Abstract
Despite rapid advances in both the early detection and treatment of cancer, the mortality from this disease remains high, which justifies the development of new products that are more selective and effective and have fewer side effects. Accordingly, a novel ester was synthesized that contains two pharmacophores with important biological activities: (I) 4-aminoantipyrine, which has anti-inflammatory and antioxidant effects, and (II) the pharmacophore 1,4-dioxo-butenyl, which has cytotoxic activity. When administered alone, this compound is non-genotoxic, and it does not cause an increasing in splenic phagocytosis. Nevertheless, it can induce cell death. When administered in combination with commercial chemotherapeutic agents, such as doxorubicin, cisplatin, and cyclophosphamide, the ester shows antigenotoxic activity and decreases phagocytosis and reduces the potential to cause cell death. These results indicate that the compound should not be used in combination with chemotherapeutic agents that exert their effect through DNA damage, an important feature of antitumor drugs.
Keywords: Cancer; 4-aminoantipyrine; 1,4-dioxo-butenyl; chemoprevention; chemotherapy
Introduction
Despite advances in the early diagnosis and treatment of cancer, this disease is the leading cause of death in developed countries and the second main cause of death in developing countries (Umar et al., 2012; Reimers et al., 2014). This lethality is due to the high complexity of the disease, the high degree of heterogeneity of tumor cells (Chen and Wang, 2016) and the capacity for uncontrolled proliferation (Helleday et al., 2008).
Chemotherapeutic agents are the main treatment strategy against cancer and generally act by causing DNA damage that induces cell-cycle arrest and programmed cell death (Helleday et al., 2008; Reimers et al., 2014). However, they are poorly selective and, therefore, induce DNA damage in healthy cells, in addition to causing several other side effects (Navarro et al., 2014; Carvalho et al., 2015; Oliveira et al., 2015). The treatment efficiency also varies depending on the type of cancer (Chabner and Roberts, 2005; Vichaya et al., 2015). As new drugs must be developed that are more effective and selective and exhibit fewer side effects, there is a growing interest in the synthesis of drugs that are designed specifically for this purpose.
Antipyrines are possible starting points for the synthesis of antitumor molecules (Khanduja et al., 1984; Nishio et al., 2005; Berno et al., 2016; Oliveira et al., 2018). In the present study, 4-aminoantipyrine was used (Jain et al., 2006) due to its antioxidant (Teng and Liu, 2013; Ghorab et al., 2014), analgesic (Costa et al., 2006), anti-inflammatory (Burdulene et al., 1999), antipyretic, and antiviral (Evstropov et al., 1992) effects.
Several previous studies of biological activity indicated that the addition of 4-aminoantipyrine to a chemical structure can enhance its biological response (Teng and Liu, 2013; Ghorab et al., 2014; Aly et al., 2011; Oliveira et al., 2018). Thus, the ester (Z)-methyl 4-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)-4-oxobut-2-enoate (IR-04) was synthesized from 4-aminoantipyrine via the carbonyl addition of a maleimide (1,4-dioxo-2-butenyl). The 1,4-dioxo-butenyl portion was added due to its strong cytotoxic effect, a key feature when synthesizing DNA damage inducers and searching for possible candidates for new antitumor drugs (Jha et al., 2010).
In general, the structure of IR-04 comprises a region (4-aminoantipyrine) that has several biologically active sites and another region (1,4-dioxo-2-butenyl moiety) with anticancer activity. Furthermore, this ester could potentially modulate plasma membrane polarity and, thus, facilitate access to the cell interior (Jha et al., 2010). These properties have guided the design and synthesis of this ester, which was evaluated for its genotoxic, phagocytic and cell death potentials, as well as its effects when combined with doxorubicin, cisplatin and cyclophosphamide.
Material and Methods
Chemistry
All reagents and solvents for synthesis and NMR measurements were purchased commercially and used without further purification. The microwave reaction was carried out in a Discover-CEM Microwave Reactor. 1H and 13C NMR spectra were recorded at room temperature on a Varian-DPX-300 (10% in DMSO-d 6 and CDCl3 solutions at 298Kfor the acid and the ester, respectively) operating at 300.132 MHz for 1H measurements and 75.476 MHz for 13C measurements. Data processing was carried out on a Solaris workstation. The 1H and 13C chemical shifts are given on the δ scale (ppm) and were referenced to internal tetramethylsilane (TMS); coupling constants J are reported in hertz (Hz). The abbreviations s, d and m were used for simplet, douplet, and multiplet, respectively.
Synthesis of (Z)-4-((1,5-dimethyl-3-oxo-2-phenyl-2,3 dihydro-1H-pyrazol-4-yl) amino)-4-oxobut-2-enoic acid (IR-01)
IR-01 was synthesized according to Oliveira et al. (2018). Briefly, a sealed tube coupled to a microwave reactor containing 4-aminoantipyrine (10.20 mmol) and maleic anhydride (10.20 mmol) was subjected to 150 W microwave irradiation at 90 °C for 10 s. Then, the tube was cooled and the product was recrystallized with ethyl acetate. Yield: 93%.
