Abstract
Background
The major limitation in the use of trastuzumab therapy is cardiotoxicity. We evaluated the safety of a strategy of continuing trastuzumab in patients with breast cancer despite mild, asymptomatic left ventricular impairment.
Methods
Charts of consecutive patients referred to a cardio-oncology clinic from January 2015 to March 2017 for decline in left ventricular ejection fraction (lvef), defined as a fall of 10 percentage points or more, or a value of less than 50% during trastuzumab therapy, were reviewed. The primary outcome of interest was change in lvef, measured before and during trastuzumab exposure and up to 3 times after initiation of cardiac medications during a median of 9 months.
Results
All 18 patients referred for decline in lvef chose to remain on trastuzumab and were included. All patients were treated with angiotensin converting–enzyme inhibitors or beta-blockers, or both. After initiation of cardiac medications, lvef increased over time by 4.6 percentage points (95% confidence interval: 1.9 percentage points to 7.4 percentage points), approaching baseline values. Of the 18 patients, 17 (94%) were asymptomatic at all future visits. No deaths occurred in the group.
Conclusions
Many patients with mildly reduced lvef and minimal heart failure symptoms might be able to continue trastuzumab without further decline in lvef, adverse cardiac events, or death when treated under the supervision of a cardiologist with close follow-up.
Keywords: Breast cancer, trastuzumab, cardiotoxicity, cardio-oncology, her2
INTRODUCTION
Overexpression of her2 (the human epidermal growth factor 2) occurs in approximately 15% of breast cancer (bca) cases1. Trastuzumab, a monoclonal antibody against the her2 receptor, has been shown to improve overall survival in women with her2-positive early-stage bca when administered for 12 months2. The main limitation in the use of trastuzumab is cardiotoxicity. Of patients receiving adjuvant therapy with trastuzumab, 4%–18% develop left ventricular (lv) dysfunction3,4, and 2%–4% develop moderate-to-severe heart failure4. A meta-analysis of randomized trials of trastuzumab for early-stage her2-positive bca with a median follow-up of 36 months demonstrated that heart failure developed in 2.5% of trastuzumab recipients2. For that reason, guidelines recommend that cardiac function be monitored serially by measurement of lv ejection fraction (lvef) during trastuzumab therapy5. Guidelines also suggest that adjuvant trastuzumab be withheld if lvef falls to 6 percentage points or more below the lower limit of normal; if lvef has fallen by 10–15 percentage points from baseline and is 1–5 percentage points below the lower limit of normal; or if lvef has fallen by 15 percentage points or more from baseline6. Notably, those recommendations are empiric, and there is little evidence to support whether such thresholds are associated with optimal cancer or overall outcomes.
Importantly, an adjuvant trastuzumab duration of less than 12 months leads to suboptimal cancer outcomes. In a meta-analysis of randomized clinical trials comparing 12 months’ trastuzumab with shorter treatment, less than 12 months’ trastuzumab therapy resulted in poorer overall and cancer-free survival7. To optimize cancer outcomes, completion of 12 months of trastuzumab therapy is therefore desirable. However, there is limited evidence about whether continuing trastuzumab in patients with a decline in lvef is safe. The purpose of the present study was to evaluate the safety of a cardio-oncology clinic–based intervention to continue trastuzumab therapy in patients with her2-positive bca despite mild, asymptomatic lv impairment.
METHODS
Study Design
In a retrospective review of the charts of patients referred to the cardio-oncology service at the Juravinski Hospital in Hamilton, Ontario, from January 2015 to March 2017, patients were eligible for inclusion if all the following criteria were fulfilled:
■ Diagnosis of her2-positive bca of any stage
■ Receipt of therapy with trastuzumab
■ Presence of signs of cardiac injury as defined by a reduced lvef meeting Canadian Trastuzumab Working Group guidance6 to withhold trastuzumab (that is, lvef 6 or more percentage points below the lower limit of normal; a fall in lvef by 10–15 percentage points from baseline, now being 1–5 percentage points below the lower limit of normal; or a fall in lvef of 15 percentage points or more from baseline) or clinical symptoms of heart failure
Patients were excluded if they were already being treated with both an angiotensin converting–enzyme inhibitor (acei) or angiotensin receptor blocker and a beta-blocker. The study protocol was approved by the local research ethics board, which waived the need for informed patient consent.
