Abstract
BACKGROUND:
The use of regional and other opioid-sparing forms of anesthesia has been associated with a decrease in the recurrence of certain malignancies. Direct suppression of human natural killer cells by opioids has been postulated to explain this observation. However, the effect of different classes of opioids on suppression of natural killer cell cytotoxicity has not been systematically characterized.
METHODS:
After confirming that freshly isolated natural killer cells from peripheral human blood express opioid receptors, cells were incubated with increasing concentrations of clinically used or receptor-specific opioid agonists. We also evaluated the effect of pretreatment with receptor-specific antagonists or naloxone. Treated natural killer cells were then coincubated with a carboxyfluorescein succinimidyl ester-labeled target tumor cell line, K562. Annexin V staining was used to compare the percent of tumor cell apoptosis in the presence of opioid-pretreated and untreated natural killer cells. Treated samples were compared to untreated samples using Kruskal-Wallis tests with a post hoc Dunn correction.
RESULTS:
Morphine, methadone, buprenorphine, loperamide, [D-Ala2, N-MePhe4, Gly-ol]-enkephalin, and U-50488 significantly decreased natural killer cell cytotoxicity. When natural killer cells were pretreated with naloxone, cyprodime, and nor-binaltorphimine before exposure to morphine, there was no difference in natural killer cytotoxicity, compared to the amount observed by untreated natural killer cells. Fentanyl, O-desmethyltramadol, and [D-Pen2,D-Pen5] enkephalin did not change natural killer cell cytotoxicity compare to untreated natural killer cells.
CONCLUSIONS:
Incubation of isolated natural killer cells with certain opioids causes a decrease in activity that is not observed after naloxone pretreatment. Suppression of natural killer cell cytotoxicity was observed with μ- and κ-receptor agonists but not δ-receptor agonists. These data suggest that the effect is mediated by μ- and κ-receptor agonism and that suppression is similar with many clinically used opioids. (Anesth Analg 2019;128:1013–21)
The use of opioid-reducing anesthetic techniques in the perioperative phase of care during tumor resection surgery has been frequently associated with the recurrence of certain malignancies, including breast, prostate, and lung adenocarcinoma.1–3 Currently, a number of ongoing prospective, randomized, and observational studies are seeking to further refine this observation.4 Several mechanisms have been proposed to explain these observations, including suppression of the hypothalamic-pituitary axis by opioids, decreased sympathetic outflow afforded by regional anesthesia, or direct suppression of the cytolytic function of circulating lymphocytes.5,6
Support for the direct inhibition of lymphocytes arises from in vitro and in vivo analysis of the suppressive effects of opioids on lymphocyte function, especially on natural killer cells.7,8 Natural killer cells are innate lymphocytes with major histocompatibility complex-independent cytolytic activity against virus-infected and cancer cells. Prior work that used chromium-release assays as a marker of cytotoxicity showed that higher doses of fentanyl produced prolonged natural killer cell immunosuppression than lower opioid doses.9 Separate publications have previously compared morphine to either tramadol or methadone with divergent results.10,11 However, studies have not yet examined the dose-dependent effects of multiple opioids, with or without naloxone, on human natural killer cytolytic function within the same assay. There is also evidence that opioids and other related agents, such as (+)-naloxone, may modulate innate immune function through alternative, nonopioid receptors, such as toll-like receptor 4.12 Further, it is not clear which of the 3 classic human opioid receptors are involved in opioid-induced immunosuppression. Although there are several possible mechanisms by which opioids may contribute to the increased recurrence of malignancies after surgery, understanding a direct mechanism is an essential component for the interpretation of ongoing and future studies examining the effects of anesthesia on cancer recurrence.
The primary aim of this study was to characterize the effect of opioids on the ability of human natural killer cells to induce apoptosis in a target tumor cell line. Secondary aims included characterization of the relative of expression on opioid receptor types on human natural killer cells, analysis of direct apoptosis induction by opioids on tumor cells, analysis of opioid dose escalations, and the effect of naloxone and other receptor-specific antagonists. We hypothesized that opioids would inhibit the cytolytic ability of natural killer cells in an antagonist-reversible manner.
METHODS
The protocol for blood donation was approved by the John Hopkins Institutional Review Board. A certified phlebotomist collected 150 mL of whole blood from healthy male volunteers between the ages of 25 and 39 into heparinized syringes. No subjects had a history of cancer, immunosuppression, human immunodeficiency virus, chronic pain, or use of opioids, steroids, or other immunosuppressant medication in the past 12 months. A relatively homogeneous cohort was chosen as natural killer cell function, even among healthy individuals, can change as a function of age and sex.13
Isolation of and Culture of Natural Killer Cells
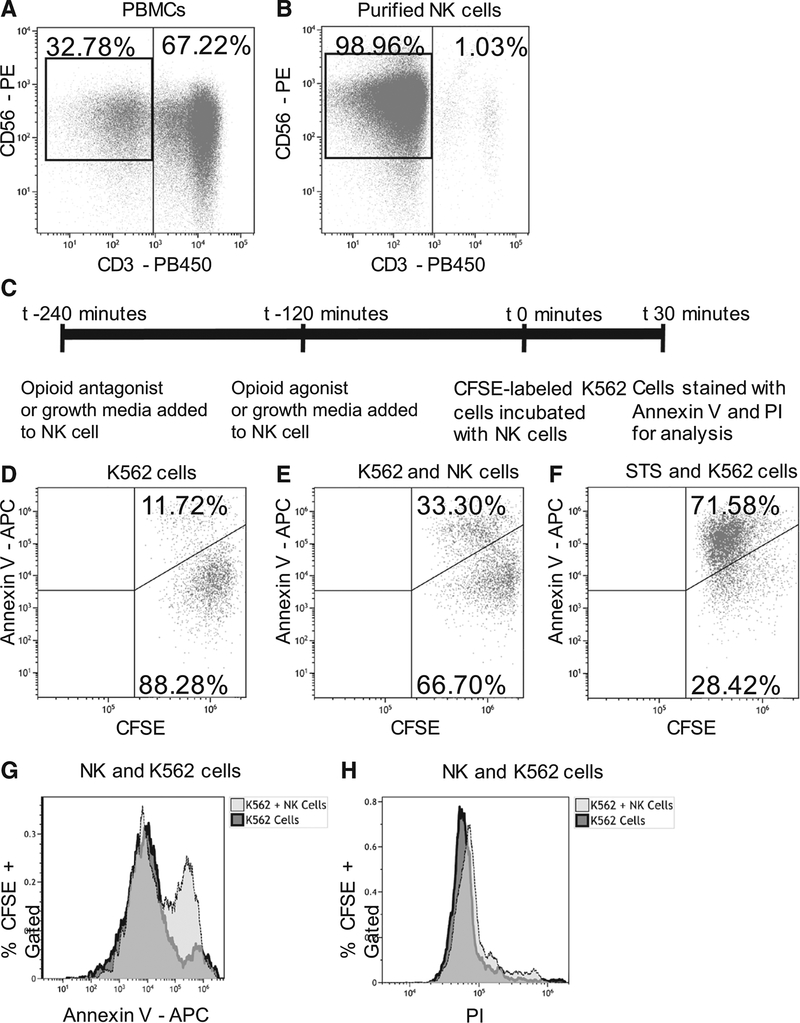
