Abstract
Background
Severe centrum semiovale perivascular spaces (CSO-PVSs) are associated with the onset of brain atrophy and dementia. This study explored the relationship between severity of CSO-PVS and development of subdural fluid (SDF) in patients with mild traumatic brain injury (TBI), with the aim of investigating independent radiological risk factors for development of SDF.
Methods
The study cohort comprised 222 patients with a mean age of 51 years (64.0% men) who presented with mild TBI from January 2013 to November 2016. In this study, mild TBI was defined as a Glasgow Coma Scale (GCS) of ≥ 13, Post-Traumatic Amnesia (PTA) of <1 day, and Loss of Consciousness (LOC) of <30 minutes. The severity of CSO-PVS was categorized as low or high-degree.
Results
Among the 222 enrolled patients, 38 (17.1%) and 90 (40.5%) had high-degree PVS in the basal ganglia (BG) and centrum semiovale, respectively. Compared with patients who did not develop SDF, the mean age of patients who developed SDF was significantly higher (47.41 years versus 60.33 years, P < 0.0001). The incidence of de novo SDF was significantly higher in men than in women (77.8% versus 59.5%, P = 0.0151). Patients who showed SDF on brain computed tomography at admission more frequently developed de novo SDF (68.5% versus 38.1%, P < 0.0001). In multivariate logistic regression analysis of risk factors, high-degree CSO-PVS, male sex, initial SDF on admission, and old age were independently associated with development of de novo SDF after mild TBI. In Cox proportional hazards models of risk factors for SDF-development free survival rate, high-degree CSO-PVS, old age, and initial subdural hemorrhage showed statistically significant differences.
Conclusions
Our study might help neurosurgeons determine the frequency of brain CT or the duration of follow-up for patients who present with mild TBI with high-degree CSO-PVS.
Introduction
Traumatic brain injury(TBI) can be classified as mild, moderate, or severe, and approximately 75% of TBI patients present with mild TBI[1]. The most common classification system for the severity of TBI is the Glasgow Coma Scale (GCS) score[2]. Generally, TBI with a GCS of ≥13 is regarded as mild, whereas ≤12 is moderate to severe. To overcome limitations in predicting the outcomes and prognosis based on GCS score, however, current approaches involve the use of three criteria: duration of post-traumatic amnesia (PTA), loss of consciousness (LOC), and GCS score[3, 4].
Post-traumatic subdural fluid (SDF) collection comprises accumulation of cerebrospinal fluid (CSF) in the subdural space, occurring in 4–6.6% of head-injured patients; it is considerably more common in older patients[5, 6]. Unlike patients with moderate to severe TBI, most patients with mild TBI rarely present with SDF or subdural hemorrhage (SDH) on admission. However, during follow-up management for patients with mild TBI, many neurosurgeons have encountered radiologic findings that include increased SDF or the transition to chronic subdural hematoma (CSDH). As a greater proportion of society enters older age, the frequency of CSDH is likely to increase further.
Several studies have reported development of CSDH following traumatic SDF[7–10]. Although patients with mild TBI occasionally show increased SDF or transition to CSDH, the specific pathophysiology is not well known. There have been many studies regarding risk factors for subsequent development or recurrence of CSDH[5, 7–12]. Among these risk factors, old age-related brain atrophy has been identified as an important factor for development of CSDH.
Enlarged perivascular spaces (PVSs), or Virchow Robin spaces, are common radiologic findings with identical signal intensities to CSF in T2-weighted magnetic resonance (MRI) sequences, especially in elderly patients. The sizes and numbers of normally microscopic PVSs increase with advancing age; thus, PVSs appear on T2-weighted MRI as round or tubular hyperintensities in basal ganglia (BG) and centrum semiovale[13, 14]. Enlarged PVSs are associated with a variety of neuropsychiatric disorders, including small vessel disease[14, 15], intracranial atherosclerosis[16, 17], multiple sclerosis[18], dementia[14, 19, 20], depression[21], systemic lupus erythematosus[22] and various others[23–25].
The aim of the present study was to investigate independent radiological risk factors for the development of SDF. To the best of our knowledge, there has been no MRI analysis of PVS severity-related factors for development of CSDH. Therefore, we closely evaluated the relationship between severe enlarged PVSs and development of SDF.
