Abstract
Neonatal hypoxic-ischemic brain injury is commonly studied by use of the Vannucci procedure in mice or rats (unilateral common carotid artery occlusion followed by hypoxia). Previously, we modified the postnatal day-seven (P7) rat procedure for use in mice and later demonstrated that genetic strain strongly influences the degree of brain injury in the P7 mouse model of hypoxia-ischemia (HI). Recently, the P9 or P10 mouse brain was recognized as the developmental equivalent of the term neonatal human brain, rather than P7. Consequently, the Vannucci procedure has again been modified and a commonly used protocol employs 10% oxygen for 50 minutes in C57Bl/6 mice. Strain differences have yet to be described for the P9-P10 mouse model. In order to determine if the strain differences we previously reported in the P7 mouse model are present in the P9 model we compared two commonly used strains, CD1 and C57Bl/6J in both the P7 model of HI [carotid ligation (in this case right) followed by exposure to 8% oxygen for 30 min] and the P9 model, [carotid ligation (in this case left) followed by exposure to 10% oxygen]. Experiments using the P7 model were performed from 2001–2012 and experiments for the P9 model were performed from 2012–2016. Five to seven days after HI mice were perfused with 4% paraformaldehyde, brains were sectioned on a Vibratome (50 um) and alternate sections stained with Perl’s iron stain or cresyl violet. Brains sections were examined microscopically and scored for degree of injury. Since brains in the P7 group were scored previously with a slightly different system, they were reanalyzed using our current scoring system, which scores injury in 11 regions –anterior, middle and posterior cortex; anterior, middle and posterior striatum; CA1, CA2, CA3 and dentate gyrus of the hippocampus and thalamus - from 0 (none) to 3 (cystic infarct) for a total score of 0 – 33. Brains in the P9 group were scored with the same system. Given the same insult, the P7 CD1 mice had greater injury than the C57Bl/6J, which agrees with our previous findings. The P9 CD1 mice also had greater injury than C57Bl/6J This study confirms that CD1 mice are more susceptible to injury than C57Bl/6J and selection of strain is important when using mouse models of HI.
Keywords: brain injury, outcome, transgenic mice, developing brain, animal model
Introduction
Hypoxic-ischemic injury (HI) is a major cause of perinatal brain injury in term infants [1]. The Vannucci model of HI has evolved since it was first established in P7 rats nearly 40 years ago, yet it remains the most widely used model of neonatal HI [2]. The model was adapted for mice, allowing for the use of genetic manipulation to study the role of single genes in the setting of HI [3]. Subsequently, we found the background strain of genetically altered mice itself had an affect on outcome. Specifically, we showed that CD1 mice were highly susceptible to injury and required 30 minutes of 8% oxygen to attain moderate injury, while the C57Bl/6J and SV129 strains were more resistant to injury, requiring 45 and 90 minutes, respectively, to achieve a similar degree of injury, but with greater mortality [4]. Age has also been modified in the Vannucci model to re-create different types of injury based on developmental stage [5] [6] [7]. In rats, for example, very young (P1-P3) rats are used to mimic very immature injury that manifests largely in white matter injury [8] [9] [10] [11]. In mice, P9 or P10 is now considered a better rodent equivalent to the term human newborn than P7 and the Vannucci model is adjusted accordingly, with different percentages of oxygen and different durations of hypoxia employed, depending on type and degree of injury being modeled [12] [13] [6, 14].
With the widespread adoption of the P9 model of murine HI, we sought to map strain influences and degree of injury in this variation of the procedure. We first re-analyzed, with our current scoring system, data using the P7 model collected over eleven years and compare wild-type (WT) CD1 and C57Bl/6J. We hypothesized that the P9 model would show susceptibility to injury based on strain as we previously found in the P7 model. For this, we summarized data using the P9 model collected over 5 years and also compared CD1 and C57Bl/6J.
Understanding susceptibility or resistance to injury based on genetic background in mice should not only improve research methods, but could also improve our understanding of human variation in injury outcome and ultimately may lead to novel targets for intervention [15] [16].
Methods
All animal research was approved by the University of California San Francisco Animal Care and Use Committee (IACUC) and performed in accordance with the standards of humane care established by the NIH.
