Abstract
Background
Gliomas are the most dreaded primary brain tumour because of their dismal cure rates. Ketogenic-type diets (kds) are high-fat, low-protein, and low-carbohydrate diets; the modified Atkins diet (mad) is a less-stringent version of a kd that still generates serum ketones in patients. The purpose of the present study was to retrospectively examine the feasibility of attaining ketosis and the safety of the mad in patients undergoing radiation and chemotherapy treatment for glioma. The rate of pseudoprogression (psp) after treatment was also assessed as a marker of radiation sensitization. To our knowledge, this dataset is the largest published relating to patients with glioma undergoing kd during radiation and chemotherapy.
Methods
We retrospectively studied 29 patients with grades ii–iv astrocytoma following the mad during standard radiation and chemotherapy. Feasibility of attaining ketosis was assessed though levels of beta hydroxybutyrate in blood. Pre- and post-radiation magnetic resonance images were evaluated for psp by a neuroradiologist blinded to patient data.
Results
In the 29 patients who started the mad during radiation, ketosis was achieved in all 29 (100%). No serious adverse events occurred secondary to the mad. Of those 29 patients, 19 had glioblastoma multiforme. Of the latter 19 patients, 11 (58%) showed psp after mad and radiation and temozolomide therapy.
Conclusions
A modified Atkins diet is feasible and safe for glioma patients during radiation and chemotherapy treatment. The mad and resulting ketosis could play a role as a radiation sensitizer.
Keywords: Modified Atkins diet, glioblastoma multiforme, ketogenic diets, pseudoprogression
INTRODUCTION
Glioblastoma multiforme (gbm) is the most common and most aggressive primary malignant brain tumour. The 5-year survival for gbm is dismal, and multiple treatment modalities have been unsuccessful. Stupp et al.1 defined the standard of care in 2005 when the combination of temozolomide and radiation therapy increased the 2-year survival to 26.5% in the combination arm from 10.3% in the radiation-only arm. Unfortunately, only 3% of patients continue to survive at 5 years.
A ketogenic-type diet (kd) is a high-fat, low-protein, and low-carbohydrate diet. Based on the Warburg hypothesis, a kd has been used in the treatment of cancers for many years. Developed by Otto Warburg in 1924, the Warburg hypothesis assumes that cancer cells are metabolically inflexible and must use the inefficient process of glycolysis of glucose to survive. If glucose has been removed from the diet, cancer cells cannot survive, but normal cells that can survive through other energy processes are spared. It has been hypothesized that glioma cells rely solely on glucose for atp production, division, and survival, but that normal brain cells can utilize ketones as a fuel source2.
This hypothesis that tumours are metabolically inflexible and are unable to use ketones as fuel has come into question. De Feyter et al.3 administered a kd to rats orthotopically implanted with glioma cells. Using magnetic resonance spectroscopy (mrs), ketones and glutamate (an energy product of ketones) were measured in tumour and in normal brain. The implanted glioma cells were able not only to uptake the ketones but also to convert the ketones into glutamate as a source of fuel3. Thus, the mechanism of tumour death seen with a kd is not purely an inability of the glioma cells to use ketones as a fuel substrate.
Ketones might work as a radiation sensitizer in tumours. Abdelwahab et al.4 implanted mice with malignant glioma cells and randomized the mice to a kd arm or a standard-diet arm to study the effect of ketosis on mouse survival and glioma control. Compared with the mice in the standard-diet control arm, the mice in the kd arm experienced a modest increase in survival. When the same trial was run again with a kd plus radiation arm and a standard diet plus radiation arm, a significant increase in survival was observed in the kd arm. The tumours were monitored with bioluminescence, and 9 of 11 mice in the kd arm were “cured” of their tumours. The mice were switched to a non-kd after 101 days, and no signs of recurrence were seen for more than 200 days in the kd arm. Therefore, only the combination of radiation and a kd had a significant effect on survival4.
