Abstract
Background
The choice of mastectomy compared with breast-conservation therapy (bct) in early-stage breast cancer (esbca) is a complicated decision-making process. Interprovincially, Canada’s mastectomy rates vary from 25% to 68%, with Saskatchewan reporting the nation’s second-highest mastectomy rate at 63%. The aim of our research was to better understand why women with esbca choose mastectomy rather than bct in Saskatchewan.
Methods
We created a survey based on a previously developed framework that organizes influencing factors into 3 constructs: clinicopathologic, physician, and individual belief factors.
Results
Treatment choice was found to be influenced by disease stage and multiple individual belief factors. Compared with their counterparts having stage i disease, women with stage ii disease were significantly more likely to undergo mastectomy [odds ratio (or): 7.48]. Patients rating “worry about cancer recurrence” and “total treatment time” as more influential in their choice were also more likely to undergo mastectomy (or: 3.4 and 1.8 respectively). Conversely, women rating “wanting to keep own breast tissue,” “tumour size,” and “surgeon’s opinion” as influential in their choice were more likely to undergo bct (or: 0.17, 0.66, and 0.69 respectively).
Conclusions
Our study demonstrates that treatment choices for Saskatchewan women with esbca are influenced primarily by disease stage and individual belief factors. Those findings suggest that women are making their treatment choices predominantly based on individual values and preferences. The use of rates of mastectomy and bct as indicators of quality of care might be misleading. Instead, a shift in attention toward patient-centred care might be more appropriate.
Keywords: Breast cancer, early-stage; mastectomy; breast-conservation therapy; decision-making; shared decision-making; patient-centred care
INTRODUCTION
Research Problem
Breast cancer (bca) is the most commonly diagnosed cancer in North America and the 2nd most common cause of cancer death in women1. In early-stage bca (esbca), it is well established that breast-conservation therapy (bct) and mastectomy are equivalent treatments for survival2–5. Treatment for esbca can therefore be viewed as preference-sensitive care, in which choosing between the treatment options should vary according to patient preferences6. Interprovincially, Canadian mastectomy rates vary greatly, ranging from 25% to 68%, with a national average of 38%7. Saskatchewan has consistently reported the nation’s 2nd highest mastectomy rate, with the most recent report showing a rate of 63%8,9. International research has been investigating why women choose mastectomy over bct10–15, but few Canadian data have been published to explain the interprovincial variations8.
Efficacy equivalency between mastectomy and bct for esbca suggests that the choice for treatment should be made in accordance with underlying patient values and preferences; however, mastectomy rates have repeatedly been used as performance and quality indicators16–19. The latter approach stems from a U.S. National Institute of Health Consensus Conference in 1999, which recommended bct as “preferable” to mastectomy because it was thought to be less invasive and cosmetically superior20. Researchers have frequently cited “underuse” of bct21 and even called bct the “standard of care” in some studies22. Viewing procedural variation through a patient-centred care lens as opposed to a standard-of-care assessment allows researchers to explore existing variations with curiosity rather than judgment. Research aimed at understanding what drives decision-making by patients will not only help guide how to evaluate quality of care in the setting of esbca, but also to identify areas of focus for quality improvement. The aim of our research was to better understand why women choose mastectomy over bct in Saskatchewan.
Canadian Statistics
In October 2012, the Canadian Institute for Health Information and the Canadian Partnership Against Cancer published a report taking a pan-Canadian perspective on patterns in surgical care8. The study cohort covered a 3-year period from 2007–2008 to 2009–2010. During that time, 65,067 women, roughly 22,000 per year, were treated surgically for bca. In that group of women, mastectomy rates varied significantly across the country. Nationally, the mastectomy rate for invasive bca was 39%—a rate that varied from 26% in Quebec to 69% in Newfoundland and Labrador. Saskatchewan had the 2nd-highest reported rate of mastectomy in Canada, with a crude rate of 65% (1094 of 1686 cases).
Data were available for a few other important factors that are expected to influence mastectomy rates. Those factors are age group, neighbourhood income quintile, and travel time to the closest cancer centre, which were included in the logistic regression model to provide adjusted mastectomy rates. However, even with incorporation of those factors to produce the adjusted rates, the discrepancies in provincial rates were only marginally reduced, with a 26 percentage point absolute difference remaining between the highest and lowest mastectomy rates (35%–61%). And Saskatchewan remained 2nd highest, with an adjusted mastectomy rate of 60%. The Canadian mastectomy rate changed to 44% from 39%8.
The report concluded that uncertainty remains about how women are exercising their choice of treatment options and recommended an examination of clinical practices by provinces to better understand the variations.
In 2015, the Canadian Partnership Against Cancer published updated data for multiple cancer system performance indicators, including a Canadian bca update9. The report considered women with unilateral invasive bca who underwent surgery between April 2008 and March 2013. Although there was some overlap of data from the previous report, it was clear that little change in treatment patterns had occurred across the country. Mastectomy rates ranged from 25.3% in Quebec to 68.3% in Newfoundland and Labrador9. Canada’s final mastectomy rate was 38.1%, compared with 39% in the previous report. In Saskatchewan, the final mastectomy rate for invasive cancer changed to 63.4% from 65% between the two reports8,9.
METHODS
Approval for the study was obtained from the University of Saskatchewan Behavioural Research Ethics Board.
Survey Conceptual Development and Design
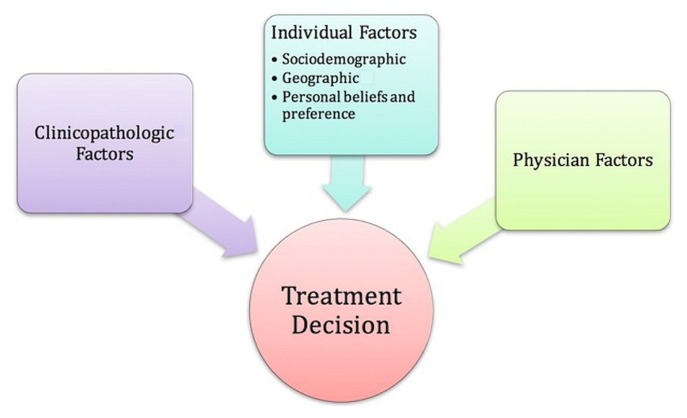
This survey is the 2nd stage of a sequential mixed-methods study23. The survey was constructed based on a previously developed conceptual framework (Figure 1). That framework organizes influencing factors potentially affecting a woman’s choice between mastectomy and bct into 3 central constructs: clinicopathologic factors, physician factors, and individual factors, with subgroups of sociodemographic, geographic, and personal belief factors. The framework provided a logical guide for organizing our inquiry while keeping the individual patient decision as the central focus.
FIGURE 1.
Conceptual framework illustrating the central constructs influencing a woman’s choice between mastectomy and breast-conserving therapy. Figure reproduced with permission from the rights owner (Gu and Groot, 201824).
The survey consisted of an online questionnaire linked with a clinicopathologic dataset from the Saskatchewan Cancer Agency (sca). For individual factors, all participants were asked to rate a series of 14 personal belief questions on a 5-point Likert scale. Five additional factors were mastectomy participant–specific, and two were bct-specific. For the physician factors, participants were asked to use a 5-point scale to reflect on their involvement in the treatment decision-making process, from completely the individual’s choice to completely the physician’s choice, with a shared decision in the middle. A detailed explanation of the survey design and the full survey can be viewed in supplementary Appendixes 1 and 2.
The questionnaire was designed in accordance with best-practice recommendations from Krosnick and Presser25. The survey underwent multiple iterations of pretesting with the research team, breast surgeons, an oncologist, and survey methodology experts. The survey was also piloted in collaboration with the local Saskatoon Breast Health Centre. Participants in that external participatory pilot survey (n = 40) were not included in the main survey. Respondents were informed that the survey was in a pilot phase, and they were asked for feedback at the end of the survey. The inclusion criteria for pilot participants were the same as for the formal survey.
