Abstract
Synaptic interactions between telencephalic neurons innervating descending motor or basal ganglia pathways are essential in the learning, planning, and execution of complex movements. Synaptic interactions within the songbird telencephalic nucleus HVC are implicated in motor and auditory activity associated with learned vocalizations. HVC contains projection neurons (PNs) (HVCRA) that innervate song premotor areas, other PNs (HVCX) that innervate a basal ganglia pathway necessary for vocal plasticity, and interneurons (HVCINT). During singing, HVCRA fire in temporally sparse bursts, possibly because of HVCINT-HVCRA interactions, and a corollary discharge can be detected in the basal ganglia pathway, likely because of synaptic transmission from HVCRA to HVCX cells. During song playback, local interactions, including inhibition onto HVCX cells, shape highly selective responses that distinguish HVC from its auditory afferents. To better understand the synaptic substrate for the motor and auditory properties of HVC, we made intracellular recordings from pairs of HVC neurons in adult male zebra finch brain slices and used spike-triggered averages to assess synaptic connectivity. A major synaptic interaction between the PNs was a disynaptic inhibition from HVCRA to HVCX, which could link song motor signals in the two outputs of HVC and account for some of the song playback-evoked inhibition in HVCX cells. Furthermore, single interneurons made divergent connections onto PNs of both types, and either PN type could form reciprocal connections with interneurons. In these two regards, the synaptic architecture of HVC resembles that described in some pattern-generating networks, underscoring features likely to be important to singing and song learning.
Keywords: HVC, in vitro intracellular, paired recordings, zebra finch, songbird, GABAA, picrotoxin, unitary synaptic coupling
Introduction
Identifying synapses in the vertebrate telencephalon that link neurons in primary motor pathways with neurons in basal ganglia pathways is important to an understanding of motor learning, planning, and execution. Songbirds learn to sing via audition-dependent vocal plasticity (Konishi, 1965; Price, 1979), and the songbird telencephalic sensorimotor nucleus HVC contains different projection neurons (PNs) that give rise to either a premotor pathway specialized for song patterning or a basal ganglia pathway necessary for audition-dependent vocal plasticity (see Fig. 1) (Nottebohm et al., 1976, 1982; Fortune and Margoliash, 1995; Foster and Bottjer, 1998; Brainard and Doupe, 2000). These features make HVC an essential site to probe for synaptic interactions important to singing and song learning.
Figure 1.
The song nucleus HVC in the zebra finch telencephalon contains two different classes of PN and at least one class of interneurons. A, A sagittal section through the telencephalon of an adult male zebra finch, stained for myelin, showing the song nucleus HVC and one of its two efferent targets, the song premotor nucleus RA. HVC fibers transiting to RA can be seen between the two nuclei. Area X, the other efferent target of HVC, is medial to this plane of section. D, Dorsal; R, rostral. B, A schematic of the song nucleus HVC, showing the three neuron classes, including PNs that innervate RA (HVCRA), PNs that innervate Area X (HVCX), and interneurons. Simultaneous dual electrode recordings were made from different pairs of HVC neurons to study their synaptic connectivity. C, Confocal images of the three HVC neuron types studied here, as revealed by intracellular staining with Neurobiotin and post hoc visualization with avidin-Alexa Fluor 488 (see Materials and Methods). HVCRA neurons (top) possessed slender and sparsely spinous dendrites and elaborated a main axon that exited at the caudal margin of HVC. HVCX neurons (middle) were characterized by thicker and more spinous dendrites and an axon that exited HVC along its rostroventral border. Interneurons (bottom) were characterized by aspinous, varicose dendrites and lacked an axon that exited HVC. Scale bar, 20 μm. D, Typical membrane potential responses of the three HVC neuron types to depolarizing current pulses (bottom trace). HVCRA neurons (top trace) fired only one or a few action potentials, even in response to large-amplitude depolarizing currents (+1.5 nA). HVCX neurons (middle trace) fired repetitively to moderate currents (+0.5 nA) with some spike-frequency accommodation. Interneurons (HVCINT) fired at high frequencies with little or no spike-frequency accommodation in response to moderate depolarizing current (+0.5 nA). Action potential widths of interneurons are narrower than in either of the PN types (data not shown). Resting potentials (in millivolts) are shown to the left of each membrane potential trace.
Neurons in HVC comprise at least three major types, including PNs (HVCRA) that gives rise to a descending premotor pathway obligatory for song, other PNs (HVCX) that innervate a basal ganglia structure within an anterior forebrain pathway (AFP) essential to vocal plasticity, and interneurons (HVCINT) (see Fig. 1) (Kirn et al., 1991; Johnson and Bottjer, 1993; Mooney, 2000). All three cell types extend axonal processes within HVC, affording the means for local synaptic processing (Katz and Gurney, 1981; Mooney, 2000). Several findings suggest that synaptic processing in HVC is extensive and likely to have important behavioral consequences, given the connections of HVC with premotor areas and the AFP. First, HVCRA neurons generate temporally sparse, high-frequency action potential bursts during singing (Hahnloser et al., 2002), and these bursts could propagate through the HVCRA ensemble via local excitatory connections and be terminated by local inhibitory interneurons. Second, AFP neurons display song motor activity (Hessler and Doupe, 1999), and this putative corollary discharge could arise because of coupling between premotor (HVCRA) and HVCX neurons (Troyer and Doupe, 2000). Finally, HVC neurons exhibit highly selective action potential responses to playback of the bird's own song (BOS), and certain features of these responses, including their temporal sparseness and sensitivity to specific syllable sequences, are thought to be refined by local circuit interactions in HVC (Margoliash, 1983; Margoliash and Fortune, 1992; Lewicki, 1996; Theunissen and Doupe, 1998; Mooney, 2000; Coleman and Mooney, 2004). Indeed, in vivo intracellular recordings show that BOS playback evokes distinct subthreshold responses and reciprocal firing patterns in the two PNs and that hyperpolarizing inhibition sculpts BOS-evoked firing patterns in HVCX cells, suggestive of interneuron-PN interactions (Mooney, 2000; Rosen and Mooney, 2003).
Although the motor and auditory properties of HVC hint at extensive local processing, knowledge of the synaptic interactions between identified HVC neuron types remains incomplete. This gap in understanding exists because axonal and dendritic processes from all three cell types as well as axonal processes from HVC afferents are interwoven with each other, complicating analysis of intrinsic connectivity (Nixdorf, 1989; Fortune and Margoliash, 1995; Foster and Bottjer, 1998; Mooney, 2000). We made intracellular recordings from pairs of identified HVC neurons in brain slices and calculated spike-triggered averages (STAs) (Perkel et al., 1967) to assess synaptic connections. We also antidromically stimulated HVCRA neurons and applied neurotransmitter receptor blockers to further characterize the intrinsic connectivity of HVC. We found extremely robust disynaptic feedforward inhibition from HVCRA to HVCX neurons, which may influence how HVC shapes and conveys song motor activity to the AFP and could account for the contrasting BOS-evoked responses in the different PNs. In addition, single interneurons can contact multiple PNs, and interneurons and PNs can form reciprocal connections. Similar architectural features contribute to synchronous oscillations in other pattern-generating networks (Selverston and Moulins, 1985) and have implications for the role of HVC in song patterning. Some results have been published in abstract form (Prather and Mooney, 2003).
Materials and Methods
These experiments use electrophysiological techniques that have been described extensively in previous published studies (Mooney, 1992; Livingston and Mooney, 1997; White et al., 1999; Livingston et al., 2000). Therefore, only a brief description of these techniques is provided here.
Subjects. Thirty-nine adult male zebra finches (>120 d posthatch) were used for these experiments, in accordance with a protocol approved by the Duke University Institutional Animal Care and Use Committee. Finches were raised in our breeding colony on a 14/10 h light/dark cycle.
Brain slices. After induction of inhalation anesthesia (halothane), the bird was decapitated, and the brain was removed rapidly and placed in oxygenated ice-cold artificial CSF (ACSF). Sagittal brain slices that included HVC were cut at 400-500 μm thickness and transferred to a holding chamber (room temperature) for 2-4 h. Individual slices were transferred to an interface-type chamber (30°C; Medical Systems, Greenvale, NY) for intracellular recordings. The ACSF consisted of (in mm) 119 NaCl, 2.5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose, equilibrated with 95%O2/5%CO2. Equiosmolar sucrose was substituted for NaCl during the tissue preparation stage.
Electrophysiological recordings. Sharp intracellular recordings were made with borosilicate glass pipettes (Sutter Instruments, Novato, CA) pulled to a final resistance of 80-200 MΩ when filled with 2 m potassium acetate and 5% Neurobiotin (Vector Laboratories, Burlingame, CA). In a few experiments, one of the two recording electrodes contained 5% Lucifer yellow (LY) in a 1 m lithium chloride solution. Cell penetration was achieved by briefly “ringing” the electrode using capacitance overcompensation, and the cell was then stabilized by passing regular hyperpolarizing current pulses through the recording electrode (-0.5 nA, 500 ms at 1 Hz). Intracellular potentials were amplified with an Axoclamp 2B amplifier (Axon Instruments, Union City, CA) in bridge mode, low-pass filtered at 1-3 kHz, and digitized at 10 kHz. To make paired recordings, we first obtained a stable recording from an HVC neuron and then lowered the second recording electrode to a point typically within ∼50 μm of the first electrode and searched for its synaptic partners. This search strategy tended to favor finding pairs in which the first cell obtained was of a type affording a more stable recording, which in our hands tended to be the HVCX cell type (Table 1) (see Results). Synaptically coupled cells usually could be identified on-line by the appearance of a hyperpolarizing or depolarizing response in one cell locked to the action potential discharge of the other cell, and also by subsequent on-line analysis of STAs (see below). Synaptically coupled cell pairs were most often encountered when the two electrode tips were in close (∼50 μm) proximity to one another, and although a detailed count of unconnected pairs was not kept for all experiments, ∼10-20% of cell pairs showed evidence of unidirectional or bidirectional synaptic coupling.