Pseudoprogression (psp) refers to radiographic evidence of an inflammatory response to the combination of radiation and temozolomide therapy. Pseudoprogression is hypothesized to be evidence of radiation sensitization and has been independently associated with a survival benefit in gbm5. Methylation of mgmt (O6-methylguanine–dna methyltransferase) is also a potent predictor of survival. At 2 years, 48.9% of patients with mgmt methylation were still living, compared with only 14.8% of patients with unmethylated mgmt6. New treatment modalities are needed for patients with gbm, especially the patient population with unmethylated mgmt. Increased radiation sensitization might help.
Given stressors such as surgery and neurologic deficits in patients recently diagnosed with gbm, health care professionals and patients alike have concerns about whether patients would be able to tolerate and complete a kd to attain ketosis. Champ et al.7 retrospectively studied 6 patients who underwent a kd treatment for glioma. Of the study patients, 4 completed the diet during radiation and temozolomide therapy for high-grade glioma. Safety and glucose levels during treatment were evaluated, determining that the kd was safe during treatment for glioma7.
A kd has been used for many years to treat pediatric epilepsy. The form of the kd used in pediatric epilepsy is a 4:1 fat–to–carbohydrate plus protein ratio. However, the modified Atkins diet (mad, <20 g carbohydrates daily) produces ketosis and has been used in the treatment of adults with epilepsy with similar efficacy8. Notably, compliance is better with the mad than with a kd9. We therefore chose to use the mad instead of the classically described kd as our study diet.
Here, we describe our retrospective experience with 29 patients who had primary glioma and who were asked to follow the mad during radiation therapy and chemotherapy. To our knowledge, this dataset of radiation and temozolomide therapy with a kd is the largest published10. We assessed the feasibility and safety of the mad in attaining ketosis. An exploratory endpoint was the assessment of psp on magnetic resonance imaging (mri) performed after radiation therapy as a marker for radiation sensitization.
METHODS
Patient Population
The study protocol was approved by the Institutional Review Board at the University of Cincinnati Medical Center. All data from medical records were coded and de-identified. The research procedures for the study were designed to meet criteria for a Privacy Waiver of Authorization. The study population included patients who were at least 18 years of age, who had glioma (World Health Organization grades ii–iv) confirmed by histology, who had been diagnosed between 2013 and 2017, and who were to undergo radiation with concurrent chemotherapy. Pathology in 1 patient with a grade ii tumour was consistent with oligodendroglioma; pathology in all the other patients was consistent with astrocytoma. The patients followed the mad during standard therapy, which included temozolomide 75 mg/m2 and 30 fractions of radiation therapy to a total dose of 59.4 Gy for a total of 6 weeks of radiation therapy (Stupp protocol)1. Patients were followed up at 4 weeks after radiation.
Diet
The goal of a kd is to switch from carbohydrate metabolism to fat metabolism and thus to produce ketone bodies and fatty acids. The ratio of macronutrients in a classic kd is defined as 4 g fat to 1 g protein and carbohydrate combined. The mad uses a ratio of 1–2 g fat to 1 g protein and carbohydrate combined, contributing approximately 20 g of net carbohydrates daily. Our mad had a ratio of 0.8–1 g fat to 1 g carbohydrate plus protein, as previously described in the literature11. Rates of dietary compliance in adults have been shown to be significantly higher with the mad than with a kd because unlike a kd, the mad allows for a slightly increased carbohydrate intake9. We therefore chose to use the mad in our study. Ketones were determined in serum by measuring levels of beta-hydroxybutyrate (bhb). Ketosis was defined as 2 or more serum bhb results greater than 0.5 mmol/L during the 6-week period of radiation therapy and temozolomide chemotherapy.
Data Collection and Statistical Analysis
Patient data were accessed from electronic medical records. Basic demographic and clinical information, including dates of diagnosis, radiation start, radiation end, therapy end, and death (if applicable), were abstracted. Patients had serial blood draws for glucose and bhb testing during radiation therapy. Only patients with 2 or more serum bhb values during the mad and radiation were included in the study. All patients underwent pre-radiation mri (pre- and post-gadolinium enhanced) and post-radiation mri at least twice within 6 months of treatment completion. Tumour histology, grade, and mgmt status were obtained from pathology reports. Height, weight, and body mass index (bmi) were obtained at the mad start and end dates, which coincided with the radiation start and end dates. Logistic regression analysis was performed using the R software application (version 3.4.2: The R Foundation, Vienna, Austria).
