Abstract
Background
Peritoneal carcinomatosis (pcm) in metastatic pancreatic ductal adenocarcinomas (mpdac) is frequently encountered in day-to-day practice, but rarely addressed in the literature. The objective of the present study was to describe the management and outcome of patients diagnosed with pcm.
Methods
Data for all consecutive patients with mpdac treated in our centre between 1 January 2014 and 31 August 2015 were analyzed retrospectively. Computed tomography imaging was centrally reviewed by a dedicated radiologist to determine the date of pcm diagnosis.
Results
The analysis included 48 patients. Median age in the group was 61 years, and 41 patients had an Eastern Cooperative Oncology Group performance status (ecog ps) of 0–1. All patients presented with pcm either synchronously (group 1) or metachronously (group 2). Those groups differed significantly by baseline ecog ps and neutrophil-to-lymphocyte ratio (nlr), with ecog ps being poorer and nlr being higher in group 1. In addition to pcm, the main sites of metastasis were liver (62.5%) and lungs (31.3%). First-line chemotherapy in 36 patients (75%) was folfirinox (fluorouracil–irinotecan–leucovorin–oxaliplatin). The median overall survival for the entire population was 10.81 months [95% confidence interval (ci): 7.16 months to 14.16 months]; it was 13.17 months (95% ci: 5.9 months to 15.4 months) for patients treated with folfirinox. Median overall survival was 7.13 months (95% ci: 4.24 months to 10.41 months) for patients in group 1 and 14.34 months (95% ci: 9.79 months to 19.91 months) for patients in group 2, p = 0.1296.
Conclusions
Compared with other metastatic sites, synchronous pcm seems to be a poor prognostic factor. It could be more frequently associated with a poor ecog ps and a nlr greater than 5 in this group of patients. In patients with mpdac and pcm, either synchronous or metachronous, folfirinox remains an efficient regimen.
Keywords: Prognosis, metastatic sites, pancreatic cancer, peritoneal carcinomatosis
INTRODUCTION
Pancreatic ductal adenocarcinoma (pdac) is the 4th leading cause of cancer deaths worldwide, and incidence rates are predicted to increase 3% per year1. More than 80% of patients with pdac have metastatic or inoperable disease at the time of diagnosis. Despite significant advances in therapeutic modalities, poor outcomes are observed, mainly because of a late diagnosis2. Since about 2010, median overall survival (os) in metastatic pdac (mpdac) has increased to 8–11 months from less than 6 months with polychemotherapy regimens such as folfirinox (fluorouracil–irinotecan–leucovorin–oxaliplatin) and gemcitabine with nab-paclitaxel3,4.
Clinical and biologic factors such as performance status (ps), age, thromboembolic events, number of metastatic sites3,5, serum albumin, carbohydrate antigen 19-9 (ca19-9)6–9, lactate dehydrogenase, and circulating markers (C-reactive protein, neutrophil and lymphocyte counts) have been used in clinical practice for prognostication4,5. Some of those factors have been used in developing various prognostic scores: the Glasgow Prognostic Score (gps) and the modified gps (mgps)10,11; the neutrophil-to-lymphocyte ratio (nlr)12–15 and the platelet-to-lymphocyte ratio; the prognostic index; and the prognostic nutritional index. However, none of those scores have been implemented in clinical practice or prospectively investigated in clinical trials. Metastatic sites include mainly liver, peritoneum, and lungs. However, the correlation between sites of metastasis and prognosis has not been sufficiently explored, even though os was reported to be higher in patients with pulmonary metastases only16.
Based on our experience, the occurrence of peritoneal carcinomatosis (pcm) correlates with poor prognosis, higher disease-related morbidity, an increased rate of complications, and decreased os. However, few published data on this topic are available17–20. Because of poor overall health status, marked symptoms, and lack of measurable target lesions, patients with pdac and pcm are not usually included in prospective phase ii–iii trials. In addition, conventional imaging is not sensitive enough for efficient pcm diagnosis and precise identification of the burden of pcm21,22. Although the Peritoneal Carcinomatosis Index is regularly evaluated in patients with colorectal cancer, it is not used in pdac because patients are almost never referred to surgeons when pcm is identified23.
