Abstract
GABAA receptors are a pivotal inhibitory influence in the nervous system, and modulators of the GABAA receptor are important anesthetics, sedatives, anticonvulsants, and anxiolytics. Current views of receptor modulation suggest that many exogenous drugs access and bind to an extracellular receptor domain. Using novel synthetic steroid analogs, we examined the access route for neuroactive steroids, potent GABAA receptor modulators also produced endogenously. Tight-seal recordings, in which direct aqueous drug access to receptor was prevented, demonstrated that steroids can reach the receptor either through plasma membrane lateral diffusion or through intracellular routes. A fluorescent neuroactive steroid accumulated intracellularly, but recordings from excised patches indicated that the intracellular reservoir is not necessary for receptor modulation, although it can apparently equilibrate with the plasma membrane within seconds. A membrane impermeant neuroactive steroid modulated receptor activity only when applied to the inner membrane leaflet, demonstrating that the steroid does not access an extracellular modulatory site. Thus, neuroactive steroids do not require direct aqueous access to the receptor, and membrane accumulation is required for receptor modulation.
Keywords: inhibition, GABA, GABAA receptor, neurosteroid, anesthesia, membrane
Introduction
Certain neuroactive steroids are among the most potent known potentiators of GABAA receptor activity. Neuroactive steroid modulation of GABAA receptors has enjoyed increasing recent attention because endogenous steroids may regulate GABAergic tone in the CNS, neurosteroid dysregulation may underlie neuropsychiatric disorders, and GABA-active steroids may be clinically useful anesthetics, anticonvulsants, and anxiolytics (Zorumski et al., 2000; Belelli and Lambert, 2005). Thus, understanding the mechanisms by which neuroactive steroids access and modulate GABAA receptors is important for understanding endogenous modulation of GABAA receptors and for developing new drugs that target GABAergic transmission.
Current models of drug-receptor interactions tend to view a molecule dissolved in extracellular aqueous medium encountering and interacting with an extracellular binding site on the ligand-gated ion channel. This model accommodates GABA and benzodiazepines, the binding sites of which are well characterized (Amin and Weiss, 1993; Buhr et al., 1996; Wingrove et al., 1997; Wagner and Czajkowski, 2001; Newell and Czajkowski, 2003). In contrast, some anesthetics bind to a hydrophobic pocket formed by membrane-spanning domains (Mihic et al., 1997; Wick et al., 1998). A clear neurosteroid binding site on GABAA receptors has been elusive, and neuroactive steroids are remarkable for their lipophilicity, with an oil-water partition coefficient of >50,000 (logP of 4.89, calculated with Advanced Chemistry Development, version 4.67; Advanced Chemistry Development, Toronto, Ontario, Canada) for the endogenous neurosteroid allopregnanolone (3α5αP). Furthermore, we have recently shown that steroid effects on GABA receptors can be much slower than predicted by simple aqueous diffusion (Shu et al., 2004). Therefore, neuroactive steroids might access receptor sites by typical (but inefficient) aqueous access, through lateral membrane diffusion, or perhaps even through an intracellular site if the steroid is capable of completely permeating the cell membrane. Investigating steroid routes of access to the receptor may be a useful indirect approach to identifying potential receptor binding sites but also needs to be generally considered in drug design. If the plasma membrane interactions are important for access to the receptor site, then steroid membrane mobility and perhaps orientation will be important considerations in the potency and effective lifetime of steroid actions. Likewise, if neuroactive steroids accumulate intracellularly, this may be an important limit on the clearance time of steroids.
We investigated the route of access of neurosteroids to GABAA receptors. Combining experiments on excised and cell-attached membrane patches with whole-cell electrophysiology and imaging of novel, GABA-active fluorescent steroid analogs, we draw several conclusions about steroid access to the GABAA receptor. First, we show that direct, extracellular aqueous access to the receptor is not necessary for potentiation. Second, we show that intracellular accumulation of GABA-active steroid is prominent, but this accumulation is not necessary for actions of the steroid on receptor function. Rather, steroid effects persist in excised patches after access to intracellular steroid is removed. Third, we show that under some conditions, intracellular steroid can resupply the membrane reservoir responsible for GABAA receptor modulation. Intracellular steroid appears to be in relatively rapid equilibrium with membrane steroid, because loss of intracellular steroid temporally tracks a slow component of decay of steroid-mediated current, and wash of cells with membrane-impermeant cyclodextrin speeds the loss of intracellular steroid. Finally, we show that a membrane-impermeant steroid analog effectively potentiates GABAA channel activity when applied to the inner but not outer membrane leaflet, excluding the possibility that aqueous steroid accumulation in the local extracellular environment near the membrane accounts for GABAA receptor effects. In summary, our results show that the plasma membrane is apparently the most direct and relevant access route governing neurosteroid interaction with the GABAA receptor.
Materials and Methods
Cells, transfection, and drugs. Rat GABAA receptor α1, β2, and γ2L subunit cDNAs were sub-cloned into pcDNAIII vector (Invitrogen, San Diego, CA) and transiently expressed in human embryonic kidney 293 (HEK293) cells using a calcium phosphate precipitation-based transfection technique (Akk et al., 2001). Most drugs and chemicals were purchased from Sigma (St. Louis, MO). (3α,5α,17β)-3-Hydroxyandrostane-17-carbonitrile (ACN) was synthesized as reported previously (Hu et al., 1993). 7-Nitrobenz-2-oxa-1,3-diazole (NBD)-3α5αP was prepared by reacting 4-chloro-7-nitrobenzo[1,2,5]oxadiazole with (3α,5α,11β)-11-amino-3-hydroxypregnan-20-one. Alexa-3α5αP was prepared by reacting (3α,5α,17β)-17-aminomethylandrostan-3-ol hydrochloride with Alexa Fluor 546 carboxylic acid, succinimidyl ester. Synthetic and spectroscopic details for NBD-3α5αP and Alexa-3α5αP will be published elsewhere. Steroid stock solutions were prepared at 10 mm in DMSO and diluted in pipette or bath solution on the day of the experiment with final DMSO concentrations typically ≤0.1% (0.5% for Fig. 4 B). For Figure 5, primary cultures of postnatal hippocampal neurons were grown as described previously (Shu et al., 2004). Cells were used 5– 8 d after plating.