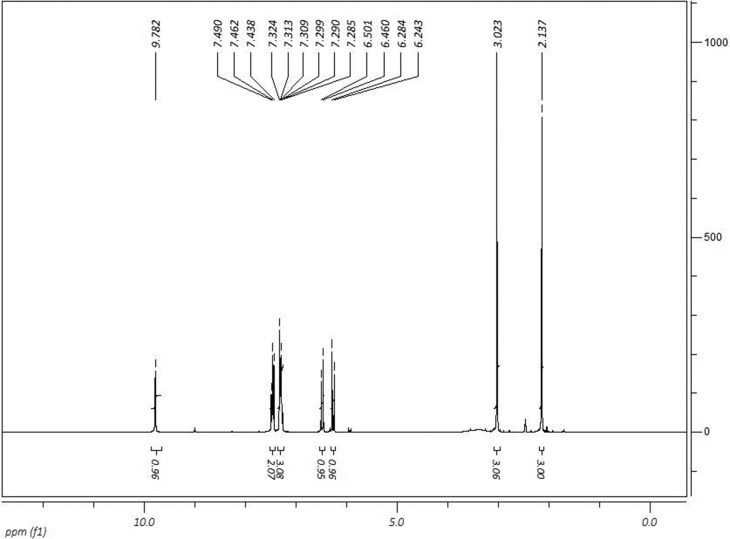
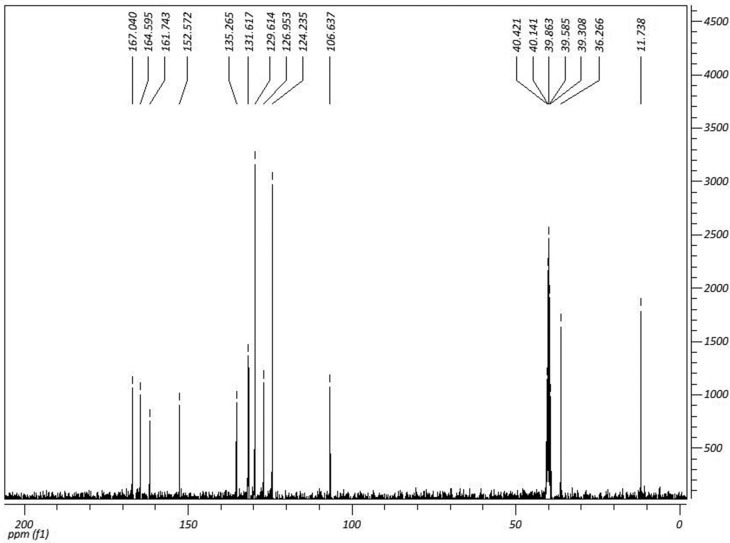
1H NMR (DMSO-d 6, 300 MHz) δ (ppm): 2.13 (s, 3H); 3.02 (s, 3H); 6.26 (d, 1H, Jcis= 12.3Hz); 6.48 (d, 1H, Jcis= 12.3Hz); 7.30 (m, 3H); 7.46 (m, 2H); 9.78 (s, 1H). 13C NMR (DMSO-d 6, 75 MHz) δ (ppm): 11.73 (CH3); 36.26 (CH3); 106.63 (C); 124.23 (CH); 126.95 (CH); 129.95 (CH); (CH); 131.61 (CH); 135.26 (C); 152.57 (C); 161.74 (C=O); 164.59 (C=O); 167.04 (C=O). 1H and 13C NMR spectra are shown in Figures 1 and 2.
Figure 1. 1H NMR spectra of (Z)-4-((1,5-dimethyl-3-oxo-2-phenyl- 2,3dihydro-1H-pyrazol-4-yl) amino)-4-oxobut-2-enoic acid (IR-01) in DMSO-D 6 at 300 MHz.
Figure 2. 13C NMR spectra of (Z)-4-((1,5-dimethyl-3-oxo-2-phenyl- 2,3dihydro-1H-pyrazol-4-yl) amino)-4-oxobut-2-enoic acid (IR-01) in DMSO-D 6 at 75 MHz.
Synthesis of the ester (Z)-methyl 4-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)-4-oxobut-2- enoate (IR-04)
In a 50 mL round-bottom flask coupled to a Dean-Stark trap, 2.70 g (8.9 mmol) of IR-01 acid, 25 mL of methanol and 2.5 mL of sulfuric acid were added. The reaction was refluxed for 3 hours. Then, the reaction mixture was washed with saturated sodium bicarbonate (2x30 mL), water (1x30 mL) and ethyl acetate (2x20 mL). The organic phase was dried over MgSO4 and concentrated by reduced pressure. The product was purified by chromatographic column using hexane/ethyl acetate (1:9) as eluent. Yield: 77%.
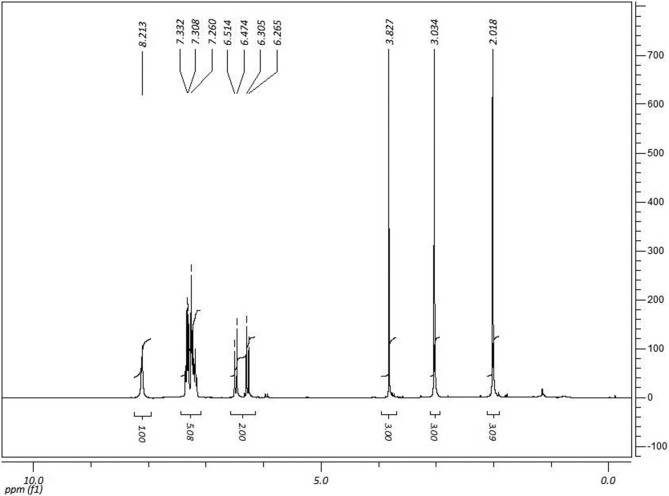
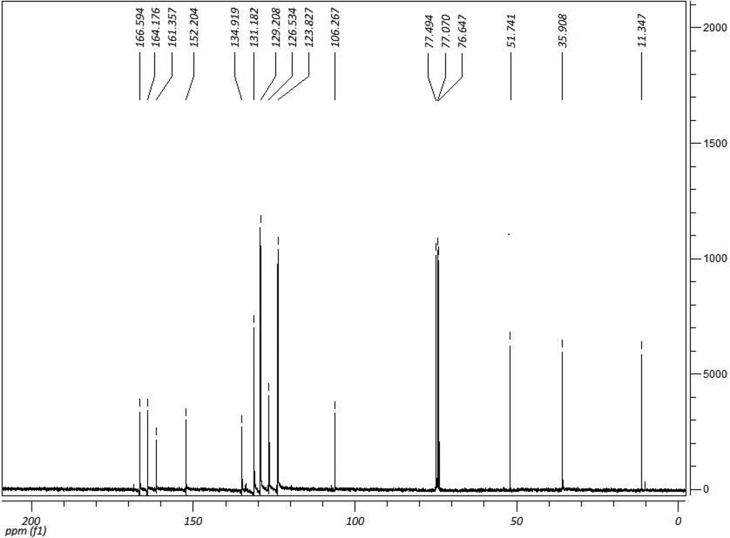
1H NMR (CDCl3, 300 MHz) δ (ppm): 2.01 (s, 3H); 3.03 (s, 3H); 3.82 (s, 3H); 6.28 (d, 1H, Jcis= 12.3Hz); 6.49 (d, 1H, Jcis= 12.3Hz); 7.30 (m, 5H); 8.21 (s, 1H). 13C NMR (CDCl3, 75 MHz) δ (ppm): 11.34 (CH3); 35.90 (CH3); 51.74 (CH3); 106.26 (C); 123.82 (CH); 126.53 (CH); 129.20 (CH); 131.18 (CH); 134.91 (C); 152.20 (C); 161.35 (C=O); 164.17 (C=O); 166.59 (C=O). 1H and 13C NMR spectra are shown in Figures 3 and 4.
Figure 3. 1H NMR spectra of (Z)-methyl 4-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)-4-oxobut-2-anoate (IR-04) in DMSO-D 6 at 300 MHz.
Figure 4. 13C NMR spectra of (Z)-methyl 4-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)-4-oxobut-2-anoate (IR-04) in DMSO-D 6 at 75 MHz.
