Abstract
Diffuse large B cell lymphoma (dlbcl) is an aggressive non-Hodgkin lymphoma, accounting for approximately 30% of lymphoma cases in Canada. Although most patients will achieve a cure, up to 40% will experience refractory disease after initial treatment, or relapse after a period of remission. In eligible patients, salvage therapy followed by high-dose therapy and autologous stem-cell transplantation (asct) is the standard of care. However, many patients are transplant-ineligible, and more than half of those undergoing asct will subsequently relapse. For those patients, outcomes are dismal, and novel treatment approaches are a critical unmet need. In this paper, we present available data about emerging treatment approaches in the latter setting and provide a perspective about the potential use of those approaches in Canada.
Keywords: Diffuse large B cell lymphoma, dlbcl, non-Hodgkin lymphoma, novel therapies
INTRODUCTION
Non-Hodgkin lymphoma (nhl) is a malignancy of the lymphatic system that encompasses more than 60 subtypes of lymphoma1. In 2017, the projected incidence of nhl was 8300 cases annually, with an age-standardized incidence rate of 20.8 cases per 100,000 Canadians2. Diffuse large B cell lymphoma (dlbcl) is an aggressive form of nhl that constitutes approximately 30% of lymphoma cases in Canada3,4. Two molecular subtypes of dlbcl—germinal centre B cell (gcb) and activated B cell (abc)—that differ in their cell of origin, oncogenic pathway, and clinical outcome have been identified5, with the abc subtype being associated with an inferior prognosis3. A highly aggressive lymphoma referred to as double- or triple-hit lymphoma, with concurrent translocations of MYC and either or both of BCL2 and BCL6, is no longer categorized as dlbcl, but rather as high-grade B cell lymphoma5,6. Double-expressor lymphoma, which involves overexpression of Myc and Bcl2, is not considered a separate entity, but has also been associated with poorer prognosis7.
In Canada, the long-term survival rate for patients with dlbcl is approximately 60% after immunochemotherapy with r-chop (rituximab with cyclophosphamide–doxorubicin–vincristine–prednisone)5,8,9. Canadian provincial guidelines10–13 and guidelines from the United States14 and Europe15 indicate that r-chop is the standard first-line therapy for patients with dlbcl. Unfortunately, after an initial response to therapy, 30%–40% of patients will experience refractory disease or will relapse and require subsequent treatment5,16,17. Although optimal treatment for patients with double- or triple-hit lymphoma is unclear, some evidence suggests that, rather than r-chop, more-intensified induction therapy could be warranted in such patients7,18.
For eligible patients who relapse or who are refractory to initial therapy, salvage chemotherapy followed by high-dose therapy and autologous stem-cell transplantation (asct) is the standard of care5,10,12,14,15. However, eligibility for that approach depends largely on response to salvage chemotherapy, performance status, age, and comorbidities18,19. Population-based studies in Canada and Denmark show that more than half the patients with relapsed or refractory (r/r) dlbcl are treated palliatively20,21. Further, eligibility for asct depends on demonstrated sensitivity to salvage chemotherapy, with 50% of patients being ineligible because of an inadequate response22. Of patients who proceed to asct, more than 50% will ultimately relapse18,23. Factors negatively affecting survival include prior treatment with rituximab, early relapse, and a high International Prognostic Index score at relapse18.
Until recent evidence emerging from chimeric antigen receptor T cell (car-t) therapy24–26, treatment for relapse after asct had been largely palliative, with median survival being approximately 6 months27. Although conventional chemotherapy can be given in the relapsed setting, clinical trials of novel agents are recommended because of poor prognosis with established therapies15,28.
Patients for whom initial therapy fails have a poor prognosis and could benefit from more effective salvage therapies. Only 30% of patients treated in recent prospective trials involving salvage therapy and asct achieved long-term remission22,29. Furthermore, patients who are ineligible for salvage therapy or transplantation, and patients who have relapsed after asct, represent a critical unmet need for which novel treatment approaches are required. Here, we present available data about emerging treatment approaches, and we provide perspectives about the potential use of those approaches in Canada.
METHODS
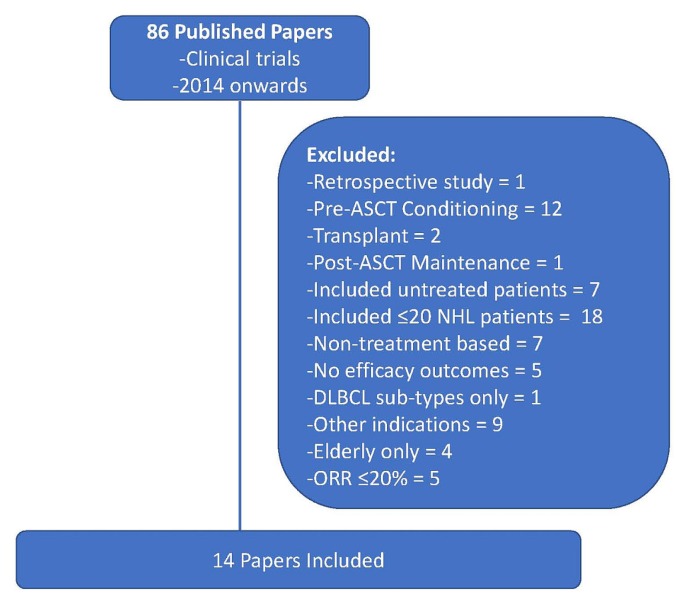
A literature search in the U.S. Library of Medicine’s PubMed database sought indexed papers published during January 2014–January 2019, using the search term “relapsed diffuse large B-cell lymphoma.” Fourteen papers were included in the analysis based on the inclusion and exclusion criteria outlined in Figure 1. One additional published paper examining car-t therapy was included because of its potential impact in this setting, although the paper had not been identified in the original search, because the title used the term “large B-cell lymphoma” rather than “diffuse large B-cell lymphoma.” In addition, eighteen relevant abstracts presented in 2018 at meetings of the American Society of Hematology, the American Society of Clinical Oncology, and the International Conference on Malignant Lymphoma were included (Tables i and ii).
FIGURE 1.
Selection of published papers in relapsed or refractory diffuse large B cell lymphoma. ASCT = autologous stem-cell transplantation; NHL = non-Hodgkin lymphoma; DLBCL = diffuse large B cell lymphoma; ORR = overall response rate.
TABLE I.
