Abstract
In rats, naturally occurring variations in maternal care contribute to the development of individual differences in the behavioral and neuroendocrine responses to stress during adulthood. The dopamine (DA) projection to the medial prefrontal cortex (mPFC) plays an important role in mediating stress responsivity and is thought to be involved also in regulating sensorimotor gating. In the present study, we compared prepulse inhibition (PPI) of acoustic startle as well as the left and right mPFC DA stress responses in the adult offspring of high- and low-licking/grooming (LG) dams. Our data indicate that the offspring of low-LG animals are impaired on measures of PPI compared with high-LG animals. We also observed in low-LG animals a significant blunting of the mPFC DA stress responses that was lateralized to the right hemisphere, whereas in high-LG animals, the left and right mPFC DA stress responses were equally attenuated. Although mPFC levels of DA transporter did not differ between the two groups of animals, mPFC levels of catechol-O-methyl transferase immunoreactivity of low-LG animals were significantly lower than those of high-LG animals. These data provide evidence that variations in maternal care can lead to lasting changes in mPFC DA responsivity to stress and suggest the possibility that such changes in mesocorticolimbic DA function can also lead to deficits in sensorimotor gating.
Keywords: maternal licking/grooming, stress, voltammetry, prepulse inhibition, catechol-O-methyl transferase, dopamine transporter
Introduction
As adults, rats that received low levels of maternal licking/grooming (LG) during the first week of postnatal life are more fearful in a novel environment and respond to stressors with more pronounced increases of both adrenocorticotropin and corticosterone levels than do the offspring of high-LG mothers (Liu et al., 1997; Caldji et al., 1998; Francis et al., 1999). Although variations in maternal care are known to produce stable, tissue-specific effects on gene expression in the hippocampus and amygdala (Meaney, 2001), the effects of such variations on medial prefrontal cortex (mPFC) function have yet to be examined. In addition to its involvement in regulating the behavioral, neuroendocrine, and autonomic responses to stress (Diorio et al., 1993; Sullivan and Gratton 1999, 2002, 2003), the mPFC plays a pivotal role in so-called executive functions. Specialized neurons within the mPFC are involved in maintaining task-relevant information “on-line” for brief periods (Fuster, 1997), essential for structuring goal-directed behaviors. Dopamine (DA) plays a modulatory role here by optimizing the activity of mPFC neurons and the functions they subserve (Williams and Goldman-Rakic, 1995; Murphy et al., 1996). Not surprisingly, disorders of higher executive processes often reflect disruptions in mPFC DA-mediated function.
Stressors will potently stimulate DA release in the mPFC (Abercrombie et al., 1989; Doherty and Gratton, 1999). Perhaps less well known is that the DA inputs to the left and right mPFC are functionally asymmetric, particularly with respect to stress responsivity (Slopsema et al., 1982; Carlson et al., 1991, 1993, 1996; Sullivan and Szechtman, 1995; Sullivan et al., 1998; Sullivan and Gratton, 1998; Andersen and Teicher, 1999; Berridge et al., 1999; Thiel and Schwarting, 2001). Furthermore, the development of such hemispheric asymmetries in mPFC function can be altered by perinatal factors and the early postnatal rearing environment (Denenberg, 1981; Denenberg et al., 1986; Brake et al., 2000). These findings along with evidence derived from clinical observations have led to the suggestion that abnormal lateralization of mPFC DA-mediated function may increase the vulnerability to a range of stress-related psychopathologies (for review, see Sullivan and Gratton, 2002). In pursuing this idea, we sought to determine whether naturally occurring variations in the level of maternal care might contribute to the development of individual differences in mPFC DA responsivity to stress during adulthood and, if so, whether such differences are lateralized to the left or right hemisphere. We used voltammetry to monitor the left and right mPFC DA stress responses in the adult offspring of high- and low-LG mothers. Changes in mPFC DA transmission were also assessed in these animals by comparing levels of the DA transporter (DAT) as well as the expression of catechol-O-methyltransferase (COMT).
The mPFC is thought to also play a role in sensorimotor gating (Swerdlow et al., 2001). Evidence of this comes from studies of prepulse inhibition (PPI) of the acoustic startle response (ASR), a coordinated contraction of the skeletal musculature elicited in response to a loud sound. PPI refers to the attenuated ASR that is observed when the startling stimulus is immediately preceded by a weaker, nonstartling tone (Geyer et al., 1990). Impaired PPI is associated with several psychiatric disorders involving central DA dysfunction, notably schizophrenia (Geyer et al., 1990, 2001), and in animals, PPI has been shown to be highly sensitive to treatments that alter DA transmission, particularly in the nucleus accumbens (Swerdlow et al., 1990a,b, 1992; Wan and Swerdlow, 1993; Wan et al., 1994; Zhang et al., 2000), but also in the mPFC (Bubser and Koch, 1994; Ellenbroek et al., 1996). There is also some evidence that sensorimotor gating, as measured by PPI of the ASR, is sensitive to variations in early environmental and rearing conditions (Cilia et al., 2001). Thus, the second objective of the present study was to determine whether the adult offspring of high- and low-LG mothers differ on measures of PPI of the ASR.
Materials and Methods
Animals
All procedures were performed in accordance with the guidelines established by the Canadian Council on Animal Care with protocols approved by the McGill University Animal Care Committee. The animals used in the present study were the male offspring of Long-Evans dams mated in our animal colony; this allowed us to avoid confounds resulting from the stress of transporting dams during the prenatal period. After mating, the dams were housed singly on a standard 12 h light/dark cycle (lights on at 8:00 A.M.) in polycarbonate maternity cages containing bedding and had ad libitum access to food and water. Cage cleaning began no earlier than postnatal day 10 (P10). Litters were left otherwise undisturbed with their respective dams until weaning on day 21, after which the male offspring from each litter were housed together in groups of three to four per cage until P45. From P45 onward, animals from each litter were housed in pairs until testing, which occurred at 3-4 months of age.
Assessment of maternal behavior
Maternal behavior was assessed using a procedure adapted from that described previously (Liu et al., 1997; Champagne et al., 2003). The frequency of maternal behaviors was scored on postpartum days 1-6. Although in the past we typically assessed maternal behaviors on postpartum days 1-10 (Liu et al., 1997; Caldji et al., 1998; Francis et al., 1999), we have established that limiting the scoring to just 6 d yielded reliable data that were highly correlated (r = 0.97) with data generated during the extended assessment procedure (Champagne et al., 2003). Observers were trained to a high level of interrater reliability (>0.90). Dams were observed in their home cage and not disturbed for the duration of the 6 d observation period. Daily observations occurred during five 75 min sessions, three of which were scheduled during the light phase (10:00 A.M., 1:00 P.M., and 5:00 P.M.) and two of which were scheduled during the dark phase (7:00 A.M. and 8:00 P.M.). Within each of these sessions, mothers were scored 25 times (every 3 min) for the presence of LG behavior (both body and anogenital licking were included). The presence of arched-back nursing (ABN) was also scored; that is when mothers were observed nursing their pups in an arched-back posture with legs extended [for a full description, see Champagne et al. (2003)]. These two behaviors are not mutually exclusive; in fact, LG behavior is frequent when the mothers are nursing, especially in an arched-back posture. Thus, each mother's LG/ABN frequency score was based on a total of 750 observations (25 observations/session × 5 sessions/day × 6 d = 750 observations/mother) and was expressed as a percentage (number of LG/ABN occurances/750 × 100).
Pups were designated as belonging to a high- or low-LG litter on the basis of their mother's LG/ABN frequency score relative to the cohort's mean frequency score and SD. The characterization of each mother's behavior thus depends on the size and reliability of the cohort's data set. To obtain reliable estimates of individual differences in maternal behavior, we observed cohorts of ∼40 mothers-litters. The size of the cohort is constrained by the rating procedure; with sequential observations within a 3 min period, a well trained observer can accurately rate the behavior of ≤40 dams. However, because the birth dates within a cohort are usually staggered over 2-3 d, observations are more typically conducted on ∼25-30 litters at any one time. High-LG mothers were defined as females whose LG/ABN frequency scores were >1 SD above the cohort mean. Low-LG mothers were defined as females whose LG/ABN frequency scores were >1 SD below the cohort mean.
