Abstract
Tonic GABAA receptor-mediated inhibition is typically generated byδ subunit-containing extrasynaptic receptors. Because the δ subunit is highly expressed in the thalamus, we tested whether thalamocortical (TC) neurons of the dorsal lateral geniculate nucleus (dLGN) and ventrobasal complex exhibit tonic inhibition. Focal application of gabazine (GBZ) (50 μm) revealed the presence of a 20 pA tonic current in 75 and 63% of TC neurons from both nuclei, respectively. No tonic current was observed in GABAergic neurons of the nucleus reticularis thalami (NRT). Bath application of 1μm GABA increased tonic current amplitude to ∼70 pA in 100% of TC neurons, but it was still not observed in NRT neurons. In dLGN TC neurons, the tonic current was sensitive to low concentrations of the δ subunit-specific receptor agonists allotetrahydrodeoxycorticosterone (100 nm) and 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP) (100 nm) but insensitive to the benzodiazepine flurazepam (5 μm). Bath application of low concentrations of GBZ (25–200 nm) preferentially blocked the tonic current, whereas phasic synaptic inhibition was primarily maintained. Under intracellular current-clamp conditions, the preferential block of the tonic current with GBZ led to a small depolarization and increase in input resistance. Using extracellular single-unit recordings, block of the tonic current caused the cessation of low-threshold burst firing and promoted tonic firing. Enhancement of the tonic current by THIP hyperpolarized TC neurons and promoted burst firing. Thus, tonic current in TC neurons generates an inhibitory tone. Its modulation contributes to the shift between different firing modes, promotes the transition between different behavioral states, and predisposes to absence seizures.
Keywords: absence epilepsy, δ-subunit, extrasynaptic receptor, thalamic reticular nucleus, thalamocortical, THIP
Introduction
GABAA receptors are pentameric ligand-gated ion channels comprising diverse subunits (α1–α6, β1–β3, γ1–γ3, δ, θ, ϵ, and π). Receptors typically comprise two α and two β subunits together with the γ2 subunit (Whiting et al., 2000). Subunit composition determines not only receptor properties and function (Korpi et al., 2002; Sieghart and Sperk, 2002) but also distribution within the cellular membrane. Receptors containing the γ2 subunit are preferentially located in the synapse (Somogyi et al., 1996), and the γ2 subunit is essential for synaptic receptor clustering (Essrich et al., 1998). After activation by presynaptically released GABA, these receptors generate “phasic” IPSCs. In some receptors, the γ2 subunit can be substituted by the δ subunit. The presence of the δ subunit results in receptor expression in the extrasynaptic or perisynaptic membrane (Nusser et al., 1998; Wei et al., 2003). Activation of these receptors is mediated by ambient extracellular GABA, probably generated by spillover from the synapse, and leads to the generation of a “tonically” active current (Mody, 2001; Semyanov et al., 2004; Farrant and Nusser, 2005).
Tonic GABAA currents have been observed primarily in cerebellar (CGCs) and dentate gyrus (DGGCs) granule cells (Kaneda et al., 1995; Brickley et al., 1996; Wall and Usowicz, 1997; Nusser and Mody, 2002; Stell and Mody, 2002). In CGCs, the δ subunit is intimately associated with the α6 subunit (Jones et al., 1997; Nusser et al., 1999), whereas in DGGCs it probably associates with the α4 subunit (Sur et al., 1999). In both cell types, the presence of a tonic current can be observed after application of GABAA antagonists. In CGCs, it has been suggested that activation of δ subunit-containing receptors mediates >95% of the total GABAA receptor-mediated inhibition, increasing coding sparseness and enhancing the information storage capacity of the cerebellum (Hamann et al., 2002; Chadderton et al., 2004).
The δ and α4 subunits are expressed in many rat brain areas, including certain nuclei of the thalamus such as the dorsal lateral geniculate nucleus (dLGN) and ventrobasal (VB) complex (Persohn et al., 1992; Wisden et al., 1992; Fritschy and Mohler, 1995; Pirker et al., 2000). Furthermore, extrasynaptic receptors have been observed in cat thalamocortical (TC) neurons of the dLGN (Soltesz et al., 1990). Experiments in δ subunit knock-out mice revealed only minor contribution of this subunit in synaptic inhibition, but tonic inhibition was not examined (Porcello et al., 2003). Therefore, we have examined whether TC neurons in the dLGN and VB exhibit tonic GABAA inhibition. We also tested whether GABAergic neurons of the nucleus reticularis thalami (NRT), which do not express α4 and δ subunits (Wisden et al., 1992; Pirker et al., 2000), exhibit tonic inhibition. Determining the presence of tonic inhibition in thalamic neurons is of importance because GABAA receptors are an integral component of physiological (Steriade et al., 1993; Blitz and Regehr, 2005) and pathophysiological (Slaght et al., 2002; Steriade, 2005) thalamic mechanisms.
Materials and Methods
Slice preparation and whole-cell patch-clamp recordings. Young (postnatal days 14–21) male and female Wistar rats were anesthetized with isoflurane and decapitated in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 and associated procedures. As described previously (Le Feuvre et al., 1997), the brains were rapidly removed, and 300-μm-thick coronal (dLGN) or horizontal (VB and NRT) slices were cut in continuously oxygenated (95% O2/5% CO2) ice-cold artificial CSF (aCSF) containing the following (in mm): 126 NaCl, 26 NaHCO3, 2.5 KCl, 2 MgCl2, 1.25 NaH2PO4, 2 CaCl2, 10 glucose, 0.045 indomethacin, and 3 kynurenic acid. Slices (three to four per hemisphere) were stored in an oxygenated incubation chamber containing aCSF of the above composition, but without indomethacin or kynurenic acid, for at least 1 h before being transferred to the recording chamber. There they were continuously perfused (∼1.5 ml/min) with warmed (32 ± 1°C) oxygenated recording aCSF of above composition, except that the concentration of MgCl2 was decreased to 1 mm and indomethacin was removed. Kynurenic acid was used in the cutting medium to increase slice viability and in the recording medium to block ionotropic glutamate receptors and therefore isolate GABAA receptor-mediated currents. Experiments were performed on only a single neuron within a given slice, after which the slice was discarded.
TC neurons of the dLGN and VB and neurons of the NRT were visualized using a Nikon (Tokyo, Japan) Eclipse E600FN microscope equipped with a 40× immersion lens and a video camera (Hamamatsu, Hamamatsu City, Japan). Whole-cell patch-clamp recordings were made from neurons held at –70 mV using pipettes (resistance, 2.5–5MΩ) containing the following (in mm): 130 CsCl, 2 MgCl2, 4 Mg-ATP, 0.3 Na-GTP, 10 Na-HEPES, and 0.1 EGTA, pH 7.25–7.30 (osmolality, ∼290 mOsm). In some experiments, the internal pipette solution also contained 0.5% biocytin. With this solution, the reversal potential of Cl– (ECl–) was ∼0 mV; therefore, GABAA receptor-mediated currents appeared inward. Pipettes were connected to the head stage of a Multiclamp 700B preamplifier controlled by Multiclamp Commander software (Axon Instruments, Union City, CA). Series resistance was compensated by ∼80% and was monitored regularly during recordings. Data were discarded if the series resistance increased by >30%. Experimental data were digitized at 20 kHz (Digidata 1322A; Axon Instruments), acquired using pClamp 9.0 software (Axon Instruments), and stored on a personal computer.
Data were filtered at 3 kHz and converted to an ASCII format to be analyzed using LabView-based software (National Instruments, Austin, TX) as described previously (Wisden et al., 2002; Stell et al., 2003; Cope et al., 2004). To determine the presence of a tonic current, the GABAA antagonist 6-imino-3-(4-methoxyphenyl)-1-(6 H)-pyridazinebutanoic acid hydrobromide [gabazine (GBZ)] was focally applied to the slice. If a tonic current is present, application of GBZ should not only block spontaneous IPSCs but result in an outward shift of the baseline current at a holding potential of –70 mV, given that ECl– =∼0 mV (see Fig. 1 A). To determine this, the baseline current was measured for 5 ms epochs sampled every 100 ms, and those points that fell on IPSCs were discarded. The average baseline current value was calculated for two 5 s periods before (I1 and I2) and one after (I3) GBZ application (see Fig. 1 A). The shift in baseline current caused by GBZ application (I3 – I2 =ΔIGBZ) was compared with that observed during control (I2 – I1 =ΔICON) (see Fig. 1 D). A tonic current was presumed to have been present if absolute values for ΔIGBZ were twice the SD of ΔICON. Cells in which ΔIGBZ was less than twice the SD of ΔICON were excluded from additional tonic current analysis, although their IPSCs were analyzed. The amplitude of ΔIGBZ was averaged across all remaining cells for a given experimental condition and compared with ΔICON using Student's paired t test. Significant effects of different drugs on the amplitude of ΔIGBZ across cell populations was determined using Student's unpaired t test. For analysis of IPSCs, populations of individual IPSCs in a cell were averaged as described previously (Stell et al., 2003; Cope et al., 2004). We then measured the peak amplitude, charge transfer (taken as the integral of the average IPSC for each cell), weighted decay time constant (defined as the integral of the average IPSC from the peak divided by peak amplitude), frequency, and the total current (charge transfer × frequency) of the IPSCs and compared these values between control and in the presence of various drugs. Significant difference between experimental conditions was determined using Student's unpaired t test. Data are presented as mean ± SD. Significance was set at p < 0.05 for all statistical tests.
