Abstract
Endothelial differentiation gene (Edg) proteins are G-protein-coupled receptors activated by lysophospholipid mediators: sphingosine-1-phosphate (S1P) or lysophosphatidic acid. We show that in the CNS, expression of Edg8/S1P5, a high-affinity S1P receptor, is restricted to oligodendrocytes and expressed throughout development from the immature stages to the mature myelin-forming cell. S1P activation of Edg8/S1P5 on O4-positive pre-oligodendrocytes induced process retraction via a Rho kinase/collapsin response-mediated protein signaling pathway, whereas no retraction was elicited by S1P on these cells derived from Edg8/S1P5-deficient mice. Edg8/S1P5-mediated process retraction was restricted to immature cells and was no longer observed at later developmental stages. In contrast, S1P activation promoted the survival of mature oligodendrocytes but not of pre-oligodendrocytes. The S1P-induced survival of mature oligodendrocytes was mediated through a pertussis toxin-sensitive, Akt-dependent pathway. Our data demonstrate that Edg8/S1P5 activation on oligodendroglial cells modulates two distinct functional pathways mediating either process retraction or cell survival and that these effects depend on the developmental stage of the cell.
Keywords: oligodendrocytes, LPA receptors, S1P receptors, retraction, survival, myelin
Introduction
Oligodendrocytes are the myelin-forming cells of the CNS. Myelination of axons allows the establishment of a rapid propagation of action potentials via saltatory conduction. During embryonic development, cells of the oligodendrocyte lineage emerge from the ventricular zone. Successive developmental stages (each being identified by a battery of antibodies) (see supplemental Table 1, available at www.jneurosci.org as supplemental material) then transform the proliferating oligodendrocyte progenitor into a mature postmitotic myelinating oligodendrocyte. These developmental stages, which are associated with different capacities of proliferation, migration, differentiation, survival, maturation, and finally myelin deposition, are cell autonomous but also depend on a variety of extrinsic factors. Growth factors (PDGF-A, FGF-2, IGF-I, CNTF, neuregulins, and neurotrophins) have been shown to affect proliferation, survival, and differentiation of oligodendrocyte precursor cells (OPCs) (Barres et al., 1992; Bansal and Pfeiffer, 1997; Flores et al., 2000; Sim et al., 2002). Guidance molecules, such as semaphorins 3A, 3F, and netrin-1, guide the migration of OPCs during development (Sugimoto et al., 2001; Spassky et al., 2002; Tsai and Miller, 2002; Jarjour et al., 2003). Maturation of oligodendrocytes into myelin-forming cells is dependent on growth factors such as members of the FGF and CNTF family (Stankoff et al., 2002a; Oh et al., 2003).
The endothelial differentiation gene (Edg) family is composed of eight G-protein-coupled receptors. Sphingosine-1-phosphate (S1P) and/or lysophosphatidic acid (LPA), which are structurally related lysophospholipid mediators, have been identified as the endogenous ligands of all known Edg receptors. The Edg receptor family can be divided in two subgroups based on ligand specificity and homology. The first subgroup binds S1P and comprises Edg1/S1P1, Edg3/S1P3, Edg5/S1P2, Edg6/S1P4, and Edg8/S1P5. The second subgroup binds LPA and contains Edg2/LPA1, Edg4/LPA2, and Edg7/LPA3 (Lee et al., 1998; Van Brocklyn et al., 1998; Chun et al., 1999; Im et al., 2001). Apart from Edg6, all other Edg receptors are expressed in the CNS. Edg2/LPA1 and Edg8/S1P5 mRNA are predominantly expressed in the CNS white matter (Allard et al., 1998; Im et al., 2001). At the cellular level, Edg2/LPA1 has been shown to be expressed on mature oligodendrocytes (Allard et al., 1998; Cervera et al., 2002; Stankoff et al., 2002b), whereas its expression on immature oligodendrocytes remains controversial (Moller et al., 1999; Dawson et al., 2003). Immunohistochemical analysis has suggested that Edg8/S1P5 is expressed in NG2-positive OPCs but not in mature oligodendrocytes (Terai et al., 2003), whereas expression of Edg8/S1P5 transcripts has been detected by reverse transcription (RT)-PCR in differentiated rat oligodendrocytes in culture (Yu et al., 2004).
Here, we report that the Edg8/S1P5 receptor is already detectable in OPCs and remains expressed throughout the development of the oligodendrocyte lineage including mature myelinating oligodendrocytes. In addition, by analyzing the effect of S1P on oligodendrocyte cultures, we provide evidence for the bivalent role for Edg8/S1P5 receptor signaling in cells of oligodendroglial lineage that is dependent on their stage of differentiation. S1P-induced activation of Edg8/S1P5 receptors in O4+ pre-oligodendrocytes resulted in retraction of oligodendroglial processes, through a Rho kinase-dependent pathway involving phosphorylation of collapsin response-mediated protein (CRMP2). In contrast, in mature oligodendrocytes, S1P-induced Edg8/S1P5 receptor activation resulted in increased cell survival, mediated through an Akt signaling pathway. These results suggest that Edg8/S1P5 receptor signaling can differentially influence specific stages of oligodendrocyte development.
Materials and Methods
Animals. Outbred OF1 mice or Wistar rats were obtained from Iffacredo (L'Arbresle, France). Transgenic 1900bp-MBP-lacZ mice (Gow et al., 1992) and Edg8/S1P5-/- mice were raised in our animal room. Homozygous Edg8/S1P5-/- mice have no apparent behavioral deficit, and neuropathological examination of Edg8/S1P5-/- brain did not show any evident myelin deficiency (supplemental Fig. S1, available at www.jneurosci.org as supplemental material)
Targeting of the Edg8/S1P5 gene and generation of mutant mice. Gene targeting was performed in embryonic day 14.1 embryonic stem (ES) cells, replacing all of the Edg8/S1P5 open reading frame downstream of the SrfI restriction site (6 bp downstream of the translation initiation codon) with an IRES-lacZ expression cassette and a positive selection cassette containing the neomycin phosphotransferase gene driven by the PGK promoter (Fig. 1 A). The 5′ and 3′ homology arms (∼4.1 kb EcoRV/SrfI and ∼3.4 kb EcoRI/HindIII restriction fragments, respectively) were cloned from a 129SVJ genomic bacterial artificial chromosome library and placed on either side of the IRES-lacZ expression cassette and positive selection cassette to generate the targeting construct. Homologous recombination in neomycin-resistant ES cells at the 5′ end of the target locus was determined by Southern blot of BamHI-digested ES cell genomic DNA, with a 437 bp 5′ external probe generated by PCR (primers: 5′-GCTACATTAGAGAGCTCTCCC-3′ and 5′-CCTCTTGGTTCTGTTTCTCCC-3′), which detects ∼7.6 and ∼4.9 kb at the wild-type and targeted locus, respectively. Approximately one in 30 G418-resistant clones had undergone homologous recombination. Homologous recombination at the 3′ end was confirmed in these ES cell clones by Southern blot of XhoI-digested genomic DNA using a 243 bp PCR fragment as a 3′ external probe (primers: 5′-CCAGAGGTGGAACTTAGGTGG-3′ and 5′-GTGCTCCAGAGACACACTCTC-3′), which detects ∼11.6 and >16 kb bands at the wild-type and targeted locus, respectively. Three targeted clones were injected into C57BL/6J-derived blastocysts. Male chimeras were crossed with C57BL/6J females to produce N1F0 offspring (C57BL/6J × 129Ola/Sv), which were subsequently back-crossed four times onto the C57BL/6J background and then intercrossed to generate an N5F1 generation used in subsequent testing. Genotyping of N1F0 offspring was confirmed by the above Southern blot procedures. Genotype analysis of mice for the generation of the N5F1 study population was performed by PCR of DNA. Primers were designed to generate PCR products specific to the wild-type locus [5′ primer exon specific, 5′-CCAACAGCTTGCAGCGATCCCC-3′; 3′ primer exon specific, 5′-GGTTGCTACTCCAGGACTGCCG-3′; 30 cycles at 94°C (30 s), 60°C (30 s), and 72°C (60 s) were used, giving a product size of 170 bp] or targeted locus [5′, neo gene-specific 5′ primer: 5′-CCGGCCGCTTGGGTGGAGAGG-3′; 3′, neo gene-specific 3′ primer: 5′-TCGGCAGGAGCAAGGTGAGATGACA-3′; 30 cycles at 94°C (30 s), 68°C (30 s), and 72°C (30 s) were used, giving a product of 299 bp]. The successful depletion of the Edg8/S1P5 transcript was confirmed by RT-PCR performed on RNA extracted from wild-type, heterozygous, and homozygous Edg8/S1P5 mutant brains (Fig. 1 B). RNA was prepared using the RNeasy kit (Qiagen, Crawley, UK). RT-PCR was performed using the One-Step RT-PCR kit (Qiagen) using Edg8/S1P5-specific primers (5′CCAACAGCTTGCAGCGATCCCC-3′ and 5′-GGTTGCTACTCCAGGACTGCCG-3′, yielding a 170 bp product) and, as a positive control, hprt-specific primers (5′-GCTGGTGAAAAGGACCTCT-3′ and 5′CACAGGACTAGAACACCTGC-3′, yielding a 250 bp product). PCR conditions were as follows: 30 s at 94°C, 30 s at 60°C, and 60 s at 72°C for 30 cycles. All experiments were conducted according to the requirements of the United Kingdom Animals (Scientific Procedures) Act (1986) and strictly conformed to the ethical standards.
Figure 1.