Imaging Review
A neuro-oncologist reviewed the magnetic resonance images for psp. In 1 patient, only computed tomography imaging was available. Imaging was also reviewed by a neuroradiologist who was blinded to patient data. The definition of psp was “New areas of contrast enhancement that developed within 6 months of completion of radiotherapy and were concerning for possible tumour progression. If these areas of enhancement either resolved or remained stable over time without clinical intervention they were determined to be psp. True tumour progression (tp) was defined as areas of enhancement that continued to grow”12. Non-psp was defined as either stable or decreased tumour size without psp.
RESULTS
Demographics
The study included 29 patients who had been diagnosed with grades ii–iv glioma during 2013–2017 and who followed the mad concomitantly with 6 weeks of radiation therapy and temozolomide therapy. The group included 1 patient with grade ii oligodendroglioma, 2 with grade ii astrocytoma, 7 with grade iii astrocytoma, and 19 with grade iv astrocytoma (gbm). These 17 men and 12 women had an age at diagnosis ranging from 30.8 years to 76.7 years (median: 52.9 years).
Feasibility
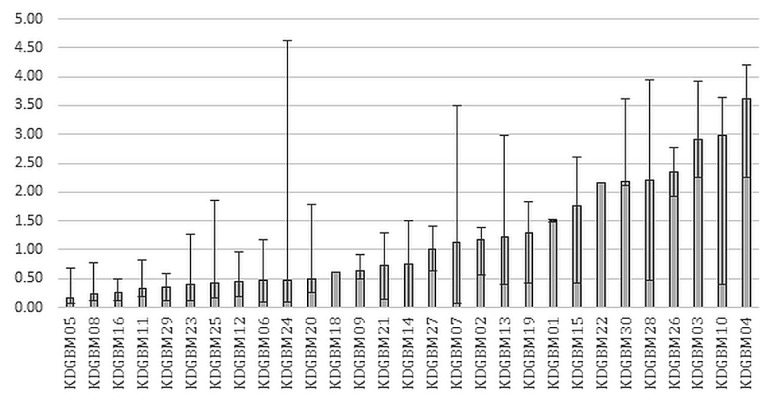
Ketosis was achieved in all 29 patients (100%), as defined by at least 1 serum bhb level of 0.5 mmol/L or greater during their 6 weeks of the mad combined with radiation and temozolomide therapy (Figure 1). Of the 29 patients, only 1 (3.4%) could not complete the mad therapy for the entire 6 weeks, feeling that the diet was too restrictive and stopping it after week 4. Nutritional ketosis has been defined in the literature as a serum bhb level greater than 1 mmol/L13. Using that definition of ketosis, 23 of the 29 patients (79%) had at least 1 value meeting the requirement. Patients were seen by a dietitian at an initial visit and then were followed weekly during radiation and chemotherapy. If a patient had difficulty attaining ketosis, the registered dietitian adjusted the diet for the patient.
FIGURE 1.
Median serum beta-hydroxybutyrate (BHB) levels for each patient during 6 weeks of a modified Atkins diet and radiation therapy. Error bars represent the maximal BHB and the minimal BHB during the diet. KDGBM = ketogenic diet in glioblastoma multiforme.
Safety and Adverse Events
No serious adverse events occurred secondary to the mad, and no grade 3 or 4 toxicities developed. Specifically, no patient developed renal insufficiency or dehydration, or required hospitalization related to the diet. Grade 2 constipation, possibly related to the diet, occurred in 1 patient. Grade 1 fatigue and grade 1 nausea were present in the patients, but of those effects, probably none were related to diet, because they are common side effects of temozolomide and radiation.