Materials and methods
Study population and data collection
This was a retrospective single-center study of patients with mild TBI. Electronic database searches were used to identify consecutive TBI patients. In total, 1324 consecutive patients with TBI presented to our hospital from January 2013 to November 2016. Among them, patients were enrolled in the present study if they (1) had a brain MRI, including T2-weighted imaging; (2) did not have a history of previous trauma or diseases such as stroke, tumors, or degenerative disease (e.g., dementia or Parkinson’s disease). Among those patients that met these criteria, mild TBI was defined as follows: (1) LOC of approximately <30 minutes or (2) an initial GCS score of 13–15, and (3) PTA < 24 hours. Among 1324 patients with TBI, 892 (67.4%) presented with mild TBI and 432 (33.6%) presented with moderate to severe TBI. Among the 892 patients with mild TBI, 225 underwent brain MRI, including T2-weighted imaging. However, three patients could not be evaluated regarding PVS severity due to poor image quality.
Several basic demographic features and risk factors were assessed in mild TBI patients. These included age at admission, sex, hypertension, diabetes mellitus, hyperlipidemia, liver cirrhosis, history of anti-thrombotic agent use, operation for drainage of SDF, initial SDF of SDH on computed tomography (CT) at admission, white matter hyperintensity graded by the Fazekas scale (0–3), and high-degree of centrum semiovale perivascular spaces (CSO-PVSs) and basal ganglia perivascular spaces (BG-PVSs). Hypertension was defined on the basis of prior diagnosis and the intake of antihypertensive drugs
The Institutional Review Board of Ilsan Paik Hospital approved this study, including the review and publishing of information obtained from patient records (IRB no.: 2017-04-029). The requirement for informed consent was waived for the use of patient medical data, as all patient information was anonymized and de-identified prior to the analysis.
CT and brain MRI acquisition and analysis
Most patients underwent follow-up brain CT every week for approximately 3 weeks after trauma, or at the onset of symptom or sign exacerbation. If SDF, SDH, or other type of traumatic intracranial hemorrhage was detected on initial brain CT, we closely monitored the patients. Brain CT was performed once per week routinely until development of SDF had stabilized. SDF diagnosis was based on published brain CT scan criteria of hypodense SDF collection after TBI, with a minimum distance between the skull and brain of at least 3 mm[26, 27].
Brain MRI was performed after SDF was stabilized on follow-up brain CT. All subjects had an axial T2-weighted fast spin-echo sequence (TR/TE, 4850/98 ms; flip angle, 90°; FOV, 220 mm; slice thickness, 5 mm; and slice gap, 6 mm) and a T2*-weighted gradient echo sequence (TR/TE, 800/26 ms; flip angle, 20°; FOV, 230 mm; slice thickness, 5 mm; and slice gap, 6 mm) based on data obtained using a 1.5-Tesla MRI scanner.
PVS severity was assessed and rated on axial T2-weighted MRI in accordance with recent published method[28, 29]. Enlarged PVSs were defined as small (<3 mm), sharply delineated structures of CSF signal intensity that followed the orientation of perforating arteries in T2-weighted MRI. After reviewing all relevant slices for the assessed anatomical area, the unilateral side of the slice with the highest number of PVSs was recorded. To confirm our hypothesis, PVS severity in the BG and centrum semiovale was categorized into low-degree (n ≤ 20) and high-degree (n > 20) groups. PVS severity was interpreted by two independent board-certified neurosurgeons. In cases of disagreement, we sought consensus between the two observers and a third interpreter[29].
Statistical analysis
Patients were dichotomized into two groups based on de novo SDF collection. We studied associations between development of SDF or SDH and independent variables, including demographic features and radiological findings. For patients with de novo SDF, subgroup analysis was performed for risk factors of operation.
The chi-squared test or Fisher’s exact test were used to compare categorical variables, whereas the t-test or Mann-Whitney U test were used to compare continuous variables. Multivariable logistic regression with stepwise selection was used to identify independent clinical and radiologic risk factors associated with the development of de novo SDF and operations in patients with mild TBI. Cohen’s kappa statistic was calculated to test interrater reliability for evaluation of CSO-PVS and BG-PVS severity on T2-weight MRI. We evaluated risk factors that affected the duration for development of de novo SDH using Kaplan-Meier method. In patients who did not show development of SDF, the duration of follow-up was limited to 90 days. A P-value <0.05 was considered to be statistically significant. SAS 9.4 (SAS Institute, Cary, NC, USA) was used for statistical analysis.