In vivo electrochemistry
Surgery. Separate groups of experimentally naive low- and high-LG rats were pretreated with atropine sulfate (0.1 mg/kg, i.p.), anesthetized with sodium pentobarbital (60 mg/kg, i.p.), and placed in a stereotaxic apparatus (Kopf, Tujunga, CA). Animals were implanted with a voltammetric electrode aimed at ventral sites within either the right or left mPFC. The coordinates were as follows: anteroposterior, +3.2 from bregma; lateral, ±0.7 mm from bregma; dorsoventral, -4.2 mm below the cortical surface. The animals were also each implanted with an Ag/AgCl reference electrode and a stainless steel ground wire in the contralateral and ipsilateral parietal cortex, respectively. Miniature pin connectors soldered to the electrochemical electrode and to the reference and ground wires were inserted into a plastic strip connector. The entire assembly was secured with acrylic dental cement anchored to three stainless steel screws threaded into the cranium. After surgery, the animals were housed individually with ad libitum access to food and water and allowed to recover for at least 4 d.
Electrochemical probe. The electrochemical probes each comprised three 30-μm-diameter carbon fibers (Avco Specialty Materials, Lowell, MA) that extended 50-100 μm beyond the epoxy-sealed tip of a pulled glass capillary. The exposed fiber bundle was repeatedly coated with a 5% solution of the ion exchange polymer Nafion (Aldrich, Milwaukee, WI). This treatment has been shown to promote the exchange of cations such as DA while impeding that of interfering anionic species, notably ascorbic acid (AA) and the primary metabolite of DA, 3,4-dihydroxyphenylacetic acid (DOPAC). Before implantation, electrodes were calibrated in 0.1 m PBS, pH 7.4, containing 250 μm AA to determine their sensitivity to DA and their selectivity for DA against AA. Only electrodes with DA/AA selectivity ratios >1000:1 and a linear response (r ≥ 0.997) to increasing concentrations of DA were used. The mean (±SEM) DA/AA selectivity ratio for the electrodes used in the present study was 3780:1 (±234:1).
Electrochemical measurements. Electrochemical recordings were performed using a computer-controlled, chronoamperometric system (FAST; Quanteon, Lexington, KY). An oxidative potential of +0.55 V, with respect to the reference electrode, was applied to the electrode for 100 ms at a rate of 5 Hz. The amplitude of the resulting oxidation current was digitized and integrated over the last 80 ms of each pulse. Every 10 digitized current measures were summed, displayed on a video monitor at 2 s intervals, and subsequently converted to molar equivalent values of DA concentration using the in vitro calibration factor for each respective electrode. The reduction current generated when the potential was stepped down to 0.0 V for 100 ms was digitized and summed in the same manner. With the Nafion-coated carbon fiber electrodes used here and a sampling rate of 5 Hz, the magnitude of the reduction current flow produced by an increase in DA concentration is typically 60-80% of the corresponding increase in oxidation current [reduction/oxidation (red/ox) = 0.6-0.8]. Whereas the oxidation of AA is virtually irreversible (red/ox = 0), that of DOPAC is almost entirely reversible (red/ox = 0.9-1.0); the red/ox ratios for norepinephrine (NE) and serotonin (5-HT) are 0.4-0.5 and 0.1-0.3, respectively (Doherty and Gratton, 1992, 1996, 1997).
Electrochemical recordings began 4 d after surgery. The animals were placed in a recording chamber and connected to the chronoamperometric system via a shielded cable and a low impedance multichannel commutator (Airflyte, Bayonne, NJ). A preamplifier configured as a current-voltage converter (gain = 1 × 108) was connected directly into the animal's head assembly to minimize electrical interference. On each of three consecutive once daily sessions, stable baseline recordings were obtained for 40 min, after which animals were stressed by placing a wooden clothespin 2 cm from the base of the tail for 15 min. Recordings were performed until the electrochemical signal returned to prestress levels.
Histology. At the conclusion of the experiment, animals were deeply anesthetized with sodium pentobarbital (75 mg/kg, i.p.) and perfused transcardially with 0.9% saline, followed by a 10% formalin solution. The brains were stored in 10% formalin and subsequently cryoprotected in a 30% sucrose-formalin solution before being sliced. Electrode tip placements were confirmed from 40 μm thionin-stained coronal sections.
Electrochemical data format and analysis. Because of the inherent differences in sensitivity between Nafion-coated electrodes, in vivo changes in oxidation current recorded with different electrodes (in different animals) cannot be assumed to be equivalent. Thus, valid comparisons are possible only if the sensitivity of each electrode is calibrated against a standard and the electrochemical data are expressed as standard equivalent values. In the present study, DA was used as the standard to calibrate electrode sensitivity. Accordingly, in vivo changes in oxidation current are expressed as micromolar equivalent values of DA concentration. Averaged data are presented as micromolar DA equivalent changes in electrochemical signal relative to the signal level at the onset of the stress period (time 0). Because the record at time 0 was the reference point for changes in electrochemical signal that followed, it was given a value of 0. A value of 0 μm, therefore, is not meant to correspond to the absolute concentration of extracellular DA. Rather, the data reflect relative changes in the DA signal elicited by stress. Statistical comparisons of group differences were based on the amplitude of electrochemical signal increases taken at 5 min intervals from onset of tail-pinch stress. The electrochemical data were analyzed using a four-way mixed factorial ANOVA with hemisphere (left or right mPFC) and maternal care (high-vs low-LG) as between-subject factors and test day (days 1-3) and time from stress onset as within-subject factors. When indicated, post hoc analyses were conducted using Tukey's honestly significant difference (HSD) test.
Acoustic startle and PPI
PPI of the ASR was studied in naive 3- to 4-month-old high- and low-LG animals using a commercially available system (SR-LAB; San Diego Instruments, San Diego, CA). The system comprised two sound-attenuating chambers, each equipped with a cylindrical Plexiglas animal enclosure (length, 16 cm; inner diameter, 8.2 cm). Ventilation was provided by a small electric fan, which also generated a 70 dB background noise. Broadband tone pulses were presented by a speaker positioned 24 cm directly above the animal enclosure. A piezoelectric accelerometer affixed to the animal enclosure frame was used to detect and transduce motion resulting from the animals' response. Tone pulse parameters were controlled by a microcomputer using a commercial software package (SR-LAB) and interface assembly that also digitized (0-4095), rectified, and recorded stabilimeter readings.
Animals were placed in the Plexiglas enclosure and allowed to acclimatize to the environment for 5 min before being tested during 37 discrete trials. On the first two trials, the magnitude of the startle response to a 50 ms duration, 120 dB tone was measured. These first two startling tones were presented to habituate the animals to the testing procedure and thus were omitted from the data analysis. On the subsequent 35 trials, the startle tone was either presented alone or 100 ms after presentation of a 30 ms duration prepulse. Prepulse intensity ranged from 3 to 15 dB above background noise (73-85 dB) and was varied randomly between trials in 3 dB steps. Measures were taken at each of the five prepulse intensities on five trials; animals were randomly presented with the startle tone alone during the other 10 trials. The same stimulus condition was never presented on more than two consecutive trials. The interval between each trial was programmed to a variable time schedule with an average duration of 15 s (range, 5-30 s). A measure of startle response amplitude was derived from the mean of 100 digitized data points collected from stimulus onset at a rate of 1 kHz. Prepulse effectiveness in suppressing the startle response was expressed as a percentage based on the mean amplitude of responses to the startle tone alone (n = 10) relative to those recorded under the five prepulse conditions (n = 5/condition) where %PPI = 100 - (startle preceded by prepulse/startle alone) × 100%.