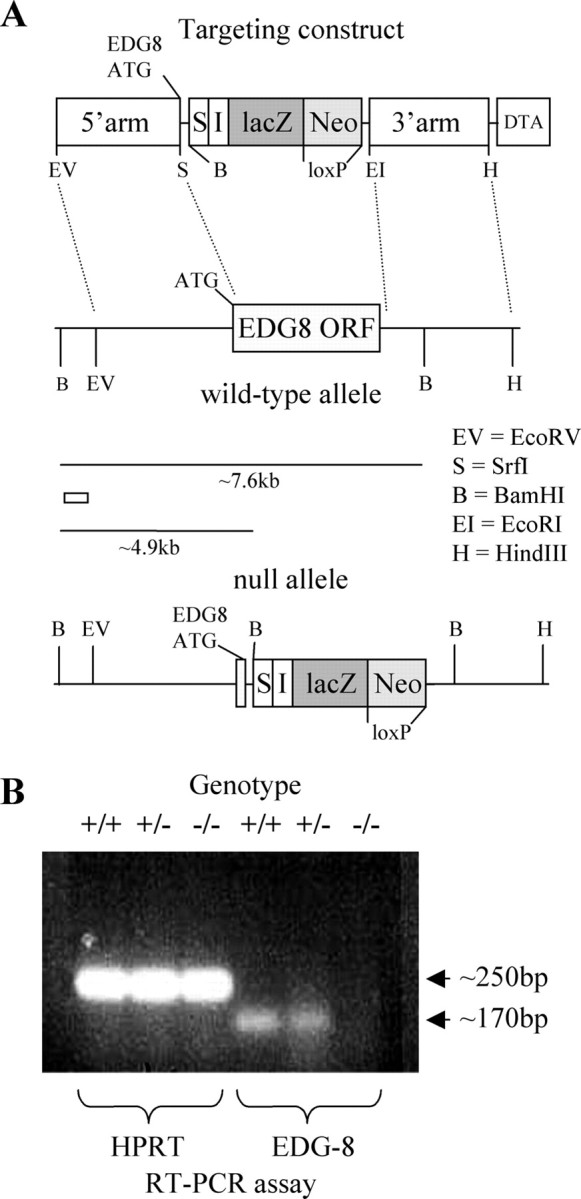
Edg8/S1P5 targeting strategy. A, Schematic representation of the homologous recombination leading the replacement of the Edg8/S1P5 open reading frame (ORF) by IRES-lacZ, as detailed in Materials and Methods. B, RT-PCR analysis was performed on 200 ng of total RNA isolated from the brains of wild-type mice and those heterozygous and homozygous for the Edg8/S1P5 null allele. HPRT primers were used as controls.
Antibodies and reagents. Antibodies were used at the following dilutions: anti-myelin basic protein (MBP) monoclonal antibody (mAb) (mouse IgG1, 1:50; Euromedex, Souffelweyersheim, France), anti-glial fibrillary acidic protein (GFAP) (mouse mAb, 1:200) (Jacque et al., 1986), A2B5 mAb (mouse IgM, 1:5; American Type Culture Collection, Manassas, VA), O4 mAb (mouse IgM, 1:10) (Sommer and Schachner, 1982), anti-proteolipid protein (PLP) (rat mAb clone AA3, 1:10) (Yamamura et al., 1991), anti-β-galactosidase (rabbit polyclonal antibody, 1:1000; Organon Technika, West Chester, PA), antigalactosylceramide (GalC) (mouse mAb IgG3, 1:10) (Ranscht et al., 1982), rat anti-NG2 antibody (1:400) (Diers-Fenger et al., 2001), antiadenomatous polyposis coli (APC) antibody (mouse mAb, 1:50; VWR International, Fontenay Sous Bois, France) (Bhat et al., 1996,), anti-MAC1 antibody (rat mAb IgG2b, 1:100; Ozyme, St. Quentin en Yvelines, France), anti-F4 - 80 antibody (rat mAb IgG2b, 1:200; Serotec, Cergy, France), anti-MAP2 antibody (mouse mAb IgG1, 1:100; Sigma, St. Louis, MO), and SMI31/32 antibodies (mouse mAb IgG1, 1:1000; Sternberger Monoclonals, Lutherville, MD). Rabbit anti-rat Edg8/S1P5 antiserum was generated against the extreme C-terminal 12 amino acids of the rat Edg8/S1P5 receptor sequence (CTANRTLVPDATD; the first cysteine is not in the sequence but was added as a linker to attach the peptide to the KLH carrier for immunization). The peptide antibody was custom-made by Invitrogen (San Diego, CA) according to standard protocols and characterized by Western blotting of protein extracts from normal mouse brain (one band at 37 kDa) and absence of staining on Edg8/S1P5-/- mouse brain protein extract (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). The antiserum was used diluted at 1:200 on cell cultures and 1:1000 on tissue sections. Alexa (488 or 594)-conjugated sheep anti-mouse IgG1 and IgG3 and Alexa-conjugated goat anti-mouse IgM, anti-rabbit IgG, and anti-rat IgG were from Molecular Probes (Eugene, OR) and used at a dilution of 1:1000. For Western blotting, we used the following dilutions: anti-phosphorylated Akt (mouse mAb IgG2b, 1:1000; Ozyme), anti-phosphorylated CRMP2 antibody (rabbit polyclonal, 1:50) (Matsui et al., 1996), rabbit anti-Edg2/LPA1 antiserum (1:1000) (Stankoff et al., 2002b), and peroxidase-conjugated anti-mouse and anti-rabbit Ig (1:5000; Dako, Glostrup, Denmark).
S1P was purchased from Sigma. Where S1P was added to the cultures, a free fatty acid bovine serum albumin (Sigma) was used. Pertussis toxin (PTX) was obtained from List Biological Laboratories (Campbell, CA) and used at a final concentration of 200 ng/ml. The specific inhibitor of Rho kinase, Y27632 (VWR International), was used at a final concentration of 10 μm. Semaphorin 3A conditioned medium was obtained from human embryonic kidney cells (HEK 293) transfected with Sema 3A expression vector, as described previously (Spassky et al., 2002).
In situ hybridization. The expression of Edg8/S1P5 mRNA in the postnatal CNS of OF1 or 1900 MBP-lacZ mice was analyzed on sagittal brain sections by in situ hybridization with a digoxigenin-labeled murine antisense riboprobe. Rat Edg8/S1P5 in pcDNA3.1 V5 His-TOPO was subcloned in pBluescript II KS+ phagemid. Sections were treated with sense and antisense Edg8/S1P5 cRNA probes generated from T3 or T7 promoters and labeled with digoxigenin-UTP (Boehringer, Mannheim, Germany). In situ hybridization and digoxigenin-labeled probe detection were performed as described previously (Spassky et al., 1998). The specificity of the staining was demonstrated by the lack of hybridization signal with the sense probe.
Cell culture. Cultures were performed in 6- or 24-well plates (Costar Corporation, Cambridge, UK) on poly-l-lysine (PLL) (Sigma)-coated glass coverslips (OSI, Maurepas, France) or directly on PLL-coated plastic plates. Oligodendrocyte cultures were maintained in a culture medium containing 0.5% fetal calf serum (FCS), 10 μm insulin, 100 μg/ml transferrin, 0.28 μg/ml albumin, 60 ng/ml progesterone, 16 μg/ml putrescine, 40 ng/ml triiodothyronine, and 30 ng/ml l-thyroxine. Different types of oligodendroglial cultures were performed:
Primary mature rat oligodendrocytes (for immunolocalization of Edg8/S1P5, analysis of the effect of S1P on cell survival and retraction of processes, and biochemical assays) were purified from 4-week-old Wistar rat brains following the procedure described by Lubetzki et al. (1986). Cytosine arabinoside (10-5μm) was added to the culture medium, to avoid proliferation of contaminating astrocytes. Three days after plating, the mature oligodendroglial population, assessed by PLP immunoreactivity, represented at least 90% of total cells.
Enriched primary cultures of O4+/GalC- pre-oligodendrocytes (for immunolocalization of Edg8/S1P5 at different stages of oligodendroglial development, and analysis of the effect of S1P on retraction process) were obtained from newborn OF1 mouse forebrains using a Percoll density gradient as described previously (Lubetzki et al., 1991). Three days after plating (i.e., at the time when S1P was added to the culture medium), O4+/GalC- pre-oligodendrocytes represented 65% of the total cell population, whereas 6% of the cells were O4+/GalC+ immature oligodendrocytes. Edg8/S1P5-deficient cultures were derived from the brains of Edg8/S1P5-/- animals, according to the same technique.
To obtained highly purified cultures of pre-oligodendrocytes (for survival assays and biochemical experiments), oligodendroglial cells were isolated following the “shaking” procedure modified from McCarthy and De Vellis (1980) using papain (30 U/ml) for the dissociation step. Two days after replating, 98% of the cells were O4+/GalC- pre-oligodendrocytes.
Immunohistochemistry. Double immunolabeling on cell cultures was performed as described previously (Charles et al., 2000). For double immunolabeling on tissue sections, after saturation in DMEM containing 10% FCS and 50% sheep serum for 1 h, primary antibodies were incubated overnight in PBS containing 0.1% Triton X-100. After washing in PBS, Alexa-conjugated secondary antibodies were incubated in the same medium for 1 h. For Edg8/S1P5 detection, an additional step of microwave treatment of sections (750 W for 2 min in 0.01 m citrate buffer) was performed before incubation with the first antibody. For immunocytochemistry combined with in situ hybridization on tissue sections, the immunolabeling step followed the in situ hybridization procedure.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide survival assay. After 3 d in the culture medium, either purified preoligodendrocytes (O4+/GalC-) or mature oligodendrocytes (PLP+) were changed to a deprivation medium (DM), without insulin and FCS. Increasing concentrations of S1P were added, and survival was quantified 24 h later, by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma) assay. Results, expressed as a percentage of controls [i.e., cells maintained in insulin (10 μm) and FCS (0.5%)-supplemented medium], are the mean ± SEM of at least three independent experiments, with at least three cultures per condition. Statistical analysis was performed using Fisher's PSLD test.
