Abstract
Extraosseous Ewing sarcoma is a rare, poorly differentiated round-cell tumour that is part of the Ewing sarcoma family of tumours. Here, we present an extremely rare case of primary extraosseous Ewing sarcoma arising in the larynx, with distant metastases.
A 53-year-old man with a history of Hodgkin lymphoma treated 4 years earlier with 8 cycles of chemotherapy presented to our medical centre with a 2-week history of hoarseness. On physical examination, he was found to have a right supraglottic mass together with a fixed right vocal cord. Computed tomography imaging of the patient’s neck showed a heterogeneously enhancing lesion measuring 5.0×3.8×3.8 cm, centred on the right thyroid cartilage and invading the right true vocal cord. Imaging by integrated fluorodeoxyglucose positron-emission tomography and computed tomography showed active subcarinal and axillary lymph nodes, multiple scattered lung nodules, and multiple bony metastases. Needle core biopsy of the laryngeal mass was diagnostic for Ewing sarcoma. The patient received radiation to the laryngeal area and then alternating cycles of vincristine–actinomycin-D–cyclophosphamide and etoposide–ifosfamide. The patient remains in remission 1 year after completing therapy.
As demonstrated in the present report, these tumours can behave very aggressively both locally and by metastasizing to distant organs. Our treatment approach provided favourable results for the patient; however, future reports are needed to further elucidate optimal management.
Keywords: Ewing sarcoma, pnets, laryngeal cancer, larynx, metastases
INTRODUCTION
Extraosseous Ewing sarcoma (ees) is a rare, poorly differentiated round-cell tumour that is part of the Ewing sarcoma family of tumours (esft), which also includes classical Ewing sarcoma (ews) of bone, peripheral neuroectodermal tumour (pnet), and Askin tumour1,2. Evidence of ees can be traced back to 1969, when Tefft, Vawter, and Mitus were the first to describe four extraosseous tumours that bore an uncanny resemblance to Ewing sarcoma and that could not be classified otherwise3.
Primary ees in the head-and-neck region is very rare, representing between 8.5% and 12% of all ees tumours according to some studies4,5. The prognosis and outcome of ews tumours in the head and neck in general is better than in other anatomic locations; however, the age of the patient and the stage of the disease at diagnosis play important roles as prognostic factors6. Primary ees of the larynx is even rarer, and to the best of our knowledge, only 6 cases have been reported in the literature thus far7–12. Because of the small number of cases, it is unclear whether, in the long run, primary ees of the larynx behaves like other ews tumours of the head and neck.
Treatment of ees generally takes a multimodal approach that can include any or all of local resection, chemotherapy, and radiotherapy. However, a set protocol has yet to be established, and most treatment regimens are extrapolated from skeletal ews13. When ees arises in the larynx, it poses an additional challenge for treatment because of its location and proximity to vital structures.
Here, we present an extremely rare case of primary ees arising in the larynx, with distant metastases. A review of the relevant literature is also presented.
CASE DESCRIPTION
A 53-year-old man, a heavy smoker and recovering alcoholic with a history of Hodgkin lymphoma treated 4 years earlier with 8 cycles of doxorubicin–bleomycin–vinblastine–dacarbazine, presented to our medical centre with a 2-week history of hoarseness. On physical examination, he was found to have a hard right-sided level ii neck mass. Flexible laryngoscopy was performed and showed a right supraglottic mass together with a fixed right vocal cord.
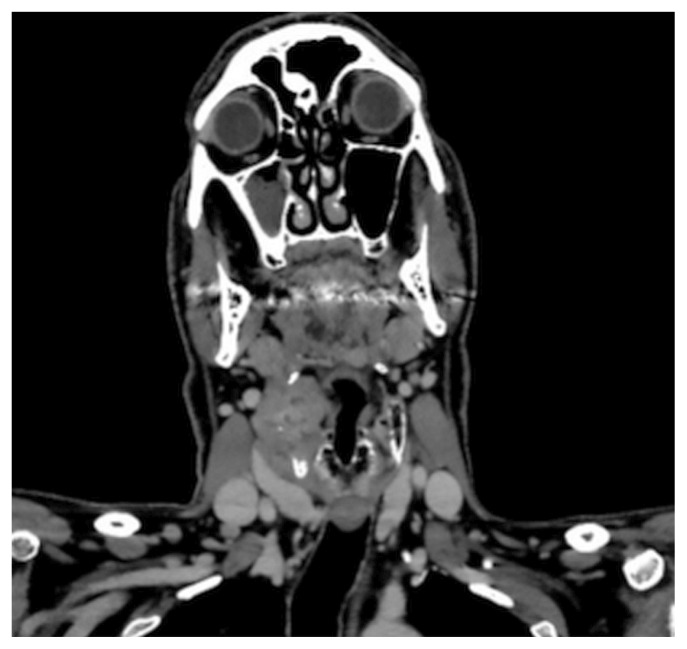
Computed tomography imaging of the patient’s neck showed a heterogeneously enhancing lesion measuring 5.0×3.8×3.8 cm centred on the right thyroid cartilage and invading the right aspect of the pre-epiglottic fat, right paraglottic space, and the right true vocal cord (Figure 1). It appeared inseparable from the right strap muscles. Prominent right cervical lymph nodes were seen, the largest (at level 2A) measuring 13 mm in the shortest axis. Subsequently, whole-body integrated positron-emission tomography and computed tomography imaging with fluorodeoxyglucose showed active subcarinal and axillary lymph nodes, multiple scattered lung nodules, and multiple bony metastases. Notably, the patient’s original lymphoma did not involve the bones or lung parenchyma.