Chemical agents, animals and experimental design
The chemotherapeutic agents were obtained commercially and administered in a single dose that was previously determined in pilot experiments (data not shown). Doxorubicin (Glenmark Pharmaceuticals Ltd., Argentina. MS Reg. No. 1.1013.0232.002-4, Lot #21130040) was diluted in distilled water and administered at a dose of 16 mg/kg body weight (b.w.) intraperitoneally (i.p.). Cisplatin (Accord Pharmaceuticals Ltd., UK. MS Reg. No. 1.5537.0002.003-7, Lot #88549) was administered at a dose of 6 mg/kg (b.w., i.p.). Cyclophosphamide (Genuxal®, Baxter Ltd., Germany. MS Reg. No. 1.00683.0168.003-1, Lot #F728) was diluted in distilled water and administered at a dose of 100 mg/kg (b.w., i.p.).
A total of 80 male adult (8-10 weeks old) mice (Swiss) with an average weight of 35 g from the State Department of Animal and Plant Health (Agência Estadual de Defesa Sanitária Animal e Vegetal - IAGRO) were kept in polypropylene boxes covered with sawdust in ventilated racks (ALESCO®) under controlled climate and light conditions (12 hours of light and 12 hours of dark, temperature of approximately 22 ± 2°C, relative humidity of 5510%), and they had ad libitum access to commercial feed (Nuvital, Nuvilab®) and filtered water.
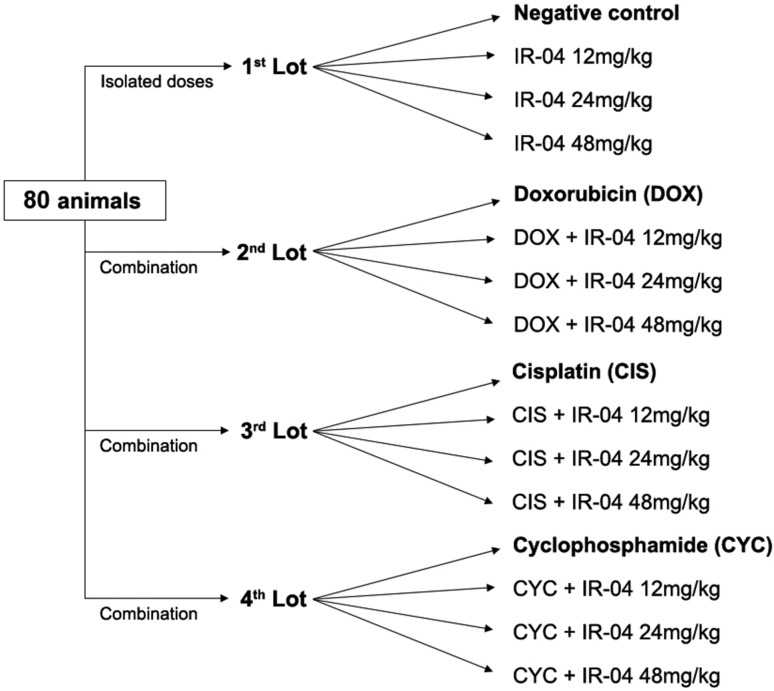
The animals were divided into four lots of 20 animals each. The first lot was used for genotoxicity assessment and was divided into 4 groups (n=5) as follows: negative control, IR-04 12, 24 and 48 mg/kg. The other three lots were used for antigenotoxicity assays with doxorubicin (n=5), cisplatin (n=5) and cyclophosphamide (n=5) as positive controls and were designed to have 3 combination groups (n=5) with IR-04 doses of 12, 24 and 48 mg/kg associated with their respective positive control (Figure 5). After the experiments, the animals were euthanized by cervical dislocation for the collection of biological materials.
Figure 5. Experimental design (doses and groups).
The study was conducted according to the guidelines of the Universal Declaration of Animal Rights. The study protocol was approved by the Ethics Committee on Animal Use of the Federal University of Mato Grosso do Sul-UFMS (protocol number 399/2012).
Biological assays
Peripheral blood Comet assay
A peripheral blood sample collected 24 hours after treatment was used to perform the Comet assay according to the protocol established by Singh et al. (1988) with modifications by Carvalho et al. (2015). In total, 100 cells/animal were visually analyzed under a fluorescence microscope (BIOVAL®, L2000A Model) with a 40x objective, 420-490 nm excitation filter and 520 nm barrier filter.
Peripheral blood Micronucleus assay
Peripheral blood samples were collected 24, 48 and 72 hours after treatment for the Micronucleus assay, according to the protocol previously described by Hayashi et al. (1990), with modifications by Carvalho et al. (2015). Approximately 20 μL of peripheral blood was collected and placed on a microscope slide previously covered with 20 μL of acridine orange (1 mg/mL). The slides were kept in a freezer (-20 °C), and subsequently, 2,000 cells/animal were analyzed under a fluorescence microscope (BIOVAL®, L Model 2000A) with a 40x objective, 420-490 nm excitation filter and 520 nm barrier filter to count the number of micronuclei.
Cell death assay
Liver and kidney tissues were collected and macerated in physiological solution, and 100 μL of this suspension was used for the smear preparation. After the slides were dried, they were fixed in Carnoy’s solution for 5 min, subsequently immersed in different concentrations of ethanol (95%, 75%, 50% and 25%), washed in McIlvaine buffer for 5 min, stained with acridine orange (1 mg/mL for 5 min) and washed again with McIlvaine buffer. The analysis was performed under a fluorescence microscope (BIOVAL® Model L 200A) with a 40x objective, 420-490 nm excitation filter and 520 nm barrier filter. The cells were classified based on DNA fragmentation patterns, and 200 cells/animal were counted (Navarro et al., 2014; Carvalho et al., 2015; Oliveira et al., 2015).
Splenic phagocytosis assay
Approximately 1/3 of the spleen was macerated in saline solution. Then, 100 μL of the cell suspension was placed on a slide previously stained with 20 μL of acridine orange (1 mg/mL) and covered with a coverslip (Hayashi et al., 1990). The analysis was performed under a fluorescence microscope with a 40x objective, 420-490 nm excitation filter and 520 nm barrier filter. For this assay, 200 cells/animal were analyzed for the presence or absence of phagocytosis, as described by Carvalho et al. (2015).
Percentage points were used to calculate the extent of phagocytosis reduction. For this purpose, the frequency of cells that exhibited evidence of phagocytosis in the positive control groups (doxorubicin, cisplatin or cyclophosphamide) was considered 100%, and the percentage for each associated group was calculated by the rule of three. This calculated value was then subtracted from 100, and the result is presented as the reduction in phagocytic activity in percentage points.
