Abstract
The gut bacterial genotoxin colibactin is linked to the development of colorectal cancer. In the final stages of colibactin’s biosynthesis, an inactive precursor (precolibactin) undergoes proteolytic cleavage by ClbP, an unusual innermembrane-bound periplasmic peptidase, to generate the active genotoxin. This enzyme presents an opportunity to monitor and modulate colibactin biosynthesis, but its active form has not been studied in vitro and limited tools exist to measure its activity. Here, we describe the in vitro biochemical characterization of catalytically active, full-length ClbP. We elucidate its substrate preferences and use this information to develop a fluorogenic activity probe. This tool will enable the discovery of ClbP inhibitors and streamline identification of colibactin-producing bacteria.
Graphical Abstract
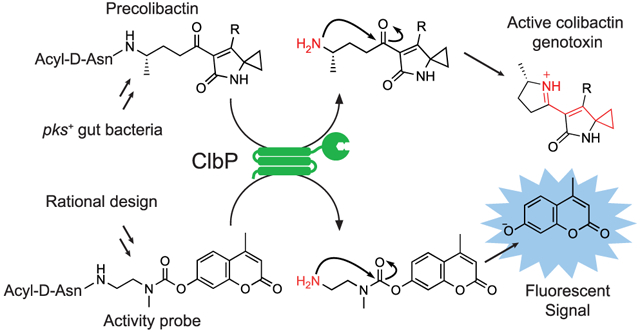
The trillions of microorganisms living on and in the human body, known as the human microbiota, have been implicated in many diseases, including cancer. In particular, commensal gut bacteria harboring the pks island are linked to colorectal cancer (CRC).1-3 The pks island encodes a hybrid nonribosomal peptide synthetase-polyketide synthase (NRPS-PKS) assembly line that synthesizes the small molecule genotoxin colibactin.4 Prior work has shown that pks+E. coli induce DNA double-strand breaks in eukaryotic cells and increase tumor load in mouse models of CRC in an inflammation-dependent fashion.2,5,6 While pks+ organisms are present in ~20% of healthy individuals, they are more prevalent in patients with inflammatory bowel disease (40%) and CRC (55%–67%).2,5 Our understanding of colibactin’s role in carcinogenesis is limited, as the active molecule has never been isolated or structurally characterized and cannot be readily detected. We sought to develop a fluorogenic probe that would allow us to measure the activity of the colibactin biosynthetic pathway in a more direct manner than current sequencing-based methods, which detect the pks genes. Such a tool could also accelerate the identification of small molecules that can inhibit colibactin production.
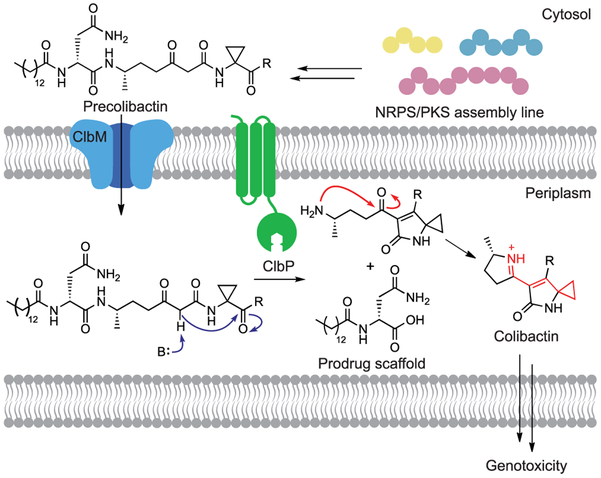
We chose the colibactin biosynthetic enzyme ClbP as a target for probe development. This periplasmic peptidase proteolytically activates colibactin in the final stages of genotoxin assembly by removing an N-myristoyl-d-Asn “prodrug scaffold” from an inactive precursor, precolibactin (Figure 1). Based on the structures of candidate precolibactins isolated from clbP deletion mutants (Figure S1), it has been hypothesized that cleavage by ClbP triggers the formation of an α,β-unsaturated iminium-conjugated cyclopropane which serves as an electrophilic warhead for DNA alkylation.7,8 This proposal has been supported by the recent identification of colibactin-derived DNA adducts.9,10 We further describe the reported structures of candidate precolibactins in a Supplementary Discussion section.
Figure 1.