Table 1.
Frequency of cell pairs encountered in HVC
|
Pair type |
Number of pairs (percentage of total) |
Number of connected pairs (percentage of pairs) [percentage total pairs] |
Type of interaction (number of observations) |
|---|---|---|---|
| HVCRA-HVCX | 46 (47.9) | 6 (13) [6.25] | HVCRA-HVCX IPSP (4) |
| HVCX-HVCRA dPSP (5) | |||
| HVCX-HVCRA IPSP (1) | |||
| Three reciprocally connected pairs | |||
| HVCX-HVCX | 19 (19.8) | 5 (26.3) [5.21] | All unidirectional IPSPs |
| HVCINT-HVCX | 12 (12.5) | 3 (25) [3.12] | HVCINT-HVCX IPSP (2) |
| HVCX-HVCINT dPSP (1) | |||
| One reciprocally connected pair | |||
| HVCRA-HVCRA | 9 (9.4) | 1 (11.1) [1] | Unidirectional dPSP |
| HVCRA-HVCINT | 6 (6.2) | 2 (33) [2] | HVCINT-HVCRA IPSP (1) |
| HVCINT-HVCRA dPSP (1) | |||
| HVCINT-HVCINT | 4 (4.2) | 1 (25) [1] | Unidirectional IPSP |
| Total |
96 (100%) |
18 (18.7) [NA] |
|
Electrophysiological data acquisition and analysis. Data acquisition and analysis for single and paired intracellular recordings were performed using a data acquisition board (AT-MIO-16E2; National Instruments, Austin, TX), controlled by custom Labview software developed by Fred Livingston, Rob Neummann, and Merri Rosen (Duke University, Durham, NC). In paired recordings, one or two action potentials were elicited in turn from each neuron in the pair by passing brief (∼10 ms) depolarizing current pulses (+0.5 to 1 nA) through the recording electrode. A software threshold peak detector was used to generate STAs of the membrane potential of the partner cell in the pair. In most cases, current amplitudes and/or the resting membrane potential of the trigger cell were adjusted to elicit only a single action potential per pulse, but in a few cells, two or three spikes were sometimes evoked. In these cases, the STA was calculated off the first spike in the series. STAs were plotted in reference to the time of the trigger spike; note that the zero time for the STA corresponds to the action potential peak and that we suspect that the resultant STAs might be slightly leftward-shifted with respect to the actual onset of transmitter release (see Fig. 5B). After collecting 10-40 pulse trials per cell, we then conducted further characterizations of the impaled cells, including their responses to more prolonged depolarizing currents (0.5 s at +0.5 nA). All HVC neurons in this study were identified to type based on their DC-evoked properties, as described previously (Dutar et al., 1998; Mooney, 2000). Briefly, HVCRA neurons fire only one to several action potentials to +0.5 nA currents of 0.5 s duration, whereas HVCX neurons fire more regularly with moderate spike-frequency adaptation, and HVCINT fire at high frequency with little or no spike-frequency adaptation (see Fig. 1 D). In addition, HVCINT can be distinguished from HVC PNs by their narrower spike widths (∼1 vs 2 ms) (Mooney, 2000; Rauske et al., 2003). In many cases, at least one cell in the pair was confirmed to morphological type through intracellular staining and post hoc morphological visualization. Relatively brief recording times (<15 min) prevented thorough filling of both cells in the recorded pair in all but a few cases.
Figure 5.
Dual intracellular recordings reveal that HVCINT provide short-latency inhibition onto HVCX cells. A, Raw membrane potential records from a synaptically coupled interneuron (bottom) and HVCX cell (top). DC-evoked spikes could evoke robust IPSPs in the HVCX cell; note that a spontaneous IPSP, presumably from another interneuron, occurred after the DC-evoked responses. B, The mean STA from all HVCINT-HVCX cell pairs compared with the mean STA from all HVCRA-HVCX cells pairs, showing the offset in the 25% rise times (horizontal dashed line) (see Table 2). The overall shapes of the STAs in the different cell pairs were very similar, but the HVCRA-HVCX STA was delayed relative to HVCINT-HVCX STA, suggesting that HVCRA cells are connected indirectly with HVCX cells. The STA conventions are as in Figures 2 and 4. C, Higher-frequency firing in an HVCINT can drive a sustained hyperpolarization in the HVCX cell. D, HVCX cells in some cases could drive EPSPs in an HVCINT cell. DC-evoked firing in the HVCX cell (bottom trace) reliably evoked suprathreshold EPSPs in the HVCINT (top trace). The HVCINT also evoked IPSPs in the HVCX cell (shown in Fig. 9D), indicating that interneurons and HVCX cells can form reciprocal synaptic connections.
In a subset of paired recordings from synaptically coupled cells, and in all cases in which we recorded from either a single cell or unconnected pairs for pharmacological experiments (see below), we also antidromically activated HVCRA neurons, and thus their axon collaterals within HVC, by passing currents (∼25-100 μA for 100 μsec) from an Isolator-10 stimulus isolation unit (Axon Instruments) to the HVC fibers that project to the robust nucleus of arcopallium (RA), using a concentric bipolar stimulating electrode (FHC, Brunswick, ME) placed midway between HVC and RA in the region of the caudal telencephalon. HVC axons innervating RA are clearly visible in the brain slice under epi-illumination, appearing as large braids of whitish fibers (see Fig. 1 A). Anatomical studies suggest that these fiber braids are composed exclusively of HVCRA axons, although a few HVCX axons do travel medial to this area but outside the plane of the slices used here (Mooney, 2000). This mid-point placement was chosen because it is unlikely to activate axons of HVC afferents. Furthermore, antidromic activation of HVCRA neurons was confirmed in some recordings by the appearance of an action potential riding on the shoulder of the stimulus artifact itself. In contrast, as HVCX axons exit rostrally and ventrally from the nucleus, they intermingle with axons arising from several afferents of HVC (i.e., NIf, Uva, and mMAN). This organization renders selective recruitment of HVCX axon collaterals by extracellular stimulation in the brain slice unlikely, and thus we did not attempt to use an antidromic stimulation approach to activate HVCX axon collaterals. Instead, we relied solely on paired recordings to deduce the nature of the synaptic connectivity that HVCX neurons make with the other HVC neuron types.
Several different features of the STA were characterized off-line, including the peak amplitude, the time to peak, and the 25% rise time (i.e., the time to reach 25% of the peak amplitude). In the small minority of cases in which the trigger neuron spiked repetitively and the STA demonstrated a biphasic peak, the first peak was used for these measurements. An ANOVA was used to compare a given STA feature across different cell pair types, followed by Tukey's post hoc test corrected for multiple comparisons. Values reported are the mean ± SEM, unless noted otherwise.
Synaptic pharmacology. To analyze the types of postsynaptic receptors activated either after antidromic stimulation of HVCRA neurons or in the case of some synaptically coupled pairs, drugs were bath applied to the whole slice after collecting evoked synaptic responses for a 5-10 min baseline period. Picrotoxin (PTX; 50 μm) was used to block inhibitory receptors of the GABAA subtype, whereas d(-)-2-amino-5-phophonopentanoic acid (d-APV; 50 μm) was used to block excitatory transmission mediated by NMDA receptors and 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo(f)quinoxaline-7-sulfonamide disodium (NBQX; 10 μm) was used to block fast excitatory transmission mediated by ionotropic glutamate receptors of the AMPA subtype. The use of an interface chamber, which we prefer for its superior slice viability compared with submersion chambers, especially when recording above room temperature, precluded collecting washouts in most cases (i.e., washout times greatly exceeded 1 h). We used other features, such as the maintenance of excitatory transmission in the presence of PTX, as well as resting membrane potential and input impedance, to determine that synaptic blockade did not reflect a general rundown of the cell or the slice. Drug effects were assessed using paired or unpaired t tests, as appropriate for normally distributed data, and a Mann-Whiney U test for other data sets.
Intracellular staining and imaging. After acquiring electrophysiological data, Neurobiotin was iontophoresed into the cell with positive current pulses (+0.5 to 1 nA, 500 ms per second, for ∼15 min). For LY staining, negative current pulses were passed through the electrode (-1 nA, 500 ms at 1 Hz). Slices then were fixed in 4% paraformaldehyde in 25 mm sodium phosphate buffer (PB) overnight at 4°C and then sunk in 30% sucrose in 25 mm PB. Slices then were resectioned at 75-100 μm thickness on a freezing microtome, and Neurobiotin was visualized with a standard avidin-fluorophore reaction using a 1:1000 dilution of avidin-Alexa Fluor 488 or 564 (Molecular Probes, Eugene, OR). Confocal images were generated with a Zeiss (Thornwood, NY) 510 laser scanning microscope, using either a 40× [1.3 numerical aperture (NA)] or 63× (1.4 NA) Zeiss NeoFluar objective and rhodamine/fluorescein filters [488 and 543 nm excitation wavelengths, emission bandpass filter of 500-540 nm, and emission long-pass filter of 560, sampled in an alternating frame arrangement; optical sections of 1 Airey unit (∼1 μm thickness), with Kalman averaging = 2].
