Abstract
Objective
Immune checkpoint inhibitors are now a standard of care for the management of many metastatic cancers, including non-small-cell lung cancer. Pembrolizumab, a selective anti–PD-1 monoclonal antibody, augments the host antitumoural response. This hyperactivation of the immune system has side effects, the so-called immune-related adverse effects. The objective of this case report was to review and point out a new pattern of immune checkpoint inhibitor–associated pneumonitis.
Case Description
A 69-year-old woman with stage iv non-small-cell lung cancer receiving pembrolizumab presented for increased dyspnea. Pembrolizumab-related obstructive bronchiolitis was diagnosed based on a new severe obstructive disorder, without bronchodilator reversibility, and mosaic attenuation on angiography, without other identifiable causes.
Summary
To our knowledge, this is the first description of a case of pembrolizumab-induced obstructive bronchiolitis. Various patterns of immune checkpoint inhibitor–associated lung disease have been described, and bronchiolitis should be included in the differential diagnosis.
Keywords: Non-small-cell lung cancer, immunotherapy, immune checkpoint inhibitors, pembrolizumab, immune-related adverse events, bronchiolitis, pneumonitis
CASE DESCRIPTION
A 69-year-old woman with stage iv right upper lobe squamous cell carcinoma with right paratracheal adenopathy and bilateral adrenal and right frontal cerebral metastasis presented to the emergency room for increasing dyspnea for a month and hypoxemic respiratory failure with a saturation of 90% at ambient air. She had received 4 cycles of carboplatin–gemcitabine as first-line chemotherapy and was currently receiving her 7th cycle of pembrolizumab, with an excellent partial tumoural response. Her tumour PD-L1 expression level was 40%.
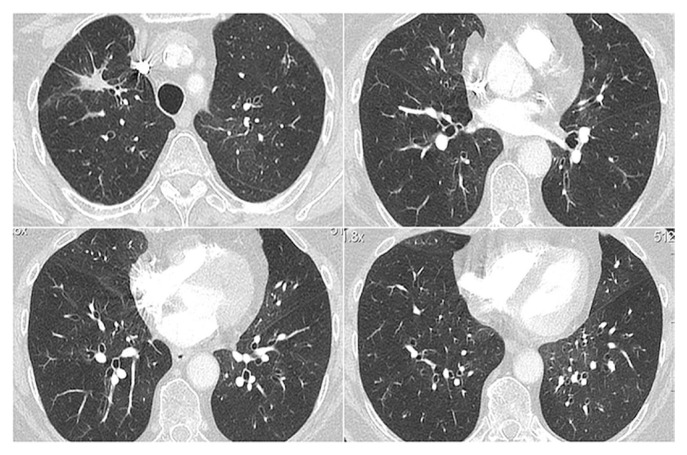
Prior history included pulmonary embolism, for which she was taking rivaroxaban. She was a former smoker and had stopped in 2011. Chest computed tomography (ct) imaging showed new mosaic attenuation, which is represented clinically by expiratory air trapping, with discrete areas of ground-glass opacities (Figure 1) without signs of pulmonary embolism. Bronchoscopy and bronchoalveolar lavage were nonspecific, with an increased neutrophil count [32% (61% macrophages, 5% lymphocytes, and 2% eosinophils)]. Microbiologic testing was negative. Pulmonary function tests revealed a new severe obstructive disorder without bronchodilator reversibility [forced expiratory volume in 1 s (fev1) of 26% (0.57 L), and fev1 to forced vital capacity ratio of 45]. A year earlier, her fev1 had been 89% (2.02 L), and her fev1 to forced vital capacity ratio, 0.78. Diffusing capacity for carbon monoxide was impossible to measure. No other causes of obstructive lung disease were identified (autoimmune disease, infection, organ transplant, smoke inhalation, medications, etc.).
FIGURE 1.
Computed tomography chest images show discrete mosaic attenuation and ground-glass opacities.
Methylprednisolone for 72 hours was prescribed at dose equivalent to prednisone 50 mg. The patient’s symptoms and saturation improved slightly. She was discharged with a tapering dose of oral corticosteroids (starting at 50 mg once daily and decreasing to 7.5 mg once daily during a 15-week period), inhaled corticosteroid, long-acting bronchodilators, and azithromycin for its anti-inflammatory effect. After 7 months, her fev1 remained reduced at 33% (0.73 L), with a fev1 to forced vital capacity ratio of 43, despite long-term low-dose prednisone.
Based on her symptoms, new severe obstructive disorder, mosaic attenuation on chest ct imaging, and lack of response to corticosteroids, we diagnosed obstructive bronchiolitis. She was deemed too ill to undergo lung biopsy. Pembrolizumab was permanently stopped because of pulmonary toxicity. Based on a good clinical response, no other oncologic treatments were started.
