Abstract
β-Amyloid protein (Aβ) has been implicated as a key molecule in the neurodegenerative cascades of Alzheimer's disease (AD). Aβ directly induces neuronal apoptosis, suggesting an important role of Aβ neurotoxicity in AD neurodegeneration. However, the mechanism(s) of Aβ-induced neuronal apoptosis remain incompletely defined. In this study, we report that Aβ-induced neuronal death is preceded by selective alterations in expression of the Bcl-2 family of apoptosis-related genes. Specifically, we observe that Aβ significantly reduces expression of antiapoptotic Bcl-w and Bcl-xL, mildly affects expression of bim, Bcl-2, and bax, but does not alter expression of bak, bad, bik, bid, or BNIP3.Aβ-induced downregulation of Bcl-w appears to contribute to the mechanism of apoptosis, because Aβ-induced neuronal death was significantly increased by Bcl-w suppression but significantly reduced by Bcl-w overexpression. Downstream of Bcl-w, Aβ-induced neuronal apoptosis is characterized by mitochondrial release of second mitochondrion-derived activator of caspase (Smac), an important precursor event to cell death. We observed that Smac release was potentiated by suppression of Bcl-w and reduced by overexpression of Bcl-w. Next, we investigated the upstream mediator of Aβ-induced Bcl-w downregulation and Smac release. We observed that Aβ rapidly activates c-Jun N-terminal kinase (JNK). Pharmacological inhibition of JNK effectively inhibited all measures of Aβ apoptosis: Bcl-w downregulation, Smac release, and neuronal death. Together, these results suggest that the mechanism of Aβ-induced neuronal apoptosis sequentially involves JNK activation, Bcl-w downregulation, and release of mitochondrial Smac, followed by cell death. Complete elucidation of the mechanism of Aβ-induced apoptosis promises to accelerate development of neuroprotective interventions for the treatment of AD.
Keywords: β-amyloid, Bcl-w, Smac, c-Jun N-terminal kinase, apoptosis, Alzheimer's disease
Introduction
Alzheimer's disease (AD) is an age-related neurodegenerative disorder believed to be initiated by neural accumulation of β-amyloid protein (Aβ) (Hardy, 1997; Selkoe, 2001). Although normally a soluble peptide (Haass et al., 1992; Shoji et al., 1992), Aβ can assemble into a variety of oligomeric forms that are thought to underlie its pathogenic effects (Pike et al., 1993; Lambert et al., 1998; Hartley et al., 1999; Walsh et al., 1999; Hoshi et al., 2003). Oligomeric Aβ is known to directly cause neurite damage (Pike et al., 1992; Ivins et al., 1998; Mattson et al., 1998) and neuronal death (Pike et al., 1991, 1993; Mattson et al., 1993; Lorenzo and Yankner, 1994; Simmons et al., 1994; Hartley et al., 1999; Hoshi et al., 2003) as well as to activate microglia (Meda et al., 1995; El Khoury et al., 1996) and astrocytes (Canning et al., 1993; Pike et al., 1994), which may indirectly injure neurons (Akiyama et al., 2000). What remain unclear are the molecular mechanisms by which Aβ induces its spectrum of deleterious neural effects.
Abundant evidence indicates that direct neurotoxic effects of Aβ involve activation of apoptosis pathways (Forloni et al., 1993; Loo et al., 1993; Estus et al., 1997). However, which signaling pathways mediate apoptosis induced by Aβ remain to be completely defined. One group of molecules theorized to function prominently in neuronal apoptosis is the Bcl-2 family. Members of the Bcl-2 family are pivotal regulators of the apoptotic process (Antonsson and Martinou, 2000) and include both proteins that promote cell survival (e.g., Bcl-2, Bcl-xL, and Bcl-w) and others that antagonize it (e.g., Bax, Bad, Bak, Bik, Bid, BNIP3, and Bim). Previous work suggests that Aβ-induced apoptosis is characterized by decreased expression of the antiapoptotic Bcl-2, Bcl-xL (Forloni et al., 1996; Paradis et al., 1996; Wei et al., 2000; Tamagno et al., 2003), and/or increased expression of the proapoptotic Bax, Bim (Paradis et al., 1996; Yin et al., 2002; Tamagno et al., 2003). Furthermore, overexpression of antiapoptotic Bcl-2 (Saille et al., 1999; Song et al., 2004) and Bcl-xL (Tan et al., 1999) or suppression of Bim (Yin et al., 2002) can attenuate Aβ toxicity.
If members of the Bcl-2 family are important regulators of Aβ-induced cell death, then identification of both upstream and downstream signaling components is critical for elucidating the mechanism of apoptosis. Upstream of the Bcl-2 family, we evaluated the potential role of c-Jun N-terminal kinase (JNK), a family of serine/threonine kinases (Ip and Davis, 1998). Activation of JNK is linked to transcriptional regulation of many genes, including members of the Bcl-2 family (Bae and Song, 2003; Tamagno et al., 2003). In addition, JNK activation is observed in cultured neurons after Aβ exposure, and its inhibition significantly attenuates Aβ toxicity (Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001). The mitochondrial localization of Bcl-2 members suggests that the downstream components may be proapoptotic molecules released from mitochondria, perhaps including second mitochondrion-derived activator of caspase (Smac)/DIABLO (direct inhibitor of apoptosis binding protein with low pI) (Du et al., 2000; Verhagen et al., 2000). In this study, we sought to identify which members of the Bcl-2 family contribute to Aβ-induced neuron death and to identify both the upstream and downstream components of this apoptotic pathway.
Materials and Methods
Cell culture. Primary cultures of cortical neurons were prepared from embryonic (gestational day 18) Sprague Dawley rat pups with minor modifications of a previously described protocol (Pike et al., 1993). In brief, dissected cerebral cortices were incubated for 5 min in 0.125% trypsin at 37°C followed by trypsin quenching with 1 vol of DMEM containing 20% fetal calf serum. Cell suspensions were centrifuged (5 min at 200 × g), resuspended in serum-free DMEM, mechanically dissociated by repeated passage through a fire-polished Pasteur pipette, and then filtered through a sterile 40 μm nylon mesh (Falcon, Franklin Lakes, NJ). Cells were plated on poly-l-lysine (0.05 mg/ml)-coated multiwell plates (Nunc, Naperville, IL) at either 5 × 104 cells/cm2 (cell viability) or 1.5 × 105 cells/cm2 [Western blot and reverse transcription-PCR (RT-PCR)] in serum-free, phenol red-free DMEM buffered by 26 mm bicarbonate, 20 mm HEPES, and supplemented with 100 μg/ml transferrin, 5 μg/ml insulin, 100 μm putrescine, and 30 nm selenium. Cultures were maintained in a humidified incubator with 5% CO2 at 37°C.
Experimental treatment of cultures. Cortical neuron cultures were used for experimentation 3-6 d in vitro after plating. Cultures were exposed to 25 μm aggregated Aβ25-35 (Biochem, Torrance, CA), Aβ1-40, or Aβ1-42 (US Peptide, Rancho Cucamonga, CA). Before addition to cultures, Aβ peptides were solubilized at 1 mm in sterile deionized water and were then incubated at 37°C for 1-3 d to yield peptide solutions in which Aβ exhibits a range of assembly and conformation states, as described previously (Pike et al., 1993, 1995). Infection with recombinant adeno-associated virus (AAV) (serotypes 2) at a multiplicity of infection of 1000 or transfection with small interfering RNA (siRNA) was performed 24 h before exposure to Aβ. The JNK inhibitor anthra[1,9-cd]pyrazol-6(2H)-one (SP600125) (Calbiochem, La Jolla, CA) or caspase-9 inhibitor benzyloxycarbonyl-Leu-Glu(OMe)-His-Asp(OMe)-fluoromethylketone (Z-LEHD-FMK) (Biovision, Mountain View, CA) was added to cultures 1-1.5 h before Aβ.