Whole blood was washed with phosphate-buffered saline (Gibco, Gaithersburg, MD) and then layered over Lympholyte-H human cell separation media (Cedarlane, Burlington, ON). Buffy coats were collected, and peripheral blood mononuclear cells were counted. Natural killer cells were isolated by a negative selection magnetic-activated cell sorting kit according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The isolated natural killer cells were then counted, and viability was confirmed with a 0.4% trypan blue exclusion dye (Gibco). Cells were stained with conjugated surface antibodies, including CD3-Pacific blue, CD45-Alexa Fluor 700, CD56-phycoerythrin (PE; Biolegend, San Diego, CA), and live/dead cell viability stain (Thermo Fisher, Waltham, MA). Flow cytometry revealed a purity of 95% for natural killer cells (Figure 1A, B).
Figure 1.
Human natural killer (NK) cell apoptosis assay. A, NK cells in total peripheral blood mononuclear cells (PBMCs; gated on live, singlets, CD45+ cells) were stained for CD3 and CD56 before negative isolation. B, Negatively isolated NK cells. Note the disappearance of the CD3+ cells compared to the PBMCs and approximately 99% purity of CD3− CD56+ cells obtained. C, Schematic representation of apoptosis assay with the time for incubation of isolated NK cells with antagonists, agonists, or control media before addition of carboxyfluorescein succinimidyl ester (CFSE)-labeled K562 target tumor cells. D, Negative control of CFSE-labeled K562 cells in growth media but without NK cells. E, Positive control of NK cells and CFSE-labeled K562 cells is coincubated for 30 min. In the case of experimental samples, NK cells were preincubated with opioid agonists and antagonists. F, Positive control of exposure of NK cells to staurosporine (STS) to ensure occurrence of apoptosis. G, Histogram overlay of negative and positive controls undergoing apoptosis. An increase in annexin V staining in CFSE-gated cells indicates that NK cells induced apoptosis. H, Histogram overlay of negative and positive controls undergoing cell death. No shift was observed in PI staining, indicating that the test assay did not induce cell death of CFSE-labeled K562 cells. Representative flow cytometry dot plots and histograms from 1 donor (from a total of 10 separate donors) are shown. APC indicates allophycocyanin; PI, propidium iodide.
Natural killer cells were maintained in 96-well plates at a concentration of 2 × 104 per 50 μL. RPMI-1640 (Gibco), with 10% fetal bovine serum (Gemini Bio Products, West Sacramento, CA) and 100 units of penicillin and streptomycin per milliliter (Gibco, Gaithersburg, MD), was used as growth medium in subsequent experiments.
Quantitative Polymerase Chain Reaction
RNA from isolated cell lines was collected in RNeasy Lysis Buffer and processed with RNeasy mini kits (Qiagen, Hilden, Germany). Quantitative polymerase chain reaction was performed with the indicated primers and SYBR Green (Thermo Fisher) on a 7500 fast real-time polymerase chain reaction instrument from Applied Biosystems (Foster City, CA). Primers were obtained from Integrated DNA Technologies (Coralville, IA) and included glyceraldehyde 3-phosphate dehydrogenase forward 5’-GGC ATG GAC TGT GGT CAT GAG-3 and glyceraldehyde 3-phosphate dehydrogenase reverse 5’-TGC ACC ACC AAC TGC TTA GC-3’, mu opioid receptor forward 5’-CGG TTC CTG GGT CAA CTT GT-3’, mu opioid receptor reverse 5’-CCG TGA TCA TGG AGG GACTG-3’, kappa opioid receptor forward 5’-TCG TGA TCA TCC GAT ACA CAA AGA-3’, kappa opioid receptor reverse 5’-GAC CGT ACT CTG AAA GGG CA-3’, delta opioid receptor forward 5’-GCC CAT CCA CAT CTT CGT CA-3’, delta opioid receptor reverse 5’-TCG AGG AAA GCG TAG AGC AC-3’, Nociceptin receptor forward 5’-CATCGT GGG GCT CTA CTT GG-3’, Nociceptin receptor reverse 5’-GAC CAG GGT ATC AGC CAG TG-3’, toll-like receptor 4 forward 5’-CAG AGT TGC TTT CAA TGG CAT C-3’, and toll-like receptor 4 reverse 5’-AGA CTG TAA TCA AGA ACC TGG AGG-3’. Gene expression was determined by using 2−ΔΔCt calculation relative to glyceraldehyde 3-phosphate dehydrogenase and then normalized to relative toll-like receptor 4 expression. All polymerase chain reactions were conducted in duplicate. Expression relative to toll-like receptor 4 was chosen because toll-like receptor 4 is expressed on the surface of both innate immune cell lineages and neural tissue which allows for comparison of relative opioid receptor expression.14
Drug Treatment
Stock solutions of morphine, methadone, fentanyl, buprenorphine, and naloxone were obtained from the hospital pharmacy and diluted in phosphate-buffered saline. All clinically used medications in this study, including naloxone, were racemic mixtures. Other drugs procured included [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (Adipogen, San Diego, CA), U-50488 (Sigma-Aldrich, St Louis, MO), [D-Pen2,D-Pen5] enkephalin (Fisher, Pittsburgh, PA), nor-binaltorphimine dihydrochloride (Sigma-Aldrich), cyprodime (Tocris, Minneapolis, MN), and O-desmethyltramadol (Sigma-Aldrich). Natural killer cells were incubated with opioid antagonists or an equivalent volume of phosphate-buffered saline for 2 hours and then with opioid agonists for an additional 2 hours. All clinically used drugs were used over a concentration range described elsewhere as clinically rel-evant.15 A 2-hour incubation was chosen because this represents a biologically plausible natural killer cell exposure duration after parenteral administration. Concentrations of drugs tested include morphine 25–250 ng/mL (8.67 × 10−2 to 8.67 μM), methadone (0.162–16.20 μM), fentanyl (2.97 × 10−3 to 0.29 μM), buprenorphine (2.13 × 10−3 to 0.21 μM), O-desmethyltramadol (4.01 × 10−3 to 0.40 μM), [D-Ala2, N-MePhe4, Gly-ol]-enkephalin (1.95 × 10−3 to 0.20 μM), U-50488 (2.71 × 10−3 to 0.27 μM), [D-Pen2,D-Pen5]enkephalin (1.55 × 10−3 to 0.16 μM), naloxone (0.31 μM), cyprodime (0.28 μM), and nor-binaltorphimine dihydrochloride (0.15 μM).