ClbP is an essential part of colibactin’s prodrug resistance mechanism. Precolibactin is synthesized by the NRPS-PKS assembly line and tailoring enzymes encoded by the pks island before hydrolysis by ClbP in the periplasm. While previous work has shown that iminium formation (red arrows) is only possible after cleavage by ClbP, the timing of lactam formation (blue arrows) has not been firmly established (see also Figure S1). ‘R’ = undefined.
Previous studies revealed that ClbP possesses three C-terminal transmembrane (TM) helices anchored in the inner membrane as well as a soluble periplasmic peptidase domain.11 Prior in vitro studies of ClbP used the truncated peptidase domain (ClbPpep), but this construct does not rescue genotoxicity in a clbP mutant and cannot process precolibactin-like synthetic substrates in whole cells.12,13 In fact, deletion of even a single TM helix blocks genotoxicity completely.11 Challenges in obtaining the full-length enzyme (ClbPFL) have hampered the discovery and characterization of ClbP inhibitors, with previous efforts limited to in silico screening with ClbPpep and indirect phenotypic measurements of activity.14 We therefore began our work by characterizing ClbPFL in vitro.
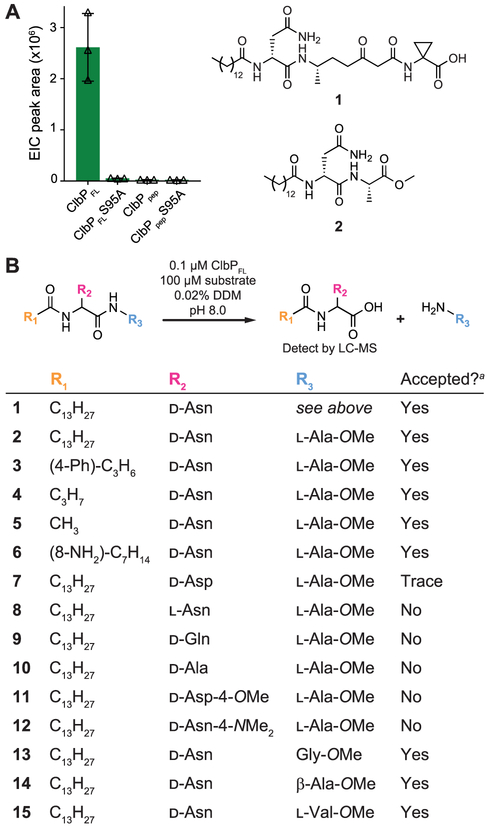
We cloned and overexpressed C-terminal His10-tagged ClbP with its TM helices intact (ClbPFL-CHis10), as well as an inactive mutant lacking the catalytic serine nucleophile (ClbPFL-S95A-CHis10), in E. coli C41 (Figure S2). We obtained purified enzyme by separating the cell components using ultracentrifugation, solubilizing the membranes in an n-dodecyl-β-d-maltoside (DDM)-containing buffer, and performing immobilized metal affinity chromatography. Because the structure of precolibactin has not yet been fully elucidated, we tested purified ClbPFL against a variety of synthetic precolibactin analogues. We found that ClbPFL cleaves isolated candidate precolibactins, such as 1,15 as well as the simplified precolibactin mimic 2 rapidly in vitro, while ClbPFL-S95A and ClbPpep showed no activity by LC-MS (Figure 2A). The kinetic parameters for cleavage of 1 and 2 by ClbPFL were comparable (Figure S3), indicating the importance of the prodrug scaffold for recognition and catalysis. We chose substrate 2 for a more detailed structure–activity relationship (SAR) study.
Figure 2.
Characterization of ClbPFL activity in vitro. (A) LC-MS detection of the prodrug scaffold ([M + H]+: 343.2597 m/z) produced by ClbP-mediated hydrolysis of 100 μM 2 in vitro. Error bars represent 1 standard deviation (SD), n = 3. (B) SAR study of ClbP substrates. a Based on detection of N-acyl-d-Asn cleavage products by LC-MS. Figures S4-S7.