In the present retrospective study, we sought descriptive data about a population of patients with mpdac and peritoneal involvement, and we report survival data stratified by the time of pcm diagnosis.
METHODS
Data Collection
This series included 48 patients with mpdac and pcm treated at the Centre Léon Bérard between 1 January 2014 and 31 August 2015. Detection of pcm was made by imaging [computed tomography (ct) or integrated positron-emission tomography and ct] and was subsequently confirmed by cytology analysis of ascites if feasible. Patient outcomes were analyzed based on the date of pcm diagnosis: group 1 included patients diagnosed at the time of presentation (synchronous); group 2 included patients who developed pcm later in the course of their disease (metachronous). A dedicated radiologist (blinded to clinical outcome) reviewed the medical imaging and determined the date of pcm diagnosis. Demographic and clinical information, including date of cancer diagnosis, American Joint Committee on Cancer stage at presentation, Eastern Cooperative Oncology Group performance status (ecog ps) at presentation, sites of metastasis at presentation, primary tumour location, treatment modalities (surgery, systemic therapy), types of systemic therapy, response rates, lines of treatments, time between diagnosis of pancreatic cancer and occurrence of ascites or peritoneal nodules (or both), symptoms of pcm, method of diagnosis of ascites, biologic parameters at diagnosis (complete blood count, comprehensive metabolic panel, ca19-9), and survival outcomes were collected. All patients were treated according to the American Society of Clinical Oncology guidelines—that is, with palliative chemotherapy and best supportive care24. The folfirinox regimen was given to patients with an ecog ps of 0–1. Gemcitabine was the preferable regimen in patients with an ecog ps of 2. Nab-paclitaxel and erlotinib were not used because those medications were not being reimbursed by national health care in France. Early best supportive care was provided by a multidisciplinary team specialized in pain and symptom management. Imaging by ct was performed every 8–10 weeks to assess tumour response. The disease control rate was defined as the percentage of patients who achieved a partial response or stable disease at the time of the first ct evaluation. All patients signed a written informed consent.
Statistical Analysis
The analysis was performed in October 2017. Descriptive statistics are used to describe patient characteristics. Between-group comparisons were performed using a chi-square or Fisher exact test for categorical data and the nonparametric Wilcoxon test for continuous data. A p value less than 0.05 was considered statistically significant. Survival data, including os and first-line progression-free survival with associated log-rank tests, were estimated using the Kaplan–Meier method. Overall survival was defined as the time from the diagnosis of metastatic cancer to the date of death or censoring at last follow-up. In group 2, os was calculated from the diagnosis of pcm to the date of death or to censoring at last follow-up. First-line progression-free survival was defined as the time from initiation of first-line chemotherapy to the date of first documented progression, death from any cause, or censoring at last follow-up. The SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.) was used for all statistical analyses.
RESULTS
Patient Characteristics
The analysis included 48 patients with mpdac and pcm. Table I presents the characteristics of the patients at the time of metastatic evolution. Median age in the group was 61 years (range: 38–83 years), and 26 of the patients (54.2%) were men. The ecog ps was 0–1 in 41 patients and 2 in 7 patients. The primary tumour was in the pancreatic head in 45.8% of the cases.
TABLE I.