Novel therapies for the treatment of relapsed or refractory diffuse large B cell lymphoma
| Treatment | Mechanism of action | Reference | Phase | Patients | |
|---|---|---|---|---|---|
|
| |||||
| (n) | Details | ||||
| Published papers | |||||
|
| |||||
| Brentuximab | Antibody–drug conjugate | Jacobsen et al., 201530 | II | 68 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Polatuzumab vedotin | Antibody drug–conjugate | Palanca-Wessels et al., 201531 | I | 95 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Ibrutinib | Bruton tyrosine kinase inhibitor | Wilson et al., 201532 | I/II | 80 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Panobinostat vs. panobinostat–rituximab | Pan histone deacetylase inhibitor (panobinostat) | Assouline et al., 201633 | II Randomized | 40 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Coltuximab ravtansine | Antibody drug–conjugate | Coiffieret al., 201634 | II | 52 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Tirabrutinib | Bruton tyrosine kinase inhibitor | Walter et al., 201635 | I | 90 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Blinatumomab | Bi-specific T cell engager antibody | Viardot et al., 201636 | II | 25 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Pinatuzumab vedotin with or without rituximab | Antibody drug–conjugate | Advani et al., 201737 | I | 91 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Lenalidomide vs. investigator choice | Immunomodulator | Czuczman et al., 201738 | II/III | 102 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Selinexor | Selective inhibitor of nuclear export | Kuruvilla et al., 201739 | I | 79 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| CUDC-907 with or without rituximab | PI3K/pan histone deacetylase inhibitor Anti-CD20 antibody | Oki et al., 201740 | I | 37 |
|
| Efficacy in DLBCL▪ Median progression-free survival: 2.9 months; overall response rate: 37%; complete response: 5 of 30 patients; overall response rate: 64% (MYC-altered disease); median duration of response: 11.2 monthsSafety▪ Most common adverse events: thrombocytopenia, diarrhea, fatigue | |||||
|
| |||||
| MOR208 | Anti-CD19 antibody | Jurczak et al., 201849 | IIA | 92 |
|
Efficacy in DLBCL
| |||||
| Abstracts | |||||
|
| |||||
| Ibrutinib–R-GDP vs. R-GDP | Bruton tyrosine kinase inhibitor (ibrutinib) | Kuruvilla et al., 201741 | II | 30 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Polatuzumab vedotin–bendamustine–rituximab vs. bendamustine–rituximab | Antibody drug–conjugate (polatuzumab vedotin) | Sehn et al., 201742 and Sehn et al., 201843 | II Randomized | 80 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| REGN1979 | Bi-specific anti-CD20/CD3 antibody | Bannerji et al., 201844 | I | 54 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Mosunetuzumab | Bi-specific anti-CD20/CD3 antibody | Budde et al., 201845 | I | 98 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Blinatumomab | Bi-specific T cell engager antibody | Coyle et al., 201846 | II | 41 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Acalabrutinib | Bruton tyrosine kinase inhibitor | Dyer et al., 201847 | I | 21 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| RG6026 | Bi-specific anti-CD20/CD3 antibody | Hutchings et al., 201848 | I | 64 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Ibrutinib–bendamustine–rituximab | Bruton tyrosine kinase inhibitor (ibrutinib) | Kedmi et al., 201850 | II | 32 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Umbralisib–ublituximab–bendamustine | PI3K inhibitor, anti-CD20 antibody, alkylator | Lunning et al., 201851 | I | 39 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Selinexor | Selective inhibitor of nuclear export | Maerevoet et al., 201852 | IIb | 110 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Ibrutinib–lenalidomide–rituximab | Bruton tyrosine kinase inhibitor, immunomodulator, anti-CD20 antibody | Ramchandren et al., 201853 | Ib/II | 55 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| MOR208–lenalidomide | Anti-CD19 antibody Immunomodulator | Salles et al., 201754 | II | 81 |
|
Efficacy in DLBCL
| |||||
|
| |||||
| Pixantrone–rituximab vs. gemcitabine–rituximab | Anthracenedione analog vs. nucleoside analog | Salles et al., 201855 | III | 312 |
|
Efficacy in DLBCL
| |||||
DLBCL = diffuse large B-cell lymphoma; PI3K = phosphoinositide 3-kinase; R-GDP = rituximab, gemcitabine–cisplatin–dexamethasone; PET = positron-emission tomography.
TABLE II.
Pivotal studies of chimeric antigen receptor T cell therapy in relapsed or refractory diffuse large B cell lymphoma