Quantitative autoradiography
Tissue used for DAT autoradiography was taken from separate groups of experimentally naive animals (n = 7-8/group). At 3-4 months of age, animals were decapitated, and the brains were rapidly removed, frozen in isopentane, and stored at -80°C. Brains were sliced at the level of the mPFC in serial 16 m coronal sections, which were mounted on glass slides (two sections per slide), dessiccated under vacuum at 4°C overnight, and then stored at -80°C. Sections were preincubated for 20 min in ice-cold 50 mm Tris HCl buffer, pH 7.0, containing 120 mm NaCl. Total binding was measured from four sections incubated for 60 min in the same ice-cold buffer with 10 nm [3H]-N-[1-(-2-benzo(b)thiophenyl)cyclohexyl]piperidine (DuPont NEN, Boston MA), a specific DAT ligand (Kd of 0.9 nm for the high-affinity DA uptake site) (Vignon et al., 1988; Katz et al., 2000). Nonspecific binding was determined from two adjacent sections by adding 1 μm GBR12935 to the binding buffer. Sections were subsequently washed in ice-cold buffer (4 × 5 min each), rinsed in ice-cold distilled water, and left to dry overnight. Brain sections were apposed to tritium-sensitive Hyperfilm (Amersham Pharmacia Biotech, Toronto, Ontario, Canada) alongside microscale calibrated tritium standards (Amersham Pharmacia Biotech) for 7 d. Autoradiograms were analyzed with a computerized image-analysis system (MCID-M4; Imaging Research, St. Catherine's, Ontario, Canada), and binding densities were converted to femtomoles per milligram of protein based on the tritium standard calibration and the specific activity of the ligand. For each animal, specific DAT binding was calculated by substracting the average nonspecific binding (n = 2 sections/animal) from the average total binding (n = 4 sections/animal). Group differences in mPFC DAT binding were analyzed for statistical significance using Student's t test.
COMT Western blotting
Separate groups of experimentally naive animals (n = 6/group) were decapitated at 3-4 months of age. The brains were removed rapidly, and the mPFC was dissected, frozen in isopentane, and stored at -80°C. Protein concentration of homogenized tissue was measured by the BCA method (Pierce, Rockford, IL). Equal amounts of protein were separated by NuPAGE Novex Bis-Tris gels (Invitrogen, Burlington, Ontario, Canada) electrophoresis and transferred to nitrocellulose membranes (Amersham, Arlington Heights, IL). The membranes were blotted using mouse anti-COMT monoclonal antibody (1:1000; catalog #611970; BD Biosciences PharMingen, San Diego, CA). COMT immunoreactivity was revealed by incubation with a goat/anti-mouse horseradish peroxidase-linked IgG (1:4000) and the ECL immunoblotting detection system (Amersham Biosciences, Arlington Heights, IL). A monoclonal antibody generated against tubulin (1:8000 dilution; Sigma, St. Louis, MO) was used for reblotting the membrane to control for loading errors. The COMT and tubulin bands were quantified by computerized densitometry (MCID-M4; Imaging Research). The results showed that the mean mPFC levels of the 24 kDa COMT isoform were significantly higher in the offspring of high-LG mothers (mean relative optical density ± SEM = 1.2 ± 0.1) than in the offspring of low-LG mothers (0.85 ± 0.1; p < 0.01). Thus, additional animals (n = 3/group) were used to determine whether these group differences might be lateralized to one hemisphere. The brains of these animals were removed rapidly, and the right and left mPFC were dissected and protein and COMT levels were measured as described above.
The data were analyzed for statistical significance using a three-way ANOVA with maternal care (high-vs low-LG) as between-subject factors and COMT isoform (24 kDa vs 28 kDa) and hemisphere (left vs right mPFC) as within-subject factors.
Results
Maternal behavior
As reported previously by Champagne et al. (2003), we observed a stable normal distribution in the frequency of LG/ABN in the cohort of animals used in the present study. There was a significant difference in the LG/ABN frequency scores of high-LG (mean ± SEM, 13.7 ± 0.1%) and low-LG (7.8 ± 0.2%) dams (Student's t test = 22.7; p < 0.0001). Such frequency scores are comparable with those observed among the cohorts of animals tested in our group since 1998 (high-LG, 14.7 ± 1.3%; low-LG, 7 ± 1.1%).
mPFC DA response to stress
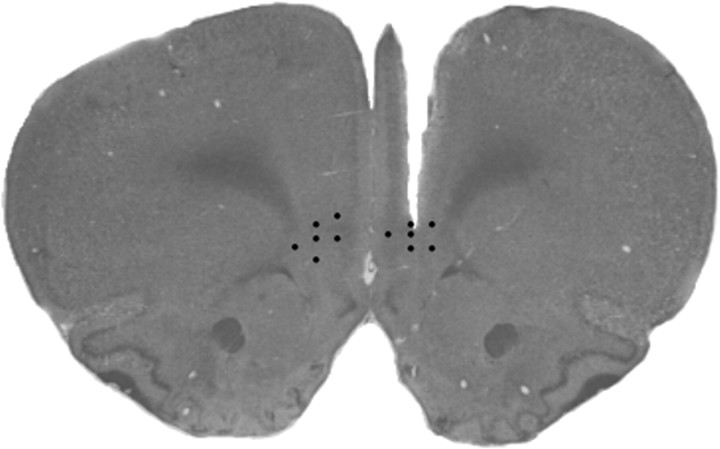
Only data from animals with histologically confirmed electrode placements within the left or right mPFC (ventral prelimbic/dorsal infralimbic) were included in the statistical analysis (Fig. 1). Animals were also excluded from the analysis when tail-pinch stress failed to elicit a short-latency increase in electrochemical signal or when the red/ox ratio of a stress-induced increase in electrochemical signal was <0.5; in the present study, the mean (±SEM) red/ox ratio at the peak of the stress response was 0.68 (±0.03). A total of 26 animals met the above criteria (high-LG: left mPFC, n = 7; right mPFC, n = 6; low-LG: left mPFC, n = 8; right mPFC, n = 5).
Figure 1.
Representative tissue damage produced by electrochemical probe implantation in the right mPFC. The filled circles represent the distribution of electrode placements in the left and right mPFC of high- and low-LG animals included in the analysis of DA stress responses.
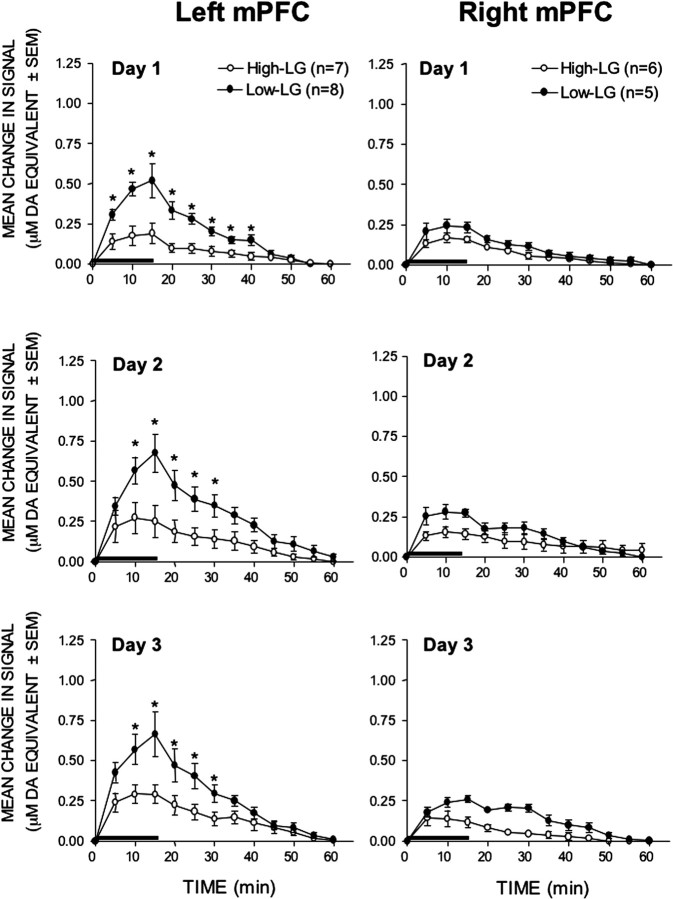
As can be seen in Figure 2, stress elicited significantly greater mPFC DA signal increases in the offspring of low-LG mothers than in their high-LG counterparts (time × maternal care × hemisphere interaction: F(1,12) = 1.969; p = 0.027). The most pronounced group differences in the mPFC stress response were observed in the left hemisphere, where stress-induced increases in DA signals were significantly greater than in the right mPFC (time × hemisphere interaction: F(1,12) = 6.729; p < 0.001). Subsequent analysis indicated, however, that this was the case only in low-LG animals; in high-LG animals, stress responses in the left and right mPFC did not differ. Post hoc analyses confirmed that, in the left hemisphere, the magnitude of the mPFC DA stress responses was greater in low-LG animals than in high-LG animals on all three test days (Tukey's HSD; p < 0.05). Although the difference was not as great as in the left mPFC, analysis of simple effects did reveal that the right mPFC DA stress responses were significantly greater in low-LG animals than in high-LG animals (F(1,9) = 8.235; p = 0.018).