FIGURE 1.
Computed tomography imaging of the patient’s neck. A coronal slice shows a 5.0×3.8×3.8 cm mass centred on the right thyroid cartilage and invading the right paraglottic space and the right true vocal cord, and appearing inseparable from the right strap muscles, together with prominent right cervical lymph nodes.
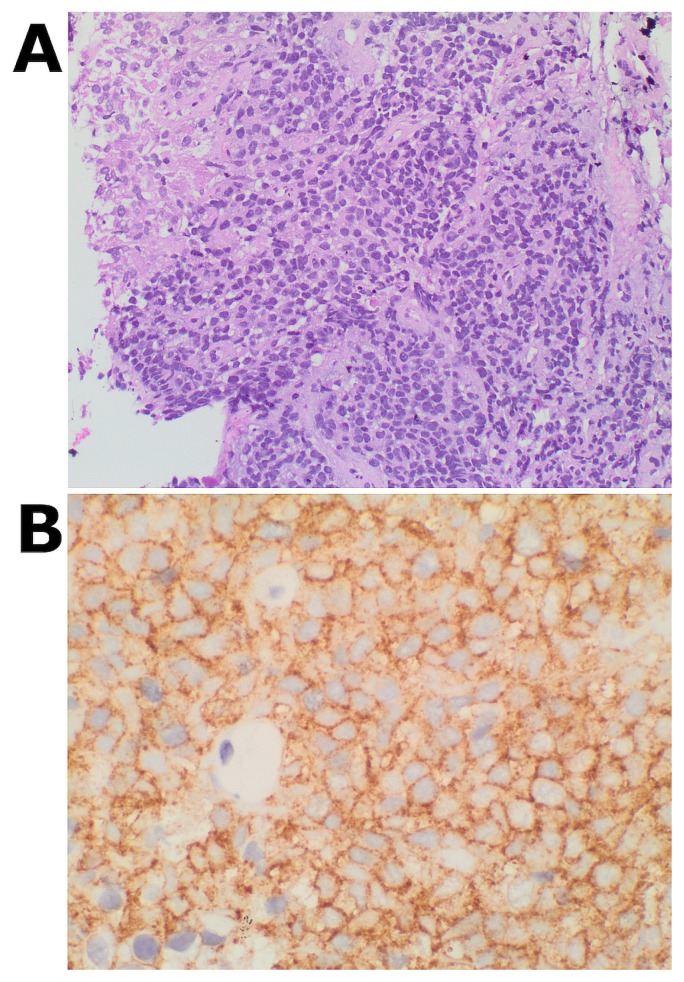
In light of the imaging findings and the patient’s history of lymphoma, a needle core biopsy of the laryngeal mass was obtained under ultrasound guidance. Microscopically, the tumour exhibited cohesive cellular proliferation of small round blue cells growing in sheets, with patchy coagulative necrosis [Figure 2(A)]. The tumour cells showed a minimal-to-moderate amount of glycogen-rich clear cytoplasm. Numerous mitotic and apoptotic figures were noted. Immunostaining for CD99 showed diffuse membranous staining [Figure 2(B)]. The microscopic features and immunoprofile excluded a diagnosis of carcinoma and lymphoma, and was highly suggestive of ews.
FIGURE 2.
Histopathology of the laryngeal tumour. (A) A sheet growth pattern of small round blue cells with clear vacuolated cytoplasm and patchy necrosis is evident. (B) Immunostaining for CD99 shows diffuse membranous staining.
Given the patient’s advanced metastatic disease, surgical treatment was not pursued, and systemic therapy was favoured. The patient received radiation to the laryngeal area and then alternating cycles of vincristine–actinomycin-D–cyclophosphamide and etoposide–ifosfamide. Currently, at 1 year after the end of therapy, the patient has no evidence of active or metastatic disease.