Patient characteristics
| Characteristic | Value |
|---|---|
| Patients (n) | 48 |
|
| |
| Sex [n (%)] | |
| Women | 22 (45.8) |
| Men | 26 (54.2) |
|
| |
| Age (years) | |
| Median | 61.0 |
| Minimum | 38 |
| Maximum | 83 |
|
| |
| ECOG PS [n (%)] | |
| 0–1 | 41 (85.4) |
| 2 | 7 (14.6) |
|
| |
| Surgery [n (%)] | 7 (14.6) |
|
| |
| Primary tumour location [n (%)] | |
| Head | 22 (45.8) |
| Body or tail, or both | 26 (54.2) |
|
| |
| Symptoms [n (%)] | |
| Pain | 32 (66.7) |
| Bowel disorders | 12 (25) |
| Jaundice | 7 (14.6) |
| Diabetes | 4 (8.3) |
|
| |
| Metastatic sites [n (%)] | |
| Liver | 30 (62.5) |
| Lung | 15 (31.3) |
| Bone | 4 (8.3) |
|
| |
| Venous thrombosis [n (%)] | 13 (27.1) |
|
| |
| Neutrophil:lymphocyte ratio [n (%)] | |
| ≥5 | 14 (31.1) |
| Missing data | 3 |
|
| |
| Serum CA19-9 | |
| Median (UI/L) | 4060 |
| Minimum (UI/L) | 1 |
| Maximum (UI/L) | 120,000 |
| Missing data (n) | 3 |
ECOG PS = Eastern Cooperative Oncology Group performance status; CA19-9 = carbohydrate antigen 19-9.
A pancreatic surgery (Whipple procedure) was performed in 7 patients, and 2 patients were treated with neoadjuvant folfirinox. Of the 7 surgical patients, 1 was diagnosed with liver metastasis during the surgery. In the remaining 6 patients, median time from surgery to the development of metastases was 12.8 months (range: 2–62 months). In addition to pcm, the main sites of metastasis were liver (62.5%) and lung (31.3%). Symptoms were present at the time of diagnosis in 45 patients, with the most frequent symptom being pain (66.7%), followed by bowel symptoms (25%). Median level of ca19-9 was 4.060 IU/L (range: 1–120.000 IU/L). In 14 patients (29.2%), the nlr exceeded 5.
PCM
Table II presents the characteristics of the metastatic disease in the patients. On imaging, 34 patients had ascites, 32 had nodules, and 19 had both. Cytology confirmation of pcm was obtained in 13 patients (27.1%).
TABLE II.
Characteristics of patients by disease group
| Variable | Patient group | p Value | |
|---|---|---|---|
|
| |||
| Synchronous carcinomatosis | Other sites of metastases | ||
| Patients (n) | 26 | 22 | |
|
| |||
| Age (years) | 0.885 | ||
| Median | 63.2 | 60.2 | |
| Minimum | 38 | 39 | |
| Maximum | 80 | 83 | |
|
| |||
| Surgery [n (%)] | 5 (19.2) | 2 (9.1) | |
|
| |||
| ECOG-PS | 0.011 | ||
| 0–1 | 19 (73.1) | 22 (100) | |
| 2 | 7 (26.9) | 0 | |
|
| |||
| Tumour location [n (%)] | |||
| Head | 10 (38.5) | 12 (54.5) | 0.265 |
| Body or tail, ot both | 16 (61.5) | 10 (45.5) | 0.265 |
|
| |||
| Metastatic site [n (%)] | |||
| Liver | 13 (50) | 17 (77.3) | 0.052 |
| Lung | 8 (30.8) | 7 (31.8) | 0.938 |
| Bone | 4 (15.4) | 0 (0) | |
|
| |||
| Serum albumin [n (%)] | |||
| <30 g/L | 2 (11.1) | 1 (5.6) | |
| Missing data | 8 | 4 | |
|
| |||
| Serum CRP [n (%)] | 1.000 | ||
| >5 mg/mL | 11 (68.8) | 11 (68.8) | |
| Missing data | 10 | 6 | |
|
| |||
| Neutrophil:lymphocyte ratio [n (%)] | 0.004 | ||
| ≥5 | 12 (50) | 2 (9.5) | |
| Missing data | 2 | 1 | |
|
| |||
| Serum CA19-9 (UI/L) | |||
| Median | 4960 | 3005 | |
| Minimum | 3 | 1 | |
| Maximum | 120,000 | 32,459 | |
| Missing data | 1 | 2 | |
|
| |||
| Neoplastic cells in ascites [n (%)] | 0.034 | ||
| Positive cytology | 10 (38.5) | 3 (13.6) | |
| Inconclusive | 2 | 0 | |
| Unrealized | 14 | 19 | |
|
| |||
| CT imaging [n (%)] | |||
| Nodules | 21 (80.8) | 11 (50) | 0.024 |
| Ascites | 18 (69.5) | 16 (72.7) | 0.791 |
|
| |||
| Lines of treatment [n (%)] | 0.028 | ||
| 1–2 | 20 (76.9) | 10 (45.5) | |
| ≥3 | 6 (23.1) | 12 (54.5) | |
|
| |||
| First-line CTx [n (%)] | |||
| FOLFIRINOX | 19 (69.2) | 17 (77.3) | |
| Gemcitabine | 7 (26.9) | 5 (22.7) | |
ECOG PS = Eastern Cooperative Oncology Group performance status; CRP = C-reactive protein; CA19-9 = carbohydrate antigen 19-9; CT = computed tomography; CTx = chemotherapy.