Morphometric analysis. The effect of S1P on process retraction was evaluated by counting the number of processes of pre-oligodendrocytes and mature oligodendrocytes in S1P-treated and control cultures. We defined three types of cells, depending on their number of processes: cells with no process, cells with one process, and cells with two or more processes. Results are expressed as the mean ± SEM of at least three independent experiments, with at least three cultures per condition. Statistical analysis was performed using the Student's t test.
Western blot analysis. After harvesting, cells were rinsed with PBS, then lysed with 1% SDS in 50 mm Tris, pH 8.0, and 150 mm NaCl buffer containing 1 mm NaVO4 and 1 mm PMSF. The lysates were collected by centrifugation for 15 min at 4°C. The protein concentration of the cell lysate was measured by the BCA protein assay (Sigma). Proteins (30 μg) were resolved by electrophoresis on 10% SDS-PAGE and electroblotted for 1 h at 4°C to nitrocellulose membranes (Amersham Biosciences, Les Ulis, France) as described previously (Barbin et al., 2004). Densitometric data were obtained using IQMac version 1.2 software (Molecular Dynamics, Sunnyvale, CA).
RNA interference. Four pooled SMART-selected 21 nucleotide RNA [small interfering RNA (siRNA)] duplexes, directed against nucleotides of the Edg8/S1P5 coding sequences, were designed and obtained from MWG Biotech (Courtaboeuf, France). Transient transfection of siRNA was performed on mature oligodendrocyte cultures, 3 d after isolation, using jetSI (Qbiogen, Illkirch, France), following the manufacturer's instructions. Cells were treated with Edg8/S1P5 siRNA (50 nm) for 48 h before S1P addition. Control cultures were treated with nonrelevant MTLR-Cy3 siRNA (60 nm) (myotubularin coupled with Cy3) (MWG Biotech).
Results
Pattern of expression of Edg8/S1P5 in postnatal brain
The expression of Edg8/S1P5 mRNA was analyzed by in situ hybridization on sagittal sections of mouse brain at postnatal day 21 (P21) (Fig. 2). Edg8/S1P5-expressing cells were detected in the white matter tracts, such as the corpus callosum, the fimbria, the anterior commissure, the bundles of white matter running through the striatum (Fig. 2 A), and the deep white matter of the cerebellum (Fig. 2 B). In the white matter tracts, Edg8/S1P5 mRNA-expressing cells were often observed in a chain-like pattern, highly suggestive of intrafascicular oligodendrocytes (Fig. 2C).
Figure 2.
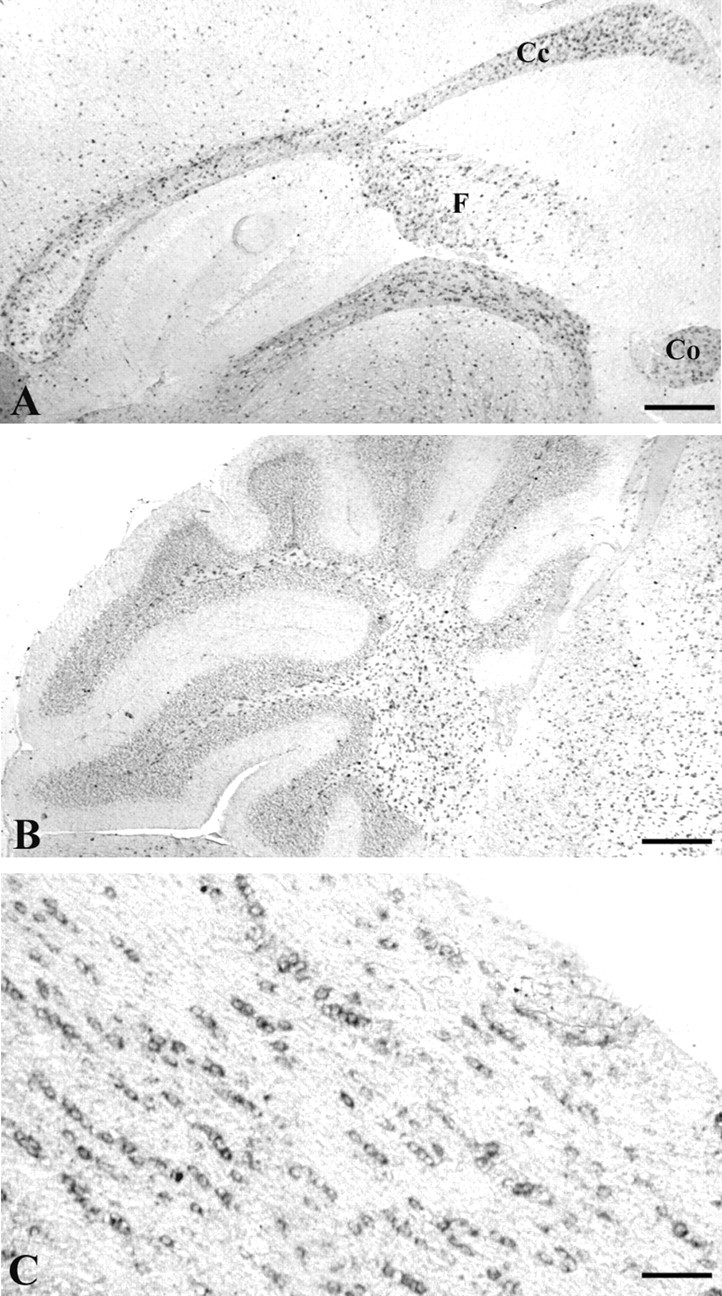
Expression of Edg8/S1P5 mRNA in vivo. In situ hybridization on sagittal sections of mouse brain at P21 with a digoxigenin-labeled Edg8/S1P5 riboprobe (A) showed that Edg8/S1P5 mRNA is detected in corpus callosum (Cc), anterior commissure (Co), fimbria (F), and in white matter of the cerebellum (B). C, In the white matter tracts, Edg8/S1P5 mRNA-expressing cells often liein a chain-like pattern, highly suggestive of intrafascicular oligodendrocytes. Scale bars: A, B, 160 μm; C, 80 μm.
To unambiguously identify the phenotype of the cells expressing Edg8/S1P5 mRNA, we took advantage of 1900bp-MBP-lacZ transgenic mice, in which the Escherichia coli lacZ reporter gene is under the control of the 1.9 kb initial portion of the MBP promoter (Gow et al., 1992). In this transgenic line, the β-galactosidase reporter is specifically expressed by mature myelinating oligodendrocytes (Stankoff et al., 1996). Sagittal sections of P21 mouse brain were hybridized with a digoxigenin-labeled Edg8/S1P5 riboprobe, followed by anti-β-galactosidase immunostaining to label oligodendrocyte cell bodies (Fig. 3). Edg8/S1P5-positive cells were also anti-β-galactosidase positive (Fig. 3A,B), and thus identified as mature oligodendrocytes.
Figure 3.
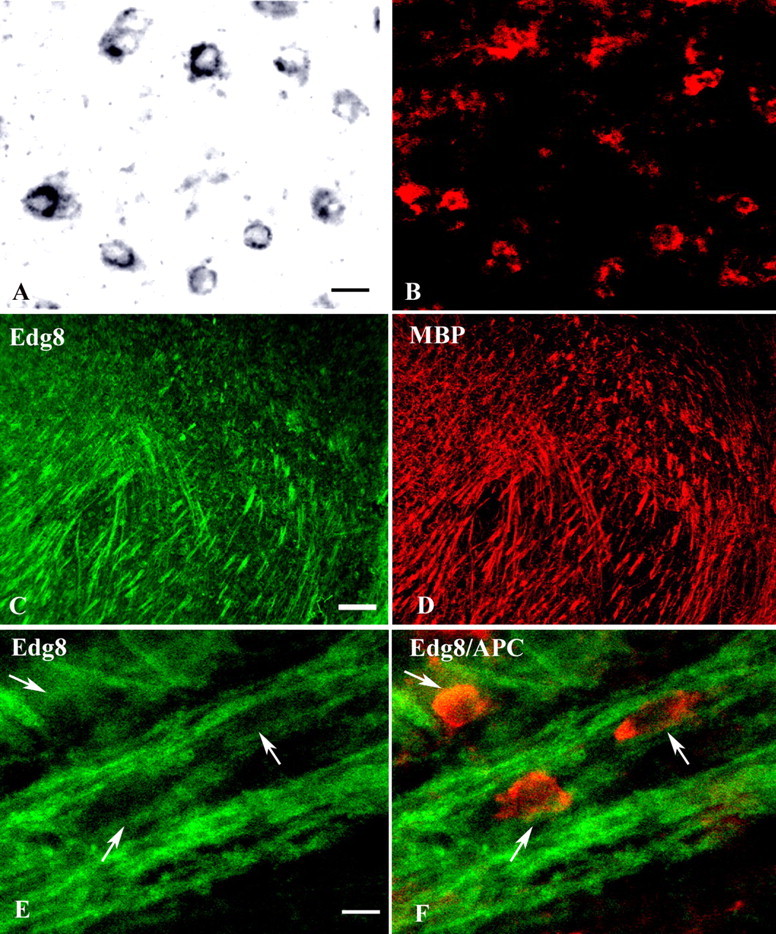
Cellular localization of Edg8/S1P5-expressing cells in adult mouse brain. A, B, Sagittal section of MBP-lacZ transgenic P21 mouse brain hybridized with a digoxigenin-labeled Edg8/S1P5 riboprobe (A) and immunostained with anti-β-galactosidase (B). Note the expression of Edg8/S1P5 (A) by β-galactosidase-positive (red) mature oligodendrocytes (B). C, D, Double immunostaining with Edg8/S1P5 Ab (C) and anti-MBP mAb (D) showing colocalization of Edg8/S1P5 and MBP immunoreactivities. Note that with both antibodies, cell bodies are not detected. E, F, Double immunostaining with Edg8/S1P5 Ab and anti-APC mAb. Edg8/S1P5 staining (green) appears restricted to myelinated fibers and is not detected in the APC+ oligodendrocyte cell bodies (red). Scale bars: A, B, E, F, 10 μm; C, D, 500 μm.