We first varied the N-acyl substituent of the prodrug scaffold. We expected that ClbP would accommodate changes at this position since E. coli overexpressing ClbPFL can hydrolyze synthetic precolibactin mimics bearing shorter fatty acyl chains.13 Thus, we synthesized a series of precolibactin mimics with varied N-acyl chain length, polarity, and steric bulk (Figure 2B, entries 3–6). LC-MS assays confirmed that ClbPFL hydrolyzed all of these substrates at the expected peptide linkage (Figure 2B, Figure S4). We also determined kinetic parameters for substrates 2, 3, and 4 using a fluorescence-based assay with o-phthaldialdehyde (OPA, Figure S5). The higher kcat and lower Km values observed for more hydrophobic, long-chain substrates indicate acyl chain length is an important, though nonessential, recognition feature (Table S1). These observations, together with the fact that ClbPpep cannot process 2 but retains some weak activity toward smaller substrates,12 suggest that the TM helices could play a role in substrate binding by interacting with the hydrophobic acyl substituent.
We next tested the ability of ClbPFL to hydrolyze substrates bearing different amino acids within the prodrug scaffold (Figure 2B, entries 7–12). When ClbPFL was incubated with substrates containing l-Asn, d-Gln, or d-Ala (8–10), no cleavage products were detected by LC-MS (Figure S6). The d-Asp containing substrate (7) was processed at low levels (>10% activity relative to 2), but we observed no hydrolysis of the corresponding d-Asp methyl ester (11) and d-Asn dimethylamide (12) substrates (Figure S6). These results indicate that this amino acid is a key recognition motif. Two closely related peptidases, ZmaM and XcnG, also hydrolyze substrates containing d-Asn at the same position in vivo, suggesting that this feature is evolutionarily conserved.16,17
We next examined ClbP’s selectivity for the second amino acid position of precolibactin mimics. Although all candidate precolibactins characterized to date have l-alanine at this location, ClbBNRPS, the module responsible for incorporating this building block accepts other amino acids in vitro.13 We incubated ClbPFL with substrates 13–15 (Figure 2B) and detected cleavage of all substrates by LC-MS (Figure S7). The observation that ClbPFL accommodates variation at this position is in agreement with the exceptionally large groove around the catalytic residues seen in the crystal structure of ClbPpep.11 The native precolibactin substrate is likely a much larger molecule than 13–15, which may explain ClbP’s promiscuity toward these substrates.
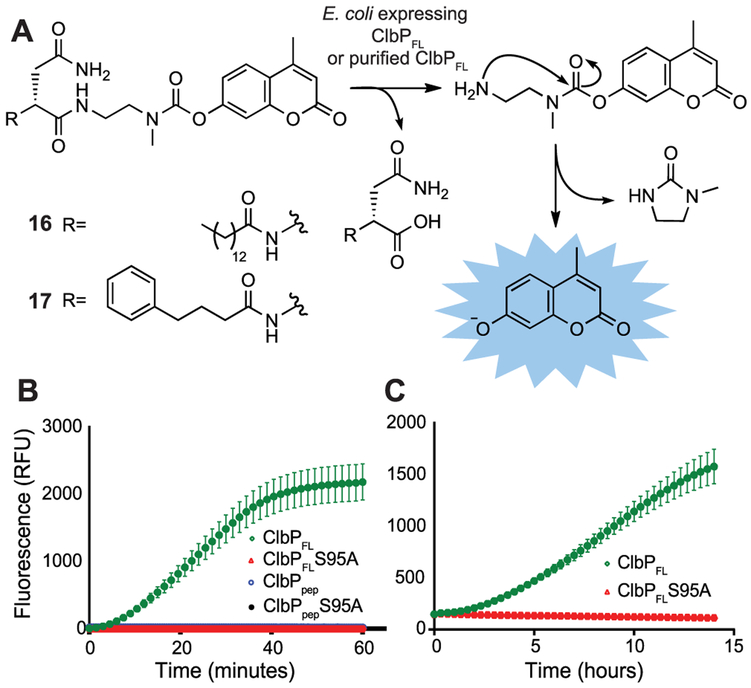
Using this information, we sought to develop a fluorogenic probe for ClbP activity. We designed a three-component probe that would incorporate a large hydrophobic acyl substituent, the key d-Asn residue, and a caged fluorophore connected to the prodrug scaffold by a self-cleaving linker. This three-component approach has been used successfully with more canonical types of peptidases.18-20 Our initial target, 16, was accessed in 9 steps from commercially available materials (see Supporting Information). Notably, the activation of 16 is analogous to precolibactin activation: hydrolysis by ClbP reveals a primary amine, which can undergo a rapid 5-exo-trig cyclization to produce the active species (Figure 3A). While ClbPFL processed 16 in vitro and in live E. coli, its low solubility and membrane permeability likely led to poor performance in some cell-based assays (Figure S8).