K562 Cell Culture and Carboxyfluorescein Succinimidyl Ester Staining
K562, a nonadherent, chronic myelogenous leukemia-derived cell line devoid of major histocompatibility I complexes, was chosen as the standardized target tumor cell for the natural killer cell apoptosis assay because it is nonadherent and can be analyzed by flow cytometry without the need for physical manipulation. Further, the use of the K562 cell line has been extensively described in natural killer cell apoptosis assays.16,17 K562 cells (ATCC, Manassas, VA) were maintained in Iscove’s Modified Dulbecco’s Medium (ATCC) with 10% fetal bovine serum and penicillin and streptomycin at a concentration of 1 × 106/mL and used between passages 0 and 2. They were tested with a universal mycoplasma detection kit (ATCC) and confirmed to be negative. Before using the cells in an apoptosis assay, we counted them and checked their viability with 0.4% trypan blue exclusion dye (Gibco). Then we labeled 2 × 106 of the K562 cells with 5 μM carboxyfluorescein succinimidyl ester (Thermo Fisher) according to the manufacturer’s instructions.
Apoptosis Assay, Annexin V Staining, and Flow Cytometry
We added 2 × 104 carboxyfluorescein succinimidyl ester-labeled K562 target cells in 50 μL of media to each well of a 96-well plate containing 2 × 104 natural killer cells. The cells were mixed with gentle pipetting, and the plate was placed in an incubator for 30 minutes (Figure 1C). Three wells containing only carboxyfluorescein succinimidyl ester-labeled K562 cells (Figure 1D) were used as a negative control. As a positive control, 3 wells that contained untreated natural killer cells and K562 cells were included (Figure 1E). Finally, as a positive control for apoptosis, 3 wells of K562 cells exposed to 100 mM staurosporine were included, which has been demonstrated to induce apoptosis in K562 cells (Figure 1F).18
After the 30-minute incubation, the cells were stained with annexin V conjugated to Allophycocyanin (Biolegend) according to the manufacturer’s instructions and 50 propidium iodide (Sigma-Aldrich). Cells were analyzed immediately on a CytoFLEX flow cytometer with a 96-well plate reader and CytEXPERT software (version 2.1; Beckman-Coulter, Indianapolis, IN). A sample of 1 × 104 carboxyfluorescein succinimidyl ester positively staining cells was collected from each well. The percent of carboxyfluorescein succinimidyl ester-gated cells that stained positive for annexin V was determined (ie, the percent of K562 cells undergoing apoptosis; Figure 1G). The percent of carboxyfluorescein succinimidyl ester-gated cells that stained positive for PI was also determined (ie, the percent of K562 cells undergoing necrosis; Figure 1H).
Analysis and Statistics
Results were analyzed with Kaluza software, version 2.0 (Beckman-Coulter). Statistical analysis was performed with Graphpad Prism, version 7.0 (La Jolla, CA), and PS Power and Sample Size Calculation, version 3.0 (Nashville, TN).
For each subject, the average of the 3 positive control wells (natural killer cells and K562 cells without any treatment) was calculated and denoted as “100% natural killer cell cytolytic efficiency.” Each treatment was performed in triplicate in 5 subjects for a total of 15 samples per data point. For each treatment condition, the average of the 3 experimental wells was determined and the ratio of 3 experimental wells to the average of the 3 control wells was determined. Given the relatively small sample size, we were not able to adequately assess normality of the data, and so nonparametric analyses were conducted. For each treatment, unpaired nonparametric analyses (Kruskal-Wallis) were conducted with a post hoc Dunn test to compare multiple treatment means to the positive control of untreated natural killer and K562 cells. Two-sided hypotheses were tested. A corrected P value of less than .05 was considered statistically significant.
We chose a sample size of 5 subjects based on observing a 25% decline in apoptosis induction between control and opioid-treated experimental groups with a 10% SD. Five subjects in the experimental and control groups are then needed to reject the null hypothesis of equal means with a power of .8.