Figure 2.
Mean ± SEM increases in DA signals recorded in the left and right mPFC of high- and low-LG animals in response to each of three consecutive once daily exposures to tail-pinch stress. The length of the horizontal bar corresponds to the duration of the stress period. *p < 0.05.
The main drawback of voltammetry is that it does not provide sufficient information to unequivocally identify the electroactive species responsible for the recorded changes in oxidation current. The most problematic species is AA because its basal extracellular concentration is higher than that of DA and because stress has been reported to elevate (striatal) levels of AA (Miele et al., 1994). Repeatedly coating the electrode surface with Nafion is one of several procedures that has been shown to substantially reduce contributions by AA and other anionic species, including the DA metabolite DOPAC (Gerhardt et al., 1984; Nagy et al., 1985; Brazell et al., 1987; Crespi and Mobius, 1992). The electrodes used in the present study had a mean (±SEM) DA/AA selectivity ratio of 3780:1 (±234:1); thus, the Nafion treatment would significantly reduce a potential contribution of AA to the increases in signal reported here.
Information on the identity of the predominant electroactive species can be derived by comparing the magnitude of the increase in reduction current to that of the increase in oxidation current. In the present study, stress elicited increases in reduction current, the average (±SEM) peak amplitude of which was 68 ± 3% (red/ox = 0.68 ± 0.03) of the corresponding increase in oxidation current. At the sampling rate used (5 Hz), such high levels of reduction current rule out increases in levels of AA (red/ox = 0-0.1) as the cause for the stress-induced signal increases reported here. However, the oxidation of DOPAC, like that of DA, is a highly reversible reaction; the current generated during the reduction of DOPAC is close to 100% of the current generated during its oxidation. Thus, the red/ox ratios of DA and DOPAC are sufficiently similar as to preclude a clear dissociation on the basis of this criterion alone. If there was a significant contribution of DOPAC though, it is unlikely to have occurred at any time soon after onset of the stress period; increases in extracellular DOPAC (and other metabolites) levels occur more gradually and are delayed relative to increases in DA levels. In the present study, electrochemical signals typically increased within 1-2 min after onset of stress, and these continued to rise steadily to peak ∼10 min later; signals would start to decline gradually at or close to the end of the 15 min stress period. Had DOPAC, rather than DA, been the predominant species detected by the electrode, increases in electrochemical signal would have been expected to peak later and would have remained elevated considerably longer after stress (for example, see Venator et al., 1999). Thus, it seems unlikely that the relatively rapid increases in signal seen in response to the stressor can be ascribed to the slow extracellular accumulation of DOPAC that follows from increased DA efflux.
The relatively high red/ox ratios observed in the present study would rule out, however, an increase in 5-HT or of its metabolite 5-hydroxyindoleacetic acid as the cause for the stressor-induced increases in signal, because the red/ox ratios of both compounds (0.1-0.2) are considerably lower than that of DA. Ruling out a contribution of NE on the basis of red/ox ratios is more difficult, because its reduced form (red/ox = 0.4-0.5) is almost as readily detected as that of DA. This and the fact that stress will stimulate NE release in the mPFC (Rossetti et al., 1990; Nakane et al., 1994) raises the possibility that NE might have contributed to the stress-induced increases in electrochemical signal reported here.
PPI of the ASR
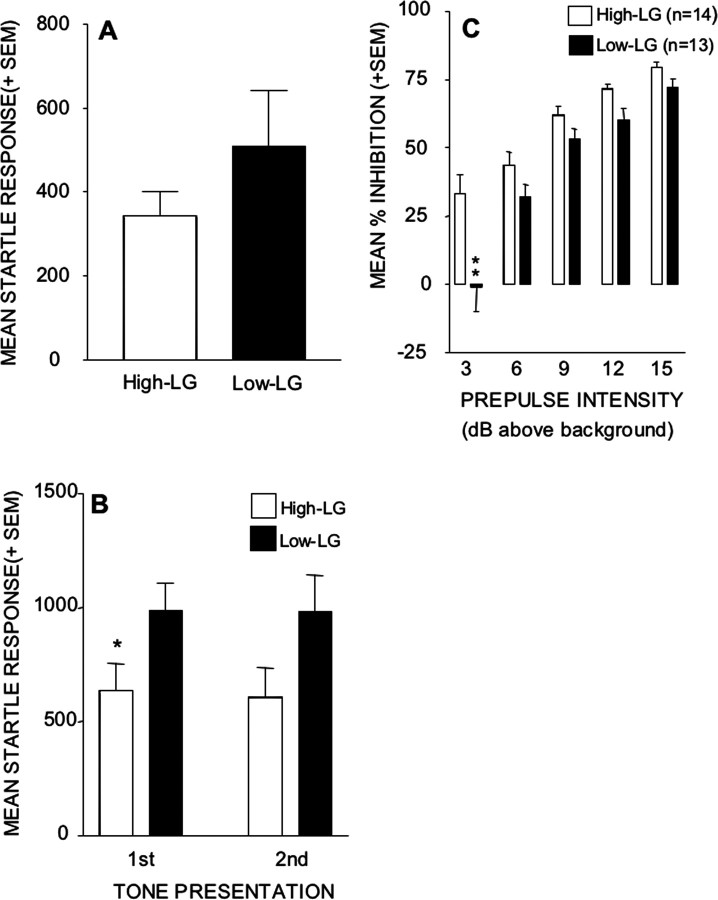
The amplitude of the startle response to the 120 dB tone presented alone was measured on 12 occasions. The startling tone was presented twice at the beginning of the test session to habituate the animals to the testing conditions. The subsequent 10 startling tones were presented randomly during the session and were used to calculate prepulse effectiveness in inhibiting the startle response. Figure 3A shows that the high- and low-LG groups of animals did not differ significantly in their response to these 10 startling tone presentations (p > 0.05; Student's t test); this rules out the possibility that group differences on PPI reflect differing responsivities to the startling tone. However, as can be seen in Figure 3B, the startle response to the first tone presentation of the session was significantly greater in the low-LG offspring than in the high-LG animals (Student's t test; p = 0.048). However, by the second tone presentation, this group difference had dissipated below statistical significance levels (p = 0.075; Student's t test).
Figure 3.
A, Mean (+SEM) amplitude of the 10 ASRs of high- and low-LG animals during no prepulse trials of PPI tests. B, Mean (+SEM) amplitude of the two ASRs of high- and low-LG animals during the habituation phase of PPI tests. *p < 0.05. C, Mean (+SEM) percentage of PPI of the ASRs of high- and low-LG animals as a function of increasing prepulse intensity (decibels above background). **p < 0.01.
The PPI data are presented in Figure 3C. These show that, overall, the prepulse was less effective in attenuating the startle response of low-LG animals than that of their high-LG counterparts. This was confirmed by the statistical analysis that revealed significant main effects of maternal care (F(1,25) = 3.94; p < 0.01) and of prepulse intensity (F(1,4) = 71.05; p < 0.0001) as well as a significant interaction of maternal care and prepulse intensity (F(4,25) = 3.94; p < 0.01). Post hoc analyses revealed that the difference between the high- and low-LG groups of animals was significant at the lowest prepulse intensity (Tukey's HSD; p < 0.01); whereas the 3 dB prepulse attenuated the startle response of high-LG animals by ∼30%, it had no effect on the startle response of low-LG animals.
mPFC DAT and COMT levels
There were no differences in mPFC DAT binding levels between high-LG (mean ± SEM = 42.5 ± 6.2 fmol/mg) and low-LG (47.5 ± 3.5 fmol/mg) animals (Student's t test = -0.72; p = 0.48). Analysis of the Western blot data, however, revealed a significant two-way interaction between maternal care and the COMT isoform (Fig. 4) (F(1,16) = 11.83; p < 0.01); post hoc analysis revealed that levels of the 24 kDa COMT isoform were significantly higher in high-LG animals than in low-LG animals (Tukey's HSD; p < 0.01). There was no effect of hemisphere nor of a maternal care by hemisphere interaction on mPFC levels of either COMT isoform.