Calculation of percent damage reduction (%DR)
The damage reduction percentage is used to assess the chemopreventive capacity of a substance when combined with a known DNA damage inducer. For this purpose, the formula proposed by Manoharan and Banerjee (1985) and Waters et al. (1990) was used:
Statistical analysis
The values are expressed as the mean ± standard error of the mean (SE) or percentage. Parametric data were analyzed by Student’s t-test, according to the nature and distribution of the data (GraphPad Prism, Version 3.2, Graph-Pad Software Inc., San Diego, CA, USA). The significance level adopted was p < 0.05.
Results
Synthesis
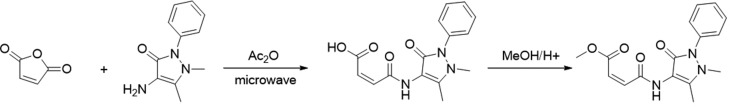
The synthesis of ester IR-04 was performed in only two steps, with 74.6% overall yield (Figure 6). The use of a microwave reactor helped to decrease the use of solvents and optimized the reaction time.
Figure 6. Synthesis of ester (Z)-methyl 4-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)amino)-4-oxobut-2-anoate.
The formed products were characterized by 1H and 13C NMR analysis with deuterated dimethyl sulfoxide for IR-01 sample and deuterated chloroform in the IR-04 sample with TMS as internal reference. Chemical shifts and integrations consistent with the proposed molecules were observed.
In the 1H-NMR spectra (Figure 3), the formation of IR-04 was indicated by the simplet that was proportional to three hydrogens of the methoxy group at 3.60 ppm. Furthermore, a methoxyl group signal was also observed in the 13C NMR spectra (Figure 4) at 51.8 ppm. Both signals are consistent with the replacement of a hydroxyl group by a methoxy group.
All other signals also showed chemical shifts and integrations related to the IR-04 ester molecule.
Biological assays
Comet assay
The results showed that none of the three tested doses of IR-04 had genotoxic activity, and all were able to reduce the extent of basal damage. When combined with the chemotherapeutic agents, IR-04 showed antigenotoxic activity, and the damage reduction percentages ranged from 110.98% to 131.81% for doxorubicin, from 136.40% to 138.94% for cisplatin, and 96.29% to 111.11% for cyclophosphamide (Table 1).
Table 1. Means ± SE of damaged cells, distribution between damage classes, and scores related to genotoxicity and anti-genotoxicity tests of IR-04 by means of the comet assay.
| Experimental Groups | Damaged cells | Classes | Score | %DR | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| LOT 1 | |||||||
| NC | 23.75 ± 2.39 | 76.25 ± 2.39 | 23.75 ± 2.39 | 0.00 ± 0.00 | 0.00 ± 0.00 | 23.75 ± 2.39ª* | |
| IR-04 12mg/kg | 10.80 ± 0.37a* | 87.20 ± 2.31ª* | 1.80 ± 0.37ª* | 0.00 ± 0.00a | 0.00 ± 0.00 | 10.80 ± 0.37ª* | |
| IR-04 24mg/kg | 9.20 ± 1.64a* | 88.80 ± 2.31ª* | 8.80 ± 0.58ª* | 0.00 ± 0.00a | 0.00 ± 0.00 | 9.20 ± 0.73ª* | |
| IR-04 48mg/kg | 2.50 ± 0.86a* | 97.50 ± 0.86ª* | 2.50 ± 0.67ª* | 0.00 ± 0.00a | 0.00 ± 0.00 | 2.50 ± 0.86ª* | |
| LOT 2 | |||||||
| DOX | 71.80 ± 2.03a | 28.40 ± 2.08a | 67.40 ± 2.08a | 4.40 ± 0.24a* | 0.00 ± 0.00 | 76.20 ± 2.01a | |
| + IR-04 12mg/kg | 3.60 ± 0.40b* | 96.20 ± 0.58b* | 3.60 ± 0.40b* | 0.00 ± 0.00b | 0.00 ± 0.00 | 3.06 ± 0.40b* | 129.16 |
| + IR-04 24mg/kg | 2.20 ± 0.37b* | 97.80 ± 0.37b* | 2.20 ± 0.37b* | 0.00 ± 0.00b | 0.00 ± 0.00 | 2.20 ± 0.37b* | 131.81 |
| + IR-04 48mg/kg | 16.50 ± 1.19b* | 83.50 ± 1.19b* | 16.50 ± 1.19b* | 0.00 ± 0.00b* | 0.00 ± 0.00 | 16.50 ± 1.19b* | 110.98 |
| LOT 3 | |||||||
| CIS | 71.25 ± 1.43a | 28.75 ± 1.43b | 23.75 ± 2.39a | 5.00 ± 0.70b* | 0.00 ± 0.00 | 76.25 ± 1.37a | |
| + IR-04 12mg/kg | 5.25 ± 0.25c* | 94.75 ± 0.25c* | 5.25 ± 0.25c* | 0.00 ± 0.00b* | 0.00 ± 0.00 | 5.25 ± 0.31c* | 138.94 |
| + IR-04 24mg/kg | 5.00 ± 0.31c* | 95.00 ± 0.31c* | 5.00 ± 0.31c* | 0.00 ± 0.00b* | 0.00 ± 0.00 | 5.00 ± 0.31c* | 136.40 |
| + IR-04 48mg/kg | 7.25 ± 1.31c* | 92.75 ± 1.31c* | 7.25 ± 1.31c* | 0.00 ± 0.00b* | 0.00 ± 0.00 | 7.25 ± 1.31c* | 137.36 |
| LOT 4 | |||||||
| CYC | 77.75 ± 1.54a | 17.80 ± 4.60a | 71.50 ± 1.32a | 6.25 ± 0.25b* | 0.00 ± 0.00 | 81.50 ± 3.57a | |
| + IR-04 12mg/kg | 17.75 ± 1.31d* | 82.25 ± 1.31d* | 17.75 ± 1.31d* | 0.00 ± 0.00b* | 0.00 ± 0.00 | 17.75 ± 1.31d* | 111.11 |
| + IR-04 24mg/kg | 16.00 ± 1.31d* | 84.0 ± 2.62d* | 5.00 ± 0.31d* | 1.80 ± 1.11b* | 0.00 ± 0.00 | 17.80 ± 3.63d* | 106.94 |
| + IR-04 48mg/kg | 20.60 ± 1.69d* | 79.4 ± 1.69d* | 19.6 ± 1.63d* | 1.00 ± 1.00b* | 0.00 ± 0.00 | 21.6 ± 2.24d* | 96.29 |
SE: Standard error of the mean; %DR: Percent damage reduction; NC: Negative control; DOX: Doxorubicin group; CIS: Cisplatin group; CYC: Cyclophosphamide group. aStatistically compared to the NC group; bStatistically compared to the DOX group; cStatistically compared to the CIS group; dStatistically compared to the CYC group; *statistically significant difference (p<0.05; ANOVA/t-Student).