Parvalbumin immunoreactivity. In some experiments, we combined intracellular staining with Neurobiotin and immunohistochemical methods to determine which cells in HVC were positive for parvalbumin (PV), a calcium-binding protein previously localized to HVC interneurons (Wild et al., 2005). After cell fills, brain slices were transferred to 4% paraformaldehyde within 30 min, where they remained for a minimum of 24 h before being placed in 30% sucrose for several hours and then sectioned at 50 μm on a freezing microtome. The sections were then incubated in streptavidin-Alexa Fluor 488 in PBS-Triton X-100 overnight, washed in PBS, and incubated overnight again with anti-PV antibody (mouse monoclonal; Swant, Bellinzona, Switzerland) at a dilution of 1:500. All immunohistochemical reactions were performed on free-floating sections at room temperature in PBS containing 0.4% Triton X-100 (PBS-TX). Primary antibody incubations were performed overnight with the inclusion of 2.5% normal horse serum and 0.1% sodium azide. PV was visualized using a biotinylated horse anti-mouse antibody (Vector Laboratories), followed by streptavidin-Alexa Fluor 564 (Molecular Probes). Secondary antibody incubations were 1-2 h in duration in PBS-TX. Between treatments, the sections were washed thoroughly in PBS.
Results
We used blind dual sharp microelectrode recording techniques to record from synaptically coupled pairs of cells in the telencephalic song nucleus HVC (Fig. 1A,B). Previous in vitro and in vivo studies have shown that in addition to their morphological differences, HVCRA, HVCX, and HVCINT are readily distinguished from each other based on their DC-evoked firing properties (Dutar et al., 1998; Mooney, 2000). In the present study, we identified all cells by their DC-evoked action potential responses and/or intracellular staining with Neurobiotin and post hoc visualization (Fig. 1C,D) (see Materials and Methods). Synaptic potentials were evident as depolarizing or hyperpolarizing membrane potential responses in one of the cells immediately after the spontaneous and/or DC-evoked action potentials of the other cell. With respect to both raw and averaged membrane potential records, we refer to spike-evoked responses that are hyperpolarizing as IPSPs, depolarizing responses as dPSPs, and depolarizing responses that demonstrably evoked spiking in the postsynaptic cell as EPSPs. In all cases, action potentials were evoked in turn from each of the cells in the pair, allowing us to assess synaptic coupling in both directions. In total, we found 79 neuron pairs that exhibited evidence of either unidirectional or bidirectional synaptic coupling, including 29 HVCRA-HVCX pairs, 20 HVCINT-HVCX pairs, 13 HVCRA-HVCINT pairs, 11 HVCX-H-VCX pairs, 5 HVCRA-HVCRA pairs, and 1 HVCINT-HVCINT pair (n = 53 slices from 30 birds). In this study, the relative abundance of connected neuronal pairs of a given class is dependent on both the true probability of connections within that class and the probability of sampling from that class. Therefore, the major focus of these results is on describing the nature of connections in each class of paired cells rather than the relative prevalence of connections among the different classes.
HVCRA-HVCX pairs
A total of 29 HVCX-HVCRA neuronal pairs exhibited evidence of synaptic coupling. In almost all (26 of 29) pairs of synaptically coupled HVCRA-HVCX neurons, DC-evoked action potentials in the HVCRA neuron triggered a synaptic response in the HVCX neuron. In the vast majority of these cases, action potentials in the HVCRA neuron evoked an IPSP in the HVCX cell (Fig. 2A, Table 2) (25 of 26 cases evoked IPSPs; 1 of 26 cases evoked a dPSP). The amplitudes of these HVCRA-HVCX IPSPs often were sufficiently large (>1 mV) to be visible without averaging. HVCRA STAs (see Materials and Methods) of the HVCX neuronal membrane potential had an average peak amplitude of -1.5 ± 0.3 mV, an average time to peak of 15.7 ± 1.0 ms, and a 25% rise time of 4.8 ± 0.4 ms (n = 25) (Fig. 2B, Table 2).
Figure 2.
Action potentials in HVCRA neurons evoke IPSPs in HVCX neurons. A, Dual intracellular recordings show that DC-evoked action potentials in the HVCRA neuron (bottom trace) can evoke IPSPs in the HVCX neuron. B, An HVCRA STA of the HVCX neuron membrane potential, plotted relative to the HVCRA action potential peak (0 ms; positive times follow the action potential), for the cell pair shown in A. A membrane hyperpolarization followed the HVCRA action potential, indicating that HVCRA neurons directly or indirectly drive IPSPs in the HVCX cell. C, In some HVCRA-HVCX cell pairs, spike doublets in the HVCRA neuron were necessary to drive IPSPs in the HVCX cell (right traces), whereas single spikes failed to evoke any response (left traces), suggestive of disynaptic coupling.
Table 2.
Characteristics of synaptic responses in connected HVC neuron pairs
|
Pair type (number of pairs with given PSP/total number of connected pairs of given type) |
Peak amplitude (mV) |
Time to peak (ms) |
25% rise time (ms) |
|---|---|---|---|
| IPSPs | |||
| HVCRA > HVCX (25 of 29) | −1.5 ± 0.3 | 15.7 ± 1.0 | 4.8 ± 0.4 |
| HVCX > HVCRA (3 of 29) | −1.1 ± 0.1 | 9.9 ± 1.3 | 2.6 ± 0.7 |
| HVCX > HVCX (11 of 11) | −2.0 ± 0.4 | 15.4 ± 2.7 | 5.0 ± 1.7 |
| HVCINT > HVCX (19 of 20) | −1.2 ± 0.2 | 10.2 ± 0.9a | 1.4 ± 0.3b |
| HVCINT > HVCRA (6 of 13) | −0.9 ± 0.2 | 10.7 ± 2.3c | 1.7 ± 1.0c |
| dPSPs | |||
| HVCRA > HVCINT (6 of 13) | 2.0 ± 0.4 | 4.7 ± 0.5 | 1.4 ± 0.1 |
| HVCX > HVCINT (5 of 20) | 1.2 ± 0.3 | 4.3 ± 0.4d | 1.0 ± 0.6d |
| HVCRA > HVCRA (4 of 4) | 2.2 ± 1.1 | 9.1 ± 2.3 | 4.0 ± 0.3 |
| HVCX > HVCRA (4 of 29) |
0.5 ± 0.1 |
8.3 ± 0.9 |
3.2 ± 0.3 |
p < 0.05 versus HVCRA > HVCX, by ANOVA.
p < 0.05 versus HVCRA > HVCX and HVCX > HVCX, by ANOVA.
p > 0.05 versus HVCINT > HVCX, by ANOVA.
p > 0.05 versus HVCRA > HVCINT, by unpaired t test.
Synaptic coupling from HVCX to HVCRA neurons was detected less frequently. In 7 of 29 pairs of synaptically coupled HVCRA-HVCX neurons, action potentials in the HVCX cell triggered synaptic responses in HVCRA neurons. In four of these cases, action potentials in the HVCX neuron evoked a dPSP in the HVCRA cell, whereas in the other three cases, IPSPs were elicited (Table 2). Notably, reciprocal connections were detected in 5 of the 29 pairs of synaptically coupled HVCRA-HVCX neurons (data not shown). In all of these cases, action potentials in the HVCRA neuron evoked IPSPs in the HVCX cell; HVCX cell action potentials evoked dEPSPs in three of these pairs and IPSPs in the other two pairs. These results indicate that bidirectional synaptic interactions, including reciprocal inhibitory interactions, can occur between HVCRA and HVCX neurons. Furthermore, these recordings suggest that a dominant pattern of synaptic connectivity between the two HVC PNs involves inhibition from HVCRA to HVCX neurons.
We suspected that the inhibitory interaction between HVCRA and HVCX neurons was mediated by interposed interneurons, given that HVCRA neurons are known to evoke ionotropic glutamate receptor-mediated EPSPs in neurons in the song nucleus RA (Mooney and Konishi, 1991). In this model, the local collaterals of HVCRA neurons excite inhibitory interneurons via ionotropic glutamatergic synapses, which in turn make inhibitory synapses on HVCX neurons. Consistent with the disynaptic model, both the mean 25% rise time and the mean time to peak of IPSPs in HVCRA-HVCX cell pairs were significantly longer than those of the IPSPs evoked in HVCX cells by interneurons (see Table 2 for statistical comparisons and the following discussion of synaptic coupling in HVCINT-HVCX cells pairs) (see also Fig. 5B). Furthermore, in some HVCRA-HVCX cell pairs, hyperpolarizing responses in HVCX neurons only were evoked when the HVCRA neuron fired a spike doublet or triplet, possibly reflecting facilitation at an intervening excitatory synapse (Fig. 2C) (n = 2 cases). These observations provide indirect evidence that HVCRA cells evoke IPSPs in HVCX neurons via a disynaptic mechanism. Another possibility is that the axon collaterals of HVCRA neurons provide monosynaptic inhibition onto HVCX neurons, perhaps via the hyperpolarizing metabotropic glutamate receptors that have been detected within HVC (Schmidt and Perkel, 1998; Dutar et al., 1999, 2000). This monosynaptic model is less likely, given that metabotropic forms of synaptic transmission typically exhibit a much slower onset than observed here for HVCRA-HVCX cell pairs.