Figure 3.
Fluorogenic probes monitor ClbP activity in whole cells and in vitro. (A) Mechanism of activation of fluorogenic probes. (B) 17 is activated by ClbPFL but not ClbPpep or ClbPFL-S95A in vitro. Points represent mean fluorescence of 6 reactions for each enzyme ± SD. (C) 17 can be activated by whole cells. Points represent mean fluorescence of 6 cultures of E. coli BL21 expressing each enzyme ± SD.
Because our SAR study indicated that ClbPFL hydrolyzed the 4-phenylbutyryl-containing substrate 3 with similar catalytic efficiency to substrate 2 (kcat/Km = 5400 ± 1800 vs 4600 ± 1100 M−1 s−1, Table S1), we synthesized an analogous probe containing the same modification (17, Figure 3) in hopes of improving probe solubility and performance. 17 was cleaved rapidly by ClbPFL in vitro and showed negligible background activity with the S95A mutant and ClbPpep (Figure 3B). It also exhibited excellent stability in DMSO stock solutions, in vitro assay conditions, and LB and MEGA growth media. In an in vitro assay containing 50 μM 17, cleavage by ClbPFL results in a >100-fold increase in fluorescence relative to the control in less than 30 min (Figure 3B). Probe 17 also shows robust and consistent cleavage in a whole-cell assay format with E. coli overexpressing ClbPFL (Figure 3C).
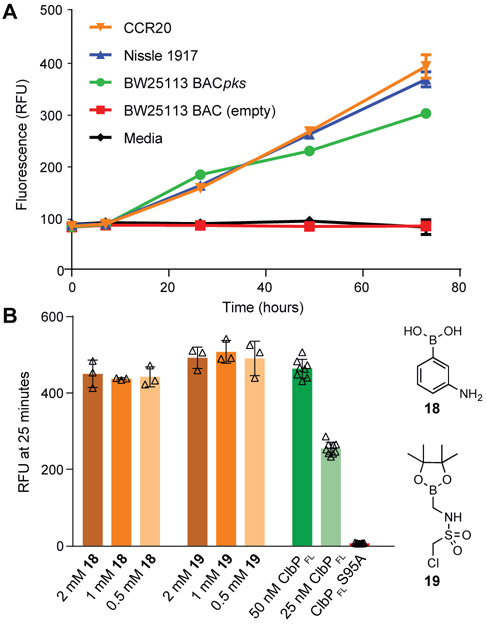
We next assessed whether our probe could detect ClbP activity in wild-type pks+ strains. For this assay to provide a reliable read out, it must be sensitive enough to be activated by native levels of ClbP. Indeed, we reliably detected activation of probe 17 after overnight incubation with the pks+ strains E. coli CCR20 and E. coli Nissle 1917, as well as with a strain heterologously expressing the pks island (BW25113 BACpks) (Figure 4A).
Figure 4.
Probe 17 can identify pks+ organisms and assess inhibition by small molecules. (a) Wild-type pks+E. coli strains were grown in MEGA medium under anaerobic conditions with 100 μM 17 (Error bars are SD, n = 3, Relative Fluorescence Units (RFU) values measure whole culture). (b) Purified ClbPFL and the indicated concentration of 18 or 19 were incubated for 1 h and fluorescence of each reaction was measured 25 min after addition of 17 (Error bars are SD, treatment n = 3, controls n = 9, 50 nM enzyme except where indicated.) Differences between treatment groups and control were not significant (Student’s two-tailed t test, P > 0.05).
Finally, we used probe 17 to test the inhibition of ClbP by small molecules. Boronic acids 18 and 19 were identified previously by in silico docking with the ClbPpep crystal structure.14 These molecules block the genotoxicity of pks+E. coli toward mammalian cells in tissue culture, but their effect on the catalytic activity of ClbP has not been reported. When ClbPFL was incubated in vitro with either 18 or 19 and probe 17, we observed a small decrease in the fluorescent signal produced, but this change was not statistically significant. This assay was also run under the same conditions with half the amount of ClbPFL present. This control showed approximately 50% activity, confirming that the assay is sensitive to modest changes in the activity of the enzyme (Figure 4B). We also used a more sensitive LC-MS assay to evaluate the inhibition of ClbPFL with substrate 2. In this format, we observed weak but statistically significant inhibition of ClbPFL (10–15% inhibition at 2 mM 18, Figure S9). The weakness of this effect suggests that these molecules abrogate genotoxicity through other cellular targets, and there remains a need for potent ClbP inhibitors. As a first step toward identifying such molecules, we screened a panel of known serine hydrolase inhibitors, including protease inhibitors, β-lactamase inhibitors, and lipase inhibitors, for ClbPFL inhibition. None of the compounds tested showed inhibition at 2 mM concentration (Figure S10). The fact that even broad-spectrum sulfonyl fluoride and fluorophosphonate inhibitors cannot block activity in vitro raises interesting questions about how ClbP’s structure may regulate access to the active site.
