Abstract
Rationale:
Policies that establish a standard for reduced nicotine content in cigarettes can decrease the prevalence of smoking in the United States. Cigarettes with nicotine yields as low as 0.05 mg produce substantial occupancy of nicotinic acetylcholine receptors (26%), but women and men respond differently to these cigarettes.
Objective:
This study aimed to measure responses to smoking cigarettes that varied widely in nicotine yields, investigating whether sex differences in the effects on craving, withdrawal, and affect would be observed at even lower nicotine yields than previously studied, and in young smokers.
Methods:
Overnight abstinent young smokers (23 men, 23 women, mean age 22.18) provided self-reports of craving, withdrawal, and affect before and after smoking cigarettes with yields of 0.027, 0.110, 0.231, or 0.763 mg nicotine, and evaluated characteristics of each cigarette.
Results:
Compared to abstinent young men, abstinent young women reported greater negative affect, psychological withdrawal and sedation, all of which were relieved equally by all cigarettes. Men but not women reported greater craving reduction, perceived nicotine content and cigarette liking with increasing nicotine dose.
Conclusions:
Men may experience less smoking-related relief of craving, and enjoy cigarettes less, if nicotine yields are reduced to very low levels. Conversely, women respond equally well to cigarettes with nicotine yields as low as 0.027 mg as to cigarettes with nicotine yields 28-fold higher (0.763 mg). These differences are relevant for policy regarding reduced nicotine in cigarettes, and may influence the efficacy and acceptability of reduced-nicotine cigarettes as smoking cessation aids.
Keywords: nicotine, sex differences, smoking, craving, affect, nicotine withdrawal
Introduction
Tobacco smoking remains a leading contributor to preventable disease and death, resulting in an estimated 6 million deaths worldwide each year (Britton, 2017) and imposing an estimated $1.4 trillion economic burden in health-related costs globally (Goodchild et al., 2017). Although nicotine is considered the primary addictive pharmacological component of tobacco smoke, non-nicotine aspects of smoking, such as the conditioned behavioral and sensory aspects, also contribute to the smoking experience (Rose and Behm, 1995). Transdermal nicotine replacement therapy helps some smokers quit (e.g., Stead et al., 2008), but most who use nicotine replacement therapies eventually relapse, partly because they miss the behavioral and sensory aspects of smoking (Rose et al., 2000). Nicotine per se may be more reinforcing in men than in women (e.g., Perkins et al., 2002), which could explain why nicotine replacement therapies are more effective in promoting cessation in men than in women (Cepeda-Benito et al., 2004; Smith et al., 2017).
Importantly, women may be more sensitive than men to conditioned reinforcement from smoking-related cues (Perkins, 2008; Perkins et al., 2001); blocking olfactory and gustatory stimuli during smoking, but not the visual stimuli, reduced hedonic ratings and puff self-administration in women but not in men (Perkins et al., 2002). Further, men but not women show smoking-induced ventral striatal dopamine release, and this observation is consistent with the notion that men smoke for the reinforcing effect of nicotine (Cosgrove et al., 2014).
Understanding the interaction between sex and the nicotine dose from smoking is an important consideration for the U.S. Food and Drug Administration, which has the authority to set a standard for reduced nicotine content in cigarettes (U.S. Congress, 2009; FDA, 2017). Studies that have not considered the influence of sex report that cigarettes that deliver 0.027 mg nicotine alleviate craving and withdrawal as much as a conventional cigarette (which deliver ~1 mg nicotine) (Faulkner et al., 2017), and that cigarettes delivering ~0.05 mg nicotine provide as much relief as conventional cigarettes when smoked ad libitum for ~90 min (Dallery et al., 2003; Tidey et al., 2013). One study found that smoking a cigarette delivering 0.05 mg nicotine alleviated craving and withdrawal less than a cigarette delivering 0.6 mg nicotine in men, but cigarettes delivering either dose alleviated such symptoms to a similar extent in women (Perkins and Karelitz, 2015). However, other than our recent report (Faulkner et al., 2017), the efficacy of cigarettes with a full range of nicotine yields (including yields as low as 0.027 mg) on craving and withdrawal has not been evaluated, nor has the influence of sex on these effects.
Cigarettes delivering low nicotine yields have also been considered as smoking cessation tools (e.g. Benowitz and Henningfield, 1994), and it is important to consider the influence of sex on the ability of reduced-nicotine cigarettes to improve public health. For example, switching to cigarettes that deliver less than conventional nicotine doses reduced nicotine dependence and cigarette consumption at 6 weeks compared to baseline in a sample of men and women (e.g., Benowitz et al., 2015; Donny et al., 2015). Notably, in a clinical trial, cigarettes delivering 0.05 mg nicotine aided smoking cessation when paired with a placebo patch as much as when paired with a nicotine patch in women, but were less effective when paired with the placebo compared to the nicotine patch in men (Vogel et al., 2014). These results led the authors to argue that cigarettes delivering very low yields of nicotine may be more effective at promoting cessation in women than in men.
Young smokers are of particular interest because smoking cessation before the age of 25 usually avoids most long-term negative consequences of smoking (Doll et al., 2004). Reducing the nicotine content of cigarettes has been considered as a way to prevent the transition of young smokers from intermittent to daily smoking, which usually occurs before age 25 (Benowitz and Henningfield, 1994). Young smokers smoke fewer cigarettes per day, and exhibit less nicotine dependence than older adult smokers (Benowitz and Henningfield, 1994). Because men typically begin smoking earlier than women (Okoli et al., 2013), young male and female smokers may respond differently to cigarettes with low nicotine yields. Yet no study has examined sex differences in how young smokers respond to smoking cigarettes over a full range of nicotine yields. The results could help inform both a nicotine-reduction policy, and the personalization and improvement of therapies for smoking cessation.