RESULTS
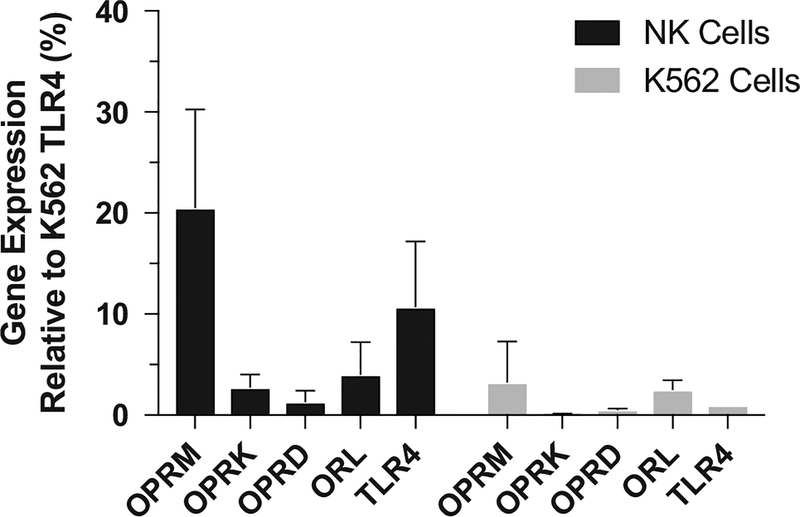
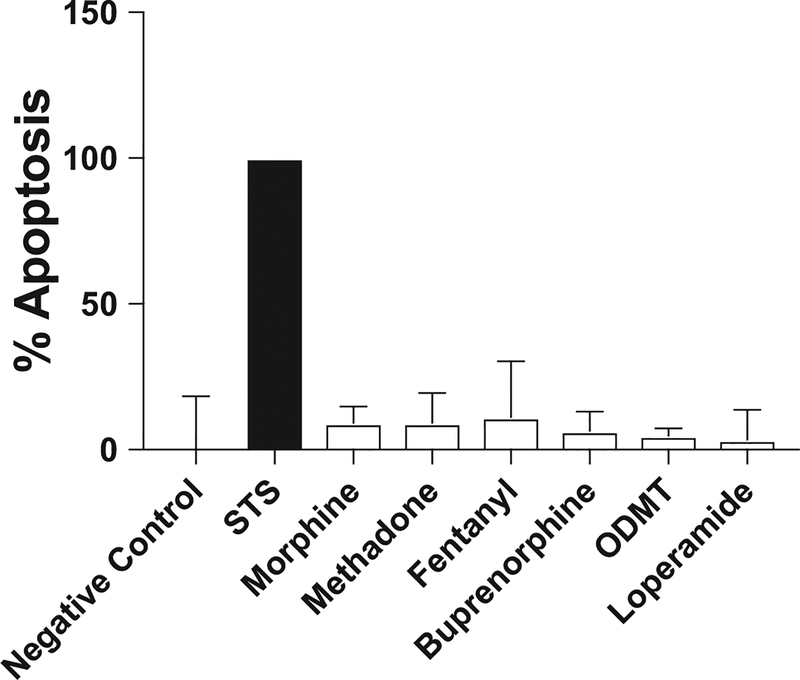
Quantitative polymerase chain reaction of freshly isolated human natural killer cells revealed expression of μ, κ, δ, and orphanin opioid receptors, as well as toll-like receptor 4. K562 cells also expressed each of these receptor types (Figure 2). K562 cells were exposed to the highest concentrations of tested opioids for 2 hours and found not to be undergoing apoptosis to a greater extent than untreated K562 cells (Figure 3). This indicates that changes in K562 cell apoptosis in this assay are due to opioid-mediated changes in natural killer cell function rather than direct effects of opioids on K562 cells.
Figure 2.
Expression of opioid receptors and toll-like receptor 4 (TLR4) on natural killer (NK) and K562 cells. Expression of the mRNA for each gene was normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCt method. Relative expression of each opioid receptor is shown as a percentage of expression of TLR4 on K562 cells. All genes were expressed on both NK cells and K562 cells, with the exception of the κ opioid receptor on K562 cells. n = 4 separate donors; mean ± SD. OPRD indicates delta opioid receptor; OPRK, kappa opioid receptor; OPRM, mu opioid receptor; ORL, nociceptin receptor.
Figure 3.
Incubation of K562 cells with opioids. The percent of K562 cells undergoing apoptosis after a 2-hour incubation with an opioid was determined. Untreated K562 cells were tested as a negative control. Staurosporine (STS; 100 nM) confirmed apoptosis as a positive control (black bar labeled STS). Each opioid was tested at the highest concentration used in subsequent apoptosis assays. Data are expressed as a percent of the positive control. Each test was repeated in triplicate. Mean and upper 95% CI are reported. ODMT (O-desmethyltramadol).
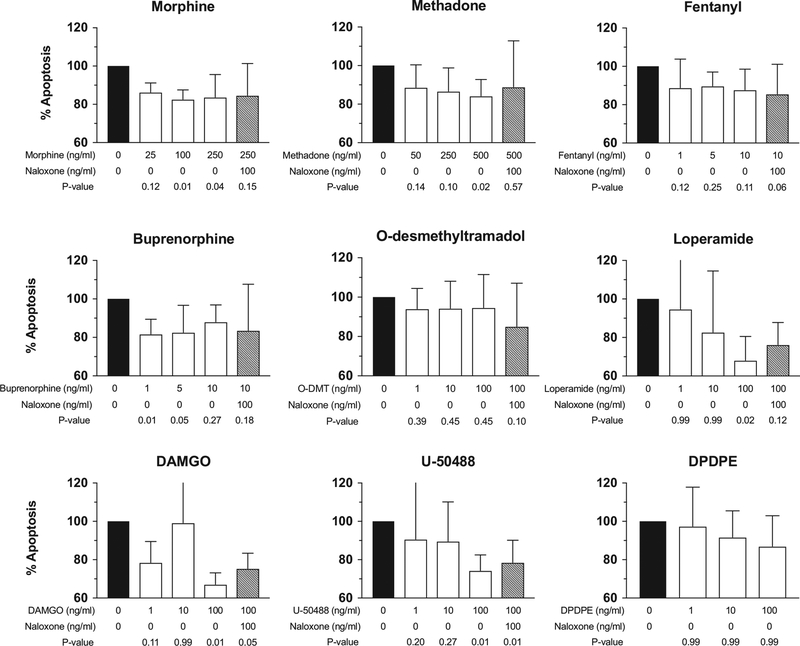
To evaluate the effect of opioids on natural killer cell cytolytic function, we chose clinically relevant concentrations of opioids that were previously reported for in vitro assays.15 The means and 95% CIs are graphically presented in Figure 4. Morphine, methadone, and buprenorphine all reduced natural killer cytolytic function, but in each case, the effect and subsequent exposure to morphine resulted in natural killer cell function that was not statistically different from controls. is not observed with naloxone pretreatment. Fentanyl and O-desmethyltramadol, also known as M1, did not produce evidence of a decrease in natural killer cytolytic function relative to positive controls. Therefore, with the exception of fentanyl and M1, clinically used opioids produced natural killer cell suppression, most commonly at high concentrations, in a naloxone-preventable manner relative to controls.