Despite over a decade of research, the colibactin genotoxin remains elusive, and evidence of its carcinogenicity relies on correlations and models that cannot fully recapitulate the complexity of the gut microbiota. To better understand this intricate pathway, we have characterized the essential colibactin-activating peptidase ClbP in vitro and developed a fluorogenic probe for its activity. Our SAR study provides a starting point for the rational design of ClbP inhibitors, while our fluorogenic assay will allow the use of high-throughput screening toward the same goal. Such inhibitors would enable more detailed studies of colibactin’s effects in physiologically relevant animal models and in complex microbiotas. The fact that our probe can detect ClbP activity in wild-type pks+ strains also opens up the possibility of activity-based diagnostics to detect these organisms.
ClbP is the most well-characterized enzyme in a larger family of prodrug-activating peptidases. Though homologues ZmaM and XcnG have been studied, ClbP is the only member of this family to be purified and characterized in vitro. Qian and coworkers have shown that additional peptidases that likely recognize N-acyl-d-Asn motifs are encoded in cryptic gene clusters.21 Our fluorogenic probe may be adaptable to these uncharacterized homologues, enabling the direct monitoring of other secondary metabolite pathways. Overall, this work shows how detailed characterization of biosynthetic enzymes can enable the development of innovative tools to interrogate natural products.
METHODS
In Vitro SAR Study with Substrates 2–15.
Substrates were prepared as 10 mM stock solutions in DMSO and then diluted to 120 μM in standard Tris assay buffer (50 mM Tris pH 8.0, 200 mM NaCl, 0.02% w/v DDM). ClbPFL-CHis10 and ClbPFL-S95A-CHis10 were defrosted from −80 °C stocks on ice and then diluted to 0.6 μM in Tris assay buffer. 25 μL of enzyme were deposited in individual wells of a 96-well plate in triplicate. Reactions were initiated by the addition of 125 μL of the appropriate substrate in Tris assay buffer to a final concentration of 0.1 μM enzyme, 100 μM substrate, 1% DMSO and incubated at 25 °C. A 20 μL sample of each reaction mixture was immediately diluted into 180 μL of cold LC-MS-grade methanol as the “t0” time point. Reactions were incubated at rt for 5 h, at which point another 20 μL of each reaction mixture were diluted into 180 μL of cold LC-MS grade methanol and mixed by pipetting (“t5”). 96-well plates containing the methanol-quenched reactions were sealed with adhesive foil and stored at −20 °C overnight. Plates were then centrifuged at 3,880 × g at 4 °C for 15 min, and 20 μL of the supernatant were diluted again into 180 μL of LC-MS-grade. Samples were analyzed by LC-MS following the procedures outlined in the General Materials and Methods (see Supporting Information), with the exception of substrates 4, 5, and 6 for which the following chromatography method was used: 0% solvent A in solvent B for 30 s, a gradient from 0% A to 50% A over 9.5 min, followed by a gradient to 90% A over 2 min, holding at 90% A for 3 min, a gradient back to 0% A over 1 min, hold at 0% A for 3 min (solvent A = acetonitrile + 0.1% formic acid; solvent B = water + 0.1% formic acid. Flow rate = 0.4 mL/min, injection volume = 10 μL). Relative activity was determined by measuring the difference in Extracted Ion Chromatogram (EIC) peak area for the expected mass of the prodrug scaffold fragment (±5 ppm) between the “t5” and “t0” samples and comparing this value for each substrate to substrate 1 which was used as a control in each sample group. For comparing substrates 2–6, a standard curve for each substrate was used to quantify the molar consumption of substrate. All substrates were assayed in triplicate.
In Vitro Activity and Inhibition Assays with Fluorescent Probes.