| Treatment | Reference (study name) | Phase | Patients | |
|---|---|---|---|---|
|
| ||||
| (n) | Details | |||
| Axicabtagene ciloleucel | Locke et al., 201956 and Neelapu et al., 201824 (ZUMA-1) | I/II | 108 |
|
Efficacy in DLBCL
| ||||
| Jacobson et al., 201857 | Real-world setting | 76 |
|
|
| Efficacy in DLBCL▪ Median follow-up: 4 months; best overall response rate: 64%a; best complete response: 41%aSafety▪ Grade 3 or greater adverse events: cytokine release syndrome, 17%; neurotoxicity, 38% | ||||
| Nastoupil et al., 201858 | Real-world setting | 211 |
|
|
Efficacy in DLBCL
| ||||
|
| ||||
| Lisocabtagene maraleucel | Abramson et al., 201825 (TRANSCEND) | I | 91 |
|
Efficacy in DLBCL
Safety
| ||||
|
| ||||
| Tisagenlecleucel | Schuster et al., 201959 (JULIET) | II | 93 |
|
Efficacy in DLBCL
| ||||
Recorded from study start to disease progression.
DLBCL = diffuse large B-cell lymphoma.
EMERGING TREATMENTS FOR R/R DLBCL
Novel Agents
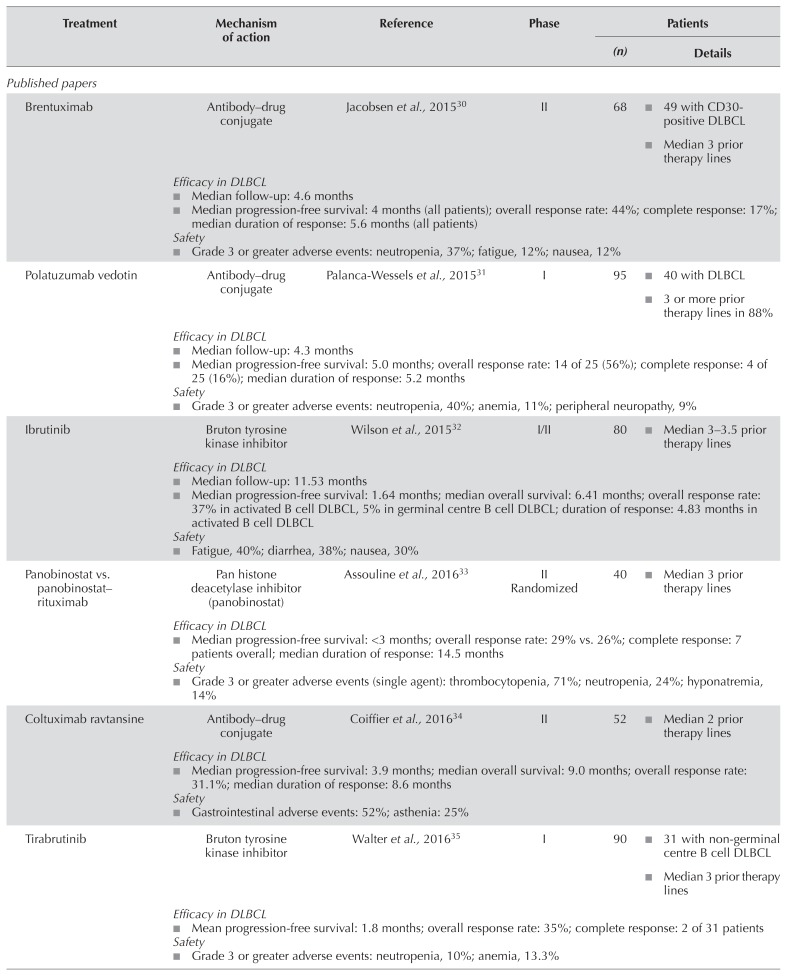
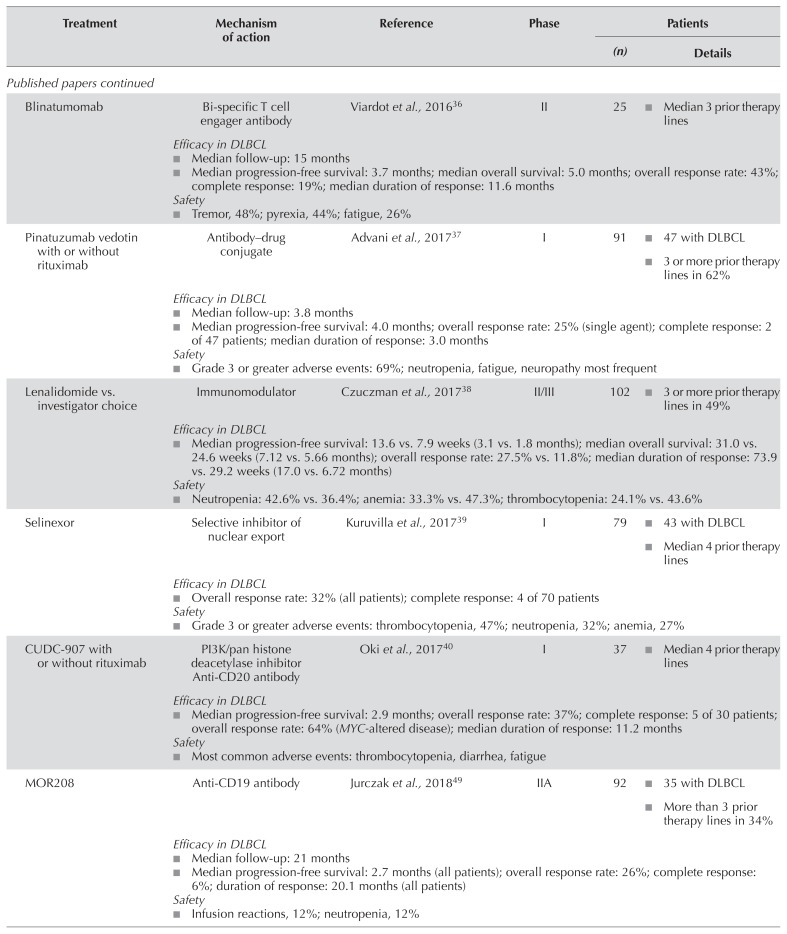
Table I summarizes the published papers and abstracts examining novel agents and combinations in r/r dlbcl. Most were nonrandomized phase i/ii trials; the one randomized phase iii trial was an ongoing study by Salles et al.55 comparing pixantrone–rituximab with gemcitabine–rituximab in patients with r/r dlbcl. In addition, the search found four randomized phase ii trials: one by Kuruvilla et al.41 comparing ibrutinib plus rituximab and gemcitabine–dexamethasone–cisplatin (r-gdp) with r-gdp alone; one by Sehn et al.60 comparing polatuzumab vedotin plus bendamustine–rituximab (br) with br alone; one by Czuczman et al.38 comparing lenalidomide with investigator’s choice; and one by Assouline et al.33 comparing panobinostat with panobinostat–rituximab. In the subsections that follow, we discuss agents and regimens with the most promising evidence for further development in r/r dlbcl.
Ibrutinib
Activation of the B cell receptor is an integral part of B cell malignancies, controlling cellular functions such as proliferation, apoptosis, differentiation, and migration61. Nuclear factor κB, a transcription factor activated from the downstream pathway of the B cell receptor, is particularly important in the survival of abc dlbcl lines62. Ibrutinib is an orally administered selective and covalent inhibitor of Bruton tyrosine kinase that reduces nuclear factor κB pathway signalling and might therefore be effective for patients with the abc subtype of dlbcl32. A phase i/ii trial by Wilson et al. examined the efficacy and safety of ibrutinib in 80 patients with r/r dlbcl, including 38 patients with abc dlbcl and 20 patients with gcb dlbcl32. Two thirds of the patients were refractory to chemotherapy and had received a median of 3 (abc dlbcl) or 3.5 (gcb dlbcl) prior regimens. An asct had been performed in 13% of the abc dlbcl group and in 30% of the gcb dlbcl group. The overall response rate (orr) was 25%, with a higher orr in the abc dlbcl group than in the gcb dlbcl group (37% vs. 5%, p = 0.0106). The duration of response (dor) in patients with the abc dlbcl subtype was 4.8 months. After a median follow-up of 11.5 months, median progression-free survival (pfs) and overall survival (os) were, respectively, 1.6 and 6.4 months in all patients. The most frequent adverse events (aes) were fatigue (40%), diarrhea (38%), and nausea (30%). Because the activity of ibrutinib monotherapy was only modest in r/r dlbcl, further trials examining combination therapy are ongoing.
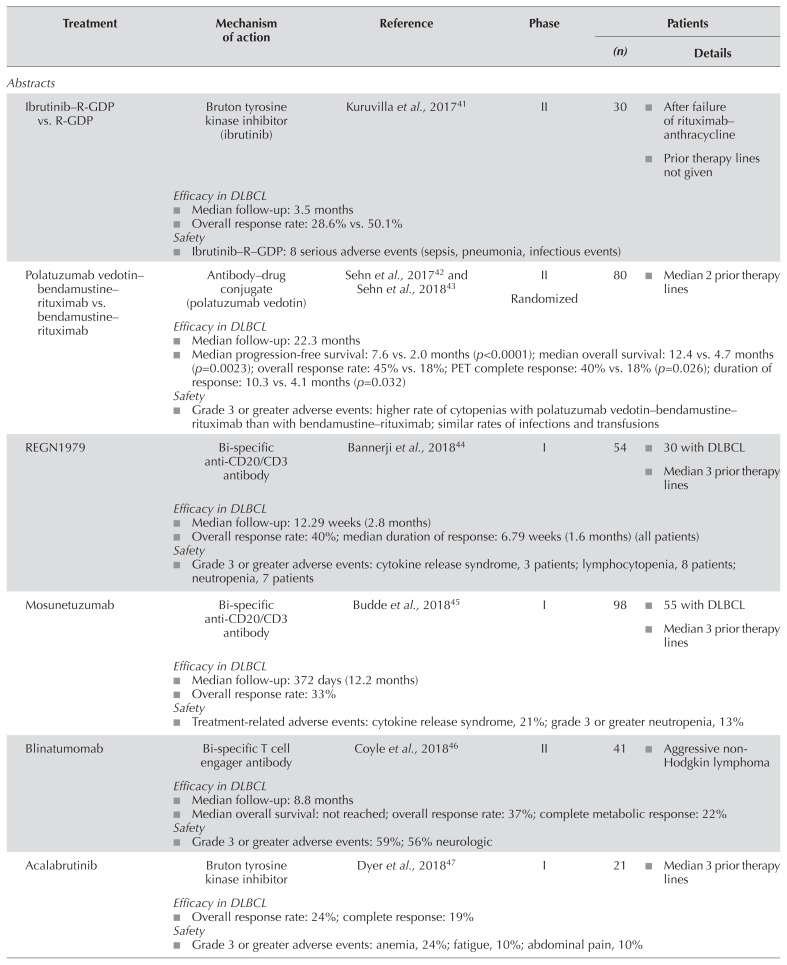
A phase ii study by Kedmi et al.50 is examining the combination of ibrutinib and br in patients with aggressive r/r nhl. The 32 patients evaluated had a median age of 69 years, with 75% being refractory to prior therapy and 19% having relapsed after asct. Preliminary results showed orr and cr rates of 45% and 30% respectively. After a median follow-up of 14 months, median pfs and os were 2.7 months and 7.1 months respectively. Serious aes with ibrutinib–br included atrial fibrillation, fatigue, and thrombocytopenia. Another ongoing randomized phase ii multi-arm trial added ibrutinib to r-gdp in 14 patients with r/r dlbcl and compared the combination with r-gdp alone41. Preliminary results suggested no advantage with the addition of ibrutinib to r-gdp (orr: 28.6% vs. 50.1% in the control arm), and toxicity was increased because of serious infectious events with the addition of ibrutinib. Patient accrual to that treatment arm has been stopped.