Figure 4.
Suppression of natural killer (NK) cytotoxic function with different classes of opioids. The cytolytic function of NK cells was determined by measuring the percentage of apoptotic K562 cells in experimental wells (white bars) stained with annexin V compared to the percentage in positive control wells containing NK cells and K562 target cells without any drug treatment (=100 % apoptosis, black bar). The gray bars indicate NK cell function after incubation with 100 ng/mL naloxone and subsequent incubation with a specific opioid agonist. Data are from 5 independent experiments conducted with NK cells from 5 separate donors in triplicate for each data point. n = 5; mean and upper limit of 95% CI are represented. Adjusted P values are reported. DAMGO indicates [D-Ala2, N-MePhe4, Gly-ol]-enkephalin; DPDPE, [D-Pen2,D-Pen5]enkephalin.
Receptor-specific agonists allowed us to pharmacologically characterize which opioid receptors were involved in natural killer cell suppression. [D-Ala2, N-MePhe4, Gly-ol] -enkephalin, U-50488, and [D-Pen2,D-Pen5]enkephalin are specific agonists for the μ-, κ-, and δ-opioid receptors, respectively. Pretreatment with naloxone before 100 nM U-50488 resulted in natural killer cytolytic function that was not statistically different from controls. No tested concentration of [D-Pen2,D-Pen5] enkephalin produced a change from the positive control. Only 100 nM [D-Ala2, N-MePhe4, Gly-ol]-enkephalin produced a decrease in natural killer cytolytic function compared to the positive controls. These data indicate that the μ- and κ-opioid receptor agonists, but not δ-opioid receptor agonists, are involved in suppression of natural killer cytolytic function.
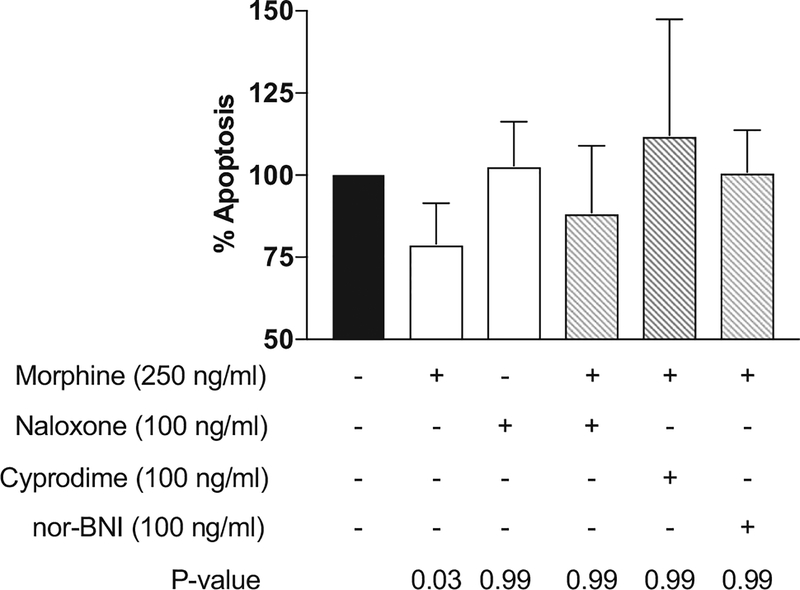
To further understand the nature of opioid agonist activity, we measured natural killer cytolytic function after incubation with both nonspecific and specific opioid receptor antagonists, including naloxone, cyprodime, and nor-binaltorphimine dihydrochloride, antagonists specific to the μ- and κ-opioid receptors, respectively. The mean natural killer cell function and upper 95% CIs after preincubation with an antagonist followed by 250 ng/mL morphine are presented in Figure 5. Cyprodime, nor-binaltorphimine dihydrochloride, and naloxone alone did not decrease natural killer cell cytotoxicity relative to positive controls (data not shown). Pretreatment with naloxone, cyprodime, or nor-binaltorphimine dihydrochloride
Figure 5.
Morphine and opioid antagonist effects on natural killer (NK) cytolytic function. The cytolytic function of NK cells was determined by measuring the percentage of apoptotic K562 cells in experimental wells (white bars) stained for annexin V compared to the percentage in positive control wells containing NK cells and K562 target cells without any drug treatment (=100% apoptosis, black bar). The gray bars indicate NK cell function after incubation with naloxone or opioid receptor-specific antagonists. Data are from 5 independent experiments conducted with NK cells from 5 separate donors in triplicate wells for each data point. n = 5; mean and upper limit of 95% CI are represented. Adjusted P values are reported. nor-BNI, nor-binaltorphimine dihydrochloride.
DISCUSSION
This study represents the first direct comparison of several opioids on the same donor’s natural killer cells in the same apoptosis assay. The findings will refine our understanding of the relationship between opioids and innate immune system function. Consistent with prior observations of single medications, our data show that, overall, opioids have the ability to decrease human natural killer cell cytotoxicity against a target tumor cell line in vitro. The potential clinical ramification of this relationship is being further explored in ongoing clinical trials.4 The current findings may serve as a partial scientific rationale for these trials.