Study Participants
Our study population included all Saskatchewan women diagnosed with and treated for esbca during 2014–2015. The definition of esbca was a diagnosis of stage i or ii cancer (per the 7th edition of the American Joint Committee on Cancer staging handbook)26. Patients with ductal carcinoma in situ, known BRCA mutation, stage iii or iv bca, male bca, and inflammatory bca were excluded.
Participants were recruited in collaboration with the Saskatchewan Cancer Agency (sca). Through the sca database, all patients treated for esbca in Saskatchewan during an inclusive 2-year period from January 2014 to December 2015 were identified. In December 2017, patients were mailed an invitation-to-participate letter from the sca; they also received a single reminder invitation 8 weeks later. Data collection ended on 30 April 2018. No incentive-based recruitment strategies were used.
The questionnaire was hosted online through Voxco [Montreal, QC (https://www.voxco.com)]. Participants were encouraged to complete the survey online in a self-administered fashion. Alternatively, participants could call the researcher and have the questionnaire administered in a telephone interview.
Data Collection and Management
Clinicopathologic data including tumour location, pathology, and staging were obtained from the sca database and linked to the questionnaire data using a unique 8-character alphanumerical identifier that patients also used to access the survey online.
Data Analysis
The statistical analysis was performed using the Stata software application (version 14.2: StataCorp LLC, College Station, TX, U.S.A.). Our primary outcome was odds of mastectomy compared with bct. Independent variables included clinicopathologic factors, individual patient factors, and physician factors. Baseline differences between groups were evaluated using the chi-square test for categorical data and the independent samples t-test for continuous data. Univariate logistic regression was applied to identify key predictors of the choice for mastectomy over bct. Multivariable logistic regression analysis was used to create the main effects model. Variables with a p value less than 0.10 in the univariate logistic regression were included in the multivariable model, and backwards elimination of variables with a p values less than 0.05 was then performed. Using an interaction term, clinically important effect modifiers were individually tested for potential inclusion in the model.
Goodness of fit was assessed using the Akaike information criterion. To reduce bias with respect to missing data, we used complete case analysis for fewer than 10% missing data points and the indicator method for more than 10% missing data points. Geocoding and mapping were performed in conjunction with the Spatial Division of the University of Saskatchewan Social Sciences Research Laboratories. Geocoding was performed by joining spatial information (latitude and longitude) to patient homes based on the first 3 characters of the postal code provided. A Web gis (geographic information system) solution developed by the local team was used to visualize routes and generate geographic information system files relating to patient home locations, hospital locations, and travel routes.
RESULTS
Of 1056 invitees, 276 (26.1%) completed the survey—150 who had undergone mastectomy (54.3%), and 126 who had undergone bct (45.7%). Of the 276 participants, 25 (9%) completed the questionnaire over the telephone.
Cohort Characteristics
Table I presents the cohort characteristics. Most participants were white (96.0%), and the mean age in both subgroups was 59 years. The only significant difference in sociodemographic and geographic characteristics between the women undergoing bct and those undergoing mastectomy was annual household income, with a larger proportion of the bct subgroup being in the middle-income bracket of $40,000–$100,000. In the mastectomy subgroup, larger proportions of the participants fell into the lower and higher income strata.
TABLE I.
Participant characteristics
| Characteristic | Participant groupa | p Valueb | |
|---|---|---|---|
|
| |||
| Mastectomy | BCT | ||
| Participants (n) | 150 | 126 | |
|
| |||
| Mean age (years) | 58.9 | 59.6 | 0.64c |
|
| |||
| Age group [n (%)] | 0.15 | ||
| <50 Years | 36 (24.0) | 27 (13.5) | |
| 50–65 Years | 62 (41.3) | 64 (50.8) | |
| 65–80 Years | 44 (29.3) | 39 (31.0) | |
| >80 Years | 8 (5.3) | 6 (4.8) | |
|
| |||
| Ethnicity [n (%)] | 0.08 | ||
| White | 145 (96.7) | 119 (94.4) | |
| First Nations, Métis, Inuit | 0 (0.0) | 4 (3.2) | |
| Other | 5 (3.3) | 3 (2.4) | |
|
| |||
| Annual household income [n (%)] | <0.01 | ||
| <$40,000 | 29 (27.4) | 13 (14.6) | |
| $40,000–$100,000 | 41 (38.8) | 56 (62.9) | |
| ≥$100,000 | 36 (34.0) | 20 (21.5) | |
|
| |||
| Employment status [n (%)] | 0.95 | ||
| Employed | 65 (43.6) | 55 (44.0) | |
| Not employed | 84 (56.4) | 70 (56.0) | |
|
| |||
| Highest level of education [n (%)] | 0.26 | ||
| Less than high school | 10 (6.7) | 8 (6.4) | |
| Completed high school | 31 (20.7 | 28 (22.2) | |
| Some technical or community college | 14 (9.3) | 11 (8.7) | |
| Completed college | 46 (30.7) | 24 (19.1) | |
| Some university | 14 (9.3) | 20 (15.9) | |
| Bachelor degree | 24 (16.0) | 18 (14.3) | |
| Master’s degree | 5 (3.3) | 7 (5.6) | |
| Professional degree or doctorate | 6 (4.0) | 10 (7.9) | |
|
| |||
| Smoking status [n (%)] | 0.94 | ||
| Never-smoker | 72 (48.0) | 62 (49.2) | |
| Ex-smoker | 66 (44.0) | 53 (42.1) | |
| Current smoker | 12 (8.0) | 11 (8.7) | |
|
| |||
| Relationship status [n (%)] | 0.69 | ||
| In a relationship | 120 (80.5) | 103 (82.4) | |
| Not in a relationship | 29 (19.5) | 22 (17.6) | |
|
| |||
| Children [n (%)] | 0.90 | ||
| Yes | 120 (86.0) | 21 (14.0) | |
| No | 109 (86.5) | 17 (13.5) | |
|
| |||
| Menopausal status [n (%)] | 0.52 | ||
| Pre-menopause | 47 (31.3) | 103 (68.7) | |
| Post-menopause | 35 (27.8) | 91 (72.2) | |
|
| |||
| Breast size [n (%)] | 0.12 | ||
| ≤A | 15 (10.0) | 3 (2.4) | |
| B | 41 (27.3) | 33 (26.2) | |
| C | 38 (25.3) | 35 (27.8) | |
| D | 45 (30.0) | 40 (31.2) | |
| E | 5 (3.3) | 4 (32.0) | |
| ≥F | 6 (4.0) | 11 (8.7) | |
|
| |||
| Residence [n (%)] | 0.34 | ||
| Urban | 85 (56.7) | 78 (62.4) | |
| Rural | 65 (43.4) | 47 (37.6) | |
|
| |||
| City of surgery [n (%)] | 0.43 | ||
| Saskatoon | 79 (52.7) | 62 (49.2) | |
| Regina | 60 (40.0) | 49 (38.9) | |
| Other | 11 (7.3) | 15 (11.9) | |
|
| |||
| Distance from surgical centre (km) | 107.4 | 101.3 | 0.79c |
Denominators for percentages vary depending on response rate.
By Pearson chi-square test unless otherwise specified. Boldface type denotes significance.
By 2-tailed t-test.
BCT = breast-conservation therapy
With respect to clinicopathologic factors (Table II), the mastectomy and bct subgroups showed no differences in terms of tumour grade, tumour type, tumour multiplicity, tumour side, tumour location, or tumour receptor status. Significant differences between the mastectomy and bct subgroups were evident in overall American Joint Committee on Cancer staging, tumour stage, mean tumour size, and nodal stage, with the mastectomy group generally having tumours of more advanced stage and larger size.