To examine the expression of Edg8/S1P5 protein in mature oligodendrocytes and myelin sheaths, adult brain sections were double-labeled with anti-Edg8/S1P5 Ab and either anti-MBP or anti-APC mAbs. Edg8/S1P5 immunoreactivity colocalized with MBP detection and appeared restricted to myelinated fibers (Fig. 3C,D). However, even within the strongly Edg8/S1P5-positive myelinated tracts, the anti-Edg8/S1P5 Ab failed to label APC-positive oligodendrocyte cell bodies [APC is an established marker of the cell body of mature oligodendrocytes (Bhat et al., 1996)] (Fig. 3E,F). This suggests that similar to MBP, Edg8/S1P5 does not accumulate in the cell body of myelinating oligodendrocytes but instead is transported into the processes and the myelin sheath. However, Edg8/S1P5 mRNA was not detected along the myelin sheath (Fig. 3A), suggesting that, in contrast to mbp transcripts (Ainger et al., 1997), only the Edg8/S1P5 protein, but not its mRNA, is transported in this manner.
To investigate the expression of Edg8/S1P5 further, we took advantage of Edg8/S1P5+/- animals, which have one allele of the Edg8/S1P5 gene replaced by an IRES-lacZ cassette (Fig. 1). Therefore, in these heterozygous mice, cells expressing the reporter β-galactosidase identify cells in which Edg8/S1P5 transcription is activated. Double-labeling of adult Edg8/S1P5+/- mouse brain sections with anti-β-galactosidase antiserum and anti-Map-2, anti-GFAP, or a mixture of anti-MAC1 and anti-F4 - 80 antibodies demonstrated that Edg8/S1P5 was not detected in neurons, astrocytes, or microglial cells, respectively (data not shown). In addition, double immunolabeling with anti-β-galactosidase and anti-NG2 (a marker of OPCs) (Levine and Stallcup, 1987; Nishiyama et al., 1996) antibodies of P5 brain sections from Edg8/S1P5+/- mice showed that among NG2-positive cells, 80% were also β-galactosidase positive, suggesting transcription of Edg8/S1P5 at the OPC stage (Fig. 4). The observation that not all NG2-positive cells were β-galactosidase positive may suggest that Edg8/S1P5 starts to be expressed at the OPC stage. Alternatively, because NG2 has also been proposed to label other brain structures such as blood vessels and microglia (Pouly et al., 1999; Jones et al., 2002), these additional NG2-positive/β-galactosidase-negative cells may represent cells different from OPCs.
Figure 4.
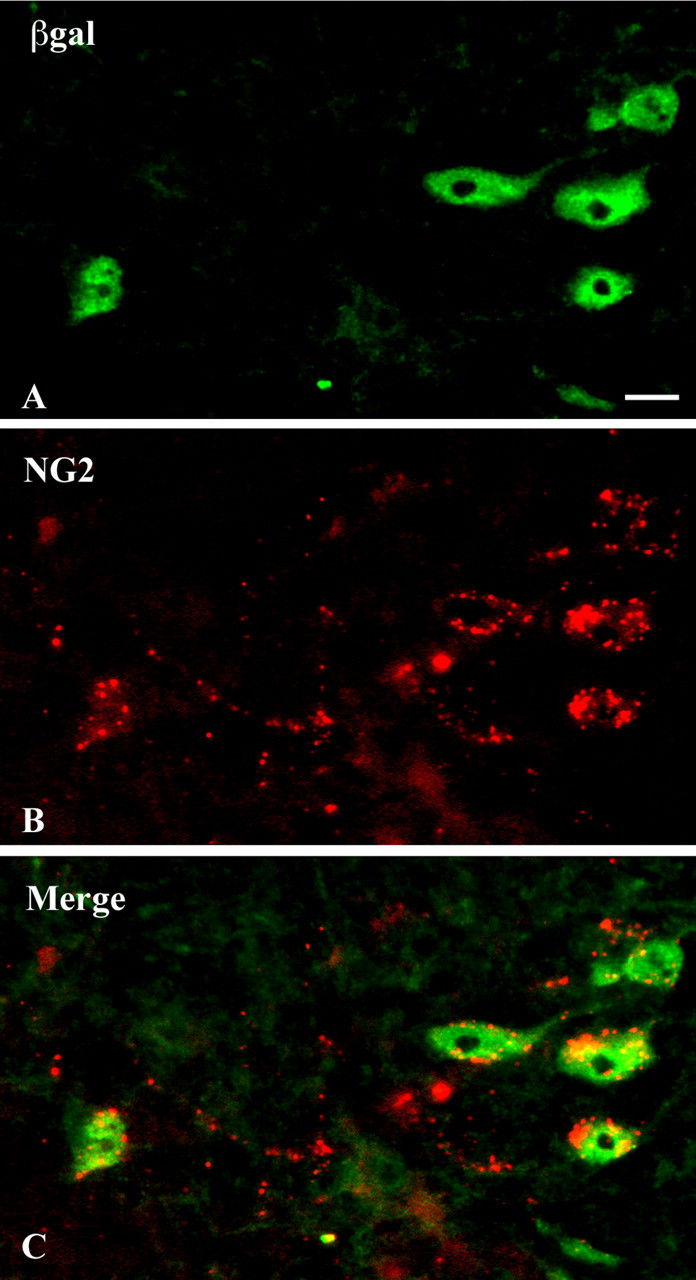
Edg8/S1P5 is expressed by NG2-expressing OPCs. A sagittal section of the brain stem of a P5 postnatal Edg8/S1P5+/- mouse, in which one Edg8/S1P5 allele is replaced by an IRES lacZ cassette (see Fig. 1), is shown. Double immunolabeling with anti-β-galactosidase antibody (green; A) and anti-NG2 antibody (red; B), merged in C, shows that Edg8/S1P5 mRNA-expressing cells (stained with anti-β-galactosidase antibody) are NG2-positive OPCs. Scale bar, 10 μm.
Expression of Edg8/S1P5 by cells of the oligodendrocyte lineage in culture
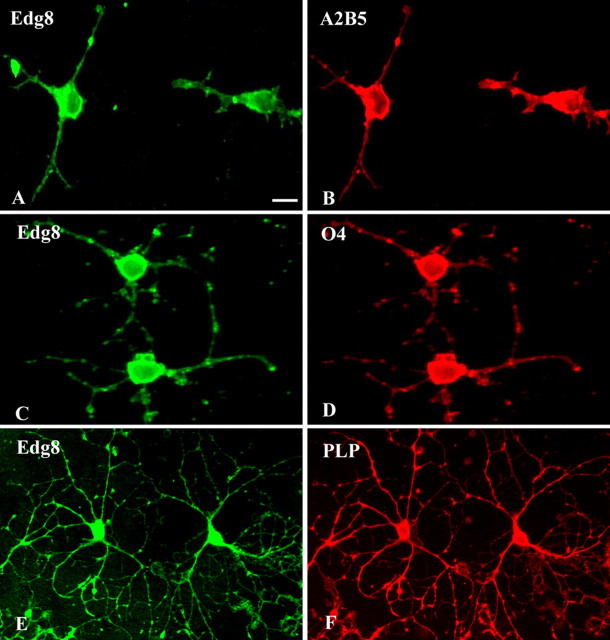
Analysis of Edg8/S1P5 expression was performed in enriched oligodendroglial cultures derived from newborn mouse brains, which contain cells at different stages of oligodendroglial development (Lubetzki et al., 1991). Edg8/S1P5-expressing cells were identified as A2B5+/GalC- OPCs (Fig. 5A,B), O4+/GalC- preoligodendrocytes (Fig. 5C,D), or differentiated GalC+ immature oligodendrocytes. In addition, immunostaining of highly enriched oligodendroglial cultures derived from adult rat brains (Lubetzki et al., 1986) showed that PLP+ mature oligodendrocytes were also Edg8/S1P5+ (Fig. 5E,F). Unlike our finding in brain sections (Fig. 3), in these cultures, Edg8/S1P5 protein was detected not only in the processes but also in the cell body of the oligodendroglial cells at all developmental stages examined. In agreement with our observations in brain sections, Edg8/S1P5 immunoreactivity was specific to oligodendroglial cells, with no detectable staining of SMI31/32+ neurons, MAC1+/F4-80+ microglial cells, or GFAP-expressing astrocytes (data not shown).
Figure 5.