Assessment of cell viability. Cell viability was assessed using calcein-AM and ethidium homodimer fluorescent staining (Molecular Probes, Eugene, OR) as described previously (Pike, 1999). Briefly, live cells were counted in four fields per well, six wells per condition, in three or more independent culture preparations. The number of live cells counted per well in vehicle-treated controls ranged from 200 to 300. Cell viability is presented graphically as a percentage of the number of live cells in the vehicle-treated control condition.
Production of recombinant AAV. AAV-Bcl-w [rAAV/cytomegalovirus (CMV)/Bcl-w] was produced and titrated as described previously (Sun et al., 2003) and was kindly provided by Dr. David A. Greenberg (Buck Institute for Age Research, Novato, CA). As a negative control, recombinant AAV vector encoding green fluorescent protein (GFP), AAV-GFP (rAAV/CMV/hrGFP), was produced using three-plasmid procedure (Stratagene, La Jolla, CA).
Design and transfection of siRNAs. siRNA that target rat Bcl-w was designed using the target finder and design tool (Ambion, Austin, TX). The target mRNA sequence of the siRNA is 5′-AAGUGCAGGAUUGGAUGGUGA-3′, corresponding to nt 362-382 of Bcl-w gene. As a negative control, a scrambled siRNA was designed consisting of the same nucleotide composition as the specific Bcl-w siRNA but lacking significant homology to the genome. As an additional negative control, a mismatched siRNA was used in which two bases in the specific Bcl-w siRNA were modified to make them noncomplementary to the target mRNA. To further ensure confidence in RNA interference (RNAi) data, a second specific Bcl-w siRNA was designed targeting the gene at 5′-AAGGGUUAUGUCUGUGGAGCU-3′, corresponding to nt 73-93 of Bcl-w. The antisense and sense template DNA oligonucleotides for each siRNA, plus T7 promoter 5′-CCTGTCTC-3′ to the 3′ end, were chemically synthesized (Integrated DNA Technologies, Coralville, IA) and were as follows: siRNA targeting Bcl-w nt 362-382 [siBcl-w (362)]: 5′-AAGTGCAGGATTGGATGGTGA-3′,5′-AATCACCATCCAATCCTGCAC-3′; scrambled siRNA (ncBcl-w): 5′-AAGGTGGTTACGAAGAGTTGG-3′, 5′-AACCAACTCTTCGTAACCACC-3′; mismatched siRNA (mmBcl-w): 5′-AAGTGCAGGATGTGATGGTGA-3′, 5′-AATCACCATCACATCCTGCAC-3′; siRNA targeting Bcl-w nt 73-93 [siBcl-w (73)]: 5′-AAGGGTTATGTCTGTGGAGCT-3′, 5′-AAAGCTCCACAGACATAACCC-3′. The synthesized template DNA was in vitro transcribed into double-strand siRNA using the Silencer siRNA construction kit (Ambion). siRNA transfection with siPORT Amine (Ambion) was performed according to the manufacturer's instructions.
RT-PCR. Total cellular RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Two micrograms of total RNA were reverse transcribed into the cDNA using Superscript first-strand synthesis system for RT-PCR (Invitrogen) following the manufacturer's instruction. Next, RT product was amplified with JumpStart TaqDNA polymerase (Sigma, St. Louis, MO). The primers (Integrated DNA Technologies) used in this experiment were as follows: 5′-CCGGGAGAACAGGGTATGAT-3′, 5′-CAGGTATGCACCCAGAGTGA-3′ for Bcl-2; 5′-AGGCTGGCGATGAGTTTGAA-3′, 5′-CGGCTCTCGGCTGCTGCATT-3′ for Bcl-x; 5′-AGCCTCAACCCCAGACACAC-3′, 5′-AAGGCCCCTACAGTTACCAG-3′ for rat Bcl-w; 5′-GGTGGCAGACTTTGTAGGTT-3′, 5′-GTGGTTCCATCTCCTTGTTG-3′ for human Bcl-w; 5′-TCAGCCCATCTTCTTCCAGATGGT-3′, 5′-CCACCAGCTCTGAACAGATCATGA-3′ for bax; 5′-ACTGCGATGAGGCCCTGTCT-3′, 5′-GGCCCAACAGAACCACACCA-3′ for bak; 5′-ATGGGAACCCCAAAGCAGCC3′, 5′-TCACTGGGAGGGAGTGGAGC3′ for bad; 5′-ATTTCATGAGGTGCCTGGAG-3′, 5′-GGCTTCCAATCAAGCTTCTG-3′ for bik; 5′-ACTCTGAGGTCAGCAACGGT-3′, 5′-CTAACCAAGTCCCTCACGTA-3′ for bid; 5′-GAATCTGGACGAAGCAGC TC-3′, 5′-AACATTTTCTGGCCGACTTG-3′ for BNIP3; 5′-GCCCCTACCTCCCTACAGAC-3′, 5′-CAGGTTCCTCCTGAGACTGC-3′ for bim (bimEL, bimL, and bimS); 5′-AGCCATGTACGTAGCCATCC-3′, 5′-CTCTCAGCTGTGGTGGTGAA-3′ for β-actin (internal control). The PCR cycles consisted of initial incubation at 94°C for 1 min, denaturation at 94°C for 30 s, annnealing at 52°C for 30 s, and extension at 72°C for 1 min, for 30 cycles, and final extension at 72°C for 7 min.
Immunocytochemistry. Neuron cultures were fixed by 20 min exposure to cold 4% paraformaldehyde/0.1 m Sorenson's buffer, pH 7.4, permeabilized with 0.2% Triton X-100, and then processed for immunochemistry as described previously (Pike and Cotman, 1993). A polyclonal antibody directed against human Bcl-w (1:1000 dilution; R&D Systems, Minneapolis, MN) was used. Briefly, neurons were blocked with Tris buffer (0.1 m Tris, 0.85% NaCl, 0.1% Triton X-100, 2% bovine serum albumin, pH 7.4) and were then incubated sequentially in primary antibody, biotinylated anti-goat antibody, and horseradish peroxidase-conjugated avidin-biotin complex (Vector Laboratories, Burlingame, CA) and visualized with 3,3-diaminobenzidine.
Preparation of mitochondrial or cytosolic extracts. Preparation of mitochondrial or cytosolic extracts was performed using the mitochondria/cytosol fractionation kit (Biovision) according to the manufacturer's instructions. Extracts were probed for levels of Smac by Western blot, as described below.
Western blot. Cultures were processed for Western blots using a standard protocol described previously (Pike, 1999). Briefly, cell lysates or extracts of mitochondria and cytosol were diluted into reducing sample buffer, electrophoresed for ∼1.5 h at 120 V in 15% polyacrylamide gels, and then transferred onto a polyvinylidene difluoride membrane (Millipore, Medford, MA) at constant voltage (100 V) for 1 h. After blocking of nonspecific binding (1 h incubation in 10 mm Tris, 100 mm NaCl, 0.1% Tween, 3% bovine serum albumin), membranes were incubated with primary antibody, which included goat anti-Bcl-w (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-Bcl-xL (R&D Systems), goat anti-Smac (Santa Cruz Biotechnology), and mouse anti-phospho-JNK (Thr183/Tyr185) antibody (Cell Signaling Technology, Beverly, MA). After rinsing (six times for 5 min each in 10 mm Tris, 100 mm NaCl, 0.1% Tween 20), membranes were incubated in the appropriate horseradish peroxidase-conjugated secondary antibody, followed by enhanced chemiluminescence detection (Amersham, Arlington Heights, IL). To detect total JNK or verify equal loading of protein among conditions, membranes were stripped (5 min in 100 mm glycine, pH 2.5, then 5 min in 62.5 mm Tris, 2% sodium dodecyl sulfate, 0.7% 2-mercaptoethanol, pH 6.7, at 60°C) and reprobed with rabbit anti-JNK antibody (Cell Signaling Technology) or mouse anti-β-tubulin antibody (Chemicon, Temecula, CA). Blots were quantified by band densitometry of scanned films using NIH Image 1.61 software. Data are presented graphically as a percentage of control values.