We separated the patients into two groups according to the time of pcm appearance. In 26 patients (54.2%), peritoneal involvement was found at the time of pdac diagnosis (group 1). In 22 patients (45.8%), other metastatic sites came first, and pcm presented later in the disease course (group 2). Two clinical characteristics of major prognostic value were significantly different between the groups: ecog ps was worse (p = 0.011) and nlr more frequently exceeded 5 (p = 0.004) in the patients with synchronous pcm (group 1). The presentation of pcm was also different, with nodules being more frequent in synchronous pcm (80.8% vs. 50%, p = 0.024). Median time to diagnosis of pcm after the occurrence of first metastases in group 2 was 10.2 months (range: 1–32 months).
Treatments
All 48 patients received at least 1 cycle of chemotherapy. In the metastatic setting, 18 patients (37.5%) received 3 or more lines of chemotherapy. The main first-line treatments were folfirinox in 36 patients (75%) and gemcitabine in 11 patients (22.9%). In 1 patient, gemcitabine plus nab-paclitaxel was used as first-line chemotherapy. Less chemotherapy was received by patients with synchronous pcm (group 1) than by patients with metachronous pcm (group 2), p = 0.025.
Outcomes
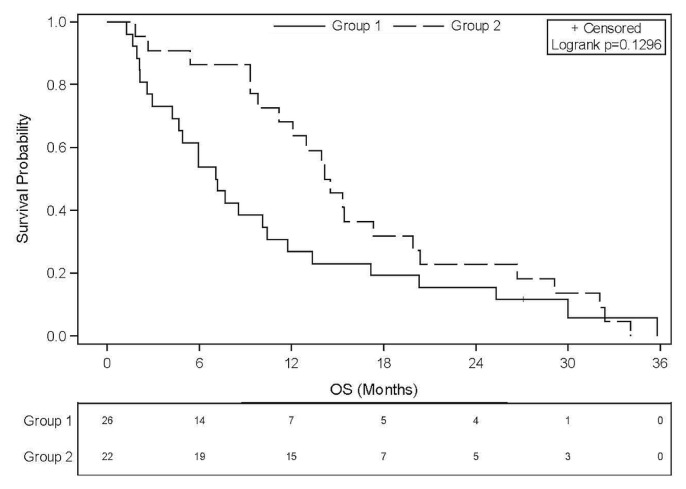
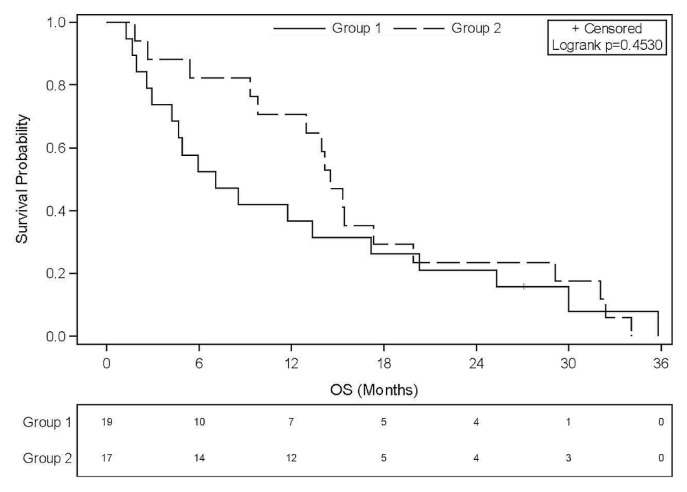
In the overall study group, median os from metastasis was 10.8 months (95% ci: 7.16 months to 14.16 months). When pcm was not present at diagnosis of metastasis, os was twice as long [14.34 months for metachronous pcm (95% ci: 9.79 months to 19.91 months) compared with 7.13 months for synchronous pcm (95% ci: 4.24 months to 10.41 months)], but the difference was not statistically significant (p = 0.1296, Figure 1). In the subgroup of patients treated with folfirinox (n = 36), median os was 13.17 months (95% ci: 5.9 months to 15.4 months). For those 36 patients, a similar trend in os favouring patients with metachronous pcm was observed, median survival being 14.5 months for those with metachronous pcm and 7.1 months for those with synchronous pcm (p = 0.4530, Figure 2). Median os in group 2, assessed from the onset of pcm, was 2.27 months (95% ci: 0.89 months to 8.31 months). Progression-free survival with first-line folfirinox was not different according to the timing of the pcm diagnosis: 7.85 months in group 1 (metachronous) compared with 7.29 months in group 2 (synchronous), p = 0.8878.
FIGURE 1.
Overall survival (OS) since diagnosis of stage IV ductal pancreatic carcinoma. Median OS in all patients was 10.8 months (95% confidence interval: 7.16 to 14.16 months). Group 1 patients were synchronously diagnosed with peritoneal carcinomatosis (PCM); group 2 patients developed PCM later in their clinical course.