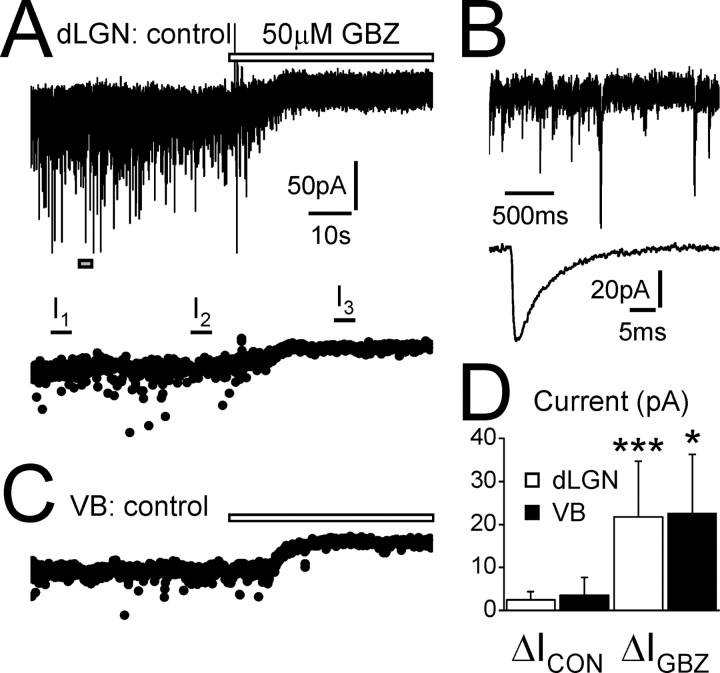
Figure 1.
The tonic GABAA current in TC neurons of the dLGN and VB. A, Recording from a dLGN TC neuron under control conditions (3 mm kynurenic acid). Focal application of the GABAA receptor antagonist GBZ (50μm; white bar) blocks inward IPSCs and reveals an outward shift in baseline current indicative of the presence of a tonic current.Note the presence of a large artifact as the GBZ is added. Below is the same trace consisting of 5 ms epochs sampled every 100 ms. I1, I2, and I3 represent equitemporal 5 s periods in which the baseline current was averaged to determine the drift of the baseline before GBZ application (I2 – I1 =ΔICON) and after GBZ application (I3 – I2 =ΔIGBZ; for additional details, see Materials and Methods). For this neuron, ΔIGBZ = 21.7 pA, and ΔICON = 2.8 pA. B, Expanded recording from the same cell as in A (indicated by the gray box) showing spontaneous IPSCs. Below is the waveform of the average IPSC for this cell. C, Trace from a VB TC neuron showing an outward shift in baseline current (ΔIGBZ = 31.0 pA) caused by focal GBZ application (white bar). D, Graph showing the comparison of ΔICON and ΔIGBZ for TC neurons of the dLGN (white columns) and VB (black columns). A significant difference between ΔICON and ΔIGBZ across the population indicates the presence of a tonic current (for additional details, see Materials and Methods). Current and time calibration in A also apply to C. Current calibration in A applies to the top trace in B.*p < 0.05, ***p < 0.001, Student's paired t test.
Intracellular sharp microelectrode and extracellular single-unit recordings. Slices for intracellular sharp microelectrode and single-unit extracellular recordings were obtained as described above, except that the thickness of slices was increased to 400 μm to better preserve network connectivity (Thomson et al., 1996). After incubation, individual slices were transferred to an interface-style recording chamber and perfused with warmed (35 ± 1°C) continuously oxygenated recording aCSF of above composition for intracellular recordings, but without kynurenic acid for extracellular recordings. Intracellular sharp microelectrode recordings were performed with pipettes filled with 1 m potassium acetate (resistance, 80–120 MΩ) attached to an Axoclamp 2A preamplifier (Axon Instruments) operating in bridge mode. For extracellular single-unit recordings, pipettes were filled with 0.5 m NaCl (resistance, 1–5 MΩ) connected to a Neurolog 104 differential amplifier (Digitimer, Welwyn Garden City, UK), and activity was bandpass filtered at 0.1–20 kHz. Data from intracellular and single-unit recordings were digitized, stored on a personal computer, and analyzed using pClamp software. Input resistance during intracellular recordings was calculated before and after drug application from small hyperpolarizing current pulses. Data are expressed as mean ± SEM.
Application and sources of drugs. During whole-cell patch-clamp recordings, the high concentration of GBZ (50 μm) used to test for the presence of a tonic current was focally applied to the slice using a pipette. All other drugs for patch-clamp, intracellular sharp electrode, and extracellular single-unit recordings were bath applied in the aCSF at concentrations indicated in the text, including the low concentrations of GBZ. Allotetrahydrodeoxycorticosterone (THDOC) was initially dissolved in DMSO before addition to the aCSF. All drugs were obtained from Sigma (Poole, UK).
Results
A GABAA receptor-mediated tonic current is present in TC neurons
Focal application of GBZ (50 μm) to TC neurons of both the dLGN and VB under control conditions (i.e., in the presence of 3 mm kynurenic acid) not only blocked the synaptically generated phasic IPSCs but, because of ECl– being ∼0 mV, also caused an outward shift in the baseline current and therefore revealed the presence of a tonically active current (Fig. 1 A, C). The amplitude of the tonic current (ΔIGBZ) was similar in cells from both nuclei, being 21.7 ± 12.9 pA in TC neurons of the dLGN (n = 12) and 22.5 ± 13.8 pA in TC neurons of the VB (n = 5) (Fig. 1 D). ΔIGBZ was significantly larger than the drift of baseline current observed during control conditions (ΔICON) in both neuron types (dLGN, 2.5 ± 1.9 pA; VB, 3.5 ± 4.2 pA; both p < 0.05) (Fig. 1 D). However, we only observed a tonic current in 75% of TC neurons in the dLGN and 62.5% of VB TC neurons. In the remaining cells, ΔIGBZ was less than twice the SD of ΔICON (see Materials and Methods). When ΔIGBZ was calculated for all TC neurons recorded under control conditions in the dLGN and VB, a tonic current was still present but of reduced amplitude (17.6 ± 13.4 pA, n = 16; and 16.4 ± 13.7 pA, n = 8, respectively).
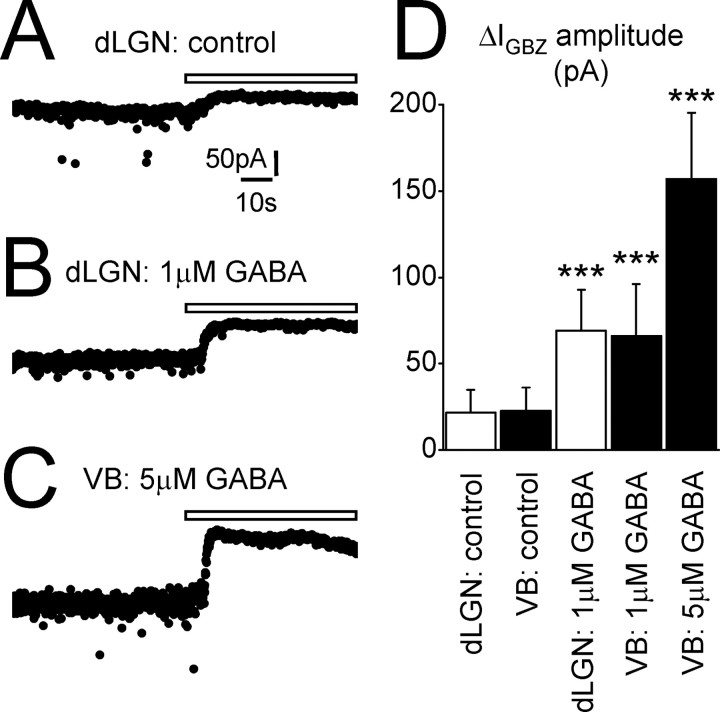
There are several possibilities why a tonic current was not observed in every neuron: (1) loss or internalization of the extrasynaptic receptors underlying the current during the slicing and/or incubation procedures; (2) differences in expression of extrasynaptic receptors between TC neuron types; (3) the dLGN also contains a population of interneurons that we may have inadvertently recorded from; (4) in some cells, GBZ might have blocked synaptic but not extrasynaptic receptors (Bai et al., 2001; Semyanov et al., 2003); or (5) in some instances, the ambient extracellular GABA concentration is too low to activate the extrasynaptic receptors. We attempted to determine which hypothesis was correct by increasing the extracellular concentration of GABA (Fig. 2). In the presence of 1 μm GABA, ΔIGBZ significantly increased to 69.1 ± 23.6 p A in dLGN TC neurons (n = 8) (Fig. 2 A, B,D) and 66.1 ± 30.0 pA in VB TC neurons (n = 10; both p < 0.05 compared with control) (Fig. 2 D). However, in both neuron types, ΔICON was similar to control conditions (dLGN, 5.8 ± 5.6 pA; VB, 5.3 ± 5.6 pA; p > 0.05). In addition, the tonic current was observed in all neurons tested in the presence of 1 μm GABA (n = 8 of 8 and 10 of 10 for the dLGN and VB, respectively). Furthermore, increasing the concentration to 5 μm (n = 4) further increased ΔIGBZ in VB TC neurons (156.9 ± 38.5 pA; p < 0.05) (Fig. 2C,D) without altering ΔICON (8.5 ± 10.2 pA; p > 0.05). Increasing the ambient GABA concentration preferentially enhanced the tonic current, because 1 μm GABA essentially did not affect the IPSC properties in either the dLGN (Table 1) or the VB (data not shown). For a few experiments in the dLGN, we also included 0.5% biocytin in the recording pipette. After development with horseradish peroxidase (Hughes et al., 2002a) and examination using a light microscope, we were able to confirm that we had only recorded from TC neurons (data not shown; n = 6 cells). Furthermore, in the dLGN, we specifically recorded from neurons with a relatively large soma so as to reduce the probability of recording from local interneurons. We cannot exclude, however, the possibility that interneurons themselves exhibit a tonic current. In conclusion, we suggest that we do not see a tonic current in all TC neurons under our control conditions because, in some instances, the ambient concentration of GABA is too low to activate the extrasynaptic receptors.