Polatuzumab Vedotin
Polatuzumab vedotin is an antibody–drug conjugate consisting of an ant i-CD79b monoclonal antibody and the microtubule-disrupting agent, monomethyl auristatin E42,60. A phase i study by Palanca-Wessels et al.31 examined the safety and efficacy of polatuzumab vedotin in 40 patients with r/r dlbcl. Median age in those patients was 67 years, 88% had received 3 or more prior therapies, 78% were refractory to their last therapy, and 33% had undergone stem-cell transplantation. The orr and cr rates were, respectively, 56% and 16%, with a median dor of 5.2 months. After a median follow-up of 4.3 months, the median pfs was 5.0 months. The most frequent grade 3 or greater aes in patients with nhl treated at the single-agent recommended dose (n = 45) were neutropenia (40%), anemia (11%), and peripheral neuropathy (9%).
The combination of polatuzumab vedotin and br is being compared with br alone in an ongoing phase ii trial, which included a randomized port ion of 80 transplantation-ineligible patients with r/r dlbcl42,43,60. Within the randomized portion, median age was 67 years, 73% had received 2 or more prior therapies, and 75% were refractory to their last treatment43. Rates of cr by positron-emission tomography were significantly higher in the polatuzumab vedotin–br group than in the group receiving br alone (40% vs. 18%, p = 0.026)43. The orr and dor—45% and 10.3 months respectively in the polatuzumab vedotin–br group—were superior to those in the group receiving br alone (18% and 4.1 months respectively). After a median follow-up of 22.3 months, the median pfs and os were also superior in the polatuzumab vedotin–br group (pfs: 7.6 months vs. 2.0 months with br alone, p < 0.0001; os: 12.4 months vs. 4.7 months with br alone, p = 0.0023).
The addition of polatuzumab vedotin appeared to provide benefit regardless of molecular subtype or double-expressor status43. Grade 3 and 4 cytopenias were more frequent in patients receiving polatuzumab vedotin–br than in those receiving br alone; infection and transfusion rates were similar in the two arms43. Based on those results, polatuzumab vedotin in combination with br is currently under priority review by the U.S. Food and Drug Administration63 and has been granted orphan designation (medicine intended for use in a rare condition) by the European Medicines Agency64 for the treatment of r/r dlbcl.
Lenalidomide
Lenalidomide is an immunomodulatory drug that is a structural and functional analog of thalidomide65. In a phase ii randomized trial, lenalidomide was compared with investigator’s choice of treatment (gemcitabine, rituximab, etoposide, or oxaliplatin) in 102 patients with r/r dlbcl38. Median age in the lenalidomide (n = 51) and investigator’s choice (n = 51) groups was 69 years and 65 years respectively. In the lenalidomide and investigator’s choice groups respectively, 49% and 62.7% of patients had received at least 3 prior lines of therapy, and 25% and 33.3% had undergone asct. The primary endpoint of the study was orr, which was required to meet a minimum threshold for the study to proceed to a randomized phase iii trial. The orr was slightly greater with lenalidomide than with investigator’s choice (27.5% vs. 11.8%), with a median dor in the lenalidomide group of 17 months. Median pfs was marginally higher in the lenalidomide cohort (pfs: 3.1 months vs. 1.8 months, p = 0.041), with no difference in os (7.13 months vs. 5.66 months, p = 0.673). Comparing the abc with the gcb disease subtype, the orr and median pfs appeared to be higher in patients with abc dlbcl (45.5% vs. 21.4% and 18.87 months vs. 1.4 months respectively). The most frequent aes of any grade in the lenalidomide group were neutropenia (42.6%), anemia (33.3%), and fatigue (33.3%). Infections were reported in 46.3% of patients in the lenalidomide group. Because the randomized phase ii results did not meet the protocol-specified threshold, the planned randomized phase iii study was not performed. Given the modest activity of lenalidomide as a single agent, combinations are now being tested.
Ibrutinib and Lenalidomide
A phase i/ii study is examining the combination of ibrutinib, lenalidomide, and rituximab in patients with non-gcb r/r dlbcl53. In 55 evaluable patients, median age is 63 years. The patients have received a median of 2 prior therapies, and 53% are refractory to their last therapy. Preliminary results include orr and cr rates of 55% and 30% respectively, with a median dor of 9 months. The median pfs and os are 5 and 17 months respectively. The most frequent grade 3 or greater aes include neutropenia (33%), rash (15%), and anemia (11%).
MOR208
The Fc-engineered humanized monoclonal mor208 antibody is directed against the antigen CD19, which is broadly expressed on the surface of B cells49. A phase ii trial examined the efficacy and safety of mor208 in 35 patients with r/r dlbcl. Before administration of the study drug, patients who had previously received asct must have been at least 4 weeks post-transplant, with full hematologic recovery. Median age in the group was 71 years, 34% of patients had received 3 or more lines of therapy, 69% were refractory to rituximab, and 6% had previously undergone asct. The orr and cr rates were 26% and 6% respectively, with a dor of 20.1 months. After a median follow-up of 21 months, the median pfs was 2.7 months. The most frequent aes of any grade in all patients with nhl (n = 92) included infusion-related reactions (12%) and neutropenia (12%).
MOR208 and Lenalidomide
An ongoing phase ii study is examining the combination of mor208 and lenalidomide in transplant-ineligible patients with r/r dlbcl54. Patients were ineligible for the trial if they had primary refractory disease or an Eastern Cooperative Oncology Group performance status greater than 2, or if they had received more than 3 prior therapies. In 81 evaluable patients, median age was 72 years, 49% had received 2 or more prior lines of therapy, 38% were refractory to rituximab, and 41% were refractory to their last line of therapy. The orr and cr rates were 58% and 33% respectively. After a median follow-up of 12 months, the dor and median os were not reached, and the median pfs was 16.2 months. The most frequent grade 3 or greater aes included neutropenia (43%), thrombocytopenia (17%), and anemia (9%).
Bi-specific Antibodies
Blinatumomab is a bi-specific T cell–engaging antibody that binds to CD3-positive T cells and CD19-positive B cells, resulting in T cell proliferation and T cell–mediated lysis of the B cells36. A phase ii dose-escalation study examined the efficacy and safety of blinatumomab in 25 patients with r/r dlbcl36 whose median age was 66 years. These patients had received a median of 3 prior lines of therapy, 65% had refractory disease, and 26% had undergone transplantation. The orr in the group was 43%, and 19% of the patients achieved a cr, with a median dor of 11.6 months. After a median follow-up of 15.0 months, the median pfs in the 21 patients evaluable for efficacy was 3.7 months. For all 25 patients, the median os was 5.0 months, with a median follow-up of 11.7 months. The most frequent aes included tremor (48%), pyrexia (44%), and fatigue (26%). Grade 3 neurologic events included encephalopathy and aphasia (9% each), and tremor, speech disorder, dizziness, somnolence, and disorientation (4% each).
An ongoing study is examining blinatumomab as second salvage therapy in patients with r/r nhl46. In 41 patients evaluated (68% with refractory disease), median age was 56 years. Preliminary results demonstrated an orr of 37% and a complete metabolic response rate of 22%. After a median follow-up of 8.8 months, 8 of 9 patients achieving a complete metabolic response were alive without relapse. Grade 3 or greater aes were reported in 59% of the patients, with 56% experiencing neurologic events of any grade. Although those results are encouraging, the rates of neurotoxicity and the prolonged continuous infusion required for this drug could limit its uptake.