Here we provide a report of a secondary analysis of published data (Faulkner et al., 2017). Data pertaining to subjective evaluations of craving, withdrawal and affect were obtained after overnight abstinence, and again after participants smoked cigarettes delivering 0.027, 0.110, 0.231 and 0.763 mg nicotine yield, in young smokers. Subjective evaluations of cigarette characteristics were also collected. The relationship between sex, smoking (pre- vs. post-smoking), and nicotine dose on each variable was assessed to determine the extent to which nicotine dose effects differ in men and women. On the basis of existing literature, it was hypothesized that men would show significant dose- related smoking-induced reductions in craving and withdrawal, and dose-related ratings of cigarette characteristics, whereas the responses of women would be independent of nicotine dose.
Methods
Procedure
Participants attended four testing sessions after overnight (~12 h) abstinence, verified by expired CO < 10 ppm (measured using a coVita monitor, Haddonfield, NJ). They were required to have negative urine screens for drugs of abuse (including Δ9- tetrahydrocannabinol) at each session. Participants rated craving, withdrawal and affect before and after smoking one research cigarette (four nicotine doses counterbalanced, double-blind). Participants smoked one cigarette ad libitum through a smoking topography device (see Smoking Topography, below), and there were no time restraints for smoking, but participants were instructed to smoke the entire cigarette (average duration of smoking = 4.16 ±.16 min). Participants also underwent MRI scanning both before and after smoking (results reported elsewhere). After smoking, participants also rated cigarette characteristics. Between testing sessions (mean inter-session interval = 3.14 ± 2.01 days), participants were allowed to smoke as usual. There was also a fifth testing session in which participants completed all measures described above before and after smoking one cigarette of their own preferred brand (results described in the Supplementary Materials).
Participants
Forty-six young smokers (23 men, 23 women) were recruited from the Los Angeles community via online and print advertisements, attended an intake session, and received a detailed explanation of study procedures (approved by UCLA’s Institutional Review Board). Inclusion criteria were being between 18 and 25 years and smoking ≥ 5 cigarettes per day for ≥ 1 year. Exclusion criteria were positive urine tests for abused drugs other than nicotine or cannabinoids, marijuana use > 8 times per month or consumption of alcohol > 15 days per month, any Axis 1 psychiatric disorders other than nicotine dependence, history of neurological injury, pregnancy and use of electronic cigarettes, cigars, chewing tobacco and snuff. Smokers who preferred menthol to non-menthol cigarettes were excluded because menthol increases the rate of nicotine accumulation in the brains of men but not in women (Zuo et al., 2015). The use of menthol cigarettes would add an important variable for which the study was not powered. Of the 46 participants who entered the study, 40 completed all testing sessions (20 men, 20 women). For detailed participant characteristics, see Table 1.
Table 1.
Participant Characteristics
| Entire Sample a | Men | Women | |
|---|---|---|---|
| Sex (n) | 23/23 | 23 | 23 |
| Age (years) b | 22.28 (2.19) | 22.51 (1.29) | 22.05 (1.97) |
| Education (years) | 13.79 (1.70) | 13.96 (2.22) | 13.61 (2.13) |
| Ethnicity (no. of participants) | |||
| White Caucasian | 19 | 10 | 9 |
| African American | 10 | 4 | 6 |
| Asian American | 8 | 5 | 3 |
| Hispanic | 6 | 2 | 4 |
| Other | 3 | 1 | 2 |
| Cigarette smoking b | |||
| Age of first use (years) | 16.37 (2.20) | 16.75 (2.41) | 15.99 (1.89) |
| Cigarettes per day | 11.59 (6.17) | 12.40 (9.25) | 10.78 (3.54) |
| Nicotine Dependence c | 3.55 (2.03) | 3.67 (1.91) | 3.43 (2.26) |
| Substance Use b | |||
| Marijuana (days used in past 30) | 1.43 (2.10) | 1.84 (2.32) | 1.02 (0.38) |
| Alcohol (drinks per week) | 2.92 (3.89) | 3.39 (4.42) | 2.45 (3.13) |
| Nicotine Metabolite Ratiob | 0.40 (0.23) | 0.41 (0.217) | 0.39 (0.26) |
Of the 46 participants, 40 (20 men, 20 women) completed all assessments, including five test sessions.
mean (SD).
Determined using the Fagerström Test for Nicotine Dependence.
Cigarettes
Research cigarettes, manufactured by 22nd Century Group Inc. (Spectrum – Clarence, NY), were provided by the National Institute on Drug Abuse. As determined by the International Organization for Standardization Method, the nicotine yields of these cigarettes were 0.027, 0.110, 0.231, and 0.763 mg, corresponding to nicotine contents of ~0.3, 2.1, 4.3 and 13.8 mg/g tobacco, respectively (see Hatsukami et al., 2013). For simplicity, research cigarettes will be identified by their nicotine yield, not content. The cigarettes were non-mentholated, were well matched on level of regular tar (9 ± 1.5 mg), and did not vary in ventilation (Hatsukami et al., 2013).
Smoking Topography
A Clinical Research Support System (CReSS) topography monitor (Borgwaldt KC, Richmond, VA) was used to record the number of puffs per cigarette, and the average volume and duration of each puff.
