Abstract
We present a collection of minimalist binary vectors for transformation through ATMT applicable to several fungi species. pLUO plasmid binary vectors consist of a reporter module containing fluorescent proteins, mCherry or eGFP, flanked by a multiple cloning site and a transcription terminator site. They also present a synthetic gene allowing resistance to Hygromicin B flanked by alternate promoters, one for yeast and another for filamentous fungi. Left and right borders were added for Agrobacterium tumefaciens recognition, and a minimal broad-host range RK2 replication origin. Transformation was validated in the pathogenic fungus Paracoccidioides lutzii. Hence, we developed an efficient and reliable molecular tool for fungal transformation: minimalist, synthetic, modular, and available in four different versions, and these can still be readily modified using a few primers and few cloning steps.
Keywords: synthetic biology, fungi, Agrobacterium tumefaciens, transformation, vectors.
Introduction
Fungi are organisms comprising a universe that has not been fully explored by mankind(Leigh et al., 2003), but have been extensively studied because of their huge impact in everyday life and their endless applications in industry, such as production of biofuels (Glass et al., 2013), foods and feedstock (Bhat, 2000), human therapeutics (Ward, 2012), among many others. Likewise, even greater efforts are being engaged in studying their pathogenicity (Almeida et al., 2007; Teixeira et al., 2013). Tools that can provide a better understanding of the molecular mechanisms that control gene expression in those organisms are useful, not only for shedding light on their functioning, but also because it can be used for genetic engineering and delivery of products. Synthetic Biology is an ever-growing field responsible for building new genetic circuits with known biological parts, and a great amount of the challenge in this area is in finding minimal synthetic vectors that provide a desirable setting for this cycle of re-designing parts (Silva-Rocha et al., 2013; Pasin et al., 2017). Fungi and synthetic biology are a promising combination that is opening brand-new doors for science, however, there is still plentiful of work to be done (Amores et al., 2016). In the pursuit of overcoming the lack of tools for fungal studies, we developed the pLUO vectors, a collection of minimal and versatile binary plasmid vectors for A. tumefaciens-mediated transformation (ATMT).
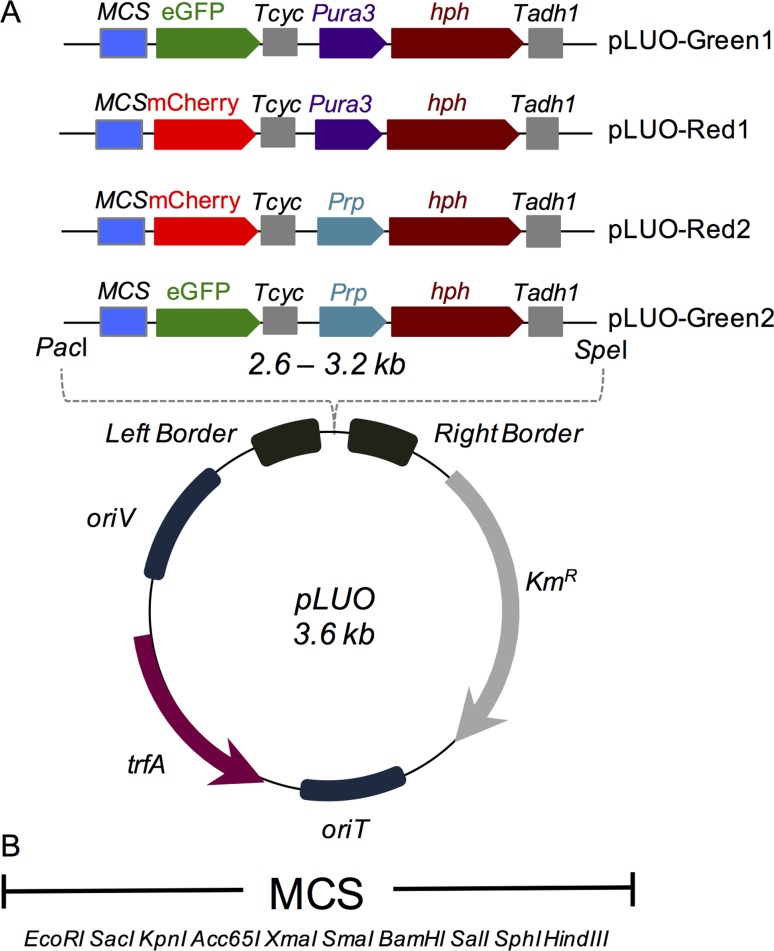
The pLUO vectors were constructed using minimal essential parts so that they could be reduced in size while still keeping their functionality. This was achieved by employing the pGLR2 plasmid as vector backbone (Benedetti et al., 2012) that is also minimum and presents a broad host range RK2 origin of replication, so it replicates in E. coli and in A. tumefaciens. pLUO vectors present a multiple cloning site (MCS) with 11 different restriction sites for several cloning options, so any given promoter can be placed to modulate a red (mCherry) or a green (eGFP) reporter protein. The selection marker is a synthetic, codon-optimized, and free from restriction sites gene, allowing resistance to Hygromicin B (hph) flanked by two different optimized promoters – so one can choose to transform it into yeast using Pura3 or into filamentous fungi using Prp2 – and a terminator (Tadh1). Two regions of 25 direct imperfect repeats were added at both ends of this cassette, the left and right borders, so that A. tumefaciens can recognize, nick and transfer the DNA from the binary vector to the host (Figure 1). The method of ATMT for fungi has been widely used for a long time due to its high yield of positive transformants (Michielse et al., 2008).