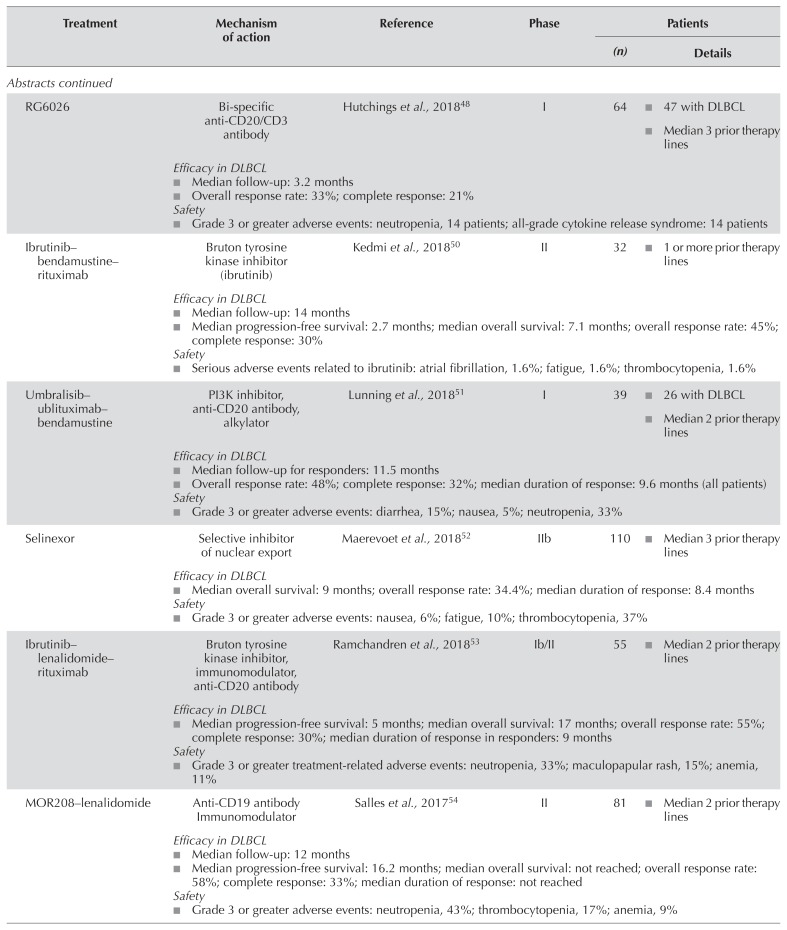
Mosunetuzumab, regn1979, and rg6026 are bi-specific CD20/CD3 antibodies that redirect cytotoxicity of endogenous T cells against malignant B cells by simultaneously binding to CD3 on T cells and to CD20 on B cells44,45,48. A phase i trial examining mosunetuzumab in patients with r/r transformed follicular lymphoma or dlbcl is ongoing45. In 55 patients evaluated in a preliminary analysis, median age is 64 years, patients have received a median of 3 prior lines of therapy, 71% are refractory to last therapy, and 24% have undergone asct. Preliminary results report orr and cr rates of 33% and 21% respectively. All patients achieving a cr remained in remission after a median follow-up of 12.2 months. A second ongoing phase i trial is examining regn1979 in patients with r/r dlbcl44. Of 30 patients treated with a median of 3 prior therapies, 76% are refractory to their last therapy, and 11% have received prior asct. Preliminary results in those patients demonstrated an orr of 40%, all responses being partial. After a median follow-up of 2.8 months, the median dor is 1.6 months. A third ongoing phase i trial is examining rg6026 in patients with r/r nhl48. In 64 evaluable patients, median age is 64 years, 61% are men, and patients have received a median of 3 prior lines of therapy. Preliminary results have demonstrated orr and cr rates of 33% and 21% respectively in patients with r/r aggressive nhl. The most common treatment-related ae with all 3 of the foregoing agents is cytokine release syndrome, but importantly, the rate of neurologic complications is very low44,45,48.
CAR-T Therapy
In car-t therapy, autologous genetically engineered T cells designed to express chimeric antigen receptors are used to target specific antigens18. Introduction of the new gene occurs through viral transfection, using either a retrovirus or a lentivirus66. The targeting domain of the car is a single-chain variable antibody fragment capable of targeting an antigen on a tumour cell. A number of car-t therapies targeting CD19, a cell-surface molecule present in most B cell leukemias and lymphomas, have been examined in patients with r/r dlbcl (Table II). In pivotal trials, the car-t agents tisagenlecleucel, axicabtagene ciloleucel, and lisocabtagene maraleucel have demonstrated orr and cr rates in the ranges 52%–83% and 40–58% respectively. Although long-term follow-up data are not yet available, the median dor reported in the zuma-1 trial was 11.1 months, with 37% of patients remaining in cr at a follow-up of 27.1 months. The dor was related to depth of response, with the dor being longer in patients who obtained a cr than in those who had an objective or partial response.
The car-t therapies are associated with a number of unique toxicities: studies show rates of grade 3 or greater cytokine release syndrome and neurologic toxicity in the ranges of 1%–22% and 12%–32% respectively24,25,56,59. Two reports have examined the efficacy and safety of car-t therapy in the real-world setting, demonstrating promising results, with comparable rates of cytokine release syndrome and neurotoxicity24,57,58. Based on results from the pivotal trials, Health Canada has approved tisagenlecleucel67 and axicabtagene ciloleucel68 for the treatment of patients with r/r dlbcl after 2 or more lines of systemic therapy.
CANADIAN PERSPECTIVE
Patients with dlbcl for whom initial therapy fails have limited treatment options, ranging from supportive care to conventional salvage therapy and asct, with the choice of therapy depending on age and comorbidities. No national guidelines in this setting have been developed, and treatment is often individualized. However, the Alberta guideline provides a list of recommended salvage regimens, including dhap (dexamethasone–cisplatin–cytarabine), ice (ifosfamide–carboplatin–etoposide), gdp (gemcitabine–dexamethasone–cisplatin), cepp (cyclophosphamide–etoposide–prednisone–procarbazine), and mep (mitomycin C–etoposide–cisplatin)12. Of the salvage therapies, gdp is most commonly used69 because it can be given on an outpatient basis; and in a randomized comparison, it demonstrated a favourable toxicity profile when compared with dhap12,29. The addition of rituximab to salvage therapies has shown some benefit, and that agent can be given in this setting where provincial funding allows70.
Patients who are not candidates for asct are usually treated with palliative intent and could receive sequential single- or multi-agent therapy depending on tolerance. Involved-field radiotherapy has a limited role in patients with r/r dlbcl, although it can be useful to treat symptomatic sites, depending on the location and burden of the disease. Notably, in patients who do not respond to standard salvage therapy or who relapse after high-dose therapy or asct, few treatment options are available. In such cases, clinical trials are highly recommended when feasible. Regardless of the approach, outcomes remain poor27, and it is crucial that patients have access to effective novel therapeutic regimens.
The development of car-t therapy has opened up a novel and promising approach for r/r dlbcl. However, further follow-up is needed to determine its long-term potential and late toxicities. Moreover, in some studies, patients with rapid progression would likely not have been eligible, and therefore selection bias might exist59. Importantly, a number of logistical and practical barriers must be overcome before this costly therapy can be routinely offered in Canada. Currently, car-t therapy is manufactured in a centralized system in the United States, with production taking an average of 10–21 days71. Subsequently, the product has to be shipped through Canadian customs to reach infusion centres. Given the aggressive nature of r/r dlbcl, patients with uncontrollable disease might be unable to wait the required length of time. Such patients might need bridging therapy, the success of which might be limited in these highly refractory patients.
Access to car-t therapy in Canada currently remains limited, with some centres having access to clinical trials and small numbers of patients being treated in U.S. centres. For example, some patients have been referred for treatment in Seattle and Boston. In Alberta, a phase ib/ii trial currently under development will examine the feasibility and cost of car-t therapy, with the manufacturing taking place in Edmonton and Calgary. A larger national trial with Canadian-developed products is also planned. Because of the infrastructure and expertise required for delivery of therapy and management of potential toxicities, car-t therapy will likely be available only at select academic centres. As a result, travel constraints, resource limitations, and provincial funding restrictions could limit the number of patients who ultimately have access. Finally, most patients currently receiving car-t therapy will experience disease progression and will require further treatment.