Figure 4.
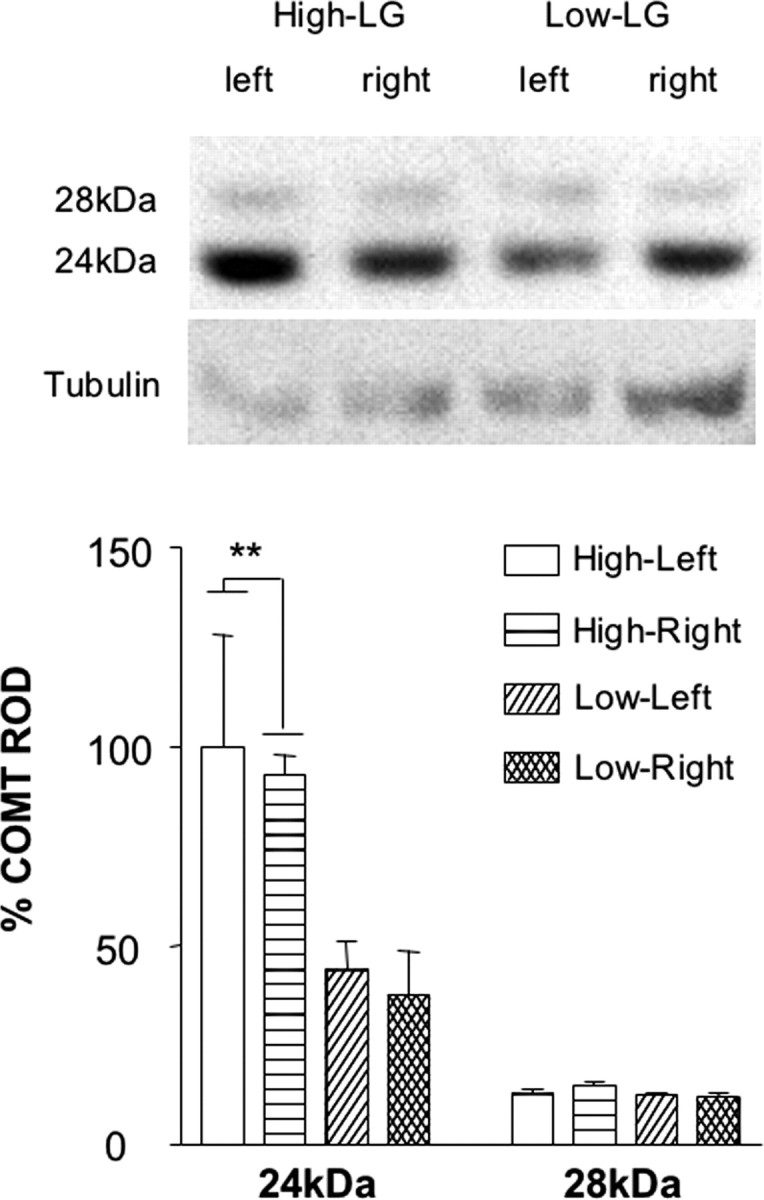
Mean (+SEM) levels of the 24 and 28 kDa isoforms of COMT in the left and right mPFC of high- and low-LG animals. **p < 0.01.
Discussion
mPFC DA response to stress
Overall, mPFC DA stress responses were stronger and longer-lasting in low-LG animals than in high-LG animals. This group difference, however, was more pronounced in the left hemisphere, where the peak DA stress responses of low-LG animals were two to three times greater than those of high-LG animals. There are two additional features of the present data that need to be considered here. The first concerns the group differences in hemispheric biases of the mPFC DA stress responses. Although stress-induced activation of mPFC DA was heavily biased toward the left hemisphere in low-LG animals, no evidence of any such bias was observed in high-LG animals. Thus, whereas stress activated DA transmission in low-LG animals more strongly in the left mPFC than in the right mPFC, in high-LG animals it did so equally in both hemispheres. This is a potentially important observation, given that such symmetric left-right mPFC DA stress responses are also seen in “normal” control animals (i.e., animals that were born and reared under standard animal facility conditions) [for examples, see Brake et al. (2000), their Fig. 2, and Stevenson et al. (2003), their Fig. 3A]. The second point concerns group differences in the relative magnitude of the mPFC DA stress responses. When compared with the normal control animals of our previous studies, both the left and right mPFC DA stress responses of high-LG animals are noticeably smaller and shorter-lasting; whereas peak mPFC DA stress responses ranging from 0.16 to 0.22 μm were recorded in high-LG animals, they typically ranged from 0.42 to 0.75 μm in the control animals of our previous studies. Thus, when compared with normal control animals, the left and right mPFC DA stress responses of high-LG animals appear to be equally attenuated. This is not the case of low-LG animals. With peak increases ranging from 0.52 and 0.72 μm, the left mPFC DA stress responses of low-LG animals are remarkably similar in magnitude to those typically seen in the left mPFC of normal control animals. Where low-LG animals differ markedly from control animals is in the right mPFC. Here, the DA stress responses of low-LG animals (range, 0.22-0.26 μm) are only 30-40% of those observed in normal controls. It would appear then that low-LG animals deviate from the “norm” in that their right mPFC DA stress response is greatly attenuated. Thus, taken together with our previous findings, the present data indicate that exposure to above average levels of maternal care leads to a symmetric (bilateral) dampening of the mPFC DA stress responses, whereas an asymmetric (right-sided) dampening of mPFC DA stress responsivity results from below average levels of maternal care.
Impaired mPFC function, particularly when it is lateralized to the right hemisphere, has been linked to a number of stress-related pathologies (for review, see Sullivan and Gratton, 2002). In animals, right-sided mPFC DA activity is associated with responding to novel stressful environments (Berridge et al., 1999), anxiolytic behavior in the plus maze (Andersen and Teicher, 1999), protection from stress ulcer pathology (Sullivan and Szechtman, 1995), and escape performance after exposure to uncontrollable shock (Carlson et al., 1993). In humans, electroencephalographic (EEG) studies reveal that right frontal biases are associated with negative emotional states and affective styles (Davidson, 1998). Left-biased frontal EEG asymmetry is associated with approach behaviors and positive affect, whereas right-sided biases are linked to withdrawal and defensive behavior. Neuro-imaging studies show that the right ventromedial mPFC is closely correlated with negative affect (Zald et al., 2002). Moreover, alterations in autonomic function and cognitive-emotional processing resulting from insult to the ventromedial mPFC appear to be accounted for almost exclusively by right hemisphere damage (Tranel et al., 2002). Taken together, not only is the mPFC intrinsically lateralized with respect to stress and emotion regulation, but at least one of its major afferent systems is similarly lateralized to optimally modulate this cortical regulation.
It is unlikely that altered DA uptake contributed to the group differences in mPFC DA stress responsivity, because mPFC DAT binding levels in high- and low-LG animals were comparable. Likewise, we found no differences in D1- or D2-like receptor binding in the mPFC as a function of maternal care (P. Chrétien and A. Gratton, unpublished data). Note, however, that such findings do not necessarily preclude the possibility of alterations in DA receptor function. Although the DAT does not appear to be involved, differing mPFC levels of COMT might explain at least some of the group differences in the mPFC DA stress response. COMT is a postsynaptic enzyme that methylates DA and, in the mPFC, appears to be the primary mechanism by which extracellular DA is inactivated (Elsworth et al., 1987; Matsumoto et al., 2003). Studies in humans suggest that a functional polymorphism (Val158Met) in the COMT gene is associated with altered performance on mPFC-mediated cognitive tasks (Egan et al., 2001). Variants in the COMT gene may be associated with susceptibility for schizophrenia (Goldberg et al., 2003; Tunbridge et al., 2004), which involves altered DA activity in the mPFC. It is worth noting also that deficits in sensorimotor gating are also seen in schizophrenic patients, in whom impaired PPI correlates with core cognitive symptoms (Light and Braff, 1999).