FIGURE 2.
Overall survival (OS) in the two groups of patients treated with FOLFIRINOX (leucovorin–fluorouracil–irinotecan–oxaliplatin) in the first line. Group 1 patients were synchronously diagnosed with peritoneal carcinomatosis (PCM); group 2 patients developed PCM later in their clinical course.
DISCUSSION
In routine clinical practice, pcm is a common occurrence in patients with mpdac, but only a few studies have specifically described the clinical course of those patients and the effect of pcm on their survival. In the prodige 4/accord 11 study, only 33 (19.5%) and 32 patients (18.7%) with pcm were treated with folfirinox and gemcitabine respectively. In the mpact study, few patients treated with gemcitabine and gemcitabine–nab-paclitaxel were identified as having pcm (3%). Furthermore, few retrospective studies on this topic are available17–20. In the present study, we aimed to describe the clinical and biologic characteristics of patients with pcm and to explore the effect of pcm on their survival.
The median os of 10.81 months (95% ci: 7.16 months to 14.16 months) for our entire population, and of 13.17 months (95% ci: 5.9 months to 15.4 months) for patients treated with folfirinox, are consistent with the literature, in which the median os in patient populations with mpdac is 11.1 months with folfirinox and 8.5 months with gemcitabine–nab-paclitaxel3,4. Furthermore, the characteristics of our patients were similar to those of the patients included in phase iii clinical trials. Their general ps (ecog ≤2) allowed for administration mainly of folfirinox chemotherapy (75% of patients). Similarly, Hicks et al.18 showed a median os of 12 months in a retrospective analysis of 180 patients treated at Memorial Sloan Kettering Cancer Center, thus comparing favourably with our results. That group found that time from diagnosis to ascites presentation was 11 months, within a timeframe comparable to that seen in our patients—that is, the median of 10.2 months (range: 1–32 months) to peritoneal involvement after the occurrence of metastases at other sites. Survival duration after the appearance of ascites was 1.8 months, but only 44% of the patients had received chemotherapy at that time18. In another series that reviewed the clinical course of 73 patients with pdac and cytologically confirmed malignant ascites, Takahara et al.17 reported shorter os in patients presenting with metachronous ascites than in those presenting with synchronous ascites (42 days vs. 115 days). However, very few patients were treated with chemotherapy, which might account for the discordance with our results.