Patient Management
The 18 consecutive patients meeting the eligibility criteria were counselled at the cardio-oncology clinic about the potential risks and benefits of both continuing and discontinuing trastuzumab. They were also informed of the evidence to suggest that aceis and beta-blockers can improve cardiac function in patients with reduced lvef, including chemotherapy-induced lv dysfunction. All patients expressed the wish to receive ongoing trastuzumab coupled with treatment using an acei or beta-blocker (or both) under cardiologist oversight and were included in the study analysis. Table I presents their characteristics.
TABLE I.
Characteristics of the study patients at baseline
| Characteristic | Value |
|---|---|
| Patients | |
|
| |
| Mean age (years) | 57.4±10.5 |
|
| |
| Mean body mass index (kg/m2) | 28.5±4.9 |
|
| |
| Mean heart rate (bpm) | 79±13 |
|
| |
| Mean blood pressure (mmHg) | |
| Systolic | 135±22 |
| Diastolic | 78±9 |
|
| |
| Sex [n (%) women] | 18 (100) |
|
| |
| Cancer stage [n (%)] | |
| IA | 2 (11) |
| IB | 2 (11) |
| IIA | 8 (44) |
| IIB | 2 (11) |
| IIIA | 2 (11) |
| IV | 2 (11) |
|
| |
| Cardiac risk factors [n (%)] | |
| Coronary artery disease | 1 (6) |
| Diabetes | 2 (11) |
| Dyslipidemia | 2 (11) |
| Hypertension | 3 (17) |
| History of smoking | 4 (22) |
| Valvular heart disease | 0 |
| Previous heart failure | 0 |
|
| |
| NYHA class on presentation [n (%)] | |
| I | 16 (89) |
| II | 2 (11) |
| Tumour | |
|
| |
| Receptor status [n (%)] | |
| ER-positive | 11 (61) |
| PgR-positive | 8 (44) |
| ER- or PgR-negative | 7 (39) |
|
| |
| Left-sided disease [n (%)] | 12 (67) |
|
| |
| Previous radiation therapy [n (%)] | 17 (94) |
|
| |
| Previous anthracycline treatment [n (%)] | 17 (94) |
|
| |
| Recurrent disease [n (%)] | 2 (11) |
NYHA = New York Heart Association; ER = estrogen receptor; PgR = progesterone receptor.
A cardiovascular risk factor was considered present for patients who were current or former smokers or who had coronary artery disease, diabetes, dyslipidemia, or hypertension (any one or a combination). Patients were prescribed an acei or a beta-blocker, or both, at their first cardio-oncology clinic appointment. The choice of medication to initiate first was at the discretion of the cardiologist after a consideration of the potential adverse effects and measurement of the patient’s resting heart rate (when >80 bpm, a beta-blocker was generally preferred). The doses of the selected drugs were increased to the maximum tolerated over 2–3 clinic visits at intervals of 3–12 weeks.
Study Outcomes
The primary outcome of interest was change in lvef as measured by transthoracic echocardiography or multigated acquisition imaging. Echocardiographic 3-dimensional lvef was recorded when available; otherwise, the Simpson bi-plane lvef was recorded. Recording of lvef occurred at up to 5 time points:
■ Before trastuzumab exposure (baseline)
■ During trastuzumab exposure (point 2)
■ Up to 3 times after trastuzumab exposure and after initiation of cardiac medications with ongoing trastuzumab treatment (points 3–5)
Patients were referred to cardio-oncology based on the change in lvef noted on transthoracic echocardiography or multigated acquisition imaging at time point 2. Timing of lvef measurements during follow-up was at the discretion of the treating cardiologist. Secondary outcomes included
■ global longitudinal strain as measured by speckle-tracking 2-dimensional echocardiography (in which a negative value is normal, but to allow for easier interpretation, values are presented as positive in this paper);
■ left atrial volume indexed to body surface area;
■ right ventricular function as measured by the tricuspid annular plane systolic excursion;
■ the proportion of patients who developed symptomatic heart failure; and
■ blood pressure (bp).