Statistical analyses. All experiments were repeated at least three times using independent culture preparations. Quantitative data were statistically analyzed by one-way ANOVA followed by pairwise comparisons using the Fisher's least significant difference test. A p value of <0.05 was considered significant.
Results
Bcl-w expression is downregulated in Aβ-induced neuronal apoptosis
In agreement with our previous observations (Pike et al., 1991, 1993, 1995), we found that micromolar levels of aggregated Aβ peptides gradually induce significant cell death in primary neuron cultures. In the presence of the active Aβ fragment Aβ25-35 (25 μm), neuron loss became significant within 12 h and worsened through 48 h (Fig. 1A). To explore the effect of Aβ on Bcl-2 family gene expression in this model system, we used RT-PCR to screen the expression of Bcl-2, Bcl-xL, Bcl-w, bax, bak, bad, bik, bid, BNIP3, and bimEL 6, 12, 24, and 48 h after Aβ exposure. The results showed that Aβ treatment induced no significant changes in the mRNA levels of some Bcl-2 members examined, including bak, bad, bik, bid, and BNIP3. Modest alterations in mRNA levels were observed for three genes: bimEL was time-dependently increased at 12-48 h, bax was slightly increased at 48 h, and Bcl-2 was mildly decreased at 24-48 h. Besides significant Bcl-xL downregulation at 12-48 h, the most significant effect was the robust, time-dependent depletion of the antiapoptotic Bcl-w, which was detectable within 6 h after Aβ (Fig. 1B) and progressed in parallel to the observed cell loss (Fig. 1A). This downregulation of Bcl-w mRNA induced by the Aβ25-35 peptide was reproduced with the full-length peptides Aβ1-40 and Aβ1-42 (Fig. 1C). To confirm that protein levels also decreased, Bcl-w levels were analyzed by Western blot 6, 12, 24, and 48 h after exposure to Aβ. The results revealed a similar time-dependent downregulation of Bcl-w (Fig. 1D), which was only 15% of basal level 48 h after exposure to Aβ (Fig. 1E).
Figure 1.
Bcl-wexpression is downregulated during Aβ-induced neuronal death. Primary neuron cultures were exposed to 25 μm aggregated Aβ peptide for the indicated times and then assayed for cell viability or Bcl-w expression. A, Aβ induces time-dependent neuronal death. Data show mean ± SEM cell viability from a representative experiment (n = 6). Significance is defined as *p < 0.05 and **p < 0.01 in comparison to vehicle-treated control group (Ctrl). B, Aβ induces time-dependent decrease in mRNA levels of Bcl-w. The mRNA expression of Bcl-2 family members (Bcl-2, Bcl-xL, Bcl-w, bax, bak, bad, bik, bid, BNIP3, and bimEL) was detected by RT-PCR followed by agarose gel electrophoresis. β-Actin served as internal control. C, Full-length Aβ peptides (25 μm) Aβ1-40 and Aβ1-42 also reduce mRNA levels of Bcl-w as detected by RT-PCR. D, Aβ induces time-dependent decrease in protein levels of Bcl-w. Protein expression of Bcl-w (top) was analyzed by Western blot. β-Tubulin (bottom) was used as a control. The picture shown is a representative of duplicated experiments. E, Relative amounts of Bcl-w were determined by densitometric scanning of Western blots from three independent experiments. Data is represented as a mean ± SEM percentage of control values. *p < 0.05 and **p < 0.01 relative to vehicle-treated control group.
Overexpression of Bcl-w reduces Aβ-induced neuron death
If downregulation of Bcl-w is a key component in the Aβ apoptotic signaling pathway, then we would predict that increased expression of Bcl-w should attenuate Aβ toxicity. To investigate this possibility, recombinant AAV vector was used to deliver the human Bcl-w gene into primary cultured neurons. Cultures infected with AAV did not show significant changes in either morphology or viability evaluated after 7 d in culture (data not shown). To evaluate AAV-mediated gene expression, Bcl-w mRNA was measured by RT-PCR 1 and 3 d after AAV-Bcl-w infection using human-specific primers. The results show that cultures infected with AAV-Bcl-w exhibited strong expression of human Bcl-w mRNA (Fig. 2B). In comparison, vehicle-treated and AAV-GFP-infected cultures show no human Bcl-w mRNA. These mRNA observations were confirmed at the protein level by Western blot, using an antibody that detects both endogenous rat Bcl-w and the exogenous human Bcl-w (Fig. 2C). Densitometry measures indicated that the magnitude of AAV-mediated Bcl-w expression 3 d after infection was nearly threefold higher than vehicle-treated cultures (Fig. 2D). To assess AAV infection efficiency in our paradigm, Bcl-w expression was detected by immunocytochemistry using anti-human Bcl-w antibody 3 d after infection (Fig. 2A). In comparison to the negative control lacking primary antibody, weak to modest Bcl-w immunoreactivity was detected in vehicle-treated and AAV-GFP-infected neuron cultures. However, nearly all cells exhibited strong Bcl-w immunoreactivity in cultures infected with AAV-Bcl-w. Together, these data demonstrate that recombinant AAV-Bcl-w had high expression efficiency when infected into our primary neuron cultures.
Figure 2.
Overexpression of Bcl-w protects against Aβ-induced neuronal death. A, Primary neuron cultures were infected AAV-Bcl-w, AAV-GFP, or reagents without vector (Vehicle) for 3 d. Bcl-w expression was detected by immunocytochemistry using anti-human Bcl-w antibody. In comparison to negative control without first antibody, only weak Bcl-w expression was detected in vehicle-treated and AAV-GFP-infected cultures. Almost all of neurons exhibited strong expression of Bcl-w in AAV-Bcl-w-infected cultures. B, Infection with AAV-Bcl-w yielded strong expression of human Bcl-w mRNA (top), as detected by RT-PCR. β-Actin served as internal control (bottom). C, Bcl-w protein expression was significantly increased in cultures infected with AAV-Bcl-w (top), as analyzed by Western blot using an antibody that recognizes both rodent and human Bcl-w. β-Tubulin was used as a control (bottom). The picture shown is a representative of duplicated experiments. D, Relative amounts of Bcl-w were determined by densitometric scanning of Western blots from three independent experiments. Data is represented as a mean ± SEM percentage of vehicle (1 d) control values. Significance is defined as **p < 0.01 relative to respective vehicle-treated control group. E, Infection with AAV-Bcl-w significantly protects neuron cultures from cell death induced by 48 h exposure to 25 μm Aβ25-35. Data show mean ± SEM cell viability from a representative experiment (n = 6). Significance is defined as **p < 0.01 in comparison to AAV-GFP condition.
To investigate the effect of Bcl-w overexpression on Aβ-induced neuronal apoptosis, cultures were infected with AAV-GFP or AAV-Bcl-w, treated 24 h later with 25 μm Aβ25-35, and assessed for cell viability 48 h after Aβ exposure. Vehicle-treated controls exhibited high levels of cell loss, an effect that was not affected by AAV-GFP infection (p > 0.05, compared with vehicle) (Fig. 2E). In contrast, cultures infected with AAV-Bcl-w showed greatly reduced vulnerability to Aβ (p < 0.01, compared with AAV-GFP infection) (Fig. 2E), a result consistent with the hypothesis that Bcl-w overexpression antagonizes Aβ-induced neuronal apoptosis.
Suppression of Bcl-w expression exacerbates Aβ-induced neuron death
To further verify the putative protective function of Bcl-w against Aβ-induced neuronal apoptosis, RNAi was used to deplete endogenous Bcl-w expression. Cultures were transfected for 1 or 3 d with one of two different siRNAs directed against Bcl-w. To confirm specificity, both mismatched and scrambled siRNAs were also used. Bcl-w mRNA was significantly reduced by both specific siRNAs but by neither of the control siRNAs (Fig. 3A). Reduced Bcl-w protein expression induced by the Bcl-w siRNA treatment was confirmed by Western blot (Fig. 3B). Densitometry measures indicated that siRNAs decreased Bcl-w levels by 60-70% 1 d after transfection and by up to 85% 3 d after transfection in comparison to scrambled siRNA control (Fig. 3C). These results demonstrate that the designed Bcl-w siRNAs had strong inhibitory effects on Bcl-w mRNA and protein levels.