Congruent with previously published observations, our data indicate that opioids suppress human natural killer cell cytotoxicity toward tumor cells.5,19 First, the changes relative to controls in natural killer cell function observed with [D-Ala2, N-MePhe4, Gly-ol]-enkephalin and U-50488, but not [D-Pen2,D-Pen5]enkephalin, implicate the μ- and κ-opioid receptors, but not the δ receptor, as causative for the decreased natural killer cytolytic function. All 3 receptors, along with the orphanin receptor and toll-like receptor 4, are expressed on human natural killer cells. In a rodent model that used [D-Ala2, N-MePhe4, Gly-ol]-enkephalin-, [D-Pen2,D-Pen5]enkephalin-, and the Dimethyl sulfoxide-soluble κ-agonist U69593, only the μ-opioid receptor was associated with decreased natural killer cytolytic function.20 The fact that a κ agonist suppressed natural killer cells in our study, but not in the rodent model, could be the result of the substituted use of U-50488, which is soluble in phosphate-buffered saline, or a difference between rodent and human immune cells and in vivo versus in vitro systems.
Similar decreases in natural killer cytolytic function relative to controls were observed with clinically used opioids, including, morphine, methadone, buprenorphine, and loperamide, but not fentanyl or O-desmethyltramadol. The concentrations that were tested have been previously determined to be reflective of clinically relevant concentrations of each medication.15 These results are consistent with prior observations.9,10,21–23 The lack of an observed change in natural killer cytolytic function relative to controls after incubation with fentanyl and O-desmethyltramadol may reflect test concentrations that were too low or too brief an incubation time, or it could be that these drugs do not suppress natural killer cell function. The administration of tramadol in the perioperative period has been reported to cause less suppression of natural killer cell cytotoxicity than mor-phine.24,25 We used O-desmethyltramadol instead of tramadol in our study because this metabolite has a significantly greater affinity for opioid receptors than the parent drug.26
Opioid antagonists were also studied, including naloxone, cyprodime, and nor-binaltorphimine dihydrochloride. None of these antagonists significantly changed natural killer cell cytotoxicity relative to controls when administered alone. Naloxone pretreatment did not result in significant differences between controls and samples treated with opioids, including morphine, methadone, and buprenorphine, loperamide, and [D-Ala2, N-MePhe4, Gly-ol]-enkephalin. Pretreatment of naloxone and subsequent exposure to U-50488 resulted in natural killer cell function that was significantly different than controls. Pretreatment with the receptor-specific antagonists cyprodime and nor-binaltorphimine dihydrochloride or naloxone followed by morphine treatment resulted in apoptosis that was not significantly different from controls This lack of reversibility of κ opioid-specific agonists could suggest an immunosuppressive effect that is not entirely the result of interaction with the opioid receptors, but rather other receptor, such as toll-like receptor 4.27,28
Buprenorphine is a partial μ agonist with a higher affinity for the μ-opioid receptor than many other opioids including morphine, methadone, fentanyl, and O-desmethyltramadol. In a rodent model, single doses of fentanyl, but not buprenorphine, were found to decrease lymphoid cell proliferation but not impact the function of natural killer cells.29 It is unclear if buprenorphine is immunologically neutral. The presented data suggest that pretreatment with low concentrations of buprenorphine produced changes in the ability of natural killer cells to induce apoptosis but that no differences relative to controls were noted at higher concentrations. The clinical significance of this observation deserves further investigation.
Associations between perioperative opioid use and clinical manifestations of immunosuppression, including perioperative infections, herpetic viral lesion recurrence, and the recurrence of several malignancies, have been described.1,30–34 Several mechanisms likely simultaneously contribute to the observed increase in the recurrence of cancer. Opioid receptor expression is increased in non-small cell lung cancer specimens.35 Additionally, opioids promote tumor angiogenesis and lymphangiogenesis, which increase both tumor growth and survival.36,37 Opioids produce alterations in the function of multiple immune cell lineages involved in surveillance and eradication of tumors, including T cells, monocytes/macrophages, and natural killer cells.38–41 This decreased function could occur in response to opioids binding directly to the opioid receptor, interacting with another nonopioid receptor, inhibiting the hypothalamic-pituitary axis, or a combination of mechanisms. Our data provide further evidence that opioids inhibit natural killer cells directly.
Our results suggest a possible mechanistic explanation for the results of prior retrospective studies, which showed an association between the recurrence of some malignancies and increased perioperative opioid use.1–3,33 The prior studies also suggest an association between the use of opioid-reducing or regional anesthesia and a lower incidence of cancer recurrence. Neither chronic opioid use nor chronic pain has been associated with suppression of natural killer cytolytic function.42,43 Prior examination of the effect of morphine on natural killer cells in a chromium-release assay suggested that the immunosuppressive effects may last for <48 hours.21 However, it is unclear how long after initial exposure other opioids cease to be immunosuppressive and whether tolerance develops in lymphocytes similar to the development of opioid tolerance in neurons. While prior work focused on the effects of chronic opioid use on natural killer cell function, the presented study seeks to investigate acute opioid administration as a model for the perioperative setting.42
This study had a number of limitations. Only 3 concentrations of each drug were tested, preventing the granularity required to establish true concentration-response curves. The data presented represent changes from a positive control baseline but not changes between treatments. Multiple hypotheses were tested in different experimental conditions, which is necessary and common in laboratory research. To provide a conservative statistical analysis, an assumption of non-normal distributions was made requiring a Kruskal-Wallis test, and a post hoc Dunn test was used to correct for multiple comparisons. However, because multiple hypotheses were simultaneously tested, there remains a considerable risk of type I error in the presented data. As such, these data should be viewed as hypothesis generating rather than conclusive evidence.