TABLE II.
Clinicopathologic characteristics
| Characteristic | Participant groupa | p Valueb | |
|---|---|---|---|
|
| |||
| Mastectomy | BCT | ||
| AJCC stage [n (%)] | <0.01 | ||
| I | 69 (46.0) | 85 (67.5) | |
| II | 81 (54.0) | 41 (32.5) | |
|
| |||
| T Stage [n (%)] | <0.01 | ||
| T1mic | 6 (4.0) | 2 (1.6) | |
| T1A | 6 (4.0) | 3 (2.4) | |
| T1B | 24 (16.0) | 35 (27.8) | |
| T1C | 46 (30.7) | 55 (43.7) | |
| T2 | 65 (43.3) | 30 (23.8) | |
| T3 | 3 (2.0) | 0 (0) | |
|
| |||
| Mean tumour size (cm) | 2.0 | 1.6 | <0.01c |
|
| |||
| N Stage [n (%)] | 0.036 | ||
| N0 | 105 (70.0) | 102 (80.1) | |
| N1 | 45 (45.0) | 24 (19.0) | |
|
| |||
| ER assay [n (%)] | 0.46 | ||
| Positive | 134 (89.3) | 114 (90.5) | |
| Negative | 16 (10.7) | 10 (7.9) | |
|
| |||
| PgR assay [n (%)] | 0.27 | ||
| Positive | 122 (81.3) | 107 (84.9) | |
| Negative | 28 (18.7) | 17 (13.5) | |
|
| |||
| HER2 assay [n (%)] | 0.32 | ||
| Positive | 13 (25.0) | 5 (12.8) | |
| Negative | 37 (71.2) | 33 (84.6) | |
| Borderline | 2 (3.8) | 1 (2.6) | |
|
| |||
| Combination of receptor assaysd [n (%)] | 0.07 | ||
| Not favourable | 30 (20.8) | 15 (12.4) | |
| Favourable | 114 (79.2) | 106 (87.6) | |
|
| |||
| Tumour grade [n (%)] | 0.07 | ||
| 1 | 31 (22.0) | 34 (27.8) | |
| 2 | 62 (44.0) | 62 (50.8) | |
| 3 | 48 (34.0) | 26 (21.3) | |
|
| |||
| Tumour description [n (%)] | 0.42 | ||
| Infiltrating ductal | 105 (70.0) | 90 (71.4) | |
| Infiltrating lobular | 20 (13.3) | 11 (8.7) | |
| Ductal and lobular | 6 (4.0) | 3 (2.4) | |
| Mucinous adenocarcinoma | 6 (4.0) | 4 (3.2) | |
| Other | 13 (8.7) | 18 (14.3) | |
|
| |||
| Tumour multiplicity counter [n (%)] | 0.34 | ||
| 01 | 110 (79.7) | 110 (87.3) | |
| 02 | 16 (11.6) | 9 (7.1) | |
| 03 | 6 (4.4) | 3 (2.4) | |
| ≥04 | 6 (4.4) | 3 (2.4) | |
|
| |||
| Tumour side [n (%)] | 0.24 | ||
| Left | 81 (54.0) | 59 (46.8) | |
| Right | 69 (46.0) | 67 (53.2) | |
|
| |||
| Tumour location [n (%)] | 0.02 | ||
| Breast othere | 112 (74.7) | 77 (61.1) | |
| Upper outer | 38 (25.3) | 48 (38.9) | |
|
| |||
| Preoperative MRI [n (%)] | 0.05 | ||
| Yes | 90 (60.0) | 61 (48.4) | |
| No | 60 (40.0) | 65 (51.6) | |
Denominators vary depending on response rate.
By Pearson chi-square test unless otherwise specified. Boldface type denotes significance.
By 2-tailed t-test.
“Not favourable” combinations: ER−, PgR−, HER2−; ER−, PgR−, HER2+; ER−, PgR+, HER2+; ER+, PgR−, HER2+; and ER+, PgR+, HER2+. “Favourable” combinations: ER+, PgR+, HER2−; ER−, PgR+, HER2−; and ER+, PgR+, HER2−.
Breast not otherwise specified, central, lower inner, lower outer, overlap, and upper inner.
BCT = breast-conservation therapy; AJCC = American Joint Committee on Cancer; ER = estrogen receptor; PgR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; MRI = magnetic resonance imaging.
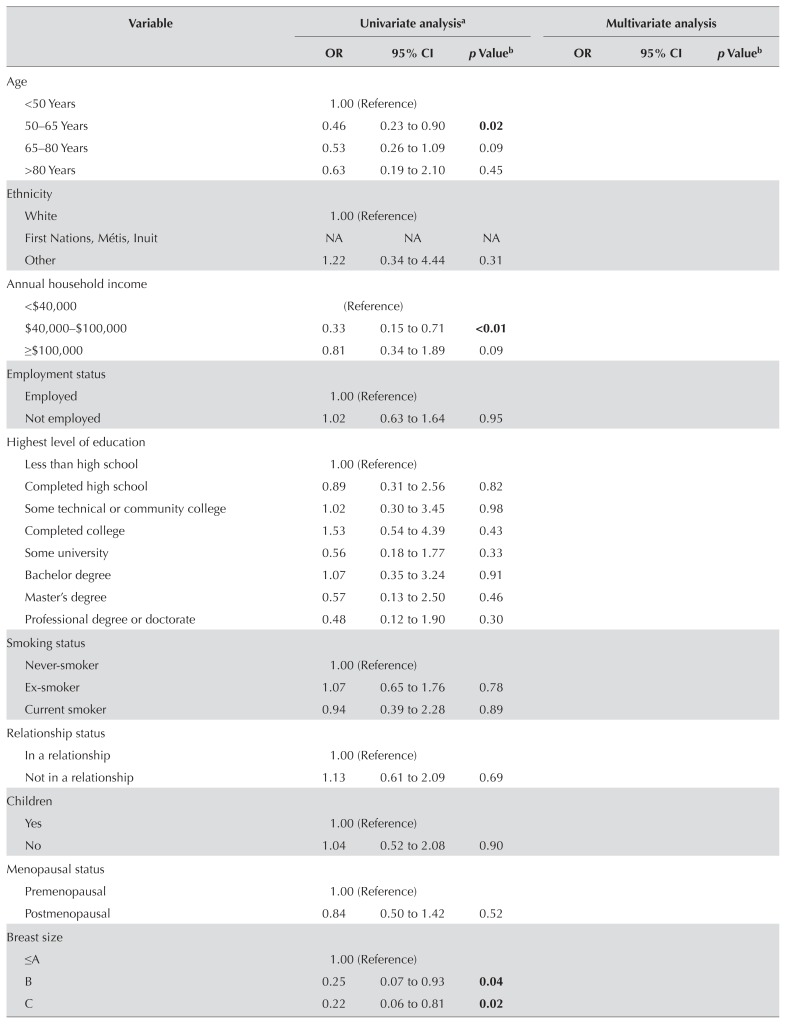
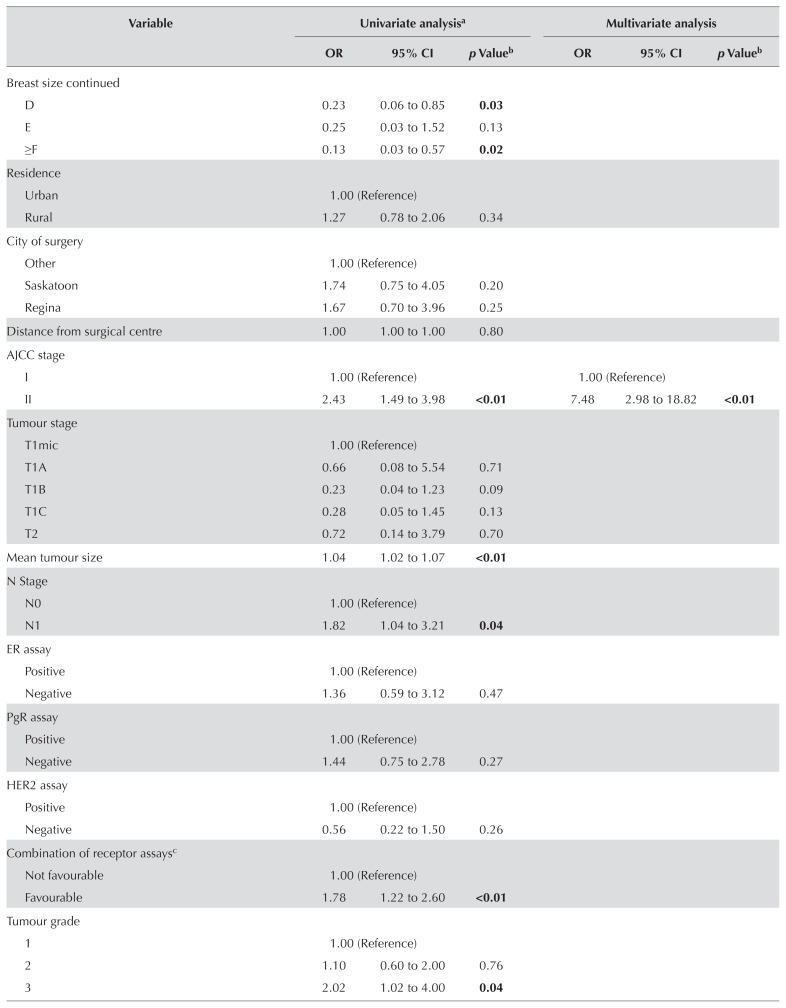
Predictors of BCT Compared with Mastectomy
Table III presents results of the univariate analysis and the multivariate logistic regression. In the final model, 6 factors were significant influencers in the therapy choice by patients, with stage of disease being the only clinicopathologic factor. The significant factors were individual belief and preference factors: worry about cancer recurrence, subjective tumour size, surgeon’s opinion, and wanting to keep breast tissue. Two interactions, stage of disease with surgeon’s opinion and stage of disease with subjective tumour size were also significant. The subsections that follow provide detailed explanations of the results for each construct from the conceptual framework.
TABLE III.
Univariate and multivariate logistic regression
| Variable | Univariate analysisa | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95% CI | p Valueb | OR | 95% CI p | Valueb | |