Figure 2.
Bath application of GABA enhances the tonic GABAA current. A, Trace recorded from a dLGN TC neuron under control conditions showing a small outward shift (ΔIGBZ = 35.4 pA) in baseline current after focal GBZ application (white bar). B, In a different TC neuron recorded in the presence of bath-applied GABA (1 μm), GBZ application causes a larger shift in baseline current (ΔIGBZ = 81.5 pA). C, In a VB TC neuron recorded in the presence of 5 μm extracellular GABA, GBZ application causes a massive shift in baseline current (ΔIGBZ = 159.0 pA). D, Graph showing the comparison of ΔIGBZ amplitude under control conditions and in the presence of 1 μm GABA in dLGN TC neurons (white columns), and under control conditions, in the presence of 1μm GABA, and in the presence of 5μm GABA in VBTC neurons (black columns). Calibration in A also applies to B and C. ***p < 0.001 compared with control, Student's unpaired t test.
Table 1.
Comparison of spontaneous IPSC properties in dLGN TC neurons under various experimental conditions
|
|
IPSC parameter |
|
|
|
|
|---|---|---|---|---|---|
| Experimental condition |
Peak amplitude (pA) |
Weighted decay time constant (ms) |
Frequency (Hz) |
Charge transfer (fC) |
Total current (pA) |
| Control (n = 21) | -54.8 ± 13.0 | 5.8 ± 1.4 | 6.7 ± 3.9 | -350.4 ± 142.1 | -2.4 ± 1.9 |
| 1 μm GABA (n = 9) | -61.0 ± 14.2 | 4.4 ± 1.0* | 4.4 ± 2.4 | -285.4 ± 46.5 | -1.2 ± 0.6 |
| 5 μm flurazepam (n = 10) | -61.3 ± 12.0 | 8.1 ± 1.9*** | 6.9 ± 1.6 | -541.1 ± 158.0** | -3.9 ± 1.8* |
| 10 nm THDOC (n = 12) | -50.5 ± 13.0 | 4.9 ± 0.9 | 7.0 ± 8.0 | -265.5 ± 91.3 | -1.9 ± 2.4 |
| 100 nm THDOC (n = 12) | -53.3 ± 14.8 | 7.7 ± 2.0* | 5.2 ± 2.1 | -456.0 ± 187.0 | -2.4 ± 1.5 |
| 100 nm THIP (n = 10) | -51.7 ± 12.9 | 4.9 ± 0.6 | 6.1 ± 2.7 | -267.4 ± 74.9 | -1.8 ± 1.1 |
| 200 nm GBZ (n = 7) | -35.8 ± 8.8** | 6.3 ± 2.1 | 1.4 ± 1.2** | -262.9 ± 130.3 | -0.4 ± 0.3** |
| 100 nm GBZ (n = 12) | -34.7 ± 5.0*** | 5.7 ± 1.3 | 1.7 ± 0.6*** | -236.8 ± 66.9* | -0.4 ± 0.2** |
| 50 nm GBZ (n = 18) | -43.4 ± 10.5** | 6.4 ± 1.3 | 3.3 ± 1.7** | -317.2 ± 123.5 | -1.1 ± 0.9* |
| 25 nm GBZ (n = 14) | -43.6 ± 7.1** | 5.6 ± 1.3 | 5.2 ± 2.7 | -273.1 ± 80.65 | -1.4 ± 0.9 |
| 50 nm GBZ + 1 μm GABA (n = 7) | -35.1 ± 6.5*** | 4.5 ± 1.0* | 3.3 ± 1.6* | -176.3 ± 55.6** | -0.6 ± 0.5* |
| 25 nm GBZ + 1 μm GABA (n = 6) |
-42.7 ± 5.3* |
3.9 ± 0.6** |
4.0 ± 1.9 |
-184. ± 126.0** |
-0.7 ± 0.2* |
Number of recorded neurons for each condition are in parentheses. Data are expressed as mean ± SD. Values significantly different from control are as indicated (*p < 0.05, **p < 0.01, ***p < 0.001, Student's unpaired t test).
We estimated what proportion of total GABAA receptor-mediated inhibition was carried by phasic and tonic currents. In the 12 dLGN TC neurons in which we observed a tonic current under control conditions, we found that IPSCs contributed only 7.7 ± 4.2% (1.5 ± 0.7 of 23.3 ± 13.2 pA) of total GABAA receptor-mediated current, with the tonic component contributing the remaining 92.3 ± 4.2% (21.7 ± 12.9 of 23.3 ± 13.2 pA). Similarly, in the five VB TC neurons in which a tonic current was observed under control conditions, 6.1 ± 5.4% (1.0 ± 0.6 of 23.5 ± 13.4 pA) of total current was mediated by IPSCs and 93.9 ± 5.4% (22.5 ± 13.8 of 23.5 ± 13.4 pA) by tonic current. Thus, the overwhelming majority of GABAA receptor-mediated inhibition in dLGN and VB TC neurons is generated by extrasynaptic receptor activation.
The tonic current in TC neurons is mediated by δ subunit-containing receptors
In both CGCs and DGGCs, tonic inhibition is generated by receptors containing the δ subunit (Brickley et al., 2001; Stell et al., 2003). However, in other cell types, tonic inhibition appears to be mediated by non-δ, probably γ2, subunit-containing receptors (Bai et al., 2001; Semyanov et al., 2003; Stell et al., 2003). The presence of the δ subunit conveys unique pharmacological properties on the receptors containing it, including insensitivity to benzodiazepine (BDZ) site ligands, sensitivity to low concentrations of certain neuroactive steroids (Nusser and Mody, 2002; Stell et al., 2003), and sensitivity to the agonist 4,5,6,7-tetrahydroisoxazolo[5,4-c]-pyridin-3-ol (THIP) (Brown et al., 2002; Maguire et al., 2005). The δ subunit is highly expressed in the dLGN; therefore, we tested whether the tonic current in TC neurons of the dLGN is sensitive to the classical BDZ flurazepam, the neurosteroid THDOC, and THIP. In addition, we simultaneously determined whether spontaneous IPSCs are sensitive to these three compounds. Application of flurazepam (5 μm; n = 6) did not result in any significant change in the size of the tonic current (ΔIGBZ = 23.4 ± 17.0 pA) compared with control (p > 0.05) (Fig. 3A,F). However, we did observe a significant increase in the weighted decay time constant, charge transfer, and total current of IPSCs (Table 1) (p < 0.05). Application of 10 nm
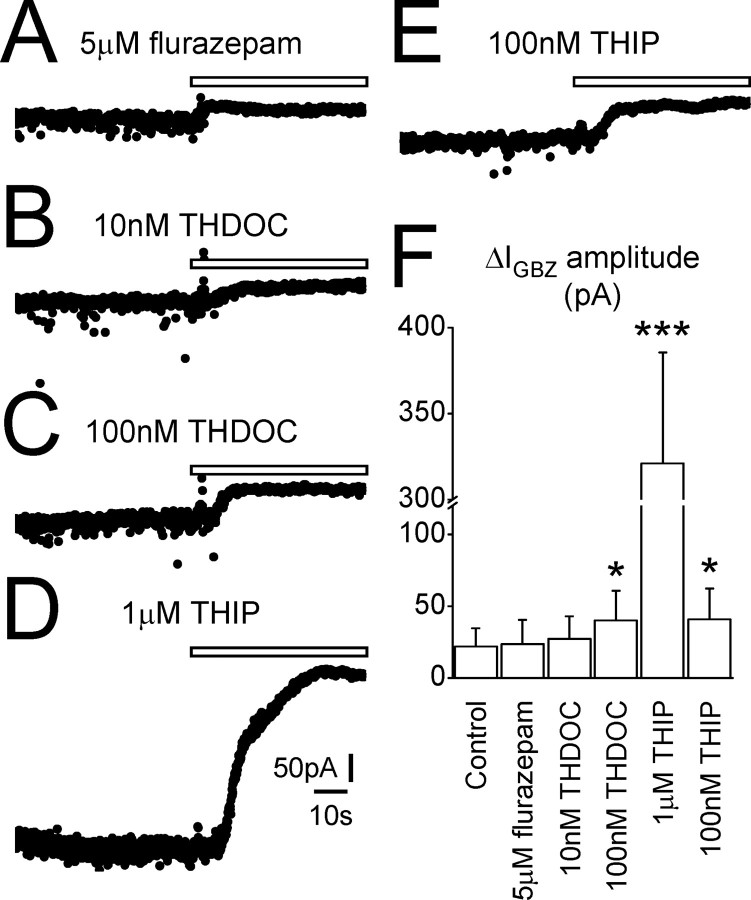
Figure 3.