With higher levels of COMT, extracellular DA would presumably be degraded more rapidly resulting in smaller, shorter-lasting DA stress responses. Thus, the finding that left and right mPFC levels of COMT in high-LG animals were equally elevated (relative to low-LG animals) would be consistent with the symmetric bilateral attenuation of the DA stress responses seen in these animals. This explanation, however, could not account for the data of low-LG animals. Given the strong leftward bias of the mPFC DA stress responses in these animals, one would expect to find hemispheric asymmetries in mPFC COMT levels as well (i.e., relatively higher COMT levels in the right hemisphere than in the left hemisphere). This was not the case. In low-LG animals, left and right mPFC COMT levels were equally reduced relative to those of high-LG animals. Although relatively lower COMT levels might arguably have contributed to enhance the DA stress response in the left mPFC of low-LG animals, it cannot account in any obvious way for the blunted DA response seen in the right mPFC of these animals. Clearly, other mechanisms are involved here.
At present, we can only speculate as to what these might be. One likely candidate would be the NE input to the mPFC, which is known to be activated by stress (Finlay et al., 1995) and appears to regulate DA-mediated function through a variety of mechanisms. In the mPFC, for instance, DA uptake appears to be mediated as much, if not more, by the NE transporter (NET) than by the DAT (Wayment et al., 2001). Thus, there is a distinct possibility that some of the group and hemispheric differences in the mPFC DA stress responses reported here reflect, at least in part, alterations in NET levels. Another potentially important mechanism involves the subpopulation of mPFC DA-sensitive glutamatergic neurons that send, by way of the corpus callosum (and perhaps the anterior commissure), direct, topographically distributed projections to the opposite mPFC where they synapse primarily on GABA neurons (Carr and Sesack, 1998, 2000). Although the circuitry remains to be fully elucidated, these callosal projection neurons are very likely involved in interhemispheric modulation of mPFC DA stress responsivity. Evidence of this has been reported in a study by Carlson et al. (1996), who showed that unilateral 6-OHDA lesions to the right mPFC will not only reduce DA content in the ipsilateral mPFC but will also result in a significant increase of DA levels in the contralateral mPFC. Furthermore, Sullivan and Szechtman (1995) showed that subcortical DA transmission is enhanced in animals with unilateral DA-depleting lesions to the right mPFC but not in animals with either bilateral or unilateral left mPFC lesions. Whether hemispheric asymmetries in mPFC DA function lead to, or emerge as a consequence of, altered development of interhemispheric collosal neurons remains to be explored (for review, see Lent and Schmidt, 1993). The important point here is that excessive hemispheric asymmetry in mPFC DA stress responsivity, such as was seen in low-LG animals, would not only have direct effects on the activity of ipsilateral mPFC neurons but also indirectly affect the activity of contralateral mPFC neurons. The implicit assumption here is that vulnerability to stress-related disorders increases with abnormal or excessive lateralization of mPFC DA-mediated function.
The DA projection to the mPFC has a protracted period of maturation that persists well into the pubertal period (Andersen et al., 2000; Andersen, 2003). Not surprisingly, numerous early developmental perturbations have been shown to have lasting effects on mPFC function. As adults, rats exposed to prenatal stress are more fearful and show lateralized changes in mPFC DA function (Fride and Weinstock, 1988). Maternal separation and social isolation result in abnormally high synaptic density within the infralimbic (IL) cortex (Ovtscharoff and Braun 2001), as well as significantly altered densities of DA and 5-HT fibers throughout the mPFC (Braun et al., 2000). Early social isolation also results in decreases in basal DA turnover, selectively within the IL cortex (Heidbreder et al., 2000). Postnatal handling stimulates the development of cerebral lateralization (Denenberg, 1981; Denenberg et al., 1986). Interestingly, postnatal handling, which increases maternal licking and grooming (Lee and Williams, 1975; Liu et al., 1997), results in significantly reduced synaptic density in the IL cortex compared with nonhandled controls (Ovtscharoff and Braun, 2001), which may in part account for the reduced anxiety and high exploration typically displayed by neonatally handled animals. A recent magnetic resonance imaging study has confirmed in monkey the impact of early rearing conditions on mPFC function, showing that maternal separation results in a significant enlargement of the ventromedial mPFC, which is entirely lateralized to the right hemisphere (Lyons et al., 2002). These findings underscore the potential importance of maternal care and early social influences for the development of the mPFC DA function and the regulation of cognitive-emotional states in response to stress. The results of the current study suggest such systems are responsive not only to abnormal rearing, such as prolonged maternal separation, but also to natural variations in maternal care.
PPI of the ASR
PPI of the ASR was found to be impaired in low-LG animals but not in their high-LG counterparts, suggesting that sensorimotor gating is sensitive to variations in early postnatal maternal care. Similar conclusions have emerged from several other studies involving manipulations of early postnatal rearing conditions (Ellenbroek et al., 1998; Ellenbroek and Cools, 2000, 2002; Cilia et al., 2001). The direct link between maternal care and PPI is reinforced with the findings that among rats reared artificially, with no maternal contact, tactile stimulation applied by stroking pups influences the development of PPI: increased stroking, which is considered as a surrogate form of LG, enhances PPI (Lovic and Fleming, 2004). However, in at least one recent study, PPI was reported to be unaffected by early postnatal handling and isolation (Pryce et al., 2001). The reasons for this discrepency are not immediately apparent, and there are sufficient procedural differences between the two studies to preclude direct comparisons. Although adult, postnatally handled animals can display the core characteristics of high-LG animals, it is not at all clear that the same can be said of postnatally isolated animals and low-LG animals. There are marked strain differences on measures of PPI (Conti et al., 2002), and this too may have contributed to the discordance between the data obtained here using Long-Evans rats and those reported by Pryce et al. (2001) using Wistar rats. Some of the inconsistency may be related to the fact that the effects of maternal care on PPI reported here were subtle. Although others have reported similar findings (Ellenbroek et al., 1995), we can only speculate as to the reason why PPI in low-LG animals was impaired only at the low prepulse intensity. One interpretation might be that different mechanisms mediate the effects of low and high prepulse intensities and that the weak prepulse-sensitive mechanism is specifically impaired in low-LG animals. Alternatively, it may be the case, as suggested by Ellenbroek et al. (1995), that group differences in PPI are gradually masked or “washed out” with increasing prepulse intensities. If nothing else, the present finding underscores the importance of testing prepulse effectiveness over a range of intensities.
Our data also suggest that, intially at least, low-LG animals were hyperresponsive to the startling tone, in that the ASR to the first tone presentation was greater in low-LG animals than in high-LG animals. However, this difference had dissipated by the second tone presentation, although there was an overall trend of a stronger ASR in low-LG animals as reported previously (Caldji et al., 1998). It could be argued that reliable group differences in the ASR might have been uncovered had we tested a range of startling stimulus intensities. Be that as it may, the critical point here is that the ASR of high- and low-LG animals were comparable, thus precluding the possibility that measures of PPI were confounded by group differences in the ASR. In support of this, we found no correlation between prepulse effectiveness and the magnitude of the ASR, confirming the independence of PPI from the ASR (Ellenbroek et al., 1999; Koch, 1999, Swerdlow et al., 2001).
A number of brain regions and pathways have been implicated in the circuitry regulating sensorimotor gating as measured by PPI (for review, see Swerdlow et al., 2001). Of these, the DA projections to the nucleus accumbens and mPFC figure prominently. Local applications of the D1-like receptor antagonist SCH 39166 or of the D2-like receptor antagonist sulpiride into the mPFC produce a dose-dependent impairment of PPI (Ellenbroek et al., 1996). It should be noted, however, that an indirect consequence of impaired mPFC DA transmission would be to enhance subcortical DA transmission (Vezina et al., 1991; Doherty and Gratton, 1996), and this too would be expected to impair PPI (Swerdlow et al., 1992). Equally important is the fact that impaired PPI has also been observed after local mPFC D1 receptor activation (Arnsten, 1997), suggesting that mPFC-mediated regulation of sensorimotor gating requires an optimal level of DA transmission; impaired function can arise from either too much or too little DA activation. Indeed such an inverted U-shaped function for DA on mPFC-mediated working memory performance is found in both monkeys and rats: excessive or deficient D1 receptor activation produces impairments in both species (Sawaguchi and Goldman-Rakic, 1991; Arnsten and Goldman-Rakic 1998; Seamans et al., 1998).