We also demonstrated that, compared with pcm that occurs later in the course of the disease, synchronous pcm plays a role as a poor prognostic factor in patients with mpdac (median os: 7.1 months; 95% ci: 4.2 months to 10.4 months; p = 0.1296). That median os of 7.1 months is also inferior to results observed in recent phase iii clinical trials in mpdac3,4. We observed that patients with synchronous pcm also had a poorer ecog ps and more often had a nlr exceeding 5, [50% in group 1 (synchronous) vs. 9.5% in group 2 (metachronous), p = 0.004]. It has already been shown that pcm is accompanied by a cellular inflammatory response. Indeed, peritoneal macrophages produce mediators such as transforming growth factor β, interleukin 6, and epidermal growth factor, which are themselves responsible for tumour growth24,25. Our study is the first to suggest a link between synchronous pcm and a high nlr in pdac. The most commonly cited prognostic factors in mpdac are ecog ps, primitive localization of the tumour, and presence of liver metastases. Those factors are frequently used for stratification in clinical trials3,4,26. The impact of a nlr exceeding 4 or 5 has also previously been reported as a reliable indicator of poor prognosis in all solid tumours and especially in pdac12,14. A high nlr, typically as a result of a relatively elevated neutrophil count and a decreased lymphocyte count in cancer patients, reflects the systemic inf lammatory response and the patient’s inflammatory state. High C-reactive protein and low serum albumin are other well-known prognostic factors in pancreatic cancer, revealing a chronic systemic inflammatory state in the patient, and implying poor prognosis10. Prognostic scores making use of many of those factors, such as the gps and mgps, were subsequently developed. Other prognostic factors—biologic and clinical—have been investigated, ca19-9 being the marker most extensively studied6–9. However, in our series, ca19-9 did not differ significantly between the groups and did not appear to be a prognostic factor.
Our study is limited by its retrospective design and the small sample size, which might explain its lack of statistical power with respect to prognostic factors. It could be interesting to prospectively study all those biologic and clinical criteria (including synchronous pcm) to identify the most significant ones so that they could be used as stratification factors in clinical trials.
We acknowledge that the characterization and medical management of pcm remain challenging in 2019. Physicians are faced with morphologic characteristics that can often be complex. The diagnosis is usually based on ct imaging identifying any or all of ascites, involvement of the greater omentum, invasion of the mesentery, and tumour implants (nodules)21,22,27. Recent studies have indicated that magnetic resonance imaging is the most accurate method for evaluating the pcm index28,29. Nonetheless, given a current lack of access to magnetic resonance imaging technology, and in the absence of consensus concerning the best method for establishing a diagnosis of pcm, the debate is still ongoing30.
Lastly, contrary to situation for pcm of colorectal origin, surgical exploration of the abdomen and pelvis is never performed in patients with pdac, precluding the use of the pcm index to determine disease extent. In the present series, we used ct and integrated positron-emission tomography and ct imaging for the pcm diagnosis, because we now know that many patients with pcm can have negative cytology results. Notably, we required all medical imaging to be reviewed by a dedicated radiologist, which further strengthens the validity of our data.
Treating patients with pcm can be difficult; dedicated palliative care guidelines for managing pcm are few31. In particular, the European Society for Medical Oncology clinical practice guideline proposes no specific recommendations in pancreatic cancer2. Chemotherapy and treatments for symptoms are the only therapeutic options available. Of those options, corticosteroids and anti-secretory agents (such as scopolamine and somatostatin analogs) are often effective for symptom relief. Scopolamine butylbromide and octreotide was compared for efficacy in a prospective series of 17 patients32. Octreotide was a more effective treatment than scopolamine in reducing the volume of secretions, but no proof of an association with improvement in quality of life and general status has been demonstrated. In 2012, the efficacy of lanreotide, a somatostatin analog, was demonstrated in a randomized placebo-controlled phase iii trial that enrolled 80 patients with pcm, including 5 patients with mpdac33. One injection of lanreotide 30 mg decreased daily episodes of vomiting after nasogastric tube removal at day 7. Lanreotide is now currently used to treat symptoms resulting from inoperable bowel obstruction from pcm.