Global longitudinal strain is an emerging echocardiographic approach to quantifying lv systolic function. In a recent systematic review, a relative reduction in global longitudinal strain of 10%–15% was found to be the most useful echocardiographic predictor for the later development of overt cardiotoxicity after chemotherapy exposure8.
Statistical Analysis
Data are summarized as counts and proportions, or as means with standard deviation. Linear mixed-effects models were used to analyze lvef, other echocardiographic data, and bp, and corresponding 95% confidence intervals (cis) are reported. In analyses in which continuous outcome variables are measured serially in individuals, measurements within any one individual tend to be correlated more closely with each other than with measurements in other participants. Linear mixed-effects models, in which patient identity is included as a random effect, are an accepted approach to address that within-person clustering. An interaction term for the interaction between the cardiovascular risk factor variable and the patient visit was created and was included in the model to evaluate whether the presence of a cardiovascular risk factor influenced change in lvef over the duration of the study period. Analyses were performed using the Stata software application (version 15: StataCorp LP, College Station, TX, U.S.A.).
RESULTS
Population
We identified 18 consecutive patients with her2-positive bca receiving trastuzumab who experienced a decline in lvef warranting trastuzumab discontinuation. Table I presents baseline clinicopathologic characteristics of our patient cohort at the time of the first cardio-oncology clinic visit. All patients were women with mean age of 57.4 ± 10.5 years (standard deviation). Stage of bca was primarily early-stage disease; 2 patients had metastatic disease (Table I). All patients had her2-positive disease based on immunohistochemistry or fluorescence in situ hybridization, or both. In 11 patients (61%), bca was hormone-positive, and 12 patients (67%) had left-sided bca. Radiation therapy had previously been given in 17 patients [94% (local, n = 9; locoregional, n = 7; palliative, n = 1)], and 17 patients (94%) had previously received anthracycline therapy for treatment of their bca.
A history of coronary artery disease was present in 1 patient (6%); of diabetes, in 2 patients (11%); of dyslipidemia, in 2 patients (11%); of hypertension, in 3 patients (17%); and of smoking, in 4 patients (22%: 1 current smoker, 3 former smokers). None of the patients had clinically significant valvular heart disease or prior congestive heart failure. The mean body mass index and heart rate of patients at their first visit was 28.5 ± 4.9 kg/m2 and 79 ± 13 bpm respectively. At the index cardio-oncology clinic appointment, New York Heart Association (nyha) class i symptoms were assessed in 16 patients (89%), and nyha class ii symptoms, in 2 patients (11%).
After expressing their wish to receive both ongoing trastuzumab for their bca and drug therapy for their lv dysfunction, the patients were treated with 1 or a combination of these medications: carvedilol (n = 11), bisoprolol (n = 2), and ramipril (n = 14, Table II). A statin for dyslipidemia (atorvastatin, rosuvastatin) had previously been prescribed for 2 patients. The patients were followed for a median of 280 days (range: 21–563 days) after starting cardiac therapy. At the last cardio-oncology clinic appointment, most patients remained on 1 or more cardiac medications: carvedilol (n = 12), bisoprolol (n = 1), ramipril (n = 9), perindopril (n = 1), candesartan (n = 1), irbesartan (n = 1), atorvastatin (n = 3), and rosuvastatin (n = 2).
TABLE II.