| Age | ||||||
| <50 Years | 1.00 (Reference) | |||||
| 50–65 Years | 0.46 | 0.23 to 0.90 | 0.02 | |||
| 65–80 Years | 0.53 | 0.26 to 1.09 | 0.09 | |||
| >80 Years | 0.63 | 0.19 to 2.10 | 0.45 | |||
|
| ||||||
| Ethnicity | ||||||
| White | 1.00 (Reference) | |||||
| First Nations, Métis, Inuit | NA | NA | NA | |||
| Other | 1.22 | 0.34 to 4.44 | 0.31 | |||
|
| ||||||
| Annual household income | ||||||
| <$40,000 | (Reference) | |||||
| $40,000–$100,000 | 0.33 | 0.15 to 0.71 | <0.01 | |||
| ≥$100,000 | 0.81 | 0.34 to 1.89 | 0.09 | |||
|
| ||||||
| Employment status | ||||||
| Employed | 1.00 (Reference) | |||||
| Not employed | 1.02 | 0.63 to 1.64 | 0.95 | |||
|
| ||||||
| Highest level of education | ||||||
| Less than high school | 1.00 (Reference) | |||||
| Completed high school | 0.89 | 0.31 to 2.56 | 0.82 | |||
| Some technical or community college | 1.02 | 0.30 to 3.45 | 0.98 | |||
| Completed college | 1.53 | 0.54 to 4.39 | 0.43 | |||
| Some university | 0.56 | 0.18 to 1.77 | 0.33 | |||
| Bachelor degree | 1.07 | 0.35 to 3.24 | 0.91 | |||
| Master’s degree | 0.57 | 0.13 to 2.50 | 0.46 | |||
| Professional degree or doctorate | 0.48 | 0.12 to 1.90 | 0.30 | |||
|
| ||||||
| Smoking status | ||||||
| Never-smoker | 1.00 (Reference) | |||||
| Ex-smoker | 1.07 | 0.65 to 1.76 | 0.78 | |||
| Current smoker | 0.94 | 0.39 to 2.28 | 0.89 | |||
|
| ||||||
| Relationship status | ||||||
| In a relationship | 1.00 (Reference) | |||||
| Not in a relationship | 1.13 | 0.61 to 2.09 | 0.69 | |||
|
| ||||||
| Children | ||||||
| Yes | 1.00 (Reference) | |||||
| No | 1.04 | 0.52 to 2.08 | 0.90 | |||
|
| ||||||
| Menopausal status | ||||||
| Premenopausal | 1.00 (Reference) | |||||
| Postmenopausal | 0.84 | 0.50 to 1.42 | 0.52 | |||
|
| ||||||
| Breast size | ||||||
| ≤A | 1.00 (Reference) | |||||
| B | 0.25 | 0.07 to 0.93 | 0.04 | |||
| C | 0.22 | 0.06 to 0.81 | 0.02 | |||
|
| ||||||
| Breast size continued | ||||||
| D | 0.23 | 0.06 to 0.85 | 0.03 | |||
| E | 0.25 | 0.03 to 1.52 | 0.13 | |||
| ≥F | 0.13 | 0.03 to 0.57 | 0.02 | |||
|
| ||||||
| Residence | ||||||
| Urban | 1.00 (Reference) | |||||
| Rural | 1.27 | 0.78 to 2.06 | 0.34 | |||
|
| ||||||
| City of surgery | ||||||
| Other | 1.00 (Reference) | |||||
| Saskatoon | 1.74 | 0.75 to 4.05 | 0.20 | |||
| Regina | 1.67 | 0.70 to 3.96 | 0.25 | |||
|
| ||||||
| Distance from surgical centre | 1.00 | 1.00 to 1.00 | 0.80 | |||
|
| ||||||
| AJCC stage | ||||||
| I | 1.00 (Reference) | 1.00 (Reference) | ||||
| II | 2.43 | 1.49 to 3.98 | <0.01 | 7.48 | 2.98 to 18.82 | <0.01 |
|
| ||||||
| Tumour stage | ||||||
| T1mic | 1.00 (Reference) | |||||
| T1A | 0.66 | 0.08 to 5.54 | 0.71 | |||
| T1B | 0.23 | 0.04 to 1.23 | 0.09 | |||
| T1C | 0.28 | 0.05 to 1.45 | 0.13 | |||
| T2 | 0.72 | 0.14 to 3.79 | 0.70 | |||
|
| ||||||
| Mean tumour size | 1.04 | 1.02 to 1.07 | <0.01 | |||
|
| ||||||
| N Stage | ||||||
| N0 | 1.00 (Reference) | |||||
| N1 | 1.82 | 1.04 to 3.21 | 0.04 | |||
|
| ||||||
| ER assay | ||||||
| Positive | 1.00 (Reference) | |||||
| Negative | 1.36 | 0.59 to 3.12 | 0.47 | |||
|
| ||||||
| PgR assay | ||||||
| Positive | 1.00 (Reference) | |||||
| Negative | 1.44 | 0.75 to 2.78 | 0.27 | |||
|
| ||||||
| HER2 assay | ||||||
| Positive | 1.00 (Reference) | |||||
| Negative | 0.56 | 0.22 to 1.50 | 0.26 | |||
|
| ||||||
| Combination of receptor assaysc | ||||||
| Not favourable | 1.00 (Reference) | |||||
| Favourable | 1.78 | 1.22 to 2.60 | <0.01 | |||
|
| ||||||
| Tumour grade | ||||||
| 1 | 1.00 (Reference) | |||||
| 2 | 1.10 | 0.60 to 2.00 | 0.76 | |||
| 3 | 2.02 | 1.02 to 4.00 | 0.04 | |||
|
| ||||||
| Tumour description | ||||||
| Ductal and lobular | 1.00 (Reference) | |||||
| Infiltrating lobular | 0.75 | 0.11 to 4.90 | 0.76 | |||
| Infiltrating ductal | 0.91 | 0.19 to 4.37 | 0.91 | |||