Results
A total of 222 consecutive patients with mild TBI satisfied the inclusion criteria for this study. These patients had a mean age of 51 years and were 64.0% men. All patients presented with several radiological findings: 66 showed minimal SDH, 56 had multiple focal brain contusions, 35 had single minimal cerebral contusions, 12 had traumatic subarachnoid hemorrhage, 11 had minimal epidural hematoma, and 42 had simple concussion. Mild TBI causes were as follows: slip in 66 (29.7%) cases, in-car accidents in 41 (18.5%) cases, out-of-car accidents in 22 (9.9%) cases, bicycle accidents in 34 (15.3%) cases, falling in 21 (9.5%) cases, assault in 16 (7.2%) cases, and by being struck by or against an object in 22 (9.9%) cases. The mean GCS score at admission was 14.83 ± 0.54 (range, 13–15); 202 of 222 patients (90.9%) had a score of 15. Thirty-eight (17.1%) and 90 (40.5%) patients had high-degree BG-PVS and CSO-PVS, respectively. The interrater kappa values were 0.74 and 0.78 for EPVS in the BG and the centrum semiovale, respectively.
Table 1 shows the demographic features of risk factors based on the development of de novo SDF among the patients in this study; 54 (24.3%) patients showed development of de novo SDF and 168 (75.7%) did not. Overall, the mean follow-up period was 71.86 ± 33.08 days (range, 7–90 days). The mean period for development of de novo SDF from trauma was 14.28 ± 17.18 days (range, 1–90 days). Thirty-nine cases (72%) of de novo SDF were diagnosed within fewer than 14 days.
Table 1. Analysis of risk factors for the development of de novo subdural fluid collection in mild traumatic brain injury patients.
| Characteristics | Development (n = 54) | Non-development (n = 168) | P-value |
|---|---|---|---|
| Age, y, mean (±SD) | 60.33 ± 16.67 | 47.41 ± 18.49 | <0.0001 |
| Sex, male, n (%) | 42 (77.8) | 100 (59.5) | 0.0151 |
| High-degree CSO-PVS, n (%) | 40 (74.1) | 50 (29.8) | <0.0001 |
| High-degree BG-PVS, n (%) | 16 (29.6) | 22 (13.1) | 0.005 |
| Initial subdural fluid | 37 (68.5) | 64 (38.1) | <0.0001 |
| Glasgow Coma Scale, n (%) | 0.505 | ||
| 13 | 6 (11.1) | 11 (6.5) | |
| 14 | 1 (1.9) | 2 (1.2) | |
| 15 | 47 (87.0) | 155 (92.3) | |
| Surgery | 12 (22.2) | 0 (0) | <0.0001 |
| Hypertension, n (%) | 18 (33.3) | 38 (22.6) | 0.1203 |
| Diabetes, n (%) | 16 (29.6) | 19 (11.3) | 0.0014 |
| Liver cirrhosis, n (%) | 3 (5.6) | 4 (2.4) | 0.2455 |
| Anti-thrombotic agent, n (%) | 8 (14.8) | 18 (10.7) | 0.4236 |
| WMH, Fazekas grade | 0.0203 | ||
| 0 | 2 (3.7) | 20 (11.9) | |
| 1 | 39 (72.2) | 123 (73.2) | |
| 2 | 12 (22.2) | 15 (8.9) | |
| 3 | 1 (1.9) | 9 (5.4) |
SD, standard deviation; CSO-PVS, centrum semiovale perivascular space; BG-PVS, basal ganglia perivascular space; WMH, white matter hyperintensity
The mean age of patients who developed SDF was significantly higher than that of patients who did not (60.33 years versus 47.41 years, P < 0.0001). The incidence of de novo SDF after mild TBI was significantly higher in males (77.8% versus 59.5%, P = 0.0151). High-degree CSO-PVS and BG-PVS were more frequent among patients who developed SDF than among those who did not (74.1% versus 29.8% for CSO-PVS, P < 0.0001; 29.6% versus 13.1% for BG-PVS, P = 0.005). Patients who showed SDF on brain CT at admission more frequently developed de novo SDF (68.5% versus 38.1%; P < 0.0001). Among vascular risk factors, diabetes mellitus was more frequent among patients who developed SDF (29.6% versus 11.3%, P = 0.0014). The development group showed more frequent moderate to severe white matter hyperintensity (Fazekas 2 or 3). There were no statistical differences in GCS score, anti-thrombotic agent use, hypertension history, or liver cirrhosis between the two groups.