The tonic GABAA current is BDZ insensitive but neuroactive steroid and THIP sensitive.A–E, Traces recorded from individual dLGNTC neurons in the presence of the classical BDZ agonist flurazepam (5 μm), the neuroactive steroid THDOC (10 and 100 nm), and the agonist THIP (1μm and 100 nm) as indicated. Focal GBZ application (white bar) causes an outward shift in baseline current in each cell (ΔIGBZ values for each neuron: 5μm flurazepam, 23.1 pA; 10 nm THDOC, 24.6 pA; 100 nm THDOC, 54.8 pA; 1 μm THIP, 343.6 pA; 100 nm THIP, 67.8 pA). F, Comparison of ΔIGBZ amplitude in dLGN TC neurons under control conditions and in the presence of 5 μm flurazepam, 10 or 100 nm THDOC, and 1 μm or 100 nm THIP. Note that only in the presence of 100 nm THDOC and the two concentrations of THIP is the tonic current significantly larger than control. Calibration in D also applies to A–C and E.*p < 0.05, ***p < 0.001, Student's unpaired t test.
THDOC (n = 10), a concentration shown previously to enhance tonic inhibition (Stell et al., 2003), did not significantly alter the size of the tonic current compared with control (ΔIGBZ = 27.1 ± 15.6 pA; p > 0.05) (Fig. 3B,F). There was also no effect on the properties of the IPSCs (Table 1). Increasing the concentration of THDOC 10-fold (100 nm; n = 6) did increase the amplitude of the tonic current (ΔIGBZ = 39.8 ± 20.8 pA; p < 0.05) (Fig. 3C,F), without altering the majority of IPSC properties (Table 1), although we did observe an increase in the weighted decay time constant (Stell et al., 2003). Application of 1 μm THIP (n = 5) caused a massive increase in the tonic current compared with control (ΔIGBZ = 321.2 ± 64.2 pA; p < 0.05) (Fig. 3D,F) and increased baseline noise so as to prevent analysis of the IPSCs. Decreasing THIP concentration 10-fold (100 nm; n = 10) also resulted in a larger tonic current compared with control (ΔIGBZ = 40.6 ± 21.8 pA; p < 0.05) (Fig. 3E,F) with no apparent effect on IPSC properties (Table 1). Therefore, although the tonic current appears to show a differential sensitivity to THDOC compared with CGCs and DGGCs, its sensitivity to THIP and insensitivity to flurazepam suggests that receptors mediating the tonic current in TC neurons contain the δ subunit.
The tonic current is not present in NRT neurons
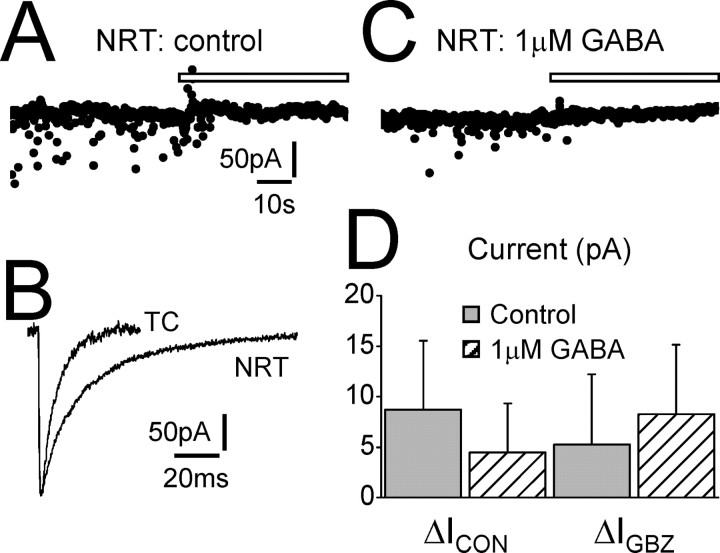
The NRT is one of the few thalamic nuclei that does not express either the δ or α4 subunits (Wisden et al., 1992; Pirker et al., 2000). However, in some neuron types, tonic inhibition appears to be generated by receptors that do not contain the δ subunit (Bai et al., 2001; Semyanov et al., 2003; Stell et al., 2003). Therefore, we tested for the presence of a tonic current in NRT neurons (Fig. 4). We did not observe an outward shift in baseline current after focal application of GBZ in NRT neurons under our control conditions (n = 8), and ΔIGBZ (5.2 ± 7.0 pA) was not significantly different from ΔICON (8.7 ± 6.8 pA; p > 0.05) (Fig. 4A,D). However, we could not exclude the possibility that, as in some TC neurons, we did not observe a tonic current because the ambient GABA concentration was too low. Increasing the ambient concentration by bath applying 1 μm GABA (n = 6) also did not result in the observation of a tonic current (ΔICON = 4.4 ± 4.9 pA; ΔIGBZ: 8.2 ± 6.9 pA; p > 0.05) (Fig. 4C,D). Therefore, NRT neurons do not appear to exhibit a tonic current under our experimental conditions.
Figure 4.
NRT neurons do not exhibit tonic GABAA current. A, Trace recorded from an NRT neuron under control conditions. Focal GBZ application (white bar) does not cause an outward shift in baseline current (ΔIGBZ = 3.4 pA; ΔICON = 8.9 pA). B, The waveform of the average IPSC for the same cell as in A. The average IPSC of a dLGN TC neuron has been scaled for comparison. Note that the IPSC of the NRT neuron has a characteristically longer decay than that of the TC neuron. C, Trace recorded from a different NRT neuron in the presence of extracellular GABA (1 μm). Focal application of GBZ still does not cause an outward shift in baseline current (ΔIGBZ = 9.8 pA; ΔICON = 6.5 pA). D, Graph showing the comparison of ΔICON and ΔIGBZ for NRT neurons under control conditions (gray columns) and in the presence of 1 μm GABA (hatched columns). Under neither condition is a tonic current apparent (Student's paired t test). Calibration in A also applies to C. Calibration in B applies only to the trace from the NRT neuron.
Preferential block of the tonic current by low concentrations of GBZ
A previous report suggested that low concentrations of GBZ selectively blocked phasic over tonic currents in DGGCs because extrasynaptic receptors have a greater affinity for GABA than synaptic ones, whereas the affinity for GBZ is similar for the two receptor types (Stell and Mody, 2002). However, those authors performed these experiments in the presence of a GABA uptake blocker, thereby increasing the extracellular concentration of GABA. Furthermore, in a few TC neurons under various experimental conditions, we observed an outward shift in baseline current but no block of IPSCs after focal GBZ application (Fig. 5A). In addition, the tonic current was typically blocked before the IPSCs (Fig. 5B). Therefore, we repeated experiments in TC neurons of the dLGN in the presence of low concentrations of bath-applied GBZ to determine whether there was a concentration that preferentially blocked tonic over phasic currents. In the presence of 200 nm (n = 7), 100 nm (n = 12), and 50 nm (n = 15) GBZ, there was no apparent shift in the baseline current, and ΔIGBZ values were not significantly different from ΔICON values (all p > 0.05) (Fig. 5C). Values of ΔIGBZ for the three GBZ concentrations were significantly smaller than control (p < 0.05) (Fig. 5E1). Only when the concentration of bath-applied GBZ was further reduced to 25 nm (n = 11) was a tonic current observed (ΔICON, 3.5 ± 1.9 pA; ΔIGBZ, 7.9 ± 5.2 pA; p < 0.05) (Fig. 5C), but it was still significantly smaller than control (p < 0.05) (Fig. 5E1). Comparison of the IPSC properties showed that peak amplitude, frequency, charge transfer, and total current in the presence of 200, 100, and 50 nm GBZ were generally significantly smaller than control (p < 0.05) (Fig. 5E2, Table 1). In the presence of 25 nm GBZ, however, only the peak amplitude was significantly smaller than control (p < 0.05) (Table 1), although there was still a trend for frequency, charge transfer, and total current to be smaller. Thus, under our experimental conditions, low concentrations of bath-applied GBZ preferentially inhibit tonic rather than phasic currents, probably because the GBZ outcompetes the low endogenous concentration of GABA in the neuropil and therefore blocks the extra-synaptic receptors. To test this, we repeated these experiments with low concentrations of bath-applied GBZ in the presence of increased extracellular GABA. A tonic current was indeed apparent after coapplication of 50 nm GBZ and 1 μm GABA (n = 7), and its amplitude was not significantly different from control (ΔIGBZ = 28.4 ± 10.3 pA; p > 0.05) (Fig. 5D,E1). Similarly, in the presence of both 25 nm GBZ and 1 μm GABA (n = 5), a tonic current was present and, in this instance, was significantly larger than control (ΔIGBZ = 42.5 ± 21.7 pA; p < 0.05) (Fig. 5D,E1). Under both experimental conditions, almost all IPSCs parameters were significantly different from control (Fig. 5E2, Table 1), but IPSCs were not abolished. These results confirm previous findings in DGGCs (Stell and Mody, 2002) in which, in the presence of enhanced extracellular GABA, low concentrations of GBZ block phasic rather than tonic currents.