DISCUSSION
Although Tefft, Vawter, and Mitus3 were the first to suggest the presence of extraosseous tumours that much resembled ews in 1969, the first case of ees of the larynx wasn’t published until 1983 by Abramowsky and Witt7. In the present report, we have described the 7th case ever reported of ees of the larynx, and the first to demonstrate distant metastases. Because such cases are few in number, conclusions about the behaviour of laryngeal ees cannot be accurately drawn. Table I summarizes the reported cases of laryngeal ees7–12. Of those previously reported cases, 2 occurred in pediatric patients (1 day of age and 9 months of age); the other 4 occurred in adults between 45 years and 74 years of age. Of those 6 previously reported cases, 4 occurred in male patients, who presented with either hoarseness, stridor, or acute respiratory distress. It is interesting to note that the older published cases relied on histopathology to make the diagnosis; only the most recent report, by Lynch et al.11, confirmed the diagnosis through nuclear staining for EWSR1-FTI1, which is now considered the “gold standard” for diagnosis14.
TABLE I.
Cases of extraosseous Ewing sarcoma of the larynx as reported in the English literature
| ID | Reference | Sex | Age | Presentation | Size (cm) | Treatment | Comments |
|---|---|---|---|---|---|---|---|
| 1 | Abramowsky and Witt, 1983 | Male | 1 Day | Stridor | 1 | Total laryngectomy | |
| 2 | Jones and McGill, 1995 | Male | 9 Months | — | — | CTx plus laryngectomy | Recurred locally |
| 3 | Yang and Hong, 2004 | Male | 74 Years | Acute respiratory distress | 3.5×2.0 | Total laryngectomy | 6 Months disease free |
| 4 | Wygoda et al., 2013 | Male | 68 Years | Hoarseness, aphonia | 2.0×1.9×1.7 | CTx plus RT | 30 Months disease free |
| 5 | Lynch et al., 2014 | Female | 45 Years | Rapidly growing lump, hoarseness | 2.9 | CTx plus RT | |
| 6 | Ijichi et al., 2016 | Female | 33 Years | Hoarseness | — | Microscopic resection plus CTx | Vocal cord lesion, 5 years disease free |
CTx = chemotherapy; RT = radiotherapy.
Histologically, esft can present in one of several different variations, including typical ews, typical adamantinoma-like esft, spindle-cell sarcoma-like esft, sclerosing esft, and large-cell or atypical ews14. Although those variations can differ in both their histologic and immunohistochemical features, typical ews is often described as having uniform sheets of small round cells in a lobular or diffuse arrangement, with a scant amount of pale, clear cytoplasm that might or might not be vacuolated14–16. In addition, mic2 (CD99) expression has been shown to be highly specific for both ews and pnet, and can be used to differentiate those tumours from other small round-cell tumours17.
In recent years, the esft has been recognized as a spectrum of small round-cell entities that were previously classified separately, ranging in their extent of differentiation from the poorly differentiated ews to the more differentiated pnet11. Before the use of molecular and cytogenetic techniques became prevalent, diagnosis of these small round-cell tumours had been based on clinical, radiologic, and histopathologic features, which posed a challenge because of their many similarities. Those features include CD99 staining and the classical t(11;22)(q24;q12) translocation, both of which are associated with most, but not all, cases of ews and pnet18,19. In relatively recent years, the presence of chimeric transcripts involving the EWSR1 gene and a member of the ets-domain transcription factor family, with FLI1 being the most common, has been shown to be a defining characteristic that unifies all esft tumours. Other less-common fusions include EWSR1-ERG, EWSR1-ETV1, EWSR1-E1AF, and EWSR1-FEV14,19.
Treatment of ees of the larynx in the literature has consisted of either surgical resection, chemoradiotherapy, or a combined approach. Total laryngectomy was used in 3 cases, and in 1 case, where the patient presented with a vocal cord lesion, treatment with microsurgery was used7–9,12. A local recurrence was reported in only 1 case, in which the patient had been treated with a combination of surgery and chemotherapy8. Our patient was not a candidate for surgery, given the advanced stage of his disease at presentation.