These two models can be distinguished by pharmacological methods: HVCRA-evoked IPSPs in HVCX neurons mediated by the disynaptic mechanism will be abolished by ionotropic glutamate receptor blockers, whereas IPSPs mediated by a monosynaptic, metabotropic glutamatergic pathway should not be affected by such treatment. To distinguish between these two outcomes, we recorded intracellularly from HVCX cells and antidromically activated HVCRA axon collaterals en masse and then bath applied ionotropic glutamate receptor blockers to the slice (Fig. 3). In control conditions, antidromic stimulation of HVCRA axons evoked robust IPSPs in all HVCX neurons that we tested (n = 5), and in all cases, these IPSPs were abolished by the bath application of a mixture of ionotropic glutamate receptor blockers, NBQX (10 μm) and d-APV (50 μm) (Fig. 3) (mean ± SD: control, -12.4 ± 4.8 mV; drug, 0.9 ± 1.7 mV; paired t test; n = 5; p = 0.00132). These results support a model in which HVCRA axon collaterals activate excitatory ionotropic glutamate receptors on inhibitory interneurons, ultimately providing a disynaptic inhibitory linkage from HVCRA to HVCX cells. Together with the results of paired recordings, these experiments also suggest that the HVCRA-interneuron excitatory coupling is sufficiently robust to enable single HVCRA neurons to drive disynaptic, feedforward inhibition in HVCX neurons.
Figure 3.

Antidromic stimulation of HVCRA axons can be used to characterize the pharmacological nature of the inhibitory interactions between HVCRA and HVCX cells. A schematic of the slice preparation (top), showing how antidromic stimulation of the HVCRA axon fiber bundle (lightning bolt) can be used to activate the HVC microcircuit while recording intracellularly from HVCX cells. In this model, local collaterals of the HVCRA axon excite HVCINT, which ultimately drive IPSPs in the HVCX cell. Consistent with this idea, electrical stimulation of the HVCRA fibers drives IPSPs in HVCX cells (bottom; control), which are blocked by the bath application of ionotropic glutamate receptor antagonists (NBQX/APV).
HVCRA-HVCINT pairs
Paired recordings provided direct evidence of the excitatory nature of the synaptic contacts that HVCRA axon collaterals make onto HVCINT, consistent with the disynaptic model of feedforward inhibition between HVCRA and HVCX cells (Fig. 4A). A total of 13 HVCRA-HVCINT cell pairs revealed evidence of unidirectional or bidirectional synaptic coupling. Seven cases of HVCRA-to-HVCINT coupling were observed, and in six of those cases, DC-evoked action potentials in the HVCRA neuron evoked a fast dPSP in the corresponding HVCINT (six of seven HVCRA neurons evoked positive STAs in the interneuron; one of seven HVCRA neurons evoked an IPSP). These dPSPs were characterized by a rapid time course and often by their large amplitudes (Fig. 4B, Table 2) (average peak amplitude, 2.0 ± 0.4 mV; average time to peak, 4.7 ± 0.5 ms; 25% rise time, 1.4 ± 0.1 ms; n = 6). The strength and excitatory nature of this connection was reflected in the observation that a single spike in the HVCRA neuron often was sufficient to drive the interneuron to spike threshold (Fig. 4A,C) (five of six HVCRA-HVCINT pairs displayed such one-for-one spike coupling). This one-to-one spike coupling could account for how a single action potential in an HVCRA neuron can evoke an IPSP in an HVCX neuron. Furthermore, we noted that spike doublets or triplets in the HVCRA neuron could trigger facilitation of EPSPs in the HVCINT (Fig. 4C) (n = 2 pairs). On average, PSPs corresponding to doublet or triplet spikes were facilitated by 46% (paired t test; p < 0.01), measured at an average inter-PSP interval of 27 ms. Such facilitation may explain the observation that spike doublets in HVCRA neurons sometimes were required to trigger IPSPs in HVCX cells. In summary, HVCRA neurons provide short-latency excitatory synaptic input to interneurons.
Figure 4.

Dual intracellular recordings provide direct evidence of the excitatory synapses that HVCRA neurons make with HVCINT. A, Depolarizing current pulses injected into the HVCRA neuron (bottom trace) elicit action potentials, which were followed at short latency by subthreshold (left) and suprathreshold (right) EPSPs in the HVCINT. B, An STA of HVCINT membrane potential triggered off of the HVCRA action potential, from the cell pair shown in A. A fast-rising, short-latency-positive STA was detected after the HVCRA action potential, consistent with the idea that the HVCRA neuron makes an excitatory synapse with the interneuron. C, Longer depolarizing currents could evoke irregular spiking in the HVCRA neuron (bottom trace), which were paralleled by dEPSPs in the HVCINT. In this case, note that the last six HVCRA spikes occurred in doublets and that the second dPSP was larger than the one immediately preceding it, suggestive of synaptic facilitation.
Paired recordings also revealed that interneurons could make synaptic contacts on HVCRA neurons. Eight of the 13 coupled HVCRA-HVCINT pairs showed evidence of synaptic transmission from the interneuron to the PN. In six pairs, the interneuron action potential evoked an IPSP in the HVCRA neuron (individual data not shown; average peak amplitude, -0.9 ± 0.2 mV; average time to peak, 10.7 ± 2.3 ms; 25% rise time, 1.7 ± 1.0 ms; n = 6) (for mean data, see Table 2), whereas in two pairs, the interneuron action potential evoked a dPSP in the HVCRA cell (data not shown). The mean 25% rise time and the mean time to peak of these interneuron-evoked IPSPs were not significantly longer than those recorded in HVCINT-HVCX pairs, consistent with the idea that they were attributable to monosynaptic connections (for statistical comparisons, see Table 2). These results indicate that the HVCRA-HVCINT coupling is robust and bidirectional, at least at the population level. Notably, reciprocal connections were detected in two HVCRA-HVCINT pairs (data not shown). In both cases, the interneuron to PN interaction was inhibitory, whereas the PN to interneuron coupling was excitatory in one case and inhibitory in the other. The latter case may reflect a disynaptic pathway, because the PN-interneuron STA was of longer latency than the interneuron-PN STA (data not shown). The strong excitatory coupling from HVCRA to HVCINT and the existence of reciprocal inhibitory connections from HVCINT to HVCRA suggests that action potential activity in HVCRA neurons could be shaped by inhibitory feedback acting on a spike-by-spike basis.
HVCINT-HVCX pairs
Paired recordings also revealed that interneurons could provide inhibitory input to HVCX cells. In almost all synaptically connected HVCINT-HVCX cell pairs, an action potential in the interneuron evoked an IPSP in the HVCX cell (Fig. 5A) (19 of 20 cases; average peak amplitude, -1.2 ± 0.2 mV; average time to peak, 10.2 ± 0.9 ms; 25% rise time, 1.4 ± 0.3 ms; n = 19) (Table 2). In one case, unidirectional coupling from the HVCX neuron to the interneuron, in the form of a dPSP, was observed (data not shown). We noted that interneuron-evoked IPSPs in HVCX cells had faster rise times and times to peak than did IPSPs recorded in HVCRA-HVCX cell pairs, although IPSPs of either type otherwise had a similar overall shape and time course (Fig. 5B) (for statistical comparisons, see Table 2). The very short onset latency of these responses (i.e., <1.4 ms) supports the view that the fast-spiking interneurons we recorded from here provide monosynaptic inhibitory input to HVCX cells and thus are plausible cellular intermediaries through which HVCRA neurons drive IPSPs in HVCX cells. Another feature we noted was that longer action potential trains in the interneuron generated a sustained hyperpolarization in the HVCX neuron, reminiscent of song-evoked hyperpolarizations that have been described in HVCX cells from in vivo recordings (Fig. 5C) (n = 9 cases). Therefore, interneurons evoke short-latency IPSPs in HVCX cells and are likely to constitute the distal arm of the disynaptic pathway linking HVCRA to HVCX cells.
Paired recordings also provided evidence of reciprocal connectivity between interneurons and HVCX cells (Figs. 5D) (see Fig. 9D, right) (n = 4 pairs). In all four reciprocally connected pairs, the HVCX neuron action potential evoked a dPSP in the interneuron; in two pairs, these PSPs were demonstrably excitatory (Fig. 5D, Table 2) (average peak amplitude, 1.2 ± 0.3 mV; average time to peak, 4.3 ± 0.4 ms; 25% rise time, 1.0 ± 0.6 ms). The rapid onset and time to peak of the dPSPs were similar to those recorded in HVCRA-HVCINT cell pairs (for statistical comparisons, see Table 2). In all of our reciprocally connected HVCINT-HVCX cell pairs, the interneuron action potential evoked an IPSP in the HVCX neuron. Therefore, HVCX neurons can form strong excitatory synapses onto interneurons that provide them with reciprocal inhibitory input, suggesting that action potential activity in HVCX neurons, as with HVCRA neurons, could be shaped on a spike-by-spike basis by inhibitory feedback.
Figure 9.