CONCLUSIONS
The results of the present study indicate that the presence of pcm is a poor prognostic factor in patients with pdac, especially when it is found at the time of pdac diagnosis. Our study also revealed a new insight about the nlr in mpdac. Confirmation of our results and comparisons with other prognostic factors in larger studies are needed. The diagnosis of and symptom control for pcm remain challenging issues in 2019, providing an opportunity to make significant progress for affected patients.
ACKNOWLEDGMENTS
We thank Sophie Darnis phd, who provided medical writing assistance.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: CM reports personal fees from Deeplink Medical and personal fees from BTG outside the submitted work. CDLF reports personal fees and nonfinancial support from Amgen, personal fees from Roche, and personal fees from Servier outside the submitted work. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–6. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 2.Ducreux M, Cuhna AS, Caramella C, et al. on behalf of the esmo Guidelines Committee. Cancer of the pancreas: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56–68. doi: 10.1093/annonc/mdv295. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, et al. folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Tabernero J, Chiorean EG, Infante JR, et al. Prognostic factors of survival in a randomized phase iii trial (mpact) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Oncologist. 2015;20:143–50. doi: 10.1634/theoncologist.2014-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiorean EG, Von Hoff DD, Reni M, et al. ca19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase iii trial (mpact) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol. 2016;27:654–60. doi: 10.1093/annonc/mdw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert M, Jarlier M, Conroy T, et al. Retrospective analysis of ca19-9 decrease in patients with metastatic pancreatic carcinoma (mpc) treated with folfirinox or gemcitabine (gem) in a randomized phase iii study (accord11/prodige4) [abstract 4115] J Clin Oncol. 2014;32 doi: 10.1200/jco.2014.32.15_suppl.4115. [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2014.32.15_suppl.4115; cited 8 July 2019] [DOI] [PubMed] [Google Scholar]
- 8.Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70:255–64. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 9.Bauer TM, El-Rayes BF, Li X, et al. Carbohydrate antigen 19-9 is a prognostic and predictive biomarker in patients with advanced pancreatic cancer who receive gemcitabine-containing chemotherapy: a pooled analysis of 6 prospective trials. Cancer. 2013;119:285–92. doi: 10.1002/cncr.27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMillan DC. The systemic inflammation–based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–40. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Glen P, Jamieson NB, McMillan DC, Carter R, Imrie CW, McKay CJ. Evaluation of an inflammation-based prognostic score in patients with inoperable pancreatic cancer. Pancreatology. 2006;6:450–3. doi: 10.1159/000094562. [DOI] [PubMed] [Google Scholar]
- 12.Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med. 2014;3:406–15. doi: 10.1002/cam4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An X, Ding PR, Li YH, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers. 2010;15:516–22. doi: 10.3109/1354750X.2010.491557. [DOI] [PubMed] [Google Scholar]
- 14.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Luo H, Qiu M, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita K, Miyamoto A, Hama N, et al. Survival impact of pulmonary metastasis as recurrence of pancreatic ductal adenocarcinoma. Dig Surg. 2015;32:464–71. doi: 10.1159/000439545. [DOI] [PubMed] [Google Scholar]