Given limited treatment options and overall dismal outcomes, r/r dlbcl remains a significant unmet medical need. The aggressive nature and chemotherapy refractoriness of the disease necessitates the development of novel therapies with unique mechanisms of action for major impact. A number of novel agents examined in phase i/ii studies have shown promise and, compared with current palliative options, might prolong response (Table I). However, the efficacy of these agents as monotherapies remains limited and ongoing studies of combination therapy are underway. For example, ibrutinib32 and lenalidomide38 have both demonstrated some utility in patients with abc dlbcl, but the associated dor is short, and therefore novel combinations are being evaluated. Bi-specific antibodies harness the immune system and are showing significant promise similar to that with car-t therapy, but without the need for cellular manipulation. The continuous infusion route of administration for blinatumomab could limit its uptake, but novel constructs with easier administration such as mosunetuzumab, regn1979, and rg6026 are under development.
Of the combination regimens, polatuzumab vedotin–br; ibrutinib–lenalidomide plus rituximab; and mor208–lenalidomide are furthest into development and have demonstrated promising efficacy42,53,54,60. Of those regimens, polatuzumab vedotin–br might be the first to receive approval from the U.S. Food and Drug Administration based on impressive results showing a clinically meaningful improvement in os42,60. On its own, br has demonstrated modest activity in r/r dlbcl and is generally well tolerated; it might therefore provide a reasonable backbone for combination regimens72,73. Given the demonstrated os benefit of adding polatuzumab vedotin to br, a phase iii trial using br as a comparator is no longer feasible. Ultimately, head-to-head trials of novel combinations to assess their comparative effectiveness in r/r dlbcl would be helpful. Data related to quality of life will also be important to help guide selection of therapy for the purpose of palliation. However, the ultimate goal is to identify effective combinations that can extend survival and improve the chance of cure.
CONCLUSIONS
Patients with r/r dlbcl who are ineligible for asct or who relapse after transplantation have a poor prognosis and are in need of effective treatment approaches27. Outcomes with car-t therapy are encouraging and require further follow-up to determine long-term benefit and toxicities. However, travel constraints, resource limitations, and provincial funding restrictions could limit accessibility. Novel agents that are well tolerated and that can extend survival are needed. However, the efficacy of novel therapies as single agents remains limited. Ongoing studies of novel combination regimens appear more promising and will likely lead to additional treatment options in the near future.
ACKNOWLEDGMENTS
The authors acknowledge support from Hoffmann–La Roche Canada Inc. for the development of this article. Medical writing assistance was provided by Anna Christofides of impact Medicom Inc.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: Medical writing support provided by Anna Christofides of impact Medicom Inc. was funded by Hoffmann–La Roche Canada Inc. SA has received honoraria for consulting purposes from Janssen, Gilead, and Hoffmann–La Roche. DM has received honoraria for consulting purposes from Hoffmann–La Roche/Genentech, AbbVie, Amgen, AstraZeneca, Celgene, Gilead, Janssen, Lundbeck, Merck, and Seattle Genetics. AP has received honoraria for consulting purposes from AstraZeneca and AbbVie. RS has received honoraria for consulting purposes from Pfizer, Boehringer Ingelheim, AstraZeneca, Hoffmann–La Roche/Genentech, Lundbeck, Eli Lilly, Bristol–Myers Squibb, Merck, AbbVie, Novartis, and Takeda. BAM is an employee of Hoffmann–La Roche Canada Inc. LHS has received honoraria for consulting purposes from Hoffmann–La Roche/Genentech, AbbVie, Amgen, Apobiologix, AstraZeneca, Acerta Pharma, Celgene, Gilead, Janssen, Kite, Karyopharm, Lundbeck, Merck, MorphoSys, Seattle Genetics, Teva, Takeda, TG Therapeutics, and Versatem Oncology. She has also received research funding from Hoffmann–La Roche/Genentech. PS has no conflicts to disclose.
REFERENCES
- 1.Lymphoma Canada. Non-Hodgkin Lymphoma [Web page] Mississauga, ON: Lymphoma Canada; n.d.. [Available at: https://www.lymphoma.ca/lymphoma/non-hodgkinlymphoma; cited 11 March 2019] [Google Scholar]
- 2.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018. Toronto, ON: Canadian Cancer Society; 2018. [Google Scholar]
- 3.Lymphoma Canada. Diffuse Large B cell Lymphoma (DLBCL) [Web page] Mississauga, ON: Lymphoma Canada; n.d.. [Available at: https://www.lymphoma.ca/DLBCL; cited 10 January 2019] [Google Scholar]
- 4.Anderson JR, Armitage JO, Weisenburger DD. Epidemiology of the non-Hodgkin’s lymphomas: distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann Oncol. 1998;9:717–20. doi: 10.1023/A:1008265532487. [DOI] [PubMed] [Google Scholar]
- 5.Sarkozy C, Sehn LH. Management of relapsed/refractory dlbcl. Best Pract Res Clin Haematol. 2018;31:209–16. doi: 10.1016/j.beha.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riedell PA, Smith SM. Double hit and double expressors in lymphoma: definition and treatment. Cancer. 2018;124:4622–32. doi: 10.1002/cncr.31646. [DOI] [PubMed] [Google Scholar]
- 8.Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined chop plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–33. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 9.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125:22–32. doi: 10.1182/blood-2014-05-577189. [DOI] [PubMed] [Google Scholar]
- 10.Crump M. Princess Margaret Cancer Centre Clinical Practice Guidelines: Lymphoma. Aggressive lymphoma. Toronto, ON: Princess Margaret Cancer Centre; 2013. [Available online at: https://www.uhn.ca/PrincessMargaret/Health_Professionals/Programs_Departments/Lymphoma_Myeloma/Documents/CPG_Lymphoma_AggressiveLymphoma.pdf; cited 10 January 2019] [Google Scholar]
- 11.Dudebout J. Cancer Centre of Southeastern Ontario Standard Management Guidelines. Aggressive B Non-Hodgkin’s Lymphoma. Kingston, ON: Queen’s University; 2016. [Google Scholar]
- 12.Alberta Health Services (ahs) Lymphoma. Clinical practice guideline lyhe-002. Edmonton, AB: ahs; 2018. [Available online at: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-lyhe002-lymphoma.pdf; cited 16 June 2019] [Google Scholar]
- 13.Cancer BC. Lymphoma, Chronic Leukemia, Myeloma [Web resource] Vancouver, BC: BC Cancer; n.d.. [Available at: http://www.bccancer.bc.ca/health-professionals/clinical-resources/cancer-management-guidelines/lymphoma-chronic-leukemiamyeloma; cited 15 March 2013] [Google Scholar]
- 14.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: B-Cell Lymphomas. Ver. 1.2019. Fort Washington, PA: nccn;; 2018. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf (free registration required); cited 15 March 2013. [Google Scholar]
- 15.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (dlbcl): esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v116–25. doi: 10.1093/annonc/mdv304. [DOI] [PubMed] [Google Scholar]
- 16.Coiffier B. Rituximab in the treatment of diffuse large B-cell lymphomas. Semin Oncol. 2002;29(suppl 2):30–5. doi: 10.1053/sonc.2002.30153. [DOI] [PubMed] [Google Scholar]