Micronucleus assay
The micronucleus assay suggested that IR-04 did not cause chromosomal damage, and that IR-04 combined with the chemotherapeutic agents doxorubicin and cisplatin prevented chromosomal damage at all dose levels with damage reduction percentages ranging from 26.17 to 80% and from 4.65 to 78.31%, respectively. In combination with cyclophosphamide, there was no chemoprevention 24 hours after the high dose of IR-04 was applied, or 48 hours after the lowest dose and the intermediate dose were applied. The DNA damage reduction percentages ranged from 0.38 to 82.60% for the combination of cyclophosphamide with IR-04 (Table 2).
Table 2. Results related to the ability of IR-04 in cause or prevent chromosomal damage through the micronucleus assay.
| Experimental Groups | Mean ± SE | % Damage reduction | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| LOT 1 | ||||||
| NC | 5.60 ± 1.07 | 8.40 ± 0.81 | 8.20 ± 1.59 | - | - | - |
| IR-04 12mg/kg | 8.80 ± 0.86ª | 7.20 ± 1.06a | 7.60 ± 1.60a | - | - | - |
| IR-04 24mg/kg | 6.75 ± 1.54a | 7.75 ± 1.25a | 7.80 ± 1.65a | - | - | - |
| IR-04 48mg/kg | 9.20 ± 1.71a | 9.20 ± 0.86a | 10.00 ± 0.89a | - | - | - |
| LOT 2 | ||||||
| DOX | 35.40 ± 1.43ª* | 37.00 ± 1.51ª* | 50.20 ± 2.95ª* | |||
| + IR-04 12mg/kg | 27.60 ± 1.40b* | 21.00 ± 1.58b* | 20.60 ± 2.69b* | 26.17 | 55.94 | 70.47 |
| + IR-04 24mg/kg | 20.50 ± 0.95b* | 27.20 ± 2.13b* | 22.40 ± 2.11b* | 50 | 34.26 | 66.19 |
| + IR-04 48mg/kg | 27.40 ± 1.74b* | 26.20 ± 1.59b* | 16.60 ± 1.80b* | 26.84 | 37.76 | 80 |
| LOT 3 | ||||||
| CIS | 49.75 ± 2.42ª* | 51.80 ± 2.63ª* | 41.60 ± 1.77ª* | |||
| + IR-04 12mg/kg | 26.00 ± 1.58c* | 23.75 ± 1.10c* | 17.00 ± 1.00c* | 10.52 | 4.65 | 6.02 |
| + IR-04 24mg/kg | 19.25 ± 1.97c* | 28.20 ± 1.24c* | 15.00 ± .2.48c* | 40.13 | 22.98 | 18.07 |
| + IR-04 48mg/kg | 23.25 ± 1.79c* | 15.00 ± 2.48c* | 10.00 ± 0.91c* | 22.58 | 50.31 | 78.31 |
| LOT 4 | ||||||
| CYC | 32.00 ± 1.47a* | 34.40 ± 1.86ª* | 22.00 ± 1.47ª* | |||
| + IR-04 12mg/kg | 20.60 ± 1.53d* | 34.50 ± 2.50d | 14.25 ± 1.37d* | 43.18 | 0.38 | 56.15 |
| + IR-04 24mg/kg | 19.75 ± 1.37d* | 32.20 ± 1.31d | 15.25 ± 1.75d* | 46.40 | 8.46 | 48.91 |
| + IR-04 48mg/kg | 30.80 ± 2.03d | 27.00 ± 1.70d* | 10.60 ± 1.03d* | 4.54 | 28.46 | 82.60 |
SE: Standard error of the mean; NC: Negative control; DOX: Doxorubicin group; CIS: Cisplatin group; CYC: Cyclophosphamide group. aStatistically compared to the NC group; bStatistically compared to the DOX group; cStatistically compared to the CIS group; dStatistically compared to the CYC group; *statistically significant difference (p<0.05; ANOVA/t-Student).
Cell death assay
IR-04 induces cell death in liver and kidney cells when administered alone. The increases caused by the 12, 24 and 48 mg/kg doses were respectively 1.87x, 2.63x and 3.30x in the liver, and 1.82x, 3.51x and 2.85x in the kidney. When this same compound was combined with doxorubicin, cisplatin and cyclophosphamide, all doses of the compound, except the highest dose in combination with doxorubicin in the liver, caused less cell death (p<0.05) when compared to the chemotherapeutic agent alone (Table 3).
Table 3. Cell death evaluation on mice’ kidneys and liver.
| Experimental Groups | Liver | Kidneys | ||
|---|---|---|---|---|
| Number of dead cells | Mean ± SE | Number of dead cells | Mean ± SE | |
| LOT 1 | ||||
| NC | 79 | 15.80 ± 1.24 | 73 | 14.60 ± 0.81 |
| IR-04 12 mg/kg | 148 | 29.60 ± 2.20a* | 138 | 26.60 ± 1.24a* |
| IR-04 24 mg/kg | 208 | 41.60 ± 0.81a* | 208 | 41.60 ± 0.92a* |
| IR-04 48 mg/kg | 261 | 52.20 ± 1.02a* | 256 | 51.20 ± 0.96a* |
| LOT 2 | ||||
| DOX | 550 | 110.00 ± 0.70a* | 546 | 109.20 ± 0.58a* |
| + IR-04 12 mg/kg | 486 | 97.20 ± 0.37b* | 481 | 96.20 ± 0.58b* |
| + IR-04 24 mg/kg | 516 | 103.20 ± 1.02b* | 511 | 102.20 ± 0.86b* |
| + IR-04 48 mg/kg | 540 | 108.00 ± 0.54b | 535 | 107.00 ± 0.54b* |
| LOT 3 | ||||
| CIS | 552 | 110.40 ± 0.50a* | 547 | 109.40 ± 0.67a* |
| + IR-04 12 mg/kg | 472 | 94.04 ± 0.87c* | 469 | 93.80 ± 1.11c* |
| + IR-04 24 mg/kg | 533 | 106.60 ± 1.20c* | 522 | 104.40 ± 1.20c* |
| + IR-04 48 mg/kg | 532 | 106.40 ± 1.36c* | 526 | 105.20 ± 1.65c* |
| LOT 4 | ||||
| CYC | 547 | 109.40 ± 0.40a* | 540 | 108.00 ± 0.54a* |
| + IR-04 12 mg/kg | 474 | 94.80 ± 0.86d* | 472 | 94.40 ± 1.20d* |
| + IR-04 24 mg/kg | 517 | 103.4 ± 0.81d* | 511 | 102.20 ± 0.70d* |
| + IR-04 48 mg/kg | 526 | 105.20 ± 1.15d* | 522 | 104.40 ± 0.87d* |
SE: Standard error of the mean; NC: Negative control; DOX: Doxorubicin group; CIS: Cisplatin group; CYC: Cyclophosphamide group. aStatistically compared to the NC group; bStatistically compared to the DOX group; cStatistically compared to the CIS group; dStatistically compared to the CYC group; *statistically significant difference (p<0.05; ANOVA/t-Student).