In multivariate logistic regression analysis of risk factors, high-degree CSO-PVS, male sex, initial SDF on admission, and old age were independently associated with development of de novo SDF after mild TBI [Table 2]. Table 3 shows the characteristics of risk factors with regard to surgery among the patients in this study. Twelve (5.4%) patients who underwent surgical treatment by burr hole drainage were men. The mean age of patients who underwent surgery was significantly higher than that of patients who did not (66.17 years versus 49.66 years, P = 0.0034). High-degree CSO-PVS and BG-PVS were both more common in the surgery group than in the non-surgery group (75.0% versus 39.0% for CSO-PVS, P = 0.0138; 41.7% versus 15.7% for BG-PVS, P = 0.0203). More patients in the surgery group demonstrated SDF on brain CT at admission (75.0% versus 43.8%, P = 0.0348).
Table 2. Multivariate analysis of risk factors for development of de novo subdural fluid collection.
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| High-degree of CSO-PVS | 5.242 | 2.52–10.91 | <0.0001 |
| Male sex | 2.33 | 1.05–5.15 | 0.0377 |
| Initial subdural fluid | 2.32 | 1.1–4.89 | 0.0272 |
| Age | 1.031 | 1.01–1.05 | 0.006 |
CSO-PVS, centrum semiovale perivascular space; OR, odds ratio; CI, confidence interval
Table 3. Characteristics of mild traumatic brain injury patients according to surgical treatment.
| Operation (Yes or No) | Yes (n = 12) | No (n = 210) | P-value |
|---|---|---|---|
| Age, y, mean (± SD) | 66.17 ± 11.87 | 49.66 ± 18.77 | 0.0034 |
| Sex, male, n (%) | 12 (100) | 130 (61.9) | 0.0075 |
| High-degree CSO-PVS, n (%) | 9 (75.0) | 82 (39.0) | 0.0138 |
| High-degree BG-PVS, n (%) | 5 (41.7) | 33 (15.7) | 0.0203 |
| Initial subdural fluid | 9 (75.0) | 92 (43.8) | 0.0348 |
| Glasgow Coma Scale, n (%) | 0.5337 | ||
| 13 | 0 (0) | 17 (8.1) | |
| 14 | 0 (0) | 3 (1.4) | |
| 15 | 12 (100) | 190 (90.5) | |
| Hypertension, n (%) | 3 (25.0) | 53 (25.2) | 0.9778 |
| Diabetes, n (%) | 3 (25.0) | 32 (15.2) | 0.3713 |
| Liver cirrhosis, n (%) | 1 (8.3) | 6 (2.9) | 0.2911 |
| Anti-thrombotic agent, n (%) | 2 (16.6) | 24 (11.4) | 0.5878 |
| WMH, Fazekas grade | 0.4972 | ||
| 0 | 1(8.3) | 21(10.0) | |
| 1 | 8 (66.7) | 154 (73.3) | |
| 2 | 3 (25.0) | 24 (11.4) | |
| 3 | 0 (0) | 10 (4.8) |
SD, standard deviation; CSO-PVS, centrum semiovale perivascular space; BG-PVS, basal ganglia perivascular space; WMH, white matter hyperintensity
In multivariate logistic regression analysis of risk factors, male sex and old age were independently associated with surgery for de novo SDF after mild TBI [Table 4].
Table 4. Multivariate analysis of risk factors for operation after mild traumatic brain injury.
| Variables | OR | 95% CI | p-value |
|---|---|---|---|
| Male sex | 8.045 | 1.01–64.57 | 0.0497 |
| Age | 1.067 | 1.02–1.11 | 0.0021 |
OR, odds ratio; CI, confidence interval
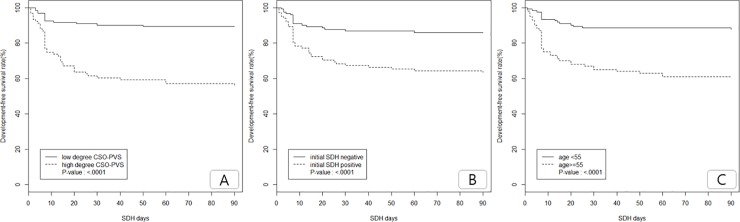
In analysis using Kaplan-Meier method for risk factors of SDF-development free survival rate, high-degree CSO-PVS, old age, and initial SDH showed statistically significant differences (log-rank test: P < 0.0001 for all) [Fig 1].