Sequential paired recordings reveal divergent and convergent inhibitory and excitatory synaptic connections in HVC. A, Sequential recordings from an HVCRA and HVCX neuron while maintaining an intracellular recording from an interneuron. The averages of both PN membrane potentials triggered off of the action potentials of the interneuron showed that a single interneuron could evoke IPSPs in both cells. B, Sequential recordings from three different HVCX neurons (HVCX 1-3) show that action potentials in a single interneuron could evoke IPSPs in all three HVCX cells. C, Sequential recordings from an interneuron and two different HVCRA neurons while maintaining a recording from a single HVCX cell show that both HVCRA cells and the interneuron can provide inhibitory input onto the same HVCX cell. D, A reciprocally connected interneuron-HVCX cell pair also receives synaptic input from HVCRA axon collaterals. Antidromic stimulation of HVCRA axons evokes an EPSP in the interneuron (top) and an IPSP in the HVCX cell (bottom). Spike-triggered averaging reveals that the interneuron evokes an IPSP in the HVCX cell, which in turn could evoke a dPSP in the interneuron. This is the same HVCX-interneuron pair shown in Figure 5D, in which action potentials in the HVCX cell evoked suprathreshold EPSPs in the interneuron. These recordings show that both HVCX and HVCRA axon collaterals can excite the same interneuron in HVC.
Pharmacology of HVCINT-mediated inhibition
Previous studies showed that IPSPs in HVC neurons are mediated by several different neurotransmitter receptors, including ionotropic GABAA receptors and metabotropic GABA and glutamate receptors (Schmidt and Perkel, 1998; Dutar et al., 1999, 2000; Rosen and Mooney, 2003). The relatively fast onset and time to peak of the IPSPs we recorded in HVCINT-HVCX cell pairs suggested that ionotropic GABAA receptors, and not metabotropic receptors, were involved. We tested this idea in several ways. First, we bath applied PTX (50 μm), a GABAA receptor blocker, while recording from synaptically coupled pairs of cells. In two HVCINT-HVCX pairs and one HVCRA-HVCX cell pair, spike-evoked IPSPs in the HVCX cell were abolished by this treatment, indicating that in both types of connections, ionotropic GABAA receptors mediated the IPSP (Fig. 6A,B) (control, -1.2 ± 0.2 mV; PTX, 0.0 ± 0.1 mV; n = 3; p < 0.05; ANOVA). Second, we evoked fast, short-latency IPSPs in HVCX cells by antidromically stimulating HVCRA neurons and then bath applied PTX (50 μm). Treatment with PTX consistently and completely abolished the electrically evoked short-latency IPSP and unmasked a short-latency dPSP (Fig. 7A, inset) (n = 12 cases; control, -13.7 ± 0.6 mV; PTX, +4.9 ± 0.3 mV; p = 0.000012). Third, paired recording revealed that interneuron-evoked IPSPs in an HVCX cell were unaffected by ionotropic glutamate receptor blockers (Fig. 6C), although EPSPs in the interneuron and IPSPs in the HVCX cell evoked by antidromic stimulation of HVCRA neurons were blocked by this treatment (Fig. 6D) (n = 1). These experiments show that GABAA receptors mediate the fast IPSPs evoked in HVCX cells by both interneurons and HVCRA cells. These experiments also reveal monosynaptic and/or polysynaptic excitatory pathways from HVCRA to HVCX cells, which are normally suppressed or otherwise masked by GABAA-mediated inhibition.
Figure 6.

The IPSPs evoked in HVCX cells by both interneurons and HVCRA cells were mediated by GABAA receptors. A, In this interneuron-HVCX cell pair, action potentials in the interneuron evoked a hyperpolarizing response in the HVCX cell, indicative of an IPSP (control). Bath application of the GABAA receptor antagonist PTX blocked the IPSP. B, A negative STA of the HVCX membrane potential evoked by action potentials in an HVCRA neuron was also blocked by the bath application of PTX. C, An IPSP evoked in an HVCX cell by DC-evoked action potentials in a simultaneously recorded interneuron did not decrement in the presence of ionotropic glutamate receptor blockers NBQX and d-APV. D, In the same pair of neurons, antidromic stimulation of the HVCRA fiber tract (arrow) evoked an EPSP in the interneuron (bottom; control) and an IPSP in the simultaneously recorded HVCX cell (top; control). Subsequent bath application of NBQX/d-APV greatly reduced the excitation onto the interneuron and abolished the IPSP in the HVCX cell. Thus, in the presence of compounds that block fast excitatory transmission, inhibition from HVCRA onto HVCX cells is abolished, although inhibition from the interneuron onto the HVCX cell persists. E, Antidromic stimulation of the HVCRA fiber tract was used to evoke an IPSP in an HVCX cell (control). Subsequent bath application of PTX abolished the IPSP, unmasking robust EPSPs, resulting in repetitive action potential discharge in the HVCX neuron (PTX; middle; 4 spikes in burst; mean burst rate, 111 Hz). The subsequent addition of the ionotropic glutamate receptor blockers NBQX and d-APV blocked all synaptic responses in the HVCX cell.
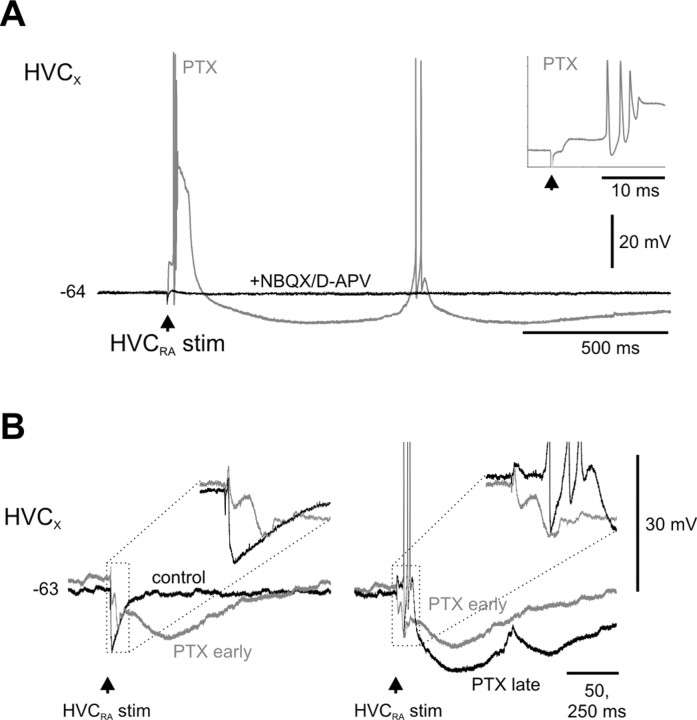
Figure 7.
Blocking GABAA-mediated inhibition in HVC could unmask additional excitatory and inhibitory synaptic pathways from HVCRA to HVCX neurons. A, In a PTX-treated brain slice, antidromic stimulation of HVCRA neurons could evoke repetitive bursting (early burst: 3 spikes, 200 Hz mean burst rate; later burst: 2 spikes, 59 Hz burst rate) and slow hyperpolarizing responses, which were blocked by the bath application of the ionotropic glutamate receptor antagonists NBQX and d-APV. B, Before PTX treatment, antidromic stimulation of the HVCRA fibers evoked a fast IPSP in an HVCX neuron (control); subsequent PTX treatment blocked the early, fast IPSP and unmasked a multiphasic IPSP that included a slow component (PTX early). At later times during the treatment (PTX late), the same stimulation evoked an initial excitatory response, followed by a prolonged, biphasic hyperpolarization. The subthreshold depolarization that occurs during the middle of the slow, biphasic hyperpolarization is believed to be similar to the event associated with the longer-latency bursting behavior in A.
Prolonged blockade of GABAA receptors in HVC also un-masked more complex synaptic interactions between HVCRA and HVCX cells. Notably, after 15-20 min of PTX application, antidromic stimulation of HVCRA neurons evoked larger EPSPs capable of eliciting a high-frequency action potential burst in the HVCX neuron (Fig. 6E, middle). In several cells (n = 3), repetitive bursting followed a single antidromic stimulus (Fig. 7A). PTX treatment also unmasked evidence for slow inhibitory signaling from HVCRA to HVCX cells. In two cells, a prolonged, multiphasic response was evoked by HVCRA stimulation shortly after applying PTX but before the emergence of the dPSP (Fig. 7B, left). In addition, prolonged hyperpolarizations sometimes followed the shorter-latency dEPSPs (Fig. 7A,B, right). The shorter-latency EPSPs, as well as associated prolonged hyperpolarizations, were completely abolished by a combination of NBQX and d-APV, indicating they were mediated in part via monosynaptic and/or polysynaptic pathways involving ionotropic glutamate receptors (Figs. 6E, right; 7A) (n = 9 cases; PTX, +8.8 ± 1.7 mV; NBQX/d-APV, -0.1 ± 0.12 mV; p < 0.001; Mann-Whitney U test). In summary, these pharmacological experiments show that fast-spiking interneurons evoke IPSPs in HVCX cells via GABAA receptors. Furthermore, HVCRA neurons drive excitatory and inhibitory responses in HVCX neurons via synaptic pathways that involve ionotropic glutamate receptors.