Nicotine, Metabolites and NMR
Twenty-nine participants provided blood samples for assay of plasma nicotine (fifteen before and after smoking), and fourteen after smoking only. Nicotine concentrations in plasma were assayed by Quest Diagnostics, as described before (Faulkner et al., 2017). All participants provided blood samples for assay of the 3’-hydroxycotinine:cotinine ratio in plasma, which was determined by liquid chromatography-tandem mass spectrometry as described before (Tanner et al., 2015).
Questionnaires
The Fagerström Test for Nicotine Dependence (FTND) (Fagerström, 2012) was administered at the beginning of the first testing session to quantify the level of nicotine dependence. The Shiffman-Jarvik Withdrawal Scale was administered to obtain data on five subscales (craving, psychological withdrawal, physiological withdrawal, stimulation/sedation and appetite), all of which were combined to calculate a ‘total withdrawal’ score (Shiffman and Jarvik, 1976). The Positive and Negative Affect Schedule (Watson et al, 1988) was also administered to quantify positive and negative affect, and the Cigarette Characteristics Questionnaire (Hatsukami et al, 2013b) was administered to evaluate ratings of flavor, strength, harshness, level of nicotine, and cigarette liking and disliking, all of which were combined to calculate a ‘total cigarette rating’ score.
Statistics
Statistical analyses were performed in the Statistical Package for Social Scientists (SPSS 22; IBM, Chicago, IL). Data from all 46 (40 completers) were included in the final analyses. For pre-post smoking measures, separate but parallel linear mixed models were performed, with relevant scores added as the dependent variable, and time (pre-post smoking), cigarette type (4 research cigarettes) and sex was added as separate factors. Because nicotine clearance, as indicated by the nicotine metabolite ratio (i.e., 3’- hydroxycotinine:cotinine ratio in plasma, NMR), influences responses to smoking reduced-nicotine cigarettes (Faulkner et al., 2017), NMR was also added to the model as a covariate of no interest to control for its influence. To examine sex differences in withdrawal measures during abstinence and in ratings of cigarette characteristics obtained post-smoking, the same models as above were used, except that time (pre- vs. post- smoking) was not included in the model.
Multiple comparisons were performed as follows. For the Shiffman-Jarvik Withdrawal Scale and Cigarette Characteristics Questionnaire, Fisher’s Least Significant Difference method was used. Following omnibus tests (linear mixed models) on a ‘total withdrawal score’ and ‘total cigarette rating’ score, respectively, separate but parallel tests for each subscale were performed after the total was deemed significant. The Bonferroni correction was used for the two outcome measures in the PANAS (critical p ≤ 0.025). For smoking topography, three variables (puffs/cigarette, average puff volume, and puff duration) were accounted for using the Bonferroni-correction (critical p ≤ 0.0125)
Results
Participant Characteristics
Forty-six daily smokers (23 women), mean age 22.28 (SD = 2.19) were included in the final analyses (Table 1). Of these, 40 (20 women) completed all 4 testing sessions. Plasma nicotine and smoking topography were measured in only 29 and 36 completing participants, respectively. There were no sex differences in FTND scores (F(1,38) = 0.568, p = 0.456), cigarettes smoked per day (F(1,38) = 0.667, p = 0.419), or NMR (F1,38) = 0.002, p = 0.968).
Pre-Smoking Measures
Withdrawal and Craving (see Table 2 and Fig. 1)
Table 2.
Effects of sex and smoking on withdrawal, craving and affect
| Main effect of sex pre-smoking | Sex-by-smoking interaction | Sex-by-smoking-by-dose interaction | |
|---|---|---|---|
| Craving | 0.054 | 0.146 | 0.026* |
| Psychological Withdrawal | 0.010* | 0.004* | 0.214 |
| Physiological Withdrawal | 0.298 | 0.287 | 0.619 |
| Stimulation/Sedation | 0.040* | 0.006* | 0.388 |
| Appetite | 0.070 | 0.031* | 0.275 |
| Positive Affect | 0.004* | 0.009* | 0.618 |
| Negative Affect | 0.032 | <0.001* | 0.447 |
Values denote p-values. Asterisks denote significant results. There was no effect of testing day on pre-smoking scores of withdrawal on any of the SJWS subscales, or on pre-smoking scores of positive or negative affect (all ps > 0.236).
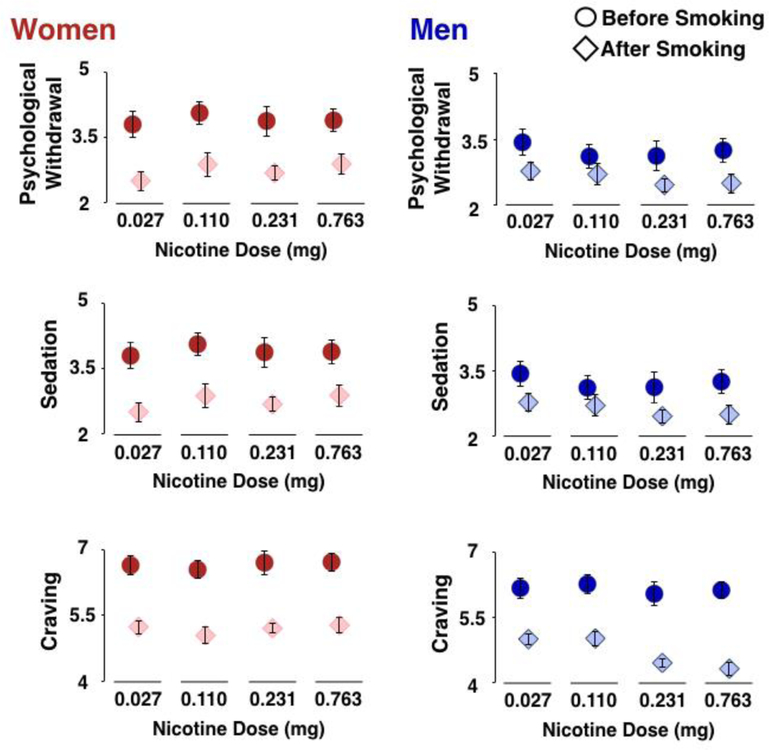
Figure 1. Psychological withdrawal (top), sedation (middle) and cigarette craving (bottom) in women (left) and men (right) both before and after smoking.