- 17.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the lnh-98.5 trial, the first randomized study comparing rituximab–chop to standard chop chemotherapy in dlbcl patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br J Haematol. 2018;182:633–43. doi: 10.1111/bjh.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vardhana SA, Sauter CS, Matasar MJ, et al. Outcomes of primary refractory diffuse large B-cell lymphoma (dlbcl) treated with salvage chemotherapy and intention to transplant in the rituximab era. Br J Haematol. 2017;176:591–9. doi: 10.1111/bjh.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arboe B, Olsen MH, Gorlov JS, et al. Treatment intensity and survival in patients with relapsed or refractory diffuse large B-cell lymphoma in Denmark: a real-life population-based study. Clin Epidemiol. 2019;11:207–16. doi: 10.2147/CLEP.S178003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitz F, Connors JM, Gascoyne RD, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after r-chop treatment. Ann Hematol. 2015;94:1839–43. doi: 10.1007/s00277-015-2467-z. [DOI] [PubMed] [Google Scholar]
- 22.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rovira J, Valera A, Colomo L, et al. Prognosis of patients with diffuse large B cell lymphoma not reaching complete response or relapsing after frontline chemotherapy or immunochemotherapy. Ann Hematol. 2015;94:803–12. doi: 10.1007/s00277-014-2271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neelapu SS, Ghobadi A, Jacobson CA, et al. 2-Year follow-up and high-risk subset analysis of zuma-1, the pivotal study of axicabtagene ciloleucel (Axi-Cel) in patients with refractory large B cell lymphoma [abstract] Blood. 2018;132(suppl 1):2967. [Google Scholar]
- 25.Abramson JS, Siddiqi T, Palomba ML, et al. High durable cr rates and preliminary safety profile for JCAR017 in r/r aggressive B-nhl (transcend nhl 001 Study): a defined composition CD19-directed car T-cell product with potential for outpatient administration [abstract 120] J Clin Oncol. 2018;36(suppl) [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.5_suppl.120; cited 16 June 2019] [Google Scholar]
- 26.Schuster SJ, Bishop MR, Tam CS, et al. on behalf of the juliet investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 27.Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international scholar-1 study. Blood. 2017;130:1800–8. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaganti S, Illidge T, Barrington S, et al. on behalf of the British Committee for Standards in Haematology. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol. 2016;174:43–56. doi: 10.1111/bjh.14136. [DOI] [PubMed] [Google Scholar]
- 29.Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: ncic-ctg ly.12. J Clin Oncol. 2014;32:3490–6. doi: 10.1200/JCO.2013.53.9593. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen ED, Sharman JP, Oki Y, et al. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory dlbcl with variable CD30 expression. Blood. 2015;125:1394–402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- 31.Palanca-Wessels MC, Czuczman M, Salles G, et al. Safety and activity of the anti-CD79B antibody–drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16:704–15. doi: 10.1016/S1470-2045(15)70128-2. [DOI] [PubMed] [Google Scholar]
- 32.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922–6. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Assouline SE, Nielsen TH, Yu S, et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood. 2016;128:185–94. doi: 10.1182/blood-2016-02-699520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coiffier B, Thieblemont C, de Guibert S, et al. A phase ii, single-arm, multicentre study of coltuximab ravtansine (sar3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol. 2016;173:722–30. doi: 10.1111/bjh.13992. [DOI] [PubMed] [Google Scholar]
- 35.Walter HS, Rule SA, Dyer MJS, et al. A phase 1 clinical trial of the selective btk inhibitor ONO/GS-4059 in relapsed and refractory mature B-cell malignancies. Blood. 2016;127:411–19. doi: 10.1182/blood-2015-08-664086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viardot A, Goebeler ME, Hess G, et al. Phase 2 study of the bispecific T-cell engager (bite) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016;127:1410–16. doi: 10.1182/blood-2015-06-651380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Advani RH, Lebovic D, Chen A, et al. Phase i study of the anti-CD22 antibody–drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2017;23:1167–76. doi: 10.1158/1078-0432.CCR-16-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czuczman MS, Trneny M, Davies A, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2017;23:4127–37. doi: 10.1158/1078-0432.CCR-16-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood. 2017;129:3175–83. doi: 10.1182/blood-2016-11-750174. [DOI] [PubMed] [Google Scholar]
- 40.Oki Y, Kelly KR, Flinn I, et al. cudc-907 in relapsed/refractory diffuse large B-cell lymphoma, including patients with MYC-alterations: results from an expanded phase i trial. Haematologica. 2017;102:1923–30. doi: 10.3324/haematol.2017.172882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuruvilla J, Crump M, Villa D, et al. Canadian Cancer Trials Group (cctg) ly.17: a randomized phase ii study evaluating novel salvage therapy pre-autologous stem cell transplant (asct) in relapsed/refractory diffuse large B cell lymphoma (r/r-dlbcl)—outcome of ibrutinib + r-gdp [abstract] Hematol Oncol. 2017;35(suppl) doi: 10.1002/hon.2437_76. [Available online at: https://onlinelibrary.wiley.com/doi/10.1002/hon.2437_76; cited 16 June 2019] [DOI] [Google Scholar]
- 42.Sehn LH, Herrera AF, Matasar MJ, et al. Addition of polatuzumab vedotin to bendamustine and rituximab (br) improves outcomes in transplant-ineligible patients with relapsed/refractory (r/r) diffuse large B-cell lymphoma (dlbcl) versus br alone: results from a randomized phase 2 study [abstract 2821] Blood. 2017;130(suppl 1) [Available online at: http://www.bloodjournal.org/content/130/Suppl_1/2821; cited 16 June 2019] [Google Scholar]
- 43.Sehn LH, Herrera AF, Matasar MJ, et al. Polatuzumab vedotin (Pola) plus bendamustine (b) with rituximab (r) or obinutuzumab (g) in relapsed/refractory (r/r) diffuse large B-cell lymphoma (dlbcl): updated results of a phase (Ph) ib/ii study [abstract 1683] Blood. 2018;132(suppl) [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/1683; cited 16 June 2019] [Google Scholar]
- 44.Bannerji R, Arnason J, Advani R, et al. Emerging clinical activity of regn1979, an anti-CD20 × anti-CD3 bispecific antibody, in patients with relapsed/refractory follicular lymphoma (fl), diffuse large B-cell lymphoma (dlbcl), and other B-cell non-Hodgkin lymphoma (B-nhl) subtypes [abstract 1690] Blood. 2018;132(suppl 1) [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/1690; cited 16 June 2019] [Google Scholar]
- 45.Budde LE, Sehn LH, Assouline S, et al. Mosunetuzumab, a full-length bispecific CD20/CD3 antibody, displays clinical activity in relapsed/refractory B-cell non-Hodgkin lymphoma (nhl): interim safety and efficacy results from a phase 1 study [abstract 399] Blood. 2018;132(suppl 1) [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/399; cited 16 June 2019] [Google Scholar]
- 46.Coyle L, Morley NJ, Rambaldi A, et al. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma [abstract 400] Blood. 2018;132(suppl) doi: 10.1080/10428194.2020.1759055. [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/400; cited 16 June 2019] [DOI] [PubMed] [Google Scholar]
- 47.Dyer MJS, De Vos S, Ruan J, et al. Acalabrutinib monotherapy in patients (pts) with relapsed/refractory (r/r) diffuse large B-cell lymphoma (dlbcl) [abstract 7547] J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.15_suppl.7547. [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.7547; cited 16 June 2019] [DOI] [Google Scholar]