The concentrations tested were within the accepted range of clinical relevance. Only 1 concentration of each of the antagonists was tested. We examined only opioid receptor agonists and antagonists without testing the possibility that they may bind to other receptors. Future testing with animal knockout models could be used to explore receptor-specific binding. We did not clearly establish a “safe concentration” for use in surgery to minimize natural killer cell suppression. Nor did we establish at what point in the perioperative period opioids can be given without immunosuppression occurring. We will need to examine the clinical ramifications of these data in vitro during clinical trials by comparing different pain treatment strategies. We also only examined natural killer cells from healthy, relatively young, male donors. It is possible that age and sex differences exist and that the presence of an active malignancy may alter natural killer cell behavior in the presence of opioids. Future work will examine sex and age differences. The response when alternative target tumor cell lines are used will be examined in future work. We will also seek to examine the effects of chronic pain and active malignancies on opioid-induced changes in natural killer cell function.
Extrapolation of the results to clinical settings must proceed with caution. The use of opioids is the sine que non for the treatment of postsurgical pain. Although the in vitro immunosuppressive effects of opioids should be a consideration, postoperative pain itself has been associated with immunosuppression.44 There are also abundant ethical concerns regarding withholding pain medications for unproven reasons. However, our data could suggest an expanded role for perioperative pain control with nonopioid measures, such as regional anesthetics.
In conclusion, in vitro exposure of human natural killer cells to many clinically used opioids resulted in suppression of cytotoxicity against a target leukemia cell line. Several differences were noted among opioids, such as a lack of suppression observed with fentanyl, O-desmethyltramadol, and [D-Pen2,D-Pen5]enkephalin. The μ- and κ-opioid receptors seem to be involved in the suppression of natural killer cell cytolytic function. The direct inhibition of natural killer cells by opioids, in addition to indirect mechanisms described elsewhere, could provide an explanation for the putative relationship between perioperative opioid use and the recurrence of cancer.
KEY POINTS.
Question: Can in vitro exposure of natural killer cells to opioids, including clinically used concentrations of morphine, methadone, buprenorphine, fentanyl, and tramadol in addition to receptor-specific agonists, inhibit the ability of natural killer cells to induce apoptosis in target tumor cells?
Findings: With the exception of fentanyl, [D-Pen2,D-Pen5]enkephalin, and the tramadol metabolite, O-desmethyltramadol, opioids decreased the cytotoxic activity of natural killer cells in vitro.
Meaning: While opioids decrease the function of natural killer cells in vitro, additional research is needed to extrapolate these findings to the perioperative setting.
ACKNOWLEDGMENTS
This article is dedicated to my loving father, Patrick Oliver Maher, MD, who graduated from the University College Dublin in 1969 and practiced anesthesia for 49 years. His vocational alacrity, ceaseless curiosity, dedication, and intelligence guided all 4 of his children into medicine, including 2 who are now practicing anesthesiologists. Thank you for everything, Dad.
Funding: D.P.M. received funding from the Blaustein Pain Research Fund Grant and National Institutes of Health (NIH) Grant 5T32GM075774. N.M.H. receives funding from NIH Grant R01 HL124477. No copyrighted material from outside sources was used in preparation of this manuscript. This study has not been previously presented.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Maher DP, Wong W, White PF, et al. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth. 2014;113(suppl 1):i88–i94. [DOI] [PubMed] [Google Scholar]
- 2.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109:180–187. [DOI] [PubMed] [Google Scholar]
- 4.Royds J, Khan AH, Buggy DJ. An update on existing ongoing prospective trials evaluating the effect of anesthetic and analgesic techniques during primary cancer surgery on cancer recurrence or metastasis. Int Anesthesiol Clin. 2016;54:e76–e83. [DOI] [PubMed] [Google Scholar]
- 5.Boland JW, Foulds GA, Ahmedzai SH, Pockley AG. A preliminary evaluation of the effects of opioids on innate and adaptive human in vitro immune function. BMJ Support Palliat Care. 2014;4:357–367. [DOI] [PubMed] [Google Scholar]
- 6.Maher DP, White PF. Proposed mechanisms for association between opioid usage and cancer recurrence after surgery. J Clin Anesth. 2016;28:36–40. [DOI] [PubMed] [Google Scholar]
- 7.Bayer BM, Daussin S, Hernandez M, Irvin L. Morphine inhibition of lymphocyte activity is mediated by an opioid dependent mechanism. Neuropharmacology. 1990;29:369–374. [DOI] [PubMed] [Google Scholar]
- 8.Fuggetta MP, Di Francesco P, Falchetti R, et al. Effect of morphine on cell-mediated immune responses of human lymphocytes against allogeneic malignant cells. J Exp Clin Cancer Res. 2005;24:255–263. [PubMed] [Google Scholar]
- 9.Beilin B, Shavit Y, Hart J, et al. Effects of anesthesia based on large versus small doses of fentanyl on natural killer cell cytotoxicity in the perioperative period. Anesth Analg. 1996;82:492–497. [DOI] [PubMed] [Google Scholar]
- 10.Van der Laan JW, Timmerman H, Van Loveren H. Comparison of the in vivo effects of morphine and methadone on natural killer cell activity in spleen, peritoneal cavity, and lungs in rats. Int J Immunopharmacol. 1996;18:401–407. [DOI] [PubMed] [Google Scholar]