Cardiac medications initiated during trastuzumab therapy
| Medication | Use [n (%)] | ||
|---|---|---|---|
|
| |||
| Before referral | First visit | Last Visit | |
| Beta-blocker | |||
| Carvedilol | 0 | 11 (61) | 12 (67) |
| Bisoprolol | 0 | 2 (11) | 1 (6) |
|
| |||
| ACEi | |||
| Ramipril | 0 | 14 (78) | 9 (50) |
| Perindopril | 0 | 0 | 1 (6) |
|
| |||
| ARB | |||
| Candesartan | 0 | 0 | 1 (6) |
| Irbesartan | 1 (6) | 1 (6) | 1 (6) |
| Losartan | 1 (6) | 0 | 0 |
|
| |||
| Statin | |||
| Atorvastatin | 2 (11) | 2 (11) | 3 (17) |
| Rosuvastatin | 1 (6) | 1 (6) | 2 (11) |
ACEi = angiotensin converting-enzyme inhibitor; ARB = angiotensin receptor blocker.
Primary Outcome
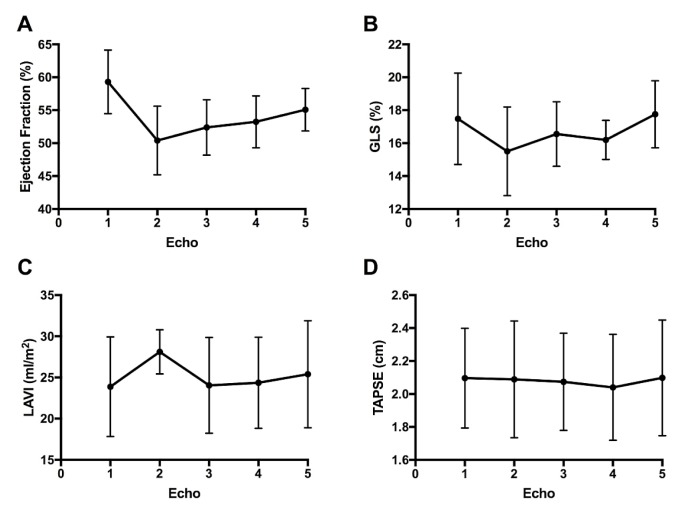
Figure 1(A) presents lvef as a function of time. Mean lvef at baseline, before trastuzumab exposure, was 59.3% ± 4.84%; during trastuzumab exposure, it was 50.4% ± 5.22% (p < 0.0001 compared with baseline); and after treatment with cardiac medications, it was 52.4% ± 4.21%, 53.2% ± 3.94%, and 55.1% ± 3.24% at 3 subsequent follow-up visits (p < 0.002 compared with baseline). The lvef observed at the final visit was significantly higher than the nadir lvef measured during trastuzumab exposure (p < 0.0001). The presence of any cardiovascular risk factor did not modify the likelihood of improvement in lvef (p > 0.2 at all time points).
FIGURE 1.
Progression of (A) left ventricular ejection fraction, (B) global longitudinal strain (GLS), (C) left atrial volume index (LAVI), and (D) tricuspid annular plane systolic excursion (TAPSE) with respect to the use of trastuzumab without and with angiotensin converting-enzyme inhibitors or beta-blockers, or both. For ease of interpretation, the presentation of GLS, a measure having negative values, here uses positive values.
Secondary Outcomes
The pattern of global longitudinal strain was similar to that for lvef [Figure 1(B)]. Global longitudinal strain worsened from baseline after trastuzumab exposure (−17.5% ± −2.78% vs. −15.5% ± −2.69%, p < 0.015). After treatment with cardiac medications, global longitudinal strain had recovered by the time that echo 5 was performed, returning to levels similar to baseline (−17.5% ± −2.78% vs. −17.8% ± −2.03%). No differences from baseline to trastuzumab exposure or to treatment with cardiac medications were observed in indexed left atrial volume or right ventricular function as measured by tricuspid annular plane systolic excursion. Figure 1(C–D) presents those echocardiographic measures as a function of time.
Patients were assessed for symptoms of heart failure at each of their cardio-oncology appointments. After the initiation of therapy with cardiac medications, 17 of the 18 patients were assessed to have symptoms consistent with nyha class i heart failure at all future visits (that is, no limitation in physical activity). Table III shows the adverse events in patients continuing trastuzumab despite lv dysfunction. No deaths were observed. During the course of trastuzumab, 1 patient was unable to continue because of the development of severe mitral regurgitation. That patient had evidence of mild mitral regurgitation at the baseline echocardiogram and subsequently developed severe mitral regurgitation with nyha class iii heart failure and acute pulmonary edema. She received inpatient heart failure therapy with improvement in her symptoms. Trastuzumab was permanently discontinued at that point.