Figure 1. Representative scheme of the minimal binary vector for fungi transformation through ATMT. (A) Design of the plasmid showing all the minimal modules that compose it, including the four versions of the expression cassette that were constructed. pLUO-Green1 (with eGFP) and pLUO-Red1 (with mCherry) have a Pura3 promoter for hph expression, while pLUO-Green2 and pLUO-Red2 uses Prp2 from T. reesei. (B) Representation of the restriction enzymes available in the MCS for several cloning options.
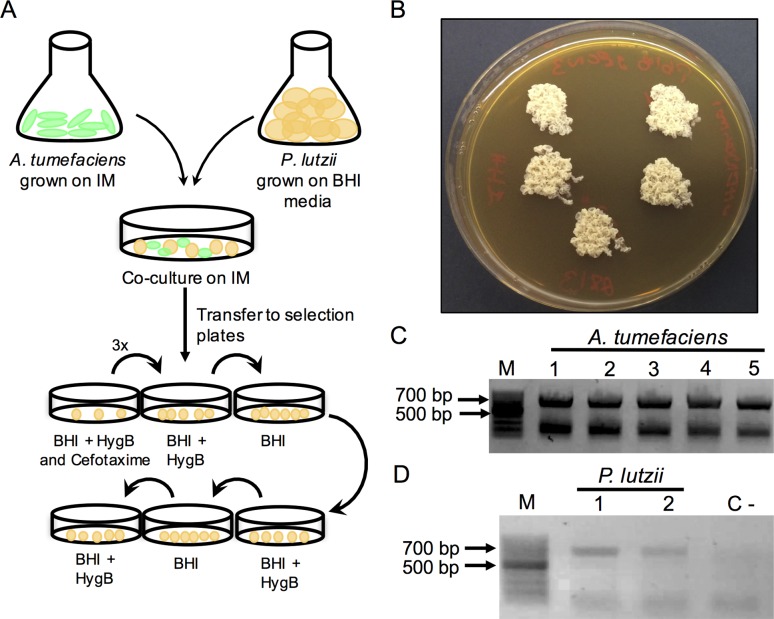
The validation of pLUO vectors was performed in the fungus P. lutzii, a dimorphic human opportunistic pathogen. Most of the vectors used for its molecular studies contained more than 15,000 base pairs (Almeida et al., 2007), making pLUO a desirable substitute, since it comprises only 6 kb. Six rounds of selection were performed as established to reach mitotic stability. Three more round were done to verify stability (Figure 2A) with the expression cassette (Figure 2B), for a total of nine rounds. The electrophoresis gel shows that the transformants were positive for the hph gene, which proves that transformation was successful (Figure 2C,D). Henceforward, this vector would be applicable as an efficient method to study gene expression in this pathogenic organism. The expression cassette tested contains an eGFP reporter and Pura3 modulating hph – the following versions were all adapted by overlapping PCR reactions. Thus, expansion of the modules can be performed by using the primers provided (Table 1), or variations, when the aim is to build additional versions that suit other target organisms. P. lutzii was used as a model to prove the functionality of this vector, but studies in other strains will be further developed using this collection of vectors.
Figure 2. Validation of pLUO vector in P. lutzii. (A) Representative image of experiment workflow. The first selection plate contains Cefotaxime 100 μg/mL to kill the remaining A. tumefaciens colonies. Afterwards, fungal colonies are selected on BHI containing only Hygromicin B for three rounds, and then alternating media with or without Hygromicyn until reaching nine rounds of mitotic stability. (B) P. lutzii colonies transformed with pLUO vector after reaching mitotic stability. (C) Eletrophoresis gel showing the colony PCR reactions from A. tumefaciens as a positive control with the hph gene amplified by its primers (resulting in a band of 705 base pairs; the lower band is an unespecific one that always appears for A. tumefaciens). (D) Eletrophoresis gel showing the PCR reactions of P. lutzii transformants. M, molecular ladder; and C, negative control of the reaction.