At 10 months’ follow-up, progression of her lung cancer was observed.
DISCUSSION
Immune checkpoint inhibitors are now a standard of care for the management of many metastatic cancers, including non-small-cell lung cancer, with a substantial improvement in prognosis. Checkpoint pathways are used by cancer cells to escape cytotoxic T cell–mediated death1–3. Pembrolizumab is a selective anti–PD-1 monoclonal antibody that blocks the interaction between PD-1 and its ligand, PD-L1, thereby disrupting the programmed cell death signal.
Immune-related adverse events with the use of these medications are now well described1,4,5 and are related to an exaggerated activation of the immune system. Almost any organs can be affected, including the lungs6–10. The adverse effects are usually mild-to-moderate in severity, and the immune checkpoint inhibitor can be continued. However, severe adverse effects are not uncommon, and death has also been reported6,7.
Lung toxicities are uncommon, but are potentially serious immune-related adverse events. The incidence is variable, but ranges between 0% and 10%5. The diagnosis is one of exclusion, with a variable and nonspecific clinical presentation. Diagnostic workup should include chest radiography, ct imaging, and pulse oximetry1. For grade 2 and greater toxicities, the clinical practice guideline from the American Society of Clinical Oncology also recommends an infection workup, including nasal swab, sputum culture and sensitivity, blood culture and sensitivity, and urine culture and sensitivity. Lung biopsy is not mandatory if the clinical picture is consistent with pneumonitis, because there is no specific pathology to confirm the diagnosis of immune-related pneumonitis6. Naidoo et al.6 have classified pneumonitis into 5 radiologic subtypes: cryptogenic organizing pneumonia-like, ground-glass opacities, interstitial, hypersensitivity, and pneumonitis not otherwise specified. Ground-glass opacities is the most common radiologic abnormality reported in the literature6,7.
Bronchiolitis is a general term used to describe a nonspecific inflammatory injury affecting the small airways. Various types of bronchiolitis are described, and the nomenclature is confusing because of the mixed use of the term to describe clinical and histopathologic abnormalities. The diagnosis usually requires a biopsy, but if there is high clinical probability with suggestive findings on pulmonary function testing, imaging, and bronchoscopy, a diagnosis of bronchiolitis obliterans can be made. Suggestive findings include obstructive syndrome without bronchodilator reversibility at pulmonary lung tests, mosaic attenuation and bronchial wall thickening with or without ground-glass opacities on chest ct imaging, and nonspecific findings on bronchoalveolar lavage.
Bronchiolitis was reported by Delaunay et al.8 in 4 of 64 patients in a 2017 publication describing immune-related pneumonitis, but we were unable to retrieve an appropriate case description. Hence, to our knowledge, pembrolizumab-induced obstructive bronchiolitis has not yet been explicitly reported as a lung immune-related adverse event.
SUMMARY
We report the first case description of pembrolizumab-induced bronchiolitis. Bronchiolitis should be included in the differential of immune checkpoint inhibitor–associated interstitial lung disease. Pulmonary function testing should be included in the initial workup, especially with anti–PD-1/PD-L1 therapy. Clinicians should have a high index of suspicion for lung toxicities in any patients complaining of new pulmonary symptoms during treatment with an immune checkpoint inhibitor.
ACKNOWLEDGMENTS
The patient was contacted and informed of this case report. She provided her written consent to the authors to publish her de-identified case.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Brahmer J, Lacchetti C, Schneider B, et al. on behalf of the National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–68. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dine J, Gordon R, Shames Y, Kasler MK, Barton-Burke M. Immune checkpoint inhibitors: an innovation in immunotherapy for the treatment and management of patients with cancer. Asia Pac J Oncol Nurse. 2017;4:127–35. doi: 10.4103/apjon.apjon_4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih K, Arkenau HT, Infante JR. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs. 2014;74:1993–2013. doi: 10.1007/s40265-014-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119–42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 5.Naidoo J, Page DB, Li BT, et al. Toxicities of the anti–PD-1 and anti–PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26:2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti–programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35:709–17. doi: 10.1200/JCO.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor–related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2:1607–16. doi: 10.1001/jamaoncol.2016.2453. [DOI] [PubMed] [Google Scholar]
- 8.Delaunay M, Cadranel J, Lusque A, et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50 doi: 10.1183/13993003.00050-2017. pii:1750050. [Erratum in: Eur Respir J 2017;50:pii:1750050] [DOI] [PubMed] [Google Scholar]
- 9.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]