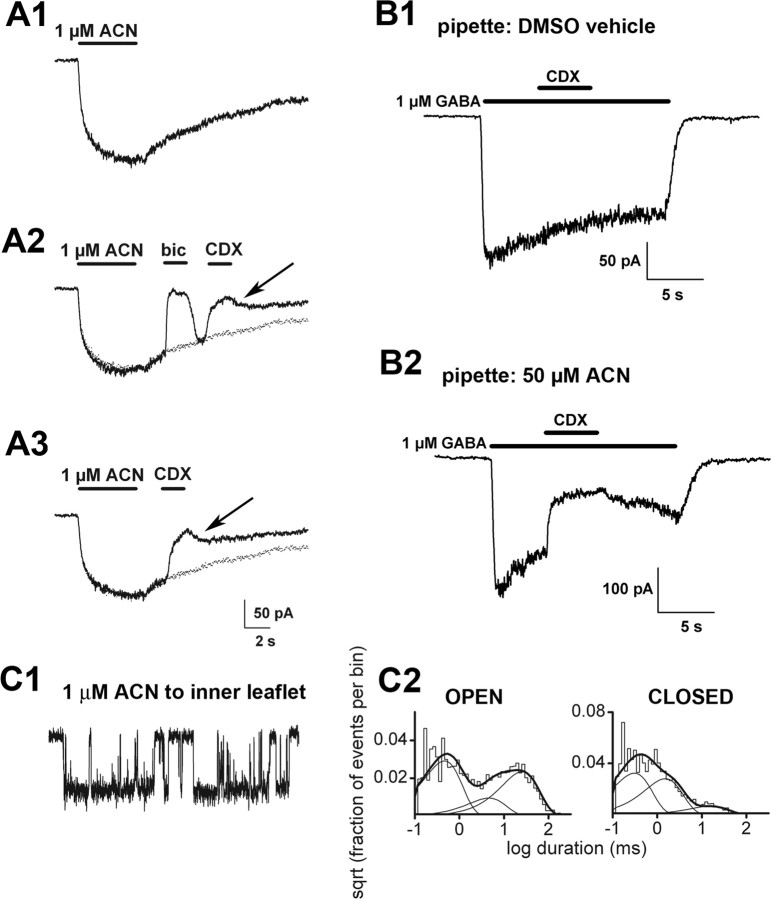
Figure 4.
Intracellular loading can result in potentiation. A, Resurgent steroid effects after cyclodextrin wash. A1, Slow direct gating of GABAA receptors in response to 1 μm ACN in a transfected HEK cell. The cell was rapidly perfused with drug-free saline after ACN removal but nevertheless had a slowly decaying off response, similar to that previously described for neurons and the natural neurosteroid 3α5αP (Shu et al., 2004). A2, In the same cell on another application of ACN, the offset current was reversibly blocked by bicuculline (bic), which is a noncompetitive antagonist with respect to steroid (Ueno et al., 1997). Therefore, in the case of bicuculline, a large part of the resurgence results from bicuculline unbinding before steroid departure. A2, A3, The offset current was partly inhibited by a brief wash with 500 μm γ-cyclodextrin (CDX), which facilitates the removal of steroid (Shu et al., 2004). The resurgent current (arrows) indicates that the replenishment of receptor-accessible steroid, possibly from the intracellular pools, which are inaccessible to cyclodextrin. To facilitate comparison of offset, the dotted trace represents a replot of the control trace in A1, scaled to account for slight rundown between sweeps. Note that the resurgence is less complete than for bicuculline and less than expected from baseline decay (dotted traces). B, Effects of intracellular steroid loading. The currents presented show representative data from a total of four cells under control conditions and five cells loaded with steroid. B1, Response to 1μm GABA in a control transfected HEK cell. Note that the GABA response is insensitive to 500 μm cyclodextrin. B2, In a cell loaded with 50 μm ACN through the whole-cell pipette, 1μm GABA gated a larger response, which was partly sensitive to 500μm γ-cyclodextrin. C1, Channel activity in an inside-out membrane patch (50μm GABA in the pipette), to which 1 μm ACN was applied by bath exchange to the intracellular face. C2, Summary of channel effects. Channel openings are shown downward. The open times were 0.42 ms (48%), 4.2 ms (15%), and 23.3 ms (37%). The closed times were 0.28 ms (48%), 1.4 ms (42%), and 14.5 ms (10%).
Figure 5.
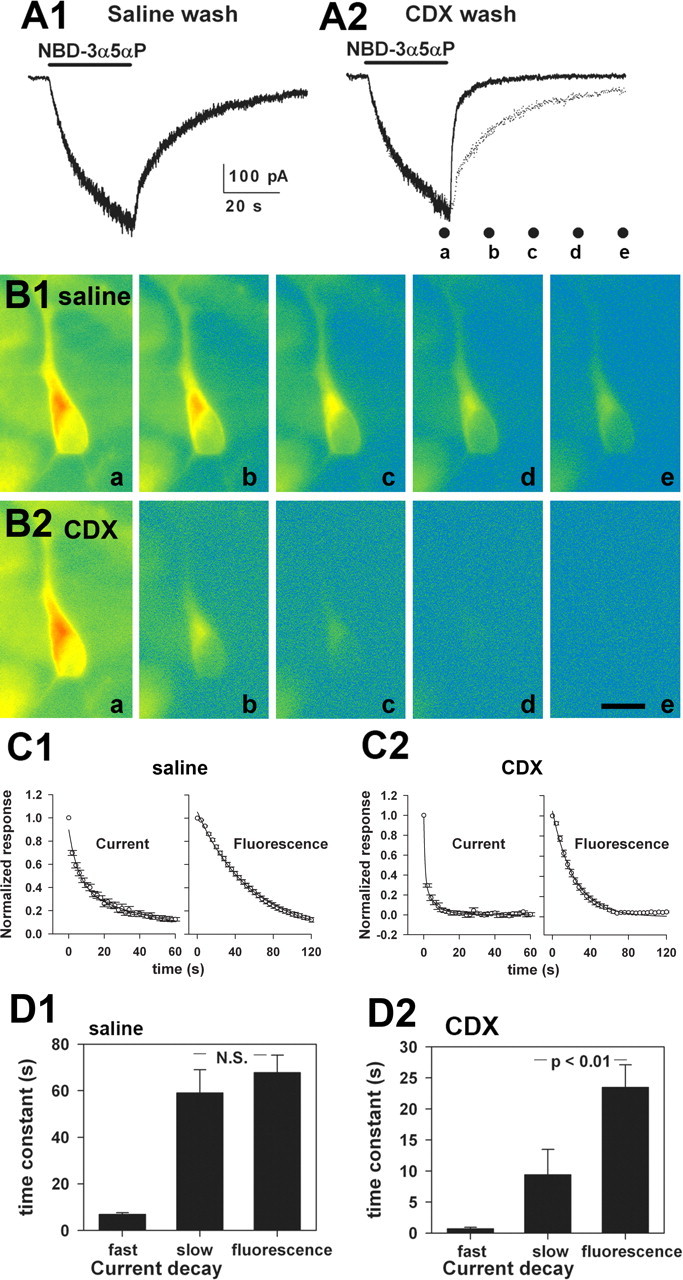
The bulk intracellular steroid pool equilibrates with the receptor-accessible pool in <1 min. A1, Directly gated current in a hippocampal neuron by 3 μm NBD-3α5αP. After 30 s of application, the cell was washed with saline. A2, The same neuron was rechallenged, but the wash included 500 μm γ-cyclodextrin (CDX). The dotted trace is a replot of A1 for comparison. The dots below the trace indicate time points at which images were taken of cells for B. B1, Pseudocolor fluorescence images (warm colors, high-fluorescence intensity; cool colors, low-fluorescence intensity) of a cell subjected to the protocol in A1. Photographs were from the time points indicated by the labeled dots in A2 (a– e). B2, The same cell was subjected to the protocol indicated in A2. Again, the photographs are from the time points indicated by the dots in A2. Scale bar, 20 μm. C1, C2, The data points show raw normalized decays of NBD-3α5αP-gated currents (left graphs; n = 8 saline-washed and 7 CDX-washed neurons) and fluorescence (right graphs; n = 8 saline-washed and 6 CDX-washed neurons). For current traces, only every 100th data point is represented for clarity. The lines represent superimposed biexponential fits (current decays) and single-exponential fits (fluorescence). Parameters are summarized in D. D1, D2, Summary of exponential fits to the decay phase of NBD-3α5αP-generated currents and decay of intracellular fluorescence from the individual cells represented in C. Current decays were well described by a biexponential fit in all cases. The fast component accounted for 57 ± 5% of the decay in saline and 59 ± 5% in γ-cyclodextrin. Fluorescence decays were fit to fluorescence intensities taken from an intracellular region near the nucleus. To diminish bleaching, images were obtained beginning at the end of the 30 s application of NBD-3α5αP, continuing every 4 s for 60–120 s. Fluorescence decays were well described by a single exponential. Both the fast and slow time constants of current decay were significantly speeded by cyclodextrin wash, as was the time constant of fluorescence wash(p<0.01; note the change in the y-axis between D1 and D2). Data show mean ± SEM from seven to eight cells.
