensory cortices (DeFelipe and Jones, 1991). Considering that our analysis failed to reveal e-l PV varicosities of presumptive thalamic origin, and although the contribution of other thalamic nuclei cannot be excluded, it is reasonable to assume that cortical pyramids are the major source of D1R+ boutons in the infragranular PFC.
Axons possess two possibly interchanging D1R components
Although axoplasmic labeling of D1Rs could potentially represent a diffusion artifact (i.e., of axolemmal D1Rs), it should be carefully interpreted given that cytoplasmic occurrence was reported for postsynaptic DARs and other receptor systems as a phase of dynamic trafficking from and toward the plasmalemma (Bloch et al., 1999; Malinow and Malenka, 2002; Heydorn et al., 2004). Whether presynaptic (and postsynaptic) D1Rs undergo similar dynamic internalization in the PFC is currently unknown. Yet such a versatile mechanism operating at the presynaptic and/or postsynaptic level would be best suited for adjusting the active component, namely dopaminoceptive D1R, at individual e-l varicosities and/or postsynaptic spines, and, in effect, the weight of volumetric modulation of single synapses. This could be vital for sequestering extrasynaptic signaling (see Introduction), considering that D1R+ boutons, similar to D1R+ spines (Smiley et al., 1994), will most likely sense relatively remote DA signals, for they were not encountered in a triadic configuration or in direct association with DAergic-like axons (Figs. 2E, 3C).
DAergic modulation of the glutamatergic synapse
In vitro electrophysiological evidence from this and other laboratories indicates that DA depresses the amplitude of EPSPs at synapses between layer V pyramids (Gao et al., 2001; Seamans et al., 2001), a presynaptic D1-LR-mediated effect that has been attributed to reduced transmitter-release probability in the glutamatergic synapse (Tsodyks and Markram, 1997). Interestingly, synapses of the same pyramids with FS interneurons show no direct D1-LR effects; instead, agonists increase the excitability of a subpopulation of these cells and elicit firing in the rising phase of EPSPs (Gao and Goldman-Rakic, 2003). This unique modulatory pattern could play a role in constraining excitatory spread by enhancing feedback inhibition.
Although these data openly suggest a presynaptic DA heteroreceptor component, the possibility of a postsynaptic mechanism retrogradely affecting presynaptic transmission cannot be ignored. Such transsynaptic actions have indeed been reported, in which D1-LRs on spines could mediate adenosine release and promote feedback inhibition via A1 heteroreceptors on the presynaptic membrane (Harvey and Lacey, 1997). Furthermore, because D1Rs and D5Rs share a common pharmacological profile (Missale et al., 1998), the subtype responsible for such selective modulatory effects cannot be physiologically verified. However, our nanogold-D5R analysis in the PFC (Paspalas and Goldman-Rakic, 2004a,b) confirms that pyramidal cells do not possess a significant D5R axonal component, and the rare occurrence of presynaptic D5Rs is often associated with type II nonglutamatergic synapses. In fact, the purported target-specific presynaptic depression and postsynaptic excitation (without presynaptic effect) by D1-LR agonists (Gao and Goldman-Rakic, 2003) reflect the D1R expression pattern in the PFC neuropil: e-l boutons express D1Rs in selective axospinous synapses not involving PV interneurons, which may often coexpress postsynaptic D1Rs (Fig. 5).
An integral part of this circuitry is undoubtedly the D1R-immunoreactive spine. Our data show that in axospinous synapses involving D1R+ boutons, almost 80% of postsynaptic spines also express D1Rs, and nearly 11% of axospinous e-l synapses in the infragranular PFC (extrapolated from total presynaptic D1R) may be subject to both presynaptic and postsynaptic D1R activation. There is also a small percentage of D1R+ boutons targeting immunonegative spines and, certainly, other dendritic spines and PV dendrites expressing postsynaptic D1Rs but receiving e-l synapses from D1R- boutons (Fig. 5). In fact, distal spines and PV dendrites are known to be a preferred target of synaptic DA afferents (Sesack et al., 1998; Goldman-Rakic et al., 1999). The heterogeneity of D1R expression at glutamatergic synapses may be suggestive of corresponding input-specific (see above, D1Rs in excitatory circuits) and/or target-specific DAergic modulation of glutamate transmission in the PFC. The selective regulation of presynaptic and/or postsynaptic elements may result in differential regulation of functionally distinct aspects of neurotransmission, including baseline efficacy, short-term plasticity, the ratio of NMDA and AMPA receptor activation, and Hebbian or other nonassociative forms of long-term synaptic plasticity (Brunel, 2003; Seamans and Yang, 2004). Finally, the rare occurrence of multipartite arrangements involving glutamatergic and PVergic paired synapses and extrasynaptic D1Rs (Fig. 4D) may represent a previously unacknowledged neural basis for cortical neuromodulation that merits closer scrutiny, especially in light of the reported PFC cognitive deficits associated with altered GABA neurotransmission and the PV cell group in particular (Lewis et al., 2004).