Edg8/S1P5 expression in oligodendrocyte cultures. In oligodendroglial cultures derived from newborn mouse (A-D) and adult rat brains (E, F), Edg8/S1P5 protein (A, C, E) is detected in A2B5+ OPCs (B) and O4+ pre-oligodendrocytes (D) as well as in PLP+ mature oligodendrocytes (F). Note that Edg8/S1P5 is detected on both cell bodies and processes. Scale bar, 10 μm
S1P promotes process retraction of pre-oligodendrocytes through activation of Edg8/S1P5
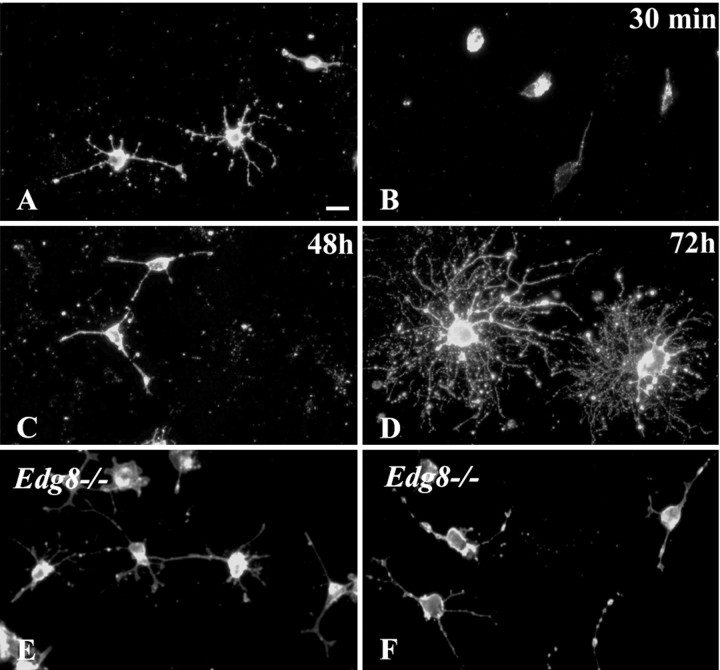
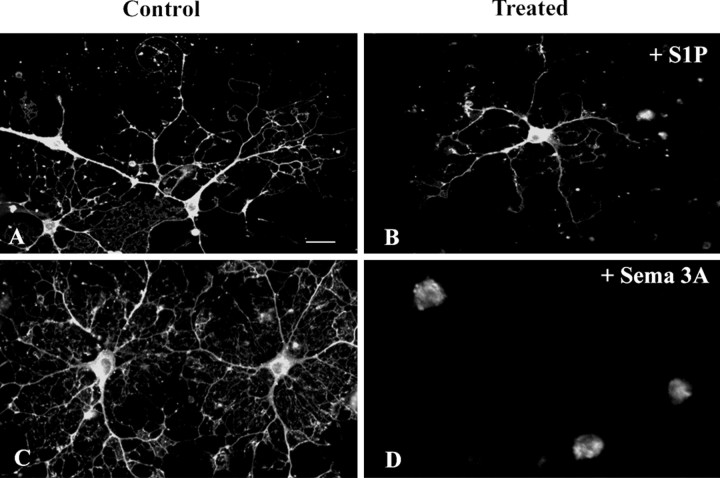
Treatment with S1P induced dramatic morphological changes of pre-oligodendrocytes. This effect was observed in cultures derived from newborn mouse brains (Lubetzki et al., 1991), 3 d after plating, when up to 65% of the cells were O4-positive (Fig. 6A) and GalC-negative pre-oligodendrocytes, with an average of four to six processes per cell. The addition of S1P (1 μm) for 30 min induced a cell rounding (Fig. 6B), with 90% of the preoligodendrocytes retracting all their processes and the remaining cells having either one process left (7%) or two or more (3%) (see Fig. 8A). The effect of S1P on process retraction was dose dependent, with an abrupt shift for concentration of S1P between 0.01 and 0.1 μm (see Fig. 8A). The retraction of processes induced by S1P treatment was transient, with process reextension after 48 h more pronounced at 72 h (Fig. 6C,D). The role of Edg8/S1P5 activation in this retraction effect was confirmed by experiments on cultures derived from Edg8/S1P5-/- brains. In these cultures, which are phenotypically identical to wild-type cultures, S1P (1 μm) did not induce retraction of oligodendroglial processes (Fig. 6E,F). This effect of S1P on the retraction of oligodendroglial processes was not observed on mature oligodendrocytes (Fig. 7A,B), suggesting a change of response to S1P between pre-oligodendrocytes and mature oligodendrocytes. The absence of retraction was not attributable to our culture conditions, because in control experiments, we confirmed that, as reported by Bagnard et al. (1998), the addition of semaphorin 3A for 24 h triggered a major retraction of processes of mature oligodendrocytes (Fig. 7C,D).
Figure 6.
S1P induces process retraction in pre-oligodendrocytes. A-D, Preoligodendrocytes derived from newborn mouse brains were treated for 30 min with S1P (1 μm) and immunostained with O4 mAb. Cell morphology was analyzed before (A) and 30 min (B), 48 h (C), and 72 h (D) after S1P withdrawal. There is a dramatic loss of processes detected 30 min after the addition of S1P, but process reextension is already observed 48 h later. Process extension is more pronounced 72 h after S1P addition. Cultures of oligodendrocyte precursors from Edg8/S1P5-deficient brain before (E) and 30 min after (F) the addition of S1P (1 μm) show that in the absence of Edg8/S1P5, no process retraction is elicited by the addition of S1P in the culture medium. Scale bar, 10 μm.
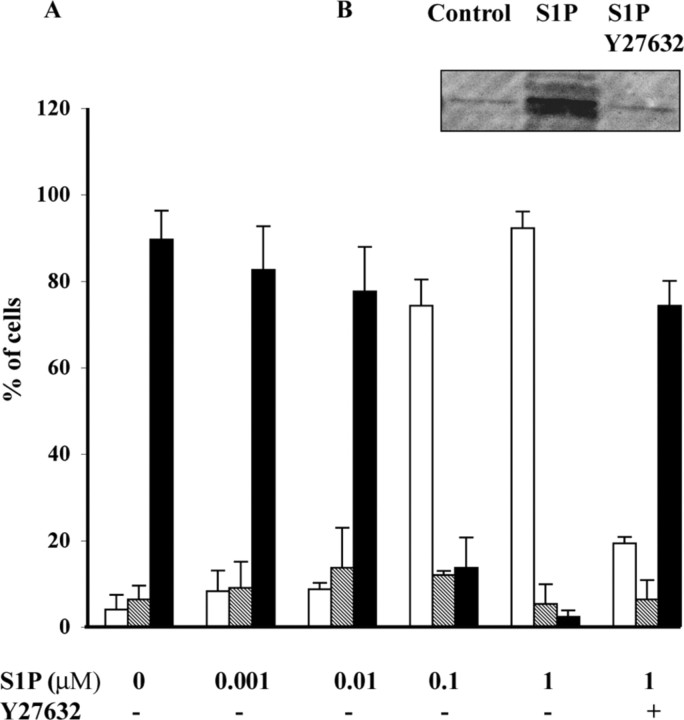
Figure 8.
S1P-induced process retraction in immature oligodendrocytes is concentration dependent and mediated through Rho kinase. A, Increasing concentrations of S1P were added to immature oligodendrocyte cultures, with or without the Rho kinase inhibitor Y27632. The number of cell processes was analyzed 30 min later. The different columns correspond to the number of processes per cell: 0, □; 1, ▧; two or more processes, ▪. B, S1P induces CRMP2 phosphorylation in immature oligodendrocytes mediated through the Rho kinase pathway. Immature oligodendrocytes were treated with S1P (1 μm) for 15 min, in the presence or absence of the Rho kinase inhibitor Y27632. Whole-celllysates were prepared and immunoblotted with antibodies that recognize the phosphorylated form of CRMP2.
Figure 7.
Absence of S1P-induced process retraction in mature oligodendrocytes. Mature oligodendrocytes purified from adult rat brains were treated with S1P (1 μm) (A, B) or Sema 3A-containing medium (C, D). The effect on morphology of oligodendrocytes, immunostained with an anti-MBP antibody, was analyzed before (A, C) and 24 h after S1P (B) or Sema 3A (D) addition. Sema 3A, but not S1P, induces rounding of mature oligodendrocytes. Scale bar, 10 μm.
S1P-induced process retraction of preoligodendrocytes is mediated through CRMP2 phosphorylation and Rho kinase
It has been reported that in neuronal cells, growth cone collapse induced by activation of LPA receptors requires a Rho kinase-mediated phosphorylation of CRMP2 (Arimura et al., 2000). To address the possibility that CRMP2 phosphorylation is involved in S1P-induced oligodendroglial process retraction, we analyzed by Western blot the phosphorylation of CRMP2 in extracts from S1P-treated cultures of either purified O4+/GalC- pre-oligodendrocytes or mature PLP+ oligodendrocytes, using anti-phosphorylated CRMP2 antibody. As shown in Figure 8B, phosphorylation of CRMP2 was detected in extracts from cultures of pre-oligodendrocytes treated for 15 min with S1P (1 μm). In contrast, CRMP2 phosphorylation was not elicited by S1P in mature oligodendrocytes, in agreement with the absence of S1P-induced process retraction in these cells.
To determine whether S1P-induced phosphorylation of CRMP2 was mediated by Rho kinase, we examined the effect of blocking Rho kinase using the specific Rho kinase inhibitor Y27632. The addition of Y27632 for 30 min before S1P addition resulted in an inhibition of CRMP2 phosphorylation (Fig. 8B), as well as a suppression of the S1P-induced process retraction (Fig. 8A). Altogether, these results indicate that S1P-induced process retraction of pre-oligodendrocytes involves CRMP2 phosphorylation by Rho kinase.
S1P is a survival factor for mature oligodendrocytes, acting through Edg8/S1P5
Because the purity of oligodendroglial cultures derived from rat brains is consistently higher than from mouse brains, we chose to investigate the role of S1P on oligodendrocyte survival in rat cultures. Mature oligodendrocytes isolated from adult rat brain were cultured for 3 d before changing to a DM, lacking insulin and FCS. After 24 h of culture under DM conditions, we observed, using a standard MTT viability assay, a 58% reduction of mature oligodendrocyte survival compared with control cultures maintained in a medium supplemented with insulin (10 μm) and FCS (0.5%). In contrast, under exactly the same culture conditions, the addition of S1P to the DM resulted in a significant increase in oligodendrocyte survival. This effect was concentration dependent and for a 1 μm concentration of S1P oligodendrocyte survival had nearly returned to that of control cultures (Fig. 9A). The addition of S1P to insulin and FCS-supplemented medium did not further increase the survival of mature oligodendrocytes [83 and 81% survival compared with control for S1P (1 μm) in DM and S1P (1 μm) plus insulin and FCS, respectively], suggesting the absence of an additive effect. In contrast to the effect observed with mature oligodendrocytes, no change in the survival of pre-oligodendrocytes was detected after S1P treatment of O4+ cell-enriched cultures (data not shown).