Electrophysiology. The single-channel currents were recorded using the patch-clamp technique in the cell-attached and inside-out configurations (Hamill et al., 1981). The bath solution contained the following (in mm): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH 7.4. The pipette solution in single-channel recordings contained the following (in mm): 120 NaCl, 5 KCl, 10 MgCl2, 0.1 CaCl2, 20 tetraethylammonium chloride, 5 4-aminopyridine, 10 glucose, and 10 HEPES, pH 7.4. The pipette potential was held at +60 to +80 mV, for a total potential across the patch membrane of approximately –100 mV (Vrest, approximately –40 to –20 mV). The channel activity was recorded with an Axopatch 200B amplifier, low-pass filtered at 10 kHz, acquired with a Digidata 1200 series interface at 50 kHz using pClamp software (Molecular Devices, Union City, CA), and stored on a personal computer hard drive. For whole-cell recordings, we used a pipette solution containing the following (in mm): 140 KCl, 4 NaCl, 5 EGTA, 0.5 CaCl2, and 10 HEPES, pH 7.25. Drugs were delivered with a multibarrel, gravity-driven local perfusion system (whole cells), or by bath exchange (patch experiments).
Kinetic analysis. The kinetic analysis has been described previously (Akk et al., 2001, 2004; Steinbach and Akk, 2001). In short, analysis was performed on clusters of openings occurring within a critical closed-time interval of 500 ms. Typical cluster duration is 2–3 s, with many seconds usually separating one cluster from the next (Akk et al., 2001). Clusters isolated from the recording were low-pass filtered at 2–3 kHz and idealized using the segmented-k-means algorithm (QuB Suite, www.qub.buffalo.edu). The intracluster open and closed times were estimated using maximum likelihood methods, which incorporate a correction for missed events (Qin et al., 1996, 1997).
Imaging. Images were obtained with a Nikon C1 confocal laser-scanning attachment to a TE300 inverted microscope (Fig. 2) or with conventional epifluorescence illumination and a SPOT RT digital camera (Diagnostic Instruments, Sterling Heights, MI) (Figs. 5, 6). For confocal data, images represent z-series projections excited from the 488 and 543 nm laser lines.
Figure 2.
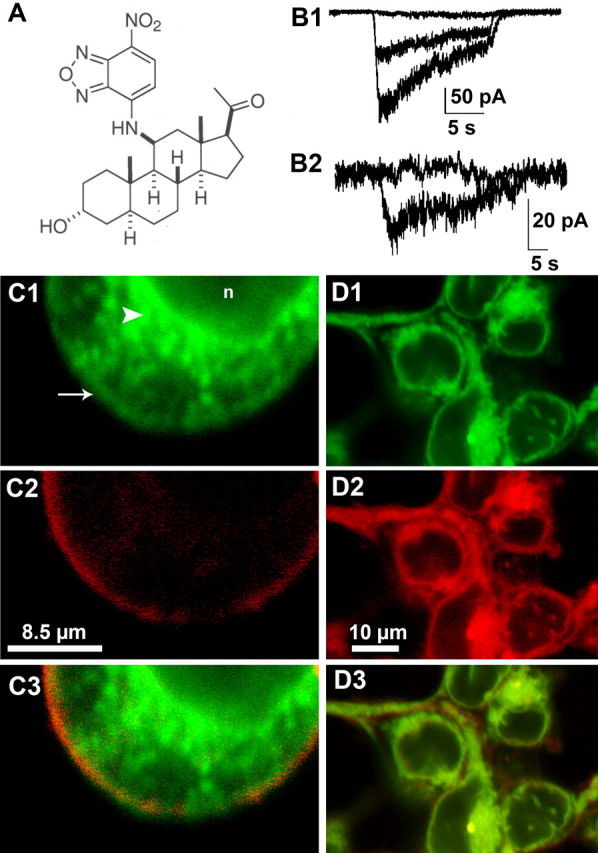
A fluorescent GABA-active stero id suggests plasma membrane and intracellular steroid accumulation. A, Structure of NBD-3α5αP. B1, Potentiation of whole-cell GABA responses in HEK cells. The superimposed traces represent the response of a transfected HEK cell to 2μm GABA alone (middle trace), GABA plus 3 μm NBD-3α5αP (largest trace), and GABA, NBD-3α5αP, and 50 μm bicuculline(flat trace). B2, Direct response of another transfected HEK cell to 3μm NBD-3α5αPalone and coapplied with 50μm bicuculline (flat trace). C, NBD-3α5αP (green fluorescence, C1, C3) colocalization with DiI (red fluorescence, C2, C3). Note some colocalization with plasma membrane (arrow) but the prominent intracellular staining (arrowhead) that is excluded from the nucleus(n). C3 is a merged image of C1 and C2. D, NBD-3α5αP (green, D1,D3) colocalization with Nile Red (red, D2, D3), a cell-permeant lipophilic marker. D3 is a merged image of D1 and D2.
Figure 6.