| Mucinous adenocarcinoma | 0.58 | 0.14 to 2.40 | 0.46 | |||
| Other | 0.26 | 0.07 to 1.72 | 0.20 | |||
|
| ||||||
| Tumour multiplicity counter | ||||||
| 01 | 1.00 (Reference) | |||||
| 02 | 1.78 | 0.75 to 4.19 | 0.19 | |||
| 03 | 2.00 | 0.48 to 8.20 | 0.96 | |||
| ≥04 | 2.00 | 0.48 to 8.20 | 0.96 | |||
|
| ||||||
| Tumour side | ||||||
| Left | 1.00 (Reference) | |||||
| Right | 0.75 | 0.47 to 1.21 | 0.24 | |||
|
| ||||||
| Tumour location | ||||||
| Breast otherd | 1.00 (Reference) | |||||
| Upper outer | 0.53 | 0.32 to 0.89 | 0.02 | |||
|
| ||||||
| Preoperative MRI | ||||||
| Yes | 1.00 (Reference) | |||||
| No | 0.3 | 0.39 to 1.01 | 0.06 | |||
|
| ||||||
| Worry about cancer recurrence | 2.75 | 2.12 to 3.33 | <0.01 | 3.44 | 2.32 to 5.11 | <0.01 |
|
| ||||||
| Age | 1.20 | 0.99 to 1.46 | 0.06 | |||
|
| ||||||
| Other individual medical history | 1.14 | 0.94 to 1.37 | 0.19 | |||
|
| ||||||
| Family history of breast cancer | 1.34 | 1.10 to 1.64 | <0.01 | |||
|
| ||||||
| Previous breast disease | 1.16 | 0.94 to 1.44 | 0.17 | |||
|
| ||||||
| Breast size | 1.00 | 0.81 to 1.24 | 0.99 | |||
|
| ||||||
| Tumour size | 0.61 | 0.50 to 0.75 | <0.01 | 0.66 | 0.47 to 0.94 | <0.01 |
|
| ||||||
| Travel distance | 1.08 | 0.85 to 1.38 | 0.51 | |||
|
| ||||||
| Surgeon’s opinion | 0.66 | 0.54 to 079 | <0.01 | 0.69 | 0.50 to 0.96 | 0.03 |
|
| ||||||
| Feminine identity | 0.63 | 0.51 to 0.79 | <0.01 | |||
|
| ||||||
| Sexuality | 0.64 | 0.50 to 0.81 | <0.01 | |||
|
| ||||||
| Wanting to keep breast tissue | 0.31 | 0.23 to 0.42 | <0.01 | 0.17 | 0.10 to 0.30 | <0.01 |
|
| ||||||
| Incorporating reconstruction | 1.11 | 0.91 to 1.38 | 0.29 | |||
|
| ||||||
| Total treatment time | 1.24 | 1.00 to 1.54 | 0.05 | 1.81 | 1.19 to 2.75 | <0.01 |
Evaluates the likelihood of mastectomy compared with breast-conserving therapy, unless otherwise specified. A higher odds ratio favours mastectomy.
Boldface type denotes significance.
“Not favourable” combinations: ER−, PgR−, HER2−; ER−, PgR−, HER2+; ER−, PgR+, HER2+; ER+, PgR−, HER2+; and ER+, PgR+, HER2+. “Favourable” combinations: ER+, PgR+, HER2−; ER−, PgR+, HER2−; and ER+, PgR+, HER2−.
Breast not otherwise specified, central, lower inner, lower outer, overlap, and upper inner.
OR = odds ratio; CI = confidence interval; NA = not applicable; AJCC = American Joint Committee on Cancer; ER = estrogen receptor; PgR = progesterone receptor; HER2 = human epidermal growth factor receptor 2; MRI = magnetic resonance imaging.
Clinicopathologic Factors
In multivariate analysis only, overall stage remained a significant factor. Compared with their counterparts having stage i disease, women with stage ii disease had a 7.5 odds [95% confidence interval (ci): 2.98 to 18.82] of undergoing mastectomy. When examining the subgroup of participants who rated tumour size as an important influencing factor, the actual tumour size between groups is amplified. In that subgroup, the mean tumour sizes in participants undergoing mastectomy and bct were 2.5 cm and 1.5 cm respectively (p < 0.01).
Individual Factors
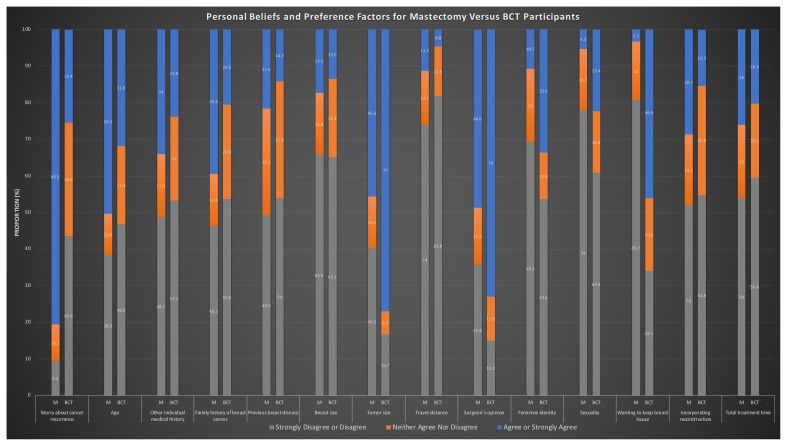
Personal Belief and Preference Factors
In the final model, factors that were rated as significantly more important in the choice for mastectomy were worry about cancer recurrence and total treatment time (Figure 2). Factors that were significantly more important in the choice for bct were “wanting to keep own breast tissue,” “tumour size,” and “surgeon’s opinion.” Supplementary Figures 1 and 2 show belief factors cited by participants undergoing mastectomy and bct.
FIGURE 2.
Participant personal beliefs and preference factors for mastectomy compared with breast-conservation therapy.
Sociodemographic Factors
No sociodemographic factors remained significant in our final main effects model.