Hypoxia-ischemia
Experiments using the P7 model were performed from 2001–2012: After ligation by electrical coagulation of the right carotid artery under isofluorane anesthesia and a one-hour recovery period with the dam, both CD1 and C57Bl/6J mice received 30 min of 8% oxygen while in chambers maintained at 36.5 degrees C. Experiments for the P9 model were performed from 2012–2016: After carotid ligation as above (with the exception of the left artery being ligated), CD1 and C57Bl/6J mice received 50 min of 10% oxygen.
Mice from all groups were anesthetized with Euthasol (Henry Schein) and perfused with 4% paraformaldehyde 5–7 days after HI (when edema has subsided and injured regions are histologically distinct) and brains post-fixed in the same fixative overnight. Brains were sectioned on a Vibratome (50 um) and alternate sections stained for Nissl (cresyl violet) and iron (Perl’s stain modified by enhancement with diaminobenzidine). Brains in the P7 groups were reanalyzed using our current scoring system, which scores injury in 11 regions–anterior, middle and posterior cortex; anterior, middle and posterior striatum; CA1, CA2, CA3 and dentate gyrus of the hippocampus and thalamus - from 0 (none) to 3 (cystic infarct), for a total score of 0 – 33 as previously described [17]. Brains in the P9 group were analyzed with the same system.
Statistical Analysis
Injury scores were analyzed by Mann-Whitney U test, Mortality was analyzed by contingency tables with Fisher’s exact test. Analysis was done with Prism v7.0 (Carlsbad, CA). Significance was considered to be p < 0.05.
Results
Injury was greater in the HI hemisphere (ipsilateral to the ligation) of the CD1 mouse brain than the C57Bl/6J brain for both P7 and P9, confirming our previous findings for P7, and documenting the strain difference for P9 (Fig. 1 and Table 1). When analyzed separately, the cortex also had greater injury in CD1 mouse brain than C57Bl/6J for both P7 and P9 (Fig. 2a and Table 1). In the hippocampus, however, injury scores were not significantly different in P7 between CD1 and C57Bl/6J (p = 0.10), but in P9 the CD1 brains were more injured than C57Bl/6J (Fig. 2b and Table 1). In the striatum, injury was greater in CD1 mouse brain than C57Bl/6J for both P7 and P9 (Fig. 2c and Table 1). In the thalamus, a region we did not analyze separately in our previous study, injury was greater in CD1 mouse brain than C57Bl/6J for both P7 and P9 (Fig. 2d and Table 1).
Fig. 1.
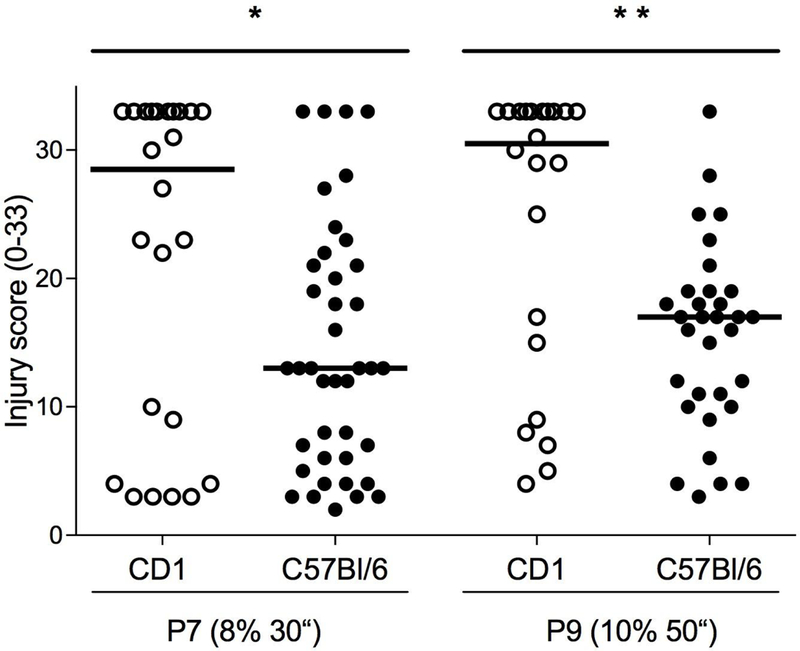
Histological injury scores for the entire hemisphere after HI (scale 0–33). CD1 compared to C57Bl/6J after HI at P7 with 30 minutes of 8% oxygen and CD1 compared to C57Bl/6J after HI at P9 with 50 minutes of 10% oxygen. Median injury score is higher in CD1 compared to C57Bl/6 in both the P7 model (* p < 0.03) and the P9 model (** p = 0.0025). Horizontal line indicates median score.