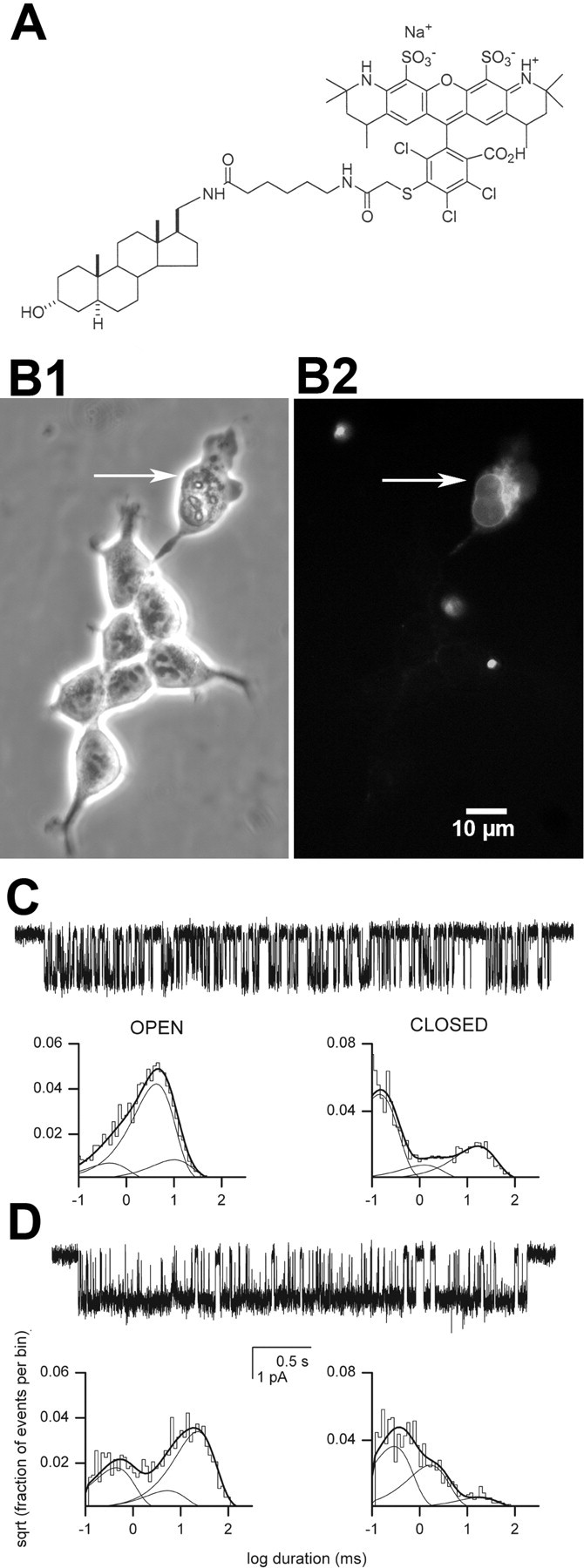
Effects of a membrane impermeant steroid. A, Structure of Alexa-3α5αP. B, Phase-contrast (left) and fluorescence (right) images of cells incubated in Alexa-3α5αP for 10 min. Incubation times >60 min failed to label healthy cells. The arrow indicates a cell with a membrane that was purposely ruptured with a sharp patch pipette before Alexa-3α5αP application. Note that only this cell and not intact cells stain with the Alexa-conjugated steroid. Several other pieces of cellular debris also fluorescently stained.C, Cell-attached patch data with GABA (50 μm) plus Alexa-3α5αP(1 μm) in the recording pipette. Channel openings are shown downward. The open times were 0.42 ms (12%), 3.9 ms (73%), and 9.3 ms (15%). The closed times were 0.13 ms (65%), 1.2 ms (10%), and 15.4 ms(24%). D, Inside-outpatch with GABA(50 μm) in the pipette and Alexa-3α5αP(1 μm) applied to the inner membrane face. The open times were 0.41 ms (31%), 4.9 ms (13%), and 20.8 ms (56%). The closed times were 0.27 ms (54%), 1.5 ms (37%), and 16.7 ms (9%). Alexa-3α5αP potentiated receptor function when applied to the intracellular but not the extracellular side of the membrane.
Results
We used cell-attached patch recording from HEK cells transfected with rat α1β2γ2 GABAA receptor subunits to test whether steroid requires direct aqueous access to receptors to modulate channel activity. Figure 1A shows channel activity in a typical patch elicited by 50 μm GABA in the cell-attached pipette. When 1 μm ACN, the neuroactive steroid (Hu et al., 1993), was included with 50 μm GABA (Fig. 1B), channel activity was potentiated in a characteristic manner, as reported previously (Akk et al., 2004). At least three effects can be distinguished. Mean open time and the fractional contribution of the longest duration openings increased, and the contribution of the longest closed time decreased. Previous studies (Akk et al., 2004) have indicated that at least two sites are likely involved in mediating these effects; the “A” site underlies the changes in the relative contributions of the long open and closed time components, whereas the “B” site underlies the increase in duration of the longest open time component. An abbreviated summary of effects on channel kinetics for all single-channel analyses performed in our studies can be found in Table 1. Complete analysis, including channel parameters unaffected by steroids, can be found in supplemental Table 1 (available at www.jneurosci.org as supplemental material).
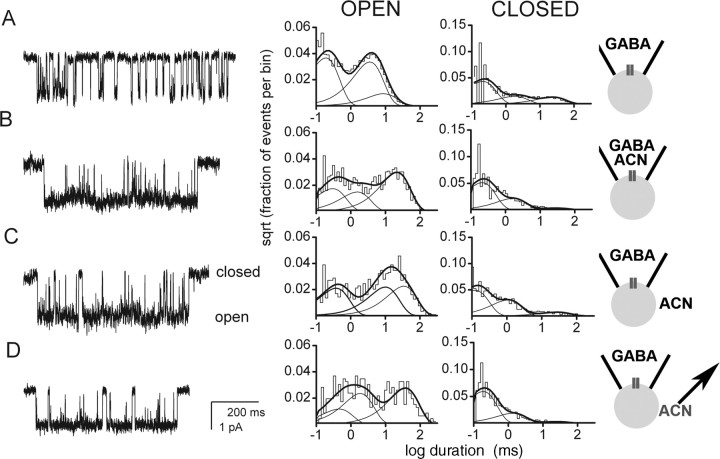
Figure 1.
Steroid access from extracellular solution is not necessary for robust GABAA receptor modulation. Cell-attached recordings from transfected HEK cells were performed under the conditions schematized to the right of the panels. Channel openings are shown as downward deflections. The graphs represent log-binned open and closed time histograms from the patch as indicated. A, Channel activity in a patch exposed to 50μm GABA in the recording pipette. The open times were 0.16 ms (49%), 3.1 ms (37%), and 7.8 ms (14%). The closed times were 0.19 ms (61%), 1.7 ms (20%), and 21.7 ms (18%). B, Channel activity in a patch exposed to the combination of GABA and 1 μm ACN in the patch pipette. The open times were 0.27 ms (29%), 1.4 ms (24%), and 19.6 ms (47%). The closed times were 0.19 ms (68%), 1.5 ms (28%), and 25.6 ms (5%). Note the increased duration and amplitude of the third component of the open time distribution and the decreased relative weight of the third component of the closed time distribution, as described previously (Akk et al., 2004). C, Potentiation of receptor function was qualitatively similar when the steroid was applied not via the patch pipette but rather to the bath solution after seal formation. The open times were 0.34 ms (33%), 9.2 ms (34%), and 21.3 ms (33%). The closed times were 0.11 ms (57%), 1.0 ms (35%), and 25.0 ms (8%). D, Channel activity from a cell pretreated with ACN. After removal of bath ACN and exposure of the channels to GABA in the pipette, potentiation of channel activity remained. The open times were 0.42 ms (19%), 1.7 ms (38%), and 34.5 ms (43%). The closed times were 0.17 ms (73%), 1.2 ms (23%), and 22.7 ms (4%).
Table 1.