Fig 1.
Subdural fluid-development free survival rate according to the severity of centrum semiovale perivascular space (CSO-PVS) (A), initial subdural hemorrhage (SDH) presence (B) and old age (C).
Discussion
Our study focused mainly on the role of PVS, a known radiological marker of brain degeneration, in patients who developed SDF or CSDH after mild TBI. There were two main findings in our study. First, high-degree CSO-PVS, male sex, initial SDF on admission, and old age (≥55 years) were independently associated with development of de novo SDF, whereas BG-PVS was not. Second, high-degree CSO-PVS, old age (≥55 years), and initial SDH significantly affected the time for development of de novo SDH.
Our study was undertaken with the hypothesis that the development of de novo SDF after mild TBI may be more common in patients with brain atrophy on MRI; however, there remains considerable controversy regarding the relationship between brain atrophy and PVS severity[15]. A few studies have shown that the severity of PVS was not associated with brain atrophy [30, 31]. However, in a recent study of stroke patients, brain atrophy was associated with the severity of BG-PVS [15, 32]. Additionally, enlarged CSO-PVS was associated with cognitive function in healthy elderly patients [19].
PVSs are defined as fluid-filled spaces that follow the typical course of a vessel as it travels through gray or white matter[33]. PVSs also act as pre-lymphatic drainage system for the removal of substances. As arteries stiffen with age, the amplitude of pulsation is reduced, and insoluble amyloid-β is deposited in the interstitial fluid drainage pathway; this impedes drainage of soluble amyloid-β further. Subsequently, these pathophysiologic courses can lead to CSO-PVS dilation [29].
The Kashima Scan Study suggested that PVS distribution may be useful for early detection and classification of small vessel diseases or hypertensive diseases in neurologically healthy populations[34]. The presence of BG-PVS may be a marker for pure hypertensive arteriopathy, whereas the presence of CSO-PVS might reflect both hypertensive arteriopathy and cerebral amyloid angiopathy (CAA)[34]. Notably, the severity of CSO-PVS is associated with CAA, which is related to atrophy or lesions in cortical or subcortical brain lobes [29]. Our study showed that a high-degree of CSO-PVS was more prevalent in patients who developed SDF or CSDH after mild TBI, suggesting that atrophy of cortical or subcortical areas of the brain may be closely related to the development of SDF.
Several clinical risk factors have been suggested to contribute to the development or recurrence of CSDH: old age, male sex, bilateral SDF, high and mixed density on brain CT, medical diseases with bleeding tendency, and brain atrophy[7, 11, 12].
Several studies have shown male preponderance for the development of SDF and CSDH[7, 35–38]. This tendency was also detected in our study, but remains controversial. An existing rationale for male dominance is men generally have greater exposure to moderate to severe traumatic injuries[7]. However, we included only patients who met mild TBI criteria in our study. Additionally, male sex was analyzed as an important risk factor for requiring surgical treatment after mild TBI. The severity of TBI may also be an important factor for the development of SDF; however, the causative mechanism or event that led to TBI could also constitute an important factor for male dominance. An analysis of the development of SDF or CSDH on the basis of the causative mechanism or event may be needed.
Among the four risk factors in multivariate analysis for development of SDF or CSDH, high-degree CSO-PVS, initial SDH presence, and old age (≥55 years) showed statistically significant effects on the development of SDF or CSDH over time. If the initial brain CT shows evidence of SDF or SDH in older patients with TBI, the prognosis of these patients can be predicted by confirming the severity of CSO-PVS through brain MRI, because it is likely to increase over time.
Recently, there have been many studies regarding the pathophysiology of CSDH development after TBI. Early theories suggested that SDF developed as a result of tears within bridging veins that traverse from the brain to the draining dural venous sinuses; by separation of the dura-arachnoid interface at the dural border cell layer; or by an arachnoid tear and CSF influx into the subdural space[39–41]. However, recent evidence suggests that there are more complex processes involved, because the pathophysiology of CSDH development cannot be explained in patients with no TBI, or in those who exhibit only mild TBI [39, 42]. Thus, the pathophysiology of the development of CSDH after any type of TBI may be influenced by complex interrelated mechanisms, including inflammation, impaired coagulation, fibrinolysis, and angiogenesis[39, 42]. Recently, Holl et al. described a pathway involved in the pathophysiology of CSDH after minor TBI, suggesting that cleavage of the dural border cell layer can activate cytokines, fibroblasts, and vascular endothelial growth factor. Finally, immature capillaries may allow extravasation into the hematoma cavity[42].