Figure 3.

Suppression of endogenous Bcl-w expression exacerbates Aβ-induced neuronal death. Primary neuron cultures were transfected for 1 or 3 d with siRNA that either specifically targeted Bcl-w [siBcl-w(73), siBcl-w(362)] or served as scrambled or mismatched controls. A, The specific Bcl-w siRNAs but not the control siRNAs decreased endogenous Bcl-w mRNA expression as detected by RT-PCR using rat-specific primers (top). β-Actin served as internal control (bottom). B, Protein levels of Bcl-w were similarly affected by the siRNA treatments, as determined by Western blot with Bcl-w antibody (top).β-Tubulin served as a control (bottom). The picture shown is a representative of duplicated experiments. C, Relative amounts of Bcl-w were determined by densitometric scanning of Western blots from three independent experiments. Data is represented as a mean ± SEM percentage of ncBcl-w (1 d) control values. Significance is defined as **p < 0.01 relative to respective ncBcl-w control group. D, Neuron cultures were exposed for 48 h to 25 μm Aβ25-35 24 h after transfection with siRNA. Data show mean ± SEM cell viability from a representative experiment (n = 6). Significance is defined as **p < 0.01 in comparison to ncBcl-w condition.
To examine the effect of Bcl-w suppression on Aβ-induced neuron death, cultures were treated with 25 μm Aβ25-35 1 d after siRNA transfection, and cell viability was assessed 48 h later. In comparison to both the mismatched and scrambled siRNAs conditions, cultures transfected with the Bcl-w siRNAs showed significantly increased Aβ-induced cell death (p < 0.01, compared with scrambled siRNA) (Fig. 3D). Together with the Bcl-w overexpression data, these findings suggest that Bcl-w plays an important role in regulating apoptosis pathways involved in Aβ-induced neuronal death.
Bcl-w inhibits Aβ-induced Smac release
How Bcl-w regulates apoptotic signaling is not known. Abundant evidence suggests that antiapoptotic members of the Bcl-2 family are localized to and function at the mitochondria (Desagher and Martinou, 2000; Tsujimoto, 2003). One important step in the mitochondrial pathway of apoptosis is release of the protein Smac from the mitochondria into the cytosol (Du et al., 2000; Verhagen et al., 2000; Adrain et al., 2001; Carson et al., 2002). The proteins involved in regulating the release of Smac have not been investigated thoroughly, although members of the Bcl-2 family are implicated (Adrain et al., 2001; Lutter et al., 2001; Fulda et al., 2002; Sun et al., 2002; Yin et al., 2002; Kandasamy et al., 2003; Yamaguchi et al., 2003). A recent study has demonstrated that Aβ-induced apoptosis of murine cerebral endothelial cells involves mitochondrial Smac release (Yin et al., 2002). Thus, we sought to determine whether (1) Aβ-induced neuron death involves mitochondrial release of Smac, (2) Smac release is important for Aβ-induced neuron death, and (3) Bcl-w functions at least in part by regulating Smac release. We observed that Smac levels are decreased in the mitochondrial fraction and increased in the cytosolic fraction 24-48 h after Aβ treatment, indicating that Smac translocates from mitochondria to cytosol during Aβ-induced neuronal death (Fig. 4A).
Figure 4.
Aβ-induced apoptosis involves Smac release from mitochondria to cytosol in neuron cultures. A, Primary neuron cultures were exposed to vehicle (Ctrl) or 25 μm Aβ25-35 for 6-48 h. Levels of Smac in mitochondrial (Mt) and cytosolic (Cyt) extracts were analyzed by Western blot. B, Neuron cultures were pretreated for 1 h with 0 or 40 μm of the caspase 9 inhibitor Z-LEHD-FMK followed by exposure to 25 μm Aβ25-35 for 48 h. Data show mean ± SEM cell viability from a representative experiment (n = 3). Significance is defined as **p < 0.01 in comparison to Aβ condition.
We were not able to directly confirm a role of Smac release in Aβ-induced neuron death using molecular approaches to suppress Smac expression, because the sequence of rat Smac gene has not been published. Rather, we pharmacologically manipulated the downstream consequence of Smac release: promotion of caspase-9 activation. In the cytochrome c/apoptotic protease activating factor-1 (Apaf-1)/caspase-9 pathway, Smac released from the mitochondria binds to and functionally inactivates the inhibitor of apoptosis proteins (IAP), which inhibits caspase-9 activation (Du et al., 2000; Chauhan et al., 2001; McNeish et al., 2003). In short, if Smac release contributes to the mechanisms of Aβ-induced apoptosis, it should do so via caspase-9. Thus, to indirectly evaluate the role of Smac release in Aβ-induced neuronal death, we determined how inhibition of caspase-9 affects Aβ-induced apoptosis. Neuron cultures were pretreated with 40 μm caspase-9 inhibitor Z-LEHD-FMK for 60 min, followed by exposure to 25 μm Aβ25-35 for 48 h. Analysis of cell viability showed that inhibition of caspase-9 significantly attenuated Aβ-induced neuron death (Fig. 4B).
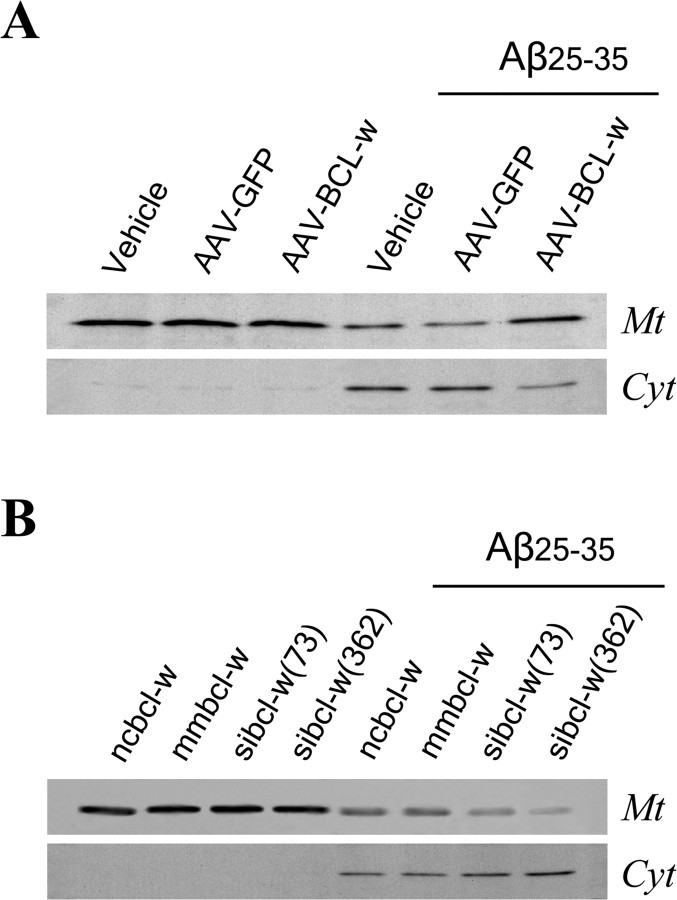
To investigate whether Bcl-w protection against Aβ-induced cell death may involve inhibition of Smac release, we determined how Aβ-induced Smac release from mitochondria is affected by alteration of Bcl-w expression levels. We observed that the overexpression of Bcl-w mediated by AAV-Bcl-w effectively but incompletely inhibited Aβ-induced Smac release (Fig. 5A), whereas suppression of Bcl-w expression by siRNA increased Smac release (Fig. 5B). Interestingly, Bcl-w siRNAs did not induce Smac release in the absence of Aβ treatment. These data suggest that Aβ-induced Smac release is regulated in part by Bcl-w and in addition, that the downregulation of Bcl-w induced by Aβ contributes to Smac release.