Interneurons that evoke IPSPs are PV positive
The interneurons that evoke IPSPs in HVCX cells are fast-spiking cells with varicose dendrites (Fig. 1C,D). Previous immunohistochemical studies indicated that fast-spiking HVC interneurons with varicose dendrites are PV positive (PV+) (Wild et al., 2005) but did not resolve whether PV+ interneurons are a source of inhibitory input onto HVC PNs. We used anti-PV antibodies and intracellular staining with Neurobiotin to determine whether the interneurons that provided inhibitory input onto HVCX cells were PV+. In two HVCINT-HVCX pairs, morphologically and physiologically identified fast-spiking interneurons that evoked IPSPs in HVCX cells were PV+, whereas the corresponding HVCX cells were PV negative (PV-) (Fig. 8A). We did note, however, that HVCX neurons were sometimes in extremely close apposition to PV+ cell bodies (Fig. 8B) (n = 2 cases). These results show that PV+ interneurons provide some of the inhibitory input onto HVC PNs that innervate basal ganglia structures in the songbird brain.
Figure 8.
Fast-spiking interneurons that evoked IPSPs in HVCX neurons are PV+. A, Top, A single optical section of a confocal image of an intracellularly stained interneuron (green) with immunohistochemical staining for PV (red). Action potentials in this interneuron evoked IPSPs in an HVCX cell (data not shown). In the bottom panel, only the red wavelength is shown, showing that the soma of the interneuron was PV+. B, An HVCX neuron (green) that received inhibitory input from a fast-spiking interneuron; a PV+ cell body was closely apposed to the HVCX neuron soma.
Homotypic synaptic interactions
Paired recordings also revealed synaptic interactions between neurons of the same type. Eleven HVCX pairs showed signs of unidirectional inhibitory synaptic coupling, all in the form of spike-evoked IPSPs (individual data not shown; average peak amplitude, -2.0 ± 0.4 mV; average time to peak, 15.4 ± 2.7 ms; 25% rise time, 5.0 ± 1.7 ms; n = 11) (for mean data, see Table 2). These IPSPs were presumably mediated by intervening interneurons, in part because recordings previously described indicated that HVCX neurons can make excitatory synapses onto HVCINT. Furthermore, the mean 25% rise time of IPSPs evoked in HVCX-HVCX cell pairs was significantly longer than that of IPSPs recorded in HVCINT-HVCX, but not HVCRA-HVCX, cell pairs (for statistical comparisons, see Table 2). Five HVCRA pairs were also recorded: four exhibited unidirectional dEPSPs (Table 2), whereas one showed an IPSP (data not shown). Finally, one synaptically coupled interneuron pair was detected that exhibited a unidirectional IPSP (data not shown) (Table 1). Therefore, inhibitory as well as excitatory connections serve to synaptically link homotypic as well as heterotypic pairs of neurons within HVC.
Divergent and convergent synaptic connections in HVC
The extensive local axonal network of both PNs and interneurons raises the possibility of divergent and convergent patterns of synaptic connectivity within HVC. We were able to detect both divergent and convergent synaptic connections by recording from one HVC neuron with a fixed electrode and moving a second electrode about the slice to sequentially record from a series of other cells that were either its presynaptic or postsynaptic partner. In four of these sequential paired recordings, the fixed electrode was in an interneuron, whereas the moveable electrode encountered a series of PNs. Three qualitative observations resulted from these recordings. First, a single interneuron can evoke IPSPs in both HVCRA and HVCX neurons (Fig. 9A). Second, a single interneuron can make divergent inhibitory synapses on several HVCX cells (Fig. 9B). Third, a single interneuron can receive convergent input from two or more HVCRA neurons (data not shown). In three other sequential paired recordings, the fixed electrode was placed in an HVCX neuron, whereas the moveable electrode recorded from HVCINT and/or HVCRA neurons. These recordings revealed that both HVCINT and HVCRA neurons can provide inhibitory input onto the same HVCX cell (Fig. 9C). Therefore, single interneurons can contact PNs of both types and can receive convergent excitatory input from HVCRA neurons.
Interneurons also receive convergent excitatory input from PNs of different types. We examined interactions between the three cell types by coupling antidromic stimulation of HVCRA neurons while using two electrodes to record from HVCINT-HVCX cell pairs. In all six pairs, the HVCINT evoked IPSPs in the HVCX cell; in two of these pairs, the HVCX cell provided reciprocal excitation to the interneuron. In all of these pairs, antidromic activation of the HVCRA axon collateral network evoked an EPSP in the HVCINT cell and an IPSP in the HVCX cell (Fig. 9D). These results show that the two PN types provide convergent excitatory input onto single interneurons, which in turn provide an inhibitory link between the HVC PNs. The various features of the local synaptic organization of HVC revealed in this study are summarized in Figure 10.
Figure 10.
The major synaptic features of the HVC microcircuit revealed in the present study by paired recordings and antidromic stimulation of HVCRA neurons are shown. HVCRA (gray circles) and HVCX (white circles) neurons form excitatory synaptic connections (arrows) on interneurons (black circles), which provide divergent inhibitory input (t-endings) on PNs of both types. Fast excitation is mediated by ionotropic glutamate receptors, whereas fast inhibition is mediated by GABAA receptors. Additional polysynaptic and possibly monosynaptic excitatory pathways and polysynaptic inhibitory pathways also provide a synaptic linkage from HVCRA to HVCX neurons (dashed lines). These monosynaptic and polysynaptic pathways are dependent on ionotropic glutamate receptors, presumably involving direct synapses between HVCRA axon collaterals and HVCX neurons and intervening synapses between HVCRA axon collaterals and other HVC interneurons.
Frequency of synaptic connections between different HVC cell types
We also performed additional paired recordings to provide an estimate both of the frequency of pair types that we encountered using these recording methods and the frequency of synaptic connections that we detected between neurons of given types. A total of 96 pairs was obtained, 18 of which displayed either unidirectional or bidirectional synaptic coupling (∼19%; note that the connected pairs from this sample contributed to the total pool of connected pairs represented in Table 2 and discussed in previous sections of Results) (Table 1). Several features of this sample are notable. First, the vast majority of pairs that we obtained [77 of 96 (∼80%)] contained at least one HVCX neuron, likely reflecting the fact that these cells are relatively numerous and large, and typically afford the most stable recordings with the sharp electrode methods used in this study. Second, almost two-thirds [61 of 96 (63%)] of all pairs consisted of HVCRA neurons, which despite their small size are highly abundant (Kirn et al., 1991; Wild et al., 2005). Perhaps as a result of these various factors, HVCRA-HVCX neuron pairs made up almost one-half this sample [46 of 96 (∼48%)]. Finally, in contrast to our overall larger sample of HVCRA and HVCX neuron pairs, this smaller sample exhibited a higher proportion of HVCX-to-HVCRA synaptic connections [5 of 6 (83%) vs 7 of 29 (24%)] and included three reciprocally connected cell pairs.
Discussion
The present study reveals several synaptic features likely to be important to the song-related motor and auditory functions of HVC. First, HVCRA neurons excite interneurons, which inhibit HVCX neurons, providing a feedforward inhibitory mechanism linking song premotor and basal ganglia projecting pathways emanating from HVC. This feedforward inhibition could help shape motor-related activity transmitted to the AFP and generate cell type-specific patterns of auditory activity. Second, interneurons innervate multiple PNs of both types and thus could coordinate their activity. Finally, HVC contains reciprocally connected PNs and interneurons, similar to other pattern-generating networks.
Feedforward inhibition and excitation from HVCRA to HVCX neurons
These studies show that HVCX cells, which innervate basal ganglia structures important to vocal plasticity (Nottebohm et al., 1976, 1982; Bottjer et al., 1984; Scharff and Nottebohm, 1991), are inhibited directly by interneurons and indirectly by HVC PNs of both types. The IPSPs from HVCRA to HVCX cells were most likely mediated via disynaptic, feedforward mechanisms, because: (1) antagonists of ionotropic glutamate receptors blocked all inhibitory synaptic transmission in HVCX cells evoked by antidromic stimulation of HVCRA fibers; (2) in paired recordings, GABAA receptor blockers abolished IPSPs evoked in HVCX cells by either HVCINT or HVCRA; (3) HVCRA neurons drive fast rise-time EPSPs mediated by ionotropic glutamate receptors on HVCINT and on their extrinsic targets in the nucleus RA; (4) interneurons drive IPSPs in HVCX cells with faster rise times than IPSPs driven in HVCX cells by HVCRA neurons; and (5) spike doublets in HVCRA cells could evoke excitatory synaptic facilitation in interneurons and were sometimes required to trigger IPSPs in HVCX cells. Similar disynaptic mechanisms likely underlie IPSPs detected in pairs of HVCX cells, because the rise times of these IPSPs were relatively slow, like those in HVCRA-HVCX cell pairs, and because HVCX cells evoke EPSPs in interneurons. Additionally, monosynaptic and polysynaptic excitatory pathways and polysynaptic inhibitory pathways link HVCRA to HVCX cells but are normally masked by fast inhibition.