Women reported greater psychological withdrawal, greater sedation, and a trend towards greater craving than men during abstinence. Women also reported greater reductions in psychological withdrawal and sedation than men due to smoking, with no effect of nicotine dose. Men reported greater reductions in craving after smoking cigarettes delivering ≥0.231 mg nicotine than after smoking cigarettes delivering ≤0.231 mg nicotine.
Compared to men, women reported greater total withdrawal (F(1,38) = 9.197, p = 0.004, Cohen’s f2 = 0.24). Examination of the subscales on the SJWS revealed that compared to men, women reported more psychological withdrawal (F(1,38) = 7.210, p = 0.010, Cohen’s f2 = 0.19), greater sedation (F(1,38) = 9.373, p = 0.04, Cohen’s f2 = 0.25). There were trends towards women endorsing greater craving and appetite, but there were no sex differences in pre-smoking physiological withdrawal or appetite (see Table 2).
Affect (see Table 2 and Fig. 2).
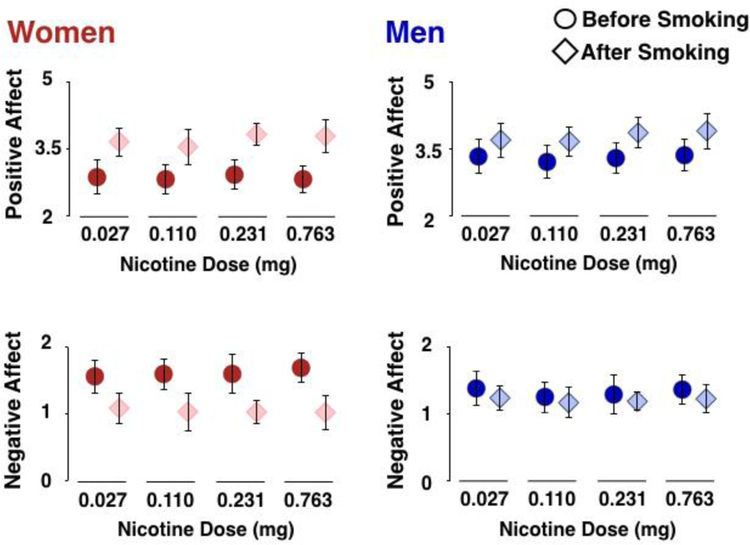
Figure 2. Positive affect (top) and negative affect (bottom) in women (left) and men (right) both before and after smoking.
Women reported less positive affect and greater negative affect than men during abstinence. Women also reported greater improvements in positive affect and greater reductions in negative affect than men due to smoking, with no effect of nicotine dose.
Compared to men, women reported less positive affect (F(1,38) = 9.576, p = 0.004, an effect that exceeded the Bonferroni-corrected criterion of p ≤ 0.025, Cohen’s f2 = 0.25). There also was a trend towards greater negative affect (F(1,38) = 4.934, p = 0.032, but this effect did not reach the Bonferroni-corrected criterion for significance (see Table 2).
Measures Obtained During Smoking and Effects of Smoking
Smoking Topography
There were no sex differences in puff count, duration volume when smoking (all ps > 0.082).
Plasma Nicotine
There was a main effect of smoking (F(1,111) = 13.160, p < 0.001, Cohen’s f2 = 0.41), and a significant smoking-by-nicotine dose interaction (F(3,93) = p = 0.002) on plasma nicotine levels. There was no main effect of sex no sex-by-smoking interaction, and no sex-by-smoking-by-nicotine dose interaction on plasma nicotine levels however (all ps > 0.537).
Withdrawal and Craving (see Table 2 and Fig.1)
Smoking the reduced-nicotine research cigarettes significantly reduced the total withdrawal score (19% reduction; F(1,263) = 105.887, p < 0.001, Cohen’s f2 = 0.39), and did so in women more than in men (27% vs 15%; sex-by-smoking interaction; F(1,274) = 5.763, p = 0.017, Cohen’s f2 = 0.03), with no effect of nicotine dose (no sex-by-smoking–by-nicotine dose interaction; F(3, 263) = 0.807, p = 0.491). Examination of the individual subscales of the SJWS revealed that smoking decreased psychological withdrawal (30% reduction; F(1,263) = 101.158, p < 0.001, Cohen’s f2 = 0.38), and did so more in women than in men (39% vs 21%; F(1,263) = 8.193, p = 0.005, Cohen’s f2 = 0.04). Further, there was no smoking-by-nicotine dose interaction and no sex-by- smoking-by-nicotine dose interaction (F(6,264) = 1.402, both p’s > 0.213; see Table 2).