- 11.Sacerdote P, Bianchi M, Gaspani L, et al. The effects of tramadol and morphine on immune responses and pain after surgery in cancer patients. Anesth Analg. 2000;90:1411–1414. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SS, Loram LC, Hutchinson MR, et al. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. J Pain. 2012;13:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phan MT, Chun S, Kim SH, et al. Natural killer cell subsets and receptor expression in peripheral blood mononuclear cells of a healthy Korean population: reference range, influence of age and sex, and correlation between NK cell receptors and cytotoxicity. Hum Immunol. 2017;78:103–112. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boland JW, Pockley AG. Clinically relevant concentrations of opioids for in vitro studies. J Opioid Manag. 2016;12:313–321. [DOI] [PubMed] [Google Scholar]
- 16.Fischer K, Andreesen R, Mackensen A. An improved flow cytometric assay for the determination of cytotoxic T lymphocyte activity. J Immunol Methods. 2002;259:159–169. [DOI] [PubMed] [Google Scholar]
- 17.Aubry JP, Blaecke A, Lecoanet-Henchoz S, et al. Annexin V used for measuring apoptosis in the early events of cellular cytotoxicity. Cytometry. 1999;37:197–204. [DOI] [PubMed] [Google Scholar]
- 18.Oliver L, Hue E, Rossignol J, et al. Distinct roles of Bcl-2 and Bcl-Xl in the apoptosis of human bone marrow mesenchymal stem cells during differentiation. PLoS One. 2011;6:e19820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. 2018;175:2717–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson CJ, Schneider GM, Lysle DT. Involvement of central mu- but not delta- or kappa-opioid receptors in immunomodulation. Brain Behav Immun. 2000;14:170–184. [DOI] [PubMed] [Google Scholar]
- 21.Yeager MP, Colacchio TA, Yu CT, et al. Morphine inhibits spontaneous and cytokine-enhanced natural killer cell cytotoxicity in volunteers. Anesthesiology. 1995;83:500–508. [DOI] [PubMed] [Google Scholar]
- 22.Carrigan KA, Saurer TB, Ijames SG, Lysle DT. Buprenorphine produces naltrexone reversible alterations of immune status. Int Immunopharmacol. 2004;4:419–428. [DOI] [PubMed] [Google Scholar]
- 23.Yardeni IZ, Beilin B, Mayburd E, Alcalay Y, Bessler H. Relationship between fentanyl dosage and immune function in the postoperative period. J Opioid Manag. 2008;4:27–33. [DOI] [PubMed] [Google Scholar]
- 24.Gaspani L, Bianchi M, Limiroli E, Panerai AE, Sacerdote P. The analgesic drug tramadol prevents the effect of surgery on natural killer cell activity and metastatic colonization in rats. J Neuroimmunol. 2002;129:18–24. [DOI] [PubMed] [Google Scholar]
- 25.Bakr MA, Amr SA, Mohamed SA, et al. Comparison between the effects of intravenous morphine, tramadol, and ketorolac on stress and immune responses in patients undergoing modified radical mastectomy. Clin J Pain. 2016;32:889–897. [DOI] [PubMed] [Google Scholar]
- 26.Lai J, Ma SW, Porreca F, Raffa RB. Tramadol, M1 metabolite and enantiomer affinities for cloned human opioid receptors expressed in transfected HN9.10 neuroblastoma cells. Eur J Pharmacol. 1996;316:369–372. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson MR, Northcutt AL, Hiranita T, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchinson MR, Zhang Y, Shridhar M, et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun 2010;24:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martucci C, Panerai AE, Sacerdote P. Chronic fentanyl or buprenorphine infusion in the mouse: similar analgesic profile but different effects on immune responses. Pain. 2004;110:385–392. [DOI] [PubMed] [Google Scholar]
- 30.Menendez ME, Ring D, Bateman BT. Preoperative opioid misuse is associated with increased morbidity and mortality after elective orthopaedic surgery. Clin Orthop Relat Res. 2015;473:2402–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mojadadi S, Jamali A, Khansarinejad B, Soleimanjahi H, Bamdad T. Acute morphine administration reduces cell-mediated immunity and induces reactivation of latent herpes simplex virus type 1 in BALB/c mice. Cell Mol Immunol. 2009;6:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Tan N, Douglas SD, Zhang T, Wang YJ, Ho WZ. Morphine inhibits CD8+ T cell-mediated, noncytolytic, anti-HIV activity in latently infected immune cells. J Leukoc Biol. 2005;78:772–776. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Qu X, Wang Y, Shen H, Liu Q, Du J. Effect of mu agonists on long-term survival and recurrence in nonsmall cell lung cancer patients. Medicine (Baltimore). 2015;94:e1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crone LA, Conly JM, Clark KM, et al. Recurrent herpes simplex virus labialis and the use of epidural morphine in obstetric patients. Anesth Analg. 1988;67:318–323. [PubMed] [Google Scholar]
- 35.Lennon FE, Mirzapoiazova T, Mambetsariev B, Salgia R, Moss J, Singleton PA. Overexpression of the μ-opioid receptor in human non-small cell lung cancer promotes Akt and mTOR activation, tumor growth, and metastasis. Anesthesiology. 2012;116:857–867. [DOI] [PubMed] [Google Scholar]
- 36.Gupta K, Kshirsagar S, Chang L, et al. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 37.Nguyen J, Luk K, Vang D, et al. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014;113(suppl 1):i4–i13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franchi S, Moretti S, Castelli M, et al. Mu opioid receptor activation modulates Toll like receptor 4 in murine macrophages. Brain Behav Immun 2012;26:480–488. [DOI] [PubMed] [Google Scholar]
- 39.Borner C, Kraus J, Bedini A, Schraven B, Hollt V. T-cell receptor/ CD28-mediated activation of human T lymphocytes induces expression of functional mu-opioid receptors. Mol Pharmacol. 2008;74:496–504. [DOI] [PubMed] [Google Scholar]
- 40.Borner C, Warnick B, Smida M, et al. Mechanisms of opioid-mediated inhibition of human T cell receptor signaling. J Immunol. 2009;183:882–889. [DOI] [PubMed] [Google Scholar]
- 41.Beilin B, Martin FC, Shavit Y, Gale RP, Liebeskind JC. Suppression of natural killer cell activity by high-dose narcotic anesthesia in rats. Brain Behav Immun. 1989;3:129–137. [DOI] [PubMed] [Google Scholar]
- 42.Tabellini G, Borsani E, Benassi M, et al. Effects of opioid therapy on human natural killer cells. Int Immunopharmacol. 2014;18:169–174. [DOI] [PubMed] [Google Scholar]
- 43.Yoon JJ, Song JA, Park SY, Choi JI. Cytotoxic activity and subset populations of peripheral blood natural killer cells in patients with chronic pain. Korean J Pain. 2018;31:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Page GG, Blakely WP, Ben-Eliyahu S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191–199. [DOI] [PubMed] [Google Scholar]