Figure 5.
Bcl-w inhibits Aβ-induced Smac release. A, Overexpression of Bcl-w inhibits Aβ-induced Smac release. Neuron cultures were infected with AAV-Bcl-w or AAV-GFP or vehicle for 24 h, followed by exposure to 25 μm Aβ25-35 for 48 h. Smac levels in mitochondrial (Mt) and cytosolic (Cyt) extracts were analyzed by Western blot. B, Suppression of Bcl-w expression increases Aβ-induced Smac release. Neuron cultures were transfected with siRNA for 24 h, followed by exposure to 25 μm Aβ 25-35 for 48 h. Smac levels in mitochondrial and cytosolic extracts were analyzed by Western blot. ncBcl-w, Scrambled siRNA as a negative control; mmBcl-w, mismatched siRNA control; siBcl-w (73), siRNA targeting Bcl-w nt 73-93; siBcl-w (362), siRNA targeting Bcl-w nt 362-382.
Aβ-induced Bcl-w downregulation and Smac release are dependent on JNK activation
The upstream signals by which Aβ triggers the downregulation of Bcl-w and subsequent mitochondrial release of Smac are unclear. Previous studies have reported that one key upstream signaling component in Aβ-induced neuronal apoptosis is activation of JNK (Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001; Jang and Surh, 2002). To investigate the role of JNK activation in Aβ-induced neuron death in our paradigm, we used Western blot analysis with phosphorylation site-specific antibodies that recognize the phosphorylated, activated form of JNK. The antibody used here detected two isoforms of JNK, 46 and 54 kDa. We observed that treatment of neuron cultures with 25 μm Aβ25-35 triggered phosphorylation of JNK that was detectable as early as 1 h after Aβ treatment and maximal within 6-12 h (Fig. 6A, top). This activation of JNK in response to Aβ did not alter total JNK protein levels (Fig. 6A, bottom).
Figure 6.
Aβ-induced Bcl-w downregulation and Smac release are dependent on JNK activation. A, JNK is activated (phosphorylated) in a time-dependent manner by Aβ. Neuron cultures were treated with 25 μm Aβ25-35 for the indicated times and then probed by Western blot with phospho-JNK (p-JNK) or pan JNK antibodies. The JNK inhibitor SP600125 (100 nm) decreases both basal levels (B) and Aβ-induced increases (C) in phosphorylated JNK, as determined by Western blot with p-JNK and pan JNK antibodies. D, Inhibition of JNK with SP600125 inhibits Aβ-induced Bcl-w downregulation. Neuron cultures were pretreated with 100 nm SP600125 for 90 min, followed by exposure to 25 μm Aβ25-35 for the indicated times, and were then analyzed for Bcl-w mRNA expression by RT-PCR. β-Actin served as internal control. E, Western Blot confirmed that SP600125 inhibits Aβ-induced Bcl-w downregulation (top). Neuron cultures were pretreated with 100 nm SP600125 for 90 min, followed by exposure to 25 μm Aβ25-35 for 48 h, and were then analyzed for Bcl-w expression by Western blot. As a control, Aβ-induced Bcl-xL downregulation was also inhibited by SP600125 (middle). β-Tubulin was used as a control (bottom). F, Relative amounts of Bcl-w were determined by densitometric scanning of Western blots from three independent experiments and are represented as a mean ± SEM percentage of control values. **p < 0.01 relative to the Aβ condition. G, JNK inhibition attenuates Aβ-induced Smac release. Neuron cultures were pretreated with 100 nm of SP600125 for 90 min, followed by exposure to 25 μm Aβ25-35 for 48 h, and were then analyzed for Smac content in mitochondrial (Mt) or cytosolic (Cyt) extracts Western blot. H, JNK inhibition blocks Aβ-induced neuronal death. Neuron cultures were pretreated with increasing concentrations (0-1 μm) of SP600125 for 90 min, followed by exposure to 25 μm Aβ25-35 for 48 h, and were then assayed for cell viability. Data show mean ± SEM cell viability from a representative experiment (n = 6). Significance is defined as **p < 0.01 in comparison to Aβ25-35 condition. Ctrl, Control.
To investigate the role of JNK signaling in Aβ-induced Bcl-w downregulation and Smac release, we used the recently developed specific inhibitor of JNK activation SP600125 (Bennett et al., 2001). Evaluation of phospho-JNK and total JNK immunoblots showed that 100 nm SP600125 significantly attenuated both the basal level of phosphorylated JNK (Fig. 6B, top) and the Aβ-induced increase in JNK phosphorylation (Fig. 6C, top) without altering total levels of JNK (Fig. 6B,C, bottom). If JNK activation contributes to the observed pathway of Aβ-induced neuron death, then the JNK inhibitor should block the Aβ effects of Bcl-w downregulation, mitochondrial release of Smac, and loss of neuronal viability. Our results are consistent with this prediction. First, pretreatment of cortical neurons with 100 nm SP600125 effectively prevented the Aβ-induced Bcl-w downregulation (Fig. 6D, top). This effect was confirmed at the protein level using Western blot (Fig. 6E, top, F). Perhaps suggesting that JNK activation is a general mechanism by which Aβ regulates expression of Bcl-2 family members (Fig. 1A), we observed that Aβ-induced Bcl-xL downregulation was also inhibited by SP600125 (Fig. 6E, middle).
Second, because Aβ-induced Bcl-w downregulation contributed to Smac release, we next assessed whether Aβ-induced JNK activation is required for the mitochondrial release of Smac. In the presence of JNK inhibitor SP600125, Aβ-induced Smac release was significantly but not completely attenuated (Fig. 6G). Finally, cell viability assays revealed that SP600125 dose-dependently inhibited neuronal apoptosis induced by Ab25-35, reaching maximal effect at 100 nm (Fig. 6H). The IC50 of this SP600125 effect was between 10 and 100 nm, a concentration range in which JNK is the only known target of SP600125 (Bennett et al., 2001).
Bcl-w does not affect Aβ-induced JNK activation
Our findings suggest that in the Aβ apoptotic signaling pathway, Bcl-w downregulation is located downstream of JNK activation. However, a recent study showed that Bcl-w suppressed cell apoptosis of the gastric cancer cell line SNU-16 by blocking JNK activation (Lee et al., 2003). To address this possibility in neurons, JNK activation was evaluated in neuron cultures after overexpression of Bcl-w. If Bcl-w functions upstream of JNK and/or regulates JNK activation, Bcl-w overexpression should significantly diminish Aβ-induced activation of JNK. In contrast to this possibility, we observed that Bcl-w overexpression mediated by AAV-Bcl-w neither reduced the ability of Aβ to activate JNK nor altered total JNK expression levels (Fig. 7). This observation confirms that Aβ-induced Bcl-w downregulation is located downstream of JNK activation.
Figure 7.
Bcl-w does not affect Aβ-induced JNK activation. Neuron cultures were exposed to 25 μm Aβ25-35 for the indicated times 24 h after AAV-Bcl-w infection. Western blots of whole-cell extracts were probed with a phospho-JNK (p-JNK) antibody (top) or JNK antibody (bottom). Results parallel those in noninfected cultures (Fig. 6 A).
Discussion
Bcl-w plays an important role in Aβ-induced neuronal apoptosis
Neural accumulation of Aβ has been implicated in the neuronal loss of AD. Although many (Forloni et al., 1993; Loo et al., 1993; Estus et al., 1997) but not all (Behl et al., 1994) previous studies have shown that Aβ-mediated neuronal death occurs via apoptosis, the underlying mechanism(s) remain incompletely defined. A key modulator of apoptosis pathways is the Bcl-2 family (Cory and Adams, 2002; Burlacu, 2003). Previous studies examining the role of the Bcl-2 family in Aβ-induced apoptosis have observed that Aβ can significantly decrease expression of antiapoptotic Bcl-2 and Bcl-xL (Forloni et al., 1996; Paradis et al., 1996; Wei et al., 2000; Tamagno et al., 2003) and increase expression of proapoptotic Bax and Bim (Paradis et al., 1996; Yin et al., 2002; Tamagno et al., 2003). Curiously, subtoxic and mildly toxic levels of Aβ can increase expression of the antiapoptotic Bcl-w (Zhu et al., 2004), perhaps suggesting induction of a protective response under certain stress conditions. In the current study, we observed modest effects of neurotoxic levels of Aβ on Bcl-2, bax, and bim expression. The most robust effects of Aβ on the Bcl-2 family were downregulation of Bcl-xL and Bcl-w.