Specificity of DA signaling
The D1R of the glutamatergic varicosity is evidently a heteroreceptor sensing short- or long-range diffuse DA signals, similar to other DARs localized beyond the chemical synapse. Still, one should not assume that volumetric (i.e., input-nonspecific) modulation of cortical functions by DA lacks target specificity.
In addition to cell type-specific expression of DAR subtypes and/or subtype isoforms (Levey et al., 1993; Mrzljak et al., 1996; Khan et al., 1998; Muly et al., 1998), previous analyses in the PFC have revealed complementary expression patterns within a single neuron (dendritic spines vs shafts) (Bergson et al., 1995) and within an individual cellular compartment with regard to cytoplasmic specializations (perisomatic microdomains) (Paspalas and Goldman-Rakic, 2004a). Accordingly, it was proposed that spatial specificity could be an additional factor for determining the signal-transduction mechanisms of nonsynaptic DARs and that even diffusion dynamics could potentially generate “hot spots” of elevated DA levels to subserve a quasi-targeted mode of extrasynaptic transmission (discussed in Paspalas and Goldman-Rakic, 2004a). These and other hitherto undiscovered qualities of the cortical DAergic system, and, of course, synaptically mediated DA signaling (Goldman-Rakic et al., 1999; Sesack et al., 2003) would ensure this high degree of input specificity or target specificity required for the subtle and differential regulation of cognitive operations by endogenous DA and, potentially, the growing list of its pharmacological analogs.
In this paper, we introduce an extrasynaptic yet highly specific D1R substratum for interplay between the DAergic system, inhibitory interneurons, and the principal excitatory constituent of the primate PFC. This interplay, both at the presynaptic and at the postsynaptic level, would also define neuron pathophysiology, for deviation from the DA/glutamate functional equilibrium may ultimately represent a key factor for many neuropsychiatric disorders affecting cognition (Goldman-Rakic, 1995, 1996; Lewis et al., 1999; Carlsson et al., 2001; Frankle et al., 2003; Laruelle et al., 2003; Sesack et al., 2003).
Footnotes
This work was supported by National Institutes of Health Grants MH44866 and MH68789. Part of this study was completed in consultation with P.S.G.-R. before her death and presented in abstract form (Paspalas and Goldman-Rakic, 2002). C.D.P. is most grateful to K. Szigeti for technical assistance and to Drs. E. Markakis, W. J. Gao, T. Koos, S. Vijayraghavan, and P. Rakic for critical comments and discussion.
Correspondence should be addressed to Dr. Constantinos D. Paspalas, Department of Neurobiology, Yale University School of Medicine, Sterling Hall of Medicine B408, 333 Cedar Street, New Haven, CT 06510. E-mail: constantinos.paspalas@yale.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251260-08$15.00/0
Deceased, July 31, 2003.
References
- Baude A, Nusser Z, Molnár E, McIlhinney RAJ, Somogyi P (1995) High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience 69: 1031-1055. [DOI] [PubMed] [Google Scholar]
- Bergson C, Mrzljak L, Smiley JF, Pappy M, Levenson R, Goldman-Rakic PS (1995) Regional, cellular, and subcellular variations in the distribution of D1 and D5 dopamine receptors in primate brain. J Neurosci 15: 7821-7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch B, Dumartin B, Bernard V (1999) In vivo regulation of intraneuronal trafficking of G protein-coupled receptors for neurotransmitters. Trends Pharmacol Sci 20: 315-319. [DOI] [PubMed] [Google Scholar]
- Brunel N (2003) Dynamics and plasticity of stimulus-selective persistent activity in cortical network models. Cereb Cortex 13: 1151-1161. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Waters N, Holm-Waters S, Tedroff J, Nilsson M, Carlsson ML (2001) Interactions between monoamines, glutamate, and GABA in schizophrenia: new evidence. Annu Rev Pharmacol Toxicol 41: 237-260. [DOI] [PubMed] [Google Scholar]
- Condé F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA (1994) Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol 341: 95-116. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F (2002) Distribution and synaptic localization of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience 110: 403-420. [DOI] [PubMed] [Google Scholar]
- DeFelipe J (1997) Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat 14: 1-19. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Farinas I (1992) The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol 39: 563-607. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG (1991) Parvalbumin immunoreactivity reveals layer IV of the monkey cerebral cortex as a mosaic of microzones of thalamic afferent terminations. Brain Res 562: 39-47. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Lewis DA (2004) Cortical connections of the lateral mediodorsal thalamus in cynomolgus monkeys. J Comp Neurol 473: 107-127. [DOI] [PubMed] [Google Scholar]
- Feldman ML (1984) Morphology of the neocortical pyramidal neuron. In: Cerebral cortex: cellular components of the cerebral cortex (Peters A, Jones EG, eds), pp 123-200. New York: Plenum.