Reciprocal connections between HVC PNs and interneurons
Although a common pattern of synaptic flow was from HVCRA to HVCX cells, HVCX neurons also could evoke depolarizing or hyperpolarizing responses in some HVCRA neurons, and reciprocally coupled heterotypic PN pairs were sometimes encountered. Consistent with the idea that HVCRA and HVCX cells are bidirectionally connected via interneurons, single interneurons could be excited by PNs of both types and also could inhibit multiple PNs of both types. Reciprocal inhibitory interactions between the two PN types may have important implications for the functioning of the HVC in response to song playback and during singing. HVCRA and HVCX cells alternate in their firing during playback of the BOS (Mooney, 2000), which could be explained if these two types of excitatory neurons were coupled via reciprocal inhibition. More generally, half-center oscillators, wherein two neurons make reciprocally inhibitory connections, can produce highly rhythmic bursts of action potential activity (Cropper and Weiss, 1996; Marder and Bucher, 2001; Cymbalyuk et al., 2002). Although some HVCRA and HVCX neurons form architecture characteristic of half-center oscillators, the importance of such an arrangement for generating rhythmical activity underlying singing is unclear, because adult song structure remains intact immediately after selective ablation of HVCX neurons (Scharff et al., 2000). Therefore, other mechanisms in HVC can generate or transmit patterned song premotor activity when HVCX neurons are reduced or absent. One generative mechanism could be reciprocal coupling between excitatory HVCRA neurons and fast-spiking inhibitory interneurons. Indeed, reciprocally connected excitatory and inhibitory neurons can form bistable networks, generating either no output or low-frequency rhythms, depending on the amount of excitatory drive applied to the excitatory cells (Borgers and Kopell, 2005).
Functional implications of divergent and convergent synaptic connections
Sequential paired recordings revealed that interneurons divergently innervate PNs of both types, an arrangement that could synchronize the firing of multiple HVC cells, as occurs in sleeping birds (Rauske et al., 2003). Although synaptically coupled cell pairs recorded here were typically in close proximity (cf. Feldmeyer et al., 1999), intracellular staining showed that interneuron processes are extensive (Fig. 1C) (Katz and Gurney, 1981; Mooney, 2000; Wild et al., 2005), raising the possibility of a more widespread influence on HVC synchrony. Although none of the paired recordings we obtained displayed evidence of electrotonic coupling, gap junctions have been detected in HVC (Gahr and Garcia-Segura, 1996), affording a potential synchronizing influence on HVC activity in addition to or in conjunction with the interneuron network (Deans et al., 2001; Galarreta and Hestrin, 2002; Long et al., 2004). Divergent patterns of interneuron-mediated inhibition have also been invoked to explain HVCRA neuronal activity during singing, which is characterized by temporally sparse action potential bursts thought to propagate sequentially through an array of these neurons (Hahnloser et al., 2002). Convergent excitatory input from HVCRA neurons onto interneurons, coupled with divergent projections from single interneurons onto multiple HVCRA neurons, could form a synaptic substrate for sequence propagation throughout the HVCRA neuronal array. An important future goal will be to determine whether single interneurons synapse on multiple HVCRA neurons, as shown here for HVCX neurons. Furthermore, reciprocal connectivity between single PNs of either type and interneurons, as seen here, could generate negative feedback, limiting spike burst duration and augmenting the temporal sparseness that characterizes HVC PN activity during singing and song playback (Mooney, 2000; Hahnloser et al., 2002). Indeed, simply adding the mean time to peak of HVCRA to interneuron and interneuron to HVCRA PSPs yields a value of ∼15 ms (Table 2). This value is similar to the spike burst duration of HVCRA neurons during singing (typically ∼6 ms in vivo) (Hahnloser et al., 2002) and could be regarded as an estimate of the mean upper limit for the time scale over which reciprocal interactions between HVCRA and interneurons might occur.
Types of interneuron-mediated inhibition
Previous in vivo and in vitro studies showed that HVCX neurons receive remarkably diverse forms of inhibition, including fast IPSPs mediated by GABAA receptors and slow IPSPs mediated by GABAB and metabotropic glutamate receptors (Schmidt and Perkel, 1998; Dutar et al., 1999, 2000; Hahnloser et al., 2002; Rosen and Mooney, 2003). This functional diversity may be reflected in part by the diverse calcium-binding protein expression patterns of HVC interneurons, which contain various combinations of PV, calbindin, and calretinin (Wild et al., 2005). The present study shows that at least some fast-spiking interneurons are PV+ and evoke GABAA receptor-mediated IPSPs in HVCX cells. This result links previous observations that fast-spiking interneurons are PV+ (Wild et al., 2005) and that PV+ cells coexpress the synthetic enzyme for GABA (Zuschratter et al., 1987). Because PV+ neurons evoke fast, GABAA-mediated IPSPs in HVCX cells, they functionally resemble PV+ interneurons in the mammalian cortex, which evoke fast GABAA-mediated inhibitory synaptic currents in pyramidal neurons (Maccaferri et al., 2000). Interneurons also are the likely source of the slow IPSPs evoked in HVCX cells by antidromically stimulating HVCRA neurons in the presence of PTX. These slow IPSPs could be blocked by antagonists of ionotropic glutamate receptors, suggesting they arise polysynaptically through HVC interneurons, rather than monosynaptically via HVCRA axon collaterals (i.e., via metabotropic glutamate receptors) (Schmidt and Perkel, 1998; Dutar et al., 1999, 2000). An important future goal will be to further characterize the correspondence between morphological, biochemical and functional properties of different HVC interneuron types.
Relevance to the auditory and motor properties of HVC
The inhibitory and excitatory linkage from HVCRA to HVCX cells could have important consequences for the processing of auditory and song motor activity in HVC. Exquisite auditory selectivity for the BOS is a hallmark of HVC neuronal responses in anesthetized songbirds (Margoliash, 1983; Theunissen and Doupe, 1998), and the HVC local circuit is thought to play a role in shaping this selectivity (Lewicki and Konishi, 1995; Lewicki and Arthur, 1996; Mooney, 2000; Rosen and Mooney, 2003; Coleman and Mooney, 2004). Intracellular recordings from urethane anesthetized zebra finches have shown that BOS playback evokes distinct subthreshold responses in the two HVC PNs, including sustained and mostly subthreshold depolarization in HVCRA neurons and prolonged hyperpolarizing responses punctuated by phasic excitation in HVCX cells (Mooney, 2000). These hyperpolarizing responses help shape the pattern of BOS-evoked firing in HVCX cells and likely arise through local inhibition onto HVCX cells (Rosen and Mooney, 2003). Indeed, inactivating HVC by local application of GABA unmasks prolonged BOS-evoked depolarizations in HVCX cells, pointing to a local source of inhibition (M. Rosen and R. Mooney, unpublished observations). Furthermore, BOS-evoked hyperpolarizations in HVCX cells closely correlate with firing in interneurons (Mooney, 2000), and these interneurons appear to be the same type shown here that drive fast IPSPs in HVCX cells, because both cells are fast spiking, have varicose dendrites, and are PV+ (Mooney, unpublished observations). However, BOS-evoked hyperpolarizations in HVCX cells involve slow G-protein-mediated potassium currents, with only a cryptic contribution from chloride-mediated currents typical of ionotropic GABAA receptors (Rosen and Mooney, 2003). Therefore, additional inhibitory pathways normally quiescent in the in vitro preparation must be active during song playback, possibly including the slow inhibitory pathways unmasked by PTX that indirectly link HVCRA to HVCX cells.
Chronic recordings in singing birds reveal that activity in the AFP is closely locked to the acoustical features of the bird's song and persists after deafening, suggesting a motor origin (Hessler and Doupe, 1999; Leonardo, 2002). The inhibitory and excitatory synaptic linkage from HVCRA to HVCX cells seen here suggests mechanisms by which HVC circuitry could shape and convey song motor activity to the AFP. First, monosynaptic excitation and lagging disynaptic inhibition from HVCRA cells could generate tightly correlated phasic excitation in HVCX cells (Pouille and Scanziani, 2001), which may enhance signal propagation in the AFP. Second, inhibition can synchronize neuronal firing (Lytton and Sejnowski, 1991; Bush and Sejnowski, 1996) and, in HVCX cells, may also trigger burst firing by deinactivation of low-threshold calcium channels (Kubota and Saito, 1991; Rosen and Mooney, 2003), two features that could facilitate transmission of excitatory signals to the AFP. Third, by analogy to mammalian basal ganglia circuitry (Afifi, 1994; Wichmann and DeLong, 1996; Reiner, 2002), motor-driven inhibition from HVCRA onto HVCX cells could disinhibit downstream targets in the AFP (Wilson, 1993; Sil'kis, 2002; Nambu, 2004). Indeed, inhibitory synapses in the AFP [i.e., between Area X and the medial nucleus of the dorsolateral thalamus (Luo and Perkel, 2002)] could effect the necessary sign inversion for such disinhibition. Fourth, high-frequency (>100 Hz) firing in the HVCRA neuron sometimes was required to evoke an IPSP in the HVCX cell, apparently because of facilitation at the HVCRA-interneuron synapse. Given the propensity for HVCRA neurons to fire in high-frequency bursts during singing (Hahnloser et al., 2002), such facilitation could be integral to shaping premotor activity in HVCX neurons and thus modulating AFP song motor activity. Finally, we noted that antidromic stimulation of HVCRA neurons evoked an IPSP that was nearly 10-fold greater in amplitude than unitary IPSPs evoked in HVCX neurons by either interneurons or HVCRA cells, suggesting that multiple interneurons converge directly onto single HVCX cells and that multiple HVCRA neurons converge indirectly onto HVCX cells. This pattern of convergence in HVC may enable the activity of a larger ensemble of HVCRA neurons to be integrated in single cells projecting to the AFP. Because HVCRA neurons fire in a temporally sparse manner during singing and song playback (Mooney, 2000; Hahnloser et al., 2002), such synaptic integration may facilitate larger time scale representations of song in the AFP.