Geographic Factors
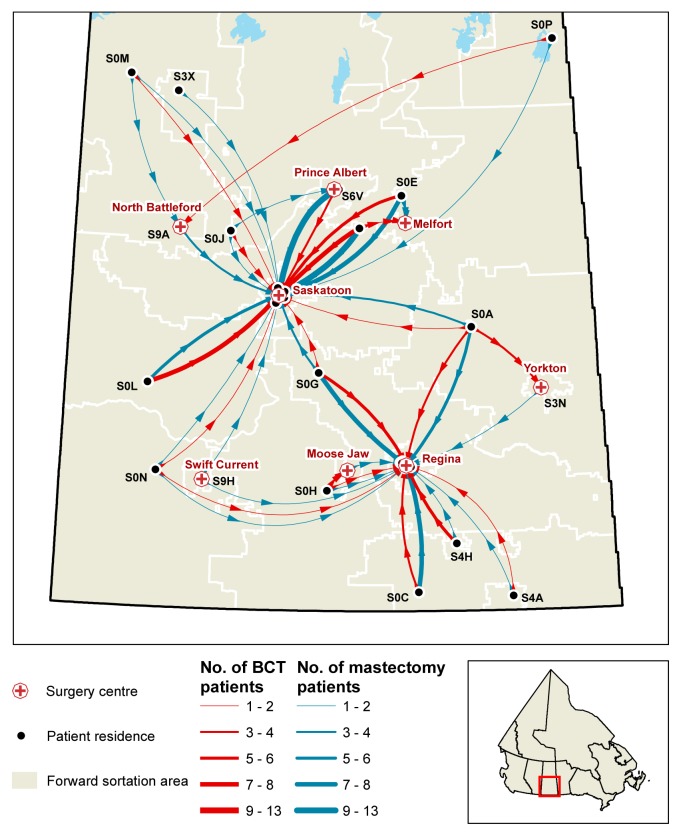
Figure 3 depicts travel from the home residences of patients to the surgical centre where they received treatment. Overall, no geographic factors were observed to significantly affect treatment choice in this cohort. In the mastectomy subgroup, the participants who rated “travel distance” as important (n = 17, 11.3%) resided a significantly greater mean distance from radiation centre than did the participants who resided closer (n = 133, 88.7%): 195 km and 105 km respectively (p < 0.01).
FIGURE 3.
Geographic information system map of travel from home residence to surgical centre.
Physician Factors
Participants were asked to reflect on the involvement of their physician in the treatment decision-making process (Table IV). Women who made the treatment decision completely on their own were more likely to undergo mastectomy. Conversely, when the decision was shared [odds ratio (or): 0.22; 95% ci: 0.09 to 0.52] or mostly the physician’s choice (or: 0.17; 95% ci: 0.06 to 0.49), participants were significantly more likely to undergo bct.
TABLE IV.
Involvement in the treatment decision-making process
| Involvement in decision-making | Participant group [n (%)] | Univariate analysisa | |||
|---|---|---|---|---|---|
|
|
|
||||
| Mastectomy (n = 150) | BCT (n = 126) | OR | 95% CI | p Valueb | |
| Completely your choice, no physician input | 30 (20.0) | 8 (6.5) | 1.00 (Reference) | ||
|
| |||||
| Mostly your choice, minimal physician input | 51 (34.0) | 32 (25.8) | 0.43 | 0.17 to 1.04 | 0.06 |
|
| |||||
| Shared decision | 47 (31.3) | 57 (45.9) | 0.22 | 0.09 to 0.52 | <0.01 |
|
| |||||
| Mostly your physician’s choice | 13 (8.7) | 20 (16.1) | 0.17 | 0.06 to 0.49 | <0.01 |
|
| |||||
| Completely physician’s choice, no individual input | 9 (6.0) | 7 (5.7) | 0.34 | 0.10 to 1.21 | 0.10 |
Evaluates the likelihood of mastectomy compared with BCT unless otherwise specified. A higher odds ratio favours mastectomy.
Boldface type denotes significance.
BCT = breast-conservation therapy; OR = odds ratio; CI = confidence interval.
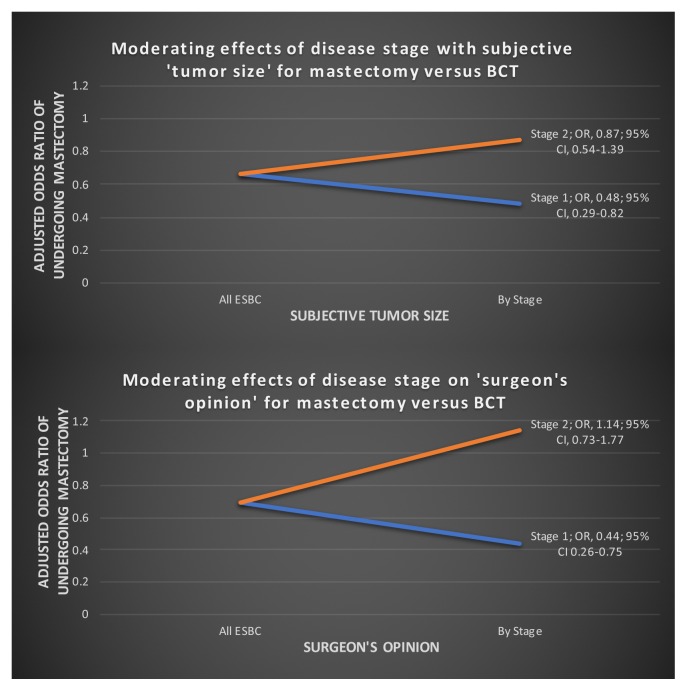
Moderating Effects of Disease Stage with Surgeon’s Opinion and of Disease Stage with Subjective Tumour Size
Two significant interaction terms were evident in our model: stage of disease with surgeon’s opinion and stage of disease with subjective tumour size (Figure 4). The stage of disease, either stage i or ii, moderated the effect of tumour size as an important influence on treatment choice. Of the participants who rated tumour size as important to their treatment choice, those with stage i disease (compared with their stage ii counterparts) were significantly more affected by their subjective tumour size. Women with stage i disease who placed more importance on tumour size were twice as likely to undergo bct (or: 0.48; 95% ci: 0.29 to 0.82).
FIGURE 4.
Moderating effects of disease stage with subjective “tumour size” and disease stage with “surgeon’s opinion” for mastectomy compared with breast-conservation therapy (BCT). OR = odds ratio; CI = confidence interval; ESBC = early-stage breast cancer.
A similar relationship was observed between earlier stage of disease and surgeon’s opinion. For participants who valued the surgeon’s opinion about their therapy choice, stage i disease had significantly more influence on their treatment choice than did stage ii disease. Women with stage i disease who placed more value on their surgeon’s opinion were also more than twice as likely to undergo bct (or: 0.44; 95% ci: 0.26 to 0.75).
DISCUSSION
The choice of mastectomy or bct is a complicated decision-making process that is influenced by a wide array of factors. Our study demonstrates that, for Saskatchewan women with esbca, treatment choice was influenced primarily by tumour stage and individual belief factors. Those findings suggest that women are making their treatment choices predominantly based on individual values and preferences.
Later stage of disease, which is determined by tumour size in esbca, was a significant factor influencing women to undergo mastectomy. That association has been consistently demonstrated in the literature10. Although a choice of either treatment should be offered, patients with larger tumours might subjectively believe that their bca is more advanced, thus subsequently influencing their choice of mastectomy. Additionally, larger tumours can result in worse cosmetic outcomes, and the breast–tumour ratio has been reported to affect treatment choice23. Larger tumours might also bias the physician toward recommending mastectomy, because such tumours have been associated with higher re-excision and local recurrence rates27–30.
An interesting finding from our study was the effect modification that clinical stage had on the subjective tumour size. When considering the influence of tumour size on treatment choice, it might be expected that a smaller tumour would influence a choice for bct and a larger tumour, for mastectomy. That influence was true for women with stage i disease, because the value placed on tumour size significantly influenced the choice for bct. However, among women with stage ii disease, placing a value on tumour size did not significantly influence therapy choice. That finding suggests that tumour size, especially a smaller tumour, is an important reason for women choosing bct, but a less important reasons for women choosing mastectomy.
Demographically, the mastectomy and bct subgroups in our study were very similar. Previously, the literature has shown mixed results for the effect of age on treatment choice, with many studies pointing toward women at the older and younger extremes favouring mastectomy10. Our study demonstrated a trend toward younger compared with older women choosing mastectomy at a higher rate. The literature has suggested that, with increased treatment knowledge, younger women are more driven by prophylaxis and engage in more reconstruction options19,31.