Potentiating actions of steroids on GABAA receptors
|
Configuration |
Preincubation |
Pipette |
Bath |
OT3 |
Fraction OT3 |
Fraction CT3 |
n |
|---|---|---|---|---|---|---|---|
| Cell-attached | 7.6 ± 3.0 | 0.19 ± 0.13 | 0.29 ± 0.02 | 8 | |||
| Inside-out | 5.9 ± 1.2 | 0.22 ± 0.13 | 0.21 ± 0.10 | 4 | |||
| Cell-attached | 1 μm ACN | 19.8 ± 3.4* | 0.42 ± 0.08* | 0.06 ± 0.03*** | 3 | ||
| Cell-attached | 1 μm ACN | 32.3 ± 7.9*** | 0.35 ± 0.12 (NS, 0.11) | 0.06 ± 0.02*** | 4 | ||
| Cell-attached | 1 μm ACN | 24.0 ± 7.7*** | 0.46 ± 0.08*** | 0.08 ± 0.03*** | 7 | ||
| Inside-out | 1 μm ACN | 15.5 ± 1.9** | 0.37 ± 0.10 (NS, 0.15) | 0.12 ± 0.02* | 5 | ||
| Inside-out | 1 μm ACN | 5 mm CDX | 6.5 ± 1.5 (NS: 1.0) | 0.18 ± 0.09 (NS, 1.0) | 0.28 ± 0.05 (NS, 0.24) | 4 | |
| Inside-out | 1 μm ACN | 19.4 ± 6.1** | 0.29 ± 0.14 (NS, 0.79) | 0.10 ± 0.01* | 4 | ||
| 1 μm | |||||||
| Cell-attached | Alexa-3α5αP | 8.5 ± 2.9 (NS, 1.0) | 0.16 ± 0.13 (NS, 1.0) | 0.23 ± 0.03* | 7 | ||
| Inside-out | 1 μm Alexa-3α5αP | 15.2 ± 5.7* | 0.49 ± 0.07** | 0.09 ± 0.01** | 4 | ||
| 10 μm | |||||||
| Cell-attached |
|
NBD-3α5αP |
|
21.3 ± 8.4** |
0.38 ± 0.11* |
0.13 ± 0.07*** |
5 |
In each case, the pipette solution contained 50 μm GABA in addition to the indicated steroid. The open and closed duration histograms each showed three exponential components when activity was elicited by 50 μm GABA, as described previously (Steinbach and Akk, 2001). Previous studies have indicated that potentiating steroids have their predominant effects on the mean duration of the long-duration open-time component (OT3), the fraction of openings in that component (fraction OT3), and on the fraction of closed periods in the long-duration component (fraction CT3) (Akk et al., 2004). The fraction of openings in the long duration component is the most variable experimental parameter, which we believe is the reason why ACN, in some conditions, increases the fraction OT3 but not significantly (e.g., lines 4,6, and 8). The columns list, respectively, the configuration of the recording, the presence or absence of preincubation of intact cells, the drugs dissolved in the pipette solution (to which the extracellular surface of the receptors is exposed), and the drugs dissolved in the solution in the bath outside the pipette during the recording are shown. The next columns list the mean (±1 SD) of the mean OT3, fraction OT3, fraction CT3, and the numbers of recordings in given condition. Statistical analysis was performed using ANOVA with pair-wise comparison to control group with two-tailed Dunnet's correction (Systat 7.0; SSPS, Chicago, IL). *p < 0.05, **p < 0.01, and ***p < 0.001. NS, Nonsignificant; CDX, methyl-β-cyclodextrin. Two control groups were used; for comparison with cell-attached records, the data obtained with 50 μm GABA for cell-attached records (line 1) was used, whereas analogous data for excised patches (line 2) was used as control for data from excised patches.
The potentiation of GABAA channel activity was equally robust when we exposed the cell-attached patch directly only to GABA and applied ACN to the bath solution after seal formation (Fig. 1C). As a control, we compared channel activity in patches directly exposed to 1 μm GABA (which corresponds to <EC2) in the cell-attached patch pipette with activity in the same patches after adding 1 mm GABA (saturating concentration) to the surrounding bath solution. We found no effect of the increased GABA in the bath on channel open or closed times (n = 3 patches), indicating our glass/membrane seal effectively excluded direct access of small molecules to the patch. Overall, the result from Figure 1C indicates that steroid does not require direct aqueous access to the receptor to effectively potentiate channel activity.
If steroid in aqueous solution is not directly important for modulating receptor activity, then removal of free steroid from the extracellular solution may not terminate drug action. To test this idea, we pretreated cells with ACN for 5 min before exchanging total bath solution three to four times with drug-free saline. We found that even after extensive washing, potentiation of GABAA channel activity continued (Fig. 1D) for as long as 30 min after wash. These results suggest that steroid can modulate the receptor without being present in the extracellular bath, most likely because of accumulation in the plasma membrane, or possibly even inside the cell.
We also tested the effect of preincubation (followed by wash) ofa5β-reduced steroid (B285) (Akk et al., 2004) and an inactive steroid (3β5αP). As expected from previous single-channel (Akk et al., 2004) and whole-cell (Wang et al., 2002) experiments, preincubation with a 5β-reduced steroid resulted in prolonged potentiation, whereas preincubation with 3β5αP was ineffective at potentiating the receptor function (see supplemental material, available at www.jneurosci.org). We then examined whether steroid applied by preincubation exhibits concentration dependence. We preincubated cells expressing GABAA receptors in 10 nm ACN for 8 –10 min, followed by five full-bath exchanges. We found no evidence of potentiation when cells were subsequently patched with GABA in the pipette solution (supplemental material, available at www.jneurosci.org). These results are consistent with the lack of effect of 10 nm ACN on single-channel currents when this low concentration of ACN is coapplied with GABA in the pipette (Akk and Steinbach, 2003).
Although canonical steroid signaling involves activation of intracellular receptors, GABA-active steroids do not typically activate intracellular receptors (Belelli and Lambert, 2005). Therefore, whether extracellularly applied GABA-active steroids permeate the plasma membrane and accumulate intracellularly is currently unknown. We synthesized a novel steroid with an NBD fluorescent tag at carbon 11 of the endogenous steroid 3α5αP (Fig. 2A). NBD-3α5αP retained activity at GABAA receptors (Fig. 2B). NBD-3α5αP potentiated bicuculline-sensitive GABA-gated currents in transfected HEK cells (Fig. 2B1) and neurons (data not shown) and directly gated bicuculline-sensitive currents (Figs. 2B2, 5A), although the fluorescent analog was ∼30-fold less potent than the parent compound 3α5αP (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Aside from potency, the effects of NBD-3α5αP on channel kinetics were practically indistinguishable from other 5α-reduced steroid potentiators such as 3α5αP (supplemental Fig. 1, available at www.jneurosci.org as supplemental material) or ACN (Akk et al., 2004).
In imaging studies, we found that 3 μm NBD-3α5αP (incubated for at least 10 min) partially colocalized with DiI, a plasma-membrane marker (Fig. 2C1–C3), but NBD-3α5αP also colocalized prominently with Nile Red, a membrane-permeant lipophilic dye (Greenspan et al., 1985) (Fig. 2D1–D3). These results indicate that GABA-active steroids likely accumulate both in the plasma membrane and intracellularly. Thus, the data from imaging studies are consistent with conclusions from cell-attached patch recordings (Fig. 1) and again suggest that either lateral membrane diffusion or intracellular access to the receptor may be important for steroid modulation of GABAA receptors.
We examined the rate of NBD-3α5αP accumulation with confocal microscopy. Accumulation at 3 μm NBD-3α5αP plateaued by 60 s of local application, and we could detect no difference in the time constant of accumulation in perimembrane regions versus intracellular compartments (τ = 20.1 ± 1.6 s for perimembrane accumulation vs 28.6 ± 4.5 s for intracellular accumulation at 3 μm drug; n = 4), consistent with the idea that steroid passes readily through the cell membrane into intracellular compartments (Fig. 5). These rates are similar to the slow rate of development of 3α5αP-gated currents in hippocampal cells, particularly when low concentrations of steroids are applied (Shu et al., 2004) (Fig. 5).