SUMMARY
To the best of our knowledge, we have presented the 1st reported case of primary ees of larynx, with distant metastasis. Treatment consisted of chemotherapy and radiation. These tumours can present in a wide range of age groups, and as demonstrated in our report, can behave very aggressively both locally and by metastasizing to distant organs. With such a scarce number of cases of ees of the larynx in the literature, it is difficult to make any solid conclusions about the behaviour and treatment of these tumours. Our treatment approach provided favourable results for our patient; however, future reports are needed to further elucidate optimal management.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Huh WW, Daw NC, Herzog CE, Munsell MF, McAleer MF, Lewis VO. Ewing sarcoma family of tumours in children younger than 10 years of age. Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26275. [Epub] [DOI] [PubMed] [Google Scholar]
- 2.Riley RD, Burchill SA, Abrams KR, et al. A systematic review of molecular and biological markers in tumours of the Ewing’s sarcoma family. Eur J Cancer. 2003;39:19–30. doi: 10.1016/S0959-8049(02)00500-2. [DOI] [PubMed] [Google Scholar]
- 3.Tefft M, Vawter GF, Mitus A. Paravertebral “round cell” tumours in children. Radiology. 1969;92:1501–9. doi: 10.1148/92.7.1501. [DOI] [PubMed] [Google Scholar]
- 4.Javery O, Krajewski K, O’Regan K, et al. A to Z of extraskeletal Ewing sarcoma family of tumors in adults: imaging features of primary disease, metastatic patterns, and treatment responses. Am J Roentgenol. 2011;197:W1015–22. doi: 10.2214/AJR.11.6667. [DOI] [PubMed] [Google Scholar]
- 5.Huh J, Kim KW, Park SJ, et al. Imaging features of primary tumours and metastatic patterns of the extraskeletal Ewing sarcoma family of tumours in adults: a 17-year experience at a single institution. Korean J Radiol. 2015;16:783–90. doi: 10.3348/kjr.2015.16.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grevener K, Haveman LM, Ranft A, et al. Management and outcome of Ewing sarcoma of the head and neck. Pediatr Blood Cancer. 2016;63:604–10. doi: 10.1002/pbc.25830. [DOI] [PubMed] [Google Scholar]
- 7.Abramowsky CR, Witt WJ. Sarcoma of the larynx in a newborn. Cancer. 1983;51:1726–30. doi: 10.1002/1097-0142(19830501)51:9<1726::AID-CNCR2820510928>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 8.Jones JE, McGill T. Peripheral neuroectodermal tumours of the head and neck. Arch Otolaryngol Head Neck Surg. 1995;121:1392–5. doi: 10.1001/archotol.1995.01890120050009. [DOI] [PubMed] [Google Scholar]
- 9.Yang YS, Hong KH. Extraskeletal Ewing’s sarcoma of the larynx. J Laryngol Otol. 2004;118:62–4. doi: 10.1258/002221504322731682. [DOI] [PubMed] [Google Scholar]
- 10.Wygoda A, Rutlowski T, Ponikiewska D, Hejduk B, Skladowski K. Ewing’s sarcoma of the larynx. Effective treatment with organ preservation. Strahlenther Onkol. 2013;189:586–9. doi: 10.1007/s00066-013-0356-8. [DOI] [PubMed] [Google Scholar]
- 11.Lynch MC, Baker A, Drabick JJ, Williams N, Goldenberg D. Extraskeletal Ewing’s sarcoma arising in the larynx. Head Neck Pathol. 2014;8:225–8. doi: 10.1007/s12105-013-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ijichi K, Tsuzuki T, Adachi M, Murakami S. A peripheral primitive neuroectodermal tumour in the larynx: a case report and literature review. Oncol Lett. 2016;11:1120–4. doi: 10.3892/ol.2015.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qureshi SS, Laskar S, Kembhavi S, et al. Extraskeletal Ewing sarcoma in children and adolescents: impact of narrow but negative surgical margin. Pediatr Surg Int. 2013;29:1303–9. doi: 10.1007/s00383-013-3409-2. [DOI] [PubMed] [Google Scholar]
- 14.Folpe AL, Goldblum JR, Rubin BP, et al. Morphologic and immunophenotypic diversity in Ewing family tumours. Am J Surg Pathol. 2005;29:1025–33. [PubMed] [Google Scholar]
- 15.Angervall L, Enzinger FM. Extraskeletal neoplasm resembling Ewing’s sarcoma. Cancer. 1975;36:240–51. doi: 10.1002/1097-0142(197507)36:1<240::AID-CNCR2820360127>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Priya D, Kumar RV, Appaji L, Aruna Kamari BS, Padma M, Kumari P. Histological diversity and clinical characteristics of Ewing sarcoma family of tumours in children: a series from a tertiary care center in South India. Indian J Cancer. 2015;52:331–5. doi: 10.4103/0019-509X.176700. [DOI] [PubMed] [Google Scholar]
- 17.Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. mic2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from mic2 expression and specific chromosome aberration. Cancer. 1991;67:1886–93. doi: 10.1002/1097-0142(19910401)67:7<1886::AID-CNCR2820670712>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.de Alava E, Pardo J. Ewing tumour: tumour biology and clinical applications. Int J Surg Pathol. 2001;9:7–17. doi: 10.1177/106689690100900104. [DOI] [PubMed] [Google Scholar]
- 19.Delattre O, Zucman J, Melot T, et al. The Ewing family of tumours—a subgroup of small-round-cell tumours defined by specific chimeric transcripts. N Engl J Med. 1994;331:294–9. doi: 10.1056/NEJM199408043310503. [DOI] [PubMed] [Google Scholar]