TABLE III.
Adverse events in patients continuing trastuzumab with treatment for left ventricular dysfunction
| Event | Occurrence [n (%)] |
|---|---|
| Cardiac event | 1 (6) |
| Pulmonary edemaa | 1 |
|
| |
| Hospitalization | 2 (11) |
| Pulmonary edemaa | 1 |
| Cellulitis | 1 |
|
| |
| Death | 0 |
The cardiac event of pulmonary edema and the related hospitalization occurred in the same patient.
Overall, the cardiac medications were well-tolerated, with no serious drug-related adverse events. These minor side effects were reported: cough (n = 5), dizziness (n = 5), fatigue (n = 2), and rash (n = 1).
The median number of trastuzumab doses received by patients taking adjuvant trastuzumab was 18 (range: 4–18 doses); the 2 patients with metastatic disease received 27 and 53 doses. The number of patients who completed their planned doses of adjuvant trastuzumab was 11 of 16 (69%). Of the 5 patients who did not complete their planned doses, 1 developed mitral regurgitation, 1 did not resume trastuzumab because of shingles and fatigue, and 2 decided not to continue cardiac medications and therefore also stopped trastuzumab therapy. In 1 patient, the reason for not resuming trastuzumab was not documented. Among the patients who completed 18 planned cycles of trastuzumab, the mean proportion of cycles administered after development of lv dysfunction and under cardiology supervision was 61%. The 2 patients with metastatic disease received 19 of 27 (70%) and 15 of 53 (28%) trastuzumab doses after development of cardiotoxicity (denominators based on the number of doses administered during the study period).
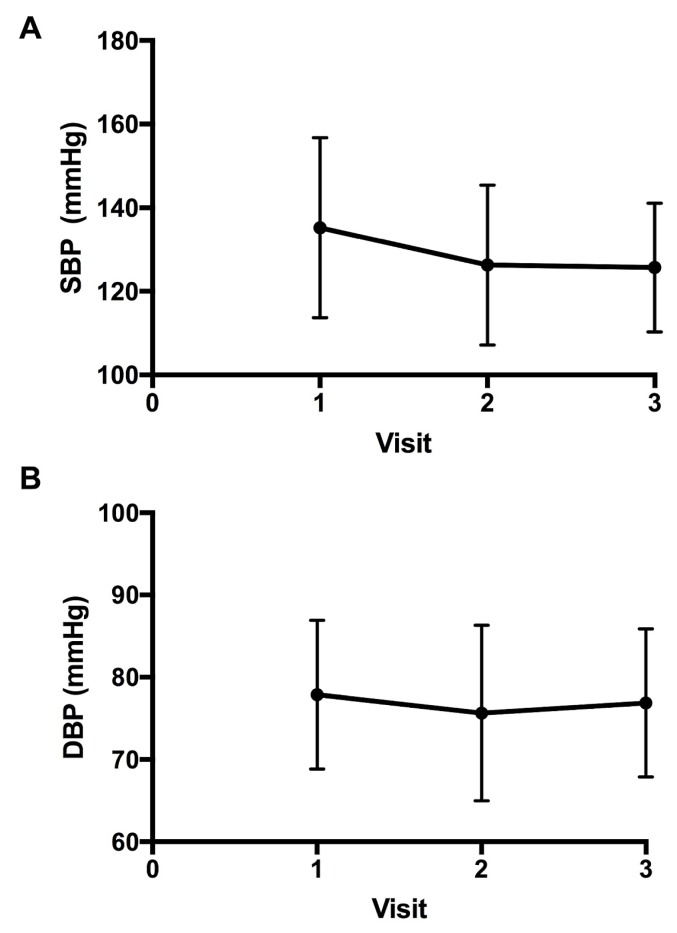
Figure 2 shows the bp for the patients over the course of the study. At the initial cardio-oncology clinic visit, mean systolic and diastolic bp readings were 135 ± 22 mmHg and 78 ± 9 mmHg respectively. After the initiation of cardiac medications, the mean systolic bp at the following 2 visits was 126 ± 19 mmHg and 126 ± 15 mmHg, and the mean diastolic bp was 76 ± 11 mmHg and 77 ± 9 mmHg. After cardiac medications were started, only the mean systolic bp was significantly reduced from the initial visit (−14 mmHg; 95% ci: −23 mmHg to −5 mmHg; p < 0.002).