Our results have meaningful implications for predicting the prognosis of male patients ≥55 years of age with high-degree CSO-PVS after mild TBI. For patients with high-degree CSO-PVS, we can provide guidance regarding the length and frequency of monitoring through radiologic examinations.
This study had several limitations. First, selection bias was likely involved, as we included only patients who underwent T2-weighted MRI. Second, a limited number of patients required surgical treatment, which led to weak statistical power with regard to evaluating risk factors for surgery. Third, this was a retrospective study. Patients were treated with a variety of methods and had various follow-up periods. To overcome these limitations, a prospective study of mild TBI patients with high-degree CSO-PVS is need to confirm our findings and further evidence for guidance of clinical practice.
Conclusions
High-degree CSO-PVS, male sex, initial SDF on admission, and old age were independently associated with development of de novo SDF. In addition, Cox proportional hazards models of risk factors affecting the duration of de novo SDH showed that high-degree CSO-PVS, old age, and initial SDH were statistically significant. Our study might help neurosurgeons determine the frequency of brain CT or the length of follow-up for patients who present with mild TBI with high-degree CSO-PVS. A large prospective study is warranted to validate our present findings.
Supporting information
(XLS)
Data Availability
All relevant data are within the paper and Supporting Information files.
Funding Statement
This work was funded by the Bio & Medical department of Hanmi medical company. Only H.W.K. received the fund (Hanmi corporation research fund). The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Andelic N. The epidemiology of traumatic brain injury. Lancet Neurol. 2013;12(1):28–9. 10.1016/S1474-4422(12)70294-6 . [DOI] [PubMed] [Google Scholar]
- 2.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4. 10.1016/s0140-6736(74)91639-0 . [DOI] [PubMed] [Google Scholar]
- 3.Jang SH, Kim SH, Lee HD. Relation between memory impairment and the fornix injury in patients with mild traumatic brain injury; a diffusion tensor tractography study. Am J Phys Med Rehabil. 2018. Epub 2018/07/12. 10.1097/PHM.0000000000000996 . [DOI] [PubMed] [Google Scholar]
- 4.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45(7):1253–60. 10.1212/wnl.45.7.1253 . [DOI] [PubMed] [Google Scholar]
- 5.Ohno K, Suzuki R, Masaoka H, Matsushima Y, Inaba Y, Monma S. Chronic subdural haematoma preceded by persistent traumatic subdural fluid collection. J Neurol Neurosurg Psychiatry. 1987;50(12):1694–7. 10.1136/jnnp.50.12.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada Y, Akai T, Okamoto K, Iida T, Takata H, Iizuka H. A comparative study of the treatment of chronic subdural hematoma—burr hole drainage versus burr hole irrigation. Surg Neurol. 2002;57(6):405–9; discussion 10. Epub 2002/08/15. 10.1016/s0090-3019(02)00720-6 . [DOI] [PubMed] [Google Scholar]
- 7.Ahn JH, Jun HS, Kim JH, Oh JK, Song JH, Chang IB. Analysis of Risk Factor for the Development of Chronic Subdural Hematoma in Patients with Traumatic Subdural Hygroma. J Korean Neurosurg Soc. 2016;59(6):622–7. Epub 2016/11/17. 10.3340/jkns.2016.59.6.622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee KS, Bae WK, Bae HG, Yun IG. The fate of traumatic subdural hygroma in serial computed tomographic scans. J Korean Med Sci. 2000;15(5):560–8. 10.3346/jkms.2000.15.5.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohno K, Suzuki R, Masaoka H, Matsushima Y, Inaba Y, Monma S. Role of traumatic subdural fluid collection in developing process of chronic subdural hematoma. Bull Tokyo Med Dent Univ. 1986;33(3):99–106. . [PubMed] [Google Scholar]
- 10.Park SH, Lee SH, Park J, Hwang JH, Hwang SK, Hamm IS. Chronic subdural hematoma preceded by traumatic subdural hygroma. J Clin Neurosci. 2008;15(8):868–72. 10.1016/j.jocn.2007.08.003 . [DOI] [PubMed] [Google Scholar]