Putative inhibitors were prepared as 50 mM stocks in DMSO, while probe 17 was prepared as a 10 mM stock in DMSO. ClbPFL-CHis10 was defrosted on ice from −80 °C stocks, diluted to 62.5 nM in standard Tris assay buffer (50 mM Tris pH 8.0, 200 mM NaCl, 0.02% w/v DDM), and incubated with the appropriate concentration of inhibitor or vehicle (DMSO) for 1 h at ambient temperature (n = 3 for each concentration). Separately, 250 μM 17 in Tris assay buffer was deposited in the wells of an opaque black 384-well flat-bottom plate (5 μL/well). Reactions were initiated by transferring 20 μL of the enzyme/inhibitor or enzyme/DMSO mix to the 384-well plate (final assay conditions: 0.1 μM enzyme, 50 μM probe 17, 0–2 mM inhibitor, 4.5% DMSO, 25 °C). Activity was monitored by measuring the fluorescence of each well once per minute for 4 h using a Bio-Tek Synergy HTX multimode plate reader (360/40 nm excitation filter, 440/20 nm emission filter).
Kinetic Comparison of Substrates 1 and 2 with ClbPFL (LC-MS).
In vitro reactions of ClbPFL-CHis10 1 and 2 were conducted as described above with assays for the SAR study with 50 nm ClbPFLCHis10. Initial reaction rates were determined at various concentrations of substrates by quenching 10 μL aliquots of the reaction mixture in 90 μL of cold LC-MS grade methanol every minute and analyzing these mixtures via LC-MS as described above. Conversion was quantified using a standard curve of the myristoyl-d-Asn cleavage product for both substrates (6 concentrations in triplicate). Rates were calculated at 7 concentrations for both substrates in 3 technical replicates and fit to the Michaelis–Menten equation using the GraphPad Prism software package.
Procedures and characterization data for all synthetic compounds can be found in the Supporting Information. All other procedures, including protocols for the purification of the enzymes discussed and additional biochemical characterization, can also be found in the Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank F. Prati for providing compound 18, R. Müller and R. Bonnet for providing bacterial strains, Y. Jiang and P. Boudreau for input on experimental design, J. Velilla Garcia and R. Gaudet for plasmids and helpful discussion, C. Chittim and M. Bollenbach for comments on the manuscript, and D. Kahne and co-workers for use of equipment. The authors acknowledge financial support from the National Institutes of Health (R01CA208834) and the Damon Runyon-Rachleff Innovation Award. M.R.W. acknowledges support from the American Cancer Society-New England Division Postdoctoral Fellowship (PF-16-122-01-CDD). S.E.J. acknowledges support from The Amgen Scholars Program.
Footnotes
The authors declare the following competing financial interest(s): A provisional patent has been filed on the fluorogenic probes described here which lists M.R.V., M.R.W., and E.P.B. as co-inventors (U.S. Provisional Application No.: 62/719,325).
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschembio.9b00069.
Detailed Materials and Methods; Supplementary Discussion; Supplementary Figures S1-S9; Synthetic procedures and small molecule characterization data (PDF)
DEDICATION
This paper is dedicated to the memory of our friend and colleague Ethan S. Winter.