FIGURE 2.
Progression of (A) systolic blood pressure (SBP) and (B) diastolic blood pressure (DBP) after initiation of cardiac medications.
DISCUSSION
In the present study, we retrospectively evaluated the outcomes of patients with bca who continued trastuzumab despite lv dysfunction, but while receiving treatment with aceis or beta-blockers, or both. Our study found that most patients with a mildly reduced ejection fraction as a consequence of trastuzumab, and minimal heart failure symptoms, were able to continue that anticancer therapy while concomitantly receiving cardiac medications, and that those patients did not experience a further decrease in lvef or experience adverse cardiac events or death.
The development of cardiotoxicity during treatment for bca presents a significant challenge that can limit therapeutic options and likely lead to poorer cancer-specific outcomes. A meta-analysis of four randomized trials showed improvements in both overall survival and disease-free survival with the use of 1 year of adjuvant trastuzumab compared with a shorter duration7. Notably, the risk of cardiotoxicity also increased with a longer duration of trastuzumab in those studies, and the odds of a cardiac event more than doubled after 1 year of trastuzumab exposure compared with a shorter treatment time7. Recently, the final analyses of two studies included in the meta-analysis, phare and persephone, were presented, drawing different conclusions about whether 6 months of trastuzumab therapy is noninferior to 12 months9,10. Although the influence of those new data on the duration of trastuzumab therapy is uncertain at the present time, managing trastuzumab cardiotoxicity with medications under the supervision of a cardiologist could prevent interruptions in cancer treatment. In our study, the reasons that patients did not complete their planned doses of adjuvant trastuzumab were largely related to patient preference. Notably, the mean percentage of adjuvant trastuzumab doses administered after development of cardiotoxicity was 61% in our study, representing a large proportion of doses that might have been withheld without cardiologist monitoring and management with cardiac medications. In the setting of advanced disease, trastuzumab is continued until unacceptable toxicity or disease progression. The 2 patients in our study with metastatic bca were able, while on cardiac medications, to safely continue trastuzumab for 19 and 15 doses, representing 70% and 28% of their doses during the study period.
The risk of trastuzumab-related cardiac dysfunction is highest in patients who have also received anthracycline therapy4,11. In our study population, 94% of patients had previously been exposed to anthracycline-based chemotherapy. However, unlike anthracycline-related cardiotoxicity, trastuzumab-related cardiotoxicity does not appear to be dose-dependent, and evidence that it is largely reversible is growing12–14. Our data suggest that treatment with aceis or beta-blockers might not only prevent further decline in lvef while the patient continues on trastuzumab, but also might restore the ejection fraction to a level close to baseline. A randomized study is needed to confirm that potential effect.
Adverse Events
Most patients in our study had nyha class i symptoms despite having a decrease in lvef warranting discontinuation of trastuzumab. That finding is consistent with evidence from the hera trial, in which 84% of discontinuations were attributable to a decrease in lvef that was either asymptomatic or mildly symptomatic3. After starting cardiac medications, all but 1 of our patients remained asymptomatic throughout the follow-up period. The exception was a patient who experienced heart failure symptoms and acute pulmonary edema requiring hospitalization. We believe that before receiving trastuzumab, this patient’s mitral valve exhibited little redundancy, and lv dilatation after administration of an anthracycline and trastuzumab precipitated mitral regurgitation by preventing mitral valve coaptation in that vulnerable valve. That experience suggests that structural abnormalities of the heart should be taken into consideration when adopting a plan of continuing trastuzumab in the setting of cardiac lv dysfunction.