Although it remains to be established empirically, there is reason to suspect that the impaired PPI observed in low-LG animals may be related, at least indirectly, to the lateralized rightsided blunting of the DA stress response seen in these animals. Previous reports that subcortical DA transmission is enhanced after right but not bilateral or left mPFC DA depletion raise the possibility that the impaired PPI seen in low-LG animals is attributable to an enhancement of subcortical DA transmission resulting from diminished DA-mediated function in the right mPFC. Here again, the fact that mPFC DA transmission in high-LG animals was equally attenuated in both hemispheres may explain why PPI was unaffected in these animals.
Conclusion and functional implications
Clearly, the functional implications of the laterializations in DA responses to stress remain to be defined. Moreover, the precise mechanism by which maternal care regulates the development of prefrontal DA systems are yet to be clarified. DA release in the mPFC is regulated, in part, by glutamate and GABA (Takahata and Moghaddam, 1998; Doherty and Gratton, 1999). Variations in maternal licking and grooming over the first week of life alter the expression of glutamic acid decaroxylase in the mPFC (T. Y. Zhang, M. J. Meaney, and A. Gratton, unpublished data), and effects on such regulatory pathways, along with the difference in COMT expression, might contribute to a complex set of developmentally regulated signals that determines individual differences in DA responses to stress in adulthood. In humans, we (Pruessner et al., 2004) reported that scores on a measure of parent-offspring relationships (the Parental Bonding Index) were highly correlated with the magnitude of the DA response to stress in the nucleus accumbens. In epidemiological studies, parental care predicts vulnerability for multiple forms of psychopathology, including those directly linked to DA function in the mPFC, such as attention deficit hyperactivity disorder (Carlson et al., 1995; Tully et al., 2004). Thus, the well established relationship between the quality of early family life and vulnerability to multiple forms of psychopathology might, in part, be mediated by parental influences on the development of individual differences in the function of selective neurotransmitter systems under conditions of stress. The results of the current studies suggest that in rodents there are direct effects of maternal care on the mesocortical DA- and mPFC-mediated function.
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research and the National Institutes for Drug Addiction. We gratefully acknowledge the contribution of Drs. Frances Champagne and Timothy Bredy.
Correspondence should be addressed to Dr. Alain Gratton, Douglas Hospital Research Centre, 6875 LaSalle Boulevard, Montréal (Verdun), Québec, H4H 1R3 Canada. E-mail:alain.gratton@mcgill.ca.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251493-10$15.00/0
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ (1989) Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem 52: 1655-1658. [DOI] [PubMed] [Google Scholar]
- Andersen SL (2003) Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 27: 3-18. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH (1999) Serotonin laterality in amygdala predicts performance in the elevated plus maze. NeuroReport 10: 3497-3500. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH (2000) Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse 37: 167-169. [DOI] [PubMed] [Google Scholar]
- Arnsten AF (1997) Catecholamine regulation of the prefrontal cortex. J Psychopharmacol 11: 151-162. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS (1998) Noise stress impairs prefrontal cortical cognitive function in monkeys: evidence for a hyperdopaminergic mechanism. Arch Gen Psychiatry 55: 362-368. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Mitton E, Clark W, Roth RH (1999) Engagement in a nonescape (displacement) behavior elicits a selective and lateralized suppression of frontal cortical dopaminergic utilization in stress. Synapse 32: 187-197. [DOI] [PubMed] [Google Scholar]
- Brake WG, Sullivan RM, Gratton A (2000) Perinatal distress leads to lateralized medial prefrontal cortical dopamine hypofunction in adult rats. J Neurosci 20: 5538-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Lange E, Metzger M, Poeggel G (2000) Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience 95: 309-318. [DOI] [PubMed] [Google Scholar]
- Brazell MP, Kasser RJ, Renner KJ, Feng J, Moghaddam B, Adams RN (1987) Electrocoating carbon fiber microelectrodes with Nafion improves selectivity for electroactive neurotransmitters. J Neurosci Methods 22: 167-172. [DOI] [PubMed] [Google Scholar]
- Bubser M, Koch M (1994) Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology 113: 487-492. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ (1998) Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci USA 95: 5335-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EA, Jacobvitz D, Sroufe LA (1995) A developmental investigation of inattentiveness and hyperactivity. Child Dev 66: 37-54. [DOI] [PubMed] [Google Scholar]
- Carlson JN, Fitzgerald LW, Keller RW, Glick SD (1991) Side and region dependent changes in dopamine activation with various durations of restraint stress. Brain Res 550: 313-318. [DOI] [PubMed] [Google Scholar]
- Carlson JN, Fitzgerald LW, Keller Jr RW, Glick SD (1993) Lateralized changes in prefrontal cortical dopamine activity induced by controllable and uncontrollable stress in the rat. Brain Res 630: 178-187. [DOI] [PubMed] [Google Scholar]
- Carlson JN, Visker KE, Keller Jr RW, Glick SD (1996) Left and right 6-hydroxydopamine lesions of the medial prefrontal cortex differentially alter subcortical dopamine utilization and the behavioral response to stress. Brain Res 711: 1-9. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR (1998) Callosal terminals in the rat prefrontal cortex: synaptic targets and association with GABA-immunoreactive structures. Synapse 29: 193-205. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR (2000) Dopamine terminals synapse on callosal projection neurons in the rat prefrontal cortex. J Comp Neurol 425: 275-283. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ (2003) Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav 79: 359-371. [DOI] [PubMed] [Google Scholar]
- Cilia J, Reavill C, Hagan JJ, Jones DN (2001) Long-term evaluation of isolation-rearing induced prepulse inhibition deficits in rats. Psychopharmacology 156: 327-337. [DOI] [PubMed] [Google Scholar]
- Conti LH, Murray JD, Ruiz MA, Printz MD (2002) Effects of corticotropin-releasing factor on prepulse inhibition of the acoustic startle response in two rat strains. Psychopharmacology 161: 296-303. [DOI] [PubMed] [Google Scholar]
- Crespi F, Mobius C (1992) In vivo selective monitoring of basal levels of cerebral dopamine using voltammetry with Nafion modified (NA-CRO) carbon fibre micro-electrodes. J Neurosci Methods 42: 149-161. [DOI] [PubMed] [Google Scholar]
- Davidson RJ (1998) Cerebral asymmetry, emotion and affective style. In: Brain asymmetry (Davidson RJ, Hughdahl K, eds), pp 361-387. Cambridge, MA: MIT.