Smoking also decreased sedation (15% reduction; F(1,263) = 18.322, p < 0.001, Cohen’s f2 = 0.07), and did so more in women than in men (24% vs 5%; F(1,263) = 7.675, p = 0.006, Cohen’s f2 = 0.03). Further, there was no smoking-by-nicotine dose interaction and no sex-by-smoking-by-nicotine dose interaction (both p’s > 0.388; see Table 2).
Smoking decreased appetite (13% reduction; F(1,263) = 20.408, p < 0.001, Cohen’s f2 = 0.08), and did so more in women than in men (7% vs 20%; F(1,263) = 4.691, p = 0.031, Cohen’s f2 = 0.02). Further, there was no smoking-by-nicotine dose interaction and no sex-by-smoking-by-nicotine dose interaction (both p’s >0.274; see Table 2).
Although there was a significant effect of smoking on cigarette craving (29% reduction; F(1,263) = 183.946, p < 0.001, Cohen’s f2 = 0.70), there was no smoking-by- sex interaction (F(1,263) = 2.127, p = 0.146). However, while there was no smoking-by- nicotine dose interaction (F(3,274) = 1.308, p = 0.272), there was a significant sex-by- smoking-by-nicotine dose interaction (F(6,264) = 2.439, p= 0.026, Cohen’s f2 = 0.14). Post-hoc tests revealed that this was driven by a smoking-by-nicotine dose interaction in men (F(3,1353) = 2.939, p = 0.035, Cohen’s f2 = 0.07) but not in women (F(3,133) = 0.236, p = 0.871).
Finally, there were no main or interactive effects of smoking or sex on physiological withdrawal (all ps > 0.130).
Affect (see Table 2 and Fig 2).
Smoking increased positive affect (12% increase; F(1,263) = 25.371, p < 0.001, Cohen’s f2 = 0.10) and decreased negative affect (14% reduction; F(1,263) = 30.052, p < 0.001, Cohen’s f2 = 0.12). Smoking increased positive affect more in women than in men (22% vs 6%; sex-by-smoking interaction: F(1,263) = 6.909, p = 0.009, Cohen’s f2 = 0.03), and decreased negative affect more in women than in men (25% vs 3%; sex-by-smoking interaction: F(1,263) = 16.677, p < 0.001,Cohen’s f2 = 0.07), both of which remained significant after Bonferroni correction,. However, there were no smoking-by-nicotine dose or sex-by-smoking-by-nicotine dose interactions however (all ps > 0.398).
Cigarette Characteristics Questionnaire (see Table 3 and Fig. 3)
Table 3.
Perceived Cigarette Characteristics
| Main effect of dose | Sex-by-dose interaction | |
|---|---|---|
| Perceived Nicotine Content | 0.031* | 0.011* |
| Liking | 0.025* | 0.011* |
| Disliking | 0.002* | 0.027* |
| Flavor | 0.301 | 0.198 |
| Strength | 0.220 | 0.283 |
| Harshness | 0.419 | 0.333 |
Values denote p-values. Asterisks denote significant results.
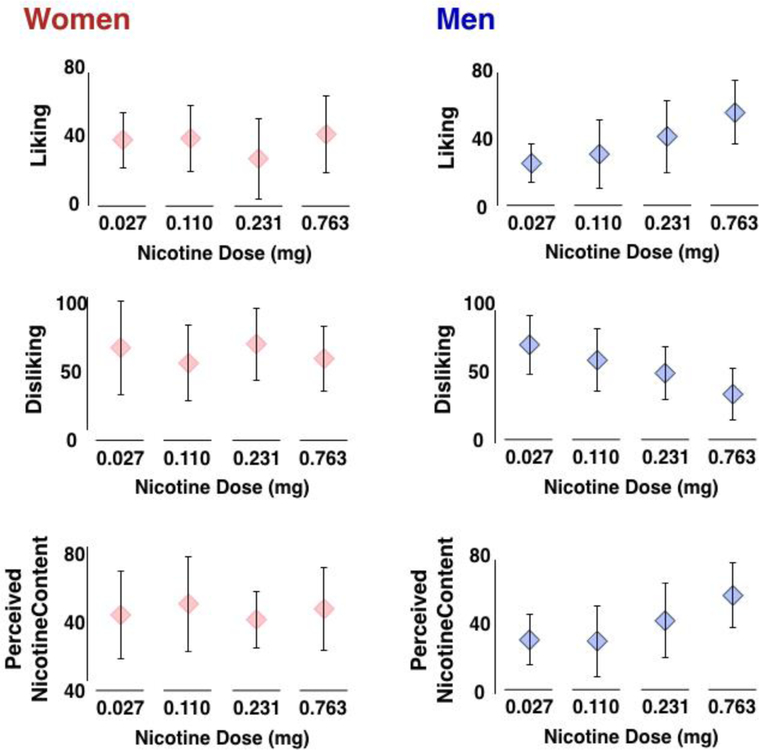
Figure 3. Cigarette liking (top), cigarette disliking (middle) and perceived nicotine content (bottom) in women (left) and men (right).
Women reported no effect of nicotine dose on all three cigarette ratings, whereas men reported greater liking, less disliking and greater perceived nicotine content as the nicotine content of the cigarette increased. These scores were obtained after smoking only.