Figure 9.
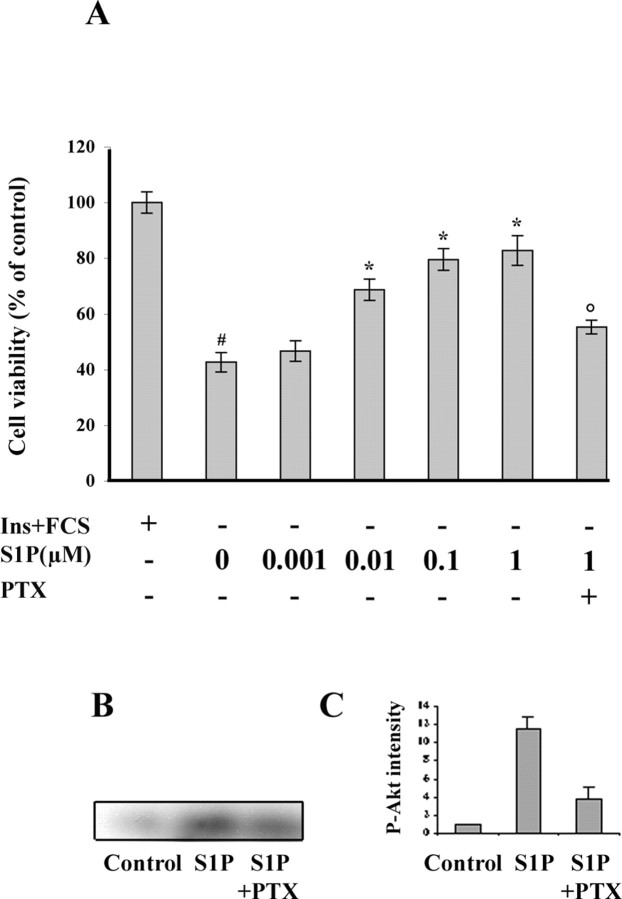
S1P induces survival of mature oligodendrocytes. Mature oligodendrocytes, isolated from 4-week-old rat brain, were maintained for 3 d in culture medium containing insulin (10 μm) and FCS (0.5%), then changed to a DM (medium without insulin and FCS) containing an increasing concentration of S1P. Cell viability was assessed 24 h later by the MTT survival assay, and the results are expressed as the percentage of controls (i.e., cells maintained in insulin and FCS-supplemented medium). Removal of insulin and FCS resulted in a 58% reduction in cell viability (#p < 0.001 vs controls). The addition of S1P to the DM resulted in a concentration-dependent increase in the oligodendroglial survival (*p < 0.001 vs S1P untreated cultures maintained in DM). The addition of 1 μm S1P rescued mature oligodendrocytes from DM-induced impaired survival and returned survival to levels that were not statistically different from control cells in supplemented medium. The survival effect of S1P (1 μm) was reversed by the addition of PTX [°p < 0.001 vs S1P (1 μm)-treated cultures without PTX]. B, S1P-induced Akt phosphorylation on mature oligodendrocytes. Mature oligodendrocytes were stimulated with 1 μm S1P for 20 min in the presence or absence of PTX. Whole-cell lysates were prepared and immunoblotted with antibodies that recognize phosphorylated Akt. C, The densitometric data are the mean ± SEM of three different experiments.
To determine whether the survival effect induced by S1P was mediated by Edg8/S1P5 receptor signaling, we performed loss-of-function experiments using siRNA. This siRNA procedure was used, rather than oligodendroglial cultures from adult Edg8/S1P5-/- brains, because of the limited number of Edg8/S1P5-/- mice available. In preliminary experiments, using nonrelevant MTLR-Cy3 siRNA, we determined that, under our experimental conditions, transfection efficiency of mature (PLP+) oligodendrocytes was close to 80% (Fig. 10A) with no noticeable changes in cell viability or morphology. Transfection with Edg8/S1P5 siRNA resulted in a significant reduction of the level of Edg8/S1P5 immunoreactivity as detected by Western blotting. This effect was highly specific because no reduction in the level of the Edg2/LPA1 receptor was observed (Fig. 10B). We next examined the effect of adding S1P (1 μm) on the survival of Edg8/S1P5 siRNA-treated mature oligodendrocytes maintained in DM. The increased survival of mature oligodendrocytes induced by the addition of S1P in cultures treated with nonrelevant MTLR siRNA was no longer observed in Edg8/S1P5 siRNA-treated cultures (Fig. 10C). These experiments demonstrate that the survival effect induced by S1P on mature oligodendrocytes is mediated by the Edg8/S1P5 receptor.
Figure 10.
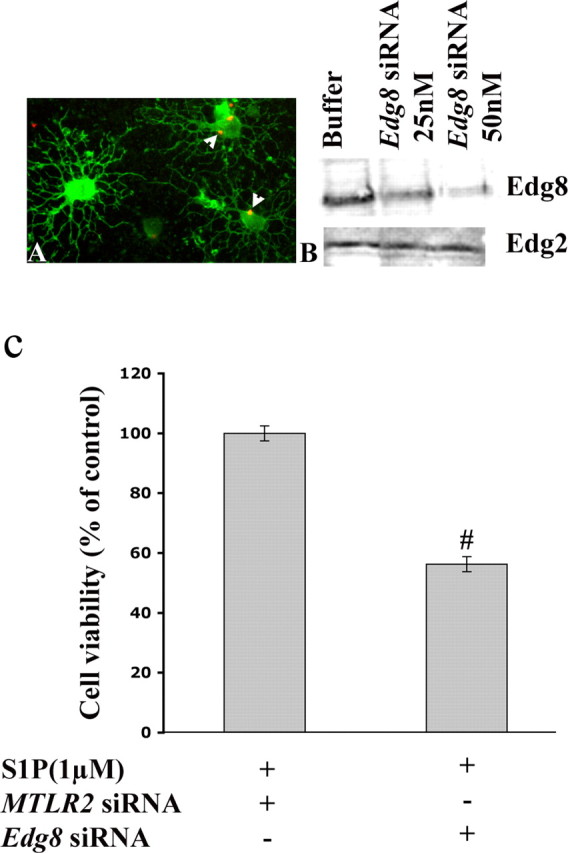
Edg8/S1P5 siRNA silencing suppresses the S1P-induced survival of mature oligodendrocytes. A, Transfection of mature oligodendrocytes with an MTLR-CY3 siRNA. Oligodendrocytes are stained with anti-GalC mAb (green). Arrowheads point to siRNA, localized in the cell body (red dots). B, Mature oligodendrocytes were transfected with increasing concentrations of Edg8/S1P5 siRNA. Forty-eight hours after transfection, whole-cell lysates were prepared and immunoblotted with antibodies that recognize either Edg8/S1P5 or Edg2/LPA1 to show the specific inhibition of expression of Edg8/S1P5. C, Mature oligodendrocytes were transfected with either Edg8/S1P5 siRNA or nonrelevant MTLR siRNA and maintained in DM (see legend to Fig. 9). After 48 h, S1P-mediated survival was analyzed by the MTT survival assay, and the results are expressed as the percentage of controls (i.e., S1P-treated cells transfected with MTLR siRNA and maintained in DM. (#p < 0.001 vs controls).
S1P-induced survival of mature oligodendrocytes is mediated through the Gα/I and Akt pathways
We next investigated the mechanism of signal transduction mediated by S1P activation of Edg8/S1P5 receptor in mature oligodendrocytes. First, we showed that S1P-induced survival was suppressed by previous treatment of the cultures with PTX, suggesting that this phenomenon is mediated through a PTX-sensitive Gα/i-protein (Fig. 9A). Because the Akt signaling cascade has been previously shown to be involved in oligodendrocyte survival (Vemuri and McMorris, 1996; Flores et al., 2000), we performed an immunoblot with an anti-phosphoAkt mAb on S1P-treated cell extracts from mature oligodendrocytes. Exposure of mature oligodendrocytes to S1P for 15 min induced a robust phosphorylation of Akt, which was mainly prevented by incubation of the cells with PTX (Fig. 9B,C). In contrast, S1P treatment of cultures enriched in O4+ cells failed to induce Akt phosphorylation of pre-oligodendrocytes (data not shown).
Discussion
Oligodendroglial expression of Edg8/S1P5
In their initial report on the identification of Edg8/S1P5, Im et al. (2001) stated that in the brain, Edg8/S1P5 mRNA was observed predominantly in white matter tracts, suggesting expression in mature oligodendrocytes, which was confirmed in cultures of differentiated oligodendrocytes (Yu et al., 2004). This is in contrast to the finding of Terai et al. (2003), who reported Edg8/S1P5 protein expressed by NG2-positive OPCs but not mature oligodendrocytes. Under our experimental conditions, we showed, by both in situ hybridization and immunolabeling, the expression of Edg8/S1P5 in mature oligodendrocytes of the mouse brain. In agreement with the report of Terai et al. (2003), we were also able to detect Edg8/S1P5 expression on pre-oligodendrocytes and the majority of OPCs. These data are consistent with an expression of Edg8/S1P5 throughout oligodendroglial development.
Activation of Edg8/S1P5 induces process retraction in pre-oligodendrocytes
We have shown that S1P stimulation of oligodendroglial precursors, but not mature oligodendrocytes, resulted in an oligodendroglial process retraction mediated by the Edg8/S1P5 receptor. In a similar manner, LPA has been shown to induce retraction of processes in immature, but not mature, CG4 oligodendroglial cell lines and OPCs (Dawson et al., 2003), an effect that is likely mediated by the Edg2/LPA1 receptor (Allard et al., 1998, 1999). It is of note that in the same study, Dawson et al. (2003) reported that in addition to LPA, S1P also induced process retraction of undifferentiated CG4 cells.