Echocardiographic Measures
Global longitudinal strain has been shown to be a predictor of subsequent cardiotoxicity after treatment with chemotherapy8. In our patients, a reduction in global longitudinal strain was evident after trastuzumab exposure, with a return to baseline levels after treatment with cardiac medications. Left atrial volume is often called the “HbA1c of the heart,” because it reflects the severity and chronicity of lv dysfunction15. No differences in indexed left atrial volume occurred throughout the study. That lack of change might be explained by the mild degree of lv dysfunction seen in our patients, which might not have caused sufficient elevation in lv filling pressure to result in left atrial dilatation. That finding is consistent with the absence of significant heart failure symptoms in all but 1 of our patients. Tricuspid annular plane systolic excursion, one of the measurements of right ventricular systolic function, was also unchanged throughout the study, suggesting that, compared with lv dysfunction, right ventricular dysfunction might be observed at a more advanced stage of cardiotoxicity. Some reports suggest that right ventricular dysfunction can occur with chemotherapy including trastuzumab16,17; however, such dysfunction was not observed in our cohort.
Cardiac Medications
In individuals with heart failure and reduced ejection fraction, aceis and beta-blockers have been shown to improve morbidity and mortality, including heart failure attributable to non-ischemic cardiomyopathy18. A small number of studies have examined whether prophylactic administration of an acei or beta-blocker can prevent trastuzumab-related lv dysfunction in patients with normal lvef. The resulting evidence was summarized in a recent systematic review of eight studies, which showed that prophylactic beta-blockers, but not aceis, attenuated development of lv dysfunction and heart failure in patients receiving anthracycline chemotherapy with or without trastuzumab19. Notably, the event rates and sample sizes in those studies of healthy patients who had not yet developed ventricular dysfunction were low, and the power of the analysis might have been insufficient to detect the effect of aceis on those outcomes. A previous meta-analysis suggested that aceis and beta-blockers both attenuated lv dysfunction in patients treated with anthracyclines; however, the analysis was performed by combining the data for both agents, and it was not possible to conclude whether one of those classes of medications was more beneficial than the other20. Our study focused on agents that have been endorsed for the general treatment of heart failure (aceis, beta-blockers); in contrast, there is little evidence for the routine use of statins to treat heart failure in the absence of coronary artery disease18. Whether statins offer any additional benefit in this patient population remains to be studied.
In our study, treatment with aceis or beta-blockers, or both, seemed to ameliorate trastuzumab-induced lv dysfunction; however, the optimal duration of those medications in patients with bca who have experienced lvef decline is unclear. Based on population data, trastuzumab therapy is associated with an increased incidence of heart failure during treatment, but not thereafter21. The patients in our study had few cardiovascular risk factors, and therefore most did not have alternative indications to remain on cardiac medications. Future studies examining long-term cardiovascular outcomes in this patient group are necessary to determine whether the heart medications should be continued after completion of trastuzumab.
Study Limitations
Our study is limited by its retrospective design and inclusion of a limited number of patients from a single centre. Ethics considerations did not permit the inclusion of a control group that continued trastuzumab despite lv dysfunction without any intervention. Additionally, the total amount of trastuzumab therapy received within our study population differed because of previous disruptions attributable to lv dysfunction and cancer stage. The follow-up was not standardized; some patients had longer follow-up in the cardio-oncology clinic.
Future Directions
The reversibility of lv dysfunction with cardiac medications seen in our study, together with the number of patients who remained asymptomatic, suggests that discontinuing adjuvant trastuzumab therapy based on current guideline thresholds might be premature. However, prospective studies with larger samples are needed to evaluate the risk–benefit ratio of ongoing trastuzumab in patients with lv dysfunction. Our results have informed the design of a prospective clinical study that is currently underway to determine the safety and tolerability of trastuzumab therapy for bca in patients with mild or moderate heart failure or lv dysfunction. If trastuzumab continuation is shown to be safe, our research might have practice-changing implications that could increase the number of patients receiving full-course adjuvant trastuzumab to maximum anticancer effect.