Socioeconomic status, or indicators of socioeconomic status, have been associated with an increased likelihood of bct; however, most research is U.S.-based, where health insurance is a complicating factor10. Given that our study is based in a publicly funded health care system, we did not expect to find such an association. Annual household income excepted, most demographic factors (employment status, relationship status, children, urban compared with rural residence, and highest level of education) were similar in our surveyed mastectomy and bct subgroups. However, income was not significant in the multivariate analysis when we controlled for disease stage; no difference between the mastectomy and bct rates was observed in both the stratified and regression analyses. That observation suggests that differences in income between the groups was likely an incidental finding or a result of lack of statistical power. Another possibility is differences in health literacy or cultural norms that were not captured by our survey questions and that can be associated with socioeconomic status, leading to differences in personal beliefs.
The literature reports mixed results about the effect of rural residence and travel distance to radiation facilities on mastectomy rates, with more studies showing decreasing bct rates with increasing distance to treatment centres10. In our study, distance to treatment centres and urban compared with rural residence did not affect treatment choice except for the subset of participants who rated travel distance as an important factor influencing the choice for mastectomy. Their mean travel distance to surgical and radiation therapy was significantly farther than it was for other mastectomy participants. That observation indicates that, although distance to the treatment centre does not affect treatment choice for most individuals, distance remains a concern for a subset of participants. We identified and mapped the postal code regions where those patients reside and fed that information back to the sca and the breast units. It is important to identify such individuals and to emphasize the local supports in place to address geographic barriers. Locally, our cancer centre offers lodging during radiation treatments and shorter-course radiotherapy regimes. All efforts should be made to provide those supports to patients so that geographic constraints do not limit implementation of their therapy choice.
Individual belief factors might be among the most important factors influencing women’s decision-making, but they are also among the most difficult to study and to understand. Many of these individual values influencing decision-making might be based on a life of experiences and complex interactions with close confidents. Nevertheless, we believe that our survey was complete in covering the range of belief factors that might influence therapy choice because it was grounded in previous qualitative research conducted locally, as well as in significant literature review.
In our final model, the odds of undergoing mastectomy were increased for patients who rated “worry about cancer recurrence” and “total treatment time” as important. In the literature, worry about cancer recurrence has consistently been reported as the belief factor that most commonly influences the choice of mastectomy10. Our previous qualitative study also found such worry to be a primary theme motivating mastectomy choice—and always attributable to a secondary underlying reason: family history of bca, having observed a poor bct outcome, and avoiding follow-up imaging23. Interestingly, follow-up questions about why women rated worry about cancer recurrence as important indicated that family history was the most common reason. Patients having increased concern about recurrence because of family history is justified, because risk of developing a second bca is increased32. Health care providers must focus on appropriate education and counselling about relative risks and treatment options for these patients.
In our multivariate model, belief factors that significantly influenced the choice of bct were “tumour size,” “surgeon’s opinion,” and “wanting to keep breast tissue.” As noted in the earlier part of this discussion, tumour size predominantly affects bct choice when the tumour is small; that association is logical, given that a small tumour might convey to the patient a belief of early disease, which therefore does not require an aggressive operation. In our study, wanting to keep breast tissue was the belief factor most strongly associated with undergoing bct (or: 0.17; 95% ci: 0.10 to 0.30). That observation is in line with previous literature demonstrating that breast tissue, body-image concerns, and feminine identity are the most important belief factors influencing the choice for bct10.
The previous literature about physician-related factors has focused largely on physician demographics such as sex, age, type of training, and practice pattern10. Evaluating those factors in our study was impractical, given that there are more than 20 surgeons operating in 8 different surgical centres in Saskatchewan. Instead, we focused on patient perceptions of their physician interaction and the perceived influence of the physician on therapy decision-making. In our survey, the odds of undergoing bct was significantly increased for patients who gave a high rating to “surgeon’s opinion”. That relationship has also been demonstrated in local and other research10,23,33 and likely reflects the fact that bct, when possible, is thought by physicians to be better for the patient and is promoted as such. Some authors have even suggested that the use of mastectomy rates as a quality indicator might actually bias the physician against the patient’s treatment wishes34. In our own study, decisional conflict scores were lower in a mastectomy group (19.8 vs. 25.2, p = 0.02). As well, when participants were asked to reflect on their involvement in the treatment decision-making process (Table III), women who made the treatment decision completely on their own were more likely to undergo mastectomy. Conversely, when the decision was shared or mostly the physician’s choice, participants were significantly more likely to undergo bct. Those findings might indicate that patients undergoing mastectomy are more confident of their treatment choice and less likely to take account of the physician’s opinion. Alternatively, another plausible explanation is that when physician engagement and guidance in the decision-making process is not adequate, the patient might be more likely to choose mastectomy.
Another interesting result from our study is the effect modification that clinical stage had on the surgeon’s opinion as an influential factor. For women with stage i disease, the value placed on the surgeon’s opinion significantly influenced their treatment choice toward bct. However, for women with stage ii disease, valuing the surgeon’s opinion did not have a significant influence on therapy choice. That finding suggests that the physician is more likely to influence a patient with stage i disease toward the choice of bct and less likely to significantly influence therapy choice for a women with stage ii disease.
A limitation of our study is that most of our survey participants were white women (96.7% and 94.4% of the mastectomy and bct subgroups respectively). Approximately 16% of the Saskatchewan population identify as Indigenous (First Nations, Métis, or Inuit)35. Our survey captured only 4 such individuals who underwent bct and none who underwent mastectomy. Future targeted research strategies will be required to understand decision-making in that ethnic group.
A strength of our study was the grounding of the survey in a clinical framework to help guide and organize the study design and analysis. To our knowledge, no previous studies on decision-making for esbca treatment have done so. We believe that organizing our survey around that framework has allowed us to more holistically examine the factors influencing therapy choice, compared with just investigating a subset of the relevant domains. Furthermore, questionnaire development was, in part, directly informed by previous exploratory work within the province, which improves the relevance and depth of understanding of the personal belief factors.
CONCLUSIONS
The choice of mastectomy or bct in esbca is a complicated decision-making process that is influenced by a wide array of factors. As research evaluating this topic is becoming more complete, a better understanding is being generated about the importance of many individual belief factors that are driving therapy choice. If patients are choosing mastectomy predominantly because of the values they hold and their individual preference, a lower rate of bct should not be of concern. We would advise against the use of mastectomy or bct rates as an indicator of quality of care in the future. Instead, a shift in attention should be made toward care that is patient-centred. For physicians, patient-centred care means providing education for patient, understanding the views and preferences of the patient, understanding their personal treatment biases, engaging in a shared decision-making process, and facilitating the treatment goals of the patient. From a quality improvement viewpoint, attention should be focused on identifying and limiting barriers to treatment options. Examples include identifying patients who face travel barriers and ensuring that they are aware of local supports, or ensuring that therapy choices are offered to patients.
Supplemental Materials
ACKNOWLEDGMENTS
We acknowledge the sca, the Saskatoon Breast Health Centre, and the Social Sciences Research Laboratories for their collaboration and help with this study.