The actions of S1P and LPA on primary neuronal cultures have been characterized by a number of groups and shown to induce neurite retraction and/or growth cone collapse (Sato et al., 1997; Hirose et al., 1998; Fukushima et al., 2002). Subsequent studies have implicated the involvement of a serine/threonine-dependent Rho kinase in the lysophospholipid-induced neurite retraction and have identified both myosin light chains and CRMP2 as major substrates for the Rho kinase-mediated retraction. This transduction cascade has therefore been proposed as a key system in lysophospholipid-induced growth cone collapse and is considered to be an important pathway for axonal growth cone remodeling (Moolenaar, 1995; Leung et al., 1996; Ishizaki et al., 1997; Arimura et al., 2000). Our results suggest that the same pathway is implicated in S1P-induced retraction of preoligodendrocyte processes and that this effect is dependent on the activity of Rho kinase and increased phosphorylation of CRMP2. Semaphorin 3A, a known chemorepellent to OPCs migrating into the optic nerve (Sugimoto et al., 2001; Spassky et al., 2002), has also been reported to induce process retraction in oligodendrocytes. However, the Sema 3A-induced retraction of oligodendroglial processes, mediated via CRMP2, was found to be restricted to mature oligodendrocytes (Ricard et al., 2000). These results suggest that S1P and Sema 3A can induce oligodendroglial process retraction in pre-oligodendrocytes and mature oligodendrocytes, respectively. These ligands exert their action by interacting with different receptors (neuropilin 1 for Sema 3A and Edg8/S1P5 for S1P) expressed by both pre-oligodendrocytes and mature oligodendrocytes, and CRMP2 is one common element of the downstream signaling pathway.
The functional consequences of the S1P-mediated process retraction in pre-oligodendrocytes remains speculative. It is possible that after migration pre-oligodendrocytes settle in a given bundle of axons before initiating the myelination process. This myelination process (i.e., the enwrapping of each axon segment by a single oligodendrocyte extension) is preceded by a major modification of the morphology of oligodendrocytes. Both in vivo and in vitro, it has been shown that this terminal differentiation of the oligodendrocyte is characterized by a drastic reduction in the number of processes, transforming the stellate, sunlike, premyelinating oligodendrocyte into a mature myelin oligodendrocyte glycoprotein (MOG) expressing myelinating oligodendrocyte with a few primary processes, at the end of which the myelin sheath will extend (Lubetzki et al., 1993; Pfeiffer et al., 1993; Solly et al., 1996). In this regard, one of the potential functions of Edg8/S1P5 receptor on oligodendrocytes could be to regulate the reduction in arborization, such that the number of oligodendrocyte processes matches the number of axonal segments in need of myelination. It is also of interest that there seems to be a certain level of redundancy in the system, with both S1P and LPA causing the retraction of processes in OPCs. This finding may explain the absence of apparent myelin pathology in transgenic lines deficient for either Edg2/LPA1 (Contos et al., 2002) or Edg8/S1P5 (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). In this respect, it will be of interest to examine the phenotype of the Edg2/LPA1-/-/Edg8/S1P5-/- double knock-out mice.
S1P and survival of mature oligodendrocytes
As mentioned above, S1P activation has no effect on process retraction in mature oligodendrocytes. However, we did observe a marked and significant increase in mature oligodendrocyte survival that was PTX sensitive (i.e., Gα/i activated) and mediated through the Akt signaling pathway. In this respect, our results are in agreement with previously identified roles of S1P in Akt activation and survival of hepatic myofibroblast or endothelial cells (Morales-Ruiz et al., 2001; Davaille et al., 2002). A variety of different agents have been shown to promote oligodendrocyte survival, and some such as NT3, IGF-I, and neuregulin (Vemuri and McMorris, 1996; Flores et al., 2000) signal through the phosphatidylinositol 3′-kinase (PI3K) transduction pathway. S1P therefore appears to be another oligodendroglial survival factor, signaling through the Akt pathway downstream of the Edg8/S1P5 receptor.
Our results show that in cells of the oligodendroglial lineage, S1P activation of Edg8/S1P5 receptor can trigger two distinct physiological effects that are dependent on the developmental stage of the cell. In pre-oligodendrocytes, S1P retraction of processes is mediated by a Rho kinase/CRMP2 pathway, whereas in mature oligodendrocytes, S1P promotes survival through a PTX-sensitive pathway. It is likely that these pathways are activated by distinct G-proteins, and Edg8/S1P5 has been shown to couple with both Gαi and G12 (Malek et al., 2001). The PI3K pathway has been shown to be activated by Gαi and the Rho kinase pathway to require G12/13 (Li et al., 2003). The functional changes in the effect of Edg8/S1P5 activation may therefore relate to changes in receptor coupling with heterotrimeric G-proteins. This could occur by a variety of potential mechanisms including developmental switches of G-protein expression (i.e., from G12/13 to Gα expression) or alternative splicing of the Edg8/S1P5 receptor resulting in changes to the coupling efficiency of the receptor to specific G-proteins, as suggested for Edg2/LPA1 (Allard et al., 1999). However, we were unable to isolate an alternative spliced variant of Edg8/S1P5 by comparison of RNA extracted from O4+-enriched pre-oligodendrocytes and mature oligodendrocytes (our unpublished data). Our findings are consistent with observations seen in Edg2/LPA1-expressing Schwann cells, in which Edg2/LPA1 activation by LPA mediates Schwann cell survival through a PI3K/mitogen-activated protein kinase-dependent pathway and cytoskeletal changes are triggered by a Rho kinase-dependent pathway (Weiner and Chun, 1999; Li et al., 2003).
In multiple sclerosis, remyelination of demyelinated axons attributable to the disease process often fails. Oligodendroglial depopulation may account for this repair deficit in a proportion of lesions characterized by oligodendrocyte loss. However, the absence of myelin repair has also been reported in lesions with surviving oligodendrocyte progenitors or differentiated oligodendrocytes, suggesting that these cells were unable to or were prevented from forming myelin (Chang et al., 2002; Franklin, 2002; Reynolds et al., 2002). Hence, understanding the signaling pathways that modulate the activation and inactivation of the myelinating process may enable the identification of novel proteins, the modulation of which may ultimately allow for improved therapies of this devastating disease. The data we present here suggest that the Edg8/S1P5 receptor could be one such protein that participates in the regulation of myelinating process. By mediating a developmentally regulated dual-signaling pathway in oligodendrocytes, leading to two distinct functional effects modulating either process retraction or cell survival, activation of the Edg8/S1P5 receptor may be important in the maturation and survival of oligodendrocytes and may ultimately play a key role in the modulation, formation, and repair of myelin.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale and Association de Recherche sur la Sclérose En Plaques. C.J. is the recipient of a GlaxoSmithKline (GSK) fellowship. We thank Drs. V. P. Collins, K. Ikenaka, B. Ranscht, B. Crook, and J. Trotter for the gift of valuable reagent, Dr. Anna Williams for careful reading of this manuscript, and members of the GSK Laboratory Animal Sciences and Discovery Pipeline Genetics teams for animal husbandry and genotyping.
Correspondence should be addressed to Dr. Catherine Lubetzki, Biologie des Interactions Neurones/Glie, Institut National de la Santé et de la Recherche Médicale Unité Mixte de Recherche-711, Hôpital de la Salpêtrière, F-75651 Paris, France. E-mail:catherine.lubetzki@psl.ap-hop-paris.fr.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251459-11$15.00/0
C.J. and S.H. contributed equally to this work.