- 11.Ko BS, Lee JK, Seo BR, Moon SJ, Kim JH, Kim SH. Clinical analysis of risk factors related to recurrent chronic subdural hematoma. J Korean Neurosurg Soc. 2008;43(1):11–5. 10.3340/jkns.2008.43.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oishi M, Toyama M, Tamatani S, Kitazawa T, Saito M. Clinical factors of recurrent chronic subdural hematoma. Neurol Med Chir (Tokyo). 2001;41(8):382–6. 10.2176/nmc.41.382 . [DOI] [PubMed] [Google Scholar]
- 13.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc Dis. 2015;39(3–4):224–31. Epub 2015/04/01. 10.1159/000375153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding J, Sigurethsson S, Jonsson PV, Eiriksdottir G, Charidimou A, Lopez OL, et al. Large Perivascular Spaces Visible on Magnetic Resonance Imaging, Cerebral Small Vessel Disease Progression, and Risk of Dementia: The Age, Gene/Environment Susceptibility-Reykjavik Study. JAMA Neurol. 2017;74(9):1105–12. Epub 2017/07/18. 10.1001/jamaneurol.2017.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Ding L, Yang L, Qin W, Yuan J, Li S, et al. Brain Atrophy Correlates with Severe Enlarged Perivascular Spaces in Basal Ganglia among Lacunar Stroke Patients. PLoS One. 2016;11(2):e0149593 Epub 2016/02/24. 10.1371/journal.pone.0149593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Brutto OH, Mera RM. Enlarged perivascular spaces in the basal ganglia are independently associated with intracranial atherosclerosis in the elderly. Atherosclerosis. 2017;267:34–8. Epub 2017/11/04. 10.1016/j.atherosclerosis.2017.10.024 . [DOI] [PubMed] [Google Scholar]
- 17.Riba-Llena I, Jimenez-Balado J, Castane X, Girona A, Lopez-Rueda A, Mundet X, et al. Arterial Stiffness Is Associated With Basal Ganglia Enlarged Perivascular Spaces and Cerebral Small Vessel Disease Load. Stroke. 2018;49(5):1279–81. Epub 2018/04/20. 10.1161/STROKEAHA.118.020163 . [DOI] [PubMed] [Google Scholar]
- 18.Wuerfel J, Haertle M, Waiczies H, Tysiak E, Bechmann I, Wernecke KD, et al. Perivascular spaces—MRI marker of inflammatory activity in the brain? Brain. 2008;131(Pt 9):2332–40. 10.1093/brain/awn171 . [DOI] [PubMed] [Google Scholar]
- 19.Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75(11):1519–23. Epub 2004/10/19. 10.1136/jnnp.2003.030858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taniguchi D, Shimura H, Watanabe M, Hattori N, Urabe T. Widespread enlarged perivascular spaces associated with dementia and focal brain dysfunction: case report. BMC Neurol. 2017;17(1):210 Epub 2017/12/08. 10.1186/s12883-017-0997-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Y, Chan YL, Deng M, Chen YK, Mok V, Wang F, et al. Enlarged perivascular spaces in the centrum semiovale are associated with poststroke depression: A 3-month prospective study. J Affect Disord. 2018;228:166–72. Epub 2017/12/19. 10.1016/j.jad.2017.11.080 . [DOI] [PubMed] [Google Scholar]
- 22.Miyata M, Kakeda S, Iwata S, Nakayamada S, Ide S, Watanabe K, et al. Enlarged perivascular spaces are associated with the disease activity in systemic lupus erythematosus. Sci Rep. 2017;7(1):12566 Epub 2017/10/05. 10.1038/s41598-017-12966-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Brutto OH, Mera RM, Atahualpa Project I. Enlarged Basal Ganglia Perivascular Spaces are Associated with Pulsatile Components of Blood Pressure. Eur Neurol. 2018;79(1–2):86–9. Epub 2018/01/10. 10.1159/000486308 . [DOI] [PubMed] [Google Scholar]
- 24.Yang S, Yuan J, Zhang X, Fan H, Li Y, Yin J, et al. Higher ambulatory systolic blood pressure independently associated with enlarged perivascular spaces in basal ganglia. Neurol Res. 2017;39(9):787–94. Epub 2017/05/06. 10.1080/01616412.2017.1324552 . [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Zhang X, Yuan J, Yin J, Hu W. Serum Uric Acid is Independently Associated with Enlarged Perivascular Spaces. Sci Rep. 2017;7(1):16435 Epub 2017/11/29. 10.1038/s41598-017-16715-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka Y, Mizuno M, Kobayashi S, Sugita K. Subdural fluid collection following craniotomy. Surg Neurol. 1987;27(4):353–6. 10.1016/0090-3019(87)90010-3 . [DOI] [PubMed] [Google Scholar]