Splenic phagocytosis assay
Only the highest dose of IR-04 increased (p<0.05) the extent of splenic phagocytosis (1.66x). When this compound was combined with the commercial chemotherapeutic agents, there was a significant decrease in the splenic phagocytosis for all doses and all combinations. For the combination with doxorubicin, the reductions were 65, 49.44 and 35.28 percentage points for the doses of 12, 24 and 48 mg/kg, respectively. The same was observed for the combination with cisplatin, with reductions of 44.27, 25.16 and 11.15 percentage points, respectively; for cyclophosphamide, the corresponding reductions were 31.13, 29.56 and 25.47 percentage points (Table 4).
Table 4. Results related to splenic phagocytosis evaluation.
| Experimental Groups | Phagocytosis | |
|---|---|---|
| Absolute values | Mean ± SE | |
| LOT 1 | ||
| NC | 140 | 28.0 ± 2.55 |
| IR-04 12 mg/kg | 136 | 27.2 ± 2.01a |
| IR-04 24 mg/kg | 140 | 28.0 ± 1.64a |
| IR-04 48 mg/kg | 232 | 46.4 ± 1.03a* |
| LOT 2 | ||
| DOX | 288 | 72.0 ± 1.08a* |
| + IR-04 12 mg/kg | 101 | 25.2 ± 0.75b* |
| + IR-04 24 mg/kg | 182 | 36.4 ± 1.16b* |
| + IR-04 48 mg/kg | 233 | 46.6 ± 1.20b* |
| LOT 3 | ||
| CIS | 314 | 62.8 ± 0.96a* |
| + IR-04 12 mg/kg | 175 | 35.0 ± 1.87c* |
| + IR-04 24 mg/kg | 235 | 47.0 ± 2.21c* |
| + IR-04 48 mg/kg | 279 | 55.8 ± 1.65c* |
| LOT 4 | ||
| CYC | 318 | 63.6 ± 1.32a* |
| + IR-04 12 mg/kg | 219 | 43.8 ± 1.65d* |
| + IR-04 24 mg/kg | 224 | 44.8 ± 1.46d* |
| + IR-04 48 mg/kg | 237 | 47.4 ± 0.67d* |
SE: Standard error of the mean; NC: Negative control; DOX: Doxorubicin group; CIS: Cisplatin group; CYC: Cyclophosphamide group. aStatistically compared to the NC group; bStatistically compared to the DOX group; cStatistically compared to the CIS group; dStatistically compared to the CYC group; *statistically significant difference (p<0.05; ANOVA/t-Student).
Discussion
IR-04 is a novel compound whose toxicogenic effects are unknown. Thus, the present study is the first to report that this 4-aminoantipyrine derivative is not genotoxic.
For the isolated administration of this compound in the comet assay, the proportion of comets was lower in the IR-04-treated groups than in the control group. This property should be further explored in future studies because this ability to prevent basal genotoxic damage may be required when searching for chemoprotective agents because compounds that have antioxidant properties generally also have chemoprotective activity (Mantovani et al., 2008; Bacanli et al., 2015). This chemoprotective effect may have occurred because the molecule that was developed for the present study contains 4-aminoantipyrine, which has previously shown to have antioxidant activity. Moreover, 4-aminoantipyrine can inhibit the formation of free radicals, which can cause DNA damage (Jain et al., 2006).
Another noteworthy characteristic of IR-04 is that, when administered alone, it can induce cell death in the liver and kidneys. Commercial anticancer drugs increase DNA damage, and therefore induce apoptosis in cancer cells (Brown and Attardi, 2005). However, normal cells can also acquire genomic/genetic lesions. Thus, compounds like IR-04 that are capable of inducing apoptosis without causing genotoxic or mutagenic damage are essential in the search for new chemotherapeutic drugs (Almeida et al., 2005).
The designed molecule, IR-04, has the radical 1,4-dioxo-2-butenyl, which has been reported to have cytotoxic and anticancer activities (Jha et al., 2010). Thus, some characteristics of this radical are desired in chemotherapeutic and/or cytotoxic compounds. This type of compound can induce DNA damage, making it a promising regulator of the cell cycle and leading to the elimination of damaged cells, including tumor cells.
A further confirmation that this compound does not cause genetic damage and toxicity is the fact that the treatment with the two lowest doses tested or with the low stimulation at the highest dose did not induce splenic phagocytosis in the animals. As reported in the literature, toxic compounds cause DNA damage and/or cell death through different mechanisms, and damaged cells, cell debris, and especially cells infected by viruses and bacteria are removed from the circulation by splenic phagocytosis (Ishii et al., 2011). Therefore, the absence of splenic phagocytosis, as observed in the present study, indicates the lack of toxicity and/or toxicogenic damage.
The combination of IR-04 with commercial chemotherapeutic agents showed that it has antigenotoxic and prevents cell death, in addition to its ability to decrease splenic phagocytosis. The hypothesized chemopreventive characteristic of the compound could explain these data. Due to its chemopreventive nature, the compound could be used to prevent DNA damage, especially chemically induced damage; thus, the proportion of cells with DNA damage that should be removed from circulation would be lower.
When IR-04 is combined with the commercial chemotherapeutic agents doxorubicin, cisplatin and cyclophosphamide, it is capable of preventing DNA damage, cell death, and decrease of splenic phagocytosis. Thus, the combination with the tested commercial chemotherapeutic agents is discouraged. This suggestion is based on all the data indicating a reduction in the extent of genetic damage, which is one of the main modes of action of these drugs (Goldstein and Kastan, 2015), and which would lead to apoptosis, the desired anticancer effect (Ishii et al., 2011).
Although the use of IR-04 is discouraged in combination with doxorubicin, cisplatin, or cyclophosphamide as a coadjuvant in anticancer treatment, when we analyzed just the induction of DNA damage that generates apoptosis, we could observe one of the desired anticancer effects. On the other hand, even though observing a decrease in DNA damage (antigenotoxic effect, reported in this study) we need to consider that cancer cells can be more susceptible than their normal correspondents when exposed to anti-cancer drugs (Oberley and Buettner, 1979; Cebrian et al., 2006). Such difference is due, for example, to tumor cells containing less antioxidant enzymes, as is the case for superoxide dismutate, GSH peroxidase, and GSH reductase (Oliveira et al., 2018).