Changes in BMI
Body mass index was recorded at the beginning of the mad and at the end of the 6 weeks of the mad plus radiation and temozolomide therapy. The median change of bmi for all patients was −1.04. Overall, 25 patients (86.2%) experienced a decrease in bmi over the course of treatment, with the most significant decrease being −2.31 (bmiinitial = 33.02, bmifinal = 30.71). Only 3 patients experienced an increase in bmi, with the most significant increase being 0.27 (bmiinitial = 24.01, bmifinal = 24.28). In 1 patient, bmi did not change. Only 1 patient who started with a healthy bmi (18.75) had a final bmi that was classified as underweight (17.79), representing a −0.96 change in bmi or a decrease of 5.12% from the initial bmi.
PsP: Grades II–IV Astrocytoma
After the mad and radiation and temozolomide therapy, psp occurred in 16 of the 29 patients (55%). Tumour progression occurred in 5 patients (17%). The tumour was stable or decreased in size in 8 patients (28%). Of the 29 study patients, 13 had methylated mgmt, with 8 of that group experiencing psp and 5 experiencing either a stable tumour or a decrease in tumour size. In 12 patients, mgmt was unmethylated, and 5 of that group experienced psp, 4 experienced tumour progression, and 2 had a stable tumour or a decrease in tumour size (Table I).
TABLE I.
Tumour response based on MGMT (O6-methylguanine–DNA methyltransferase) status, all patients (grades II–IV astrocytoma)
| Tumour response | MGMT status | ||
|---|---|---|---|
| Methylated | Unmethylated | Unknown | |
| Pseudoprogression | 8 | 6 | 2 |
| Progression | 0 | 4 | 1 |
| Stable or decrease | 5 | 2 | 1 |
PsP: GBM
Of the 29 patients, 19 had gbm. In that subgroup, 11 (58%) showed psp after the mad and radiation and temozolomide therapy. Tumour progression occurred in 4 patients (21%), and 4 patients (21%) showed either a stable tumour or a decrease in tumour size. Of 6 patients with methylated mgmt, 4 experienced psp, and 2 showed either a stable tumour or a decrease in tumour size. Of 10 patients with unmethylated mgmt, 5 (50%) experienced psp, 3 experienced tumour progression, and the remaining 2 had a stable tumour or a decrease in tumour size (Table II).
TABLE II.
Tumour response based on MGMT (O6-methylguanine–DNA methyltransferase) status, patients with glioblastoma multiforme (grade IV astrocytoma)
| Tumour response | MGMT status | ||
|---|---|---|---|
| Methylated | Unmethylated | Unknown | |
| Pseudoprogression | 4 | 5 | 2 |
| Progression | 0 | 3 | 1 |
| Stable or decrease | 2 | 2 | 0 |
Serum BHB
The average number of serum bhb levels obtained during radiation and the 4-week follow-up was 5. The average serum bhb in the patients overall was 1.3 mmol/L, and each patient had 1 serum bhb level of at least 0.5 mmol/L. Of the 29 patients, 14 had a serum bhb level greater than 1 mmol/L, and of those 14 patients, 8 (57.1%) experienced psp. Of 7 gbm patients with a serum bhb level greater than 1 mmol/L, 5 (71.4%) experienced psp; of 16 with a serum bhb level less than 1 mmol/L, 6 experienced psp (37.5%). Logistic regression for experiencing compared with not experiencing psp when the patient had a serum bhb level greater than 1 mmol/L resulted in an odds ratio of 6.0 (95% ci: 0.5 to 6.3).
Overall Survival in GBM
Only patients who were deceased by the time of the 2-year survival analysis or who had been diagnosed at least 2 years before the analysis were analyzed for overall survival—that is, patients who were alive, but who had been diagnosed less than 2 years earlier were excluded. The 2-year survival for all gbm patients was 26.7% (4 of 15), which compares with the 26.5% (95% ci: 21.2% to 31.7%) reported in the Stupp et al. landmark trial1. When examining patients with methylated mgmt, survival in the present study was 50% (2 of 4), which compares with 46.0% (95% ci: 31.2% to 60.8%) in a trial by Hegi et al.14 comparing survival in patients with methylated and unmethylated mgmt. When examining patients with unmethylated mgmt, who often do very poorly with standard-of-care treatment (temozolomide and radiation), Hegi et al.14 found that 13.8% of patients (95% ci: 4.8% to 22.7%) survived 2 years. In our study, patients with unmethylated mgmt had an overall survival of 22% (2 of 9). Calculating overall survival rates with such small numbers is intriguing, but must be viewed with caution.