Table 1. Primers used in this study.
| Name | Sequence (5’- 3’) | Target DNA |
|---|---|---|
| 5’_GFP_HindIII | GCGCAAGCTTCGGTATCGATCATGAGTAAAG | pMCB17-apx |
| 3’_GFP | TTAAGCCGGCGCGCC | pMCB17-apx |
| 5’_Tcyc_GFP | GGCGCGCCGGCTTAACTCCTCCCACATCCGC | S. cerevisae |
| 3’_Tcyc_SpeI_BamHI | GCGCGGATCCACTAGTAAGCCTTCGAGCGTCCC | S. cerevisae |
| 5’_hph | CTGACACTAGCGCCACC | pGL4.14 |
| 3’_hph | GTTTAAACTCGACCTACCTCC | pGL4.14 |
| 5’_Pura3_XbaI | GCGCTCTAGAGTGCACCATACCACAGC | pRS42614 |
| 3’_Pura3_hph | TGGCGCTAGTGTCAGTGAGATTTATCTTCGTTTCCTGC | pRS42614 |
| 5’_Tadh1_hph | AGGTCGAGTTTAAACGGTAGATACGTTGTTGACAC | S. cerevisae |
| 3’_Tadh1_SpeI_EcoRI | GCGCGAATTCACTAGTGTGGTCAATAAGAGCGACC | S. cerevisae |
| 5’_LB_GFP_PacI | GCGCTTAATTAATGGCAGGATATATTGTGGTGTAAACATAACAATTTCACACAGGACCTAGG | pLUO |
| 3’_RB_Tadh1_SpeI | GCGCACTAGTGTTTACCCGCCAATATATCCTGTCAGTGGTCAATAAGAGCGACC | pLUO |
| 5’_Tcyc | CTCCTCCCACATCCGC | pLUO |
| 3’_Tcyc | AAGCCTTCGAGCGTCC | pLUO |
| 5’_HindIII_mCherry | GCGCAAGCTTGGTATGGTGAGCAAGGGC | pMR117 |
| 3’_mCherry_Tcyc | GGTTAGAGCGGATGTGGGAGGAGTTACTTGTACAGCTCGTCC | pMR117 |
| 5’_Tcyc_Prp2 | GGTTTTGGGACGCTCGAAGGCTTCGGCTGCGTGAACAGACG | T. reesei |
| 3’_Prp2_hph | GGTGGCGCTAGTGTCAGGTGGTTTGAGTTGGGTTGAGATAGG | T. reesei |
Sites for restriction enzymes are underlined.
For the construction of the plasmids, the eGFP protein was amplified from pMCB17-apx (Fernandez-Abalos et al., 1998) and its terminator was from the cyc1 gene from S. cerevisae genome. These fragments were fused using overlapping PCR with Phusion® High Fidelity DNA Polymerase (NEB), adding restriction sites for HindIII, SpeI and BamHI. The fragment was cloned into the high-copy number vector pUC19 (Yanisch-Perron et al., 1985) using HindIII and BamHI for the digestion reaction, and then transformed into chemocompetent E. coli DH10B. The hph gene is from pGL4.14 (Promega) and its terminator was amplified from the adh1 gene of S. cerevisae. The variations of promoters modulating the hph gene were the following: the ura3 promoter (Pura3) for yeast was amplified from the pRS426 shuttle vector (Christianson et al., 1992), and Prp2 for filamentous fungi was amplified from the T. reesei genome (He et al., 2013). For construction of the yeast cassette, the three fragments – Pura3, hph, Tadh1 – were fusioned by overlapping PCR, and the restriction sites for XbaI, SpeI and EcoRI were added by primers. Then, this fragment was digested with XbaI and EcoRI and inserted into pUC19 containing the reporter module eGFP_Tcyc, digested with the same enzymes. The entire expression cassette was amplified from pUC19 using primers to include the borders and, then, cloned into the pGLR2 vector using PacI and SpeI. The variations in the cassette were all built by a few reactions of overlapping PCR using the first one as template. All enzymes used in this work were from New England Biolabs and all primers are shown in Table 1.
The strain of A. tumefaciens was LBA1100 with a disarmed octopine-type pTiB6 plasmid (Menino et al., 2012) and was transformed with the vectors by electroporation. P. lutzii transformation through ATMT was done as described in Menino et al. (2012). Colonies of P. lutzii were randomly selected and plated into solid BHI media containing 75 μg/mL Hygromicin B three consecutive times. Subsequently, they were serially transferred to media with or without the selection marker for three times each, totalizing nine rounds of selection, growing for 15-20 days between each round. All plasmids and sequences can be made available upon request to the authors.
Acknowledgments
LCN, LMS, RAG and RSR were supported by FAPESP (Project numbers: 2016/03763-3, 2016/01946-3, 2014/22561-7 and 2012/22921-8). FR and BF were supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER) (NORTE-01-0145-FEDER-000013). The authors are thankful to Maria Cristina Roque Barreira for insightful discussions and support.