According to this point of view, the antigenotoxic effect can be assumed as positive/beneficial for normal cells that are being affected by non-selective chemotherapeutic medicines. Moreover, it can be assumed that the reduction of genotoxic damage observed is associated with the capacity of IR-04 to initiate cellular cycle arrest. Future studies will be necessary to test this hypothesis. However, the literature reports that antioxidant, antigenotoxic, and/or chemopreventive compounds are capable to cause cell cycle arrest (Kaur et al., 2006; Mölzer et al., 2013; Srivastava et al., 2016), and thus increase the cellular repair time, facilitating the repair of DNA damage and allowing to follow the regular cycle. In contrast, if the repair does not occur, or comes late, it leads the damaged cell to cellular death by apoptosis after the treatment with IR-04 in combination with doxorubicin, cisplatin, and cyclophosphamide.
Alkylating agents, such as cyclophosphamide, are capable of making covalent bonds with nucleophilic components such as the DNA. Thus, at the end of the process, the substitution of base pairs guanine-cytosine for adenine-thymine may occur. In addition, other mechanisms could generate cross-linking between two strands of DNA, resulting in breaks, such as the opening or removing guanine residues and/or alkylation of a second guanine. Thus, cyclophosphamide acts both on DNA synthesis and causes damage to the molecule (Hall and Tilby, 1992). Doxorubicin, an anthracycline antibiotic, is also capable of interfering with DNA transcription and replication because of its intercalating proprieties. Also, doxorubicin forms a tripartite complex with topoisomerase II and DNA, resulting in DNA breaks and apoptosis (Thorn et al., 2011). Cisplatin, on the other hand, reacts with the DNA causing intra- and interfilament cross-links. These processes result in DNA strand break, coding error, and further apoptosis. In the present study, it was verified that IR-04 was able to reduce DNA damage caused by these three commercial chemotherapies that have different mechanisms of action (Dasari and Tchounwou, 2014). However, our results are not sufficient to predict how the IR-04 ester interfered in each mechanism of action and also presented a pattern of chemoprevention in combination with the chemotherapeutics. Our results are distinct from those presented by Oliveira et al. (2018). The authors evaluated the IR-01 acid, derived from the same pharmacophoric groups as IR-04, and did not find a pattern of chemopreventive response, which suggested interference in the mechanisms of action of the chemotherapeutic agents. Given the above, new studies regarding the mechanisms of action and evaluation of the combination of IR-01/IR-04 molecules with cyclophosphamide, cisplatin and doxorubicin are needed to clarify the effects of these compounds, particularly the induction of cell death.
In conclusion, IR-04 is an important compound in the search for anticancer drugs that have greater selectivity, because IR-04 induces cell death without using the DNA damage pathway. However, IR-04 should not be considered an adjuvant for chemotherapy in combination with doxorubicin, cisplatin, and cyclophosphamide due to the possibility of decreasing the genotoxic effects and cell death potential of these drugs.
Acknowledgments
This study received finding from the Mato Grosso do Sul Foundation for the Development of Education, Science and Technology (Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul FUNDECT), the Brazilian Federal Agency for the Support and Evaluation of Graduate Education (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES, Finance Code 001), and the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Cientifico e Tecnológico - CNPq).
Footnotes
Associate Editor: Daisy Maria Fávero Salvadori
Conflict of interest
The authors declare that they have no competing interests.
Author contributions
RJO, AB, DP de L and RSG conceived and designed the study; FPANP, CRB, JRP and ACDM conducted the biological experiments; IOMF da S and RV de L conducted the chemistry synthesis; RJO, FPANP, RSG and ACMBA-S analyzed the data; RJO and FPANP and wrote the manuscript. All authors read and approved the final version.
References
- Almeida VL, Leitão A, Reina LCB, Montanari CA, Donnici CL, Lopes MTP. Cancer and cell cycle-specific and cell cycle nonspecific anticancer DNA-interactive agents: An introduction. Quím Nova. 2005;28:118–129. [Google Scholar]
- Aly HM, Saleh NM, Elhady HA. Design and synthesis of some new thiophene, thienopyrimidine and thienothiadiazine derivatives of antipyrine as potential antimicrobial agents. Eur J Med Chem. 2011;46:4566–4572. doi: 10.1016/j.ejmech.2011.07.035. [DOI] [PubMed] [Google Scholar]
- Bacanli M, Basaran AA, Basaran N. The antioxidant and antigenotoxic properties of citrus phenolics limonene and naringin. Food Chem Toxicol. 2015;81:160–170. doi: 10.1016/j.fct.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Berno CR, Rós BT, Silveira IO, Coelho HR, Antoniolli AC, Beatriz A, Lima DP, Monreal AC, Sousa FG, Silva Gomes R, Oliveira RJ. 4-Aminoantipyrine reduces toxic and genotoxic effects of doxorubicin, cisplatin, and cyclophosphamide in male mice. Mutat Res Genet Toxicol Environ Mutagen. 2016;805:19–24. doi: 10.1016/j.mrgentox.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- Burdulene D, Palaima A, Stumbryavichyute Z, Talaikite Z. Synthesis and antiinflammatory activity of 4-aminoantipyrine derivatives of succinamides. Pharm Chem J. 1999;33:191–193. [Google Scholar]
- Carvalho PC, Santos EA, Schneider BU, Matuo R, Pesarini JR, Cunha-Laura AL, Monreal AC, Lima DP, Antoniolli AC, Oliveira RJ. Diaryl sulfide analogs of combretastatin A-4: Toxicogenetic, immunomodulatory and apoptotic evaluations and prospects for use as a new chemotherapeutic drug. Environ Toxicol Pharmacol. 2015;40:715–721. doi: 10.1016/j.etap.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Cebrian A, Pharoah PD, Ahmed S, Smith PL, Luccarini C, Luben R, Redman K, Munday H, Easton DF, Dunning AM, Ponder BA. Tagging single-nucleotide polymorphisms in antioxidant defense enzymes and susceptibility to breast cancer. Cancer Res. 2006;66:1225–1233. doi: 10.1158/0008-5472.CAN-05-1857. [DOI] [PubMed] [Google Scholar]