- Frankle WG, Lerma J, Laruelle M (2003) The synaptic hypothesis of schizophrenia. Neuron 39: 205-216. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Bacon SJ (1996) Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey. I. Cell morphology and morphometrics. J Comp Neurol 364: 567-608. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Goldman-Rakic PS (2003) Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci USA 100: 2836-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS (2001) Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA 98: 295-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere M, Goldman-Rakic PS (1988) Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol 277: 195-213. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1987) Circuitry of primate prefrontal cortex and regulation of behavior by representational memory. In: Handbook of physiology: the nervous system, Vol 5 (Plum F, Mountcastle V, eds), pp 373-417. Bethesda, MD: American Physiological Society. [Google Scholar]
- Goldman-Rakic PS (1995) Cellular basis of working memory. Neuron 14: 477-485. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS (1996) Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA 93: 13473-13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Bergson C, Krimer LS, Lidow MS, Williams SM, Williams GV (1999) The primate mesocortical dopamine system. In: Handbook of chemical neuroanatomy: the primate nervous system, Pt III (Bloom FE, Björklund A, Hökfelt T, eds), pp 403-428. Amsterdam: Elsevier.
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV (2004) Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology 174: 3-16. [DOI] [PubMed] [Google Scholar]
- González-Burgos G, Kröner S, Krimer LS, Seamans JK, Urban NN, Henze DA, Lewis DA, Barrionuevo G (2002) Dopamine modulation of neuronal function in the monkey prefrontal cortex. Physiol Behav 77: 537-543. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Islas C, Hablitz JJ (2003) Dopamine enhances EPSCs in layer II-III pyramidal neurons in rat prefrontal cortex. J Neurosci 23: 867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR (2002) Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol 88: 3150-3166. [DOI] [PubMed] [Google Scholar]
- Harvey J, Lacey MG (1997) A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J Neurosci 17: 5271-5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Emson PC, Lawson DE, Heizmann CW, Streit P (1989) Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res 76: 467-472. [DOI] [PubMed] [Google Scholar]
- Henze DA, González-Burgos GR, Urban NN, Lewis DA, Barrionuevo G (2000) Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol 84: 2799-2809. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Søndergaard BP, Hadrup N, Holst B, Haft CR, Schwartz TW (2004) Distinct in vitro interaction pattern of dopamine receptor subtypes with adaptor proteins involved in post-endocytotic receptor targeting. FEBS Lett 556: 276-280. [DOI] [PubMed] [Google Scholar]
- Houser CR, Vaughn JE, Hendry SHC, Jones EG, Peters A (1984) GABA neurons in the cerebral cortex. In: Cerebral cortex: functional properties of cortical cells, Vol 2 (Jones EG, Peters A, eds), pp 63-89. New York: Plenum. [Google Scholar]
- Kägi U, Berchtold MW, Heizmann CW (1987) Ca2+-binding parvalbumin in rat testis. J Biol Chem 262: 7314-7320. [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y (1998) Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience 85: 677-701. [DOI] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS (1998) Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci USA 95: 7731-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzer MF, Goldman-Rakic PS (1995) Intrinsic circuit organization of the major layers and sublayers of the dorsolateral prefrontal cortex in the rhesus monkey. J Comp Neurol 359: 131-143. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Kegeles LS, Abi-Dargham A (2003) Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann NY Acad Sci 1003: 138-158. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Gaspar P (1998) Subpopulations of cortical GABAergic interneurons differ by their expression of D1 and D2 dopamine receptor subtypes. Brain Res Mol Brain Res 58: 231-236. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ (1993) Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci USA 90: 8861-8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt JB, Lewis DA, Yoshioka T, Lund JS (1993) Topography of pyramidal neuron intrinsic connections in macaque monkey prefrontal cortex (areas 9 and 46). J Comp Neurol 338: 360-376. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo T-UW (1999) Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry 46: 616-626. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hasminoto T (2004) Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology 174: 143-150. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Williams GV, Goldman-Rakic PS (1998) The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci 19: 136-140. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103-126. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Pucak ML, Lewis DA (1998) Synaptic targets of pyramidal neurons providing intrinsic horizontal connections in monkey prefrontal cortex. J Comp Neurol 390: 211-224. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Sesack SR, Lewis DA (1999) Parvalbumin-immunoreactive axon terminals in macaque monkey and human prefrontal cortex: laminar, regional, and target specificity of type I and type II synapses. J Comp Neurol 408: 11-22. [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998) Dopamine receptors: from structure to function. Physiol Rev 78: 189-225. [DOI] [PubMed] [Google Scholar]
- Mrzljak Z, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS (1996) Localization of D4 receptors in GABAergic neurons of the primate brain. Nature 381: 245-248. [DOI] [PubMed] [Google Scholar]
- Muly EC, Szigeti K, Goldman-Rakic PS (1998) D1 receptor in interneurons of macaque prefrontal cortex: distribution and subcellular localization. J Neurosci 18: 10553-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novikoff AB (1980) DAB cytochemistry: artifact problems in its current uses. J Histochem Cytochem 28: 1036-1038. [DOI] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS (2002) Target-selective expression of D1R immunoreactivity in excitatory-like axons of the macaque prefrontal cortex. Soc Neurosci Abstr 28: 336.5. [Google Scholar]
- Paspalas CD, Goldman-Rakic PS (2004a) Microdomains for dopamine volume neurotransmission in primate prefrontal cortex. J Neurosci 24: 5292-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paspalas CD, Goldman-Rakic PS (2004b) Receptor compartmentalization for defining input-specificity of dopamine volumetric signaling: a study of D1, D2 and D5 receptor subtypes in primate prefrontal cortex. Soc Neurosci Abstr 30: 277.9. [Google Scholar]
- Peters A, Palay SL, Webster HD (1991) The fine structure of the nervous system: neurons and their supporting cells. New York: Oxford UP.
- Pickel VM (2000) Extrasynaptic distribution of monoamine transporters and receptors. Prog Brain Res 125: 267-276. [DOI] [PubMed] [Google Scholar]
- Pucak ML, Levitt JB, Lund JS, Lewis DA (1996) Patterns of intrinsic and associational circuitry in monkey prefrontal cortex. J Comp Neurol 376: 614-630. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moral M, Cavada C (2003) Expression of dopamine D1 receptor with calbindin and parvalbumin in the thalamus of macaque monkeys. Soc Neurosci Abstr 29: 160.6. [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS (1991) D1 dopamine receptor in prefrontal cortex: involvement in working memory. Science 251: 947-950. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR (2004) The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1-57. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Durstewitz D, Christie BR, Stevens CF, Sejnowski TJ (2001) Dopamine D1/D5 receptor modulation of excitatory synaptic inputs to layer V prefrontal cortex neurons. Proc Natl Acad Sci USA 98: 301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Melchitzky DS, Lewis DA (1998) Dopamine innervation of a subclass of local circuit neurons in monkey prefrontal cortex: ultrastructural analysis of tyrosine hydroxylase and parvalbumin immunoreactive structures. Cereb Cortex 8: 614-622. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A (2003) Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci 1003: 36-52. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JDB, Ohishi H, Nusser Z, Kaneko T, Somogyi P (1996) Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature 381: 523-525. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Levey AI, Ciliax BJ, Goldman-Rakic PS (1994) D1 dopamine receptor immunoreactivity in human and monkey cerebral cortex: predominant and extrasynaptic localization in dendritic spines. Proc Natl Acad Sci USA 91: 5720-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsodyks MV, Markram H (1997) The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA 94: 719-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NN, González-Burgos G, Henze DA, Lewis DA, Barrionuevo G (2002) Selective reduction by dopamine of excitatory synaptic inputs to pyramidal neurons in primate prefrontal cortex. J Physiol (Lond) 539: 707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376: 572-575. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS, Leranth C (1992) The synaptology of parvalbumin-immunoreactive neurons in the primate prefrontal cortex. J Comp Neurol 320: 353-369. [DOI] [PubMed] [Google Scholar]
- Zoli M, Jansson A, Syková E, Agnati LF, Fuxe K (1999) Volume transmission in the CNS and its relevance for neuropsychopharmacology. Trends Pharmacol Sci 20: 142-150. [DOI] [PubMed] [Google Scholar]