DISCUSSION
The mad was followed by almost all patients with high-grade glioma. Patients tolerated the diet well, with no clinically significant side effects, including clinically significant weight loss. If we define feasibility as serum bhb greater than 1 mmol/L, then during the study, 79% of patients achieved ketosis. The level of ketosis required in humans to increase ketones in the brain and in tumours is unknown.
One study tested mrs on 4 healthy subjects who fasted for 3 days. The subjects drank only water and, at the end of the fasting, were admitted to the hospital for intravenous fluids. These subjects underwent brain mrs on days 2 and 3. On day 2 of fasting, serum bhb was 1.67 ± 0.34 mmol/L, and on day 3, it was 3.15 ± 0.67 mmol/L. Those levels correlated in a linear fashion with bhb in the brain15.
The level of systemic ketosis required to obtain ketosis in a brain tumour has to be studied further. A very high level of bhb might be needed to attain the type of response that Abdelwahab et al.4 obtained in mice undergoing radiation. Achieving such a level might not be feasible in humans, especially in patients with compromised performance status and a terminal illness. Currently, we are performing an institutional research board–approved prospective mrs evaluation of bhb levels in the brains of patients with gbm.
The biology through which a kd works in tumours is still poorly understood. The akt/pik3/mtor pathway has been implicated in the inhibition of cell death and is activated by glucose and glycolysis. That pathway is found in many gliomas16. Ketone metabolism has been used to control childhood epilepsy for many years, an approach that is thought to inhibit the akt/pik3/mtor pathway and other mechanisms17. Inhibitors of the pik3/mtor pathway have been shown to have radiation-sensitizing effects in combination with radiation and temozolomide18. Perhaps a kd does not work through the Warburg hypothesis, as previously thought, but as in Abdelwahab et al.’s mice, creates a survival benefit through radiation sensitization. The pik3/mtor pathway might be an important pathway through which ketones sensitize gbm to radiation.
Patients with mgmt methylation experience more chemotherapy–radiation sensitization and more psp. In our study, compared with historical controls (44%), 66.67% of the gbm patients with mgmt methylation (4 of 6) experienced psp. We also note that 50% of the gbm patients without mgmt methylation (5 of 10) experienced psp (it was only 18% in historical controls). Historically, psp and mgmt methylation status have been independent predictors of increased survival19. Radiation therapy combined with the mad could have a radiation-sensitizing effect.
The mad was safe in all patients and feasible in 79% of patients if serum bhb greater than 1 mmol/L is used as a ketosis marker. A nonsignificant trend (perhaps because of the small patient numbers in our study) suggests that psp could be a dose-dependent phenomenon, with increased likelihood that a patient will experience psp if serum bhb exceeds 1 mmol/L. The literature shows that levels of serum ketosis and brain ketosis correlate in a linear fashion15. What is unclear is the level of serum ketosis that produces a level of brain and brain tumour ketosis that will generate a significant oncologic response. Also, consistent achievement of higher ketone levels in this seriously ill patient population during a period of 6–10 weeks could be difficult. That difficulty should be taken into consideration when designing and interpreting future studies about kds and brain tumours.
Limitations
The main limitation of our study is the small number of patients and the retrospective nature of the study. Because patients were not part of a prospective clinical study and because the patients had a terminal illness, we limited blood draws to those times when the patients were scheduled for visits as part of their standard care. We therefore monitored bhb levels only once weekly during the course of radiation. Ideally, more frequent checking of bhb (as often as twice daily) would be ideal for future studies.