- 48.Hutchings M, Iacoboni G, Morschhauser F, et al. CD20-Tcb (rg6026), a novel “2:1” format T-cell–engaging bispecific antibody, induces complete remissions in relapsed/refractory B-cell non-Hodgkin’s lymphoma: preliminary results from a phase i first in human trial [abstract 226] Blood. 2018;132(suppl 1) doi: 10.1182/blood-2018-09-873596. [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/226; cited 16 June 2019] [DOI] [Google Scholar]
- 49.Jurczak W, Zinzani PL, Gaidano G, et al. Phase iia study of the CD19 antibody mor208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29:1266–72. doi: 10.1093/annonc/mdy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kedmi M, Ribakovsy E, Benjamini O, et al. Ibrutinib, bendamustine, rituximab combination for relapsed and refractory aggressive B cell lymphoma—interim analysis of phase ii clinical trial [abstract 4186] Blood. 2018;132 [Google Scholar]
- 51.Lunning MA, Bierman P, Bociek R, et al. Combination of umbralisib, ublituximab, and bendamustine is safe and highly active in patients with advanced diffuse large B-cell lymphoma and follicular lymphoma [abstract 4197] Blood. 2018;132(suppl 1) [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/4197; cited 16 June 2019] [Google Scholar]
- 52.Maerevoet M, Vermaat J, Canales MA, et al. Single agent oral selinexor demonstrates deep and durable responses in relapsed/refractory diffuse large B-cell lymphoma (dlbcl) in both gcb and non-gcb subtypes: the phase 2b Sadal Study [abstract 1677] Blood. 2018;132(suppl 1) [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/1677; cited 16 June 2019] [Google Scholar]
- 53.Ramchandren R, Johnson P, Ghosh N, et al. The ir regimen (ibrutinib, lenalidomide, and rituximab) is active with a manageable safety profile in patients with relapsed/refractory non-germinal center-like diffuse large B-cell lymphoma [abstract 402] Blood. 2018;132(suppl) [Available online at: https://biblio.ugent.be/publication/8618785; cited 16 June 2019] [Google Scholar]
- 54.Salles GA, Duell J, González-Barca E, et al. Single-arm phase ii study of mor208 combined with lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma: L-Mind [abstract 227] Blood. 2018;132(suppl) [Google Scholar]
- 55.Salles GA, Jurczak, Andorsky DJ, et al. Results of a phase 3 randomised multicenter study comparing pixantrone + rituximab with gemcitabine + rituximab in patients with relapsed aggressive B-cell non-Hodgkin lymphoma not eligible for stem cell transplantation [abstract 4189] Blood. 2018;132(suppl) [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/4189; cited 16 June 2019] [Google Scholar]
- 56.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (zuma-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobson CA, Hunter B, Armand P, et al. Axicabtagene ciloleucel in the real world: outcomes and predictors of response, resistance and toxicity [abstract 92] Blood. 2018;132(suppl 1) [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/92; cited 16 June 2019] [Google Scholar]
- 58.Nastoupil LJ, Jain MD, Spiegel JY, et al. Axicabtagene ciloleucel (Axi-cel) CD19 chimeric antigen receptor (car) T-cell therapy for relapsed/refractory large B-cell lymphoma: real world experience [abstract 91] Blood. 2018;132(suppl 1) doi: 10.1182/blood-2018-08-868117. [Available online at: http://www.bloodjournal.org/content/132/Suppl_1/91; cited 16 June 2019] [DOI] [Google Scholar]
- 59.Schuster SJ, Bishop MR, Tam CS, et al. on behalf of the juliet investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 60.Sehn LH, Kamdar M, Herrera AF, et al. Randomized phase 2 trial of polatuzumab vedotin (Pola) with bendamustine and rituximab (br) in relapsed/refractory (r/r) fl and dlbcl [abstract 7507] J Clin Oncol. 2018;36(suppl) doi: 10.1200/JCO.2018.36.15_suppl.7507. [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.7507; cited 16 June 2019] [DOI] [Google Scholar]
- 61.Maffei R, Fiorcari S, Martinelli S, Potenza L, Luppi M, Marasca R. Targeting neoplastic B cells and harnessing microenvironment: the “double face” of ibrutinib and idelalisib. J Hematol Oncol. 2015;8:60. doi: 10.1186/s13045-015-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winter AM, Landsburg DJ, Mato AR, et al. A multi-institutional outcomes analysis of patients with relapsed or refractory dlbcl treated with ibrutinib. Blood. 2017;130:1676–9. doi: 10.1182/blood-2017-05-786988. [DOI] [PubMed] [Google Scholar]
- 63.F. Hoffmann–La Roche Ltd. FDA grants priority review to Roche’s polatuzumab vedotin in previously treated aggressive lymphoma [Web media release] Basel, Switzerland: F. Hoffmann–La Roche Ltd; 2019. [Available at: https://www.roche.com/media/releases/med-cor-2019-02-19.htm; cited 20 February 2019] [Google Scholar]
- 64.European Medicines Agency (ema) Public Summary of Opinion on Orphan Designation: Polatuzumab Vedotin for the Treatment of Diffuse Large B-Cell Lymphoma. London, UK: ema; 2019. [Available online at: https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/18/2013-publicsummary-opinion-orphan-designation-polatuzumabvedotin-treatment-diffuse-large-b-cell_en.pdf; cited 20 February 2019] [Google Scholar]
- 65.Celgene Inc. Revlimid: lenalidomide capsules [product monograph] Mississauga, ON: Celgene; 2018. [Available online at: https://media.celgene.com/content/uploads/sites/23/Revlimid-Product_Monograph_-_English_Version.pdf; cited 16 June 2019] [Google Scholar]
- 66.Porter DL. Advances in car T-cell therapy for chronic lymphocytic leukemia. Clin Adv Hematol Oncol. 2018;16:118–20. [PubMed] [Google Scholar]
- 67.Novartis Pharmaceuticals Canada Inc. Kymriah: tisagenlecleucel [product monograph] Dorval, QC: Novartis Pharmaceuticals Canada; 2018. [Available online at: https://pdf.hres.ca/dpd_pm/00047188.PDF; cited 16 June 2019] [Google Scholar]
- 68.Marques Lopes J. Lymphoma News Today. Pensacola, FL: BioNews Services llc; 2019. Yescarta approved in Canada for adults with relapsed or refractory large B-cell lymphoma [Web article] [Web media outlet, https://lymphomanewstoday.com/] [Available at: https://lymphomanewstoday.com/2019/02/26/yescarta-approved-in-canada-for-adultswith-relapsed-or-refractory-large-b-cell-lymphoma; cited 25 April 2019] [Google Scholar]
- 69.Moccia AA, Hitz F, Hoskins P, et al. Gemcitabine, dexamethasone, and cisplatin (gdp) is an effective and well-tolerated out-patient salvage therapy for relapsed/refractory diffuse large B-cell lymphoma (dlbcl) and Hodgkin lymphoma (hl) [abstract 113] Blood. 2010;116 doi: 10.1080/10428194.2016.1193852. [Available online at: http://www.bloodjournal.org/content/116/21/113; cited 16 June 2019] [DOI] [PubMed] [Google Scholar]
- 70.Baetz T, Chen BE, Couban S, et al. Effect of the addition of rituximab to salvage chemotherapy prior to autologous stem cell transplant in aggressive CD20+ lymphoma: a cohort comparison from the ncic Clinical Trials Group Study ly.12. Leuk Lymphoma. 2017;58:64–9. doi: 10.1080/10428194.2016.1187274. [DOI] [PubMed] [Google Scholar]
- 71.Munshi PN, Ujjani C. The acceleration of car-t therapy in non-Hodgkin lymphoma. Hematol Oncol. 2018 doi: 10.1002/hon.2568. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Vacirca JL, Acs PI, Tabbara IA, Rosen PJ, Lee P, Lynam E. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B cell lymphoma. Ann Hematol. 2014;93:403–9. doi: 10.1007/s00277-013-1879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arcari A, Chiappella A, Spina M, et al. Safety and efficacy of rituximab plus bendamustine in relapsed or refractory diffuse large B-cell lymphoma patients: an Italian retrospective multicenter study. Leuk Lymphoma. 2016;57:1823–30. doi: 10.3109/10428194.2015.1106536. [DOI] [PubMed] [Google Scholar]