Table 1:
Injury scores for each region. Median (range)
| Injured region |
P7 |
P9 |
||||
|---|---|---|---|---|---|---|
| CD1 (n=24) |
C57Bl/6 (n= 40) |
p value | CD1 (n = 22) |
C57Bl/6 (n = 32) |
p value | |
| Whole hemi-sphere | 28.5 (3–33) | 13 (2–33) | *<0.03 | 30.5 (4–33) | 17 (3–33) | **<0.003 |
| Cortex | 9 (3–9) | 5 (2–9) | *<0.01 | 9 (0–9) | 4 (1–9) | ***<0.0001 |
| Hippo-campus | 10 (0–12) | 6 (0–12) | >0.10 | 11 (0–12) | 7 (0–12) | **<0.005 |
| Striatum | 7.5 (0–9) | 2 (0–9) | *<0.02 | 6 (0–9) | 5 (0–9) | *<0.03 |
| Thalamus | 2 (0–3) | 1 (0–3) | *<0.03 | 2 (0–3) | 1 (0–3) | **<0.003 |
Fig. 2.
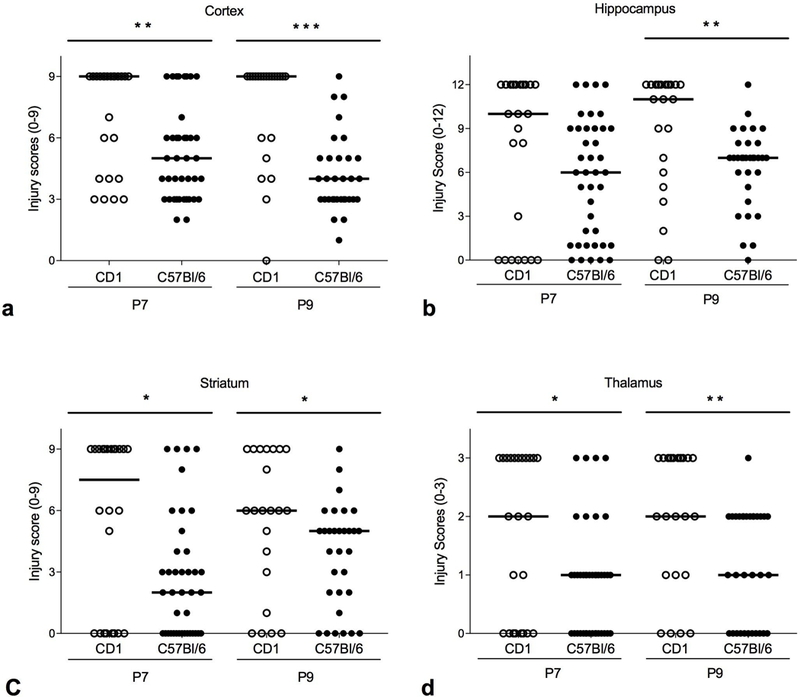
Histological injury scores by region. CD1 compared to C57Bl/6J after HI at P7 with 30 minutes of 8% oxygen and CD1 compared to C57Bl/6J after HI at P9 with 50 minutes of 10% oxygen. a Cortex (scale 0–9). Median injury score is higher in CD1 compared to C57Bl/6J in both the P7 model (* p < 0.03) and the P9 model (** p = 0.003). b Hippocampus (scale 0–12). Median injury score is not different between CD1 and C57Bl/6J in the P7 model (p > 0.10), but injury is higher in CD1 compared to C57Bl/6J in the P9 model (** < 0.005). c Striatum (scale 0–9). Median injury score is higher in CD1 compared to C57Bl/6J in both the P7 model (* p < 0.02) and the P9 model (* p = <0.03). d Thalamus (scale 0–3). Median injury score is higher in CD1 compared to C57Bl/6J in both the P7 model (* p < 03) and the P9 model (* p < <0.003). Horizontal line indicates median score.
Comparison of brain injury scores by age at HI
Injury scores were not significantly different in either CD1 P7 compared to P9 or in C57Bl/6J P7 compared to P9, either overall or regionally.