Footnotes
This work was supported by National Institutes of Health Grants DC02524 and F32 DC006152-01. We thank Dr. Stephen Shea for helpful comments on preliminary versions of this manuscript. We also acknowledge the technical support of David Kloetzer and Stefan Nenkov.
Correspondence should be addressed to Dr. Richard Mooney, Department of Neurobiology, Duke University School of Medicine, Durham, NC 27710. E-mail: mooney@neuro.duke.edu.
Copyright © 2005 Society for Neuroscience 0270-6474/05/251952-13$15.00/0
References
- Afifi AK (1994) Basal ganglia: functional anatomy and physiology. Part 1. J Child Neurol 9: 249-260. [DOI] [PubMed] [Google Scholar]
- Borgers C, Kopell N (2005) Effects of noisy drive on rhythms in networks of excitatory and inhibitory neurons. Neural Comput, in press. [DOI] [PubMed]
- Bottjer SW, Miesner EA, Arnold AP (1984) Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224: 901-903. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ (2000) Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762-766. [DOI] [PubMed] [Google Scholar]
- Bush P, Sejnowski T (1996) Inhibition synchronizes sparsely connected cortical neurons within and between columns in realistic network models. J Comput Neurosci 3: 91-110. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Mooney R (2004) Synaptic transformations underlying highly selective auditory representations of learned birdsong. J Neurosci 24: 9251-9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cropper EC, Weiss KR (1996) Synaptic mechanisms in invertebrate pattern generation. Curr Opin Neurobiol 6: 833-841. [DOI] [PubMed] [Google Scholar]
- Cymbalyuk GS, Gaudry Q, Masino MA, Calabrese RL (2002) Bursting in leech heart interneurons: cell-autonomous and network-based mechanisms. J Neurosci 22: 10580-10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL (2001) Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron 31: 477-485. [DOI] [PubMed] [Google Scholar]
- Dutar P, Vu HM, Perkel DJ (1998) Multiple cell types distinguished by physiological, pharmacological, and anatomic properties in nucleus HVc of the adult zebra finch. J Neurophysiol 80: 1828-1838. [DOI] [PubMed] [Google Scholar]
- Dutar P, Vu HM, Perkel DJ (1999) Pharmacological characterization of an unusual mGluR-evoked neuronal hyperpolarization mediated by activation of GIRK channels. Neuropharmacology 38: 467-475. [DOI] [PubMed] [Google Scholar]
- Dutar P, Petrozzino JJ, Vu HM, Schmidt MF, Perkel DJ (2000) Slow synaptic inhibition mediated by metabotropic glutamate receptor activation of GIRK channels. J Neurophysiol 84: 2284-2290. [DOI] [PubMed] [Google Scholar]
- Feldmeyer D, Egger V, Lubke J, Sakmann B (1999) Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single “barrel” of developing rat somatosensory cortex. J Physiol (Lond) 521: 169-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D (1995) Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata). J Comp Neurol 360: 413-441. [DOI] [PubMed] [Google Scholar]
- Foster EF, Bottjer SW (1998) Axonal connections of the high vocal center and surrounding cortical regions in juvenile and adult male zebra finches. J Comp Neurol 397: 118-138. [PubMed] [Google Scholar]
- Gahr M, Garcia-Segura LM (1996) Testosterone-dependent increase of gap-junctions in HVC neurons of adult female canaries. Brain Res 712: 69-73. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S (2002) Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA 99: 12438-12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS (2002) An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: 65-70. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ (1999) Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci 19: 10461-10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW (1993) Induced cell death in a thalamic nucleus during a restricted period of zebra finch vocal development. J Neurosci 13: 2452-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Gurney ME (1981) Auditory responses in the zebra finch's motor system for song. Brain Res 221: 192-197. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Alvarez-Buylla A, Nottebohm F (1991) Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J Neurosci 11: 1756-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M (1965) The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol 22: 770-783. [PubMed] [Google Scholar]
- Kubota M, Saito N (1991) Sodium- and calcium-dependent conductances of neurones in the zebra finch hyperstriatum ventrale pars caudale in vitro. J Physiol (Lond) 440: 131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A (2002) Neural dynamics underlying complex behavior in a songbird. PhD thesis, California Institute of Technology.
- Lewicki MS (1996) Intracellular characterization of song-specific neurons in the zebra finch auditory forebrain. J Neurosci 16: 5855-5863. [PubMed] [Google Scholar]
- Lewicki MS, Arthur BJ (1996) Hierarchical organization of auditory temporal context sensitivity. J Neurosci 16: 6987-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki MS, Konishi M (1995) Mechanisms underlying the sensitivity of songbird forebrain neurons to temporal order. Proc Natl Acad Sci USA 92: 5582-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston FS, Mooney R (1997) Development of intrinsic and synaptic properties in a forebrain nucleus essential to avian song learning. J Neurosci 17: 8997-9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston FS, White SA, Mooney R (2000) Slow NMDA-EPSCs at synapses critical for song development are not required for song learning in zebra finches. Nat Neurosci 3: 482-488. [DOI] [PubMed] [Google Scholar]
- Long MA, Landisman CE, Connors BW (2004) Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus. J Neurosci 24: 341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Perkel DJ (2002) Intrinsic and synaptic properties of neurons in an avian thalamic nucleus during song learning. J Neurophysiol 88: 1903-1914. [DOI] [PubMed] [Google Scholar]
- Lytton WW, Sejnowski TJ (1991) Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J Neurophysiol 66: 1059-1079. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P (2000) Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol (Lond) 524: 91-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D (2001) Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986-R996. [DOI] [PubMed] [Google Scholar]
- Margoliash D (1983) Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci 3: 1039-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D, Fortune ES (1992) Temporal and harmonic combination-sensitive neurons in the zebra finch's HVc. J Neurosci 12: 4309-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R (1992) Synaptic basis for developmental plasticity in a birdsong nucleus. J Neurosci 12: 2464-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R (2000) Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J Neurosci 20: 5420-5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Konishi M (1991) Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci USA 88: 4075-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu A (2004) A new dynamic model of the cortico-basal ganglia loop. Prog Brain Res 143: 461-466. [DOI] [PubMed] [Google Scholar]
- Nixdorf BE (1989) Ultrastructural analysis of the development and maturation of synapses and subsynaptic structures in the ectostriatum of the zebra finch. J Comp Neurol 290: 472-486. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM (1976) Central control of song in the canary, Serinus canarius J Comp Neurol 165: 457-486. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA (1982) Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol 207: 344-357. [DOI] [PubMed] [Google Scholar]
- Perkel DH, Gerstein GL, Moore GP (1967) Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J 7: 391-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M (2001) Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science 293: 1159-1163. [DOI] [PubMed] [Google Scholar]
- Prather J, Mooney R (2003) Synaptic coupling of HVC neurons revealed by pairwise recordings. Soc Neurosci Abstr 29: 294.11. [Google Scholar]
- Price PH (1979) Developmental determinants of structure in zebra finch song. J Comp Physiol Psychol 93: 260-277. [Google Scholar]
- Rauske PL, Shea SD, Margoliash D (2003) State and neuronal class-dependent reconfiguration in the avian song system. J Neurophysiol 89: 1688-1701. [DOI] [PubMed] [Google Scholar]
- Reiner A (2002) Functional circuitry of the avian basal ganglia: implications for basal ganglia organization in stem amniotes. Brain Res Bull 57: 513-528. [DOI] [PubMed] [Google Scholar]
- Rosen MJ, Mooney R (2003) Inhibitory and excitatory mechanisms underlying auditory responses to learned vocalizations in the songbird nucleus HVC. Neuron 39: 177-194. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F (1991) A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci 11: 2896-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F (2000) Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 25: 481-492. [DOI] [PubMed] [Google Scholar]
- Schmidt MF, Perkel DJ (1998) Slow synaptic inhibition in nucleus HVc of the adult zebra finch. J Neurosci 18: 895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston AI, Moulins M (1985) Oscillatory neural networks. Annu Rev Physiol 47: 29-48. [DOI] [PubMed] [Google Scholar]
- Sil'kis IG (2002) A possible mechanism for the dopamine-evoked synergistic disinhibition of thalamic neurons via the “direct” and “indirect” pathways in the basal ganglia. Neurosci Behav Physiol 32: 205-212. [DOI] [PubMed] [Google Scholar]
- Theunissen FE, Doupe AJ (1998) Temporal and spectral sensitivity of complex auditory neurons in the nucleus HVc of male zebra finches. J Neurosci 18: 3786-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ (2000) An associational model of birdsong sensori-motor learning I. Efference copy and the learning of song syllables. J Neurophysiol 84: 1204-1223. [DOI] [PubMed] [Google Scholar]
- White SA, Livingston FS, Mooney R (1999) Androgens modulate NMDA receptor-mediated EPSCs in the zebra finch song system. J Neurophysiol 82: 2221-2234. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR (1996) Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol 6: 751-758. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Howie GJ, Mooney R (2005) Calcium binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata). J Comp Neurol 483: 76-90. [DOI] [PubMed] [Google Scholar]
- Wilson CJ (1993) The generation of natural firing patterns in neostriatal neurons. Prog Brain Res 99: 277-297. [DOI] [PubMed] [Google Scholar]
- Zuschratter W, Braun S, Scheich H (1987) Co-localization of parvalbumin, calbindin and GABA in avian vocal motor system. Neuroscience [Suppl] 22: S114. [Google Scholar]