- Chabner BA, Roberts TG., Jr Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- Chen C, Wang J. A physical mechanism of cancer heterogeneity. Sci Rep. 2016;6:20679. doi: 10.1038/srep20679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D, Marques AP, Reis RL, Lima JL, Fernandes E. Inhibition of human neutrophil oxidative burst by pyrazolone derivatives. Free Radic Biol Med. 2006;40:632–640. doi: 10.1016/j.freeradbiomed.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evstropov AN, Yavorovskaya VE, Vorob’ev ES, Khudonogova ZP, Gritsenko LN, Shmidt EV, Medvedeva SG, Filimonov VD, Prishchep TP, Saratikov AS. Synthesis and antiviral activity of antipyrine derivatives. Pharm Chem J. 1992;26:426–430. [Google Scholar]
- Ghorab MM, El-Gazzar MG, Alsaid MS. Synthesis, characterization and anti-breast cancer activity of new 4-aminoantipyrine-based heterocycles. Int J Mol Sci. 2014;15:7539–7553. doi: 10.3390/ijms15057539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M, Kastan MB. The DNA damage response: Implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–143. doi: 10.1146/annurev-med-081313-121208. [DOI] [PubMed] [Google Scholar]
- Hall AG, Tilby MJ. Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies. Blood Rev. 1992;6:163–173. doi: 10.1016/0268-960x(92)90028-o. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Morita T, Kodama Y, Sofuny T, Ishidate M., Jr The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat Res. 1990;245:245–249. doi: 10.1016/0165-7992(90)90153-b. [DOI] [PubMed] [Google Scholar]
- Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. doi: 10.1038/nrc2342. [DOI] [PubMed] [Google Scholar]
- Ishii PL, Prado CK, Mauro MO, Carreira CM, Mantovani MS, Ribeiro LR, Dichi JB, Oliveira RJ. Evaluation of Agaricus blazei in vivo for antigenotoxic, anticarcinogenic, phagocytic and immunomodulatory activities. Regul Toxicol Pharmacol. 2011;59:412–422. doi: 10.1016/j.yrtph.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Jain SC, Juhi S, Bhagat S, Errigton W, Olsen CE. A facile synthesis of novel Spiro- [Indole-pyrazolinyl-thiazolidine]-2,4’-dione. Synth Commun. 2006;33:563–577. [Google Scholar]
- Jha A, Mukherjee C, Prasad AK, Parmar VS, Vadaparti M, Das U, De Clercq E, Balzarini J, Stables JP, Shrivastav A, Sharma RK, Dimmock JR. Derivatives of aryl amines containing the cytotoxic 1,4-dioxo-2-butenyl pharmacophore. Bioorg Med Chem Lett. 2010;20:1510–1515. doi: 10.1016/j.bmcl.2010.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- Khanduja KL, Dogra SC, Kaushal S, Sharma RR. The effect of anti-cancer drugs on pharmacokinetics of antipyrine in vitamin A deficiency. Biochem Pharmacol. 1984;33:449–452. doi: 10.1016/0006-2952(84)90239-9. [DOI] [PubMed] [Google Scholar]
- Manoharan K, Banerjee MR. Beta-carotene reduces sister chromatid exchanges induced by chemical carcinogens in mouse mammary cells in organ culture. Cell Biol Int Rep. 1985;9:783–789. doi: 10.1016/0309-1651(85)90096-7. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mölzer C, Pfleger B, Putz E, Roßmann A, Schwarz U, Wallner M, Bulmer AC, Wagner KH. In vitro DNA-damaging effects of intestinal and related tetrapyrroles in human cancer cells. Exp Cell Res. 2013;319:536–545. doi: 10.1016/j.yexcr.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro SD, Beatriz A, Meza A, Pesarini JR, Gomes RS, Karaziack CB, Cunha-Laura AL, Monreal AC, Romão W, Lacerda V, Júnior, et al. A new synthetic resorcinolic lipid 3-heptyl-3,4,6-trimethoxy-3H-isobenzofuran-1-one: evaluation of toxicology and ability to potentiate the mutagenic and apoptotic effects of cyclophosphamide. Eur J Med Chem. 2014;75:132–142. doi: 10.1016/j.ejmech.2014.01.057. [DOI] [PubMed] [Google Scholar]
- Nishio M, Matsuda M, Ohyanagi F, Sato Y, Okumura S, Tabata D, Morikawa A, Nakagawa K, Horai T. Antipyrine test predicts pharmacodynamics in docetaxel and cisplatin combination chemotherapy. Lung Cancer. 2005;49:245–251. doi: 10.1016/j.lungcan.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: A review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- Oliveira RJ, Navarro SD, Lima DP, Mauro MO, Silva AF, Souza TR, Ribeiro LR. A novel cytosporone 3-Heptyl-4,6-dihydroxy-3H-isobenzofuran-1-one: Synthesis; toxicological, apoptotic and immunomodulatory properties; and potentiation of mutagenic damage. BMC Cancer. 2015;15:1–15. doi: 10.1186/s12885-015-1532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira RJ, Santos NCL, Pesarini JR, Oliveira BC, Berno CR, Araújo FHS, Silveira IOMF, Nascimento RO, Brochado Antoniolli-Silva ACM, Duenhas Monreal AC, et al. Assessment of genetic integrity, splenic phagocytosis and cell death potential of (Z)-4-((1,5-dimethyl-3-oxo-2-phenyl-2,3dihydro-1H-pyrazol-4-yl) amino)-4-oxobut-2-enoic acid and its effect when combined with commercial chemotherapeutics. Genet Mol Biol. 2018;41:154–166. doi: 10.1590/1678-4685-GMB-2017-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers MS, Engels CC, Kuppen PJ, van de Velde CJ, Liefers GJ. How does genome sequencing impact surgery? Nat Rev Clin Oncol. 2014;11:610–618. doi: 10.1038/nrclinonc.2014.101. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Somasagara RR, Hegde M, Nishana M, Tadi SK, Srivastava M, Choudhary B, Raghavan SC. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci Rep. 2016;6:24049. doi: 10.1038/srep24049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Y, Liu R. Insights into potentially toxic effects of 4-aminoantipyrine on the antioxidant enzyme copper-zinc superoxide dismutase. J Hazard Mater. 2013;262:318–324. doi: 10.1016/j.jhazmat.2013.08.047. [DOI] [PubMed] [Google Scholar]
- Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet Genomics. 2011;21:440–446. doi: 10.1097/FPC.0b013e32833ffb56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar A, Dunn BK, Greenwald P. Future directions in cancer prevention. Nat Rev Cancer. 2012;12:835–848. doi: 10.1038/nrc3397. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, Heijnen CJ, Walker AK. Mechanisms of chemotherapy-induced behavioral toxicities. Front Neurosci. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MD, Brady AL, Stack HF, Brockman HE. Antimutagenicity profiles for some model compounds. Mutat Res. 1990;238:57–85. doi: 10.1016/0165-1110(90)90039-e. [DOI] [PubMed] [Google Scholar]