Sex Differences
Some studies using HI have shown an affect associated with sex [18–20]. Here, when we compared injury scores of male and female mice, we found CD1 males to be more injured than females at P7, but no difference between CD1 males and females at P9 (Fig. 3a and Table 2). However, there are far fewer females in the P7 group compared to males. In C57Bl/6J mice, there were no differences associated with sex at either P7 or P9 (Fig. 3b and Table 2; note: data was missing for five P9 C57Bl/6J mice).
Fig. 3.
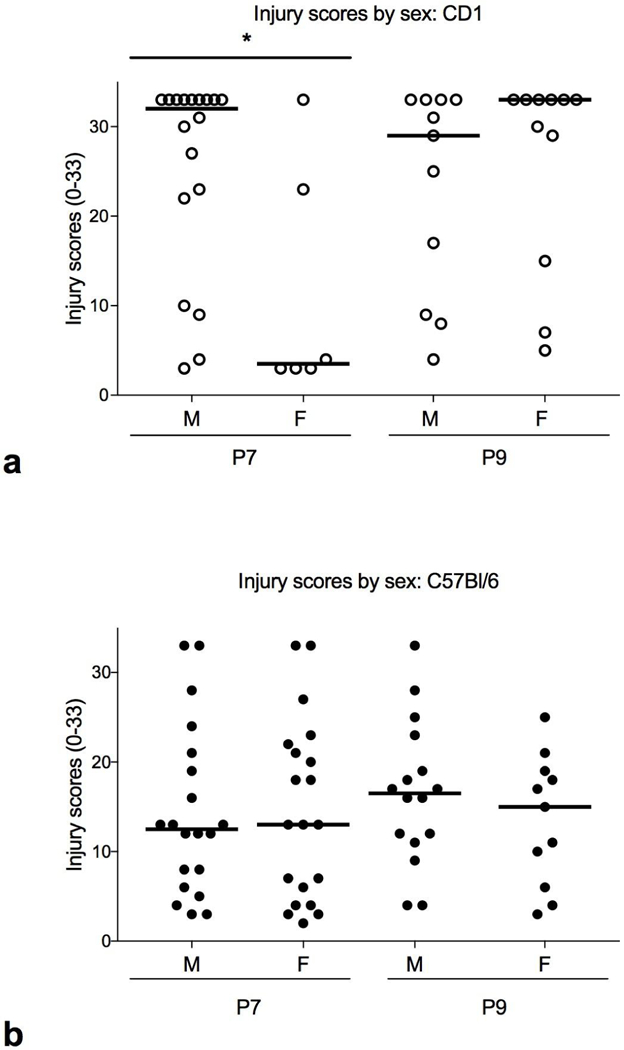
Histological Injury scores by sex (scale 0–33). a CD1. Male mice have greater injury than females at P7 (* p < 0.03) but not at P9 (p=0.5). b C57Bl/6J. There is no difference between male and female C57Bl/6J mice at P7 or P9.
Table 2:
Injury scores by sex. Median (range) n
| Strain | P7 |
P9 |
||||
|---|---|---|---|---|---|---|
| Male | Female |
p value |
Male | Female |
p value |
|
| CD1 | 32 (3–33) n = 18 |
3.5 (3–33) n = 6 |
*<0.03 | 29 (4–33) n = 11 |
33 (5–33) n = 11 |
>0.5 |
| C57Bl/6 | 12.5 (3–33) n = 20 |
13 (2–33) n = 20 |
>0.97 | 16.5 (4–33) n = 16 |
15 (3–25) n = 11 |
>0.4* |
Sex was not determined in five P9 C57Bl/6J mice
Weight
Prior to HI, CD1 mice were heavier than C57Bl/6J at P7 (mean CD1 = 4.54 ± 0.59 g vs C57Bl/6J = 4.20 ± 0.55 g; p < 0.016) and at P9 (mean CD1 = 5.91 ± 0.68 g vs. C57Bl/6J = 5.25 ± 0.85 g; p=0.003).
Mortality
The highest mortality was among the P7 mice. Fifteen of 39 CD1 mice died and 26 of 66 C57Bl/6J mice died. Among the P9 mice, only 4 of 26 CD1 mice died and none of the C57Bl/6J died. There were no differences in mortality between male and female (Table 3).