There was a significant main effect of dose on the total cigarette rating (F(3, 92) = 4.257, p = 0.007, Cohen’s f2 = 0.14), and a significant sex-by-nicotine dose interaction on these ratings (F(3, 92) = 3.835, p = 0.012, Cohen’s f2 = 0.12). Examination of the individual subscales revealed a sex-by-nicotine dose interaction on perceived nicotine content (F(3,93) = 3.8876, p = 0.011, Cohen’s f2 = 0.13). Post-hoc analysis revealed that this interaction was driven by a main effect of dose in men (F(3,44) = 4.358, p = 0.009, Cohen’s f2 = 0.30) but not in women (F(3,44) = 1.532, p = 0.168).
Further, there was a significant sex-by-nicotine dose interaction on cigarette liking (F(3,93) = 3.952, p = 0.011, Cohen’s f2 = 0.13) and disliking (F(3,93) = 3.200, p = 0.027, Cohen’s f2 = 0.10). Post-hoc analyses revealed that these interactions were driven by a main effect of dose in men (liking - F(3,44) = 6.070, p = 0.001, Cohen’s f2 = 0.41; disliking - F(3,44) = 8.356, p > 0.001, Cohen’s f2 = 0.57) but not in women (liking - F(3,44) = 1.954, p = 0.133; disliking - F(3,44) = 2.046, p = 0.120). There were no main or interactive effects of sex and nicotine dose on ratings of flavor, strength, or harshness (all ps > 0.220).
Discussion
This study of young smokers provides evidence that young women have greater abstinence-induced psychological and affective withdrawal symptoms than young men, and that smoking reduced-nicotine cigarettes relieves these symptoms in women more than in men. Further, the results indicate that smoking-induced reductions in craving and ratings of cigarette characteristics depend on nicotine dose in men but not in women. These findings have implications both for policy regarding regulation of cigarette constituents and therapeutic approaches to improve smoking cessation. One potential implication of this finding is that a policy to reduce the nicotine content of cigarettes to reduce the prevalence of smoking may be more successful in women than in men.
The findings that men, but not women, exhibit dose-dependent reductions in cigarette craving and less relief of negative affect than women due to smoking reduced nicotine cigarettes are consistent with the previous observation that men (but not women) reported less reduction in withdrawal and negative affect due to smoking a cigarette delivering 0.05 mg nicotine compared to a conventional cigarette (delivering ~1 mg nicotine) (Perkins and Karelitz, 2015). The current study found no effect of nicotine dose on negative affect. Previously, smoking cigarettes delivering 0.6 mg nicotine was more effective in this regard than smoking cigarettes delivering 0.05 mg nicotine (Perkins and Karelitz, 2015). This difference could be due to the fact that the male smokers in the current study reported substantially less negative affect during abstinence (~16% of the maximal score) as compared to participants in the study by Perkins and Karelitz (2015) (~25% of the maximal score). This difference could reflect the younger age (mean age = 22.28 vs 27.18), lower nicotine dependence (mean FTND = 3.55 vs 4.54), or fewer cigarettes smoked per day (11.59 vs 14.91) in the present study as compared to the study by Perkins and Karelitz (2015).
The results add to previous findings by showing that the sensitivity to nicotine dose observed in men (but not in women) extends to ratings of cigarette characteristics such as the perception of nicotine content and cigarette liking. That women could not distinguish between cigarettes with different nicotine contents, and reported similar liking for each cigarette, despite a >25-fold difference between the highest and lowest nicotine yield, contributes to the existing literature showing that women may respond more favorably to a reduction in the nicotine content of cigarettes than men. For example, in a study of smokers who were motivated to quit, switching to cigarettes delivering 0.05 mg nicotine helped women quit smoking more than men (Vogel et al., 2014).
In this study, women reported greater negative affect, psychological withdrawal and sedation after overnight abstinence than did men. These findings mirror prior observations in our laboratory showing that, relative to men, women report significantly greater psychological withdrawal on the SJWS, and greater negative affect during abstinence (Xu et al., 2008). Such results indicate that the psychological and affective aspects of withdrawal may particularly hinder smoking cessation in women, who report more difficulty maintaining abstinence during stressful life events than men (McKee et al., 2003). Furthermore, the results of Xu et al (2008) also indicate that smoking relieves these symptoms in women more than in men, and the present findings indicate that such relief does not depend on nicotine dose. The greater effectiveness of reduced-nicotine cigarettes as smoking cessation aids in women than in men (Vogel et al., 2014) may partly reflect the fact that, when smoked for at least 6 weeks by both male and female smokers, these cigarettes can reduce nicotine dependence (e.g., Donny et al., 2015) and relieve specific withdrawal symptoms that may particularly contribute to greater difficulty in smoking cessation by women.
Male-female differences in the response to reduced-nicotine cigarettes may be related to nicotine pharmacokinetics. In a previous report on the same sample of research participants, we showed that normal metabolizers of nicotine only (not slow metabolizers of nicotine) had greater reductions in cigarette craving and the appetite subscales of the SJWS as nicotine dose increased (Faulkner et al., 2017), indicating less sensitivity to nicotine dose in slow compared to normal metabolizers. Despite the fact that women are typically faster metabolizers than men (Benowitz et al., 2006; Johnstone et al., 2006), this investigation showed less sensitivity to nicotine dose in women than in men, suggesting that variation between men and women reported here is not explained by variation in rates of nicotine clearance.