Figure 5.
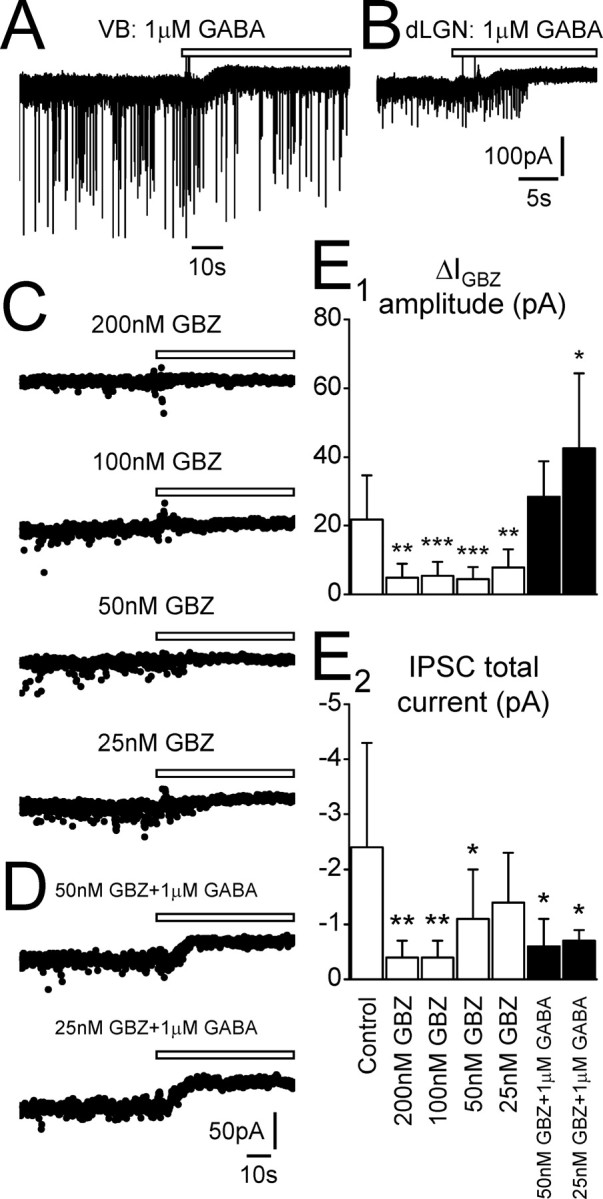
Low concentrations of GBZ preferentially block the tonic rather than phasic current. A, Recording from a VB TC neuron in the presence of 1 μm GABA. Focal application of GBZ (white bar) results in an outward shift in baseline current (ΔIGBZ = 24.9 pA), but IPSCs are not blocked. B, Recording from a dLGN TC neuron in the presence of 1μm GABA. In this neuron, GBZ application caused an outward shift in baseline current (ΔIGBZ = 31.2 pA) before IPSCs are blocked. C, Traces recorded from individual dLGN TC neurons in the presence of bath-applied GBZ (25–200 nm, as indicated). Focal application of GBZ (white bars) does not result in an outward shift in the presence of 200 nm GBZ (ΔIGBZ = 3.9 pA; ΔICON = 1.2 pA), 100 nm GBZ (ΔIGBZ = 6.5 pA; ΔICON = 5.7 pA), or 50 nm GBZ (ΔIGBZ = 0.5 pA; ΔICON = 4.6 pA). An outward shift is only observed when bath-applied GBZ is decreased to 25 nm (ΔIGBZ = 10.7 pA; ΔICON = 2.4 pA). D, Traces recorded from individual dLGNTC neurons in the presence of bath-applied GBZ (50 and 25 nm) and 1 μm GABA. Increasing the extracellular GABA concentration results in an outward shift in baseline current in response to focal GBZ application, even in the presence of bath-applied GBZ (50 nm GBZ plus 1 μm GABA, ΔIGBZ = 27.9 pA; 25 nm GBZ plus 1 μm GABA, ΔIGBZ = 30.5 pA). E1, Comparison of ΔIGBZ amplitude for dLGN TC neurons under control conditions and in the presence of differing concentrations of bath-applied GBZ with and without 1 μm GABA. E2, Comparison of dLGN TC neuron IPSC total current under control conditions and in the presence of differing concentrations of bath-applied GBZ with and without 1 μm GABA. *p < 0.05, **p < 0.01, ***p < 0.001, Student's unpaired t test. Calibration in B applies to A.
Preferential block or enhancement of the tonic current modulates TC neuron firing
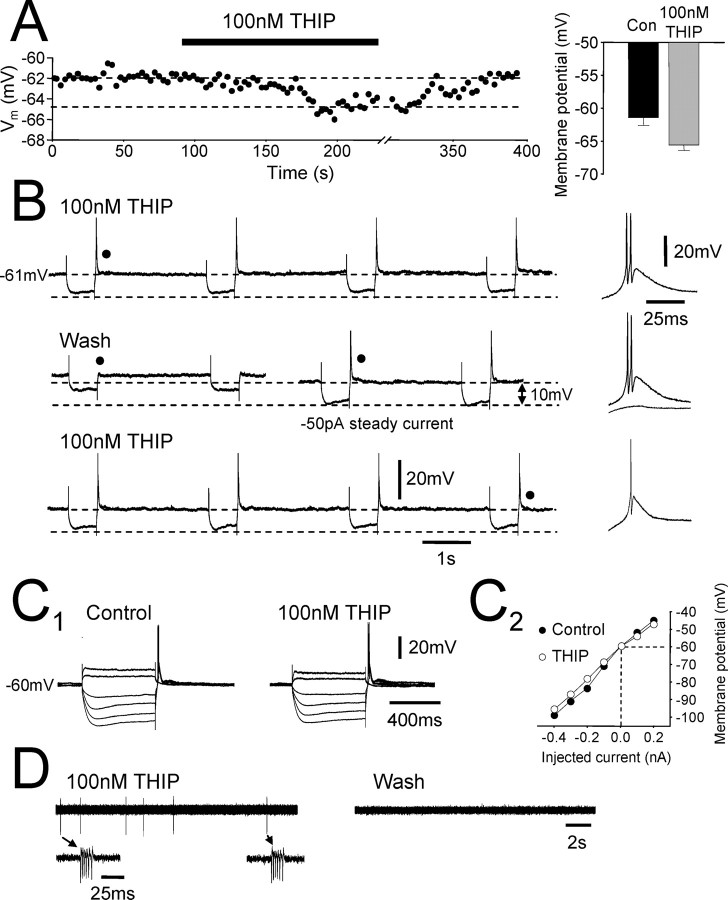
We attempted to determine the effect of the tonic current on the membrane properties of TC neurons by bath applying concentrations of GBZ shown to preferentially block tonic over phasic currents (see above). To do this, we performed intracellular sharp microelectrode current-clamp recordings with microelectrodes containing 1 m potassium acetate; therefore, the intracellular Cl– concentration was unaltered. Under control conditions, TC neurons of the dLGN exhibited tonic firing in response to depolarizing current pulses and low-threshold burst firing during the termination of hyperpolarizing current pulses (Fig. 6B2, C1) (Jahnsen and Llinás, 1984). Application of 25 nm GBZ (n = 7) led to a small but consistent depolarization (1.2 ± 0.2 mV) in all neurons tested (Fig. 6A) and a small increase in input resistance (data not shown). However, this concentration of GBZ only partially blocks the tonic current. Therefore, we repeated these experiments with a concentration of GBZ (50 nm) that blocks the tonic current but does not affect the majority of phasic current. Application of 50 nm GBZ (n = 5) resulted in a depolarization of 2.0 ± 0.8 mV (Fig. 6B1, B3). In one case, steady current injection of –180 pA lead to repetitive low-threshold burst firing under control conditions (Fig. 6B2). After the bath application of 50 nm GBZ, and for the same holding current, this small depolarization caused the burst firing to cease, and the neuron entered a region of quiescent membrane potential. In addition to the small depolarization, bath application of 50 nm GBZ caused a 36.3 ± 8.7% increase in apparent input resistance (Fig. 6C), resulting in an increase in neuronal excitability. In the presence of 50 nm GBZ, therefore, small current pulses that were previously unable to generate action potentials were now able to do so (Fig. 6C1), and the frequency of action potentials in response to larger current inputs increased (Fig. 6D). Therefore, preferential block of the tonic current modulates the firing properties of these neurons.