To determine whether intracellular pools of steroid are important for effects on GABAA receptors, we excised inside-out membrane patches from HEK cells, which had been preincubated with bath ACN, followed by subsequent ACN removal. We found that potentiation of channel activity long outlived patch excision into a steroid-free bath solution (Fig. 3A1), although there was a period of rapid decrease in potentiation within the first minute of excision (Fig. 3A1). Mean open time of patches pre-exposed to ACN subsequently slowly fell, whereas patches exposed continuously to either GABA alone (Fig. 3B) or to the combination of GABA and ACN (data not shown) retained fairly stable activity up to 13 min after patch excision. Therefore, neither extracellular water-solubilized steroid nor cytoplasmic steroid appears necessary for potentiation of channel activity. Rather, steroid retained by the plasma membrane is sufficient to potentiate channel activity. In support of this, the channel open time profile of steroid pretreated patches returned to that of GABA alone after a brief treatment with cyclodextrin to facilitate steroid removal (Fig. 3A2) (Shu et al., 2004). Although second messenger effects could mediate long-lived steroid effects, we have previously excluded a role for pertussis-toxin-sensitive G-proteins in slow steroid effects (Shu et al., 2004). Furthermore, second messenger effects are unlikely to persist in excised membrane patches.
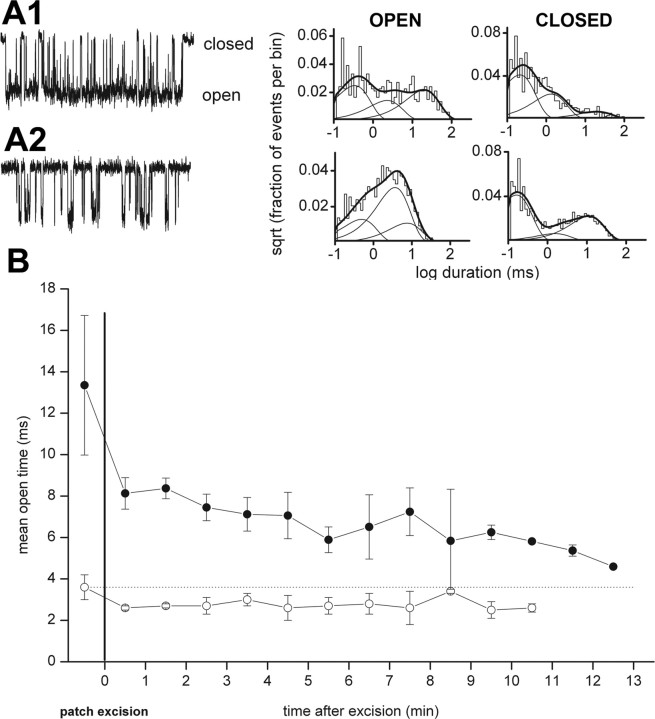
Figure 3.
Patch excision after steroid preincubation and removal does not affect potentiation, suggesting that membrane-accumulated steroid is sufficient for receptor potentiation. A, Channel activity in an inside-out patch from a cell to which ACN was preapplied and then removed before seal formation. The receptors were exposed to 50 μm GABA in the patch pipette. A1, Channel record from 1 min after excision. Histograms pertain to data from 0–2 min after excision. The open times were 0.31 ms (42%), 2.1 ms (23%), and 18.5 ms (35%). The closed times were 0.20 ms (58%), 1.2 ms (32%), and 18.5 ms (9%). A2, The addition of 5 mm methyl-β-cyclodextrin to the bath to remove steroid resulted in an immediate loss of steroid-mediated potentiation. The open times were 0.46 ms (24%), 3.4 ms (57%), and 6.9 ms (19%). The closed times were 0.15 ms (60%), 1.5 ms (9%), and 10.4 ms (31%). B, Time course of the effect of patch excision on channel activity from cells pretreated with ACN(filled circles) or in the presence of GABA alone (open circles). Mean open time was averaged over 1 min blocks. Note the prolonged potentiation of channel activity after ACN pre-exposure and removal. Data show mean ± SD from three to six patches.
Although intracellularly accumulated steroid is not directly necessary for receptor potentiation by steroid, we investigated whether intracellular pools of steroid might be indirectly important as steroid reservoirs that could supply the plasma membrane and rate limit of the decay of steroid actions at GABAA receptors. Indirect evidence for this idea comes from experiments in which we used 500 μm γ-cyclodextrin as a molecular sponge to remove steroid effects from extracellularly accessible reservoirs (Shu et al., 2004). We found that when cyclodextrin was included in the wash after exposure of transfected HEK cells to 1 μm ACN alone to directly gate GABA channels, currents were rapidly reduced by 500 μm cyclodextrin (Fig. 4A2,A3), similar to previous results in native cells (Shu et al., 2004). If the wash with cyclodextrin was sufficiently brief, we observed a small resurgent current (Fig. 4A2,A3 arrows) (n = 5 cells), indicating replenishing reservoirs inaccessible to the brief cyclodextrin wash that might represent intracellular pools. As a positive control for speed and completeness of solution exchange, we examined the effect of bicuculline (Fig. 4A2). Resurgence was also observed with bicuculline application during the decay of steroid effects, but the resurgence was stronger, reflecting the rapid kinetics of bicuculline unbinding and removal before steroid departure (Ueno et al., 1997).
The idea that such resurgent current reflects intracellular steroid accessing GABAA receptors would seem to contradict previous results suggesting that steroid is ineffective when loaded through the patch pipette (Lambert et al., 1990). However, efficiency of delivery by this method could not be verified previously. In fact, we found that NBD-3α5αP only weakly loaded HEK cells when included at concentrations up to 10 μm in the whole-cell patch pipette (supplemental Fig. 2, available at www.jneurosci.org as supplemental material), compared with the bright intracellular fluorescence observed in cells when NBD-3α5αP was extracellularly applied by the same whole-cell pipette (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). To compensate for poor loading, we directly loaded a high concentration of ACN (50 μm) by whole-cell pipette. GABA application to ACN-loaded cells exhibited a cyclodextrin-sensitive current not present in control vehicle-loaded cells (Fig. 4B). In six cells loaded with ACN, γ-cyclodextrin produced a 63 ± 4% depression of current compared with 22 ± 3% (n = 4; attributable to desensitization) in control cells (p < 0.001), suggesting that intracellular steroid, when continuously supplied through a whole-cell pipette, can access GABAA receptors on the cell surface, where it becomes rapidly accessible to extracellularly applied cyclodextrin. Similar results were also obtained by loading the 5β-reduced steroid B285. In four cells loaded with 50 μm B285, cyclodextrin reduced currents by 49 ± 5% compared with 13 ± 7% in three control cells. Note that although the experiments in Figure 4 do not address whether direct gating of receptors (Fig. 4A) and potentiation (Fig. 4B) occur through the same sites, the results indicate that both effects occur through a cyclodextrin-accessible compartment and that both sites can be replenished by steroid from the intracellular (cyclodextrin-inaccessible) compartment.
To test more directly the idea that intracellularly applied steroid is capable of modulating GABAA receptors, we challenged the intracellular face of inside-out excised patches with 1 μm ACN while exposing the extracellular face to 50 μm GABA in the pipette solution. Figure 4C shows that channel activity was potentiated in a manner indistinguishable from the effects of extracellularly applied ACN (Table 1) (supplemental Table 1, available at www.jneurosci.org as supplemental material).