Like Bcl-2 and Bcl-xL, Bcl-w functions as a negative regulator of apoptosis (Gibson et al., 1996). Similar to the other antiapoptotic Bcl-2 members, Bcl-w is widely expressed in mammalian tissues including CNS (Print et al., 1998; Hamner et al., 1999; O'Reilly et al., 2001). Notably, expression of Bcl-w increases during brain development, reaching its highest levels in the mature brain (Hamner et al., 1999). This is in contrast to Bcl-2 and Bcl-xL, which show highest neural expression during development and relatively lower levels in adult brain (Michaelidis et al., 1996; Pinon et al., 1997; Hamner et al., 1999). Furthermore, Bcl-w knock-out mice exhibit apparently normal brain development (Print et al., 1998). Together, these observations suggest that Bcl-w function may be most important in the adult brain. On the basis of both cell culture (Hamner et al., 2001; Middleton et al., 2001) and adult animal models (Sun et al., 2003), the neural function of Bcl-w includes increasing resistance to neuronal apoptosis. A protective role of Bcl-w is also supported by recent findings by Zhu et al. (2004) in both Alzheimer brain tissue and cell culture. Consistent with these observations, our data show that Aβ-induced neuronal death was reduced by overexpression of Bcl-w and potentiated by suppression of Bcl-w. Paired with our observation that toxic levels of Aβ greatly deplete Bcl-w levels before the onset of cell death, these data strongly suggest an important role of Bcl-w in the mechanism of Aβ-induced neuronal apoptosis. However, our observations that (1) overexpression of Bcl-w did not completely block Aβ-induced apoptosis and (2) Aβ altered expression of several Bcl-2 members suggest that Bcl-w likely regulates neuronal apoptosis in concert with other Bcl-2 members including Bcl-xL.
Bcl-w may modulate apoptosis by regulating Smac release
Smac release from mitochondria appears to be a general feature of apoptosis involving the mitochondrial pathway of cell death (Du et al., 2000; Verhagen et al., 2000; Adrain et al., 2001; Carson et al., 2002). Once released into the cytosol, Smac promotes caspase-9 activation in the cytochrome c/Apaf-1/caspase-9 pathway by binding to the IAP and removing their inhibitory activity. One means by which the Bcl-2 family modulates apoptosis pathways appears to be by regulating Smac release. Specifically, previous studies have reported that Smac release is regulated by Bcl-2, Bcl-xL, Bid, Bax, Bak, and Bim (Adrain et al., 2001; Lutter et al., 2001; Fulda et al., 2002; Sun et al., 2002; Yin et al., 2002; Kandasamy et al., 2003; Yamaguchi et al., 2003). The role of Bcl-w in modulating the mitochondrial pathway of cell death is less clear. Previous work has shown that recombinant rat Bcl-w protein decreases Bax- or Ca2+-induced release of cytochrome c from isolated brain mitochondria (Yan et al., 2000). Whether Smac release is regulated by Bcl-w has yet to be determined. Consistent with recent observations in murine cerebral endothelial cells (Yin et al., 2002), our data show that Aβ-induced apoptosis in neurons is characterized by mitochondrial release of Smac into the cytosol. Importantly, we observed that this Aβ-induced translocation of Smac is effectively inhibited by overexpression of Bcl-w and increased by suppression of Bcl-w expression. Vulnerability to Aβ-induced neuronal death correlated with both Bcl-w levels and Smac release. These findings suggest that Bcl-w may regulate mitochondrial Smac release in neurons. Furthermore, the data implicate the downregulation of Bcl-w and subsequent Smac release as key components in the pathway of Aβ-induced neuronal apoptosis. However, because suppression of Bcl-w expression by RNAi failed to both independently induce Smac release and decrease basal measures of neuron viability, Bcl-w function likely overlaps with Bcl-xL and Bcl-2.
Aβ-induced Bcl-w downregulation and Smac release are dependent on JNK activation
JNKs are a family of serine/threonine kinases involved in a variety of cellular responses, including cell proliferation and death (Ip and Davis, 1998). Activation of JNK signaling has been closely linked to a variety of apoptotic stimuli, and inhibition or loss of the JNK pathway provides protection against neuronal apoptosis in multiple paradigms, including Aβ neurotoxicity (Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001). Unclear are the cell signaling components downstream of JNK activation that mediate Aβ-induced apoptosis.
Based on our finding that Bcl-w downregulation plays a key role in Aβ neurotoxicity, we evaluated the possibility that JNK activation contributes to Bcl-w downregulation. To begin exploring this idea, we investigated how Aβ-induced Bcl-w downregulation is affected by SP600125, a specific inhibitor of JNK (Bennett et al., 2001). We found that SP600125 effectively prevents Aβ-induced Bcl-w downregulation, indicating that this critical step in the Aβ cell-death pathway is dependent on JNK activation. Our observation that inhibition of JNK also blocks Aβ-induced downregulation of Bcl-xL suggests that JNK signaling may be a shared mechanism of regulation of the Bcl-2 family. This possibility is consistent with other recent findings. For example, transforming growth factor-β induced cell death in the developing chick retina is mediated via activation of JNK and downregulation of the antiapoptotic protein Bcl-xL (Schuster et al., 2002). Also, JNK inhibition with SP600125 prevents the troglitazone-induced changes in the levels of Bax, Bad, and Bcl-2 in human hepatoma cells (HepG2) (Bae and Song, 2003) and the Aβ-induced decrease of Bcl-2 and increase of Bax in the SK-N-BE cell line (Tamagno et al., 2003).
Because our studies suggest that Aβ-induced downregulation of Bcl-w contributes to both Smac release and neuronal death, we also examined the involvement of JNK activation in the mitochondrial release of Smac. Our results demonstrate that Aβ-induced Smac release is JNK dependent. In agreement with this finding is a recent report that activated JNK is required for the 2-methoxyestradiol- or proteasome inhibitor (PS-341)-induced Smac release in multiple myeloma cells (Chauhan et al., 2003). Interestingly, although both Bcl-w overexpression and JNK inhibition effectively attenuated Aβ-induced Smac release, neither intervention fully protected against neuronal death. These data suggest the involvement of other Bcl-2 members in Aβ neurotoxicity and or a role for cell death mechanisms in addition to the observed JNK/Bcl-w/Smac pathway.
The mechanism by which JNK activation reduces Bcl-w expression likely reflects its established role as a transcriptional regulator. JNK is a serine threonine protein kinase that phosphorylates c-Jun (Derijard et al., 1994; Kallunki et al., 1994), a component of the transcription factor-1 (AP-1) (Karin, 1995; Ip and Davis, 1998). In complex with other DNA binding proteins, c-Jun/AP-1 regulates the transcription of numerous genes, suggesting the possibility that activated JNK-Jun/AP-1 pathway mediates Aβ-induced Bcl-w downregulation. Supporting this interpretation, previous studies have shown that Aβ increases the phosphorylation of JNK and c-Jun and DNA binding activity of AP-1 (Morishima et al., 2001; Troy et al., 2001; Xu et al., 2001; Giri et al., 2003). In the present study, the rapid and sustained activation of JNK followed by downregulation of both Bcl-w and Bcl-xL position JNK activation as the relatively upstream component. Furthermore, pharmacological inhibition of JNK effectively prevented Aβ-induced downregulation of Bcl-w. This combination of temporal and pharmacological findings suggest that Bcl-w downregulation is dependent on and occurs downstream of JNK activation.