Table 3:
Mortality. n (%)
| Strain | P7 |
P9 |
||||
|---|---|---|---|---|---|---|
| Male | Female |
p value |
Male | Female |
p value |
|
| CD1 | 10 (36) | 5 (45) | 0.72 | 2 (18) | 2 (18) | 1 |
| C57Bl/6 | 15 (43) | 11 (35) | 0.62 | 0 (0) | 0 (0) | 1 |
Discussion
Given the same insult at P7, CD1 mice were more susceptible to HI injury than the commonly used inbred strain C57Bl/6J, confirming our previous results [4]. The current results also validate the re-analysis of HI data acquired over many years. Our previous study did not analyze brain regions separately, which we do here, showing the cortex, striatum and thalamus to be the regions where the difference in injury is the greatest, with injury in the hippocampus demonstrating only a trend to difference.
We also looked for differences associated with sex and found male CD1 mice at P7 to have greater injury than females. However, there were relatively few females in this group, which likely influences this result. We have not seen differences due to sex in previous studies with CD1 mice and HI at P7 [21] [22]. Thus, while additional data from female CD1 mice might reduce the overall median score of the CD1 P7 group, our experience indicates significant sex differences are unlikely. However, it is also unknown how additional data from female CD1 mice affect the comparison of the CD1 and C57Bl/6 brains in the P7 model overall. It is a limitation of this study that the data shown here for P7 comes from archived slides, and no new mice were used to equalize the numbers.
There were no sex-related differences in injury in the C57Bl/6J mice at P7. Others, however, have seen sex differences in neonatal brain injury [23]. Recently, in a stroke model with P9 C57Bl/6J mice, saline-treated males had greater tissue loss than females three months after the insult, but not at three or eight days [24]. Outcome at adulthood is important, and the possibility of worsened injury, especially, warrants further study.
We also demonstrated that CD1 mice were more susceptible to HI injury than C57Bl/6 in an age-adjusted P9 version of the Vannucci model. In P9, the differences are seen in the entire hemisphere as well as in all regions analyzed: cortex, striatum, thalamus and hippocampus. In a recent study using P9 mutant mice with a C57BI/6J background, we compared 50 min of 10% oxygen to 60 min of 10% oxygen in order to see if increased duration of hypoxia increased injury and if so, how a greater degree of injury would affect outcome relative to WT. Injury scores increased in WT mice with the additional minutes of hypoxia, demonstrating that injury is indeed increased with a small increase in duration of hypoxia in C57Bl/6J mice [17].
Other studies have compared CD1 to C57Bl/6. For example, Li et al administered chronic hypoxia to both strains at P3 and found C57Bl/6 and the neural progenitor cells derived from them to have a ‘blunted’ response to hypoxia by several measures [15]. A model of ischemic brain injury and seizures with carotid ligation alone (no hypoxia) also showed CD1 to be far more susceptible to injury than C57Bl/6 [25] and the strain C3HeB/FeJ [26]. Substrains of C57Bl/6 have also been compared in the P7 HI model. Wolf et al showed that C57Bl/6J had decreased lesion size and score compared to C57Bl/6N [27].
Anatomical variations between mouse strains, vascular differences in particular, are often proposed to be the cause for differences in experimental outcome in stroke and HIE [28]. An adult mouse study, for example, showed C57Bl/6 had the poorest development of the circle of Willis of seven strains studied (not including CD1), as well as the highest mortality and greatest susceptibility to injury after bilateral common carotid artery occlusion [29]. Recently, another study of adult mice examined 32 inbred strains for variations in the circle of Willis, as well as the diameters of major cerebral arteries [30]. They found strain-related differences not only in vessel diameters, but also strain-related anomalies such as unilateral or bilateral absence of P1 (first segment of posterior communicating artery) and presence of accessory MCAs (middle-cerebral arteries). They also corrected for weight and age, strengthening the argument for genetic causality. The same group has previously explored the genetic basis for these vascular differences and identified four SNPs associated with infarct volume in mice after MCAO, two of which were relevant to human stroke susceptibility [16].