References
- Ainger K, Avossa D, Diana AS, Barry C, Barbarese E, Carson JH (1997) Transport and localization elements in myelin basic protein mRNA. J Cell Biol 138: 1077-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard J, Barron S, Diaz J, Lubetzki C, Zalc B, Schwartz JC, Sokoloff P (1998) A rat G protein-coupled receptor selectively expressed in myelin-forming cells. Eur J Neurosci 10: 1045-1053. [DOI] [PubMed] [Google Scholar]
- Allard J, Barron S, Trottier S, Cervera P, Daumas-Duport C, Leguern E, Brice A, Schwartz JC, Sokoloff P (1999) Edg-2 in myelin-forming cells: isoforms, genomic mapping, and exclusion in Charcot-Marie-Tooth disease. Glia 26: 176-185. [DOI] [PubMed] [Google Scholar]
- Arimura N, Inagaki N, Chihara K, Menager C, Nakamura N, Amano M, Iwamatsu A, Goshima Y, Kaibuchi K (2000) Phosphorylation of collapsin response mediator protein-2 by Rho-kinase. Evidence for two separate signaling pathways for growth cone collapse. J Biol Chem 275: 23973-23980. [DOI] [PubMed] [Google Scholar]
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J (1998) Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development 125: 5043-5053. [DOI] [PubMed] [Google Scholar]
- Bansal R, Pfeiffer SE (1997) FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res 50: 215-228. [DOI] [PubMed] [Google Scholar]
- Barbin G, Aigrot MS, Charles P, Foucher A, Grumet M, Schachner M, Zalc B, Lubetzki C (2004) L1 cell adhesion molecule and CNS myelination. Neuron Glia Biol 1: 65-72. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC (1992) Cell death and control of cell survival in the oligodendrocyte lineage. Cell 70: 31-46. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM (1996) Expression of the APC tumor suppressor protein in oligodendroglia. Glia 17: 169-174. [DOI] [PubMed] [Google Scholar]
- Cervera P, Tirard M, Barron S, Allard J, Trottier S, Lacombe J, Daumas-Duport C, Sokoloff P (2002) Immunohistological localization of the myelinating cell-specific receptor LP(A1). Glia 38: 126-136. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD (2002) Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med 346: 165-173. [DOI] [PubMed] [Google Scholar]
- Charles P, Hernandez MP, Stankoff B, Aigrot MS, Colin C, Rougon G, Zalc B, Lubetzki C (2000) Negative regulation of central nervous system myelination by polysialylated-neural cell adhesion molecule. Proc Natl Acad Sci USA 97: 7585-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Contos JJ, Munroe D (1999) A growing family of receptor genes for lysophosphatidic acid (LPA) and other lysophospholipids (LPs). Cell Biochem Biophys 30: 213-242. [DOI] [PubMed] [Google Scholar]
- Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J (2002) Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol 22: 6921-6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davaille J, Li L, Mallat A, Lotersztajn S (2002) Sphingosine 1-phosphate triggers both apoptotic and survival signals for human hepatic myofibroblasts. J Biol Chem 277: 37323-37330. [DOI] [PubMed] [Google Scholar]
- Dawson J, Hotchin N, Lax S, Rumsby M (2003) Lysophosphatidic acid induces process retraction in CG-4 line oligodendrocytes and oligodendrocyte precursor cells but not in differentiated oligodendrocytes. J Neurochem 87: 947-957. [DOI] [PubMed] [Google Scholar]
- Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J (2001) AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia 34: 213-228. [DOI] [PubMed] [Google Scholar]
- Flores AI, Mallon BS, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin WB (2000) Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci 20: 7622-7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3: 705-714. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Weiner J, Kaushal D, Contos JJ, Rehen SK, Kingsbury MA, Kim KY, Chun J (2002) Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol Cell Neurosci 20: 271-282. [DOI] [PubMed] [Google Scholar]
- Gow A, Friedrich Jr VL, Lazzarini RA (1992) Myelin basic protein gene contains separate enhancers for oligodendrocyte and Schwann cell expression. J Cell Biol 119: 605-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S (1998) Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol 141: 1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im DS, Clemens J, Macdonald TL, Lynch KR (2001) Characterization of the human and mouse sphingosine-1-phosphate receptor, S1P5 (Edg8/S1P5): structure-activity relationship of sphingosine1-phosphate receptors. Biochemistry 40: 14053-14060. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S (1997) p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett 404: 118-124. [DOI] [PubMed] [Google Scholar]
- Jacque CM, Suard IM, Collins VP, Raoul MM (1986) Interspecies identification of astrocytes after intracerebral transplantation. Dev Neurosci 8: 142-149. [DOI] [PubMed] [Google Scholar]
- Jarjour AA, Manitt C, Moore SW, Thompson KM, Yuh SJ, Kennedy TE (2003) Netrin-1 is a chemorepellent for oligodendrocyte precursor cells in the embryonic spinal cord. J Neurosci 23: 3735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH (2002) NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci 22: 2792-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T (1998) Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552-1555. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L (1996) The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 16: 5313-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JM, Stallcup WB (1987) Plasticity of developing cerebellar cells in vitro studied with antibodies against the NG2 antigen. J Neurosci 7: 2721-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gonzales M, Meinkoth JL, Field J, Kazanietz MG, Tennekoon GI (2003) Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J Biol Chem 278: 9585-9591. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Lombrail P, Hauw JJ, Zalc B (1986) Multiple sclerosis: rat and human oligodendrocytes are not the target for CSF immunoglobulins. Neurology 36: 524-528. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Goujet-Zalc C, Gansmuller A, Monge M, Brillat A, Zalc B (1991) Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem 56: 671-680. [DOI] [PubMed] [Google Scholar]
- Lubetzki C, Demerens C, Anglade P, Villarroya H, Frankfurter A, Lee VM, Zalc B (1993) Even in culture, oligodendrocytes myelinate solely axons. Proc Natl Acad Sci USA 90: 6820-6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek RL, Toman R, Edsall LC, Wong S, Chiu J, Letterle CA, Van Brocklyn JR, Milstien S, Spiegel S, Lee NH (2001) Nrg-1 belongs to the endothelial differentiation gene family of G protein-coupled sphingosine-1-phosphate receptors. J Biol Chem 276: 5692-5699. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K (1996) Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 15: 2208-2216. [PMC free article] [PubMed] [Google Scholar]
- McCarthy KD, De Vellis J (1980) Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85: 890-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T, Musante D, Ransom BR (1999) Lysophosphatidic acid-induced calcium signals in cultured rat oligodendrocytes. NeuroReport 10: 2929-2932. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH (1995) Lysophosphatidic acid signaling. Curr Opin Cell Biol 7: 203-210. [DOI] [PubMed] [Google Scholar]
- Morales-Ruiz M, Lee MJ, Zollner S, Gratton JP, Scotland R, Shiojima I, Walsh K, Hla T, Sessa WC (2001) Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J Biol Chem 276: 19672-19677. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB (1996) Colocalization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res 43: 299-314. [DOI] [PubMed] [Google Scholar]
- Oh LY, Denninger A, Colvin JS, Vyas A, Tole S, Ornitz DM, Bansal R (2003) Fibroblast growth factor receptor 3 signaling regulates the onset of oligodendrocyte terminal differentiation. J Neurosci 23: 883-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol 3: 191-197. [DOI] [PubMed] [Google Scholar]
- Pouly S, Becher B, Blain M, Antel JP (1999) Expression of a homologue of rat NG2 on human microglia. Glia 27: 259-268. [DOI] [PubMed] [Google Scholar]
- Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W (1982) Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA 79: 2709-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R, Dawson M, Papadopoulos D, Polito A, Di Bello IC, Pham-Dinh D, Levine J (2002) The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol 31: 523-536. [DOI] [PubMed] [Google Scholar]
- Ricard D, Stankoff B, Bagnard D, Aguera M, Rogemond V, Antoine JC, Spassky N, Zalc B, Lubetzki C, Belin MF, Honnorat J (2000) Differential expression of collapsin response mediator proteins (CRMP/ULIP) in subsets of oligodendrocytes in the postnatal rodent brain. Mol Cell Neurosci 16: 324-337. [DOI] [PubMed] [Google Scholar]
- Sato K, Tomura H, Igarashi Y, Ui M, Okajima F (1997) Exogenous sphingosine-1-phosphate induces neurite retraction possibly through a cell surface receptor in PC12 cells. Biochem Biophys Res Commun 240: 329-334. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Zhao C, Penderis J, Franklin RJ (2002) The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J Neurosci 22: 2451-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solly SK, Thomas JL, Monge M, Demerens C, Lubetzki C, Gardinier MV, Matthieu JM, Zalc B (1996) Myelin/oligodendrocyte glycoprotein (MOG) expression is associated with myelin deposition. Glia 18: 39-48. [DOI] [PubMed] [Google Scholar]
- Sommer I, Schachner M (1982) Cells that are O4 antigen-positive and O1 antigen-negative. Neurosci Lett 29: 183-188. [DOI] [PubMed] [Google Scholar]
- Spassky N, Goujet-Zalc C, Parmantier E, Olivier C, Martinez S, Ivanova A, Ikenaka K, Macklin W, Cerruti I, Zalc B, Thomas JL (1998) Multiple restricted origin of oligodendrocytes. J Neurosci 18: 8331-8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, De Castro F, Le Bras B, Heydon K, Queraud-LeSaux F, Bloch-Gallego E, Chedotal A, Zalc B, Thomas JL (2002) Directional guidance of oligodendroglial migration by class 3 semaphorins and netrin-1. J Neurosci 22: 5992-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankoff B, Demerens C, Goujet-Zalc C, Monge M, Peyron F, Mikoshiba K, Zalc B, Lubetzki C (1996) Transcription of myelin basic protein promoted by regulatory elements in the proximal 5′ sequence requires myelinogenesis. Mult Scler 2: 125-132. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Aigrot M, Noel F, Wattilliaux A, Zalc B, Lubetzki C (2002a) Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci 22: 9221-9227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankoff B, Barron S, Allard J, Barbin G, Noel F, Aigrot MS, Premont J, Sokoloff P, Zalc B, Lubetzki C (2002b) Oligodendroglial expression of Edg 2 receptor: developmental analysis and pharmacological responses to lysophosphatidic acid. Mol Cell Neurosci 20: 415-428. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Taniguchi M, Yagi T, Akagi Y, Nojyo Y, Tamamaki N (2001) Guidance of glial precursor cell migration by secreted cues in the developing optic nerve. Development 128: 3321-3330. [DOI] [PubMed] [Google Scholar]
- Terai K, Soga T, Takahashi M, Kamohara M, Ohno K, Yatsugi S, Okada M, Yamaguchi T (2003) Edg8/S1P5 receptors are preferentially expressed in oligodendrocyte lineage cells of the rat CNS. Neuroscience 116: 1053-1062. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Miller R (2002) Glial cell migration directed by axon guidance cues. Trends Neurosci 25: 173-175. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S (1998) Dual actions of sphingosine-1-phosphate: extracellular through the Gicoupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol 142: 229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri GS, McMorris FA (1996) Oligodendrocytes and their precursors require phosphatidylinositol 3-kinase signaling for survival. Development 122: 2529-2537. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Chun J (1999) Schwann cell survival mediated by the signaling phospholipid lysophosphatidic acid. Proc Natl Acad Sci USA 96: 5233-5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura T, Konola JT, Wekerle H, Lees MB (1991) Monoclonal antibodies against myelin PLP: identification and characterization of two major determinants. J Neurochem 57: 1671-1680. [DOI] [PubMed] [Google Scholar]
- Yu N, Lariosa-Willingham K, Lin FF, Webb M, Rao TS (2004) Characterization of lysophosphatidic acid and sphingosine-1-phosphate-mediated signal transduction in rat cortical oligodendrocytes. Glia 45: 17-27. [DOI] [PubMed] [Google Scholar]