Sex differences in neurochemical responses to smoking may be related to the findings reported here. Men show more smoking-induced dopamine release in the right ventral striatum but women do not, and instead show dopamine release in the dorsal putamen, suggesting that men smoke for reinforcement whereas women smoke for other reasons (Cosgrove et al., 2012). This interpretation is broadly consistent with our finding that women report similar levels of cigarette liking and experience similar reductions in craving regardless of nicotine content. Other neurochemical sex differences have been reported, but how they are linked to the present findings is not clear. Notably, male smokers have lower striatal dopamine D2-type receptor availability than male non-smokers, whereas no effect of smoking status is seen in females (Brown et al., 2012), yet female smokers have greater midbrain dopamine D2-type receptor availability than female non-smokers, suggesting that midbrain dopamine receptor activity may protect women from nicotine dependence (Okita et al., 2016). In addition, male smokers have higher dopamine and serotonin transporter availabilities than male non-smokers, with no effect of smoking status in females (Staley et al., 2001). Sex differences have also been observed in functional connectivity of large-scale neural networks (Wetherill et al., 2014) and in brain structure of smokers compared to non-smokers (Duriez et al., 2014; Franklin et al., 2014), but they have not been explicitly tied to smoking- related behaviors.
Information from this study can guide policy regarding effects of reduction in nicotine content of cigarettes on young smokers. Our results indicate that reductions in the nicotine content of cigarettes may be tolerated well by young females, but for young male smokers, reducing nicotine content to very low levels may produce transient increases in cigarette craving. Therefore, young male smokers may seek nicotine from other sources, such as electronic cigarettes, to alleviate increases in craving induced by switching to reduced nicotine cigarettes. Conversely, it could be argued that the lack of nicotine dose effects in young women indicates that if conventional cigarettes were replaced with cigarettes delivering as little as ~0.027 mg nicotine, women would initially continue to smoke because they like these cigarettes as much as those delivering much higher doses. However, in older, adult male and female smokers, switching to cigarettes that deliver ~0.05 mg nicotine for 6 weeks reduces both the number of cigarettes smoked per day and the effect of overnight abstinence on cigarette craving at week 6 compared to baseline measures (Donny et al., 2015). Further, the dose effects on the perception of nicotine content and on cigarette liking in men indicate that reducing the nicotine content of cigarettes may render them less reinforcing (at least initially) and thereby less acceptable to young male smokers than conventional cigarettes delivering ~1 mg nicotine.
This investigation had some limitations. Menstrual cycle phase and hormone levels in female participants was not evaluated or considered here. Further, this study included only young smokers, who typically display lower levels of nicotine dependence, smoke fewer cigarettes per day, and have shorter smoking histories than older adult smokers (CDC, 2016). The low levels of dependence and small number of cigarettes smoked per day could partly explain the few effects of nicotine dose observed in this study, as the specific amount of nicotine intake may have less influence on smoking- induced relief in low-dependent smokers. Therefore, the results of this study may not be generalizable to the wider population of smokers. Also, plasma nicotine levels were not obtained from most participants.
In conclusion, this investigation demonstrated clear sex differences in the affective and psychological aspects of withdrawal and in responses to smoking reduced nicotine cigarettes. All cigarettes, even those with negligible nicotine content, effectively alleviated craving and withdrawal and improved affect in young women, which may explain why reduced-nicotine cigarettes may be more effective in promoting smoking cessation in women than in men. Reducing the nicotine content of cigarettes may initially lead to young male smokers to experience transient increases in cigarette craving, and to experience transient reductions in the reinforcing effects of smoking.
Supplementary Material
Acknowledgments
This research was funded by the National Institute on Drug Abuse R01 DA036487 (EDL), the Iris Cantor-UCLA Women’s Health Center/UCLA National Center of Excellent in Women’s Health Pilot Research Project UL1TR000124 (EDL and NP), endowments from the Thomas P and Katherine K Pike Chair in Addiction Studies (EDL) and the Marjorie M Greene Trust, and the Canada Research Chairs Program (Dr. Tyndale, the Canada Research Chair in Pharmacogenomics)
Footnotes
Declarations of competing interest: RF Tyndale has consulted for Apotex on issues unrelated to smoking
References
- Benowitz NL & Henningfield JE (1994). Establishing a nicotine threshold for addiction. The implications for tobacco regulation. New England Journal of Medicine, 331, 123–125. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Lessov-Schlaggar CN, Swan GE, & Jacob P (2006). Female sex and oral contraceptive use accelerate nicotine metabolism. Clin. Pharm. & Therap, 79(5), 480–488. [DOI] [PubMed] [Google Scholar]
- Bialous SA & Yach D (2001). Whose standard is it anyway? How the tobacco industry determines the International Organization for Standardization (ISO) standards for tobacco and tobacco products. Tobacco Control, 10, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J (2017). Death, disease, and tobacco. Lancet, 389(10082), 1861–1862. [DOI] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, & London ED (2012): Sex difference in striatal dopamine D2/D3 receptor availability in smokers and nonsmokers. Int. J. Neuropsychopharmacol 15(7):989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, & Erath S (2004). Meta-Analysis of the Efficacy of Nicotine Replacement Therapy for Smoking Cessation: Differences Between Men and Women. J. Cons. & Clin Psych, 72(4), 712–722. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim S-J, McGovern E, Nabulsi N, Gao H, Morris ED (2014). Sex Differences in the Brain’s Dopamine Signature of Cigarette Smoking. J. Neuro, 34(50), 16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Houtsmuller EJ, Pickworth WB, & Stitzer ML (2003). Effects of cigarette nicotine content and smoking pace on subsequent craving and smoking. Psychopharm, 165(2), 172–180. [DOI] [PubMed] [Google Scholar]