There is need for more work on the genetic underpinnings of variation in neonatal models, given the variation in injury (and mortality) attributable to genetic strain, differing mechanisms of cell injury and death as well as attempts at repair [31] [32]
An advantage of the P9 model of HI in C57Bl/6J is lower mortality than at P7, especially when attempting to achieve a moderate to severe degree of injury. It is not known why mortality is high in C57Bl/6J at P7, while the brain is relatively resistant to injury, but cardiovascular differences may be involved. The neonatal rodent brain develops rapidly, and two days from P7 to P9 encompasses substantial growth and maturation. Given that we rarely know precisely when our murine research subjects are born, several hours could conceivably make a difference in a mouse’s response to HI. Optimal duration of hypoxia should be determined for different strains and ages prior to use in experiments. More importantly, background strain must be carefully considered and controlled for when using mutant mice.
Acknowledgements
This work was funded by NIH RO1 33997 and R35 NS097299 (to DMF).
Footnotes
Disclosure Statement
The authors have no conflict of interest to disclose.
References
- 1.Hagberg H, David Edwards A, Groenendaal F: Perinatal brain damage: The term infant. Neurobiol Dis 2016, 92(Pt A):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice JEr, Vannucci RC, Brierley JB: The influence of immaturity on hypoxic-ischemic brain damage in the rat. Annals of Neurology 1981, 9(2):131–141. [DOI] [PubMed] [Google Scholar]
- 3.Ditelberg JS, Sheldon RA, Epstein CJ, Ferriero DM: Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatr Res 1996, 39(2):204–208. [DOI] [PubMed] [Google Scholar]
- 4.Sheldon RA, Sedik C, Ferriero DM: Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res 1998, 810(1–2):114–122. [DOI] [PubMed] [Google Scholar]
- 5.Grafe MR: Developmental changes in the sensitivity of the neonatal rat brain to hypoxic/ischemic injury. Brain Res 1994, 653(1–2):161–166. [DOI] [PubMed] [Google Scholar]
- 6.Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, Hagberg H, Blomgren K: The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ 2005, 12(2):162–176. [DOI] [PubMed] [Google Scholar]
- 7.Lafemina MJ, Sheldon RA, Ferriero DM: Acute hypoxia-ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatr Res 2006, 59(5):680–683. [DOI] [PubMed] [Google Scholar]
- 8.Sheldon RA, Chuai J, Ferriero DM: A rat model for hypoxic-ischemic brain damage in very premature infants. Biol Neonate 1996, 69(5):327–341. [DOI] [PubMed] [Google Scholar]
- 9.Towfighi J, Mauger D, Vannucci RC, Vannucci SJ: Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Research Developmental Brain Research 1997, 100(2):149–160. [DOI] [PubMed] [Google Scholar]
- 10.McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM: Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci 2003, 23(8):3308–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM: Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. Journal of Neuroscience 2002, 22(2):455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Zhu C, Hagberg H, Korhonen L, Sandberg M, Lindholm D, Blomgren K: X-linked inhibitor of apoptosis (XIAP) protein protects against caspase activation and tissue loss after neonatal hypoxia-ischemia. Neurobiol Dis 2004, 16(1):179–189. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Xu F, Wang X, Shibata M, Uchiyama Y, Blomgren K, Hagberg H: Different apoptotic mechanisms are activated in male and female brains after neonatal hypoxia-ischaemia. J Neurochem 2006, 96(4):1016–1027. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson Y, Wang X, Schwendimann L, Rousset CI, Jacotot E, Gressens P, Thoresen M, Mallard C, Hagberg H: Combined effect of hypothermia and caspase-2 gene deficiency on neonatal hypoxic-ischemic brain injury. Pediatr Res 2012, 71(5):566–572 [DOI] [PubMed] [Google Scholar]