Our data suggest that intracellular steroid can equilibrate with the plasma membrane pool (Fig. 4). We next addressed whether the loss of intracellular fluorescence tracked the time course of current decay after steroid application, which would imply the intracellular steroid pool is in rapid equilibrium with the membrane, receptor-relevant steroid pool. For direct comparison with previously published results (Shu et al., 2004), we used primary cultures of dissociated hippocampal neurons (Fig. 5) and examined currents directly gated by fluorescent NBD-3α5αP. Kinetics of onset were slow, similar to the natural steroid 3α5αP (Shu et al., 2004). Current decays were described by a biexponential fit (Fig. 5A–D). We also measured intracellular fluorescence loss during washout of 3 μm NBD-3α5αP (Fig. 5B–D). After NBD-3α5αP application, the slow time constant of current decline tracked the loss of fluorescence closely (Fig. 5D), implying that the intracellular pool equilibrates with the plasma membrane pool sufficiently rapidly to rate limit current decay. We could detect no difference in the time constant of dye loss near the membrane versus intracellularly (data not shown), further indicating that the two pools are in relatively rapid equilibrium. Next, we used γ-cyclodextrin to speed the rate of removal of the membrane pool. In this case, we found that intracellular fluorescence was lost more quickly (Fig. 5B2,C2) than with saline wash alone (Fig. 5B1,C1). Because cyclodextrin is membrane impermeant, this is strong evidence that the intracellular and plasma membrane pools are normally in sufficiently rapid equilibrium that loss of the membrane pool to cyclodextrin speeds overall intracellular loss. However, in this case, current decay was faster than dye loss (Fig. 5D), implying that the equilibration with the membrane pool of the bulk intracellular pool is slower than τ = 9s, the slow time constant of current decay with cyclodextrin wash.
The rate constant of dye loss (Fig. 5) and current decays (Figs. 4, 5) appeared faster than loss of steroid in preincubation experiments (Fig. 1D), in which steroid effects persisted for many minutes. At least part of this difference may be a result of differences in experimental protocol. Cells were actively washed with saline or cyclodextrin for experiments in Figures 4 and 5, whereas patch experiments were performed in static bath (Fig. 1D). To determine whether this experimental difference helps explain the difference in longevity of effects, we examined dye loss in cells incubated in static bath versus active saline perfusion (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). The results show that active perfusion speeds intracellular steroid loss and is consistent with the idea of constant equilibration between membrane and cytoplasmic compartments of steroid. Together, results of Figures 3, 4, 5 and supplemental Figure 3 (available at www.jneurosci.org as supplemental material) suggest a picture in which steroids prefer to remain in the plasma membrane compartment, or shuttle between plasma membrane and intracellular hydrophobic domains, unless steroid is actively removed from the membrane by strong external perfusion with or without cyclodextrin.
The results suggest that plasma-membrane accumulation and lateral membrane diffusion of steroid are most directly important for potentiation of GABA responses but that the intracellular pool may be rate limiting in certain situations. However, none of the results directly determine which side of the plasma membrane is most closely associated with GABAA receptor modulation. Rapid cyclodextrin accessibility (Figs. 4, 5) (Shu et al., 2004) may suggest that steroid embedded in the outer membrane leaflet is most critical. However, Figure 3A2 shows that cyclodextrins can also rapidly remove steroid when applied to the inner leaflet. Steroids may rapidly flip-flop across the plasma-membrane leaflets (Hamilton, 2003). In this case, removal of steroid by cyclodextrin from one leaflet may simply alter the equilibrium between outer-leaflet steroid and inner-leaflet steroid, thus rapidly reducing the concentration in both leaflets. To better localize the relevant membrane routes of steroid access to the receptor, we synthesized an Alexa Fluor 546-conjugated steroid (Fig. 6A), which is expected to be membrane impermeant (Revankar et al., 2005). We found that Alexa-3α5αP failed to stain live cells when extracellularly applied (Fig. 6B) (n = 3). In contrast, when the plasma membrane of cells was compromised by mechanical rupture, we detected bright intracellular fluorescence (Fig. 6B). These results confirm the expectation that Alexa-3α5αP is membrane impermeant. In excised membrane patches, we found that application of Alexa-3α5αP to the inner membrane leaflet potentiated channel activity by modifying the same characteristic kinetic parameters as other 5α-reduced steroids (Fig. 6D, Table 1) (supplemental Table 1, available at www.jneurosci.org as supplemental material). In contrast, steroid exposure of the outer leaflet of the patch membrane in cell-attached recordings (Fig. 6C, Table 1) or the addition of Alexa-3α5αP to the bath solution in whole-cell recordings (data not shown) failed to potentiate GABA responses. These results suggest that the inner membrane leaflet may be particularly important for steroid access to the GABAA receptor. Because Alexa-3α5αP is membrane impermeant, these results also indicate that accumulation of steroid in an aqueous extracellular unstirred layer (Barry and Diamond, 1984) does not play an important role in GABAA receptor modulation and strongly suggest that one or more relevant potentiating sites are readily accessible from the inner leaflet.
Discussion
Our main result is that steroids can reach the site(s) involved in potentiation of the GABAA receptor function by diffusion through the plasma membrane rather than by direct binding to a site on the receptor from the external medium. Second, we show that intracellular accumulation of GABA-active steroids is prominent and that some of these intracellular pools may resupply the plasma membrane with steroid capable of modulating the GABAA receptor. Finally, we show that a membrane-impermeant steroid is active when applied to the inner face of the membrane but not the outer membrane, suggesting that steroids may more easily access at least one site on the GABAA receptor from the inner plasma-membrane leaflet.
Our results have implications for the effects of natural and exogenous steroids for nervous system function. After relatively brief steroid exposure, accumulated steroid in the plasma membrane and/or intracellular compartments could continue to modulate receptors for long after the initial exposure and limit access of the steroids to enzymes eventually responsible for terminating steroid actions (Belelli and Herd, 2003). It is worth noting that endogenous conditions may be more akin to “static bath” conditions like those in our patch experiments (Figs. 1, 3). We show that active perfusion of cells speeds up steroid loss (supplemental Fig. 3, available at www.jneurosci.org as supplemental material). Presumably, effective steroid lifetime in the brain is much longer than the ∼60 s observed in Figure 5, reflecting the strong preference of steroids to remain in the lipophilic plasma membrane and intracellular compartments. Furthermore, because neurosteroids are synthesized by neurons (Mellon et al., 2001), strong membrane and intracellular retention may suggest an important autocrine action of neurosteroids.
Our results highlight slow effects of steroid modulation of GABAA receptors. Our previous results suggest that direct gating is particularly slow (Shu et al., 2004), and the present results indicate that membrane and intracellular interactions can affect the time course of steroid actions. Although fast effects of steroids on GABAA receptors have been observed in previous experiments, it seems likely that full, steady-state effects of low steroid concentrations may not be apparent for tens of seconds, much slower than a diffusion-limited reaction. It seems likely that both slow access and slow equilibration of specific receptor states contribute to the time course of the effects. In addition, our results also suggest that the different lipid and/or sterol content of membranes may influence the kinetics of steroid actions and that onset and offset of steroid effects may therefore differ by cell type. Finally, the precise experimental protocol used may dictate the kinetics of steroid effects (supplemental Fig. 3, available at www.jneurosci.org as supplemental material).
Previous studies have suggested that intracellular application of neuroactive steroids is ineffective at modulating GABAA receptors (Lambert et al., 1990). This result may be partly responsible for the common view that modulators bind to extracellular receptor domains directly from aqueous solution, although results from other ion channels have also suggested the importance of the membrane in the access route for modulators of voltage-gated and mechanosensitive channels (Hille, 1977; Lee and Mac-Kinnon, 2004; Suchyna et al., 2004).