CONCLUSIONS
To our knowledge, this dataset is the largest published of patients with glioma undergoing a kd during radiation and chemotherapy treatment. We found that the mad is feasible and safe during radiation and chemotherapy treatment for such patients. Further study is needed to determine if a kd or the mad affects treatment response and survival in this patient population. Also, further study is required to elucidate the “dose” of serum ketosis that is required to obtain ketosis in the brains and tumours of patients with high-grade glioma, and the “dose” of ketosis in the brain that is required to obtain a clinical response (that is, survival, psp, and so on). In an ongoing prospective trial, we are using mrs to investigate ketone uptake in patients with high-grade glioma after the mad during radiation and temozolomide therapy. An intriguing hypothesis about how a kd works in tumours is that ketones might play a role as a radiation sensitizer. That role could be important in all gliomas, but especially in non-methylated gbms, for which options for meaningful treatment are limited.
Highlights.
■ A kd is feasible and safe during the treatment of gliomas.
■ A kd might play a role as a radiation sensitizer for gliomas.
■ The level of ketosis achieved might be important in the rate of psp.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, et al. on behalf of the European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups and the ncic Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Maurer GD, Brucker DP, Bähr O, et al. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11:315. doi: 10.1186/1471-2407-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Feyter HM, Behar KL, Rao JU, et al. A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth. Neuro Oncol. 2016;18:1079–87. doi: 10.1093/neuonc/now088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelwahab MG, Fenton KE, Preul MC, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7:e36197. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandes AA, Franceschi E, Tosoni A, et al. mgmt promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26:2192–7. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP, et al. on behalf of the European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups and the ncic Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase iii study: 5-year analysis of the eortc-ncic trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.Champ CE, Palmer JD, Volek JS, et al. Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme. J Neurooncol. 2014;117:125–31. doi: 10.1007/s11060-014-1362-0. [DOI] [PubMed] [Google Scholar]
- 8.Miranda MJ, Mortensen M, Povlsen JH, Nielsen H, Beniczky S. Danish study of a modified Atkins diet for medically intractable epilepsy in children: can we achieve the same results as with the classical ketogenic diet? Seizure. 2011;20:151–5. doi: 10.1016/j.seizure.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Ye F, Li XJ, Jiang WL, Sun HB, Liu J. Efficacy of and patient compliance with a ketogenic diet in adults with intractable epilepsy: a meta-analysis. J Clin Neurol. 2015;11:26–31. doi: 10.3988/jcn.2015.11.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz KA, Noel M, Nikolai M, Chang HT. Investigating the ketogenic diet as treatment for primary aggressive brain cancer: challenges and lessons learned. Front Nutr. 2018;5:11. doi: 10.3389/fnut.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDonald TJW, Cervenka MC. Ketogenic diets for adult neurological disorders. Neurotherapeutics. 2018;15:1018–31. doi: 10.1007/s13311-018-0666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bronk JK, Guha-Thakurta N, Allen PK, Mahajan A, Grosshans DR, McGovern SL. Analysis of pseudoprogression after proton or photon therapy of 99 patients with low grade and anaplastic glioma. Clin Transl Radiat Oncol. 2018;9:30–4. doi: 10.1016/j.ctro.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789–96. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 15.Pan JW, Rothman TL, Behar KL, Stein DT, Hetherington HP. Human brain beta-hydroxybutyrate and lactate increase in fasting-induced ketosis. J Cereb Blood Flow Metab. 2000;20:1502–7. doi: 10.1097/00004647-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Rathmell JC, Fox CJ, Plas DR, Hammerman PS, Cinalli RM, Thompson CB. akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival. Mol Cell Biol. 2003;23:7315–28. doi: 10.1128/MCB.23.20.7315-7328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mtor) pathway. Epilepsia. 2011;52:e7–11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi EJ, Cho BJ, Lee DJ, et al. Enhanced cytotoxic effect of radiation and temozolomide in malignant glioma cells: targeting pi3k-akt-mtor signaling, hsp90 and histone deacetylases. BMC Cancer. 2014;14:17. doi: 10.1186/1471-2407-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Li J, Cheng G, Zhang J, Li X. IDH mutation and mgmt promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg. 2016;151:31–6. doi: 10.1016/j.clineuro.2016.10.004. [DOI] [PubMed] [Google Scholar]