Figure 6.
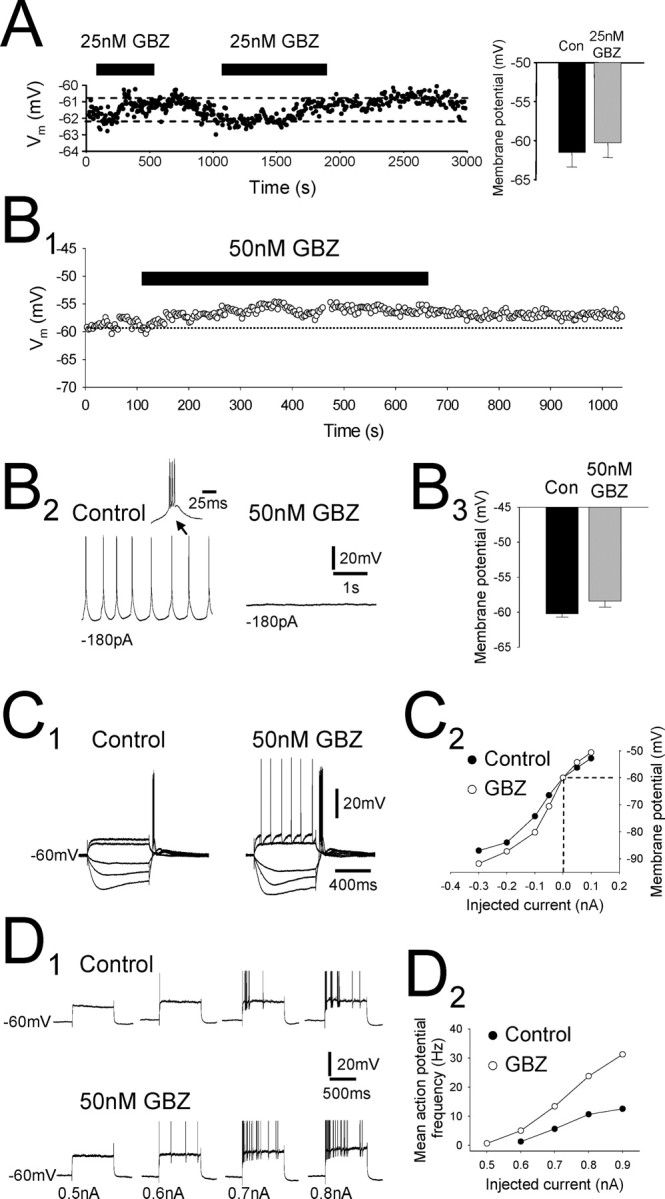
Preferential block of the tonic current modulates the membrane properties and firing of individual TC neurons. A, Intracellular current-clamp recording from a dLGN TC neuron consisting of the membrane potential sampled every 3 s. Injected holding current was constant throughout the experiment. Bath application of 25 nm GBZ (black bars) causes a small (∼1 mV) depolarization of the membrane potential that could be reversed during wash. Bar graph to the right shows membrane potential before (black column) and after (gray column) 25 nm GBZ application for all recorded neurons. B1, In another dLGN TC neuron, application of 50 nm GBZ caused a larger depolarization (∼3 mV) in membrane potential. B2, In the same neuron, low-threshold repetitive burst firing (left) was apparent under control conditions but was blocked after the bath application of 50 nm GBZ (right). Inset is one of the low-threshold bursts. B3, Graph showing the membrane potential of TC neurons before (black column) and after (gray column) 50 nm GBZ application for all recorded neurons. C1, Response of a dLGN TC neuron to depolarizing (100 and 200 pA) and hyperpolarizing (–50, –100, and –200 pA) current steps both before (left) and after (right) bath application of 50 nm GBZ. Note the appearance of action potentials on the largest depolarizing current step after GBZ application. C2, Current–voltage plot for the neuron in C1. Values were taken from the maximum deflection of the membrane potential during the current steps. GBZ application causes an increase in apparent input resistance in response to hyperpolarizing current steps. D1, Response of a dLGN TC neuron to depolarizing current steps of increasing magnitude (top). Bath application of 50 nm GBZ increased the number of action potentials observed in response to almost all of the current steps (bottom). D2, Plot of mean action potential frequency against current step amplitude for the same cell as in D1. GBZ application caused a large increase in the frequency of action potentials, especially for the largest current steps. In D, action potentials have been truncated for clarity.
To determine the effect of blocking the tonic current on the spontaneous firing properties of TC neurons, we performed extracellular single-unit recordings in the dLGN in the absence of kynurenic acid. Under control conditions, most TC neurons fired rarely and irregularly, but, when they did so, firing was predominantly of the low-threshold burst firing type (83.3%; n = 5 of 6 units) (Fig. 7A1,B) (Hughes et al., 2004). In contrast, units recorded in the presence of 50 nm GBZ predominantly exhibited tonic rather than low-threshold burst firing (94.4%; n = 17 of 18 units) (Fig. 7A2,B). In a single unit that exhibited low-threshold burst firing under control conditions, bath application of 50 nm GBZ caused a cessation of firing such that the unit became silent (Fig. 7A1). In five units that exhibited tonic firing in the presence of 50 nm GBZ, removal of GBZ from the aCSF caused a cessation of firing (Fig. 7A2), which in one case was reversed by readdition of 50 nm GBZ. In summary, we observed that, under control conditions, 83.3 and 16.7% of units exhibited low-threshold burst firing or tonic firing, respectively (Fig. 7B). In the presence of 50 nm GBZ, however, only 5.6% of units exhibited low-threshold burst firing and 94.4% tonic firing. Together with the intracellular current-clamp experiments, these data indicate that the block of the tonic current shifts the membrane potential of TC neurons from a region conducive to low-threshold burst firing to one conducive to tonic firing.
Figure 7.

Block of the tonic current promotes tonic over low-threshold burst firing. A1, Extracellular single-unit recording of a dLGN TC neuron under control conditions showing repetitive low-threshold burst firing (top trace) that is blocked by bath application of 50 nm GBZ (bottom trace). Inset is one of the low-threshold bursts. A2, Under control conditions, another dLGN TC neuron unit was silent (top trace). Bath application of 50 nm GBZ instigated tonic firing (middle trace). Wash of GBZ returned the unit to a silent state (bottom trace). B, Graph showing percentage of units exhibiting low-threshold burst firing (black columns) and tonic firing (gray columns) under control conditions and in the presence of 50 nm GBZ.
To determine the effect of enhancing the tonic current on the membrane potential and spontaneous firing properties of TC neurons, we repeated the above intracellular current-clamp and extracellular single-unit experiments in the presence of 100 nm THIP, a concentration that selectively increases the amplitude of the tonic current without affecting IPSCs (see above). Bath application of THIP (n = 5) caused a small hyperpolarization (–4.1 ± 0.6 mV) in all neurons tested (Fig. 8A,B). In one case, removal of THIP from the aCSF resulted in a small depolarization and a shift away from membrane potentials conducive to burst firing (Fig. 8B). In addition to the hyperpolarization, THIP application caused a concomitant decrease in input resistance (12.9 ± 2.5%) (Fig. 8B,C). Single-unit recordings showed that, in the presence of THIP, all recorded units (100%, n = 6 of 6) preferentially exhibited burst firing (Fig. 8D). After the wash of THIP, all units were silenced (Fig. 8D). Thus, enhancing the GABAA receptor-mediated tonic current hyperpolarizes TC neurons and shifts them into a membrane potential region conducive to burst firing.
Figure 8.
Enhancement of the tonic current hyperpolarizes TC neurons and promotes burst firing. A, Intracellular current-clamp recording from a dLGN TC neuron. Bath application of 100 nm THIP (black bar) caused a small hyperpolarization (∼3 mV) that could be reversed during washout. The bar graph to the right shows the membrane potential before (black column) and after (gray column) 100 nm THIP application for all recorded neurons. B, Recording from another dLGN TC neuron showing membrane potential responses to hyperpolarizing current steps (–100 pA) during 100 nm THIP application (top trace), wash (middle traces), and reapplication of THIP (bottom trace). Note how removal of THIP from the aCSF results in a small depolarization and increase in input resistance that is reversed after reapplication of THIP. To the right are the membrane responses at the offset of the current steps, as indicated (•). C1, Response of a dLGN TC neuron to depolarizing (100 and 200 pA) and hyperpolarizing (–400, –300, –200, and –100 pA) current steps under control conditions (left) and after bath application of 100 nm THIP (right). C2, Current–voltage plot for the neuron in C1. Values were taken from the maximum deflection of the membrane potential during the current steps. THIP application causes a small decrease in apparent input resistance in response to hyperpolarizing current steps. D, Extracellular single-unit recording of a dLGNTC neuron in the presence of 100 nm THIP (left) and after wash (right). Only burst firing is apparent in the presence of THIP (see insets), whereas its removal from the aCSF results in the silencing of the unit.