- 17.Takahara N, Isayama H, Nakai Y, et al. Pancreatic cancer with malignant ascites: clinical features and outcomes. Pancreas. 2015;44:380–5. doi: 10.1097/MPA.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 18.Hicks AM, Chou J, Capanu M, Lowery MA, Yu KH, O’Reilly EM. Pancreas adenocarcinoma: ascites, clinical manifestations, and management implications. Clin Colorectal Cancer. 2016;15:360–8. doi: 10.1016/j.clcc.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by eus-fna. Gastrointest Endosc. 2010;71:260–5. doi: 10.1016/j.gie.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Thomassen I, Lemmens VE, Nienhuijs SW, Luyer MD, Klaver YL, de Hingh IH. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: a population-based study. Pancreas. 2013;42:72–5. doi: 10.1097/MPA.0b013e31825abf8c. [DOI] [PubMed] [Google Scholar]
- 21.Diop AD, Fontarensky M, Montoriol PF, Da Ines D. ct imaging of peritoneal carcinomatosis and its mimics. Diagn Interv Imaging. 2014;95:861–72. doi: 10.1016/j.diii.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Vicens RA, Patnana M, Le O, et al. Multimodality imaging of common and uncommon peritoneal diseases: a review for radiologists. Abdom Imaging. 2015;40:436–56. doi: 10.1007/s00261-014-0224-8. [DOI] [PubMed] [Google Scholar]
- 23.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–32. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceelen WP, Bracke ME. Peritoneal minimal residual disease in colorectal cancer: mechanisms, prevention, and treatment. Lancet Oncol. 2009;10:72–9. doi: 10.1016/S1470-2045(08)70335-8. [DOI] [PubMed] [Google Scholar]
- 25.Capobianco A, Cottone L, Monno A, Manfredi AA, Rovere-Querini P. The peritoneum: healing, immunity, and diseases. J Pathol. 2017;243:137–47. doi: 10.1002/path.4942. [DOI] [PubMed] [Google Scholar]
- 26.Hammel P, Huguet F, van Laethem JL, et al. on behalf of the LAP07 Trial Group. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the lap07 randomized clinical trial. JAMA. 2016;315:1844–53. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 27.Raptopoulos V, Gourtsoyiannis N. Peritoneal carcinomatosis. Eur Radiol. 2001;11:2195–206. doi: 10.1007/s003300100998. [DOI] [PubMed] [Google Scholar]
- 28.Dohan A, Hoeffel C, Soyer P, et al. Evaluation of the peritoneal carcinomatosis index with ct and mri: evaluation of the peritoneal carcinomatosis index with ct and mri. Br J Surg. 2017;104:1244–9. doi: 10.1002/bjs.10527. [DOI] [PubMed] [Google Scholar]
- 29.Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced mri can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol. 2012;19:1394–401. doi: 10.1245/s10434-012-2236-3. [DOI] [PubMed] [Google Scholar]
- 30.Torkzad M, Casta N, Bergman A, Ahlström H, Påhlman L, Mahteme H. Comparison between mri and ct in prediction of peritoneal carcinomatosis index (pci) in patients undergoing cytoreductive surgery in relation to the experience of the radiologist: mri and ct for prediction of pci. J Surg Oncol. 2015;111:746–51. doi: 10.1002/jso.23878. [DOI] [PubMed] [Google Scholar]
- 31.Laval G, Marcelin-Benazech B, Guirimand F, et al. Recommendations for bowel obstruction with peritoneal carcinomatosis. J Pain Symptom Manage. 2014;48:75–91. doi: 10.1016/j.jpainsymman.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Ripamonti C, Mercadante S, Groff L, Zecca E, De Conno F, Casuccio A. Role of octreotide, scopolamine butylbromide, and hydration in symptom control of patients with inoperable bowel obstruction and nasogastric tubes: a prospective randomized trial. J Pain Symptom Manage. 2000;19:23–34. doi: 10.1016/S0885-3924(99)00147-5. [DOI] [PubMed] [Google Scholar]
- 33.Mariani P, Blumberg J, Landau A, et al. Symptomatic treatment with lanreotide microparticles in inoperable bowel obstruction resulting from peritoneal carcinomatosis: a randomized, double-blind, placebo-controlled phase iii study. J Clin Oncol. 2012;30:4337–43. doi: 10.1200/JCO.2011.40.5712. [DOI] [PubMed] [Google Scholar]