- 15.Li Q, Michaud M, Stewart W, Schwartz M, Madri JA: Modeling the neurovascular niche: murine strain differences mimic the range of responses to chronic hypoxia in the premature newborn. J Neurosci Res 2008, 86(6):1227–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du R, Zhou J, Lorenzano S, Liu W, Charoenvimolphan N, Qian B, Xu J, Wang J, Zhang X, Wang X et al. : Integrative Mouse and Human Studies Implicate ANGPT1 and ZBTB7C as Susceptibility Genes to Ischemic Injury. Stroke 2015,46(12):3514–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheldon RA, Windsor C, Lee BS, Arteaga Cabeza O, Ferriero DM: Erythropoietin Treatment Exacerbates Moderate Injury after Hypoxia-Ischemia in Neonatal Superoxide Dismutase Transgenic Mice. Dev Neurosci 2017, 39(1–4):228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu C, Sun Y, Gao J, Wang X, Plesnila N, Blomgren K: Inhaled nitric oxide protects males but not females from neonatal mouse hypoxia-ischemia brain injury. Transi Stroke Res 2013, 4(2):201–207 [DOI] [PubMed] [Google Scholar]
- 19.Huang HZ, Wen XH, Liu H: Sex differences in brain MRI abnormalities and neurodevelopmental outcomes in a rat model of neonatal hypoxia-ischemia. IntJ Neurosci 2016, 126(7):647–657 [DOI] [PubMed] [Google Scholar]
- 20.Diaz J, Abiola S, Kim N, Avaritt O, Flock D, Yu J, Northington FJ, Chavez-Valdez R: Therapeutic Hypothermia Provides Variable Protection against Behavioral Deficits after Neonatal Hypoxia-Ischemia: A Potential Role for Brain-Derived Neurotrophic Factor. Dev Neurosci 2017, 39(1–4):257–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon RA, Aminoff A, Lee CL, Christen S, Ferriero DM: Hypoxic preconditioning reverses protection after neonatal hypoxia-ischemia in glutathione peroxidase transgenic murine brain. Pediatr Res 2007, 61(6):666–670. [DOI] [PubMed] [Google Scholar]
- 22.Sheldon RA, Jiang X, Francisco C, Christen S, Vexler ZS, Tauber MG, Ferriero DM: Manipulation of antioxidant pathways in neonatal murine brain. Pediatr Res 2004, 56(4):656–662. [DOI] [PubMed] [Google Scholar]
- 23.Charriaut-Marlangue C, Besson VC, Baud O: Sexually Dimorphic Outcomes after Neonatal Stroke and Hypoxia-Ischemia. Int J Moi Sci 2017, 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charriaut-Marlangue C, Leconte C, Csaba Z, Chafa L, Pansiot J, Talatizi M, Simon K, Moretti R, Marchand-Leroux C, Baud O et al. : Sex differences in the effects of PARP inhibition on microglial phenotypes following neonatal stroke. Brain Behav Immun 2018, 73:375–389. [DOI] [PubMed] [Google Scholar]
- 25.Comi AM, Trescher WH, Abi-Raad R, Johnston MV, Wilson MA: Impact of age and strain on ischemic brain injury and seizures after carotid ligation in immature mice. Int J Dev Neurosci 2009, 27(3):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comi AM, Johnston MV, Wilson MA: Strain variability, injury distribution, and seizure onset in a mouse model of stroke in the immature brain. Dev Neurosci 2005, 27(2–4):127–133. [DOI] [PubMed] [Google Scholar]
- 27.Wolf S, Hainz N, Beckmann A, Maack C, Menger MD, Tschernig T, Meier C: Brain damage resulting from postnatal hypoxic-ischemic brain injury is reduced in C57BL/6J mice as compared to C57BL/6N mice. Brain Res 2015. 1650:224–231. [DOI] [PubMed] [Google Scholar]
- 28.Maeda K, Hata R, Hossmann KA: Differences in the cerebrovascular anatomy of C57black/6 and SV129 mice. Neuroreport 1998, 9(7):1317–1319. [DOI] [PubMed] [Google Scholar]
- 29.Yang G, Kitagawa K, Matsushita K, Mabuchi T, Yagita Y, Yanagihara T, Matsumoto M: C57BL/6 strain is most susceptible to cerebral ischemia following bilateral common carotid occlusion among seven mouse strains: selective neuronal death in the murine transient forebrain ischemia. Brain Res 1997, 752(1–2):209–218. [DOI] [PubMed] [Google Scholar]
- 30.Qian B, Rudy RF, Cai T, Du R: Cerebral Artery Diameter in Inbred Mice Varies as a Function of Strain. Front Neuroanat 2018, 12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keum S, Lee HK, Chu PL, Kan MJ, Huang MN, Gallione CJ, Gunn MD, Lo DC, Marchuk DA: Natural genetic variation of integrin alpha L (Itgal) modulates ischemic brain injury in stroke. PLoS Genet 2013, 9(10):e1003807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parry SM, Peeples ES: The impact of hypoxic-ischemic brain injury on stem cell mobilization, migration, adhesion, and proliferation. Neural Regen Res 2018, 13(7):1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]