- Dempsey D, Tutka P, Jacob P, Allen F, Schoedel K, Tyndale RF, & Benowitz NL (2004). Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin. Pharm. & Therap, 76(1), 64–72. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J & Sutherland I (2004). Mortality in relation to smoking: 50 years’ observations on male British doctors. British Medical Journal, 328, 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, Hatsukami DK (2015). Randomized Trial of Reduced-Nicotine Standards for Cigarettes. New Eng. J. Med, 373(14), 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriez Q, Crivello F, & Mazoyer B (2014). Sex-related and tissue-specific effects of tobacco smoking on brain atrophy: assessment in a large longitudinal cohort of healthy elderly. Front Aging Neurosci, 6:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström K, Russ C, Yu C-R, Yunis C, & Foulds J (2012). The Fagerström Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nic& Tob Res, 14(12), 1467–1473. [DOI] [PubMed] [Google Scholar]
- Faulkner P, Ghahremani DG, Tyndale RF, Cox CM, Kazanjian AS, Paterson N, London ED (2017). Reduced-Nicotine Cigarettes in Young Smokers: Impact of Nicotine Metabolism on Nicotine Dose Effects. Neuropsychopharm, 42(8), 1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, et al. (2014). The Effects of Chronic Cigarette Smoking on Gray Matter Volume: Influence of Sex. PLoS ONE, 9(8): e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (2017). FDA announces comprehensive regulatory plan to shift trajectory of tobacco-related disease, death Retrieved from https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm568923.htm
- Goodchild M, Nargis N, & Tursan d’Espaignet E (2017). Global economic cost of smoking-attributable diseases. Tob Con [DOI] [PMC free article] [PubMed]
- Hatsukami DK, Zhang Y, O’Connor RJ, & Severson HH (2013). Subjective responses to oral tobacco products: scale validation. Nic & Toba Res 15(7), 1259–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Valentine G, Gueorguieva R, & Sofuoglu M (2016). Intravenous Nicotine Self-Administration in Smokers: Dose–Response Function and Sex Differences. Neuropsychopharm, 41(8), 2034–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks L, Day I, Walton R (2006). Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharm & Therap, 80(4), 319–330. [DOI] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM (2003). Sex differences in the effects of stressful life events on changes in smoking status. Addiction, 98(6): 847–855. [DOI] [PubMed] [Google Scholar]
- Okoli C, Greaves L & Fagyas V (2013). Sex differences in smoking initiation among children and adolescents. Public Health, 127(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Perkins KA (2008) Sex Differences in Nicotine Reinforcement and Reward: Influences on the Persistence of Tobacco Smoking. In: Caggiula A, Bevins R (eds) The Motivational Impact of Nicotine and its Role in Tobacco Use. Nebraska Symposium on Motivation, vol. 55 Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Gerlach D, Vender J, Meeker J, Hutchison S, Grobe J (2001). Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nic & Tob Res, 3(2): 141–150. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, & Caggiula AR (2002). Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharm, 163(2), 194–201. [DOI] [PubMed] [Google Scholar]
- Perkins KA, & Karelitz JL (2015). Sex differences in acute relief of abstinence- induced withdrawal and negative affect due to nicotine content in cigarettes. Nic & Tob Res, 17(4), 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, & Johnson M (2000). Dissociating nicotine and nonnicotine components of cigarette smoking. Pharm Bioc & Behav, 67(1), 71–81. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, & Jarvik ME (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharm, 50(1), 35–39. [DOI] [PubMed] [Google Scholar]
- Smith PH, Zhang J Weinberger AH, Mazure CM, McKee SA (2017). Gender differences in the real-world effectiveness of smoking cessation medications: Findings from the 2010–2011 Tobacco Use Supplement to the Current Population Survey. Drug Alc Dep, 178: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB (2001). Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse, 15(41): 275–284. [DOI] [PubMed] [Google Scholar]
- Tanner JA, Novalen M, Jatlow P, Huestis MA, Murphy SE, Kapiro J et al. (2015). Nicotine metabolite ratio (3-hydroxycotinine/cotinine) in plasma and urine by different analytical methods and laboratories: implications for clinical implementation. Cancer, Epidemiology, Biomarkers and Prevention, 24, 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Colby SM, & Xavier EMH (2014). Effects of smoking abstinence on cigarette craving, nicotine withdrawal, and nicotine reinforcement in smokers with and without schizophrenia. Nic & Tob Res, 16(3), 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, & Sellers EM (2001). Variable CYP2A6-mediated nicotine metabolism alters smoking behavior and risk. Drug Met & Disp, 29(4 Pt 2), 548–552. [PubMed] [Google Scholar]
- United States Congress (2009). Family Smoking Prevention and Tobacco Control and Federal Reform Act. Publ Law No. 111–31, 1776 (Jun 22, 2009), Washington, D.C. [Google Scholar]
- Vogel RI, Hertsgaard LA, Dermody SS, Luo X, Moua L, Allen S, Hatsukami DK (2014). Sex differences in response to reduced nicotine content cigarettes. Addictive Beavh, 39(7), 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers & Soc Psych, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Shin J, & Franklin TR (2014). Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addictive Behav, 39(4): 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Azizian A, Monterosso J, Domier CP, Brody AL, Fong TW, & London DD (2008). Gender effects on mood and cigarette craving during early abstinence and resumption of smoking. Nic & Tob Res, 10(11), 1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.