We found that membrane-permeable steroid applied to the inner face of excised patches is just as effective as extracellularly applied steroid (Fig. 4C). Furthermore, with high concentrations of steroid in the whole-cell pipette, intracellularly applied steroid also effectively potentiates whole-cell responses (Fig. 4B). The difference in effectiveness of intracellular steroid on excised patches versus whole cells can be accounted for primarily by poor loading through the point source of a whole-cell patch pipette (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). Although we do not completely understand the basis for this poor loading, this likely explains the failure of previous experiments to detect an effect of steroid (up to 3 μm) loaded through a whole-cell patch pipette on GABA receptors (Lambert et al., 1990).
We used an Alexa Fluor-conjugated neuroactive steroid to test whether extracellular access to the receptor is sufficient to promote potentiation. The charge on the Alexa Fluor molecule prevents membrane permeability (Revankar et al., 2005). Perhaps surprisingly, we found that impermeant steroid applied to the inner face of the membrane was effective in potentiating GABAA channel activity (n = 4) (Fig. 6C, Table 1), whereas Alexa-3α5αP applied to the outer leaflet in cell-attached patches (n = 7) (Fig. 6D, Table 1) or in whole-cell recordings (data not shown) was ineffective. This could suggest that the relevant site on the GABAA receptor is near the inner membrane leaflet. However, there are several caveats to this conclusion. First, there are likely to be several sites on the GABAA receptor (Akk et al., 2004), and differential accessibility of these sites from the outer versus inner leaflet is possible. We also do not know how deeply the steroid embeds in the membrane or the orientation of the molecule when embedded. The failure of cells to fluoresce when Alexa-3α5αP was applied to the outside of intact cells suggests that the Alexa molecule may interact only superficially with the outer leaflet. It is possible that the relevant receptor site is near the extracellular side of the bilayer but that Alexa-3α5αP permeates in the correct orientation only when applied to the inner leaflet. Future biophysical studies are needed to test these alternatives.
In summary, we show that plasma membrane access is directly relevant to GABAA receptor activity. This may help lead to pinpointing sites on the receptor at which steroids act. Perhaps more importantly, our results demonstrate that independent of steroid interactions with the receptor binding site, properties of steroid-membrane interactions (orientation, depth of embedding, rate of flip-flop) and degree of intracellular retention will influence the potency and lifetime of steroid actions at the GABAA receptor.
Footnotes
This work was supported by National Institutes of Health Grants GM47969 (J.H.S., C.F.Z., D.F.C.), AA14707 (G.A.), and AA12952 (S.M.) and by grants from the McDonnell Foundation (S.M.) and the Bantly Foundation (C.F.Z.). J.H.S. is the Russell and Mary Shelden Professor of Anesthesiology. We thank Bei-Wen Ma for help with tissue culture and Ann Benz for help with screening of the novel steroids.
Correspondence should be addressed to Gustav Akk, Department of Anesthesiology, Washington University in St. Louis, Campus Box 8054, 660 South Euclid Avenue, St. Louis, MO 63110. E-mail: akk@morpheus.wustl.edu.
DOI:10.1523/JNEUROSCI.4173-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/2511605-09$15.00/0
G.A. and H.-J.S. contributed equally to this work.
References
- Akk G, Steinbach JH (2003) Low doses of ethanol and a neuroactive steroid positively interact to modulate rat GABAA receptor function. J Physiol (Lond) 546: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes J, Steinbach JH (2001) Pregnenolone sulfate block of GABAA receptors: mechanism and involvement of a residue in the M2 region of the α subunit. J Physiol (Lond) 532: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G, Bracamontes JR, Covey DF, Evers A, Dao T, Steinbach JH (2004) Neuroactive steroids have multiple actions to potentiate GABAA receptors. J Physiol (Lond) 558: 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J, Weiss DS (1993) GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature 366: 565–569. [DOI] [PubMed] [Google Scholar]
- Barry PH, Diamond JM (1984) Effects of unstirred layers on membrane phenomena. Physiol Rev 64: 763–872. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB (2003) The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci 23: 10013–10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ (2005) Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci 6: 565–575. [DOI] [PubMed] [Google Scholar]
- Buhr A, Baur R, Malherbe P, Sigel E (1996) Point mutations of the α1β2γ2 γ-aminobutyric acid(A) receptor affecting modulation of the channel by ligands of the benzodiazepine binding site. Mol Pharmacol 49: 1080–1084. [PubMed] [Google Scholar]
- Greenspan P, Mayer EP, Fowler SD (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100: 965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: 85–100. [DOI] [PubMed] [Google Scholar]
- Hamilton JA (2003) Fast flip-flop of cholesterol and fatty acids in membranes: implications for membrane transport proteins. Curr Opin Lipidol 14: 263–271. [DOI] [PubMed] [Google Scholar]
- Hille B (1977) Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol 69: 497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zorumski CF, Covey DF (1993) Neurosteroid analogues: structure-activity studies of benz[e]indene modulators of GABAA receptor function. 1. The effect of 6-methyl substitution on the electrophysiological activity of 7-substituted benz[e]indene-3-carbonitriles. J Med Chem 36: 3956–3967. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Peters JA, Sturgess NC, Hales TG (1990) Steroid modulation of the GABAA receptor complex: electrophysiological studies. Ciba Found Symp 153: 56–71; discussion 71–82. [DOI] [PubMed] [Google Scholar]
- Lee SY, MacKinnon R (2004) A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430: 232–235. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA (2001) Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev 37: 3–12. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL (1997) Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature 389: 385–389. [DOI] [PubMed] [Google Scholar]
- Newell JG, Czajkowski C (2003) The GABAA receptor α1 subunit Pro174-Asp191 segment is involved in GABA binding and channel gating. J Biol Chem 278: 13166–13172. [DOI] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F (1996) Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J 70: 264–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F (1997) Maximum likelihood estimation of aggregated Markov processes. Proc Biol Sci 264: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307: 1625–1630. [DOI] [PubMed] [Google Scholar]
- Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S (2004) Slow actions of neuroactive steroids at GABAA receptors. J Neurosci 24: 6667–6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach JH, Akk G (2001) Modulation of GABAA receptor channel gating by pentobarbital. J Physiol (Lond) 537: 715–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchyna TM, Tape SE, Koeppe Jr RE, Andersen OS, Sachs F, Gottlieb PA (2004) Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature 430: 235–240. [DOI] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH (1997) Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci 17: 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DA, Czajkowski C (2001) Structure and dynamics of the GABA binding pocket: a narrowing cleft that constricts during activation. J Neurosci 21: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, He Y, Eisenman LN, Fields C, Zeng CM, Mathews J, Benz A, Fu T, Zorumski E, Steinbach JH, Covey DF, Zorumski CF, Mennerick S (2002) 3β-Hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J Neurosci 22: 3366–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick MJ, Mihic SJ, Ueno S, Mascia MP, Trudell JR, Brozowski SJ, Ye Q, Harrison NL, Harris RA (1998) Mutations of γ-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor? Proc Natl Acad Sci USA 95: 6504–6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove PB, Thompson SA, Wafford KA, Whiting PJ (1997) Key amino acids in the γ subunit of the γ-aminobutyric acidA receptor that determine ligand binding and modulation at the benzodiazepine site. Mol Pharmacol 52: 874–881. [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Isenberg KE, Covey DF (2000) Potential clinical uses of neuroactive steroids. Curr Opin Investigational Drugs 1: 360–369. [PubMed] [Google Scholar]