Footnotes
Associate Editor: Célia Maria de Almeida Soares
Conflict of interest
The authors declare no competing financial interest.
Author Contributions
RSR and FR conceived and designed the study. LCN and LMS conducted the cloning experiments and built the vectors. RAG and BHF performed the ATMT experiments. LCN wrote the manuscript. All authors read and approved the final version.
References
- Almeida AJ, Carmona JA, Cunha C, Carvalho A, Rappleye CA, Goldman WE, Hooykaas PJ, Leão C, Ludovico P, Rodrigues F. Towards a molecular genetic system for the pathogenic fungus Paracoccidioides brasiliensis. Fungal Genet. Biol. 2007;44:1387–1398. doi: 10.1016/j.fgb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Amores G, Guazzaroni ME, Arruda L, Silva-Rocha R. Recent progress on systems and synthetic biology approaches to engineer fungi as microbial cell factories. Curr Genomics. 2016;17:85–98. doi: 10.2174/1389202917666151116212255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti IM, De Lorenzo V, Silva-Rocha R. Quantitative, non-disruptive monitoring of transcription in single cells with a broad-host range GFP-luxCDABE dual reporter system. PLoS One. 2012;7:e52000. doi: 10.1371/journal.pone.0052000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnol Adv. 2000;18:355–383. doi: 10.1016/s0734-9750(00)00041-0. [DOI] [PubMed] [Google Scholar]
- Christianson TW, Sikorski RS, Dante M, Shero JH, Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992;110:119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Fernandez-Abalos JM, Fox H, Pitt C, Wells B, Doonan JH. Plant-adapted green fluorescent protein is a versatile vital reporter for gene expression, protein localization and mitosis in the filamentous fungus, Aspergillus nidulans . Mol Microbiol. 1998;27:121–130. doi: 10.1046/j.1365-2958.1998.00664.x. [DOI] [PubMed] [Google Scholar]
- Glass NL, Schmoll M, Cate JHD, Coradetti S. Plant cell wall deconstruction by ascomycete fungi. Annu Rev Microbiol. 2013;67:477–498. doi: 10.1146/annurev-micro-092611-150044. [DOI] [PubMed] [Google Scholar]
- He R, Zhang C, Guo W, Wang L, Zhang D, Chen S. Construction of a plasmid for heterologous protein expression with a constitutive promoter in Trichoderma reesei . Plasmid. 2013;70:425–429. doi: 10.1016/j.plasmid.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Leigh J, Seif E, Rodriguez N, Jacob Y, Lang BF. Fungal evolution meets fungal genomics. In: Arora DK, editor. Handbook of Fungal Biotechonology. 2nd edition. CRC Press; Boca Raton: 2003. pp. 145–161. [Google Scholar]
- Menino JF, Almeida AJ, Rodrigues F. Gene knockdown in Paracoccidioides brasiliensis using antisense RNA. Methods Mol Biol. 2012;845:187–198. doi: 10.1007/978-1-61779-539-8_12. [DOI] [PubMed] [Google Scholar]
- Michielse CB, Hooykaas PJJ, van den Hondel CAMJJ, Ram AFJ. Agrobacterium-mediated transformation of the filamentous fungus Aspergillus awamori. Nat Protoc. 2008;3:1671–1678. doi: 10.1038/nprot.2008.154. [DOI] [PubMed] [Google Scholar]
- Pasin F, Bedoya LC, Bernabé-Orts JM, Gallo A, Simón-Mateo C, Orzaez D, García JA. Multiple t-DNA delivery to plants using novel mini binary vectors with compatible replication origins. ACS Synth Biol. 2017;6:1962–1968. doi: 10.1021/acssynbio.6b00354. [DOI] [PubMed] [Google Scholar]
- Silva-Rocha R, Martínez-García E, Calles B, Chavarría M, Arce-Rodríguez A, De Las Heras A, Páez-Espino D, Durante-Rodríguez G, Kim J, Nikel PI, De Lorenzo V. The Standard European Vector Architecture (SEVA): A coherent platform for the analysis and deployment of complex prokaryotic phenotypes. Nucleic Acids Res. 2013;41:666–675. doi: 10.1093/nar/gks1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MM, Theodoro RC, Oliveira FFM, Machado GC, Hahn RC, Bagagli E, San-Blas G, Felipe MSS. Paracoccidioides lutzii sp. nov.: Biological and clinical implications. Med. Mycol. 2013;52:19–28. doi: 10.3109/13693786.2013.794311. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C, Vieira J, Messing J. Improved Ml3 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Ward OP. Production of recombinant proteins by filamentous fungi. Biotechnol Adv. 2012;30:1119–1139. doi: 10.1016/j.biotechadv.2011.09.012. [DOI] [PubMed] [Google Scholar]