Discussion
Tonic GABAA receptor-mediated inhibition has been shown previously in a variety of cell types, including DGGCs, CGCs, and pyramidal cells and interneurons of the hippocampus (Kaneda et al., 1995; Brickley et al., 1996; Wall and Usowicz, 1997; Bai et al., 2001; Brickley et al., 2001; Nusser and Mody, 2002; Stell and Mody, 2002; Semyanov et al., 2003). The receptors that underlie tonic inhibition are exclusively located extrasynaptically, but their subunit composition is cell type dependent. In both CGCs and DGGCs, extrasynaptic receptors primarily contain the δ subunit and either the α6 or α4 subunits. This has been confirmed both pharmacologically, the tonic current being both neuroactive steroid and THIP sensitive but BDZ site ligand insensitive (Nusser and Mody, 2002; Stell et al., 2003; Maguire et al., 2005), and anatomically (Nusser et al., 1998; Wei et al., 2003). However, in hippocampal neurons, receptors appear not to contain the δ subunit because tonic inhibition is sensitive to BDZ site ligands (Bai et al., 2001; Semyanov et al., 2003). The α4 and δ subunits are also highly expressed in certain thalamic nuclei (Persohn et al., 1992; Wisden et al., 1992; Fritschy and Mohler, 1995; Sur et al., 1999; Pirker et al., 2000), and previous work in δ subunit knock-out mice revealed minimal contribution of the δ subunit to synaptic inhibition in thalamic neurons (Porcello et al., 2003), implying the existence of a tonic current. Here we show for the first time its presence in TC neurons of both the dLGN and VB. Furthermore, we have shown that the tonic current sets an inhibitory tone in these neurons, the blocking or enhancement of which contributes to the transition between different modes of firing.
Properties of the tonic GABAA current
Using the BDZ site ligand flurazepam, the neuroactive steroid THDOC, and THIP, we confirmed that, as in DGGCs and CGCs, tonic inhibition in TC neurons is mediated by δ subunit-containing receptors, most likely containing the α4 subunit (Sur et al., 1999). Importantly, we found that the sensitivity of the tonic current to neurosteroids was lower in TC neurons than in both DGGCs and CGCs (Stell et al., 2003) and that THDOC did not selectively modulate the tonic current. We suggest this is probably attributable to differences in receptor subunit composition between the cell types. Although much work on receptor properties has focused on the contribution of α subunits, it is becoming increasing apparent that the different β subunits also convey specific receptor properties (Benke et al., 1994; Wallner et al., 2003; Smith et al., 2004). In both the dLGN and VB, the β2 subunit is preferentially expressed, whereas in DGGCs, it is the β3 subunit. CGCs express both the β2 and β3 subunits at a high level (Persohn et al., 1992; Wisden et al., 1992; Pirker et al., 2000).
Not all TC neurons exhibited a tonic current under control conditions, probably because, in some instances, the ambient GABA concentration was too low to activate the receptors. When the extracellular GABA concentration was enhanced exogenously, tonic current amplitude increased and tonic inhibition became evident in every neuron tested. These experiments indicate that there is no difference in tonic inhibition among potential TC neuron subclasses, for instance in the dLGN (Lam et al., 2005). We also suggest that, because in all likelihood the concentration of ambient GABA in our slices under control conditions is lower than that which exists in vivo (Lerma et al., 1986), our apparent tonic current amplitude in vitro may be an underestimation of its in vivo counterpart. Furthermore, given that the concentration of ambient GABA in vivo is likely to depend on the activity of GABA-releasing neurons, we might expect that tonic current amplitude would dynamically fluctuate. Thus, under conditions in which GABA-releasing neurons are more excited (Steriade et al., 1993; Blitz and Regehr, 2005; Steriade, 2005), not only synaptic inhibition but also tonic inhibition in TC neurons may be enhanced.
We were unable to observe a tonic current in NRT neurons, even in the presence of enhanced extracellular GABA. Similar to previous studies, we observed that, although the frequency of IPSCs in NRT neurons is lower than that in TC neurons, the charge transfer is correspondingly higher (Zhang et al., 1997; Huntsman and Huguenard, 2000). Under our experimental conditions, this resulted in the total current attributable to IPSCs being similar in both neuron types (∼3 and 2.5 pA, respectively). Thus, both neuron types receive a similar amount of synaptic inhibition, albeit with different biophysical and temporal properties. The physiological significance of this difference remains to be elucidated.
In contrast to a previous study (Stell and Mody, 2002), we found that very low concentrations of GBZ were able to preferentially block the tonic over phasic current, despite extrasynaptic receptors having a higher affinity for GABA than synaptic ones. We believe two factors explain this observation. First, under our experimental conditions, the low concentration of GBZ outcompetes ambient GABA, leading to extrasynaptic receptor blockade. This was confirmed by the fact that low concentrations of GBZ did not block the tonic current in the presence of increased ambient GABA. Second, the low GBZ concentration incompletely penetrates the synapse and/or is outcompeted by the high concentration of vesicularly released GABA in the synapse, resulting in only partial receptor blockade and maintenance of phasic inhibition. This second factor may be further compounded by the existence of synaptic glomeruli, complex triadic synaptic arrangements surrounded by glial sheaths, on some if not all TC neurons in the rat dLGN (Ohara et al., 1983; Lam et al., 2005). However, we stress that the subcellular distribution of extrasynaptic receptors in the thalamus is unknown. Furthermore, not all GABAergic inputs associate with glomeruli (Ohara et al., 1983).
Physiological significance
TC neurons can exhibit three modes of firing: low-threshold bursting driven by low-threshold Ca2+ spikes; tonic firing consisting of repetitive single action potentials; and high-threshold bursting mediated by high-threshold, probably T-type channel-dependent, Ca2+ spikes (Jahnsen and Llinás, 1984; Crunelli et al., 1989; Hughes et al., 2004). Tonic and high-threshold bursting occur at relatively depolarized membrane potentials, whereas low-threshold bursting occurs at hyperpolarized membrane potentials, with a region of quiescent membrane potential in between. The type of firing observed in vivo is behavioral state dependent: low-threshold bursting is characteristic of sleep states, particularly spindle oscillations and slow-wave sleep, whereas tonic and high-threshold bursting are typical of awake or relaxed states (Steriade et al., 1993; Steriade, 1997; Hughes et al., 2002b, 2004). Our intracellular recordings show that preferential block of the tonic current leads to a small depolarization and an increase in input resistance, commensurate with the block of a small, outward current. From the single-unit recordings, this was translated as a shift away from burst firing into the quiescent region or, in units that were initially quiescent, the promotion of tonic firing. Contrastingly, enhancement of the tonic current with THIP hyperpolarized neurons and promoted burst firing. Although block or enhancement of the tonic current was never sufficient to cause a complete shift between firing states, if we assume that our observed tonic current is smaller than what would be observed in vivo, it is conceivable that modulation in vivo may be sufficient to cause a complete transition. Therefore, endogenous agents that decrease the tonic current may promote wakefulness, and those that enhance it may promote sleep. THIP is a potent hypnotic that promotes slow-wave sleep in both humans and rats (Lancel and Faulhaber, 1996; Faulhaber et al., 1997). We suggest that part of its hypnotic action is mediated by increasing the tonic current in TC neurons, thereby shifting them into a membrane potential region in which low-threshold burst firing can occur and sleep is promoted.
Pathophysiological significance
During absence seizures, NRT neurons fire strong, high-frequency bursts in response to cortical input (Slaght et al., 2002; Steriade, 2005), leading to enhanced phasic inhibition and a tonic hyperpolarization in TC neurons (Steriade and Contreras, 1995; Pinault et al., 1998). The present data suggest that this hyperpolarization can occur by the strong NRT bursts enhancing the ambient GABA concentration and increasing the tonic current. However, a reversal of this hyperpolarization by Cl–-filled electrodes was not observed (Pinault et al., 1998), although this may be explained by technical issues of in vivo experimentation and the position of the extrasynaptic receptors. Agents, such as THIP, that enhance the tonic current in TC neurons while leaving the NRT population unchanged and therefore responsive to cortical drive may therefore promote absence seizures. Indeed, THIP has been used as a model of these seizures (Fariello and Golden, 1987). Interestingly, mutations in the δ subunit have been implicated in human idiopathic generalized epilepsies (Dibbens et al., 2004).
In conclusion, TC neurons exhibit a tonic GABAA receptor-mediated current that maintains an inhibitory tone and contributes to the transition between different firing modes. The modulation of this inhibitory tone in vivo may lead to the promotion of one behavioral state over another or the instigation of absence seizures.
Footnotes
This work was supported by Wellcome Trust Grant 71436. We thank Tim Gould for his excellent technical assistance. Dr. I. Mody (University of California, Los Angeles, Los Angeles, CA) kindly provided the Lab View-based analysis software.
Correspondence should be addressed to Dr. David W. Cope, School of Biosciences, Cardiff University, Museum Avenue, Cardiff CF10 3US, UK. E-mail: copedw@cf.ac.uk.