REFERENCES
- (1).Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, Darfeuille-Michaud A, Pezet D, and Bonnet R (2013) High Prevalence of Mucosa-Associated E. coli Producing Cyclomodulin and Genotoxin in Colon Cancer. PLoS One 8, e56964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan T-J, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, and Jobin C (2012) Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 338, 120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, Shields CED, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, and Sears CL (2018) Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Nougayrede J-P, Homburg S, Taieb F, Boury M, Brzuskiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, and Oswald E (2006) Escherichia coli Induces DNA Double-Strand Breaks in Eukaryotic Cells. Science 313, 848–851. [DOI] [PubMed] [Google Scholar]
- (5).Cougnoux A, Dalmasso G, Martinez R, Buc E, Delmas J, Gibold L, Sauvanet P, Darcha C, Déchelotte P, Bonnet M, Pezet D, Wodrich H, Darfeuille-Michaud A, and Bonnet R (2014) Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 63, 1932–1942. [DOI] [PubMed] [Google Scholar]
- (6).Tomkovich S, Yang Y, Winglee K, Gauthier J, Mühlbauer M, Sun X, Mohamadzadeh M, Liu X, Martin P, Wang GP, Oswald E, Fodor AA, and Jobin C (2017) Locoregional Effects of Microbiota in a Preclinical Model of Colon Carcinogenesis. Cancer Res. 77, 2620–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Healy AR, Nikolayevskiy H, Patel JR, Crawford JM, and Herzon SB (2016) A Mechanistic Model for Colibactin-Induced Genotoxicity. J. Am. Chem. Soc. 138, 15563–15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Balskus EP (2015) Colibactin: understanding an elusive gut bacterial genotoxin. Nat. Prod. Rep. 32, 1534–40. [DOI] [PubMed] [Google Scholar]
- (9).Wilson MR, Jiang Y, Villalta PW, Stornetta A, Boudreau PD, Carrá A, Brennan CA, Chun E, Ngo L, Samson LD, Engelward BP, Garrett WS, Balbo S, and Balskus EP (2019) The human gut bacterial genotoxin colibactin alkylates DNA. Science 363, eaar7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Xue M, Shine EE, Wang W, Crawford JM, and Herzon SB (2018) Characterization of natural colibactin–nucleobase adducts by tandem MS and isotopic labeling. Support for DNA alkylation by cyclopropane ring opening. Biochemistry 57, 6391–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Cougnoux A, Gibold L, Robin F, Dubois D, Pradel N, Darfeuille-Michaud A, Dalmasso G, Delmas J, and Bonnet R (2012) Analysis of structure-function relationships in the colibactin-maturating enzyme ClbP. J. Mol. Biol. 424, 203–214. [DOI] [PubMed] [Google Scholar]
- (12).Dubois D, Baron O, Cougnoux A, Delmas J, Pradel N, Boury M, Bouchon B, Bringer M-A, Nougayrede J-P, Oswald E, and Bonnet R (2011) ClbP Is a Prototype of a Peptidase Subgroup Involved in Biosynthesis of Nonribosomal Peptides. J. Biol. Chem. 286, 35562–35570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Brotherton CA, and Balskus EP (2013) A Prodrug Resistance Mechanism Is Involved in Colibactin Biosynthesis and Cytotoxicity. J. Am. Chem. Soc. 135, 3359–3362. [DOI] [PubMed] [Google Scholar]
- (14).Cougnoux A, Delmas J, Gibold L, Faïs T, Romagnoli C, Robin F, Cuevas-Ramos G, Oswald E, Darfeuille-Michaud A, Prati F, Dalmasso G, and Bonnet R (2016) Small-molecule inhibitors prevent the genotoxic and protumoural effects induced by colibactin-producing bacteria. Gut 65, 278–285. [DOI] [PubMed] [Google Scholar]
- (15).Zha L, Jiang Y, Henke MT, Wilson MR, Wang JX, Kelleher NL, and Balskus EP (2017) Colibactin assembly line enzymes use S-adenosylmethionine to build a cyclopropane ring. Nat. Chem. Biol. 13, 1063–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kevany BM, Rasko DA, and Thomas MG (2009) Characterization of the complete zwittermicin A biosynthesis gene cluster from Bacillus cereus. Appl Environ. Microbiol. 75, 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Reimer D, Pos KM, Thines M, Grün P, and Bode HB (2011) A natural prodrug activation mechanism in nonribosomal peptide synthesis. Nat. Chem. Biol. 7, 888–890. [DOI] [PubMed] [Google Scholar]
- (18).Prost M, Canaple L, Samarut J, and Hasserodt J (2014) Tagging live cells that express specific peptidase activity with solid-state fluorescence. ChemBioChem 15, 1413–1417. [DOI] [PubMed] [Google Scholar]
- (19).Thorn-Seshold O, Vargas-Sanchez M, McKeon S, and Hasserodt J (2012) A robust, high-sensitivity stealth probe for peptidases. Chem. Commun. (Cambridge, U. K.) 48, 6253–5. [DOI] [PubMed] [Google Scholar]
- (20).Meyer Y, Richard J-A, Delest B, Noack P, Renard P-Y, and Romieu A (2010) A comparative study of the self-immolation of para-aminobenzylalcohol and hemithioaminal-based linkers in the context of protease-sensitive fluorogenic probes. Org. Biomol. Chem. 8, 1777. [DOI] [PubMed] [Google Scholar]
- (21).Li Y, Zhong Z, Hou P, Zhang W, and Qian P (2018) Resistance to nonribosomal peptide antibiotics mediated by d-stereospecific peptidases. Nat. Chem. Biol. 14, 381–387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.