In apparent contrast to our finding that Bcl-w functions downstream of JNK activation in regulating neuronal apoptosis is the recent observation that Bcl-w can function upstream of JNK in apoptosis pathways. In the gastric cancer cell line SNU-16, Bcl-w reduces apoptosis by blocking JNK activation (Lee et al., 2003). However, our results showed that Bcl-w overexpression does not reduce the ability of Aβ to activate JNK, reinforcing the conclusion that regulation of Bcl-2 family members occurs downstream of JNK.
Together, these data elucidate a pathway of neuronal death that is mediated, at least in part, by JNK-dependent downregulation of the antiapoptotic protein Bcl-w and likely involves subsequent mitochondrial release of the proapoptotic factor Smac. We speculate that Bcl-xL and other Bcl-2 family members may be regulated in a similar manner to cooperatively regulate apoptosis. These observations not only establish neuronal roles of Bcl-w, but also provide new insight into the mechanism(s) underlying Aβ-induced neuronal death. Continued understanding of the degenerative signaling cascades triggered by Aβ will yield basic knowledge about neuronal apoptosis pathways as well as identify potential molecular targets for neuroprotective AD therapies.
Footnotes
This work was supported by National Institute on Aging Grant AG15961 to C.J.P. We thank Dr. David A. Greenberg for providing AAV vector and Dr. Jinhui Wang and Allison Quaglino for kind assistance.
Correspondence should be addressed to Dr. Christian J. Pike, Andrus Gerontology Center, University of Southern California, 3715 McClintock Avenue, Los Angeles, CA 90089-0191. E-mail: cjpike@usc.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251149-10$15.00/0
References
- Adrain C, Creagh EM, Martin SJ (2001) Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J 20: 6627-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. (2000) Inflammation and Alzheimer's disease. Neurobiol Aging 21: 383-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonsson B, Martinou JC (2000) The Bcl-2 protein family. Exp Cell Res 256: 50-57. [DOI] [PubMed] [Google Scholar]
- Bae MA, Song BJ (2003) Critical role of c-Jun N-terminal protein kinase activation in troglitazone-induced apoptosis of human HepG2 hepatoma cells. Mol Pharmacol 63: 401-408. [DOI] [PubMed] [Google Scholar]
- Behl C, Davis JB, Klier FG, Schubert D (1994) Amyloid beta peptide induces necrosis rather than apoptosis. Brain Res 645: 253-264. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, O'Kane TM, Wu ZL, Dobrzanski P, Murthy S, Vaught JL, Scott RW (2001) CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Abeta-induced cortical neuron apoptosis. J Neurochem 77: 849-863. [DOI] [PubMed] [Google Scholar]
- Burlacu A (2003) Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med 7: 249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning DR, McKeon RJ, DeWitt DA, Perry G, Wujek JR, Frederickson RC, Silver J (1993) Beta-amyloid of Alzheimer's disease induces reactive gliosis that inhibits axonal outgrowth. Exp Neurol 124: 289-298. [DOI] [PubMed] [Google Scholar]
- Carson JP, Behnam M, Sutton JN, Du C, Wang X, Hunt DF, Weber MJ, Kulik G (2002) Smac is required for cytochrome c-induced apoptosis in prostate cancer LNCaP cells. Cancer Res 62: 18-23. [PubMed] [Google Scholar]
- Chauhan D, Hideshima T, Rosen S, Reed JC, Kharbanda S, Anderson KC (2001) Apaf-1/cytochrome c-independent and Smac-dependent induction of apoptosis in multiple myeloma (MM) cells. J Biol Chem 276: 24453-24456. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, Hideshima T, Podar K, Mitsiades C, Mitsiades N, Munshi N, Kharbanda S, Anderson KC (2003) JNK-dependent release of mitochondrial protein, Smac, during apoptosis in multiple myeloma (MM) cells. J Biol Chem 278: 17593-17596. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM (2002) The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2: 647-656. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu IH, Barrett T, Su B, Deng T, Karin M, Davis RJ (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76: 1025-1037. [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10: 369-377. [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102: 33-42. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD (1996) Scavenger receptor-mediated adhesion of microglia to betaamyloid fibrils. Nature 382: 716-719. [DOI] [PubMed] [Google Scholar]
- Estus S, Tucker HM, van Rooyen C, Wright S, Brigham EF, Wogulis M, Rydel RE (1997) Aggregated amyloid-β protein induces cortical neuronal apoptosis and concomitant “apoptotic” pattern of gene induction. J Neurosci 17: 7736-7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forloni G, Chiesa R, Smiroldo S, Verga L, Salmona M, Tagliavini F, Angeretti N (1993) Apoptosis mediated neurotoxicity induced by chronic application of beta amyloid fragment 25-35. NeuroReport 4: 523-526. [DOI] [PubMed] [Google Scholar]
- Forloni G, Bugiani O, Tagliavini F, Salmona M (1996) Apoptosis-mediated neurotoxicity induced by beta-amyloid and PrP fragments. Mol Chem Neuropathol 28: 163-171. [DOI] [PubMed] [Google Scholar]
- Fulda S, Meyer E, Debatin KM (2002) Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 21: 2283-2294. [DOI] [PubMed] [Google Scholar]
- Gibson L, Holmgreen SP, Huang DC, Bernard O, Copeland NG, Jenkins NA, Sutherland GR, Baker E, Adams JM, Cory S (1996) bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene 13: 665-675. [PubMed] [Google Scholar]
- Giri RK, Selvaraj SK, Kalra VK (2003) Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J Immunol 170: 5281-5294. [DOI] [PubMed] [Google Scholar]
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, Ostaszewski BL, Lieberburg I, Koo EH, Schenk D, Teplow DB, Selkoe DJ (1992) Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359: 322-325. [DOI] [PubMed] [Google Scholar]
- Hamner S, Skoglosa Y, Lindholm D (1999) Differential expression of bcl-w and bcl-x messenger RNA in the developing and adult rat nervous system. Neuroscience 91: 673-684. [DOI] [PubMed] [Google Scholar]
- Hamner S, Arumae U, Li-Ying Y, Sun YF, Saarma M, Lindholm D (2001) Functional characterization of two splice variants of rat bad and their interaction with Bcl-w in sympathetic neurons. Mol Cell Neurosci 17: 97-106. [DOI] [PubMed] [Google Scholar]
- Hardy J (1997) Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci 20: 154-159. [DOI] [PubMed] [Google Scholar]
- Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ (1999) Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci 19: 8876-8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi M, Sato M, Matsumoto S, Noguchi A, Yasutake K, Yoshida N, Sato K (2003) Spherical aggregates of beta-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3beta. Proc Natl Acad Sci USA 100: 6370-6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip YT, Davis RJ (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)-from inflammation to development. Curr Opin Cell Biol 10: 205-219. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Bui ET, Cotman CW (1998) Beta-amyloid induces local neurite degeneration in cultured hippocampal neurons: evidence for neuritic apoptosis. Neurobiol Dis 5: 365-378. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ (2002) Beta-amyloid induces oxidative DNA damage and cell death through activation of c-Jun N terminal kinase. Ann NY Acad Sci 973: 228-236. [DOI] [PubMed] [Google Scholar]
- Kallunki T, Su B, Tsigelny I, Sluss HK, Derijard B, Moore G, Davis R, Karin M (1994) JNK2 contains a specificity-determining region responsible for efficient c-Jun binding and phosphorylation. Genes Dev 8: 2996-3007. [DOI] [PubMed] [Google Scholar]
- Kandasamy K, Srinivasula SM, Alnemri ES, Thompson CB, Korsmeyer SJ, Bryant JL, Srivastava RK (2003) Involvement of proapoptotic molecules Bax and Bak in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced mitochondrial disruption and apoptosis: differential regulation of cytochrome c and Smac/DIABLO release. Cancer Res 63: 1712-1721. [PubMed] [Google Scholar]
- Karin M (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem 270: 16483-16486. [DOI] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL (1998) Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA 95: 6448-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HW, Lee SS, Lee SJ, Um HD (2003) Bcl-w is expressed in a majority of infiltrative gastric adenocarcinomas and suppresses the cancer cell death by blocking stress-activated protein kinase/c-Jun NH2-terminal kinase activation. Cancer Res 63: 1093-1100. [PubMed] [Google Scholar]
- Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW (1993) Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci USA 90: 7951-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A, Yankner BA (1994) Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci USA 91: 12243-12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Perkins GA, Wang X (2001) The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol 2: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Tomaselli KJ, Rydel RE (1993) Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res 621: 35-49. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Partin J, Begley JG (1998) Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res 807: 167-176. [DOI] [PubMed] [Google Scholar]
- McNeish IA, Bell S, McKay T, Tenev T, Marani M, Lemoine NR (2003) Expression of Smac/DIABLO in ovarian carcinoma cells induces apoptosis via a caspase-9-mediated pathway. Exp Cell Res 286: 186-198. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos Jr L, Baron P, Villalba M, Ferrari D, Rossi F (1995) Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 374: 647-650. [DOI] [PubMed] [Google Scholar]
- Michaelidis TM, Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer M, Thoenen H (1996) Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron 17: 75-89. [DOI] [PubMed] [Google Scholar]
- Middleton G, Wyatt S, Ninkina N, Davies AM (2001) Reciprocal developmental changes in the roles of Bcl-w and Bcl-x(L) in regulating sensory neuron survival. Development 128: 447-457. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME (2001) β-Amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci 21: 7551-7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly LA, Print C, Hausmann G, Moriishi K, Cory S, Huang DC, Strasser A (2001) Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ 8: 486-494. [DOI] [PubMed] [Google Scholar]
- Paradis E, Douillard H, Koutroumanis M, Goodyer C, LeBlanc A (1996) Amyloid β peptide of Alzheimer's disease downregulates Bcl-2 and up-regulates bax expression in human neurons. J Neurosci 16: 7533-7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ (1999) Estrogen modulates neuronal Bcl-xL expression and beta-amyloid-induced apoptosis: relevance to Alzheimer's disease. J Neurochem 72: 1552-1563. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cotman CW (1993) Cultured GABA-immunoreactive neurons are resistant to toxicity induced by beta-amyloid. Neuroscience 56: 269-274. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz AJ, Glabe CG, Cotman CW (1991) In vitro aging of beta-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res 563: 311-314. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cummings BJ, Cotman CW (1992) Beta-amyloid induces neuritic dystrophy in vitro: similarities with Alzheimer pathology. NeuroReport 3: 769-772. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW (1993) Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. J Neurosci 13: 1676-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ, Cummings BJ, Monzavi R, Cotman CW (1994) Beta-amyloid-induced changes in cultured astrocytes parallel reactive astrocytosis associated with senile plaques in Alzheimer's disease. Neuroscience 63: 517-531. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Walencewicz-Wasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW (1995) Structure-activity analyses of β-amyloid peptides: contributions of the β25-35 region to aggregation and neurotoxicity. J Neurochem 64: 253-265. [DOI] [PubMed] [Google Scholar]
- Pinon LG, Middleton G, Davies AM (1997) Bcl-2 is required for cranial sensory neuron survival at defined stages of embryonic development. Development 124: 4173-4178. [DOI] [PubMed] [Google Scholar]
- Print CG, Loveland KL, Gibson L, Meehan T, Stylianou A, Wreford N, de Kretser D, Metcalf D, Kontgen F, Adams JM, Cory S (1998) Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc Natl Acad Sci USA 95: 12424-12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saille C, Marin P, Martinou JC, Nicole A, London J, Ceballos-Picot I (1999) Transgenic murine cortical neurons expressing human Bcl-2 exhibit increased resistance to amyloid beta-peptide neurotoxicity. Neuroscience 92: 1455-1463. [DOI] [PubMed] [Google Scholar]
- Schuster N, Dunker N, Krieglstein K (2002) Transforming growth factor-beta induced cell death in the developing chick retina is mediated via activation of c-jun N-terminal kinase and downregulation of the antiapoptotic protein Bcl-X(L). Neurosci Lett 330: 239-242. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2001) Alzheimer's disease: genes, proteins, and therapy. Physiol Rev 81: 741-766. [DOI] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, Younkin SG (1992) Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science 258: 126-129. [DOI] [PubMed] [Google Scholar]
- Simmons LK, May PC, Tomaselli KJ, Rydel RE, Fuson KS, Brigham EF, Wright S, Lieberburg I, Becker GW, Brems DN (1994) Secondary structure of amyloid beta peptide correlates with neurotoxic activity in vitro. Mol Pharmacol 45: 373-379. [PubMed] [Google Scholar]
- Song YS, Park HJ, Kim SY, Lee SH, Yoo HS, Lee HS, Lee MK, Oh KW, Kang SK, Lee SE, Hong JT (2004) Protective role of Bcl-2 on beta-amyloid-induced cell death of differentiated PC12 cells: reduction of NF-kappaB and p38 MAP kinase activation. Neurosci Res 49: 69-80. [DOI] [PubMed] [Google Scholar]
- Sun XM, Bratton SB, Butterworth M, MacFarlane M, Cohen GM (2002) Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J Biol Chem 277: 11345-11351. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Clark KR, Peel A, Mao XO, Chang Q, Simon RP, Greenberg DA (2003) Adeno-associated virus-mediated delivery of BCL-w gene improves outcome after transient focal cerebral ischemia. Gene Ther 10: 115-122. [DOI] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Guglielmotto M, Santoro G, Bardini P, Marra L, Tabaton M, Danni O (2003) Multiple signaling events in amyloid beta-induced, oxidative stress-dependent neuronal apoptosis. Free Radic Biol Med 35: 45-58. [DOI] [PubMed] [Google Scholar]
- Tan J, Town T, Placzek A, Kundtz A, Yu H, Mullan M (1999) Bcl-X(L) inhibits apoptosis and necrosis produced by Alzheimer's beta-amyloid1-40 peptide in PC12 cells. Neurosci Lett 272: 5-8. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA (2001) Beta-amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J Neurochem 77: 157-164. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y (2003) Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 195: 158-167. [DOI] [PubMed] [Google Scholar]
- Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102: 43-53. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, Benedek GB, Selkoe DJ, Teplow DB (1999) Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem 274: 25945-25952. [DOI] [PubMed] [Google Scholar]
- Wei H, Leeds PR, Qian Y, Wei W, Chen R, Chuang D (2000) Beta-amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur J Pharmacol 392: 117-123. [DOI] [PubMed] [Google Scholar]
- Xu J, Chen S, Ahmed SH, Chen H, Ku G, Goldberg MP, Hsu CY (2001) Amyloid-β peptides are cytotoxic to oligodendrocytes. J Neurosci 21: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Bhalla K, Wang HG (2003) Bax plays a pivotal role in thapsigargin-induced apoptosis of human colon cancer HCT116 cells by controlling Smac/Diablo and Omi/HtrA2 release from mitochondria. Cancer Res 63: 1483-1489. [PubMed] [Google Scholar]
- Yan C, Chen J, Chen D, Minami M, Pei W, Yin XM, Simon RP (2000) Overexpression of the cell death suppressor Bcl-w in ischemic brain: implications for a neuroprotective role via the mitochondrial pathway. J Cereb Blood Flow Metab 20: 620-630. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Lee JM, Chen SD, Xu J, Hsu CY (2002) Amyloid-β induces Smac release via AP-1/Bim activation in cerebral endothelial cells. J Neurosci 22: 9764-9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang Y, Ogawa O, Lee HG, Raina AK, Siedlak SL, Harris PL, Fujioka H, Shimohama S, Tabaton M, Atwood CS, Petersen RB, Perry G, Smith MA (2004) Neuroprotective properties of Bcl-w in Alzheimer disease. J Neurochem 89: 1233-1240. [DOI] [PubMed] [Google Scholar]