CONCLUSIONS
Our study suggests that continuing trastuzumab in patients with cardiotoxicity, while treating adverse cardiac effects with aceis or beta-blockers under the supervision of a cardiologist, might be a safe approach in selected patients so that they can continue adjuvant trastuzumab therapy. That approach warrants further investigation in clinical studies.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 2.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the Herceptin adjuvant trial. J Clin Oncol. 2007;25:3859–65. doi: 10.1200/JCO.2006.09.1611. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. on behalf of the Breast Cancer International Research Group. Adjuvant trastuzumab in her2-positive breast cancer. N Engl J Med. 2011;365:1273–83. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. on behalf of the esc Scientific Document Group. 2016 esc position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the esc Committee for Practice Guidelines: the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (esc) Eur Heart J. 2016;37:2768–801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 6.Mackey JR, Clemons M, Côté MA, et al. Cardiac management during adjuvant trastuzumab therapy: recommendations of the Canadian Trastuzumab Working Group. Curr Oncol. 2008;15:24–35. doi: 10.3747/co.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gyawali B, Niraula S. Duration of adjuvant trastuzumab in her2 positive breast cancer: overall and disease free survival results from meta-analyses of randomized controlled trials. Cancer Treat Rev. 2017;60:18–23. doi: 10.1016/j.ctrv.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–68. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 9.Earl HM, Hiller L, Vallier AL, et al. on behalf of the persephone Steering Committee and Trial Investigators. 6 Versus 12 months of adjuvant trastuzumab for her2-positive early breast cancer (persephone): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–612. doi: 10.1016/S0140-6736(19)30650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pivot X, Romieu G, Debled M, et al. phare randomized trial final results comparing 6 to 12 months of trastuzumab in adjuvant early breast cancer [abstract GS2-07] Cancer Res. 2019;79 [Available online at: http://cancerres.aacrjournals.org/content/79/4_Supplement/GS2-07; cited 3 July 2019] [Google Scholar]
- 11.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 12.Ewer MS, Vooletich MT, Durand JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23:7820–6. doi: 10.1200/JCO.2005.13.300. [DOI] [PubMed] [Google Scholar]
- 13.Ewer MS, Lippman SM. Type ii chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900–2. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 14.Saidi A, Alharethi R. Management of chemotherapy induced cardiomyopathy. Curr Cardiol Rev. 2011;7:245–9. doi: 10.2174/157340311799960681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas PS. The left atrium: a biomarker of chronic diastolic dysfunction and cardiovascular disease risk. J Am Coll Cardiol. 2003;42:1206–7. doi: 10.1016/S0735-1097(03)00956-2. [DOI] [PubMed] [Google Scholar]
- 16.Grover S, Leong DP, Chakrabarty A, et al. Left and right ventricular effects of anthracycline and trastuzumab chemotherapy: a prospective study using novel cardiac imaging and biochemical markers. Int J Cardiol. 2013;168:5465–7. doi: 10.1016/j.ijcard.2013.07.246. [DOI] [PubMed] [Google Scholar]
- 17.Calleja A, Poulin F, Khorolsky C, et al. Right ventricular dysfunction in patients experiencing cardiotoxicity during breast cancer therapy. J Oncol. 2015;2015 doi: 10.1155/2015/609194. 609194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, et al. 2017 acc/aha/hfsa focused update of the 2013 accf/aha guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Gujral DM, Lloyd G, Bhattacharyya S. Effect of prophylactic betablocker or ace inhibitor on cardiac dysfunction & heart failure during anthracycline chemotherapy ± trastuzumab. Breast. 2018;37:64–71. doi: 10.1016/j.breast.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Kalam K, Marwick TH. Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2900–9. doi: 10.1016/j.ejca.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 21.Goldhar HA, Yan AT, Ko DT, et al. The temporal risk of heart failure associated with adjuvant trastuzumab in breast cancer patients: a population study. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djv301. pii:djv301. [DOI] [PubMed] [Google Scholar]