- 27.Zanini MA, de Lima Resende LA, de Souza Faleiros AT, Gabarra RC. Traumatic subdural hygromas: proposed pathogenesis based classification. J Trauma. 2008;64(3):705–13. Epub 2008/03/12. 10.1097/TA.0b013e3180485cfc . [DOI] [PubMed] [Google Scholar]
- 28.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12(8):822–38. Epub 2013/07/23. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo HW, Jo KI, Yeon JY, Kim JS, Hong SC. Clinical features of high-degree centrum semiovale-perivascular spaces in cerebral amyloid angiopathy. J Neurol Sci. 2016;367:89–94. 10.1016/j.jns.2016.05.040 . [DOI] [PubMed] [Google Scholar]
- 30.Zhu YC, Tzourio C, Soumare A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41(11):2483–90. Epub 2010/09/25. 10.1161/STROKEAHA.110.591586 . [DOI] [PubMed] [Google Scholar]
- 31.Burnett MS, Witte RJ, Ahlskog JE. Swiss cheese striatum: clinical implications. JAMA Neurol. 2014;71(6):735–41. Epub 2014/04/16. 10.1001/jamaneurol.2014.286 . [DOI] [PubMed] [Google Scholar]
- 32.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, et al. Enlarged perivascular spaces and cerebral small vessel disease. Int J Stroke. 2015;10(3):376–81. Epub 2013/05/23. 10.1111/ijs.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berkowitz AL, Westover MB, Bianchi MT, Chou SH. Aspirin for secondary prevention after stroke of unknown etiology in resource-limited settings. Neurology. 2014;83(11):1004–11. 10.1212/WNL.0000000000000779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yakushiji Y, Charidimou A, Hara M, Noguchi T, Nishihara M, Eriguchi M, et al. Topography and associations of perivascular spaces in healthy adults: the Kashima scan study. Neurology. 2014;83(23):2116–23. Epub 2014/11/02. 10.1212/WNL.0000000000001054 . [DOI] [PubMed] [Google Scholar]
- 35.Baechli H, Nordmann A, Bucher HC, Gratzl O. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. 2004;27(4):263–6. Epub 2004/05/19. 10.1007/s10143-004-0337-6 . [DOI] [PubMed] [Google Scholar]
- 36.Sambasivan M. An overview of chronic subdural hematoma: experience with 2300 cases. Surg Neurol. 1997;47(5):418–22. Epub 1997/05/01. 10.1016/s0090-3019(97)00188-2 . [DOI] [PubMed] [Google Scholar]
- 37.Sousa EB, Brandao LF, Tavares CB, Borges IB, Neto NG, Kessler IM. Epidemiological characteristics of 778 patients who underwent surgical drainage of chronic subdural hematomas in Brasilia, Brazil. BMC Surg. 2013;13:5 Epub 2013/03/05. 10.1186/1471-2482-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spallone A, Giuffre R, Gagliardi FM, Vagnozzi R. Chronic subdural hematoma in extremely aged patients. Eur Neurol. 1989;29(1):18–22. Epub 1989/01/01. 10.1159/000116370 . [DOI] [PubMed] [Google Scholar]
- 39.Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017;14(1):108 Epub 2017/06/01. 10.1186/s12974-017-0881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park CK, Choi KH, Kim MC, Kang JK, Choi CR. Spontaneous evolution of posttraumatic subdural hygroma into chronic subdural haematoma. Acta Neurochir (Wien). 1994;127(1–2):41–7. Epub 1994/01/01. 10.1007/bf01808545 . [DOI] [PubMed] [Google Scholar]
- 41.Stone JL, Lang RG, Sugar O, Moody RA. Traumatic subdural hygroma. Neurosurgery. 1981;8(5):542–50. Epub 1981/05/01. 10.1227/00006123-198105000-00005 . [DOI] [PubMed] [Google Scholar]
- 42.Holl DC, Volovici V, Dirven CMF, Peul WC, van Kooten F, Jellema K, et al. Pathophysiology and Nonsurgical Treatment of Chronic Subdural Hematoma: From Past to Present to Future. World Neurosurg. 2018. Epub 2018/05/18. 10.1016/j.wneu.2018.05.037 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and Supporting Information files.