Footnotes
Supplemental material available at http://www.current-oncology.com
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflict of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Steering Committee on Cancer Statistics. Canadian Cancer Statistics 2012. Toronto, ON: Canadian Cancer Society; 2012. [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Arriagada R, Le MG, Rochard F, Contesso G. Conservative treatment versus mastectomy in early breast cancer: patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J Clin Oncol. 1996;14:1558–64. doi: 10.1200/JCO.1996.14.5.1558. [DOI] [PubMed] [Google Scholar]
- 4.Van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–50. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group. Effects of radiotherapy and surgery in early breast cancer. An overview of the randomized trials. N Engl J Med. 1995;333:1444–55. doi: 10.1056/NEJM199511303332202. [Erratum in: N Engl J Med 1996;334:1003] [DOI] [PubMed] [Google Scholar]
- 6.Charles C, Gafni A, Whelan T. Decision-making in the physician–patient encounter: revisiting the shared treatment decision-making model. Soc Sci Amp Med. 1999;49:651–61. doi: 10.1016/S0277-9536(99)00145-8. [DOI] [PubMed] [Google Scholar]
- 7.Canadian Partnership Against Cancer (cpac) Breast Cancer Control in Canada: A System Performance Special Focus Report. Toronto, ON: cpac; 2012. [Available online at: http://www.cancerview.ca/idc/groups/public/documents/webcontent/breast_cancer_control_rep.pdf; cited 1 June 2016] [Google Scholar]
- 8.Canadian Institute for Health Information (cihi) Breast Cancer Surgery in Canada 2007–2008 to 2009–2010. Toronto, ON: cihi; 2012. [Available online at: https://secure.cihi.ca/estore/productFamily.htm?pf=PFC1936&lang=en&media=0; cited 1 June 2016] [Google Scholar]
- 9.Canadian Partnership Against Cancer (cpac) The 2015 Cancer System Performance Report. Toronto, ON: cpac; 2015. [Google Scholar]
- 10.Gu J, Groot G, Boden C, Busch A, Holtslander L, Lim H. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. 2018;18:e539–54. doi: 10.1016/j.clbc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Mac Bride MB, Neal L, Dilaveri CA, et al. Factors associated with surgical decision making in women with early-stage breast cancer: a literature review. J Womens Health (Larchmt) 2013;22:236–42. doi: 10.1089/jwh.2012.3969. [DOI] [PubMed] [Google Scholar]
- 12.Lee CN, Chang Y, Adimorah N, et al. Decision making about surgery for early-stage breast cancer. J Am Coll Surg. 2012;214:1–10. doi: 10.1016/j.jamcollsurg.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastaglia B, Kristjanson LJ. Factors influencing women’s decisions for choice of surgery for stage i and stage ii breast cancer in Western Australia. J Adv Nurs. 2001;35:836–47. doi: 10.1046/j.1365-2648.2001.01921.x. [DOI] [PubMed] [Google Scholar]
- 14.Olaya W, Wong JH, Morgan JW, Roy-Chowdhury S, Lum SS. Disparities in the surgical management of women with stage i breast cancer. Am Surg. 2009;75:869–72. [PubMed] [Google Scholar]
- 15.Temple WJ, Russell ML, Parsons LL, et al. Conservation surgery for breast cancer as the preferred choice: a prospective analysis. J Clin Oncol. 2006;24:3367–73. doi: 10.1200/JCO.2005.02.7771. [DOI] [PubMed] [Google Scholar]
- 16.Del Turco MR, Ponti A, Bick U, et al. Quality indicators in breast cancer care. Eur J Cancer. 2010;46:2344–56. doi: 10.1016/j.ejca.2010.06.119. [DOI] [PubMed] [Google Scholar]
- 17.Biganzoli L, Marotti L, Hart CD, et al. Quality indicators in breast cancer care: an update from the eusoma working group. Eur J Cancer. 2017;86:59–81. doi: 10.1016/j.ejca.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Stordeur S, Vrijens F, Devriese S, Beirens K, Van Eycken E, Vlayen J. Developing and measuring a set of process and outcome indicators for breast cancer. Breast. 2012;21:253–60. doi: 10.1016/j.breast.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee MC, Rogers K, Griffith K, et al. Determinants of breast conservation rates: reasons for mastectomy at a comprehensive cancer center. Breast J. 2009;15:34–40. doi: 10.1111/j.1524-4741.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- 20.Consensus conference. Treatment of early stage breast cancer. National Institutes of Health. Conn Med. 1991;55:101–7. [PubMed] [Google Scholar]
- 21.Cyran EM, Crane LA, Palmer L. Physician sex and other factors associated with type of breast cancer surgery in older women. Arch Surg. 2001;136:185–91. doi: 10.1001/archsurg.136.2.185. [DOI] [PubMed] [Google Scholar]
- 22.Reitsamer R, Menzel C, Glueck S, Hitzl W, Peintinger F. Predictors of mastectomy in a certified breast center—the surgeon is an independent risk factor. Breast J. 2008;14:324–9. doi: 10.1111/j.1524-4741.2008.00592.x. [DOI] [PubMed] [Google Scholar]
- 23.Gu J, Groot G, Holtslander L, Engler-Stringer R. Understanding women’s choice of mastectomy versus breast conserving therapy in early-stage breast cancer. Clin Med Insights Oncol. 2017;11 doi: 10.1177/1179554917691266. 1179554917691266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu J, Groot G. Creation of a new clinical framework—why women choose mastectomy versus breast conserving therapy. BMC Med Res Methodol. 2018;18:77. doi: 10.1186/s12874-018-0533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krosnick JA, Pressor S. Question and questionnaire design. In: Marsden P, Wright J, editors. Handbook of Survey Research. 2nd ed. Bingley, UK: Emerald Group Publishing; 2010. pp. 263–313. [Google Scholar]
- 26.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Ver. 2.2019. Fort Washington, PA: nccn; 2019. [Current version FACTORS INFLUENCING DECISION-MAKING FOR MASTECTOMY COMPARED WITH BCT, Gu et al. available online at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (free registration required); cited 13 July 2019. [Google Scholar]
- 27.Schiller DE, Le LW, Cho BCJ, Youngson BJ, McCready DR. Factors associated with negative margins of lumpectomy specimen: potential use in selecting patients for intraoperative radiotherapy. Ann Surg Oncol. 2008;15:833–42. doi: 10.1245/s10434-007-9711-2. [DOI] [PubMed] [Google Scholar]
- 28.Chagpar AB, Martin RC, 2nd, Hagendoorn LJ, Chao C, Mc-Masters KM. Lumpectomy margins are affected by tumor size and histologic subtype but not by biopsy technique. Am J Surg. 2004;188:399–402. doi: 10.1016/j.amjsurg.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Mirza NQ, Vlastos G, Meric F, et al. Predictors of locoregional recurrence among patients with early-stage breast cancer treated with breast-conserving therapy. Ann Surg Oncol. 2002;9:256–65. doi: 10.1007/BF02573063. [DOI] [PubMed] [Google Scholar]
- 30.Cefaro GA, Genovesi D, Marchese R, et al. Predictors of local recurrence after conservative surgery and whole-breast irradiation. Breast Cancer Res Treat. 2006;98:329–35. doi: 10.1007/s10549-006-9169-0. [DOI] [PubMed] [Google Scholar]
- 31.Balch CM, Jacobs LK. Mastectomies on the rise for breast cancer: “The tide is changing”. Ann Surg Oncol. 2009;16:2669–72. doi: 10.1245/s10434-009-0634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collaborative Group on Hormonal Factors in Breast Cancer. Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–99. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 33.Rippy EE, Ainsworth R, Sathananthan D, Kollias J, Bochner M, Whitfield R. Inf luences on decision for mastectomy in patients eligible for breast conserving surgery. Breast. 2014;23:273–8. doi: 10.1016/j.breast.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Katz SJ, Hawley ST. From policy to patients and back: surgical treatment decision making for patients with breast cancer. Health Aff (Millwood) 2007;26:761–9. doi: 10.1377/hlthaff.26.3.761. [DOI] [PubMed] [Google Scholar]
- 35.Statistics Canada. Table 2. Number and distribution of the population reporting an Aboriginal identity and percentage of Aboriginal people in the population, Canada, provinces and territories, 2011 [Web page, archived] Ottawa, ON: Statistics Canada; 2015. [Available at: https://www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-011-x/2011001/tbl/tbl02-eng.cfm; cited 1 June 2016] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.