- Denenberg VH (1981) Hemispheric laterality in animals and the effects of early experience. Behav Brain Sci 4: 1-49. [Google Scholar]
- Denenberg VH, Gall JS, Berrebi A, Yutzey DA (1986) Callosal mediation of cortical inhibition in the lateralized rat brain. Brain Res 397: 327-332. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ (1993) The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13: 3839-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1992) High-speed chronoamperometric measurements of mesolimbic and nigrostriatal dopamine release associated with repeated daily stress. Brain Res 586: 295-302. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1996) Medial prefrontal cortical D1 receptor modulation of the meso-accumbens dopamine response to stress: an electrochemical study in freely-behaving rats. Brain Res 715: 86-97. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1997) NMDA receptors in nucleus accumbens modulate stress-induced dopamine release in nucleus accumbens and ventral tegmental area. Synapse 26: 225-234. [DOI] [PubMed] [Google Scholar]
- Doherty MD, Gratton A (1999) Effects of medial prefrontal cortical injections of GABA receptor agonists and antagonists on the local and nucleus accumbens dopamine responses to stress. Synapse 32: 288-300. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98: 6917-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR (2000) The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology 23: 99-106. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Cools AR (2002) Early maternal deprivation and prepulse inhibition: the role of the postdeprivation environment. Pharmacol Biochem Behav 73: 177-184. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Geyer MA, Cools AR (1995) The behavior of Apo-Sus rats in animal models with construct validity for schizophrenia. J Neurosci 15: 7604-7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Budde S, Cools AR (1996) Prepulse inhibition and latent inhibition: the role of dopamine in the medial prefrontal cortex. Neuroscience 75: 535-542. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, van den Kroonenberg PT, Cools AR (1998) The effects of an early stressful life event on sensorimotor gating in adult rats. Schizophr Res 30: 251-260. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, van Luijtelaar G, Frenken M, Cools AR (1999) Sensory gating in rats: lack of correlation between auditory evoked potential gating and prepulse inhibition. Schizophr Bull 25: 777-788. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Leahy DJ, Roth RH, Redmond Jr DE (1987) Homovanillic acid concentrations in brain, CSF and plasma as indicators of central dopamine function in primates. J Neural Transm 68: 51-62. [DOI] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, Abercrombie ED (1995) Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience 64: 619-628. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ (1999) Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann NY Acad Sci 896: 66-84. [DOI] [PubMed] [Google Scholar]
- Fride E, Weinstock M (1988) Prenatal stress increases anxiety related behavior and alters cerebral lateralization of dopamine activity. Life Sci 42: 1059-1065. [DOI] [PubMed] [Google Scholar]
- Fuster JM (1997) Network memory. Trends Neurosci 20: 451-459. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN (1984) Nafioncoated electrodes with high selectivity for CNS electrochemistry. Brain Res 290: 390-395. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR, Mansbach RS, Braff DL (1990) Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull 25: 485-498. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156: 117-154. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR (2003) Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60: 889-896. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P (2000) Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience 100: 749-768. [DOI] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Terry P (2000) Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology 148: 90-98. [DOI] [PubMed] [Google Scholar]
- Koch M (1999) The neurobiology of startle. Prog Neurobiol 59: 107-128. [DOI] [PubMed] [Google Scholar]
- Lee MH, Williams DI (1975) Long term changes in nest condition and pup grouping following handling of rat litters. Dev Psychobiol 8: 91-95. [DOI] [PubMed] [Google Scholar]
- Lent R, Schmidt SL (1993) The ontogenesis of the forebrain commissures and the determination of brain asymmetries. Prog Neurobiol 40: 249-276. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL (1999) Human and animal studies of schizophreniarelated gating deficits. Curr Psychiatry Rep 1: 31-40. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ (1997) Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659-1662. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS (2004) Artificially reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task—reversal of effects with maternal-like licking stimulation. Behav Brain Res 148: 209-219. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Afarian H, Schatzberg AF, Sawyer-Glover A, Moseley ME (2002) Experience-dependent asymmetric variation in primate prefrontal morphology. Behav Brain Res 136: 51-59. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Akil M, Lipska BK, Hyde TM, Herman MM, Kleinman JE, Weinberger DR (2003) Catechol O-methyltransferase mRNA expression in human and rat brain: evidence for a role in cortical neuronal function. Neuroscience 116: 127-137. [DOI] [PubMed] [Google Scholar]
- Meaney MJ (2001) Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci 24: 1161-1192. [DOI] [PubMed] [Google Scholar]
- Miele M, Boutelle MG, Fillenz M (1994) The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience 62: 87-91. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Arnsten AF, Goldman-Rakic PS, Roth RH (1996) Increased dopamine turnover in the prefrontal cortex impairs spatial working memory performance in rats and monkeys. Proc Natl Acad Sci USA 93: 1325-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy G, Moghaddam B, Oke A, Adams RN (1985) Simultaneous monitoring of voltammetric and ion-selective electrodes in mammalian brain. Neurosci Lett 55: 119-124. [DOI] [PubMed] [Google Scholar]
- Nakane H, Shimizu N, Hori T (1994) Stress-induced norepinephrine release in the rat prefrontal cortex measured by microdialysis. Am J Physiol 267: R1559-R1566. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff Jr W, Braun K (2001) Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus. Neuroscience 104: 33-40. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A (2004) Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci 24: 2825-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Bahr NI, Feldon J (2001) Comparison of the effects of infant handling, isollation, and nonhandling on acoustic startle, prepulse inhibition. Locomotion and HPA activity in the adult rat. Behav Neurosci 115: 71-83. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Portas C, Pani L, Carboni S, Gessa GL (1990) Stress increases noradrenaline release in the rat frontal cortex: prevention by diazepam. Eur J Pharmacol 176: 229-231. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS (1991) D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science 251: 947-950. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG (1998) D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci 18: 1613-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopsema JS, VanDerGugten J, DeBruin JPC (1982) Regional concentrations of noradrenaline and dopamine in the frontal cortex of the rat: dopaminergic innervation of the prefrontal subareas and lateralization of prefrontal dopamine. Brain Res 250: 197-200. [DOI] [PubMed] [Google Scholar]
- Stevenson C, Sullivan RM, Gratton A (2003) Effects of basolateral amygdala dopamine depletion on the nucleus accumbens and medial prefrontal cortical dopamine responses to stress. Neuroscience 116: 285-293. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (1998) Relationships between stress-induced increases in medial prefrontal cortical dopamine and plasma corticosterone levels in rats: role of cerebral laterality. Neuroscience 83: 81-91. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (1999) Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J Neurosci 19: 2834-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (2002) Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology 27: 99-114. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A (2003) Behavioral and neuroendocrine relevance of hemispheric asymmetries in benzodiazepine receptor binding induced by postnatal handling in the rat. Brain Cognit 51: 218-220. [Google Scholar]
- Sullivan RM, Szechtman H (1995) Asymmetrical influence of mesocortical dopamine depletion on stress ulcer development and subcortical dopamine systems in rats: implications for psychopathology. Neuroscience 65: 757-766. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Talangbayan H, Einat H, Szechtman H (1998) Effects of quinpirole on central dopamine systems in sensitized and non-sensitized rats. Neuroscience 83: 781-789. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL (1990a) Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology 100: 413-416. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Masten VL, Geyer MA (1990b) Schizophrenic-like sensorimotor gating abnormalities in rats following dopamine infusion into the nucleus accumbens. Psychopharmacology 101: 414-420. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Geyer MA (1992) Regionally selective effects of intracerebral dopamine infusion on sensorimotor gating of the startle reflex in rats. Psychopharmacology 108: 189-195. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL (2001) Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology 156: 194-215. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B (1998) Glutamatergic regulation of basal and stimulus-activated dopamine release in the prefrontal cortex. J Neurochem 71: 1443-1449. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Schwarting RK (2001) Dopaminergic lateralisation in the forebrain: relations to behavioral asymmetries and anxiety in male Wistar rats. Neuropsychobiology 43: 192-199. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL (2002) Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex 38: 589-612. [DOI] [PubMed] [Google Scholar]
- Tully LA, Arseneault L, Caspi A, Moffitt TE, Morgan J (2004) Does maternal warmth moderate the effects of birth weight on twins' attention-deficit/hyperactivity disorder (ADHD) symptoms and low IQ? J Consult Clin Psychol 72: 218-226. [DOI] [PubMed] [Google Scholar]
- Tunbridge E, Burnet PW, Sodhi MS, Harrison PJ (2004) Catechol-O-methyltransferase (COMT) and proline dehydrogenase (PRODH) mRNAs in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and major depression. Synapse 51: 112-118. [DOI] [PubMed] [Google Scholar]
- Venator DK, Lewis DA, Finlay JM (1999) Effects of partial dopamine loss in the medial prefrontal cortex on local baseline and stress-evoked extracellular dopamine concentrations. Neuroscience 93: 497-505. [DOI] [PubMed] [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin JP (1991) Opposed behavioral outputs of increased dopamine transmission in prefrontocortical and subcortical areas: a role for cortical D1 receptor. Eur J Neurosci 3: 1001-1007. [DOI] [PubMed] [Google Scholar]
- Vignon J, Pinet V, Cerruti C, Kamenka JM, Chicheportiche R (1988) [3H]N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine ([3H]BTCP): a new phencyclidine analog selective for the dopamine uptake complex. Eur J Pharmacol 148: 427-436. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR (1993) Intra-accumbens infusion of quinpirole impairs sensorimotor gating of acoustic startle in rats. Psychopharmacology 113: 103-109. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Geyer MA, Swerdlow NR (1994) Accumbens D2 modulation of sensorimotor gating in rats: assessing anatomical localization. Pharmacol Biochem Behav 49: 155-163. [DOI] [PubMed] [Google Scholar]
- Wayment HK, Schenk JO, Sorg BA (2001) Characterization of extracellular dopamine clearance in the medial prefrontal cortex: role of monoamine uptake and monoamine oxidase inhibition. J Neurosci 21: 35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376: 572-575. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV (2002) Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proc Natl Acad Sci USA 99: 2450-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Forkstam C, Engel JA, Svensson L (2000) Role of dopamine in prepulse inhibition of acoustic startle. Psychopharmacology 149: 181-188. [DOI] [PubMed] [Google Scholar]