DOI:10.1523/JNEUROSCI.3362-05.2005
Copyright © 2005 Society for Neuroscience 0270-6474/05/2511553-11$15.00/0
References
- Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA (2001) Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol 59: 814–824. [DOI] [PubMed] [Google Scholar]
- Benke D, Fritschy J-M, Trzeciak A, Bannwarth W, Mohler H (1994) Distribution, prevalence, and drug binding profile of γ-aminobutyric acid type A receptor subtypes differing in the β-subunit variant. J Biol Chem 269: 27100–27107. [PubMed] [Google Scholar]
- Blitz DM, Regehr WG (2005) Timing and specificity of feed-forward inhibition within the LGN. Neuron 45: 917–928. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M (1996) Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 497: 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M (2001) Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409: 88–92. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA (2002) Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol 136: 965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton P, Margrie TW, Häusser M (2004) Integration of quanta in cerebellar granule cells during sensory processing. Nature 428: 856–860. [DOI] [PubMed] [Google Scholar]
- Cope DW, Wulff P, Oberto A, Aller MI, Capogna M, Ferraguti F, Halbsguth C, Hoeger H, Jolin HE, Jones A, Mckenzie ANJ, Ogris W, Poeltl A, Sinkkonen ST, Vekovischeva OY, Korpi ER, Sieghart W, Sigel E, Somogyi P, Wisden W (2004) Abolition of zolpidem sensitivity in mice with a point mutation in the GABAA receptor γ2 subunit. Neuropharmacology 47: 17–34. [DOI] [PubMed] [Google Scholar]
- Crunelli V, Lightowler S, Pollard CE (1989) A T-type Ca2+ current underlies low-threshold Ca2+ potentials in cells of the cat and rat lateral geniculate nucleus. J Physiol (Lond) 413: 543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibbens LM, Feng H-J, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC (2004) GABRD encoding a protein fro extra- or perisynaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum Mol Genet 13: 1315–1319. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson JA, Fritschy J-M, Lüscher B (1998) Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci 1: 563–571. [DOI] [PubMed] [Google Scholar]
- Fariello RG, Golden GT (1987) The THIP-induced model of bilateral synchronous spike and wave in rodents. Neuropharmacology 26: 161–165. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nature Rev Neurosci 6: 215–229. [DOI] [PubMed] [Google Scholar]
- Faulhaber J, Steiger A, Lancel M (1997) The GABAA agonist THIP produces slow wave sleep and reduces spindling activity in NREM sleep in humans. Psychopharmacology (Berl) 130: 285–291. [DOI] [PubMed] [Google Scholar]
- Fritschy J-M, Mohler H (1995) GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359: 154–194. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D (2002) Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33: 625–633. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Blethyn KL, Cope DW, Crunelli V (2002a) Properties and origin of spikelets in thalamocortical neurones in vitro. Neuroscience 110: 395–401. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Blethyn KL, Crunelli V (2002b) Cellular mechanisms of the slow (<1 Hz) oscillation in thalamocortical neurons in vitro. Neuron 33: 947–958. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Lörincz M, Cope DW, Blethyn KL, Kékesi KA, Parri HR, Juhász G, Crunelli V (2004) Synchronized oscillations at α and θ frequencies in the lateral geniculate nucleus. Neuron 42: 253–268. [DOI] [PubMed] [Google Scholar]
- Huntsman MM, Huguenard JR (2000) Nucleus-specific differences in GABAA-receptor-mediated inhibition are enhanced during thalamic development. J Neurophysiol 83: 350–358. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R (1984) Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol (Lond) 349: 205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Mäkela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AR, Sieghart W, Somogyi P, Smith AJH, Wisden W (1997) Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci 17: 1350–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M, Farrant M, Cull-Candy SG (1995) Whole-cell and single-channel currents activated by GABA and glycine in granule cells of the rat cerebellum. J Physiol (Lond) 485: 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Gründer G, Lüddens H (2002) Drug interactions at GABAA receptors. Prog Neurobiol 67: 113–159. [DOI] [PubMed] [Google Scholar]
- Lam Y-W, Cox CL, Varela C, Sherman SM (2005) Morphological correlates of triadic circuitry in the lateral geniculate nucleus of cats and rats. J Neurophysiol 93: 748–757. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J (1996) The GABAA agonist THIP (gaboxadol) increases non-REM sleep and enhances delta activity in the rat. NeuroReport 7: 2241–2245. [DOI] [PubMed] [Google Scholar]
- Le Feuvre Y, Fricker D, Leresche N (1997) GABAA receptor-mediated IPSCs in rat thalamic sensory nuclei: patterns of discharge and tonic modulation by GABAB autoreceptors. J Physiol (Lond) 502: 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R (1986) In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res 384: 145–155. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I (2005) Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 8: 797–804. [DOI] [PubMed] [Google Scholar]
- Mody I (2001) Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res 26: 907–913. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I (2002) Selective modulation of tonic and phasic inhibition in dentate gyrus granule cells. J Neurophysiol 87: 2624–2628. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P (1998) Segregation of different GABAA receptors to synaptic and extrasynaptic membrane of cerebellar granule cells. J Neurosci 18: 1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P (1999) Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the α6 subunit gene. Eur J Neurosci 11: 1685–1697. [DOI] [PubMed] [Google Scholar]
- Ohara PT, Lieberman AR, Hunt SP, Wu J-Y (1983) Neural elements containing glutamic acid decarboxylase (GAD) in the dorsal lateral geniculate nucleus of the rat: immunohistochemical studies by light and electron microscopy. Neuroscience 8: 189–211. [DOI] [PubMed] [Google Scholar]
- Persohn E, Malherbe P, Richards JG (1992) Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J Comp Neurol 326: 193–216. [DOI] [PubMed] [Google Scholar]
- Pinault D, Leresche N, Charpier S, Deniau J-M, Marescaux C, Vergnes M, Crunelli V (1998) Intracellular recordings in thalamic neurones during spontaneous spike and wave discharges in rats with absence epilepsy. J Physiol (Lond) 509: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G (2000) GABAA receptors: immunocytochemical distributions of 13 subunits in the adult rat brain. Neuroscience 101: 815–850. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR (2003) Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking δ subunit. J Neurophysiol 89: 1378–1386. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM (2003) GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci 6: 484–490. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA (2004) Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci 27: 262–269. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G (2002) Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem 2: 795–816. [DOI] [PubMed] [Google Scholar]
- Slaght SJ, Leresche N, Deniau J-M, Crunelli V, Charpier S (2002) Activity of thalamic reticular neurons during spontaneous genetically determined spike and wave discharges. J Neurosci 22: 2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Oxley B, Malpas S, Pillai GV, Simpson PB (2004) Compounds exhibiting selective efficacy for different β subunits of human recombinant γ-aminobutyric acidA receptors. J Pharmacol Exp Ther 311: 601–609. [DOI] [PubMed] [Google Scholar]
- Soltesz I, Roberts JDB, Takagi H, Richards JG, Mohler H, Somogyi P (1990) Synaptic and nonsynaptic localization of benzodiazepine/GABAA receptor/Cl– channel complex using monoclonal antibodies in the dorsal lateral geniculate nucleus of the cat. Eur J Neurosci 2: 414–429. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy J-M, Benke D, Roberts JDB, Sieghart W (1996) The γ2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the α1 and β2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacol 35: 1425–1444. [DOI] [PubMed] [Google Scholar]
- Stell BM, Mody I (2002) Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci 22: RC223(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc Natl Acad Sci USA 100: 14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M (1997) Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cereb Cortex 7: 583–604. [DOI] [PubMed] [Google Scholar]
- Steriade M (2005) Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci 28: 317–324. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D (1995) Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci 15: 623–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ (1993) Thalamocortical oscillations in the sleeping and aroused brain. Science 262: 679–685. [DOI] [PubMed] [Google Scholar]
- Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM (1999) Preferential coassembly of α4 and δ subunits of the γ-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol 56: 110–115. [DOI] [PubMed] [Google Scholar]
- Thomson AM, West DC, Hahn J, Deuchars J (1996) Single axon IPSPs elicited in pyramidal cells by three classes of interneurones in slices of rat neocortex. J Physiol (Lond) 496: 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM (1997) Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci 9: 533–548. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW (2003) Ethanol enhances α4β3δ and α6β3δγ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA 100: 15218–15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I (2003) Perisynaptic localization of δ subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci 23: 10650–10661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PJ, Wafford KA, McKernan RM (2000) Pharmacologic subtypes of GABAA receptors based on subunit composition. In: GABA in the nervous system: the view at fifty years (Martin DL, Olsen RW, eds), pp 113–126. Philadelphia: Lippincott Williams and Wilkins.
- Wisden W, Laurie DJ, Monyer H, Seeburg PH (1992) The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. 1. Telencephalon, diencephalon, mesencephalon. J Neurosci 12: 1040–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Cope D, Klausberger T, Hauer B, Sinkkonen ST, Tretter V, Lujan R, Jones A, Korpi ER, Mody I, Sieghart W, Somogyi P (2002) Ectopic expression of the GABAA receptor α6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology 43: 530–549. [DOI] [PubMed] [Google Scholar]
- Zhang SJ, Huguenard JR, Prince DA (1997) GABAA receptor-mediated Cl– currents in rat thalamic reticular and relay neurons. J Neurophysiol 78: 2280–2286. [DOI] [PubMed] [Google Scholar]